The Flavonoid Naringenin Alleviates Collagen-Induced Arthritis through Curbing the Migration and Polarization of CD4+ T Lymphocyte Driven by Regulating Mitochondrial Fission
Abstract
1. Introduction
2. Results
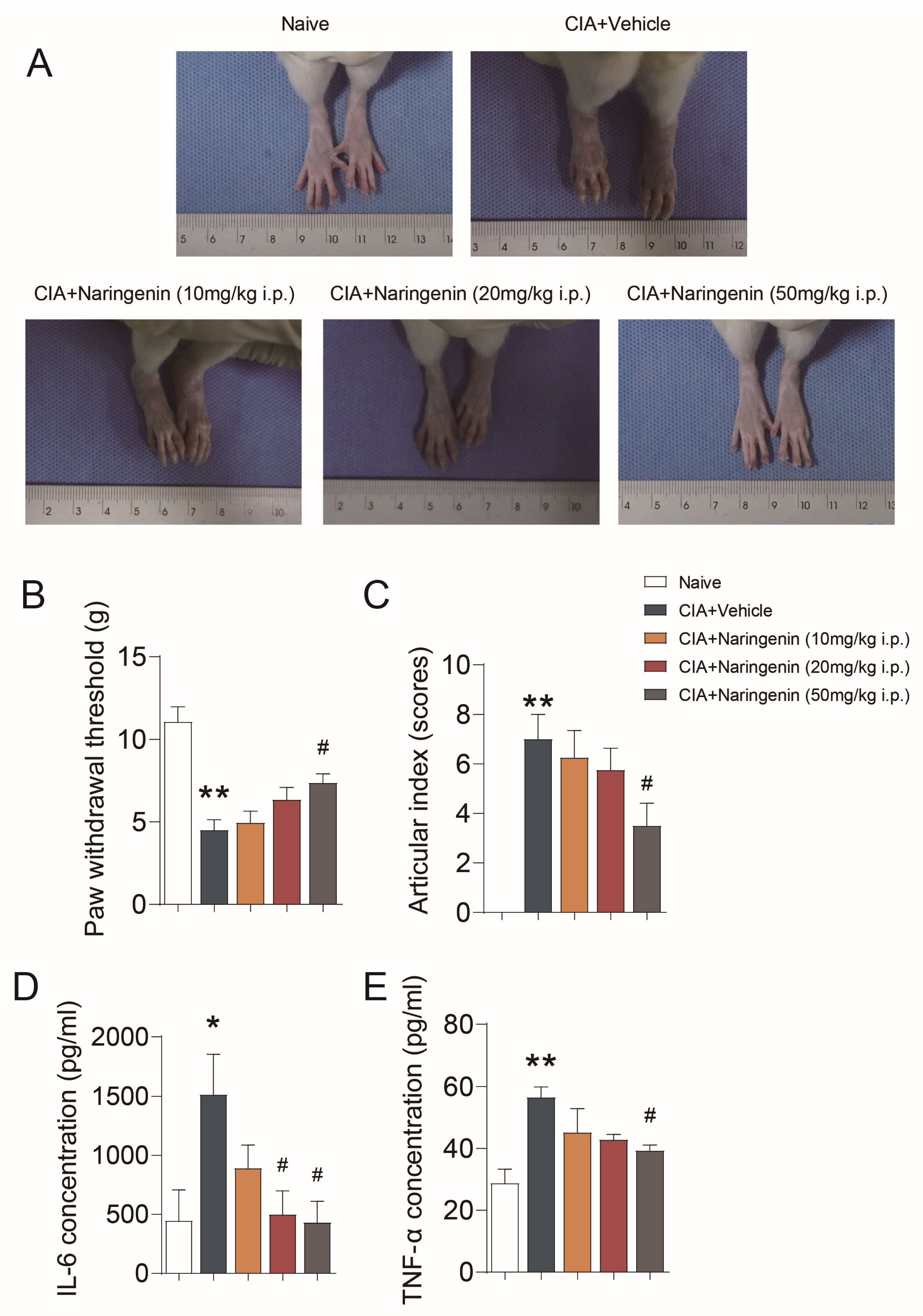
2.1. NAR Improved Pain-like Behaviors and Inflammation in CIA Model Rats
2.2. NAR Alleviated the Periarticular Inflammation Scores and Synovial Infiltration of CD4+ T Lymphocytes
2.3. NAR Ameliorated CD4+ T Lymphocyte Polarization in the Spleen of CIA Model Rats
2.4. NAR Affected the Mitochondrial Distribution in Spleen CD4+ T Cells
2.5. NAR Improved Mitochondrial Fission of CD4+ T Lymphocytes
2.6. NAR Inhibited the Polarization and Migration of Primary CD4+ T Lymphocytes In Vitro by Interfering with Mitochondrial Fission
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Model Preparation and Animal Grouping
- Establishment of a rat model of collagen-induced arthritis (CIA): The rats were fed adaptively until 8 weeks of age, and the model of collagen-induced arthritis was started. A solution of Bovine Type II Collagen-glacial acetic acid at a concentration of 4 mg/mL was prepared in advance and put in the refrigerator at 4 °C under light. The next day, the emulsion (2 mg/mL) was obtained by 1:1 mixing the solution of Bovine Type II Collagen-glacial acetic acid with FIA on ice using a high-speed agitator. The primary immunization was performed on day 0 by subcutaneous injection (200 μL/rat) 3 cm from the tail root of the rats. The second immunization was performed on day 7 by subcutaneous injection (100 μL/rat) 2 cm from the tail root of the rats.
- Establishment of a mouse model of the CIA: The preparation process of Bovine-Type II collagen-glacial acetic acid solution was the same as that of rats. The next day, a high-speed agitator was used to emulsify the Bovine-Type II collagen-glacial acetic acid solution with Freund’s complete adjuvant (FCA) (4 mg/mL) and Freund’s incomplete adjuvant (FIA) at a ratio of 1:1 (FCA was used for the primary immunization, the secondary immunization used FIA) to obtain emulsion, and the final concentration of emulsion was 2 mg/mL. On day 0, the emulsion was injected into the tail of mice for primary immunization (100 μL/mouse), and on day 21, the emulsion was injected into different parts of the back of mice for secondary immunization (100 μL/mouse).
- Rats were divided into five groups: A naive group, a CIA+vehicle group, and CIA+NAR low (10 mg/kg), medium (20 mg/kg), and high dose (50 mg/kg) groups; eight rats per group. Rats were injected intraperitoneally with 40 mg/kg pentobarbital sodium to induce anesthesia and were sacrificed by CO2 (100% concentration, for 12 min) on day 42, and the peripheral blood and knee joints were harvested.
- Mice were divided into three groups: A naive group, a CIA + vehicle group and a CIA + NAR (50 mg/kg); 10 mice per group. The mice were sacrificed by CO2 (100% concentration, for 3 min) on day 42, and the spleen was harvested.
- Preparation and administration of NAR: According to Zhou’s method [59], NAR was dissolved in solvent (10% DMSO, 10% tween-80, 80% normal saline), and the doses of 10 mg/kg, 20 mg/kg, and 50 mg/kg, respectively, prepared for intraperitoneal injection before clinical use.
4.3. Mechanical Pain Threshold
4.4. Articular Index Scores
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Histologic Assessment of Arthritis
4.7. Assessment of Joint Infiltration by CD4+ T Lymphocytes
4.8. Magnetic Beads Sorting CD4+ T Lymphocytes
4.9. Evaluation of Spleen CD4+ T Lymphocyte Polarization
4.10. Mitochondrial Distribution and Fission of Spleen CD4+ T Lymphocytes
4.11. In Vitro Induction of Primary CD4+ T Lymphocytes
- Cell polarization induction: Twelve mice in the control group were selected. CD4+ T lymphocytes were isolated using MACS cell separation beads and plated at a density of 1.5 × 105 cells/well at 37 °C, 5% CO2 in 24-well plates. Finally, cells were incubated with CXCL12 (100 ng/mL) for 6 h at 37 °C [66].
- Cell grouping: CD4+ T lymphocytes were divided into the following four groups: CD4+ T lymphocyte, CD4+ T lymphocyte+CXCL12, CD4+ T lymphocyte + CXCL12 + Mdivi-1, and CD4+ T lymphocyte + CXCL12 + NAR. NAR treatment was applied overnight at 100 µM [59], while Mdivi-1 treatment was applied overnight at 50 µM, using 0.1% DMSO as vehicle control [67].
4.12. Cell Migration Assay
4.13. Detection of Protein Expression by Western Blot
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- McInnes, I.B.; Schett, G. Pathogenetic insights from the treatment of rheumatoid arthritis. Lancet 2017, 389, 2328–2337. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Cross, M.; Smith, E.; Hoy, D.; Carmona, L.; Wolfe, F.; Vos, T.; Williams, B.; Gabriel, S.; Lassere, M.; Johns, N.; et al. The global burden of rheumatoid arthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1316–1322. [Google Scholar] [CrossRef] [PubMed]
- Jacobelli, J.; Chmura, S.A.; Buxton, D.B.; Davis, M.M.; Krummel, M.F. A single class II myosin modulates T cell motility and stopping, but not synapse formation. Nat. Immunol. 2004, 5, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Dustin, M.L.; Bromley, S.K.; Kan, Z.; Peterson, D.A.; Unanue, E.R. Antigen receptor engagement delivers a stop signal to migrating T lymphocytes. Proc. Natl. Acad. Sci. USA 1997, 94, 3909–3913. [Google Scholar] [CrossRef]
- Thelen, M. Dancing to the tune of chemokines. Nat. Immunol. 2001, 2, 129–134. [Google Scholar] [CrossRef]
- Vita, A.A.; Aljobaily, H.; Lyons, D.O.; Pullen, N.A. Berberine Delays Onset of Collagen-Induced Arthritis through T Cell Suppression. Int. J. Mol. Sci. 2021, 22, 3522. [Google Scholar] [CrossRef]
- Broadley, I.; Pera, A.; Morrow, G.; Davies, K.A.; Kern, F. Expansions of Cytotoxic CD4(+)CD28(−) T Cells Drive Excess Cardiovascular Mortality in Rheumatoid Arthritis and Other Chronic Inflammatory Conditions and Are Triggered by CMV Infection. Front. Immunol. 2017, 8, 195. [Google Scholar] [CrossRef]
- Galligan, C.L.; Siebert, J.C.; Siminovitch, K.A.; Keystone, E.C.; Bykerk, V.; Perez, O.D.; Fish, E.N. Multiparameter phospho-flow analysis of lymphocytes in early rheumatoid arthritis: Implications for diagnosis and monitoring drug therapy. PLoS ONE 2009, 4, e6703. [Google Scholar] [CrossRef]
- Petrasca, A.; Phelan, J.J.; Ansboro, S.; Veale, D.J.; Fearon, U.; Fletcher, J.M. Targeting bioenergetics prevents CD4 T cell-mediated activation of synovial fibroblasts in rheumatoid arthritis. Rheumatology 2020, 59, 2816–2828. [Google Scholar] [CrossRef]
- Reynolds, G.; Gibbon, J.R.; Pratt, A.G.; Wood, M.J.; Coady, D.; Raftery, G.; Lorenzi, A.R.; Gray, A.; Filer, A.; Buckley, C.D.; et al. Synovial CD4+ T-cell-derived GM-CSF supports the differentiation of an inflammatory dendritic cell population in rheumatoid arthritis. Ann. Rheum. Dis. 2016, 75, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Pettit, A.R.; Ji, H.; von Stechow, D.; Müller, R.; Goldring, S.R.; Choi, Y.; Benoist, C.; Gravallese, E.M. TRANCE/RANKL knockout mice are protected from bone erosion in a serum transfer model of arthritis. Am. J. Pathol. 2001, 159, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Redlich, K.; Hayer, S.; Ricci, R.; David, J.P.; Tohidast-Akrad, M.; Kollias, G.; Steiner, G.; Smolen, J.S.; Wagner, E.F.; Schett, G. Osteoclasts are essential for TNF-alpha-mediated joint destruction. J. Clin. Investig. 2002, 110, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Ledderose, C.; Bromberger, S.; Slubowski, C.J.; Sueyoshi, K.; Aytan, D.; Shen, Y.; Junger, W.G. The purinergic receptor P2Y11 choreographs the polarization, mitochondrial metabolism, and migration of T lymphocytes. Sci. Signal. 2020, 13, eaba3300. [Google Scholar] [CrossRef]
- Lauffenburger, D.A.; Horwitz, A.F. Cell migration: A physically integrated molecular process. Cell 1996, 84, 359–369. [Google Scholar] [CrossRef]
- Ridley, A.J.; Schwartz, M.A.; Burridge, K.; Firtel, R.A.; Ginsberg, M.H.; Borisy, G.; Parsons, J.T.; Horwitz, A.R. Cell migration: Integrating signals from front to back. Science 2003, 302, 1704–1709. [Google Scholar] [CrossRef]
- Samstag, Y.; Eibert, S.M.; Klemke, M.; Wabnitz, G.H. Actin cytoskeletal dynamics in T lymphocyte activation and migration. J. Leukoc. Biol. 2003, 73, 30–48. [Google Scholar] [CrossRef]
- Kleele, T.; Rey, T.; Winter, J.; Zaganelli, S.; Mahecic, D.; Perreten, L.H.; Ruberto, F.P.; Nemir, M.; Wai, T.; Pedrazzini, T.; et al. Distinct fission signatures predict mitochondrial degradation or biogenesis. Nature 2021, 593, 435–439. [Google Scholar] [CrossRef]
- Toyama, E.Q.; Herzig, S.; Courchet, J.; Lewis, T.J.; Losón, O.C.; Hellberg, K.; Young, N.P.; Chen, H.; Polleux, F.; Chan, D.C.; et al. Metabolism. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science 2016, 351, 275–281. [Google Scholar] [CrossRef]
- Adebayo, M.; Singh, S.; Singh, A.P.; Dasgupta, S. Mitochondrial fusion and fission: The fine-tune balance for cellular homeostasis. FASEB J. 2021, 35, e21620. [Google Scholar] [CrossRef]
- Campello, S.; Lacalle, R.A.; Bettella, M.; Mañes, S.; Scorrano, L.; Viola, A. Orchestration of lymphocyte chemotaxis by mitochondrial dynamics. J. Exp. Med. 2006, 203, 2879–2886. [Google Scholar] [CrossRef] [PubMed]
- Dou, W.; Zhang, J.; Sun, A.; Zhang, E.; Ding, L.; Mukherjee, S.; Wei, X.; Chou, G.; Wang, Z.T.; Mani, S. Protective effect of naringenin against experimental colitis via suppression of Toll-like receptor 4/NF-κB signalling. Br. J. Nutr. 2013, 110, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Yan, N.; Wen, L.; Peng, R.; Li, H.; Liu, H.; Peng, H.; Sun, Y.; Wu, T.; Chen, L.; Duan, Q.; et al. Naringenin Ameliorated Kidney Injury through Let-7a/TGFBR1 Signaling in Diabetic Nephropathy. J. Diabetes Res. 2016, 2016, 8738760. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Jin, L.; Zhang, F.; Zhang, C.; Liang, W. Naringenin as a potential immunomodulator in therapeutics. Pharmacol. Res. 2018, 135, 122–126. [Google Scholar] [CrossRef]
- Ravishankar, D.; Rajora, A.K.; Greco, F.; Osborn, H.M. Flavonoids as prospective compounds for anti-cancer therapy. Int. J. Biochem. Cell Biol. 2013, 45, 2821–2831. [Google Scholar] [CrossRef]
- Jin, L.; Zeng, W.; Zhang, F.; Zhang, C.; Liang, W. Naringenin Ameliorates Acute Inflammation by Regulating Intracellular Cytokine Degradation. J. Immunol. 2017, 199, 3466–3477. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, G.; Guan, H.; Li, M.; Liu, Y.; Tian, B.; He, Z.; Fu, Q. Naringenin nanocrystals for improving anti-rheumatoid arthritis activity. Asian J. Pharm. Sci. 2021, 16, 816–825. [Google Scholar] [CrossRef]
- Hajizadeh, A.; Abtahi, F.S.; Tehrani, A.A.; Azizi, S.; Bani, H.S. Effects of Naringenin on Experimentally Induced Rheumatoid Arthritis in Wistar Rats. Arch. Razi Inst. 2021, 76, 903–912. [Google Scholar] [CrossRef]
- Li, Y.R.; Chen, D.Y.; Chu, C.L.; Li, S.; Chen, Y.K.; Wu, C.L.; Lin, C.C. Naringenin inhibits dendritic cell maturation and has therapeutic effects in a murine model of collagen-induced arthritis. J. Nutr. Biochem. 2015, 26, 1467–1478. [Google Scholar] [CrossRef]
- Atik, N.; Kunii, M.; Avriyanti, E.; Furumoto, N.; Inami, K.; Yoshimura, S.; Harada, R.; Harada, A. The role of PKD in cell polarity, biosynthetic pathways, and organelle/F-actin distribution. Cell Struct. Funct. 2014, 39, 61–77. [Google Scholar] [CrossRef]
- Yu, Y.; Peng, X.D.; Qian, X.J.; Zhang, K.M.; Huang, X.; Chen, Y.H.; Li, Y.T.; Feng, G.K.; Zhang, H.L.; Xu, X.L.; et al. Fis1 phosphorylation by Met promotes mitochondrial fission and hepatocellular carcinoma metastasis. Signal Transduct. Target. Ther. 2021, 6, 401. [Google Scholar] [CrossRef] [PubMed]
- Ihenacho, U.K.; Meacham, K.A.; Harwig, M.C.; Widlansky, M.E.; Hill, R.B. Mitochondrial Fission Protein 1: Emerging Roles in Organellar Form and Function in Health and Disease. Front. Endocrinol. 2021, 12, 660095. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Tan, C.; Liu, C.; Chen, D. Mitochondrial fission mediated by Drp1-Fis1 pathway and neurodegenerative diseases. Rev. Neurosci. 2022. [Google Scholar] [CrossRef]
- Chemin, K.; Gerstner, C.; Malmström, V. Effector Functions of CD4+ T Cells at the Site of Local Autoimmune Inflammation-Lessons From Rheumatoid Arthritis. Front. Immunol. 2019, 10, 353. [Google Scholar] [CrossRef] [PubMed]
- Guderud, K.; Sunde, L.H.; Flåm, S.T.; Mæhlen, M.T.; Mjaavatten, M.D.; Lillegraven, S.; Aga, A.B.; Evenrød, I.M.; Norli, E.S.; Andreassen, B.K.; et al. Rheumatoid Arthritis Patients, Both Newly Diagnosed and Methotrexate Treated, Show More DNA Methylation Differences in CD4(+) Memory Than in CD4(+) Naïve T Cells. Front. Immunol. 2020, 11, 194. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Monticelli, S. The many faces of CD4 T cells: Roles in immunity and disease. Semin. Immunol. 2013, 25, 249–251. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; McIlraith, M.; Davis, L.S.; Lipsky, P.E. Rheumatoid synovium is enriched in CD45RBdim mature memory T cells that are potent helpers for B cell differentiation. Arthritis Rheum. 1992, 35, 1455–1465. [Google Scholar] [CrossRef]
- Klarenbeek, P.L.; de Hair, M.J.; Doorenspleet, M.E.; van Schaik, B.D.; Esveldt, R.E.; van de Sande, M.G.; Cantaert, T.; Gerlag, D.M.; Baeten, D.; van Kampen, A.H.; et al. Inflamed target tissue provides a specific niche for highly expanded T-cell clones in early human autoimmune disease. Ann. Rheum. Dis. 2012, 71, 1088–1093. [Google Scholar] [CrossRef]
- Xiong, G.; Lei, T.; Dong, S.; Xu, L.; Li, M.; Wang, R. Roles of CD3, CD4 and CD8 in synovial lymphocytes of rheumatoid arthritis. Pol. J. Pathol. 2022, 73, 21–26. [Google Scholar] [CrossRef]
- Dejaco, C.; Duftner, C.; Klauser, A.; Schirmer, M. Altered T-cell subtypes in spondyloarthritis, rheumatoid arthritis and polymyalgia rheumatica. Rheumatol. Int. 2010, 30, 297–303. [Google Scholar] [CrossRef]
- Krummel, M.F.; Bartumeus, F.; Gérard, A. T cell migration, search strategies and mechanisms. Nat. Rev. Immunol. 2016, 16, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Groom, J.R. Regulators of T-cell fate: Integration of cell migration, differentiation and function. Immunol. Rev. 2019, 289, 101–114. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Paul, W.E. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science 2010, 327, 1098–1102. [Google Scholar] [CrossRef] [PubMed]
- Ley, K.; Laudanna, C.; Cybulsky, M.I.; Nourshargh, S. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007, 7, 678–689. [Google Scholar] [CrossRef]
- Sánchez-Madrid, F.; Del, P.M. Leukocyte polarization in cell migration and immune interactions. EMBO J. 1999, 18, 501–511. [Google Scholar] [CrossRef]
- Ledderose, C.; Liu, K.; Kondo, Y.; Slubowski, C.J.; Dertnig, T.; Denicoló, S.; Arbab, M.; Hubner, J.; Konrad, K.; Fakhari, M.; et al. Purinergic P2X4 receptors and mitochondrial ATP production regulate T cell migration. J. Clin. Investig. 2018, 128, 3583–3594. [Google Scholar] [CrossRef]
- Ma, L.; Li, Y.; Peng, J.; Wu, D.; Zhao, X.; Cui, Y.; Chen, L.; Yan, X.; Du, Y.; Yu, L. Discovery of the migrasome, an organelle mediating release of cytoplasmic contents during cell migration. Cell Res. 2015, 25, 24–38. [Google Scholar] [CrossRef]
- Ramírez-Santiago, G.; Robles-Valero, J.; Morlino, G.; Cruz-Adalia, A.; Pérez-Martínez, M.; Zaldivar, A.; Torres-Torresano, M.; Chichón, F.J.; Sorrentino, A.; Pereiro, E.; et al. Clathrin regulates lymphocyte migration by driving actin accumulation at the cellular leading edge. Eur. J. Immunol. 2016, 46, 2376–2387. [Google Scholar] [CrossRef]
- Williams, H.P.; Harwood, A.J. Cell polarity and Dictyostelium development. Curr. Opin. Microbiol. 2003, 6, 621–627. [Google Scholar] [CrossRef]
- Simula, L.; Nazio, F.; Campello, S. The mitochondrial dynamics in cancer and immune-surveillance. Semin. Cancer Biol. 2017, 47, 29–42. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Shangguan, X.; Zhou, W.; Cao, Y.; Zheng, Q.; Tu, J.; Hu, G.; Liang, Z.; Jiang, C.; Deng, L.; et al. Glucose limitation activates AMPK coupled SENP1-Sirt3 signalling in mitochondria for T cell memory development. Nat. Commun. 2021, 12, 4371. [Google Scholar] [CrossRef] [PubMed]
- Uzhachenko, R.; Shanker, A.; Dupont, G. Computational properties of mitochondria in T cell activation and fate. Open Biol. 2016, 6, 160192. [Google Scholar] [CrossRef] [PubMed]
- Simula, L.; Pacella, I.; Colamatteo, A.; Procaccini, C.; Cancila, V.; Bordi, M.; Tregnago, C.; Corrado, M.; Pigazzi, M.; Barnaba, V.; et al. Drp1 Controls Effective T Cell Immune-Surveillance by Regulating T Cell Migration, Proliferation, and cMyc-Dependent Metabolic Reprogramming. Cell Rep. 2018, 25, 3059–3073. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Lendahl, U.; Nistér, M.; Zhao, J. Regulation of Mammalian Mitochondrial Dynamics: Opportunities and Challenges. Front. Endocrinol. 2020, 11, 374. [Google Scholar] [CrossRef] [PubMed]
- Downey, C. Serious infection during etanercept, infliximab and adalimumab therapy for rheumatoid arthritis: A literature review. Int. J. Rheum. Dis. 2016, 19, 536–550. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Huang, Y.; Xiao, S.; Sun, X.; Fan, Y.; Zhang, Z. Impact of Janus kinase inhibitors on risk of cardiovascular events in patients with rheumatoid arthritis: Systematic review and meta-analysis of randomised controlled trials. Ann. Rheum. Dis. 2019, 78, 1048–1054. [Google Scholar] [CrossRef]
- Liu, Z.; Niu, X.; Wang, J. Naringenin as a natural immunomodulator against T cell-mediated autoimmune diseases: Literature review and network-based pharmacology study. Crit. Rev. Food Sci. Nutr. 2022, 1–18. [Google Scholar] [CrossRef]
- Niu, X.; Sang, H.; Wang, J. Naringenin attenuates experimental autoimmune encephalomyelitis by protecting the intact of blood-brain barrier and controlling inflammatory cell migration. J. Nutr. Biochem. 2021, 89, 108560. [Google Scholar] [CrossRef]
- Zhou, Y.; Cai, S.; Moutal, A.; Yu, J.; Gómez, K.; Madura, C.L.; Shan, Z.; Pham, N.; Serafini, M.J.; Dorame, A.; et al. The Natural Flavonoid Naringenin Elicits Analgesia through Inhibition of NaV1.8 Voltage-Gated Sodium Channels. ACS Chem. Neurosci. 2019, 10, 4834–4846. [Google Scholar] [CrossRef]
- Bonin, R.P.; Bories, C.; De Koninck, Y. A simplified up-down method (SUDO) for measuring mechanical nociception in rodents using von Frey filaments. Mol. Pain 2014, 10, 26. [Google Scholar] [CrossRef]
- Woods, J.M.; Katschke, K.J.; Volin, M.V.; Ruth, J.H.; Woodruff, D.C.; Amin, M.A.; Connors, M.A.; Kurata, H.; Arai, K.; Haines, G.K.; et al. IL-4 adenoviral gene therapy reduces inflammation, proinflammatory cytokines, vascularization, and bony destruction in rat adjuvant-induced arthritis. J. Immunol. 2001, 166, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.O.; Feldmann, M.; Maini, R.N. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc. Natl. Acad. Sci. USA 1992, 89, 9784–9788. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Huang, W.; Han, L.; Qian, K.; Du, Y.; Zeng, L.; Lin, C. Prevention and treatment of Duanteng Yimu Decoction on collagen-induced arthritis model mice. Tradit. Chin. Drug Res. Pharmacol. 2020, 1, 48–53. [Google Scholar]
- Flaherty, S.; Reynolds, J.M. Mouse Naïve CD4+ T Cell Isolation and In vitro Differentiation into T Cell Subsets. J. Vis. Exp. 2015, 52739. [Google Scholar] [CrossRef]
- Acuto, O.; Cantrell, D. T cell activation and the cytoskeleton. Annu. Rev. Immunol. 2000, 18, 165–184. [Google Scholar] [CrossRef]
- Layseca-Espinosa, E.; Baranda, L.; Alvarado-Sánchez, B.; Portales-Pérez, D.; Portillo-Salazar, H.; González-Amaro, R. Rolipram inhibits polarization and migration of human T lymphocytes. J. Investig. Dermatol. 2003, 121, 81–87. [Google Scholar] [CrossRef]
- Wu, Q.; Gao, C.; Wang, H.; Zhang, X.; Li, Q.; Gu, Z.; Shi, X.; Cui, Y.; Wang, T.; Chen, X.; et al. Mdivi-1 alleviates blood-brain barrier disruption and cell death in experimental traumatic brain injury by mitigating autophagy dysfunction and mitophagy activation. Int. J. Biochem. Cell Biol. 2018, 94, 44–55. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.-P.; Wen, J.-J.; Zhao, X.-X.; Gao, Y.-C.; Ma, X.; Song, S.-Y.; Jin, Y.; Shao, T.-J.; Yu, J.; Wen, C.-P. The Flavonoid Naringenin Alleviates Collagen-Induced Arthritis through Curbing the Migration and Polarization of CD4+ T Lymphocyte Driven by Regulating Mitochondrial Fission. Int. J. Mol. Sci. 2023, 24, 279. https://doi.org/10.3390/ijms24010279
Jiang Y-P, Wen J-J, Zhao X-X, Gao Y-C, Ma X, Song S-Y, Jin Y, Shao T-J, Yu J, Wen C-P. The Flavonoid Naringenin Alleviates Collagen-Induced Arthritis through Curbing the Migration and Polarization of CD4+ T Lymphocyte Driven by Regulating Mitochondrial Fission. International Journal of Molecular Sciences. 2023; 24(1):279. https://doi.org/10.3390/ijms24010279
Chicago/Turabian StyleJiang, Yue-Peng, Jun-Jun Wen, Xiao-Xuan Zhao, Yuan-Cheng Gao, Xiao Ma, Si-Yue Song, Yan Jin, Tie-Juan Shao, Jie Yu, and Cheng-Ping Wen. 2023. "The Flavonoid Naringenin Alleviates Collagen-Induced Arthritis through Curbing the Migration and Polarization of CD4+ T Lymphocyte Driven by Regulating Mitochondrial Fission" International Journal of Molecular Sciences 24, no. 1: 279. https://doi.org/10.3390/ijms24010279
APA StyleJiang, Y.-P., Wen, J.-J., Zhao, X.-X., Gao, Y.-C., Ma, X., Song, S.-Y., Jin, Y., Shao, T.-J., Yu, J., & Wen, C.-P. (2023). The Flavonoid Naringenin Alleviates Collagen-Induced Arthritis through Curbing the Migration and Polarization of CD4+ T Lymphocyte Driven by Regulating Mitochondrial Fission. International Journal of Molecular Sciences, 24(1), 279. https://doi.org/10.3390/ijms24010279





