Microcapsule-Based Dose-Dependent Regulation of the Lifespan and Behavior of Adipose-Derived MSCs as a Cell-Mediated Delivery System: In Vitro Study
Abstract
1. Introduction
2. Results
2.1. Estimation of the Spreading and Uptake Capacity of hAMSCs during Phagocytosis of FITC-Labeled Microcapsules
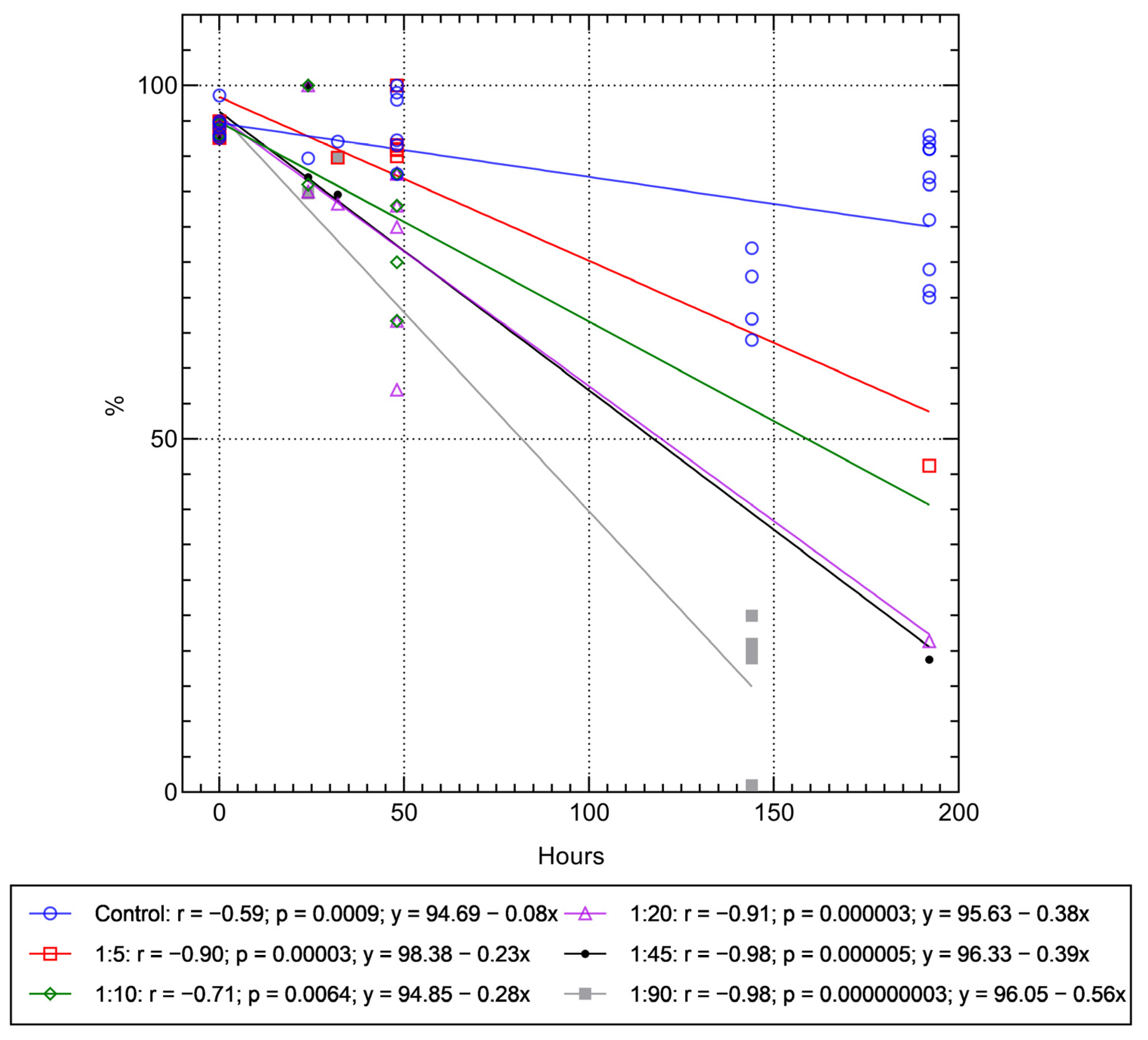
2.2. Assessment of Viability of hAMSCs Loaded with FITC-Labeled Microcapsules during Cultivation after Phagocytosis
2.3. Cell-IQ Monitoring Mobility and Division Rate of hAMSCs Loaded with FITC-Labeled Microcapsules
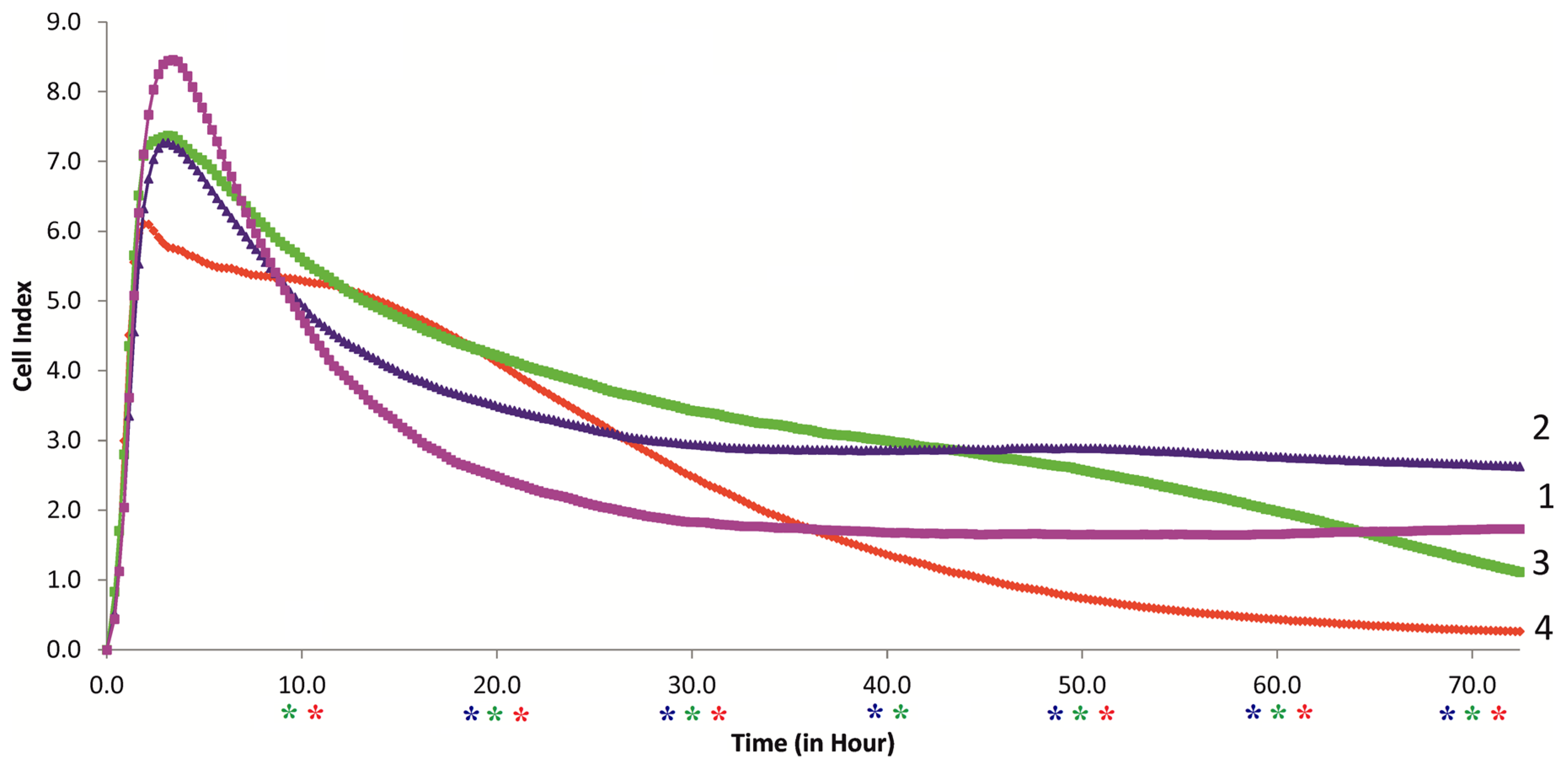
2.4. RTCA Monitoring of the Behavior of hAMSCs Loaded with FITC-Labeled Microcapsules, and Their Secretory Activity
3. Discussion
- There is no direct relationship between the amplitude and sign of the surface charge of particles and their internalization by MSCs, in contrast to some tumor lines (e.g., HeLa, Jurkat) [43] and healthy (U937 macrophages and HL-60 neutrophils) cells [39]. This suggests the presence of other non-electrostatic uptake mechanisms in MSCs;
- The initial zeta potentials of the outermost layer capsules of PAH and PSS (+10.13 mV and −17 mV, respectively) become weakly negative (−5.5 and −8.97 mV, respectively) after introduction into the culture medium [40].
4. Materials and Methods
4.1. Materials
4.2. Isolation and Cultivation of Human Adipose-Derived MSCs
4.3. Synthesis of Microcapsules
4.4. Analysis of Cell Viability and Chemokine Secretion in Response to Microcapsule Ingestion
4.5. Cell-IQ Visualization of Microcapsule Internalization, Cell Motility, and Division
4.6. RTCA Monitoring of MSC Behavior
4.7. Statistical Analysis
5. Conclusions
- hAMSCs internalize all (PAH-PSS)6 microcapsules present in the intercellular environment, with the number of particles per cell ranging from 5 to 90.
- Strong (r > 0.7) linear, dose- and time-dependent (up to 8 days) regression was observed between the in vitro decrease in cell viability and the number of microvesicles absorbed (5–90 microcapsules per cell). According to the regression equations, the approximate time-to-complete-death of hAMSCs at different concentrations of microcapsules in culture can be 428 h (1:5 ratio), 339 h (1:10), 252 h (1:20), 247 h (1:45), and 170 h (1:90 ratio).
- By varying the number of microcontainers loaded into the cells (from 1:10 to 1:90), a dose-dependent exponential decrease in both the movement rate (y = 68.39e−0.474x; R2 = 0.99) and the division rate of hAMSCs (y = 12.25e−1.65x; R2 = 0.88) was observed with high coefficients of determination. At a concentration of 90 capsules per cell, the hAMSCs hardly moved or divided on the real-time phase contrast display of Cell-IQ.
- RTCA monitoring of the effect of PAH-PSS microvesicles (from 1:5 to 1:20) on hAMSCs also showed a dose- and time-dependent decrease in cell longevity after a 50 h study, at ratios of 1:10 and 1:20.
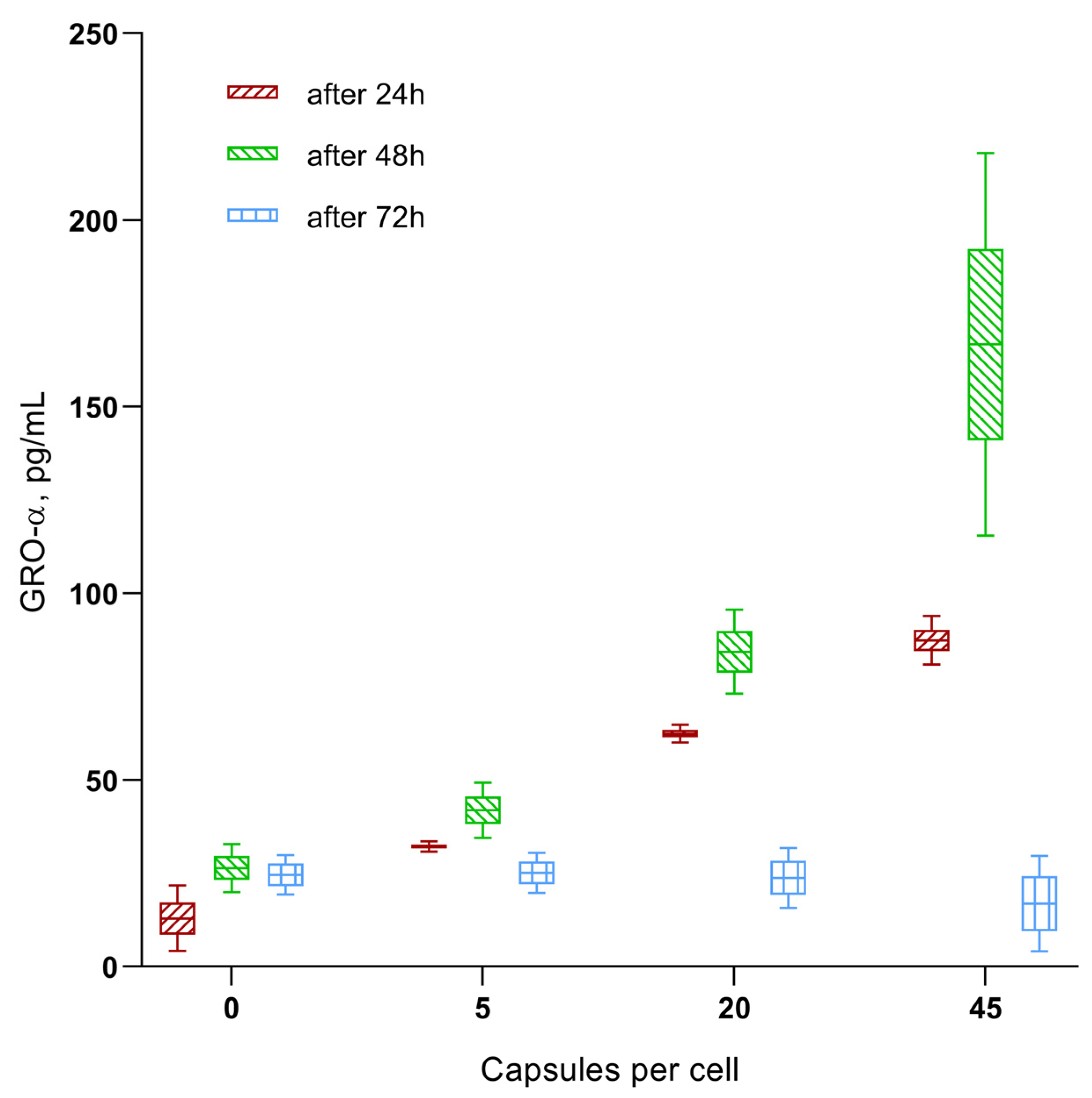
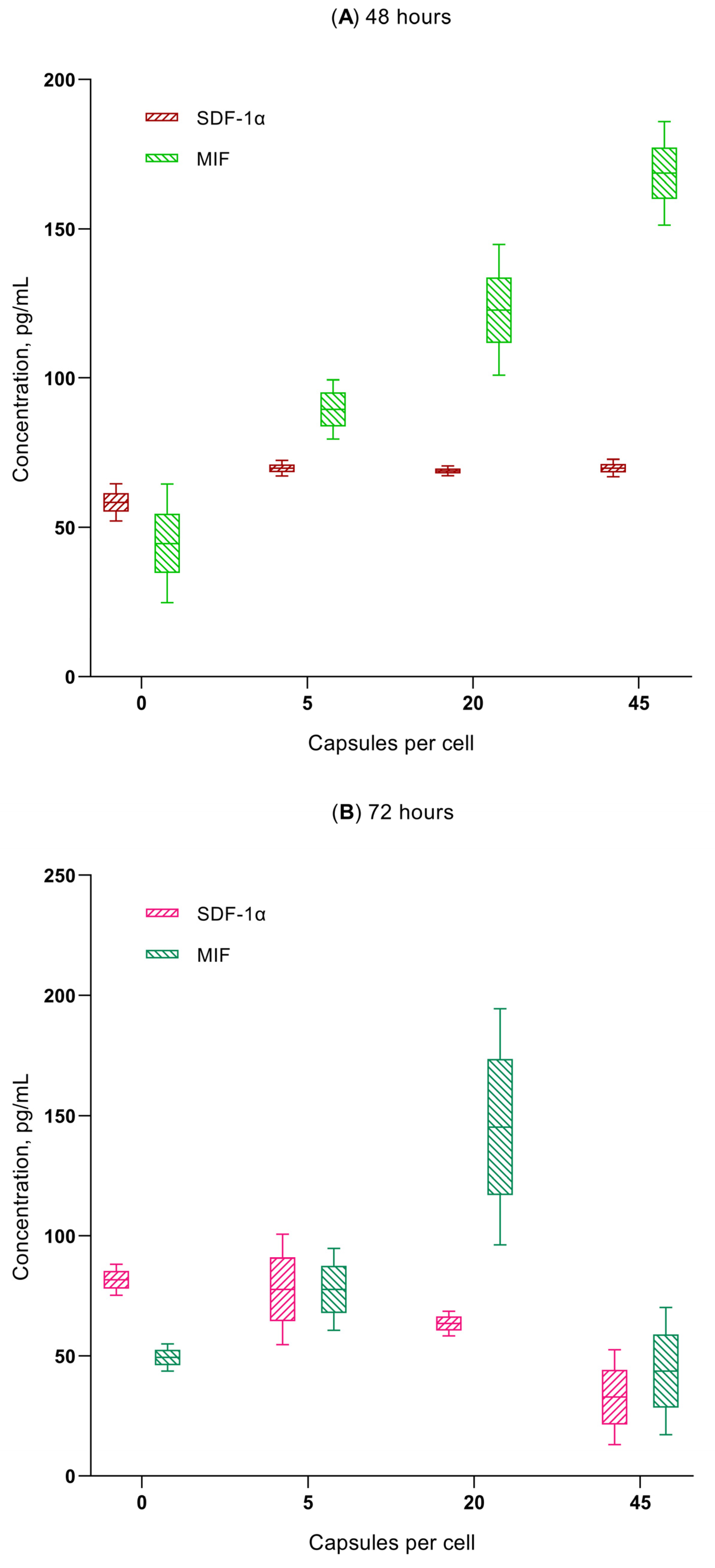
- Microcapsule uptake (1:5, 1:20, and 1:45) results in a dose-dependent (up to 0.18–0.2 ng/mL) increase in secretion of the chemokines GRO-α (CXCL1), MIF, and SDF-1α (CXCL12) in hAMSCs culture, which are capable of stimulating the activity of both stem and tumor cells (see Discussion). This is classified as average (0.1–1 ng/mL) secretory activity according to [73].
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banskota, S.; Yousefpour, P.; Chilkoti, A. Cell-Based Biohybrid Drug Delivery Systems: The Best of the Synthetic and Natural Worlds. Macromol. Biosci. 2017, 17, 1600361. [Google Scholar] [CrossRef] [PubMed]
- Schrepfer, S.; Deuse, T.; Reichenspurner, H.; Fischbein, M.; Robbins, R.; Pelletier, M. Stem Cell Transplantation: The Lung Barrier. Transplant. Proc. 2007, 39, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Wu, T.; Zhang, D.; Zhang, Z. Cell or Cell Membrane-Based Drug Delivery Systems. Theranostics 2015, 5, 863–881. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Su, J.; Liu, G.; Chen, J.; Zhang, X.; Zhang, R.; Jiang, M.; Qiu, M. Advances of blood cell-based drug delivery systems. Eur. J. Pharm. Sci. 2017, 96, 115–128. [Google Scholar] [CrossRef]
- Timin, A.; Litvak, M.M.; Gorin, D.; Atochina-Vasserman, E.N.; Atochin, D.; Sukhorukov, G.B. Cell-Based Drug Delivery and Use of Nano-and Microcarriers for Cell Functionalization. Adv. Health Mater. 2018, 7, 1700818. [Google Scholar] [CrossRef]
- Li, L.; Guan, Y.; Liu, H.; Hao, N.; Liu, T.; Meng, X.; Fu, C.; Li, Y.; Qu, Q.; Zhang, Y.; et al. Silica Nanorattle–Doxorubicin-Anchored Mesenchymal Stem Cells for Tumor-Tropic Therapy. ACS Nano 2011, 5, 7462–7470. [Google Scholar] [CrossRef]
- Litvinova, L.S.; Shupletsova, V.V.; Khaziakhmatova, O.G.; Daminova, A.G.; Kudryavtseva, V.L.; Yurova, K.A.; Malashchenko, V.V.; Todosenko, N.M.; Popova, V.; Litvinov, R.I.; et al. Human Mesenchymal Stem Cells as a Carrier for a Cell-Mediated Drug Delivery. Front. Bioeng. Biotechnol. 2022, 10, 796111. [Google Scholar] [CrossRef]
- Cheng, S.; Nethi, S.K.; Rathi, S.; Layek, B.; Prabha, S. Engineered Mesenchymal Stem Cells for Targeting Solid Tumors: Therapeutic Potential beyond Regenerative Therapy. J. Pharmacol. Exp. Ther. 2019, 370, 231–241. [Google Scholar] [CrossRef]
- Aboody, K.S.; Najbauer, J.; Danks, M.K. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther. 2008, 15, 739–752. [Google Scholar] [CrossRef]
- Lang, T.; Yin, Q.; Li, Y. Progress of Cell-Derived Biomimetic Drug Delivery Systems for Cancer Therapy. Adv. Ther. 2018, 1, 1800053. [Google Scholar] [CrossRef]
- Jiang, W.; Xu, J. Immune modulation by mesenchymal stem cells. Cell Prolif. 2020, 53, e12712. [Google Scholar] [CrossRef] [PubMed]
- Morandi, F.; Raffaghello, L.; Bianchi, G.; Meloni, F.; Salis, A.; Millo, E.; Ferrone, S.; Barnaba, V.; Pistoia, V. Immunogenicity of Human Mesenchymal Stem Cells in HLA-Class I-Restricted T-Cell Responses Against Viral or Tumor-Associated Antigens. Stem Cells 2008, 26, 1275–1287. [Google Scholar] [CrossRef] [PubMed]
- Krueger, T.E.G.; Thorek, D.L.J.; Denmeade, S.R.; Isaacs, J.T.; Brennen, W.N. Concise Review: Mesenchymal Stem Cell-Based Drug Delivery: The Good, the Bad, the Ugly, and the Promise. Stem Cells Transl. Med. 2018, 7, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Timin, A.S.; Peltek, O.O.; Zyuzin, M.V.; Muslimov, A.R.; Karpov, T.E.; Epifanovskaya, O.S.; Shakirova, A.I.; Zhukov, M.V.; Tarakanchikova, Y.V.; Lepik, K.V.; et al. Safe and Effective Delivery of Antitumor Drug Using Mesenchymal Stem Cells Impregnated with Submicron Carriers. ACS Appl. Mater. Interfaces 2019, 11, 13091–13104. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.-J.; Moniri, M.R.; Zeng, Z.-R.; Zhou, J.X.; Rayat, J.; Warnock, G.L. Potential implications of mesenchymal stem cells in cancer therapy. Cancer Lett. 2011, 305, 8–20. [Google Scholar] [CrossRef]
- Karnoub, A.E.; Dash, A.B.; Vo, A.P.; Sullivan, A.; Brooks, M.W.; Bell, G.W.; Richardson, A.L.; Polyak, K.; Tubo, R.; Weinberg, R.A. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 2007, 449, 557–563. [Google Scholar] [CrossRef]
- Kooreman, N.G.; Wu, J.C. Tumorigenicity of pluripotent stem cells: Biological insights from molecular imaging. J. R. Soc. Interface 2010, 7, S753–S763. [Google Scholar] [CrossRef]
- Kim, H.; Shin, K.; Park, O.K.; Choi, D.; Kim, H.D.; Baik, S.; Lee, S.H.; Kwon, S.-H.; Yarema, K.J.; Hong, J.; et al. General and Facile Coating of Single Cells via Mild Reduction. J. Am. Chem. Soc. 2018, 140, 1199–1202. [Google Scholar] [CrossRef]
- Furman, N.E.T.; Lupu-Haber, Y.; Bronshtein, T.; Kaneti, L.; Letko, N.; Weinstein, E.; Baruch, L.; Machluf, M. Reconstructed Stem Cell Nanoghosts: A Natural Tumor Targeting Platform. Nano Lett. 2013, 13, 3248–3255. [Google Scholar] [CrossRef]
- Lepik, K.V.; Muslimov, A.R.; Timin, A.S.; Sergeev, V.S.; Romanyuk, D.S.; Moiseev, I.S.; Popova, E.V.; Radchenko, I.L.; Vilesov, A.D.; Galibin, O.V.; et al. Mesenchymal Stem Cell Magnetization: Magnetic Multilayer Microcapsule Uptake, Toxicity, Impact on Functional Properties, and Perspectives for Magnetic Delivery. Adv. Health Mater. 2016, 5, 3182–3190. [Google Scholar] [CrossRef]
- Son, D.; Choi, T.; Yeo, H.; Kim, J.; Han, K. The Effect of Centrifugation Condition on Mature Adipocytes and Adipose Stem Cell Viability. Ann. Plast. Surg. 2014, 72, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Hoareau, L.; Bencharif, K.; Girard, A.-C.; Gence, L.; Delarue, P.; Hulard, O.; Festy, F.; Roche, R. Effect of centrifugation and washing on adipose graft viability: A new method to improve graft efficiency. J. Plast. Reconstr. Aesthetic Surg. 2013, 66, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Kho, D.; MacDonald, C.; Johnson, R.; Unsworth, C.P.; O’Carroll, S.J.; Du Mez, E.; Angel, C.E.; Graham, E.S. Application of xCELLigence RTCA Biosensor Technology for Revealing the Profile and Window of Drug Responsiveness in Real Time. Biosensors 2015, 5, 199–222. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L.M.; Widder, M.W.; McAleer, M.K.; Mayo, M.W.; Greis, A.P.; van der Schalie, W.H. Preparation and Testing of Impedance-based Fluidic Biochips with RTgill-W1 Cells for Rapid Evaluation of Drinking Water Samples for Toxicity. J. Vis. Exp. 2016, 109, e53555. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Suryaprakash, S.; Lao, Y.-H.; Leong, K.W. Engineering mesenchymal stem cells for regenerative medicine and drug delivery. Methods 2015, 84, 3–16. [Google Scholar] [CrossRef]
- Timin, A.S.; Muslimov, A.R.; Lepik, K.V.; Saprykina, N.N.; Sergeev, V.S.; Afanasyev, B.V.; Vilesov, A.D.; Sukhorukov, G.B. Triple-responsive inorganic–organic hybrid microcapsules as a biocompatible smart platform for the delivery of small molecules. J. Mater. Chem. B 2016, 4, 7270–7282. [Google Scholar] [CrossRef]
- Sherman, L.S.; Shaker, M.; Mariotti, V.; Rameshwar, P. Mesenchymal stromal/stem cells in drug therapy: New perspective. Cytotherapy 2017, 19, 19–27. [Google Scholar] [CrossRef]
- Brini, A.T.; Coccè, V.; Ferreira, L.M.J.; Giannasi, C.; Cossellu, G.; Giannì, A.B.; Angiero, F.; Bonomi, A.; Pascucci, L.; Falchetti, M.L.; et al. Cell-mediated drug delivery by gingival interdental papilla mesenchymal stromal cells (GinPa-MSCs) loaded with paclitaxel. Expert Opin. Drug Deliv. 2016, 13, 789–798. [Google Scholar] [CrossRef]
- Lenna, S.; Bellotti, C.; Duchi, S.; Martella, E.; Columbaro, M.; Dozza, B.; Ballestri, M.; Guerrini, A.; Sotgiu, G.; Frisoni, T.; et al. Mesenchymal stromal cells mediated delivery of photoactive nanoparticles inhibits osteosarcoma growth in vitro and in a murine in vivo ectopic model. J. Exp. Clin. Cancer Res. 2020, 39, 1–15. [Google Scholar] [CrossRef]
- Ouyang, S.; Zhang, Y.; Yao, S.; Feng, L.; Li, P.; Zhu, S. The efficiency of MSC-based targeted AIE nanoparticles for gastric cancer diagnosis and treatment: An experimental study. Bioeng. Transl. Med. 2021, 7, e10278. [Google Scholar] [CrossRef]
- Balducci, L.; Blasi, A.; Saldarelli, M.; Soleti, A.; Pessina, A.; Bonomi, A.; Coccè, V.; Dossena, M.; Tosetti, V.; Ceserani, V.; et al. Immortalization of human adipose-derived stromal cells: Production of cell lines with high growth rate, mesenchymal marker expression and capability to secrete high levels of angiogenic factors. Stem Cell Res. Ther. 2014, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Cocce, V.; Balducci, L.; Falchetti, M.L.; Pascucci, L.; Ciusani, E.; Brini, A.T.; Sisto, F.; Piovani, G.; Alessandri, G.; Parati, E.; et al. Fluorescent Immortalized Human Adipose Derived Stromal Cells (hASCs-TS/GFP+) for Studying Cell Drug Delivery Mediated by Microvesicles. Anti-Cancer Agents Med. Chem. 2017, 17, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Nold, P.; Hartmann, R.; Feliu, N.; Kantner, K.; Gamal, M.; Pelaz, B.; Hühn, J.; Sun, X.; Jungebluth, P.; del Pino, P.; et al. Optimizing conditions for labeling of mesenchymal stromal cells (MSCs) with gold nanoparticles: A prerequisite for in vivo tracking of MSCs. J. Nanobiotechnol. 2017, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Timin, A.S.; Gould, D.J.; Sukhorukov, G.B. Multi-layer microcapsules: Fresh insights and new applications. Expert Opin. Drug Deliv. 2017, 14, 583–587. [Google Scholar] [CrossRef]
- Toma, C.; Wagner, W.; Bowry, S.; Schwartz, A.; Villanueva, F. Fate Of Culture-Expanded Mesenchymal Stem Cells in The Microvasculature: In vivo observations of cell kinetics. Circ. Res. 2009, 104, 398–402. [Google Scholar] [CrossRef]
- Carrillo-Carrion, C.; Bocanegra, A.I.; Arnaiz, B.; Feliu, N.; Zhu, D.; Parak, W.J. Triple-Labeling of Polymer-Coated Quantum Dots and Adsorbed Proteins for Tracing their Fate in Cell Cultures. ACS Nano 2019, 13, 4631–4639. [Google Scholar] [CrossRef]
- Gribova, V.; Auzely-Velty, R.; Picart, C. Polyelectrolyte Multilayer Assemblies on Materials Surfaces: From Cell Adhesion to Tissue Engineering. Chem. Mater. 2011, 24, 854–869. [Google Scholar] [CrossRef]
- Rammal, H.; Beroud, J.; Gentils, M.; Labrude, P.; Menu, P.; Kerdjoudj, H.; Velot, E. Reversing charges or how to improve Wharton’s jelly mesenchymal stem cells culture on polyelectrolyte multilayer films. Bio-Med. Mater. Eng. 2013, 23, 299–309. [Google Scholar] [CrossRef]
- Brueckner, M.; Jankuhn, S.; Jülke, E.-M.; Reibetanz, U. Cellular interaction of a layer-by-layer based drug delivery system depending on material properties and cell types. Int. J. Nanomed. 2018, 13, 2079–2091. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.; Bai, K.; Lin, M.; Sukhorukov, G.; Wang, W. Microcapsules functionalized with neuraminidase can enter vascular endothelial cells in vitro. J. R. Soc. Interface 2014, 11, 20141027. [Google Scholar] [CrossRef]
- An, Z.; Kavanoor, K.; Choy, M.L.; Kaufman, L.J. Polyelectrolyte microcapsule interactions with cells in two- and three-dimensional culture. Colloids Surf. B Biointerfaces 2009, 70, 114–123. [Google Scholar] [CrossRef]
- Pavlov, A.M.; De Geest, B.G.; Louage, B.; Lybaert, L.; De Koker, S.; Koudelka, Z.; Sapelkin, A.; Sukhorukov, G.B. Magnetically Engineered Microcapsules as Intracellular Anchors for Remote Control Over Cellular Mobility. Adv. Mater. 2013, 25, 6945–6950. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, M.R.; Holzapfel, V.; Musyanovych, A.; Nothelfer, K.; Walther, P.; Frank, H.; Landfester, K.; Schrezenmeier, H.; Mailänder, V. Uptake of functionalized, fluorescent-labeled polymeric particles in different cell lines and stem cells. Biomaterials 2006, 27, 2820–2828. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, L.; Jiang, C. Charge-reversal nanoparticles: Novel targeted drug delivery carriers. Acta Pharm. Sin. B 2016, 6, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Grumelli, D.E.; Garay, F.; Barbero, C.A.; Calvo, E.J. Dynamics of Ion Exchange between Self-assembled Redox Polyelectrolyte Multilayer Modified Electrode and Liquid Electrolyte. J. Phys. Chem. B 2006, 110, 15345–15352. [Google Scholar] [CrossRef]
- Hsu, S.-H.; Ho, T.-T.; Tseng, T.-C. Nanoparticle uptake and gene transfer efficiency for MSCs on chitosan and chitosan-hyaluronan substrates. Biomaterials 2012, 33, 3639–3650. [Google Scholar] [CrossRef]
- Raic, A.; Friedrich, F.; Kratzer, D.; Bieback, K.; Lahann, J.; Lee-Thedieck, C. Potential of electrospun cationic BSA fibers to guide osteogenic MSC differentiation via surface charge and fibrous topography. Sci. Rep. 2019, 9, 20003–20015. [Google Scholar] [CrossRef]
- Shrestha, S.; Jiang, P.; Sousa, M.H.; Morais, P.C.; Mao, Z.; Gao, C. Citrate-capped iron oxide nanoparticles impair the osteogenic differentiation potential of rat mesenchymal stem cells. J. Mater. Chem. B 2016, 4, 245–256. [Google Scholar] [CrossRef]
- Yun, W.S.; Aryal, S.; Ahn, Y.J.; Seo, Y.J.; Key, J. Engineered iron oxide nanoparticles to improve regenerative effects of mesenchymal stem cells. Biomed. Eng. Lett. 2020, 10, 259–273. [Google Scholar] [CrossRef]
- Hamdan, M.; Blanco, L.; Khraisat, A.; Tresguerres, I.F. Influence of Titanium Surface Charge on Fibroblast Adhesion. Clin. Implant Dent. Relat. Res. 2006, 8, 32–38. [Google Scholar] [CrossRef]
- Ferrier, J.; Ross, S.M.; Kanehisa, J.; Aubin, J.E. Osteoclasts and osteoblasts migrate in opposite directions in response to a constant electrical field. J. Cell. Physiol. 1986, 129, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Curtis, A.S.G. Surface modified superparamagnetic nanoparticles for drug delivery: Interaction studies with human fibroblasts in culture. J. Mater. Sci. Mater. Med. 2004, 15, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lan, C.Q. Effects of shear stress on microalgae—A review. Biotechnol. Adv. 2018, 36, 986–1002. [Google Scholar] [CrossRef] [PubMed]
- Alías, C.B.; López, M.G.-M.; Fernández, F.A.; Sevilla, J.F.; Sánchez, J.G.; Grima, E.M. Influence of power supply in the feasibility of Phaeodactylum tricornutum cultures. Biotechnol. Bioeng. 2004, 87, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Kim, J.H.; Lee, J.K.; Choi, S.J.; Kim, J.-S.; Jeun, S.-S.; Oh, W.; Yang, Y.S.; Chang, J.W. Overexpression of CXC Chemokine Receptors Is Required for the Superior Glioma-Tracking Property of Umbilical Cord Blood-Derived Mesenchymal Stem Cells. Stem Cells Dev. 2009, 18, 511–520. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, W.; He, H.; Fan, B.; Deng, R.; Hong, Y.; Liang, X.; Zhao, H.; Li, X.; Zhang, F. Macrophage migration inhibitory factor rejuvenates aged human mesenchymal stem cells and improves myocardial repair. Aging 2019, 11, 12641–12660. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Li, J.; Zhong, L.; Chen, X.; Chen, S. SDF-1 mediates mesenchymal stem cell recruitment and migration via the SDF-1/CXCR4 axis in bone defect. J. Bone Miner. Metab. 2020, 39, 126–138. [Google Scholar] [CrossRef]
- Wei, Z.-W.; Xia, G.-K.; Wu, Y.; Chen, W.; Xiang, Z.; Schwarz, R.E.; Brekken, R.A.; Awasthi, N.; He, Y.-L.; Zhang, C.-H. CXCL1 promotes tumor growth through VEGF pathway activation and is associated with inferior survival in gastric cancer. Cancer Lett. 2015, 359, 335–343. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, X.; Li, W.; Li, M.; Tu, T.; Ba, X.; Wu, Y.; Huang, Z.; Fan, G.; Zhou, G.; et al. Macrophage migration inhibitory factor promotes osteosarcoma growth and lung metastasis through activating the RAS/MAPK pathway. Cancer Lett. 2017, 403, 271–279. [Google Scholar] [CrossRef]
- Morein, D.; Erlichman, N.; Ben-Baruch, A. Beyond Cell Motility: The Expanding Roles of Chemokines and Their Receptors in Malignancy. Front. Immunol. 2020, 11, 952. [Google Scholar] [CrossRef]
- World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.I.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Khlusov, I.A.; Litvinova, L.S.; Shupletsova, V.V.; Khaziakhmatova, O.G.; Malashchenko, V.V.; Yurova, K.A.; Shunkin, E.O.; Krivosheev, V.V.; Porokhova, E.D.; Sizikova, A.E.; et al. Costimulatory Effect of Rough Calcium Phosphate Coating and Blood Mononuclear Cells on Adipose-Derived Mesenchymal Stem Cells In Vitro as a Model of In Vivo Tissue Repair. Materials 2020, 13, 4398. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Petrov, A.I.; Volodkin, D.V.; Sukhorukov, G.B. Protein-Calcium Carbonate Coprecipitation: A Tool for Protein Encapsulation. Biotechnol. Prog. 2005, 21, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.; Said, A.H.; Hartmann, R.; Assmann, M.S.M.; Feliu, N.; Lenz, P.; Parak, W.J. Quantitative Particle Uptake by Cells as Analyzed by Different Methods. Angew. Chem. Int. Ed. 2020, 59, 5438–5453. [Google Scholar] [CrossRef]
- Freshney, R.I. Primary Culture. In Culture of Animal Cells: A Manual of Basic Technique and Specialized Applications; Freshney, R.I., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 163–186. ISBN 978-0-470-52812-9. [Google Scholar]
- Hassanzadeh, A.; Altajer, A.H.; Rahman, H.S.; Saleh, M.M.; Bokov, D.O.; Abdelbasset, W.K.; Marofi, F.; Zamani, M.; Yaghoubi, Y.; Yazdanifar, M.; et al. Mesenchymal Stem/Stromal Cell-Based Delivery: A Rapidly Evolving Strategy for Cancer Therapy. Front. Cell Dev. Biol. 2021, 9, 686453. [Google Scholar] [CrossRef]
- Chiu, A.Y.; Rao, M.S. Cell-Based Therapy for Neural Disorders—Anticipating Challenges. Neurotherapeutics 2011, 8, 744–752. [Google Scholar] [CrossRef]
- Hu, Y.-L.; Huang, B.; Zhang, T.-Y.; Miao, P.-H.; Tang, G.-P.; Tabata, Y.; Gao, J.-Q. Mesenchymal Stem Cells as a Novel Carrier for Targeted Delivery of Gene in Cancer Therapy Based on Nonviral Transfection. Mol. Pharm. 2012, 9, 2698–2709. [Google Scholar] [CrossRef]
- Salmasi, Z.; Hashemi, M.; Mahdipour, E.; Nourani, H.; Abnous, K.; Ramezani, M. Mesenchymal stem cells engineered by modified polyethylenimine polymer for targeted cancer gene therapy, in vitro and in vivo. Biotechnol. Prog. 2020, 36, e3025. [Google Scholar] [CrossRef] [PubMed]
- van den Broek, L.J.; Kroeze, K.L.; Waaijman, T.; Breetveld, M.; Sampat-Sardjoepersad, S.C.; Niessen, F.B.; Middelkoop, E.; Scheper, R.J.; Gibbs, S. Differential Response of Human Adipose Tissue-Derived Mesenchymal Stem Cells, Dermal Fibroblasts, and Keratinocytes to Burn Wound Exudates: Potential Role of Skin-Specific Chemokine CCL27. Tissue Eng. Part A 2014, 20, 197–209. [Google Scholar] [CrossRef] [PubMed]
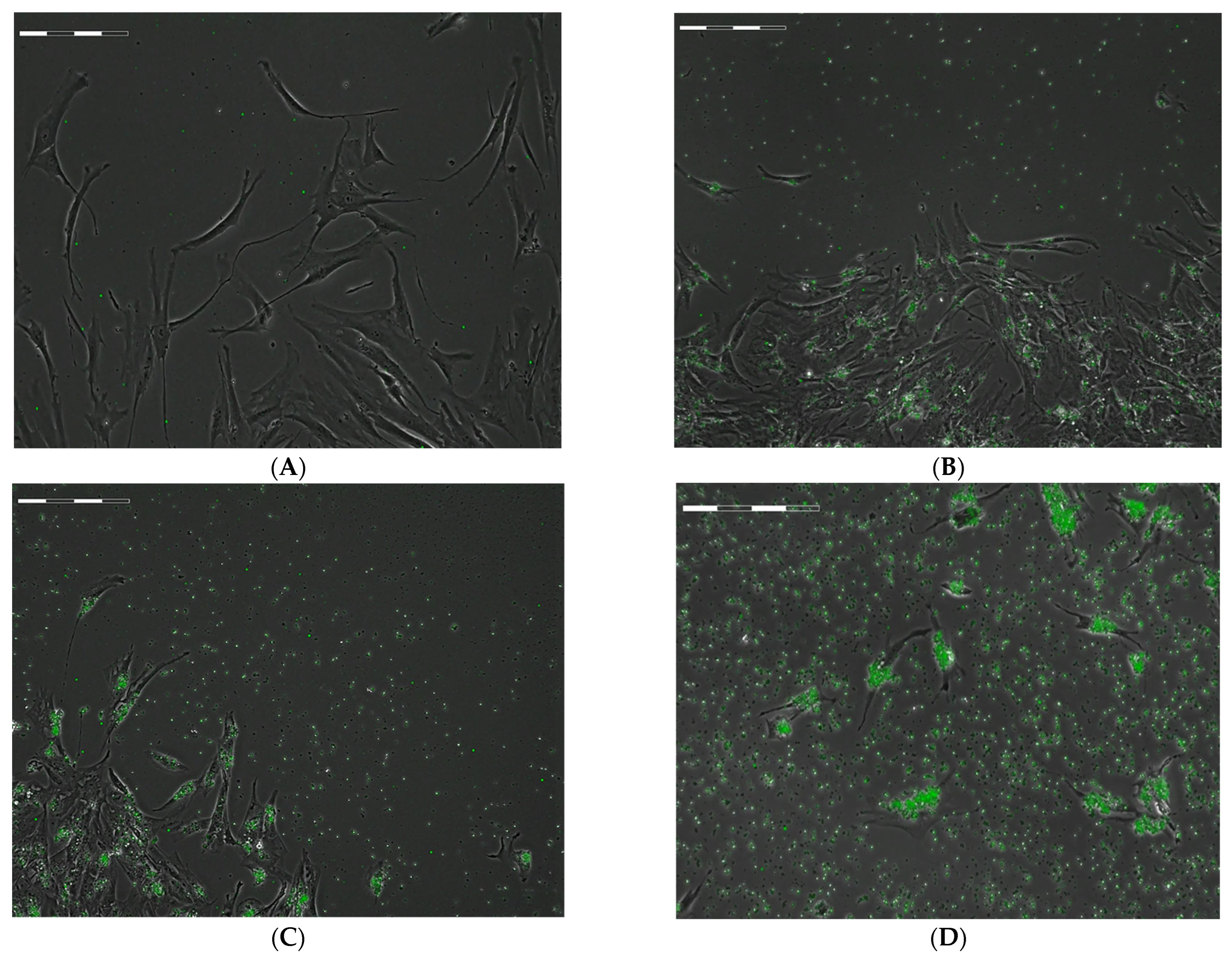
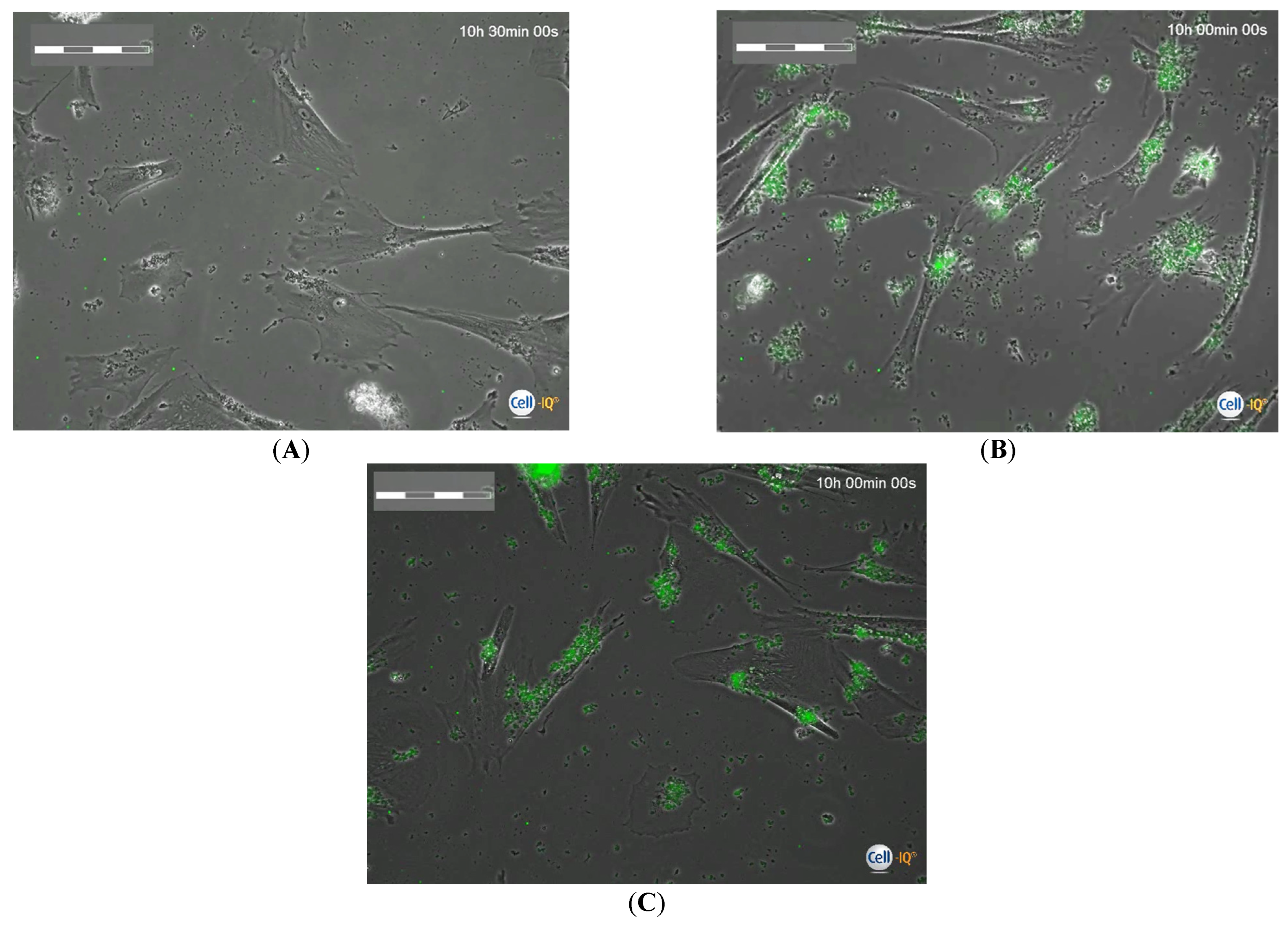
| Group Number | Calculated Number of Microcapsules in Intercellular Medium Per Cell, n = 3 | Cell Migration Rate, µm/Hour | Total Distance Moved by Cells, µm | Cell Division Rate Per 1 H of Observation | Time of First Cell Division after 24 H Phagocytosis, Hours | Time of Final Cell Division after 24 H Phagocytosis, Hours |
|---|---|---|---|---|---|---|
| 1 | Unloaded Control | 41.05 (36.26–48.9) n1 = 30 | 3352 (2782–3796) | 1.65 (1.48–1.77) n1 = 138 (126–144) | 7.48 (5.82–14.98) | 92.90 (92.90–92.90) |
| 2 | 1:10 | 27.52 (19.42–37.30) n1 = 50 P1 = 0.003 | 1738 (756–2806) | 0.27 (0.26–0.28) n1 = 17 (15–21) P1 < 0.05 | 28.73 (9.57–39.58) | 89.58 (87.92–92.50) P1 < 0.05 |
| 3 | 1:20 | 15.9 (10.61–22.36) n1 = 32 P1 < 0.001 P2 < 0.001 | 1074 (689–1684) P1 < 0.05 | 0.22 (0.12–0.29) n1 = 14 (8–18) P1 < 0.05 | 20.42 (13.75–29.17) | 82.92 (80.42–92.08) P1 < 0.05 |
| 4 | 1:45 | 11.47 (7.64–14.82) n1 = 58 P1 < 0.001 P2 < 0.001 | 413 (275–534) P1–3 < 0.05 | 0.05 (0–0.13) n1 = 2 (1–4) P1 < 0.05 P2 < 0.05 | 40.42 (30.0–42.92) P1,3 < 0.05 | 68.75 (42.92–70.83) P1–3 < 0.05 |
| 5 | 1:90 | 5.95 (4.39–8.87) n1 = 38 P1 < 0.001 P2 < 0.001 P3 < 0.001 | 192 (107–359) P1–3 < 0.05 | 0 P1–4 < 0.05 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khlusov, I.; Yurova, K.; Shupletsova, V.; Khaziakhmatova, O.; Malashchenko, V.; Kudryavtseva, V.; Khlusova, M.; Sukhorukov, G.; Litvinova, L. Microcapsule-Based Dose-Dependent Regulation of the Lifespan and Behavior of Adipose-Derived MSCs as a Cell-Mediated Delivery System: In Vitro Study. Int. J. Mol. Sci. 2023, 24, 292. https://doi.org/10.3390/ijms24010292
Khlusov I, Yurova K, Shupletsova V, Khaziakhmatova O, Malashchenko V, Kudryavtseva V, Khlusova M, Sukhorukov G, Litvinova L. Microcapsule-Based Dose-Dependent Regulation of the Lifespan and Behavior of Adipose-Derived MSCs as a Cell-Mediated Delivery System: In Vitro Study. International Journal of Molecular Sciences. 2023; 24(1):292. https://doi.org/10.3390/ijms24010292
Chicago/Turabian StyleKhlusov, Igor, Kristina Yurova, Valeria Shupletsova, Olga Khaziakhmatova, Vladimir Malashchenko, Valeriya Kudryavtseva, Marina Khlusova, Gleb Sukhorukov, and Larisa Litvinova. 2023. "Microcapsule-Based Dose-Dependent Regulation of the Lifespan and Behavior of Adipose-Derived MSCs as a Cell-Mediated Delivery System: In Vitro Study" International Journal of Molecular Sciences 24, no. 1: 292. https://doi.org/10.3390/ijms24010292
APA StyleKhlusov, I., Yurova, K., Shupletsova, V., Khaziakhmatova, O., Malashchenko, V., Kudryavtseva, V., Khlusova, M., Sukhorukov, G., & Litvinova, L. (2023). Microcapsule-Based Dose-Dependent Regulation of the Lifespan and Behavior of Adipose-Derived MSCs as a Cell-Mediated Delivery System: In Vitro Study. International Journal of Molecular Sciences, 24(1), 292. https://doi.org/10.3390/ijms24010292







