L-Carnitine Functionalization to Increase Skeletal Muscle Tropism of PLGA Nanoparticles
Abstract
:1. Introduction
2. Results
2.1. Preparation and Characterization of SC-Nanoparticles
2.2. Cytotoxicity Assay
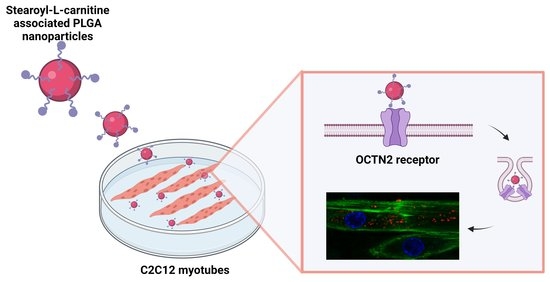
2.3. Nanoparticle Distribution in Myoblasts and Myotubes
2.4. Immunofluorescence Detection of OCTN2 Receptor
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation and Characterization of Free Base form of PTM
4.3. Preparation of Nanoparticles
4.4. Characterization of Nanoparticles
4.5. Determination of the Associated SC Percentage
4.6. PTM-B Release from Nanoparticles
4.7. Cell Culture and Treatment
4.8. Cytotoxicity Assay
4.9. Fluorescence and Transmission Electron Microscopy Analysis
4.10. Immunofluorescence Microscopy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ravi Kiran, A.V.V.V.; Kusuma Kumari, G.; Krishnamurthy, P.T.; Khaydarov, R.R. Tumor Microenvironment and Nanotherapeutics: Intruding the Tumor Fort. Biomater. Sci. 2021, 9, 7667–7704. [Google Scholar] [CrossRef]
- Shi, Y.; van der Meel, R.; Chen, X.; Lammers, T. The EPR Effect and beyond: Strategies to Improve Tumor Targeting and Cancer Nanomedicine Treatment Efficacy. Theranostics 2020, 10, 7921–7924. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F. To Exploit the Tumor Microenvironment: Since the EPR Effect Fails in the Clinic, What Is the Future of Nanomedicine? J. Control Release 2016, 244, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.K.; O’Reilly, R.K. Insights into Active Targeting of Nanoparticles in Drug Delivery: Advances in Clinical Studies and Design Considerations for Cancer Nanomedicine. Bioconjug. Chem. 2019, 30, 2300–2311. [Google Scholar] [CrossRef]
- Duan, D.; Goemans, N.; Takeda, S.; Mercuri, E.; Aartsma-Rus, A. Duchenne muscular dystrophy. Nat. Rev. Dis. Primers 2021, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Lanni, S.; Pearson, C.E. Molecular genetics of congenital myotonic dystrophy. Neurobiol. Dis. 2019, 132, 104533. [Google Scholar] [CrossRef]
- Mercuri, E.; Bönnemann, C.G.; Muntoni, F. Muscular Dystrophies. Lancet 2019, 394, 2025–2038. [Google Scholar] [CrossRef]
- Andreana, I.; Repellin, M.; Carton, F.; Kryza, D.; Briançon, S.; Chazaud, B.; Mounier, R.; Arpicco, S.; Malatesta, M.; Stella, B.; et al. Nanomedicine for Gene Delivery and Drug Repurposing in the Treatment of Muscular Dystrophies. Pharmaceutics 2021, 13, 278. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; Qaisar, R. Nanomedicine for Treating Muscle Dystrophies: Opportunities, Challenges, and Future Perspectives. Int. J. Mol. Sci. 2022, 23, 12039. [Google Scholar] [CrossRef]
- Sleboda, D.A.; Stover, K.K.; Roberts, T.J. Diversity of Extracellular Matrix Morphology in Vertebrate Skeletal Muscle. J. Morphol. 2020, 281, 160–169. [Google Scholar] [CrossRef]
- Engin, A.B.; Nikitovic, D.; Neagu, M.; Henrich-Noack, P.; Docea, A.O.; Shtilman, M.I.; Golokhvast, K.; Tsatsakis, A.M. Mechanistic Understanding of Nanoparticles’ Interactions with Extracellular Matrix: The Cell and Immune System. Part Fibre. Toxicol. 2017, 14, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, D.; Yue, F.; Qiu, J.; Deng, M.; Kuang, S. Polymeric Nanoparticles Functionalized with Muscle-Homing Peptides for Targeted Delivery of Phosphatase and Tensin Homolog Inhibitor to Skeletal Muscle. Acta Biomater. 2020, 118, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-Y.; Yuan, Z.; Cao, Z.; Wang, B.; Qiao, C.; Li, J.; Xiao, X. A Muscle-Targeting Peptide Displayed on AAV2 Improves Muscle Tropism on Systemic Delivery. Gene 2009, 16, 953–962. [Google Scholar] [CrossRef] [Green Version]
- Pochini, L.; Scalise, M.; Galluccio, M.; Indiveri, C. OCTN Cation Transporters in Health and Disease: Role as Drug Targets and Assay Development. J. Biomol. Screen 2013, 18, 851–867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kou, L.; Sun, R.; Ganapathy, V.; Yao, Q.; Chen, R. Recent Advances in Drug Delivery via the Organic Cation/Carnitine Transporter 2 (OCTN2/SLC22A5). Expert Opin. Targets 2018, 22, 715–726. [Google Scholar] [CrossRef] [PubMed]
- Juraszek, B.; Nałąecz, K.A. SLC22A5 (OCTN2) Carnitine Transporter-Indispensable for Cell Metabolism, a Jekyll and Hyde of Human Cancer. Molecules 2020, 25, 14. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Jin, Y.; Deng, Y.; Zou, Y.; Han, S.; Zhou, C.; Zhou, Y.; Liu, Y. Efficient Oral Delivery of Poorly Water-Soluble Drugs Using Carnitine/Organic Cation Transporter 2-Mediated Polymeric Micelles. ACS Biomater. Sci. Eng. 2020, 6, 2146–2158. [Google Scholar] [CrossRef]
- Kou, L.; Yao, Q.; Sun, M.; Wu, C.; Wang, J.; Luo, Q.; Wang, G.; Du, Y.; Fu, Q.; Wang, J.; et al. Cotransporting Ion Is a Trigger for Cellular Endocytosis of Transporter-Targeting Nanoparticles: A Case Study of High-Efficiency SLC22A5 (OCTN2)-Mediated Carnitine-Conjugated Nanoparticles for Oral Delivery of Therapeutic Drugs. Adv. Health Mater. 2017, 6. [Google Scholar] [CrossRef]
- Kou, L.; Yao, Q.; Sivaprakasam, S.; Luo, Q.; Sun, Y.; Fu, Q.; He, Z.; Sun, J.; Ganapathy, V. Dual Targeting of L-Carnitine-Conjugated Nanoparticles to OCTN2 and ATB0,+ to Deliver Chemotherapeutic Agents for Colon Cancer Therapy. Drug Deliv. 2017, 24, 1338–1349. [Google Scholar] [CrossRef] [Green Version]
- Ingoglia, F.; Visigalli, R.; Rotoli, B.M.; Barilli, A.; Riccardi, B.; Puccini, P.; Dall’Asta, V. Functional Activity of L-Carnitine Transporters in Human Airway Epithelial Cells. Biochim. Biophys. Acta 2016, 1858, 210–219. [Google Scholar] [CrossRef]
- Rotoli, B.M.; Visigalli, R.; Barilli, A.; Ferrari, F.; Bianchi, M.G.; Di Lascia, M.; Riccardi, B.; Puccini, P.; Dall’Asta, V. Functional Analysis of OCTN2 and ATB0,+ in Normal Human Airway Epithelial Cells. PLoS ONE 2020, 15, e0228568. [Google Scholar] [CrossRef] [Green Version]
- Strohman, R.C.; Paterson, B.; Fluck, R.; Przybyla, A. Cell Fusion and Terminal Differentiation of Myogenic Cells in Culture. J. Anim. Sci. 1974, 38, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- López-Martínez, A.; Soblechero-Martín, P.; de-la-Puente-Ovejero, L.; Nogales-Gadea, G.; Arechavala-Gomeza, V. An Overview of Alternative Splicing Defects Implicated in Myotonic Dystrophy Type I. Genes 2020, 11, 1109. [Google Scholar] [CrossRef]
- Mulders, S.A.; van den Broek, W.J.; Wheeler, T.M.; Croes, H.J.; van Kuik-Romeijn, P.; de Kimpe, S.J.; Furling, D.; Platenburg, G.J.; Gourdon, G.; Thornton, C.A.; et al. Triplet-repeat oligonucleotide-mediated reversal of RNA toxicity in myotonic dystrophy. Proc. Natl. Acad. Sci. USA 2009, 106, 13915–13920. [Google Scholar] [CrossRef] [Green Version]
- Warf, M.B.; Nakamori, M.; Matthys, C.M.; Thornton, C.A.; Berglund, J.A. Pentamidine Reverses the Splicing Defects Associated with Myotonic Dystrophy. Proc. Natl. Acad. Sci. USA 2009, 106, 18551–18556. [Google Scholar] [CrossRef] [Green Version]
- Coonrod, L.A.; Nakamori, M.; Wang, W.; Carrell, S.; Hilton, C.L.; Bodner, M.J.; Siboni, R.B.; Docter, A.G.; Haley, M.M.; Thornton, C.A.; et al. Reducing levels of toxic RNA with small molecules. ACS Chem. Biol. 2013, 8, 2528–2537. [Google Scholar] [CrossRef] [Green Version]
- Andreana, I.; Bincoletto, V.; Milla, P.; Dosio, F.; Stella, B.; Arpicco, S. Nanotechnological approaches for pentamidine delivery. Drug Deliv. Transl. Res. 2022, 12, 1911–1927. [Google Scholar] [CrossRef]
- Repellin, M.; Carton, F.; Boschi, F.; Galiè, M.; Perduca, M.; Calderan, L.; Jacquier, A.; Carras, J.; Schaeffer, L.; Briançon, S.; et al. Repurposing Pentamidine Using Hyaluronic Acid-Based Nanocarriers for Skeletal Muscle Treatment in Myotonic Dystrophy. Nanomedicine 2022, 47, 102623. [Google Scholar] [CrossRef]
- Chakraborty, M.; Llamusi, B.; Artero, R. Modeling of Myotonic Dystrophy Cardiac Phenotypes in Drosophila. Front. Neurol. 2018, 9, 473. [Google Scholar] [CrossRef] [PubMed]
- Baroni, A.; Neaga, I.; Delbosc, N.; Wells, M.; Verdy, L.; Ansseau, E.; Vanden Eynde, J.J.; Belayew, A.; Bodoki, E.; Oprean, R.; et al. Bioactive Aliphatic Polycarbonates Carrying Guanidinium Functions: An Innovative Approach for Myotonic Dystrophy Type 1 Therapy. ACS Omega 2019, 4, 18126–18135. [Google Scholar] [CrossRef] [PubMed]
- Fessi, H.; Puisieux, F.; Devissaguet, J.P.; Ammoury, N.; Benita, S. Nanocapsule Formation by Interfacial Polymer Deposition Following Solvent Displacement. Int. J. Pharm. 1989, 55, R1–R4. [Google Scholar] [CrossRef]
- Stella, B.; Andreana, I.; Zonari, D.; Arpicco, S. Pentamidine-Loaded Lipid and Polymer Nanocarriers as Tunable Anticancer Drug Delivery Systems. J. Pharm. Sci. 2020, 109, 1297–1302. [Google Scholar] [CrossRef]
- Amat di San Filippo, C.; Wang, Y.; Longo, N. Functional Domains in the Carnitine Transporter OCTN2, Defective in Primary Carnitine Deficiency. J. Biol. Chem. 2003, 278, 47776–47784. [Google Scholar] [CrossRef] [Green Version]
- Guglielmi, V.; Carton, F.; Vattemi, G.; Arpicco, S.; Stella, B.; Berlier, G.; Marengo, A.; Boschi, F.; Malatesta, M. Uptake and Intracellular Distribution of Different Types of Nanoparticles in Primary Human Myoblasts and Myotubes. Int. J. Pharm. 2019, 560, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.; Vurro, F.; Cisterna, B.; Boschi, F.; Marengo, A.; Montanari, E.; Meo, C.D.; Matricardi, P.; Berlier, G.; Stella, B.; et al. Uptake and Intracellular Fate of Biocompatible Nanocarriers in Cycling and Noncycling Cells. Nanomedicine 2019, 14, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Menon, J.U.; Kona, S.; Wadajkar, A.S.; Desai, F.; Vadla, A.; Nguyen, K.T. Effects of Surfactants on the Properties of PLGA Nanoparticles. J. Biomed. Mater. Res. A 2012, 100, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shen, M.; Li, W.; Li, W.; Zhang, F. Controlled-Release of Fluazinam from Biodegradable PLGA-Based Microspheres. J. Env. Sci. Health B 2019, 54, 810–816. [Google Scholar] [CrossRef]
- Reuter, S.E.; Evans, A.M. Carnitine and Acylcarnitines. Clin. Pharm. 2012, 51, 553–572. [Google Scholar] [CrossRef]
- Su, X.; Han, X.; Mancuso, D.J.; Abendschein, D.R.; Gross, R.W. Accumulation of Long-Chain Acylcarnitine and 3-Hydroxy Acylcarnitine Molecular Species in Diabetic Myocardium: Identification of Alterations in Mitochondrial Fatty Acid Processing in Diabetic Myocardium by Shotgun Lipidomics. Biochemistry 2005, 44, 5234–5245. [Google Scholar] [CrossRef]
- Costanzo, M.; Carton, F.; Marengo, A.; Berlier, G.; Stella, B.; Arpicco, S.; Malatesta, M. Fluorescence and Electron Microscopy to Visualize the Intracellular Fate of Nanoparticles for Drug Delivery. Eur. J. Histochem. 2016, 60, 2640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulkarni, S.A.; Feng, S.-S. Effects of Particle Size and Surface Modification on Cellular Uptake and Biodistribution of Polymeric Nanoparticles for Drug Delivery. Pharm. Res. 2013, 30, 2512–2522. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, L.; Wang, J.; Feng, Q.; Liu, D.; Yin, Q.; Xu, D.; Wei, Y.; Ding, B.; Shi, X.; et al. Tunable Rigidity of (Polymeric Core)–(Lipid Shell) Nanoparticles for Regulated Cellular Uptake. Adv. Mater. 2015, 27, 1402–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoudi, M. Debugging Nano-Bio Interfaces: Systematic Strategies to Accelerate Clinical Translation of Nanotechnologies. Trends Biotechnol. 2018, 36, 755–769. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.-S.; Chang, K.L.B.; Hwang, D.-F.; Kong, Z.-L. In Vitro Cytotoxicitiy of Silica Nanoparticles at High Concentrations Strongly Depends on the Metabolic Activity Type of the Cell Line. Env. Sci. Technol. 2007, 41, 2064–2068. [Google Scholar] [CrossRef]
- Kislinger, T.; Gramolini, A.O.; Pan, Y.; Rahman, K.; MacLennan, D.H.; Emili, A. Proteome Dynamics during C2C12 Myoblast Differentiation. Mol. Cell Proteom. 2005, 4, 887–901. [Google Scholar] [CrossRef] [Green Version]
- Casadei, L.; Vallorani, L.; Gioacchini, A.M.; Guescini, M.; Burattini, S.; D’Emilio, A.; Biagiotti, L.; Falcieri, E.; Stocchi, V. Proteomics-Based Investigation in C2C12 Myoblast Differentiation. Eur. J. Histochem. 2009, 53, e31. [Google Scholar] [CrossRef] [Green Version]
- Forterre, A.; Jalabert, A.; Berger, E.; Baudet, M.; Chikh, K.; Errazuriz, E.; De Larichaudy, J.; Chanon, S.; Weiss-Gayet, M.; Hesse, A.-M.; et al. Proteomic Analysis of C2C12 Myoblast and Myotube Exosome-like Vesicles: A New Paradigm for Myoblast-Myotube Cross Talk? PLoS ONE 2014, 9, e84153. [Google Scholar] [CrossRef]
- Briolay, A.; Jaafar, R.; Nemoz, G.; Bessueille, L. Myogenic Differentiation and Lipid-Raft Composition of L6 Skeletal Muscle Cells Are Modulated by PUFAs. Biochim. Biophys. Acta 2013, 1828, 602–613. [Google Scholar] [CrossRef]
- Fröhlich, E.; Meindl, C.; Roblegg, E.; Griesbacher, A.; Pieber, T.R. Cytotoxity of Nanoparticles Is Influenced by Size, Proliferation and Embryonic Origin of the Cells Used for Testing. Nanotoxicology 2012, 6, 424–439. [Google Scholar] [CrossRef]
- Rahman, M.; Laurent, S.; Tawil, N.; Yahia, L.; Mahmoudi, M. Nanoparticle and Protein Corona. In Protein-Nanoparticle Interactions: The Bio-Nano Interface; Rahman, M., Laurent, S., Tawil, N., Yahia, L., Mahmoudi, M., Eds.; Springer Series in Biophysics; Springer: Berlin/Heidelberg, Germany, 2013; pp. 21–44. ISBN 978-3-642-37555-2. [Google Scholar]
- Wu, X.; Huang, W.; Prasad, P.D.; Seth, P.; Rajan, D.P.; Leibach, F.H.; Chen, J.; Conway, S.J.; Ganapathy, V. Functional Characteristics and Tissue Distribution Pattern of Organic Cation Transporter 2 (OCTN2), an Organic Cation/Carnitine Transporter. J. Pharm. Exp. 1999, 290, 1482–1492. [Google Scholar]
- Georges, B.; Le Borgne, F.; Galland, S.; Isoir, M.; Ecosse, D.; Grand-Jean, F.; Demarquoy, J. Carnitine Transport into Muscular Cells. Inhibition of Transport and Cell Growth by Mildronate. Biochem. Pharm. 2000, 59, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Furuichi, Y.; Sugiura, T.; Kato, Y.; Takakura, H.; Hanai, Y.; Hashimoto, T.; Masuda, K. Muscle Contraction Increases Carnitine Uptake via Translocation of OCTN2. Biochem. Biophys. Res. Commun. 2012, 418, 774–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinuzzi, A.; Vergani, L.; Rosa, M.; Angelini, C. L-Carnitine Uptake in Differentiating Human Cultured Muscle. Biochim. Biophys. Acta 1991, 1095, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Peretti, E.; Miletto, I.; Stella, B.; Rocco, F.; Berlier, G.; Arpicco, S. Strategies to Obtain Encapsulation and Controlled Release of Pentamidine in Mesoporous Silica Nanoparticles. Pharmaceutics 2018, 10, 195. [Google Scholar] [CrossRef] [Green Version]
- Costanzo, M.; Malatesta, M. Embedding Cell Monolayers to Investigate Nanoparticle-Plasmalemma Interactions at Transmission Electron Microscopy. Eur. J. Histochem. 2019, 63, 3026. [Google Scholar] [CrossRef] [Green Version]
- Carton, F.; Calderan, L. Malatesta M. Incubation under fluid dynamic conditions markedly improves the structural preservation in vitro of explanted skeletal muscles. Eur. J. Histochem. 2017, 61, 2862. [Google Scholar] [CrossRef] [Green Version]
- Carton, F.; Malatesta, M. In Vitro Models of Biological Barriers for Nanomedical Research. Int. J. Mol. Sci. 2022, 23, 8910. [Google Scholar] [CrossRef]






| Nanoparticle Composition | Mean Diameter (nm ± S.D.) | PDI | Zeta Potential (mV ± S.D.) |
|---|---|---|---|
| PLGA | 94 ± 1 | 0.170 | −39.2 ± 1.8 |
| 5% SC-PLGA | 82 ± 1 | 0.198 | −23.7 ± 1.1 |
| 10% SC-PLGA | 73 ± 1 | 0.184 | −29.6 ± 1.0 |
| 5% SC-PTM-B-PLGA | 98 ± 11 | 0.399 | −18.4 ± 2.4 |
| 10% SC-PTM-B-PLGA | 128 ± 10 | 0.222 | −28.8 ± 3.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreana, I.; Malatesta, M.; Lacavalla, M.A.; Boschi, F.; Milla, P.; Bincoletto, V.; Pellicciari, C.; Arpicco, S.; Stella, B. L-Carnitine Functionalization to Increase Skeletal Muscle Tropism of PLGA Nanoparticles. Int. J. Mol. Sci. 2023, 24, 294. https://doi.org/10.3390/ijms24010294
Andreana I, Malatesta M, Lacavalla MA, Boschi F, Milla P, Bincoletto V, Pellicciari C, Arpicco S, Stella B. L-Carnitine Functionalization to Increase Skeletal Muscle Tropism of PLGA Nanoparticles. International Journal of Molecular Sciences. 2023; 24(1):294. https://doi.org/10.3390/ijms24010294
Chicago/Turabian StyleAndreana, Ilaria, Manuela Malatesta, Maria Assunta Lacavalla, Federico Boschi, Paola Milla, Valeria Bincoletto, Carlo Pellicciari, Silvia Arpicco, and Barbara Stella. 2023. "L-Carnitine Functionalization to Increase Skeletal Muscle Tropism of PLGA Nanoparticles" International Journal of Molecular Sciences 24, no. 1: 294. https://doi.org/10.3390/ijms24010294