Mycosynthesis of Metal-Containing Nanoparticles—Synthesis by Ascomycetes and Basidiomycetes and Their Application
Abstract
1. Introduction
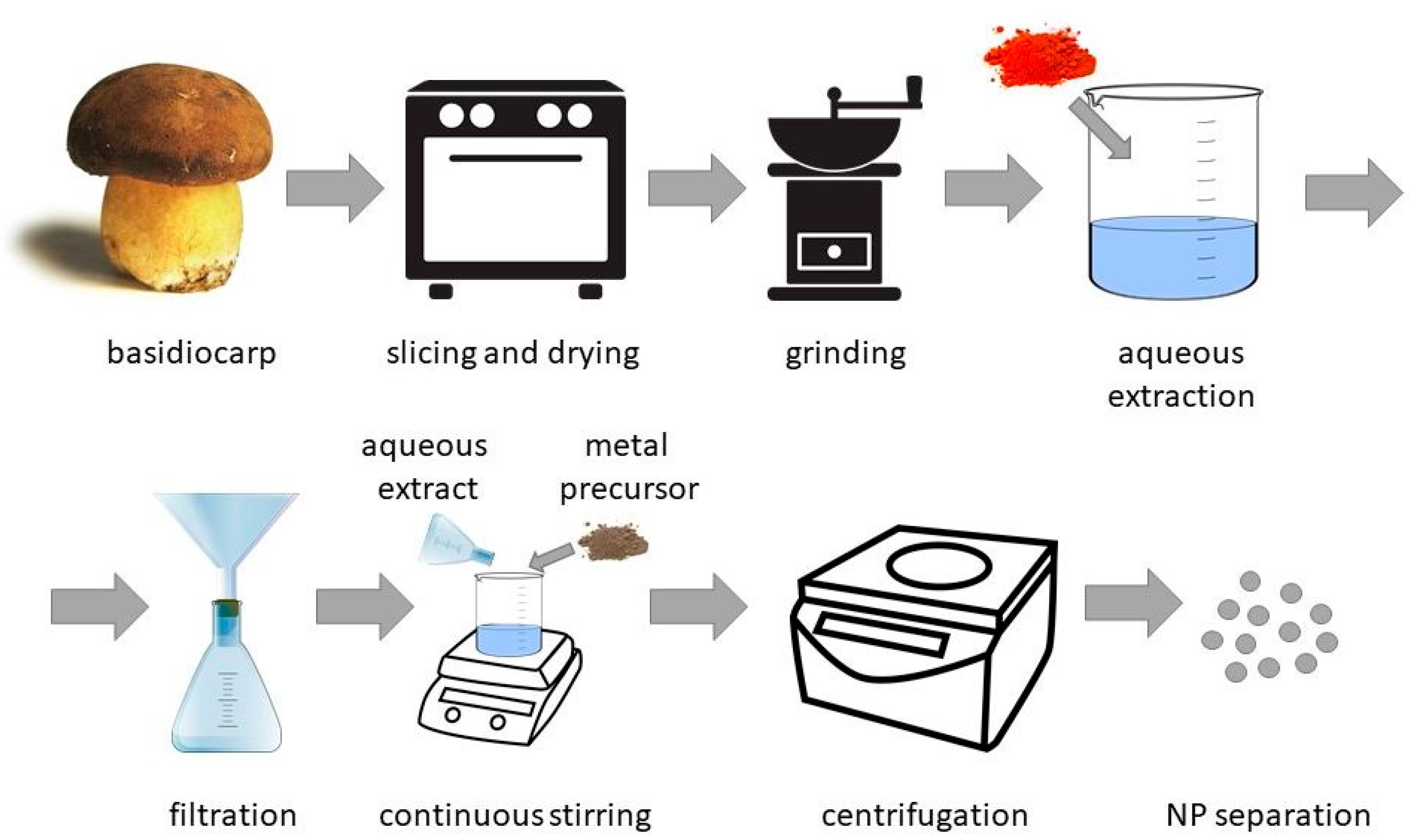
2. Synthesis of Metal-Containing Nanoparticles by Fungi
| Species of Fungus | NP Type | Average Size (nm) and Shape | Synthesis Used | Source |
|---|---|---|---|---|
| Aspergillus japonicus | Fe3O4 | 82 cubic | cell-free filtrate extracellular synthesis in presence of a living fungus | [34] |
| Aspergillus niger | Fe | 18 spherical | cell-free filtrate and supercritical condition of liquids | [35] |
| Au | 13 spherical, elliptical | cell-free filtrate | [36] | |
| Phaenerochaete chrysosporium | Ag | 50 to 200 hexagonal pyramids | Synthesis with mycelial mat | [37] |
| Saccharomyces cerevisiae | Pd | 32 hexagonal | aqueous extract | [38] |
| Trichoderma viridae | Ag | 5 to 40 spherical, rod | cell-free filtrate | [39] |
| Verticillium sp. | Au | 20 spherical, triangular, hexagonal, quasi-hexagonal | intracellular synthesis in living fungus | [23] |
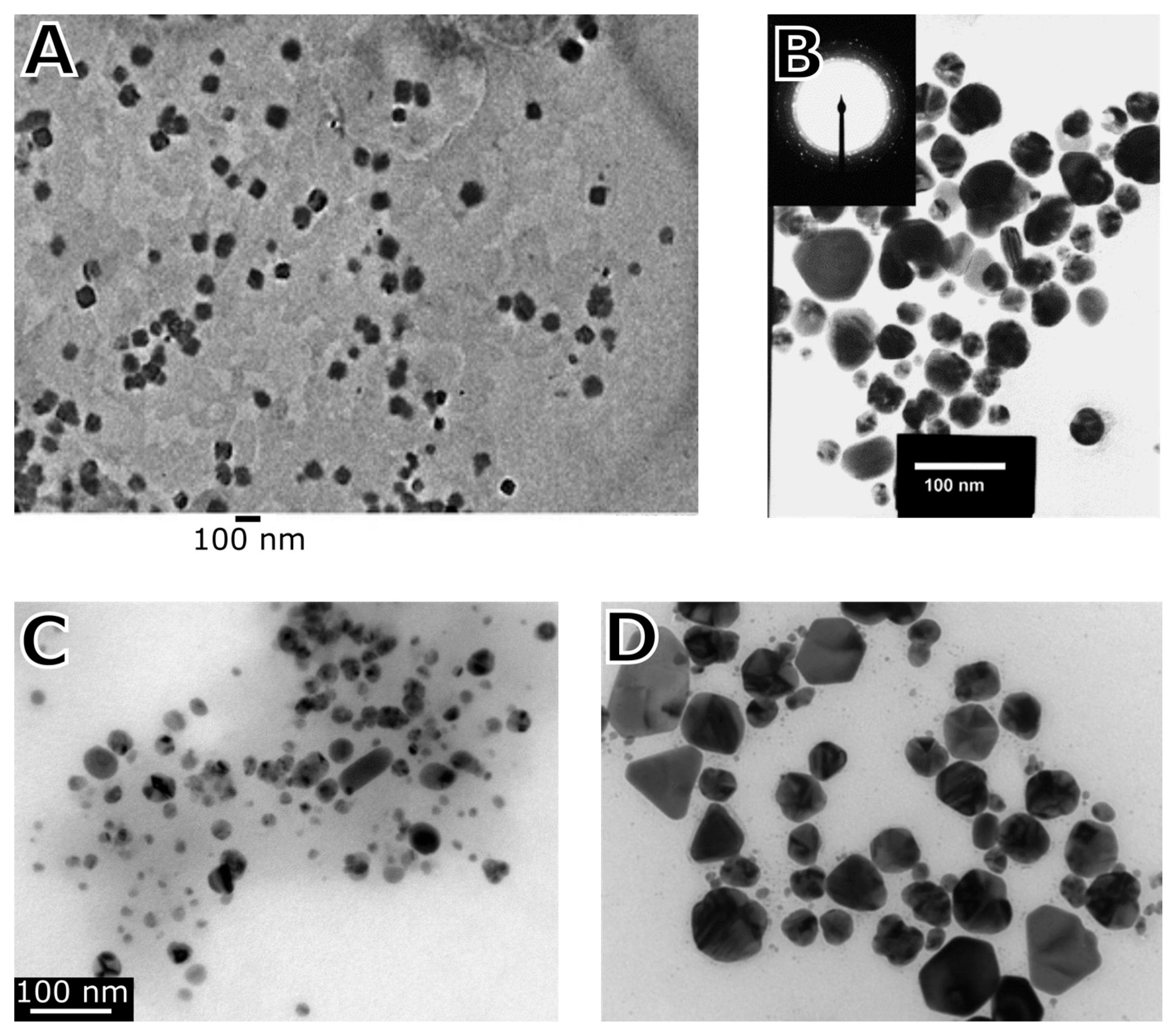
2.1. Synthesis of Nanoparticles by Ascomycetes
2.1.1. Aspergillus
2.1.2. Fusarium
2.1.3. Penicillium
2.1.4. Trichoderma
2.1.5. Verticillium
2.1.6. Yeasts
2.1.7. Other Ascomycetes
2.2. Synthesis of the Nanoparticles with Basidiomycetes
2.2.1. Pleurotus
2.2.2. Agaricus
2.2.3. Ganoderma
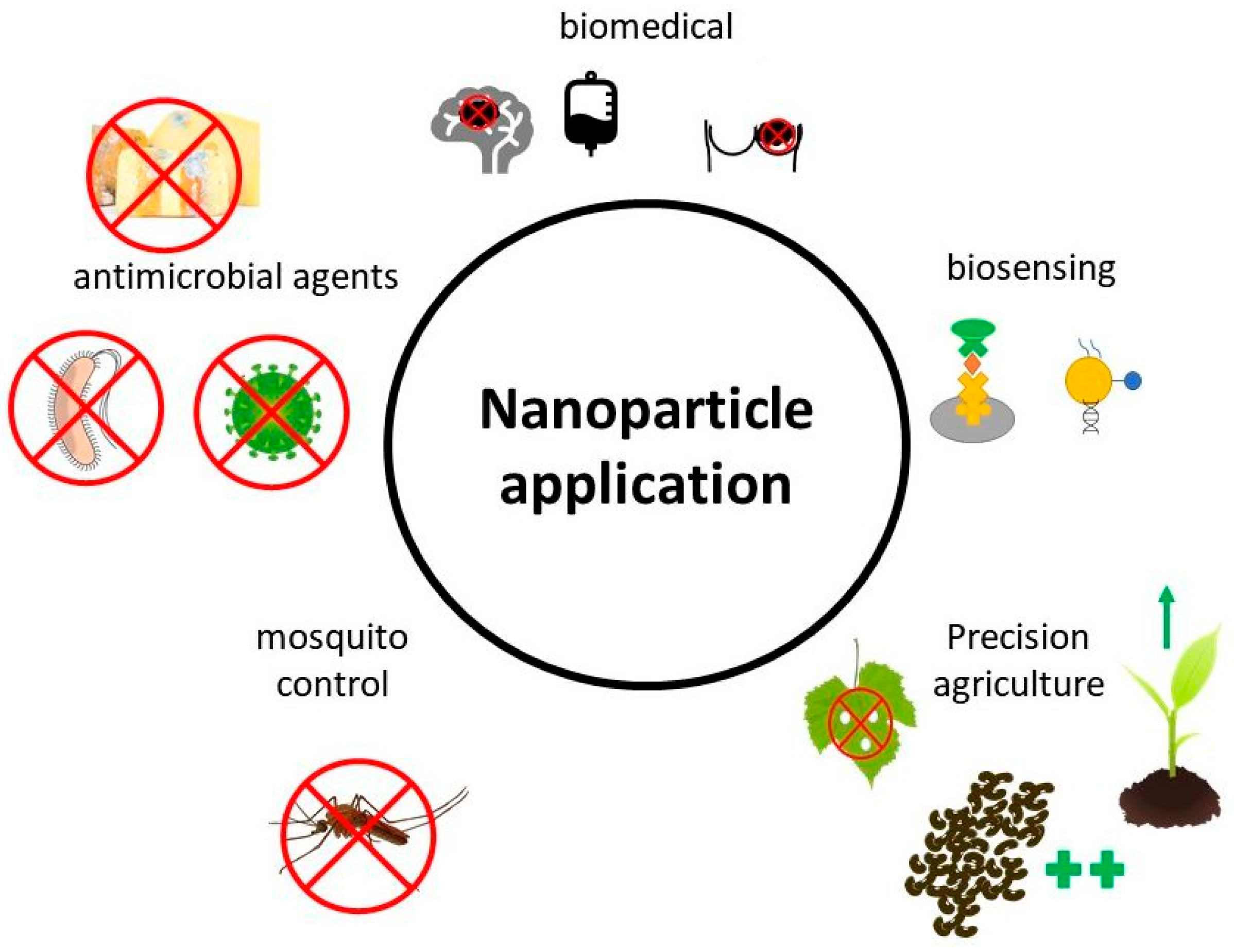
3. Application of Fungal-Synthesized Nanoparticles
3.1. Biomedical Applications
3.2. Antimicrobial Agents
3.2.1. Antibacterial Agents
3.2.2. Antifungal Agents
3.2.3. Antiviral Agents
3.3. Catalysis
3.4. Biosensing
3.5. Mosquito Control
3.6. Precision Agriculture—Nanofertilizer and Nanopesticide Applications
4. Conclusions—Advantages, Limitations, and Future Prospects of Fungal Synthesis of Metal-Containing Nanoparticles
4.1. Advantages and Limitations
4.2. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Afzal, S.; Aftab, T.; Singh, N.K. Impact of Zinc Oxide and Iron Oxide Nanoparticles on Uptake, Translocation, and Physiological Effects in Oryza Sativa L. J. Plant Growth Regul. 2022, 41, 1445–1461. [Google Scholar] [CrossRef]
- Mughal, B.; Zaidi, S.Z.J.; Zhang, X.; Hassan, S.U. Biogenic Nanoparticles: Synthesis, Characterisation and Applications. Appl. Sci. 2021, 11, 2598. [Google Scholar] [CrossRef]
- Dogru, E.; Demirbas, A.; Altinsoy, B.; Duman, F.; Ocsoy, I. Formation of Matricaria Chamomilla Extract-Incorporated Ag Nanoparticles and Size-Dependent Enhanced Antimicrobial Property. J. Photochem. Photobiol. B 2017, 174, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Kusiak-Nejman, E.; Wojnarowicz, J.; Morawski, A.W.; Narkiewicz, U.; Sobczak, K.; Gierlotka, S.; Lojkowski, W. Size-Dependent Effects of ZnO Nanoparticles on the Photocatalytic Degradation of Phenol in a Water Solution. Appl. Surf. Sci. 2021, 541, 148416. [Google Scholar] [CrossRef]
- Gahlawat, G.; Choudhury, A.R. A Review on the Biosynthesis of Metal and Metal Salt Nanoparticles by Microbes. RSC Adv. 2019, 9, 12944–12967. [Google Scholar] [CrossRef] [PubMed]
- Horváthová, H.; Dercová, K.; Tlčíková, M.; Hurbanová, M. Biological Synthesis of Nanoparticles: Iron-Based Plant Bionanoparticles and Their Use for Remediation of the Contaminated Environment. Chem. Listy 2022, 116, 405–415. [Google Scholar] [CrossRef]
- Patra, C.R.; Mukherjee, S.; Kotcherlakota, R. Biosynthesized Silver Nanoparticles: A Step Forward for Cancer Theranostics? Nanomedicine 2014, 9, 1445–1448. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: An Overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef]
- Noman, E.; Al-Gheethi, A.; Talip, B.A.; Mohamed, R.; Kassim, A.H. Inactivating Pathogenic Bacteria in Greywater by Biosynthesized Cu/Zn Nanoparticles from Secondary Metabolite of Aspergillus Iizukae; Optimization, Mechanism and Techno Economic Analysis. PLoS ONE 2019, 14, e0221522. [Google Scholar] [CrossRef]
- Yadav, A.; Kon, K.; Kratosova, G.; Duran, N.; Ingle, A.P.; Rai, M. Fungi as an Efficient Mycosystem for the Synthesis of Metal Nanoparticles: Progress and Key Aspects of Research. Biotechnol. Lett. 2015, 37, 2099–2120. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A. Fabrication of Metal Nanoparticles from Fungi and Metal Salts: Scope and Application. Nanoscale Res. Lett. 2016, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Wold, W.S.M.; Suzuki, I. The Citric Acid Fermentation by Aspergillus Niger: Regulation by Zinc of Growth and Acidogenesis. Can. J. Microbiol. 1976, 22, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Toghueo, R.M.K.; Boyom, F.F. Endophytic Penicillium Species and Their Agricultural, Biotechnological, and Pharmaceutical Applications. 3 Biotech 2020, 10, 107. [Google Scholar] [CrossRef]
- Manoharachary, C.; Deshaboina, N. Biodiversity, Taxonomy and Plant Disease Diagnostics of Plant Pathogenic Fungi from India. Indian Phytopathol. 2021, 74, 413–423. [Google Scholar] [CrossRef]
- Müller, J.; Polak, A. Classification and Taxonomy of Fungi Pathogenic for Warm-Blooded Hosts. In Antifungal Agents; Birkhäuser Basel: Basel, Switzerland, 2003; pp. 1–12. [Google Scholar]
- Owaid, M.N.; Ibraheem, I.J. Mycosynthesis of Nanoparticles Using Edible and Medicinal Mushrooms. Eur. J. Nanomed. 2017, 9, 5–23. [Google Scholar] [CrossRef]
- Elsakhawy, T.; Omara, A.E.-D.; Abowaly, M.; El-Ramady, H.; Badgar, K.; Llanaj, X.; Törős, G.; Hajdú, P.; Prokisch, J. Green Synthesis of Nanoparticles by Mushrooms: A Crucial Dimension for Sustainable Soil Management. Sustainability 2022, 14, 4328. [Google Scholar] [CrossRef]
- Fouda, H.; Sofy, M. Effect of Biological Synthesis of Nanoparticles from Penicillium Chrysogenum as Well as Traditional Salt and Chemical Nanoparticles of Zinc on Canola Plant Oil Productivity and Metabolic Activity. Egypt J. Chem. 2021, 65, 507–516. [Google Scholar] [CrossRef]
- Dhanjal, D.S.; Mehra, P.; Bhardwaj, S.; Singh, R.; Sharma, P.; Nepovimova, E.; Chopra, C.; Kuca, K. Mycology-Nanotechnology Interface: Applications in Medicine and Cosmetology. Int. J. Nanomed. 2022, 17, 2505–2533. [Google Scholar] [CrossRef]
- Chan, Y.S.; Mat Don, M. Biosynthesis and Structural Characterization of Ag Nanoparticles from White Rot Fungi. Mater. Sci. Eng. C 2013, 33, 282–288. [Google Scholar] [CrossRef]
- Kaur, G.; Kalia, A.; Sodhi, H.S. Size Controlled, Time-Efficient Biosynthesis of Silver Nanoparticles from Pleurotus Florida Using Ultra-Violet, Visible Range, and Microwave Radiations. Inorg. Nano-Met. Chem. 2020, 50, 35–41. [Google Scholar] [CrossRef]
- Navaladian, S.; Viswanathan, B.; Varadarajan, T.K.; Viswanath, R.P. Microwave-Assisted Rapid Synthesis of Anisotropic Ag Nanoparticles by Solid State Transformation. Nanotechnology 2008, 19, 045603. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Ramani, R.; Parischa, R.; Ajayakumar, P.v; Alam, M.; et al. Bioreduction of AuCl4− Ions by the Fungus, Verticillium Sp. and Surface Trapping of the Gold Nanoparticles Formed. Angew. Chem. Int. Ed. 2001, 40, 3585–3588. [Google Scholar] [CrossRef]
- Aisida, S.O.; Akpa, P.A.; Ahmad, I.; Zhao, T.; Maaza, M.; Ezema, F.I. Bio-Inspired Encapsulation and Functionalization of Iron Oxide Nanoparticles for Biomedical Applications. Eur. Polym. J. 2020, 122, 109371. [Google Scholar] [CrossRef]
- Aisida, S.O.; Batool, A.; Khan, F.M.; Rahman, L.; Mahmood, A.; Ahmad, I.; Zhao, T.; Maaza, M.; Ezema, F.I. Calcination Induced PEG-Ni-ZnO Nanorod Composite and Its Biomedical Applications. Mater. Chem. Phys. 2020, 255, 123603. [Google Scholar] [CrossRef]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; ul Ain, N.; Ao, Q. Role of Capping Agents in the Application of Nanoparticles in Biomedicine and Environmental Remediation: Recent Trends and Future Prospects. J. Nanobiotechnol. 2020, 18, 172. [Google Scholar] [CrossRef]
- Ezealigo, U.S.; Ezealigo, B.N.; Aisida, S.O.; Ezema, F.I. Iron Oxide Nanoparticles in Biological Systems: Antibacterial and Toxicology Perspective. JCIS Open 2021, 4, 100027. [Google Scholar] [CrossRef]
- Aisida, S.O.; Ugwu, K.; Nwanya, A.C.; Akpa, P.A.; Madiba, I.G.; Bashir, A.K.H.; Botha, S.; Ejikeme, P.M.; Zhao, T.; Ahmad, I.; et al. Dry Gongronema Latifolium Aqueous Extract Mediated Silver Nanoparticles by One-Step in-Situ Biosynthesis for Antibacterial Activities. Surf. Interfaces 2021, 24, 101116. [Google Scholar] [CrossRef]
- Onyedikachi, O.A.; Aisida, S.O.; Agbogu, A.; Rufus, I.; Ahmad, I.; Maaza, M.; Ezema, F.I. Zinc Ferrite Nanoparticles Capped with Gongronema Latifolium for Moderate Hyperthermia Applications. Appl. Phys. A 2022, 128, 95. [Google Scholar] [CrossRef]
- Batool, A.; Aisida, S.O.; Rufus, I.; Mahmood, A.; Ahmad, I.; Zhao, T.; Ezema, F.I. Tailoring the Microstructural, Optical, and Magnetic Properties of MgFe 2 O 4 Nanoparticles Capped Polyethylene Glycol Through a Bio-Inspired Method. J. Macromol. Sci. Part B 2022, 61, 860–870. [Google Scholar] [CrossRef]
- Aisida, S.O.; Onwujiobi, C.; Ahmad, I.; Zhao, T.; Maaza, M.; Ezema, F.I. Biogenic Synthesis of Zinc Oxide Nanorods for Biomedical Applications and Photodegradation of Rhodamine B. Mater. Today Commun. 2022, 33, 104660. [Google Scholar] [CrossRef]
- Bhardwaj, A.K.; Shukla, A.; Maurya, S.; Singh, S.C.; Uttam, K.N.; Sundaram, S.; Singh, M.P.; Gopal, R. Direct Sunlight Enabled Photo-Biochemical Synthesis of Silver Nanoparticles and Their Bactericidal Efficacy: Photon Energy as Key for Size and Distribution Control. J. Photochem. Photobiol. B 2018, 188, 42–49. [Google Scholar] [CrossRef]
- Gade, A.K.; Bonde, P.; Ingle, A.P.; Marcato, P.D.; Durán, N.; Rai, M.K. Exploitation of Aspergillus Niger for Synthesis of Silver Nanoparticles. J. Biobased Mater. Bioenergy 2008, 2, 243–247. [Google Scholar] [CrossRef]
- Bhargava, A.; Jain, N.; Barathi, L.M.; Akhtar, M.; Yun, Y.-S.; Panwar, J. Synthesis, Characterization and Mechanistic Insights of Mycogenic Iron Oxide Nanoparticles. J. Nanoparticle Res. 2013, 15, 2031. [Google Scholar] [CrossRef]
- Abdeen, M.; Sabry, S.; Ghozlan, H.; El-Gendy, A.A.; Carpenter, E.E. Microbial-Physical Synthesis of Fe and Fe3O4 Magnetic Nanoparticles Using Aspergillus Niger YESM1 and Supercritical Condition of Ethanol. J. Nanomater. 2016, 2016, 9174891. [Google Scholar] [CrossRef]
- Bhambure, R.; Bule, M.; Shaligram, N.; Kamat, M.; Singhal, R. Extracellular Biosynthesis of Gold Nanoparticles Using Aspergillus Niger—Its Characterization and Stability. Chem. Eng. Technol. 2009, 32, 1036–1041. [Google Scholar] [CrossRef]
- Vigneshwaran, N.; Kathe, A.A.; Varadarajan, P.v.; Nachane, R.P.; Balasubramanya, R.H. Biomimetics of Silver Nanoparticles by White Rot Fungus, Phaenerochaete Chrysosporium. Colloids Surf. B Biointerfaces. 2006, 53, 55–59. [Google Scholar] [CrossRef]
- Sriramulu, M.; Sumathi, S. Biosynthesis of Palladium Nanoparticles Using Saccharomyces Cerevisiae Extract and Its Photocatalytic Degradation Behaviour. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 025018. [Google Scholar] [CrossRef]
- Fayaz, A.M.; Balaji, K.; Girilal, M.; Yadav, R.; Kalaichelvan, P.T.; Venketesan, R. Biogenic Synthesis of Silver Nanoparticles and Their Synergistic Effect with Antibiotics: A Study against Gram-Positive and Gram-Negative Bacteria. Nanomedicine 2010, 6, 103–109. [Google Scholar] [CrossRef]
- Tyagi, S.; Tyagi, P.K.; Gola, D.; Chauhan, N.; Bharti, R.K. Extracellular Synthesis of Silver Nanoparticles Using Entomopathogenic Fungus: Characterization and Antibacterial Potential. SN Appl. Sci. 2019, 1, 1545. [Google Scholar] [CrossRef]
- Baker, S.E.; Bennett, J.W. An Overview of the Genus Aspergillus. In The Aspergilli: Genomics, medical aspects, biotechnology, and research methods; Goldman, G.H., Osmani, S.A., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 3–13. ISBN 9780429129162. [Google Scholar]
- Humber, R.A. Evolution of Entomopathogenicity in Fungi. J. Invertebr. Pathol. 2008, 98, 262–266. [Google Scholar] [CrossRef]
- Dobbeler, P. Biodiversity of Bryophilous Ascomycetes. Biodivers. Conserv. 1997, 6, 721–738. [Google Scholar] [CrossRef]
- Singh, P.; Kim, Y.-J.; Zhang, D.; Yang, D.-C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Akbar, M.; Haroon, U.; Ali, M.; Tahir, K.; Chaudhary, H.J.; Munis, M.F.H. Mycosynthesized Fe2O3 Nanoparticles Diminish Brown Rot of Apple Whilst Maintaining Composition and Pertinent Organoleptic Properties. J. Appl. Microbiol. 2022, 132, 3735–3745. [Google Scholar] [CrossRef] [PubMed]
- Lan Chi, N.T.; Veeraragavan, G.R.; Brindhadevi, K.; Chinnathambi, A.; Salmen, S.H.; Alharbi, S.A.; Krishnan, R.; Pugazhendhi, A. Fungi Fabrication, Characterization, and Anticancer Activity of Silver Nanoparticles Using Metals Resistant Aspergillus Niger. Environ. Res. 2022, 208, 112721. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, A.J.; Espínola, F.; Ruiz, E.; Cuartero, M.; Castro, E. Biotechnological Use of the Ubiquitous Fungus Penicillium Sp. 8L2: Biosorption of Ag(I) and Synthesis of Silver Nanoparticles. J. Environ. Manag. 2022, 316, 115281. [Google Scholar] [CrossRef]
- Rashwan, D.; Nagy, R.; El-deen, M.; Elhakim, H.A.; Mohamed, M.; Afify, M.; abd el Hamed, M.; abd el razik, M. Green Synthesis of Zinc Oxide Nanocomposite Using Fusarium Oxysporum and Evaluation of the Anticancer Effect on Hepatocellular Carcinoma. Egypt J. Chem. 2021, 65, 197–207. [Google Scholar] [CrossRef]
- Soni, N.; Prakash, S. Microbial Synthesis of Spherical Nanosilver and Nanogold for Mosquito Control. Ann. Microbiol. 2014, 64, 1099–1111. [Google Scholar] [CrossRef]
- Bhadani, R.v; Gajera, H.P.; Hirpara, D.G.; Savaliya, D.D.; Anuj, S.A. Biosynthesis and Characterization of Extracellular Metabolites-Based Nanoparticles to Control the Whitefly. Arch. Microbiol. 2022, 204, 311. [Google Scholar] [CrossRef]
- Soltani Nejad, M.; Samandari Najafabadi, N.; Aghighi, S.; Pakina, E.; Zargar, M. Evaluation of Phoma Sp. Biomass as an Endophytic Fungus for Synthesis of Extracellular Gold Nanoparticles with Antibacterial and Antifungal Properties. Molecules 2022, 27, 1181. [Google Scholar] [CrossRef]
- Mousa, S.A.; El-Sayed, E.-S.R.; Mohamed, S.S.; Abo El-Seoud, M.A.; Elmehlawy, A.A.; Abdou, D.A.M. Novel Mycosynthesis of Co3O4, CuO, Fe3O4, NiO, and ZnO Nanoparticles by the Endophytic Aspergillus Terreus and Evaluation of Their Antioxidant and Antimicrobial Activities. Appl. Microbiol. Biotechnol. 2021, 105, 741–753. [Google Scholar] [CrossRef]
- Senapati, S.; Syed, A.; Khan, S.; Pasricha, R.; Khan, M.I.; Kumar, R.; Ahmad, A. Extracellular Biosynthesis of Metal Sulfide Nanoparticles Using the Fungus Fusarium Oxysporum. Curr. Nanosci. 2014, 10, 588–595. [Google Scholar] [CrossRef]
- Syed, A.; al Saedi, M.H.; Bahkali, A.H.; Elgorban, A.M.; Kharat, M.; Pai, K.; Ghodake, G.; Ahmad, A. Biological Synthesis of α-Ag2S Composite Nanoparticles Using the Fungus Humicola Sp. and Its Biomedical Applications. J. Drug Deliv. Sci. Technol. 2021, 66, 102770. [Google Scholar] [CrossRef]
- Pereira, L.; Dias, N.; Carvalho, J.; Fernandes, S.; Santos, C.; Lima, N. Synthesis, Characterization and Antifungal Activity of Chemically and Fungal-Produced Silver Nanoparticles against Trichophyton Rubrum. J. Appl. Microbiol. 2014, 117, 1601–1613. [Google Scholar] [CrossRef] [PubMed]
- El-Sayyad, G.S.; Mosallam, F.M.; El-Batal, A.I. One-Pot Green Synthesis of Magnesium Oxide Nanoparticles Using Penicillium Chrysogenum Melanin Pigment and Gamma Rays with Antimicrobial Activity against Multidrug-Resistant Microbes. Adv. Powder Technol. 2018, 29, 2616–2625. [Google Scholar] [CrossRef]
- El-Batal, A.I.; El-Sayyad, G.S.; Mosallam, F.M.; Fathy, R.M. Penicillium Chrysogenum-Mediated Mycogenic Synthesis of Copper Oxide Nanoparticles Using Gamma Rays for In Vitro Antimicrobial Activity Against Some Plant Pathogens. J. Clust. Sci. 2020, 31, 79–90. [Google Scholar] [CrossRef]
- Mohammed Fayaz, A.; Balaji, K.; Kalaichelvan, P.T.; Venkatesan, R. Fungal Based Synthesis of Silver Nanoparticles—An Effect of Temperature on the Size of Particles. Colloids Surf. B Biointerfaces 2009, 74, 123–126. [Google Scholar] [CrossRef]
- Mishra, A.; Kumari, M.; Pandey, S.; Chaudhry, V.; Gupta, K.C.; Nautiyal, C.S. Biocatalytic and Antimicrobial Activities of Gold Nanoparticles Synthesized by Trichoderma Sp. Bioresour. Technol. 2014, 166, 235–242. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Brar, S.K.; Kaur, S.; Verma, M. Green Approach for Nanoparticle Biosynthesis by Fungi: Current Trends and Applications. Crit. Rev. Biotechnol. 2012, 32, 49–73. [Google Scholar] [CrossRef]
- Bhainsa, K.C.; D’Souza, S.F. Extracellular Biosynthesis of Silver Nanoparticles Using the Fungus Aspergillus Fumigatus. Colloids Surf. B Biointerfaces 2006, 47, 160–164. [Google Scholar] [CrossRef]
- Vigneshwaran, N.; Ashtaputre, N.M.; Varadarajan, P.V.; Nachane, R.P.; Paralikar, K.M.; Balasubramanya, R.H. Biological Synthesis of Silver Nanoparticles Using the Fungus Aspergillus Flavus. Mater. Lett. 2007, 61, 1413–1418. [Google Scholar] [CrossRef]
- Lotfy, W.A.; Alkersh, B.M.; Sabry, S.A.; Ghozlan, H.A. Biosynthesis of Silver Nanoparticles by Aspergillus Terreus: Characterization, Optimization, and Biological Activities. Front. Bioeng. Biotechnol. 2021, 9, 633468. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.C.; Kharwar, R.N.; Gange, A.C. Biosynthesis of Antimicrobial Silver Nanoparticles by the Endophytic Fungus Aspergillus Clavatus. Nanomedicine 2010, 5, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.C.; Singh, S.K.; Solanki, R.; Prakash, S. Biofabrication of Anisotropic Gold Nanotriangles Using Extract of Endophytic Aspergillus Clavatus as a Dual Functional Reductant and Stabilizer. Nanoscale Res. Lett. 2010, 6, 16. [Google Scholar] [CrossRef]
- Rajesh Kumar, R.; Poornima Priyadharsani, K.; Thamaraiselvi, K. Mycogenic Synthesis of Silver Nanoparticles by the Japanese Environmental Isolate Aspergillus Tamarii. J. Nanoparticle Res. 2012, 14, 860. [Google Scholar] [CrossRef]
- Nayak, B.K.; Anitha, K. Combined Effects of Antibiotics and AgNPs Biosynthesized from Aspergillus Ustus Studied against Few Pathogenic Bacteria. Int. J. Pharmtech. Res. 2014, 6, 1976–1980. [Google Scholar]
- Phanjom, P.; Ahmed, G. Biosynthesis of Silver Nanoparticles by Aspergillus Oryzae (MTCC No. 1846) and Its Characterizations. Nanosci. Nanotechnol. 2015, 5, 14–21. [Google Scholar] [CrossRef]
- Bharathidasan, R.; Panneerselvam, A. Biosynthesis and Characterization of Silver Nanoparticles Using Endophytic Fungi Aspergillus Concius, Penicillium Janthinellum and Phomosis Sp. Int. J. Pharm. Sci. Res. 2012, 3, 3163–3169. [Google Scholar]
- Rajput, S.; Werezuk, R.; Lange, R.M.; McDermott, M.T. Fungal Isolate Optimized for Biogenesis of Silver Nanoparticles with Enhanced Colloidal Stability. Langmuir 2016, 32, 8688–8697. [Google Scholar] [CrossRef]
- Durán, N.; Marcato, P.D.; Alves, O.L.; De Souza, G.I.H.; Esposito, E. Mechanistic Aspects of Biosynthesis of Silver Nanoparticles by Several Fusarium Oxysporum Strains. J. Nanobiotechnol. 2005, 3, 8. [Google Scholar] [CrossRef]
- Bansal, V.; Rautaray, D.; Bharde, A.; Ahire, K.; Sanyal, A.; Ahmad, A.; Sastry, M. Fungus-Mediated Biosynthesis of Silica and Titania Particles. J. Mater. Chem. 2005, 15, 2583–2589. [Google Scholar] [CrossRef]
- Bansal, V.; Poddar, P.; Ahmad, A.; Sastry, M. Room-Temperature Biosynthesis of Ferroelectric Barium Titanate Nanoparticles. J. Am. Chem. Soc. 2006, 128, 11958–11963. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.A.; Ansary, A.A.; Abroad, A.; Khan, M.I. Extracellular Biosynthesis of CdSe Quantum Dots by the Fungus, Fusarium Oxysporum. J. Biomed. Nanotechnol. 2007, 3, 190–194. [Google Scholar] [CrossRef]
- Gupta, K.; Chundawat, T.S. Green Synthesis, Characterization and Antimicrobial Activity of Copper Nanoparticles Derived from Fusarium Oxysporum. In Proceedings of the AIP Conference Proceedings 2369; AIP Publishing: New York, NY, USA, 2021; p. 020082. [Google Scholar]
- Naimi-Shamel, N.; Pourali, P.; Dolatabadi, S. Green Synthesis of Gold Nanoparticles Using Fusarium Oxysporum and Antibacterial Activity of Its Tetracycline Conjugant. J. Mycol. Med. 2019, 29, 7–13. [Google Scholar] [CrossRef]
- Ingle, A.; Rai, M.; Gade, A.; Bawaskar, M. Fusarium Solani: A Novel Biological Agent for the Extracellular Synthesis of Silver Nanoparticles. J. Nanoparticle Res. 2009, 11, 2079–2085. [Google Scholar] [CrossRef]
- El-Rafie, M.H.; Shaheen, T.I.; Mohamed, A.A.; Hebeish, A. Bio-Synthesis and Applications of Silver Nanoparticles onto Cotton Fabrics. Carbohydr. Polym. 2012, 90, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Sogra Fathima, B.; Balakrishnan, R.M. Biosynthesis and Optimization of Silver Nanoparticles by Endophytic Fungus Fusarium Solani. Mater. Lett. 2014, 132, 428–431. [Google Scholar] [CrossRef]
- Basavaraja, S.; Balaji, S.D.; Lagashetty, A.; Rajasab, A.H.; Venkataraman, A. Extracellular Biosynthesis of Silver Nanoparticles Using the Fungus Fusarium Semitectum. Mater. Res. Bull. 2008, 43, 1164–1170. [Google Scholar] [CrossRef]
- Ingle, A.; Gade, A.; Pierrat, S.; Sonnichsen, C.; Rai, M. Mycosynthesis of Silver Nanoparticles Using the Fungus Fusarium Acuminatum and Its Activity Against Some Human Pathogenic Bacteria. Curr. Nanosci. 2008, 4, 141–144. [Google Scholar] [CrossRef]
- Shelar, G.B.; Chavan, A.M. Fusarium Semitectum Mediated Extracellular Synthesis of Silver Nanoparticles and Their Antibacterial Activity. Int. J. Biomed. Adv. Res. 2014, 5, 20–24. [Google Scholar]
- Bawaskar, M.; Gaikwad, S.; Ingle, A.; Rathod, D.; Gade, A.; Duran, N.; Marcato, P.D.; Rai, M. A New Report on Mycosynthesis of Silver Nanoparticles by Fusarium Culmorum. Curr. Nanosci. 2010, 6, 376–380. [Google Scholar] [CrossRef]
- Hamad, M.T. Biosynthesis of Silver Nanoparticles by Fungi and Their Antibacterial Activity. Int. J. Environ. Sci. Technol. 2019, 16, 1015–1024. [Google Scholar] [CrossRef]
- Kathiresan, K.; Manivannan, S.; Nabeel, M.A.; Dhivya, B. Studies on Silver Nanoparticles Synthesized by a Marine Fungus, Penicillium Fellutanum Isolated from Coastal Mangrove Sediment. Colloids Surf. B Biointerfaces 2009, 71, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Kamalakannan, S.; Gobinath, C.; Ananth, S. Synthesis and Characterization of Fungus Mediated Silver Nanoparticle for Toxicity on Filarial Vector, Culex Quinquefasciatus. Int. J. Pharm. Sci. Rev. Res. 2014, 24, 124–132. [Google Scholar]
- Datta, M.; Desay, D. Green Synthesis of Silver Antimicrobials for Its Potential Application in Control of Nosocomial Infections. Asian J. Pharm. Clin. Res. 2015, 8, 219–223. [Google Scholar]
- Devi, L.S.; Bareh, D.A.; Joshi, S.R. Studies on Biosynthesis of Antimicrobial Silver Nanoparticles Using Endophytic Fungi Isolated from the Ethno-Medicinal Plant Gloriosa Superba L. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2014, 84, 1091–1099. [Google Scholar] [CrossRef]
- Honary, S.; Barabadi, H.; Gharaei-Fathabad, E.; Naghibi, F. Green Synthesis of Silver Nanoparticles Induced by the Fungus Penicillium Citrinum. Trop. J. Pharm. Res. 2013, 12, 7–11. [Google Scholar] [CrossRef]
- Maliszewska, I.; Juraszek, A.; Bielska, K. Green Synthesis and Characterization of Silver Nanoparticles Using Ascomycota Fungi Penicillium Nalgiovense AJ12. J. Clust. Sci. 2014, 25, 989–1004. [Google Scholar] [CrossRef]
- Nayak, R.R.; Pradhan, N.; Behera, D.; Pradhan, K.M.; Mishra, S.; Sukla, L.B.; Mishra, B.K. Green Synthesis of Silver Nanoparticle by Penicillium Purpurogenum NPMF: The Process and Optimization. J. Nanoparticle Res. 2011, 13, 3129–3137. [Google Scholar] [CrossRef]
- Pradhan, N.; Nayak, R.R.; Pradhan, A.K.; Sukla, L.B.; Mishra, B.K. In Situ Synthesis of Entrapped Silver Nanoparticles by a Fungus-Penicillium Purpurogenum. Nanosci. Nanotechnol. Lett. 2011, 3, 659–665. [Google Scholar] [CrossRef]
- Taha, Z.K.; Hawar, S.N.; Sulaiman, G.M. Extracellular Biosynthesis of Silver Nanoparticles from Penicillium Italicum and Its Antioxidant, Antimicrobial and Cytotoxicity Activities. Biotechnol. Lett. 2019, 41, 899–914. [Google Scholar] [CrossRef]
- Honary, S.; Barabadi, H.; Gharaei-Fathabad, E.; Naghibi, F. Green Synthesis of Copper Oxide Nanoparticles Using Penicillium Aurantiogriseum, Penicillium Citrinum and Penicillium Waksmanii. Dig. J. Nanomater. Biostruct. 2012, 7, 999–1005. [Google Scholar]
- Barabadi, H.; Honary, S.; Ali Mohammadi, M.; Ahmadpour, E.; Rahimi, M.T.; Alizadeh, A.; Naghibi, F.; Saravanan, M. Green Chemical Synthesis of Gold Nanoparticles by Using Penicillium Aculeatum and Their Scolicidal Activity against Hydatid Cyst Protoscolices of Echinococcus Granulosus. Environ. Sci. Pollut. Res. 2017, 24, 5800–5810. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Tripathy, S.K.; Wahab, R.; Jeong, S.H.; Hwang, I.; Yang, Y.B.; Kim, Y.S.; Shin, H.S.; Yun, S.I. Microbial Synthesis of Gold Nanoparticles Using the Fungus Penicillium Brevicompactum and Their Cytotoxic Effects against Mouse Mayo Blast Cancer C 2C 12 Cells. Appl. Microbiol. Biotechnol. 2011, 92, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Golnaraghi Ghomi, A.R.; Mohammadi-Khanaposhti, M.; Vahidi, H.; Kobarfard, F.; Ameri Shah Reza, M.; Barabadi, H. Fungus-Mediated Extracellular Biosynthesis and Characterization of Zirconium Nanoparticles Using Standard Penicillium Species and Their Preliminary Bactericidal Potential: A Novel Biological Approach to Nanoparticle Synthesis. Iran. J. Pharm. Res. 2019, 18, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Alghuthaymi, M.A.; Abd-Elsalam, K.A.; AboDalam, H.M.; Ahmed, F.K.; Ravichandran, M.; Kalia, A.; Rai, M. Trichoderma: An Eco-Friendly Source of Nanomaterials for Sustainable Agroecosystems. J. Fungi 2022, 8, 367. [Google Scholar] [CrossRef]
- Mukherjee, P.; Roy, M.; Mandal, B.P.; Dey, G.K.; Mukherjee, P.K.; Ghatak, J.; Tyagi, A.K.; Kale, S.P. Green Synthesis of Highly Stabilized Nanocrystalline Silver Particles by a Non-Pathogenic and Agriculturally Important Fungus T. Asperellum. Nanotechnology 2008, 19, 075103. [Google Scholar] [CrossRef]
- Devi, T.P.; Kulanthaivel, S.; Kamil, D.; Borah, J.L.; Prabhakaran, N.; Srinivasa, N. Biosynthesis of Silver Nanoparticles from Trichoderma Species. Indian J. Exp. Biol. 2013, 51, 543–547. [Google Scholar]
- Ahluwalia, V.; Kumar, J.; Sisodia, R.; Shakil, N.A.; Walia, S. Green Synthesis of Silver Nanoparticles by Trichoderma Harzianum and Their Bio-Efficacy Evaluation against Staphylococcus Aureus and Klebsiella Pneumonia. Ind. Crops Prod. 2014, 55, 202–206. [Google Scholar] [CrossRef]
- Vahabi, K.; Mansoori, G.A.; Karimi, S. Biosynthesis of Silver Nanoparticles by Fungus Trichoderma Reesei (A Route for Large-Scale Production of AgNPs). Insciences J. 2011, 1, 65–79. [Google Scholar] [CrossRef]
- Gemishev, O.T.; Panayotova, M.I.; Mintcheva, N.N.; Djerahov, L.P.; Tyuliev, G.T.; Gicheva, G.D. A Green Approach for Silver Nanoparticles Preparation by Cell-Free Extract from Trichoderma Reesei Fungi and Their Characterization. Mater. Res. Express 2019, 6, 95040. [Google Scholar] [CrossRef]
- Bilesky-José, N.; Maruyama, C.; Germano-Costa, T.; Campos, E.; Carvalho, L.; Grillo, R.; Fraceto, L.F.; de Lima, R. Biogenic α-Fe2O3 Nanoparticles Enhance the Biological Activity of Trichoderma against the Plant Pathogen Sclerotinia Sclerotiorum. ACS Sustain. Chem. Eng. 2021, 9, 1669–1683. [Google Scholar] [CrossRef]
- Consolo, V.F.; Torres-Nicolini, A.; Alvarez, V.A. Mycosinthetized Ag, CuO and ZnO Nanoparticles from a Promising Trichoderma Harzianum Strain and Their Antifungal Potential against Important Phytopathogens. Sci. Rep. 2020, 10, 20499. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Jeevithan, E.; Hu, X.; Chelliah, R.; Oh, D.H.; Wang, M.H. Enhanced Anti-Lung Carcinoma and Anti-Biofilm Activity of Fungal Molecules Mediated Biogenic Zinc Oxide Nanoparticles Conjugated with β-D-Glucan from Barley. J. Photochem. Photobiol. B 2020, 203, 111728. [Google Scholar] [CrossRef] [PubMed]
- Natesan, K.; Ponmurugan, P.; Gnanamangai, B.M.; Manigandan, V.; Joy, S.P.J.; Jayakumar, C.; Amsaveni, G. Biosynthesis of Silica and Copper Nanoparticles from Trichoderma, Streptomyces and Pseudomonas Spp. Evaluated against Collar Canker and Red Root-Rot Disease of Tea Plants. Arch. Phytopathol. Plant Prot. 2021, 54, 56–85. [Google Scholar] [CrossRef]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Parishcha, R.; Ajaykumar, P.V.; Alam, M.; Kumar, R.; et al. Fungus-Mediated Synthesis of Silver Nanoparticles and Their Immobilization in the Mycelial Matrix: A Novel Biological Approach to Nanoparticle Synthesis. Nano Lett. 2001, 1, 515–519. [Google Scholar] [CrossRef]
- Gericke, M.; Pinches, A. Biological Synthesis of Metal Nanoparticles. Hydrometallurgy 2006, 83, 132–140. [Google Scholar] [CrossRef]
- Apte, M.; Sambre, D.; Gaikawad, S.; Joshi, S.; Bankar, A.; Kumar, A.R.; Zinjarde, S. Psychrotrophic Yeast Yarrowia Lipolytica NCYC 789 Mediates the Synthesis of Antimicrobial Silver Nanoparticles via Cell-Associated Melanin. AMB Express 2013, 3, 32. [Google Scholar] [CrossRef]
- Waghmare, S.R.; Mulla, M.N.; Marathe, S.R.; Sonawane, K.D. Ecofriendly Production of Silver Nanoparticles Using Candida Utilis and Its Mechanistic Action against Pathogenic Microorganisms. 3 Biotech 2015, 5, 33–38. [Google Scholar] [CrossRef]
- Eugenio, M.; Müller, N.; Frasés, S.; Almeida-Paes, R.; Lima, L.M.T.R.; Lemgruber, L.; Farina, M.; de Souza, W.; Sant’Anna, C. Yeast-Derived Biosynthesis of Silver/Silver Chloride Nanoparticles and Their Antiproliferative Activity against Bacteria. RSC Adv. 2016, 6, 9893–9904. [Google Scholar] [CrossRef]
- Jalal, M.; Ansari, M.; Alzohairy, M.; Ali, S.; Khan, H.; Almatroudi, A.; Raees, K. Biosynthesis of Silver Nanoparticles from Oropharyngeal Candida Glabrata Isolates and Their Antimicrobial Activity against Clinical Strains of Bacteria and Fungi. Nanomaterials 2018, 8, 586. [Google Scholar] [CrossRef]
- Elahian, F.; Reiisi, S.; Shahidi, A.; Mirzaei, S.A. High-Throughput Bioaccumulation, Biotransformation, and Production of Silver and Selenium Nanoparticles Using Genetically Engineered Pichia Pastoris. Nanomedicine 2017, 13, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.S.; Joshi, S.R. Antimicrobial and Synergistic Effects of Silver Nanoparticles Synthesized Using Soil Fungi of High Altitudes of Eastern Himalaya. Mycobiology 2012, 40, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Suchitra, D.; Nageswara Rao AB, N.; Ravindranath, A.; Sakunthala Madhavendra, S.; Jayathirtha Rao, V. Silver Nanoparticle Synthesis from Lecanicillium Lecanii and Evalutionary Treatment on Cotton Fabrics by Measuring Their Improved Antibacterial Activity with Antibiotics against Staphylococcus Aureus (ATCC 29213) and E. Coli (ATCC 25922) Strains. Int. J. Pharm. Pharm. Sci. 2011, 3, 190–195. [Google Scholar]
- Niknejad, F.; Nabili, M.; Daie Ghazvini, R.; Moazeni, M. Green Synthesis of Silver Nanoparticles: Advantages of the Yeast Saccharomyces Cerevisiae Model. Curr. Med. Mycol. 2015, 1, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Salunke, B.K.; Sawant, S.S.; Lee, S.-I.; Kim, B.S. Comparative Study of MnO2 Nanoparticle Synthesis by Marine Bacterium Saccharophagus Degradans and Yeast Saccharomyces Cerevisiae. Appl. Microbiol. Biotechnol. 2015, 99, 5419–5427. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qu, Y.; Shen, W.; Wang, J.; Li, H.; Zhang, Z.; Li, S.; Zhou, J. Biogenic Synthesis of Gold Nanoparticles by Yeast Magnusiomyces Ingens LH-F1 for Catalytic Reduction of Nitrophenols. Colloids Surf. A Physicochem Eng. Asp. 2016, 497, 280–285. [Google Scholar] [CrossRef]
- Kowshik, M.; Deshmukh, N.; Vogel, W.; Urban, J.; Kulkarni, S.K.; Paknikar, K.M. Microbial Synthesis of Semiconductor CdS Nanoparticles, Their Characterization, and Their Use in the Fabrication of an Ideal Diode. Biotechnol. Bioeng. 2002, 78, 583–588. [Google Scholar] [CrossRef]
- Dameron, C.T.; Reese, R.N.; Mehra, R.K.; Kortan, A.R.; Carroll, P.J.; Steigerwald, M.L.; Brus, L.E.; Winge, D.R. Biosynthesis of Cadmium Sulphide Quantum Semiconductor Crystallites. Nature 1989, 338, 596–597. [Google Scholar] [CrossRef]
- Birla, S.S.; Tiwari, V.V.; Gade, A.K.; Ingle, A.P.; Yadav, A.P.; Rai, M.K. Fabrication of Silver Nanoparticles by Phoma Glomerata and Its Combined Effect against Escherichia Coli, Pseudomonas Aeruginosa and Staphylococcus Aureus. Lett. Appl. Microbiol. 2009, 48, 173–179. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Gade, A.K.; Duarte, M.C.T.; Duran, N. Three Phoma Spp. Synthesised Novel Silver Nanoparticles That Possess Excellent Antimicrobial Efficacy. IET Nanobiotechnol. 2015, 9, 280–287. [Google Scholar] [CrossRef]
- Banu, A.N.; Balasubramanian, C. Myco-Synthesis of Silver Nanoparticles Using Beauveria Bassiana against Dengue Vector, Aedes Aegypti (Diptera: Culicidae). Parasitol. Res. 2014, 113, 2869–2877. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Ganachari, S.; Deshpande, R.; Bedre, M.; Abbaraju, V. Biosynthesis and Characterization of Silver Nanoparticles Using Extract of Fungi Acremonium Diospyri. Int. J. Sci. Res. Conf. Proc. 2012, 1, 314–316. [Google Scholar]
- Raheman, F.; Deshmukh, S.; Ingle, A.; Gade, A.; Rai, M. Silver Nanoparticles: Novel Antimicrobial Agent Synthesized from an Endophytic Fungus Pestalotia Sp. Isolated from Leaves of Syzygium Cumini (L). Nano Biomed. Eng. 2011, 3, 174–178. [Google Scholar] [CrossRef]
- Balaji, D.S.; Basavaraja, S.; Deshpande, R.; Mahesh, D.B.; Prabhakar, B.K.; Venkataraman, A. Extracellular Biosynthesis of Functionalized Silver Nanoparticles by Strains of Cladosporium Cladosporioides Fungus. Colloids Surf. B Biointerfaces 2009, 68, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Chandankere, R.; Chelliah, J.; Subban, K.; Shanadrahalli, V.C.; Parvez, A.; Zabed, H.M.; Sharma, Y.C.; Qi, X. Pleiotropic Functions and Biological Potentials of Silver Nanoparticles Synthesized by an Endophytic Fungus. Front. Bioeng. Biotechnol. 2020, 8, 00095. [Google Scholar] [CrossRef] [PubMed]
- Hamedi, S.; Shojaosadati, S.A.; Shokrollahzadeh, S.; Hashemi-Najafabadi, S. Extracellular Biosynthesis of Silver Nanoparticles Using a Novel and Non-Pathogenic Fungus, Neurospora Intermedia: Controlled Synthesis and Antibacterial Activity. World J. Microbiol. Biotechnol. 2014, 30, 693–704. [Google Scholar] [CrossRef]
- Qian, Y.; Yu, H.; He, D.; Yang, H.; Wang, W.; Wan, X.; Wang, L. Biosynthesis of Silver Nanoparticles by the Endophytic Fungus Epicoccum Nigrum and Their Activity against Pathogenic Fungi. Bioprocess Biosyst. Eng. 2013, 36, 1613–1619. [Google Scholar] [CrossRef]
- Saha, S.; Sarkar, J.; Chattopadhyay, D.; Patra, S.; Chakraborty, A.; Acharya, K. Production Of Silver Nanoparticles By A Phytopathogenic Fungus Bipolaris Nodulosa And Its Antimicrobial Activity. Dig. J. Nanomater. Biostruct. 2010, 5, 887–895. [Google Scholar]
- Castro-Longoria, E.; Vilchis-Nestor, A.R.; Avalos-Borja, M. Biosynthesis of Silver, Gold and Bimetallic Nanoparticles Using the Filamentous Fungus Neurospora Crassa. Colloids Surf. B Biointerfaces 2011, 83, 42–48. [Google Scholar] [CrossRef]
- Bhargava, A.; Jain, N.; Khan, M.A.; Pareek, V.; Dilip, R.V.; Panwar, J. Utilizing Metal Tolerance Potential of Soil Fungus for Efficient Synthesis of Gold Nanoparticles with Superior Catalytic Activity for Degradation of Rhodamine B. J. Environ. Manag. 2016, 183, 22–32. [Google Scholar] [CrossRef]
- Castro-Longoria, E.; Moreno-Velasquez, S.D.; Moreno-Velasquez, S.D.; Arenas-Berumen, E. Production of Platinum Nanoparticles and Nanoaggregates Using Neurospora Crassa. J. Microbiol. Biotechnol. 2012, 22, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Gadd, G.M. Biosynthesis of Copper Carbonate Nanoparticles by Ureolytic Fungi. Appl. Microbiol. Biotechnol. 2017, 101, 7397–7407. [Google Scholar] [CrossRef]
- Suryavanshi, P.; Pandit, R.; Gade, A.K.; Derita, M.G.; Zachino, S.A.; Rai, M. Colletotrichum Sp.- Mediated Synthesis of Sulphur and Aluminium Oxide Nanoparticles and Its in Vitro Activity against Selected Food-Borne Pathogens. Lwt-Food Sci. Technol. 2017, 81, 188–194. [Google Scholar] [CrossRef]
- Berger, R.G.; Bordewick, S.; Krahe, N.-K.; Ersoy, F. Mycelium vs. Fruiting Bodies of Edible Fungi—A Comparison of Metabolites. Microorganisms 2022, 10, 1379. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, V.K.; Yadav, N.; Rai, N.K.; Ellah, N.H.A.; Bohara, R.A.; Rehan, I.F.; Marraiki, N.; Batiha, G.E.-S.; Hetta, H.F.; Singh, M.P. Pleurotus Sajor-Caju-Mediated Synthesis of Silver and Gold Nanoparticles Active against Colon Cancer Cell Lines: A New Era of Herbonanoceutics. Molecules 2020, 25, 3091. [Google Scholar] [CrossRef] [PubMed]
- Rabeea, M.A.; Owaid, M.N.; Aziz, A.A.; Jameel, M.S.; Dheyab, M.A. Mycosynthesis of Gold Nanoparticles Using the Extract of Flammulina Velutipes, Physalacriaceae, and Their Efficacy for Decolorization of Methylene Blue. J. Environ. Chem. Eng. 2020, 8, 103841. [Google Scholar] [CrossRef]
- El-Ramady, H.; Abdalla, N.; Fawzy, Z.; Badgar, K.; Llanaj, X.; Törős, G.; Hajdú, P.; Eid, Y.; Prokisch, J. Green Biotechnology of Oyster Mushroom (Pleurotus ostreatus L.): A Sustainable Strategy for Myco-Remediation and Bio-Fermentation. Sustainability 2022, 14, 3667. [Google Scholar] [CrossRef]
- Ghahremani-Majd, H.; Dashti, F. Chemical Composition and Antioxidant Properties of Cultivated Button Mushrooms (Agaricus bisporus). Hortic. Environ. Biotechnol. 2015, 56, 376–382. [Google Scholar] [CrossRef]
- Vetchinkina, E.; Loshchinina, E.; Kupryashina, M.; Burov, A.; Pylaev, T.; Nikitina, V. Green Synthesis of Nanoparticles with Extracellular and Intracellular Extracts of Basidiomycetes. PeerJ 2018, 6, e5237. [Google Scholar] [CrossRef]
- Owaid, M.N.; Naeem, G.A.; Muslim, R.F.; Oleiwi, R.S. Synthesis, Characterization and Antitumor Efficacy of Silver Nanoparticle from Agaricus Bisporus Pileus, Basidiomycota. Walailak J. Sci. Technol. (WJST) 2018, 17, 75–87. [Google Scholar] [CrossRef]
- Mohana, S.; Sumathi, S. Multi-Functional Biological Effects of Palladium Nanoparticles Synthesized Using Agaricus Bisporus. J. Clust. Sci. 2020, 31, 391–400. [Google Scholar] [CrossRef]
- Naeem, G.A.; Jaloot, A.S.; Owaid, M.N.; Muslim, R.F. Green Synthesis of Gold Nanoparticles from Coprinus Comatus, Agaricaceae, and the Effect of Ultraviolet Irradiation on Their Characteristics. Walailak J. Sci. Technol. (WJST) 2021, 18, 9396. [Google Scholar] [CrossRef]
- Dandapat, S.; Kumar, M.; Ranjan, R.; Sinha, M.P. Ganoderma Applanatum Extract Mediated Synthesis of Silver Nanoparticles. Braz. J. Pharm. Sci. 2022, 58, e19173. [Google Scholar] [CrossRef]
- Sedefoglu, N.; Zalaoglu, Y.; Bozok, F. Green Synthesized ZnO Nanoparticles Using Ganoderma Lucidum: Characterization and In Vitro Nanofertilizer Effects. J. Alloys Compd. 2022, 918, 165695. [Google Scholar] [CrossRef]
- Manimaran, K.; Murugesan, S.; Ragavendran, C.; Balasubramani, G.; Natarajan, D.; Ganesan, A.; Seedevi, P. Biosynthesis of TiO2 Nanoparticles Using Edible Mushroom (Pleurotus Djamor) Extract: Mosquito Larvicidal, Histopathological, Antibacterial and Anticancer Effect. J. Clust. Sci. 2021, 32, 1229–1240. [Google Scholar] [CrossRef]
- Khan, A.U.; Malik, N.; Khan, M.; Cho, M.H.; Khan, M.M. Fungi-Assisted Silver Nanoparticle Synthesis and Their Applications. Bioprocess. Biosyst. Eng. 2018, 41, 1–20. [Google Scholar] [CrossRef]
- Naraian, R.; Abhishek, A.K.B. Green Synthesis and Characterization of Silver NPs Using Oyster Mushroom Extract For Antibacterial Efficacy. J. Chem. Environ. Sci. Its Appl. 2020, 7, 13–18. [Google Scholar] [CrossRef]
- Bhardwaj, K.; Sharma, A.; Tejwan, N.; Bhardwaj, S.; Bhardwaj, P.; Nepovimova, E.; Shami, A.; Kalia, A.; Kumar, A.; Abd-Elsalam, K.A.; et al. Pleurotus Macrofungi-Assisted Nanoparticle Synthesis and Its Potential Applications: A Review. J. Fungi 2020, 6, 351. [Google Scholar] [CrossRef]
- Bhat, R.; Deshpande, R.; Ganachari, S.V.; Huh, D.S.; Venkataraman, A. Photo-Irradiated Biosynthesis of Silver Nanoparticles Using Edible Mushroom Pleurotus Florida and Their Antibacterial Activity Studies. Bioinorg. Chem. Appl. 2011, 2011, 650979. [Google Scholar] [CrossRef]
- Manimaran, K.; Balasubramani, G.; Ragavendran, C.; Natarajan, D.; Murugesan, S. Biological Applications of Synthesized ZnO Nanoparticles Using Pleurotus Djamor Against Mosquito Larvicidal, Histopathology, Antibacterial, Antioxidant and Anticancer Effect. J. Clust. Sci. 2021, 32, 1635–1647. [Google Scholar] [CrossRef]
- Sarkar, J.; Kalyan Roy, S.; Laskar, A.; Chattopadhyay, D.; Acharya, K. Bioreduction of Chloroaurate Ions to Gold Nanoparticles by Culture Filtrate of Pleurotus Sapidus Quél. Mater. Lett. 2013, 92, 313–316. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Sharma, R.; Nepovimova, E.; Cruz-Martins, N.; Dhanjal, D.S.; Singh, R.; Chopra, C.; Verma, R.; Abd-Elsalam, K.A.; et al. Potential Usage of Edible Mushrooms and Their Residues to Retrieve Valuable Supplies for Industrial Applications. J. Fungi 2021, 7, 427. [Google Scholar] [CrossRef] [PubMed]
- Eskandari-Nojedehi, M.; Jafarizadeh-Malmiri, H.; Rahbar-Shahrouzi, J. Hydrothermal Green Synthesis of Gold Nanoparticles Using Mushroom (Agaricus Bisporus) Extract: Physico-Chemical Characteristics and Antifungal Activity Studies. Green Process. Synth. 2018, 7, 38–47. [Google Scholar] [CrossRef]
- Loshchinina, E.A.; Vetchinkina, E.P.; Kupryashina, M.A.; Kursky, V.F.; Nikitina, V.E. Nanoparticles Synthesis by Agaricus Soil Basidiomycetes. J. Biosci. Bioeng. 2018, 126, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Chopra, H.; Bibi, S.; Singh, I.; Hasan, M.M.; Khan, M.S.; Yousafi, Q.; Baig, A.A.; Rahman, M.M.; Islam, F.; bin Emran, T.; et al. Green Metallic Nanoparticles: Biosynthesis to Applications. Front. Bioeng. Biotechnol. 2022, 10. [Google Scholar] [CrossRef]
- Poudel, M.; Pokharel, R.; Sudip, K.C.; Awal, S.; Pradhananga, R. Biosynthesis of Silver Nanoparticles Using Ganoderma Lucidum and Assessment of Antioxidant and Antibacterial Activity. Int. J. Appl. Sci. Biotechnol. 2017, 5, 523. [Google Scholar] [CrossRef]
- Nguyen, V.P.; le Trung, H.; Nguyen, T.H.; Hoang, D.; Tran, T.H. Synthesis of Biogenic Silver Nanoparticles with Eco-Friendly Processes Using Ganoderma Lucidum Extract and Evaluation of Their Theranostic Applications. J. Nanomater. 2021, 2021, 6135920. [Google Scholar] [CrossRef]
- Gurunathan, S.; Raman, J.; Abd Malek, S.N.; John, P.; Sabaratnam, V. Green Synthesis of Silver Nanoparticles Using Ganoderma Neo-Japonicum Imazeki: A Potential Cytotoxic Agent against Breast Cancer Cells. Int. J. Nanomed. 2013, 8, 4399–4413. [Google Scholar] [CrossRef]
- Murillo-Rábago, E.I.; Vilchis-Nestor, A.R.; Juarez-Moreno, K.; Garcia-Marin, L.E.; Quester, K.; Castro-Longoria, E. Optimized Synthesis of Small and Stable Silver Nanoparticles Using Intracellular and Extracellular Components of Fungi: An Alternative for Bacterial Inhibition. Antibiotics 2022, 11, 800. [Google Scholar] [CrossRef]
- Nguyen, V.P.; le Trung, H.; Nguyen, T.H.; Hoang, D.; Tran, T.H. Advancement of Microwave-Assisted Biosynthesis for Preparing Au Nanoparticles Using Ganoderma Lucidum Extract and Evaluation of Their Catalytic Reduction of 4-Nitrophenol. ACS Omega 2021, 6, 32198–32207. [Google Scholar] [CrossRef]
- Clarance, P.; Luvankar, B.; Sales, J.; Khusro, A.; Agastian, P.; Tack, J.-C.; Al Khulaifi, M.M.; AL-Shwaiman, H.A.; Elgorban, A.M.; Syed, A.; et al. Green Synthesis and Characterization of Gold Nanoparticles Using Endophytic Fungi Fusarium Solani and Its In-Vitro Anticancer and Biomedical Applications. Saudi J. Biol. Sci. 2020, 27, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Arun, G.; Eyini, M.; Gunasekaran, P. Green Synthesis of Silver Nanoparticles Using the Mushroom Fungus Schizophyllum Commune and Its Biomedical Applications. Biotechnol. Bioprocess Eng. 2014, 19, 1083–1090. [Google Scholar] [CrossRef]
- Balakumaran, M.D.; Ramachandran, R.; Kalaichelvan, P.T. Exploitation of Endophytic Fungus, Guignardia Mangiferae for Extracellular Synthesis of Silver Nanoparticles and Their in Vitro Biological Activities. Microbiol. Res. 2015, 178, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Husseiny, S.M.; Salah, T.A.; Anter, H.A. Biosynthesis of Size Controlled Silver Nanoparticles by Fusarium Oxysporum, Their Antibacterial and Antitumor Activities. Beni. Suef. Univ. J. Basic. Appl. Sci. 2015, 4, 225–231. [Google Scholar] [CrossRef]
- Guilger-Casagrande, M.; de Lima, R. Synthesis of Silver Nanoparticles Mediated by Fungi: A Review. Front. Bioeng. Biotechnol. 2019, 7, 287. [Google Scholar] [CrossRef]
- Durán, N.; Marcato, P.D.; De Souza, G.I.H.; Alves, O.L.; Esposito, E. Antibacterial Effect of Silver Nanoparticles Produced by Fungal Process on Textile Fabrics and Their Effluent Treatment. J. Biomed. Nanotechnol. 2007, 3, 203–208. [Google Scholar] [CrossRef]
- Srivastava, S.; Bhargava, A.; Pathak, N.; Srivastava, P. Production, Characterization and Antibacterial Activity of Silver Nanoparticles Produced by Fusarium Oxysporum and Monitoring of Protein-Ligand Interaction through in-Silico Approaches. Microb. Pathog. 2019, 129, 136–145. [Google Scholar] [CrossRef]
- Elegbede, J.A.; Lateef, A.; Azeez, M.A.; Asafa, T.B.; Yekeen, T.A.; Oladipo, I.C.; Adebayo, E.A.; Beukes, L.S.; Gueguim-Kana, E.B. Fungal Xylanases-Mediated Synthesis of Silver Nanoparticles for Catalytic and Biomedical Applications. IET Nanobiotechnol. 2018, 12, 857–863. [Google Scholar] [CrossRef]
- Fatima, F.; Wahid, I. Eco-Friendly Synthesis of Silver and Copper Nanoparticles by Shizophyllum Commune Fungus and Its Biomedical Applications. Int. J. Environ. Sci. Technol. 2021, 19, 7915–7926. [Google Scholar] [CrossRef]
- Khan, M.; Khan, A.U.; Rafatullah, M.; Alam, M.; Bogdanchikova, N.; Garibo, D. Search for Effective Approaches to Fight Microorganisms Causing High Losses in Agriculture: Application of P. Lilacinum Metabolites and Mycosynthesised Silver Nanoparticles. Biomolecules 2022, 12, 174. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S.; Kiran, K.; Banerjee, S.; Pande, V.; Dandapat, A. Myco-Nanotechnological Approach to Synthesize Silver Oxide Nanocuboids Using Endophytic Fungus Isolated from Citrus Pseudolimon Plant. Colloids Surf B Biointerfaces 2021, 206, 111948. [Google Scholar] [CrossRef]
- Islam, S.N.; Raza, A.; Naqvi, S.M.A.; Parveen, S.; Ahmad, A. Unveiling the Antisporulant Activity of Mycosynthesized Gold-Selenide Nanoparticles against Black Fungus Aspergillus Niger. Surf. Interfaces 2022, 29, 101769. [Google Scholar] [CrossRef]
- Sharma, J.L.; Dhayal, V.; Sharma, R.K. White-Rot Fungus Mediated Green Synthesis of Zinc Oxide Nanoparticles and Their Impregnation on Cellulose to Develop Environmental Friendly Antimicrobial Fibers. 3 Biotech 2021, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Win, T.T.; Khan, S.; Fu, P. Fungus- (Alternaria Sp.) Mediated Silver Nanoparticles Synthesis, Characterization, and Screening of Antifungal Activity against Some Phytopathogens. J. Nanotechnol. 2020, 2020, 8828878. [Google Scholar] [CrossRef]
- Wang, D.; Xue, B.; Wang, L.; Zhang, Y.; Liu, L.; Zhou, Y. Fungus-Mediated Green Synthesis of Nano-Silver Using Aspergillus Sydowii and Its Antifungal/Antiproliferative Activities. Sci. Rep. 2021, 11, 10356. [Google Scholar] [CrossRef]
- Thakor, R.; Mistry, H.; Patel, H.; Jhala, D.; Parmar, N.; Bariya, H. Biogenic Synthesis of Silver Nanoparticles Mediated by the Consortium Comprising the Marine Fungal Filtrates of Penicillium Oxalicum and Fusarium Hainanense along with Their Antimicrobial, Antioxidant, Larvicidal and Anticancer Potency. J. Appl. Microbiol. 2022, 133, 857–869. [Google Scholar] [CrossRef]
- Sumanth, B.; Lakshmeesha, T.R.; Ansari, M.A.; Alzohairy, M.A.; Udayashankar, A.C.; Shobha, B.; Niranjana, S.R.; Srinivas, C.; Almatroudi, A. Mycogenic Synthesis of Extracellular Zinc Oxide Nanoparticles from <em>Xylaria Acuta</Em> and Its Nanoantibiotic Potential. Int. J. Nanomed. 2020, 15, 8519–8536. [Google Scholar] [CrossRef]
- Gajbhiye, M.; Kesharwani, J.; Ingle, A.; Gade, A.; Rai, M. Fungus-Mediated Synthesis of Silver Nanoparticles and Their Activity against Pathogenic Fungi in Combination with Fluconazole. Nanomedicine 2009, 5, 382–386. [Google Scholar] [CrossRef]
- Gaikwad, S.; Ingle, A.; Gade, A.; Rai, M.; Falanga, A.; Incoronato, N.; Russo, L.; Galdiero, S.; Galdiero, M. Antiviral Activity of Mycosynthesized Silver Nanoparticles against Herpes Simplex Virus and Human Parainfluenza Virus Type 3. Int. J. Nanomed. 2013, 8, 4303–4314. [Google Scholar] [CrossRef]
- Pei, X.; Qu, Y.; Shen, W.; Li, H.; Zhang, X.; Li, S.; Zhang, Z.; Li, X. Green Synthesis of Gold Nanoparticles Using Fungus Mariannaea Sp. HJ and Their Catalysis in Reduction of 4-Nitrophenol. Environ. Sci. Pollut. Res. 2017, 24, 21649–21659. [Google Scholar] [CrossRef]
- Qu, Y.; Li, X.; Lian, S.; Dai, C.; Jv, Z.; Zhao, B.; Zhou, H. Biosynthesis of Gold Nanoparticles Using Fungus Trichoderma Sp. WL-Go and Their Catalysis in Degradation of Aromatic Pollutants. IET Nanobiotechnol. 2019, 13, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.M.; Rajan, R.; Tom, T.C.; Kumar, V.S.; Kurup, G.G.; Shanmuganathan, R.; Pugazhendhi, A. Biogenic Design of ZnS Quantum Dots—Insights into Their in-Vitro Cytotoxicity, Photocatalysis and Biosensing Properties. Ceram. Int. 2019, 45, 24193–24201. [Google Scholar] [CrossRef]
- Priyanka, U.; Gowda, K.M.A.; Elisha, M.G.; Teja, B.S.; Nitish, N.; Mohan, B.R. Biologically Synthesized PbS Nanoparticles for the Detection of Arsenic in Water. Int. Biodeterior. Biodegrad. 2017, 119, 78–86. [Google Scholar] [CrossRef]
- Prakash, S.; Singh, G.; Soni, N.; Sharma, S. Pathogenicity of Fusarium Oxysporum against the Larvae of Culex Quinquefasciatus (Say) and Anopheles Stephensi (Liston) in Laboratory. Parasitol. Res. 2010, 107, 651–655. [Google Scholar] [CrossRef]
- Soni, N.; Prakash, S. Effect of Chrysosporium Keratinophilum Metabolites against Culex Quinquefasciatus after Chromatographic Purification. Parasitol. Res. 2010, 107, 1329–1336. [Google Scholar] [CrossRef]
- Raliya, R.; Tarafdar, J.C.; Biswas, P. Enhancing the Mobilization of Native Phosphorus in the Mung Bean Rhizosphere Using ZnO Nanoparticles Synthesized by Soil Fungi. J. Agric. Food Chem. 2016, 64, 3111–3118. [Google Scholar] [CrossRef]
- Shobha, B.; Lakshmeesha, T.R.; Ansari, M.A.; Almatroudi, A.; Alzohairy, M.A.; Basavaraju, S.; Alurappa, R.; Niranjana, S.R.; Chowdappa, S. Mycosynthesis of ZnO Nanoparticles Using Trichoderma Spp. Isolated from Rhizosphere Soils and Its Synergistic Antibacterial Effect against Xanthomonas Oryzae Pv. Oryzae. J. Fungi 2020, 6, 181. [Google Scholar] [CrossRef]
- Zaki, S.A.; Ouf, S.A.; Albarakaty, F.M.; Habeb, M.M.; Aly, A.A.; Abd-Elsalam, K.A. Trichoderma Harzianum-Mediated ZnO Nanoparticles: A Green Tool for Controlling Soil-Borne Pathogens in Cotton. J. Fungi 2021, 7, 952. [Google Scholar] [CrossRef]
- Elgorban, A.M.; Aref, S.M.; Seham, S.M.; Elhindi, K.M.; Bahkali, A.H.; Sayed, S.R.; Manal, M.A. Extracellular Synthesis of Silver Nanoparticles Using Aspergillus Versicolor and Evaluation of Their Activity on Plant Pathogenic Fungi. Mycosphere 2016, 7, 844–852. [Google Scholar] [CrossRef]
- El-Aziz, A.R.M.; Al-Othman, M.R.; Mahmoud, M.; Metwaly, H.A. Biosynthesis of Silver Nanoparticles Using Fusarium Solani and Its Impact on Grain Borne Fungi. Dig. J. Nanomater. Biostruct. 2015, 10, 655–662. [Google Scholar]
- Gherbawy, Y.A.; Shalaby, I.M.; El-Sadek, M.S.A.; Elhariry, H.M.; Abdelilah, B.A. The Anti-Fasciolasis Properties of Silver Nanoparticles Produced by Trichoderma Harzianum and Their Improvement of the Anti-Fasciolasis Drug Triclabendazole. Int. J. Mol. Sci. 2013, 14, 21887–21898. [Google Scholar] [CrossRef] [PubMed]
- Guilger-Casagrande, M.; Germano-Costa, T.; Pasquoto-Stigliani, T.; Fraceto, L.F.; de Lima, R. Biosynthesis of Silver Nanoparticles Employing Trichoderma Harzianum with Enzymatic Stimulation for the Control of Sclerotinia Sclerotiorum. Sci. Rep. 2019, 9, 14351. [Google Scholar] [CrossRef] [PubMed]
- Sawake, M.M.; Moharil, M.P.; Ingle, Y.V.; Jadhav, P.V.; Ingle, A.P.; Khelurkar, V.C.; Paithankar, D.H.; Bathe, G.A.; Gade, A.K. Management of Phytophthora Parasitica Causing Gummosis in Citrus Using Biogenic Copper Oxide Nanoparticles. J. Appl. Microbiol. 2022, 132, 3142–3154. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-W.; Rawn, C.J.; Rondinone, A.J.; Love, L.J.; Roh, Y.; Everett, S.M.; Lauf, R.J.; Phelps, T.J. Large-Scale Production of Magnetic Nanoparticles Using Bacterial Fermentation. J. Ind. Microbiol. Biotechnol. 2010, 37, 1023–1031. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-W.; Ivanov, I.N.; Duty, C.E.; Love, L.J.; Rondinone, A.J.; Wang, W.; Li, Y.-L.; Madden, A.S.; Mosher, J.J.; Hu, M.Z.; et al. Scalable Economic Extracellular Synthesis of CdS Nanostructured Particles by a Non-Pathogenic Thermophile. J. Ind. Microbiol. Biotechnol. 2013, 40, 1263–1271. [Google Scholar] [CrossRef]
- Moon, J.-W.; Phelps, T.J.; Fitzgerald, C.L., Jr.; Lind, R.F.; Elkins, J.G.; Jang, G.G.; Joshi, P.C.; Kidder, M.; Armstrong, B.L.; Watkins, T.R.; et al. Manufacturing Demonstration of Microbially Mediated Zinc Sulfide Nanoparticles in Pilot-Plant Scale Reactors. Appl. Microbiol. Biotechnol. 2016, 100, 7921–7931. [Google Scholar] [CrossRef]
- Ramos-Ruiz, A.; Sesma-Martin, J.; Sierra-Alvarez, R.; Field, J.A. Continuous Reduction of Tellurite to Recoverable Tellurium Nanoparticles Using an Upflow Anaerobic Sludge Bed (UASB) Reactor. Water Res. 2017, 108, 189–196. [Google Scholar] [CrossRef]
- Birla, S.S.; Gaikwad, S.C.; Gade, A.K.; Rai, M.K. Rapid Synthesis of Silver Nanoparticles from Fusarium Oxysporum by Optimizing Physicocultural Conditions. Sci. World J. 2013, 2013, 796018. [Google Scholar] [CrossRef]
- Abu-Tahon, M.A.; Ghareib, M.; Abdallah, W.E. Environmentally Benign Rapid Biosynthesis of Extracellular Gold Nanoparticles Using Aspergillus Flavus and Their Cytotoxic and Catalytic Activities. Process Biochem. 2020, 95, 1–11. [Google Scholar] [CrossRef]
- Hamedi, S.; Ghaseminezhad, M.; Shokrollahzadeh, S.; Shojaosadati, S.A. Controlled Biosynthesis of Silver Nanoparticles Using Nitrate Reductase Enzyme Induction of Filamentous Fungus and Their Antibacterial Evaluation. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1588–1596. [Google Scholar] [CrossRef]
- Eldomany, E.; Essam, T.M.; Ahmed, A.E.; Farghali, A. Biosynthesis Physico-Chemical Optimization of Gold Nanoparticles as Anti-Cancer and Synergetic Antimicrobial Activity Using Pleurotus Ostreatus Fungus. J. Appl. Pharm. Sci. 2018, 8, 119–128. [Google Scholar] [CrossRef][Green Version]
| Species of Fungus | NP Type | Average Size (nm) | Synthesis Used | Additional Process Used | Source |
|---|---|---|---|---|---|
| Aspergillus niger | Ag | 20 | extracellular fungal culture | [33] | |
| Fe Fe3O4 | 18 50 | fungal homogenate solution | supercritical condition of ethanol | [35] | |
| Aspergillus terreus | Co3O4 CuO Fe3O4 NiO ZnO | 10 19 32 43 30 | cell-free filtrates | - | [52] |
| Beauveria spp. | Ag | 25 | extracellular fungal culture | - | [50] |
| Fusarium oxysporum | MnS NiS PbS ZnS | variable polydispersed fractal type structure | cell-free filtrates | - | [53] |
| Humicola sp. | Ag2S | 20 | cell-free filtrates | - | [54] |
| Penicillium chrysogenum | Ag | 19 to 60 | cell-free filtrates | - | [55] |
| MgO | 10 | fungal melanin pigment | irradiation with gamma rays | [56] | |
| CuO | 10 | cell-free filtrates | irradiation with gamma rays | [57] | |
| ZnO | 54 | cell-free filtrates | - | [18] | |
| Phoma sp. | Au | 10 to 100 | fresh biomass | - | [50] |
| Trichoderma viride | Ag | 2 to 4 | cell-free filtrates | - | [58] |
| Au | 20 to 30 | cell-free filtrates | - | [59] |
| Species of Fungus | NP Type | Average Size (nm) | Synthesis Used | Additional Process Used | Source |
|---|---|---|---|---|---|
| Agaricus bisporus | Ag | 44 | Fresh caps aqueous extract | - | [143] |
| Pd | 13 | Aqueous extract | - | [144] | |
| Coprinus comatus | Au | 38 to 59 | Fruit body aqueous extract | Ultraviolet irradiation 1 to 3 h | [145] |
| Flammulina velutipes | Au | 74 | Fresh fruit body aqueous extract | An additional catalyst to decrease color | [139] |
| Ganoderma applanatum | Ag | 59 | Fruiting body aqueous extract | - | [146] |
| Ganoderma lucidum | ZnO | 30 to 50 | Alcohol extract | - | [147] |
| Ag Au | 3 to 70 20 to 75 | Intra- and extracellular aqueous extracts | - | [142] | |
| Grifola frondosa | Ag Au | 3 to 70 10 to 55 | Intra- and extracellular aqueous extracts | - | [142] |
| Lentinus edodes | Ag Au | 3 to 70 10 to 75 | Intra- and extracellular aqueous extracts | - | [142] |
| Pleurotus djamor | TiO2 | 31 | Fresh fruit body aqueous extract | - | [148] |
| Pleurotus florida | Ag | 10 | Fruit body aqueous extract | Microwave, visible, and UV irradiation | [21] |
| Pleurotus ostreatus | Ag Au | 3 to 70 2 to 75 | Intra- and extracellular aqueous extracts | - | [142] |
| Pleurotus sajo-caju | Ag Ag | 23 37 | Aqueous extract | - | [138] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šebesta, M.; Vojtková, H.; Cyprichová, V.; Ingle, A.P.; Urík, M.; Kolenčík, M. Mycosynthesis of Metal-Containing Nanoparticles—Synthesis by Ascomycetes and Basidiomycetes and Their Application. Int. J. Mol. Sci. 2023, 24, 304. https://doi.org/10.3390/ijms24010304
Šebesta M, Vojtková H, Cyprichová V, Ingle AP, Urík M, Kolenčík M. Mycosynthesis of Metal-Containing Nanoparticles—Synthesis by Ascomycetes and Basidiomycetes and Their Application. International Journal of Molecular Sciences. 2023; 24(1):304. https://doi.org/10.3390/ijms24010304
Chicago/Turabian StyleŠebesta, Martin, Hana Vojtková, Veronika Cyprichová, Avinash P. Ingle, Martin Urík, and Marek Kolenčík. 2023. "Mycosynthesis of Metal-Containing Nanoparticles—Synthesis by Ascomycetes and Basidiomycetes and Their Application" International Journal of Molecular Sciences 24, no. 1: 304. https://doi.org/10.3390/ijms24010304
APA StyleŠebesta, M., Vojtková, H., Cyprichová, V., Ingle, A. P., Urík, M., & Kolenčík, M. (2023). Mycosynthesis of Metal-Containing Nanoparticles—Synthesis by Ascomycetes and Basidiomycetes and Their Application. International Journal of Molecular Sciences, 24(1), 304. https://doi.org/10.3390/ijms24010304








