The Bio-Aging of Biofilms on Behalf of Various Oral Status on Different Titanium Implant Materials
Abstract
:1. Introduction
2. Results
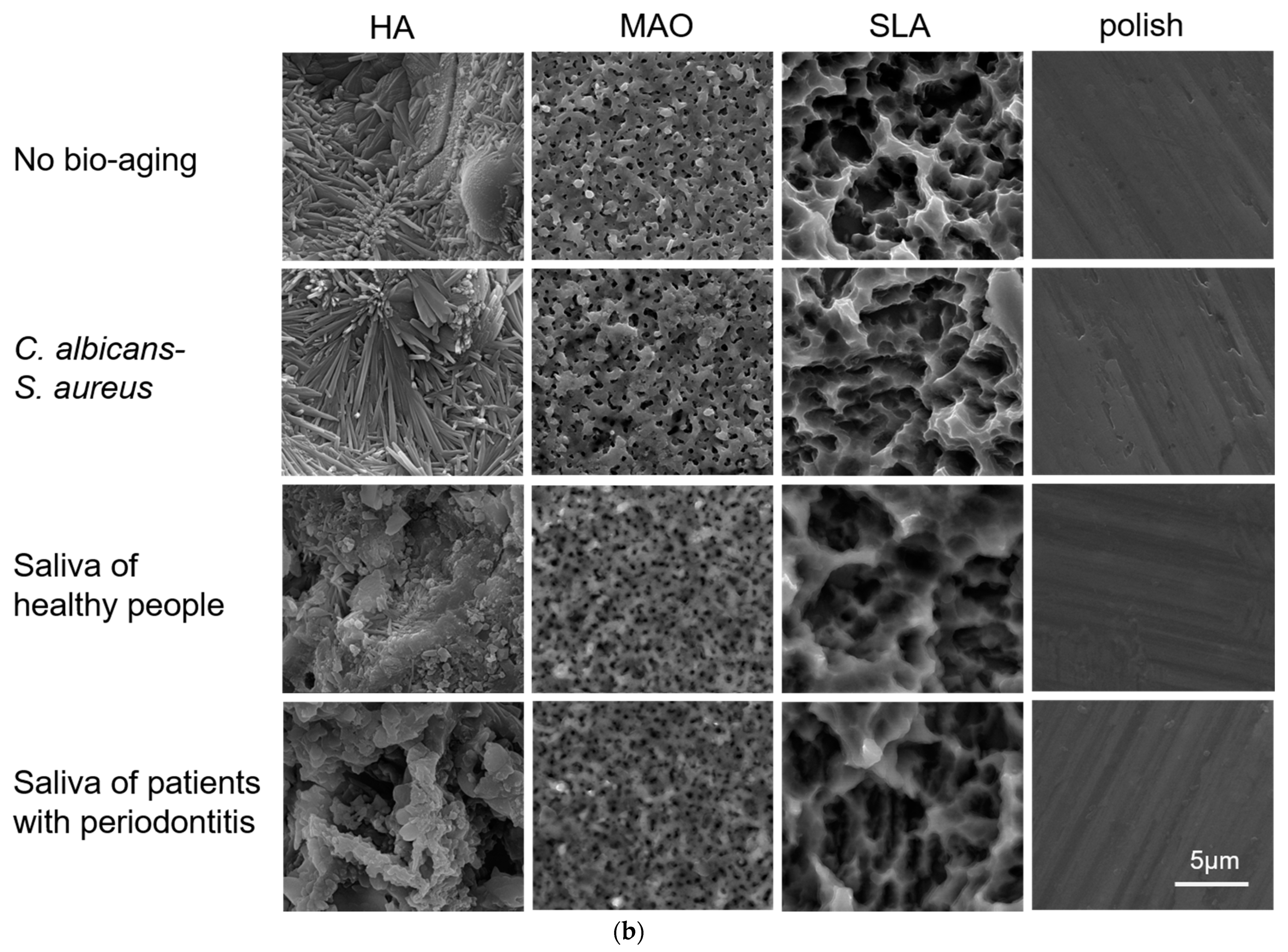
2.1. Surface Morphology
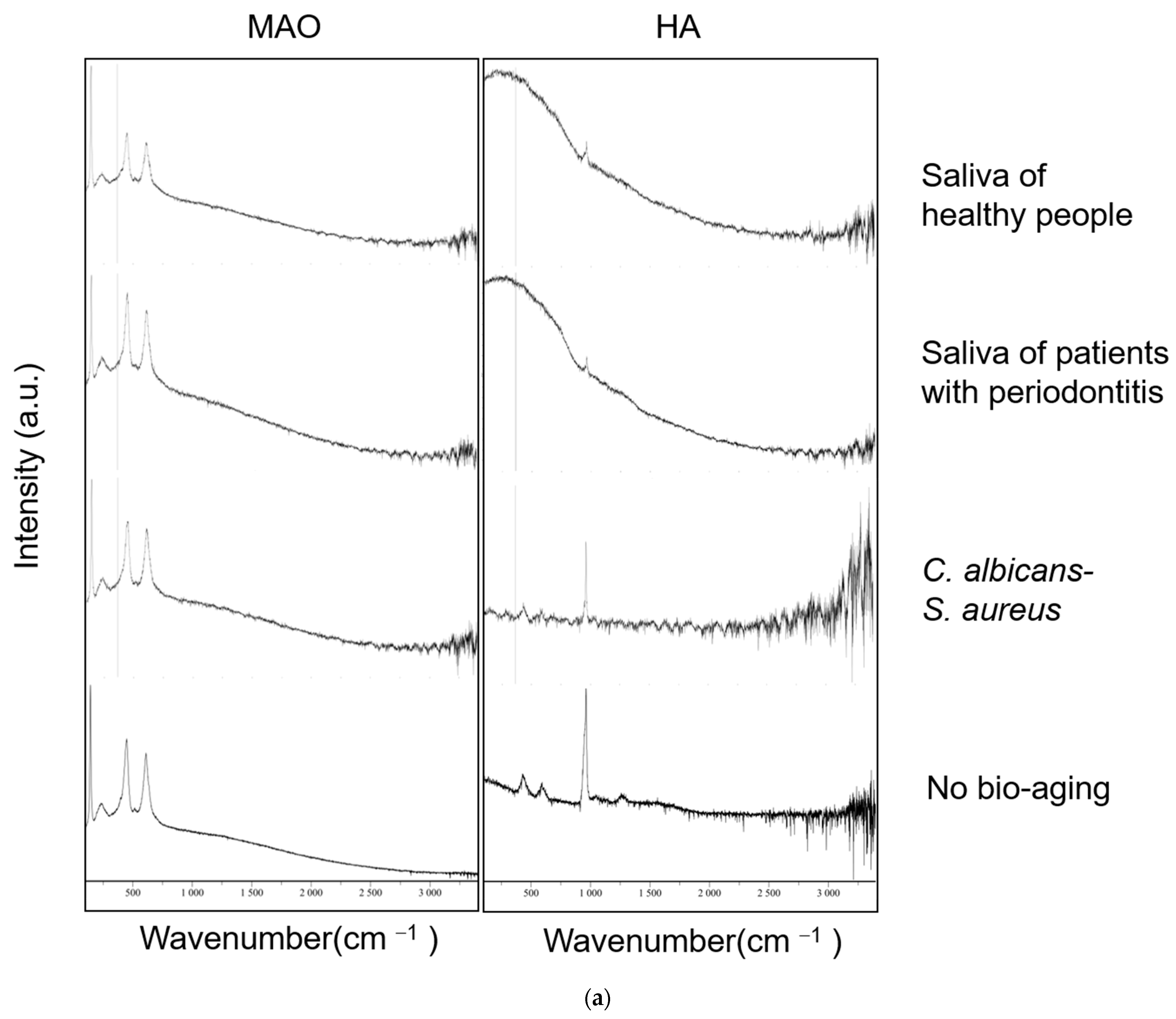
2.2. Surface Characteristic
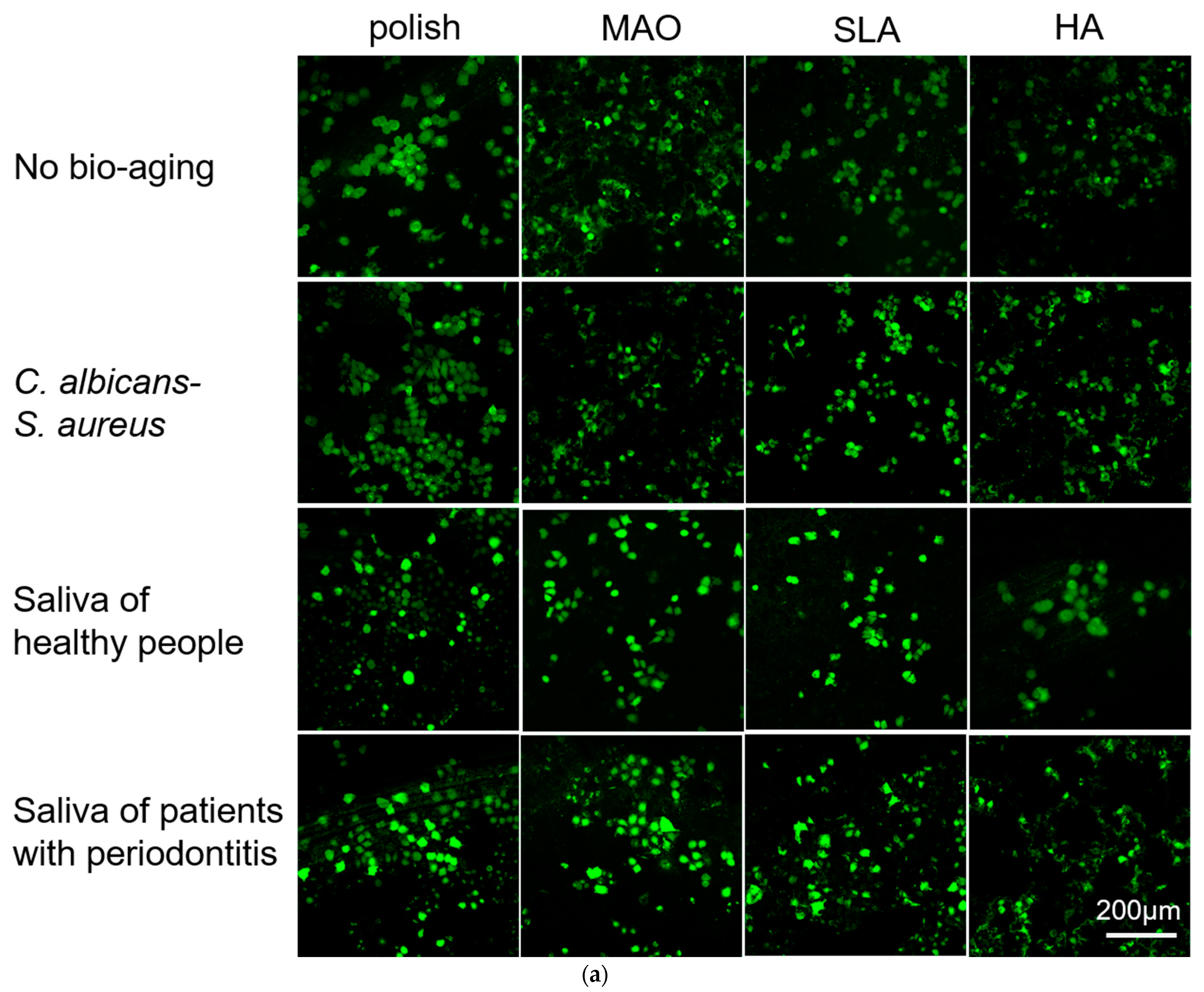
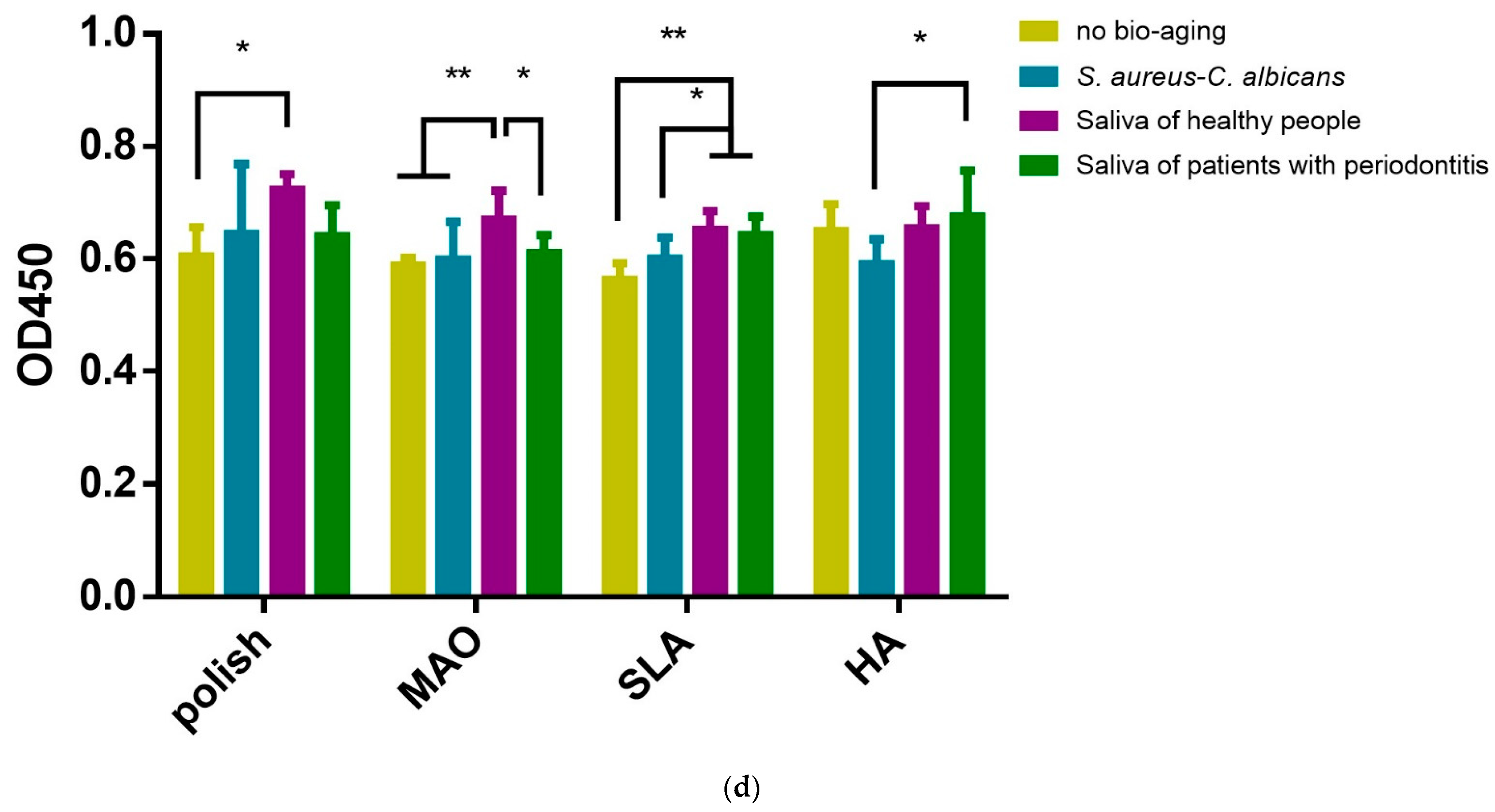
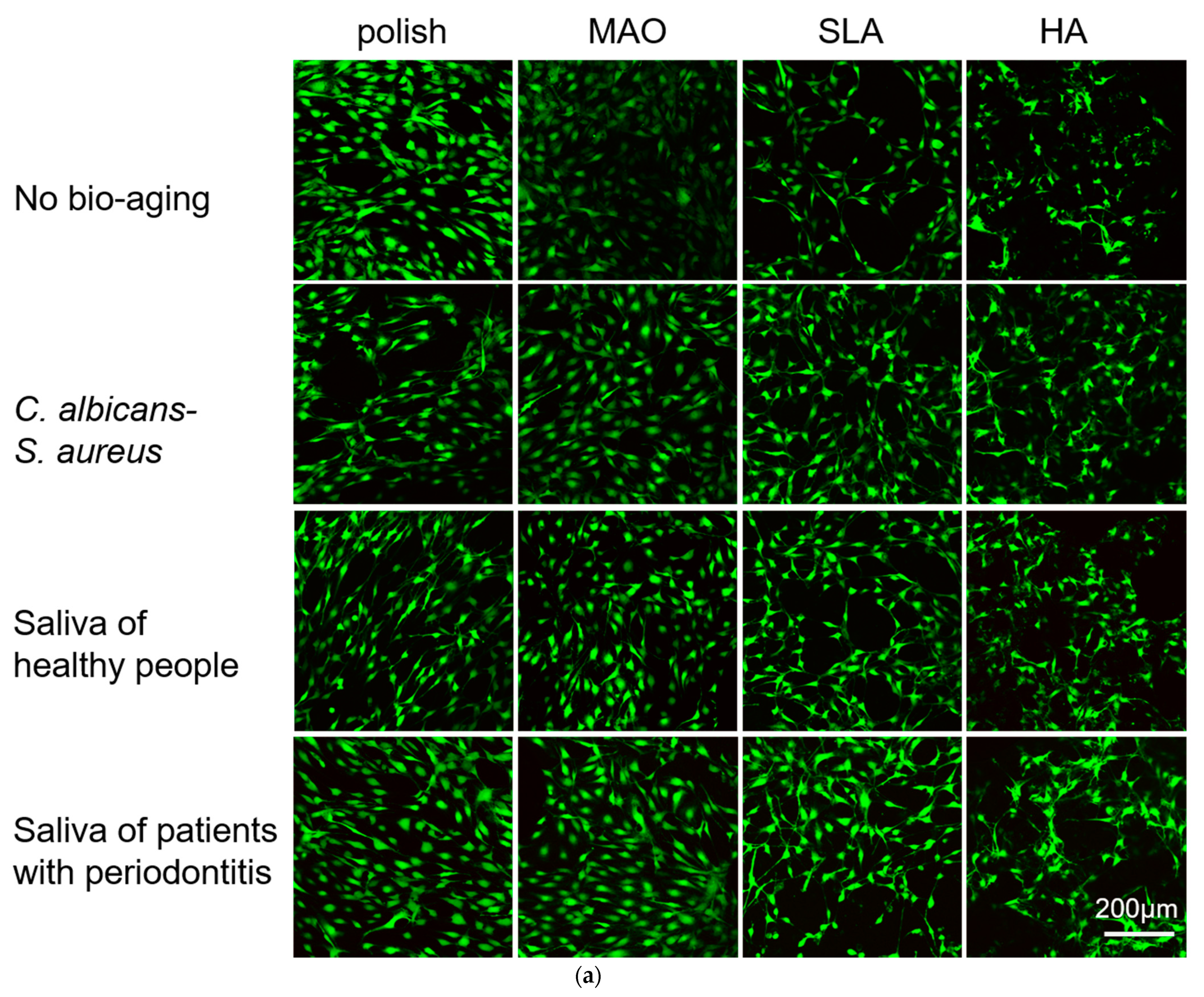
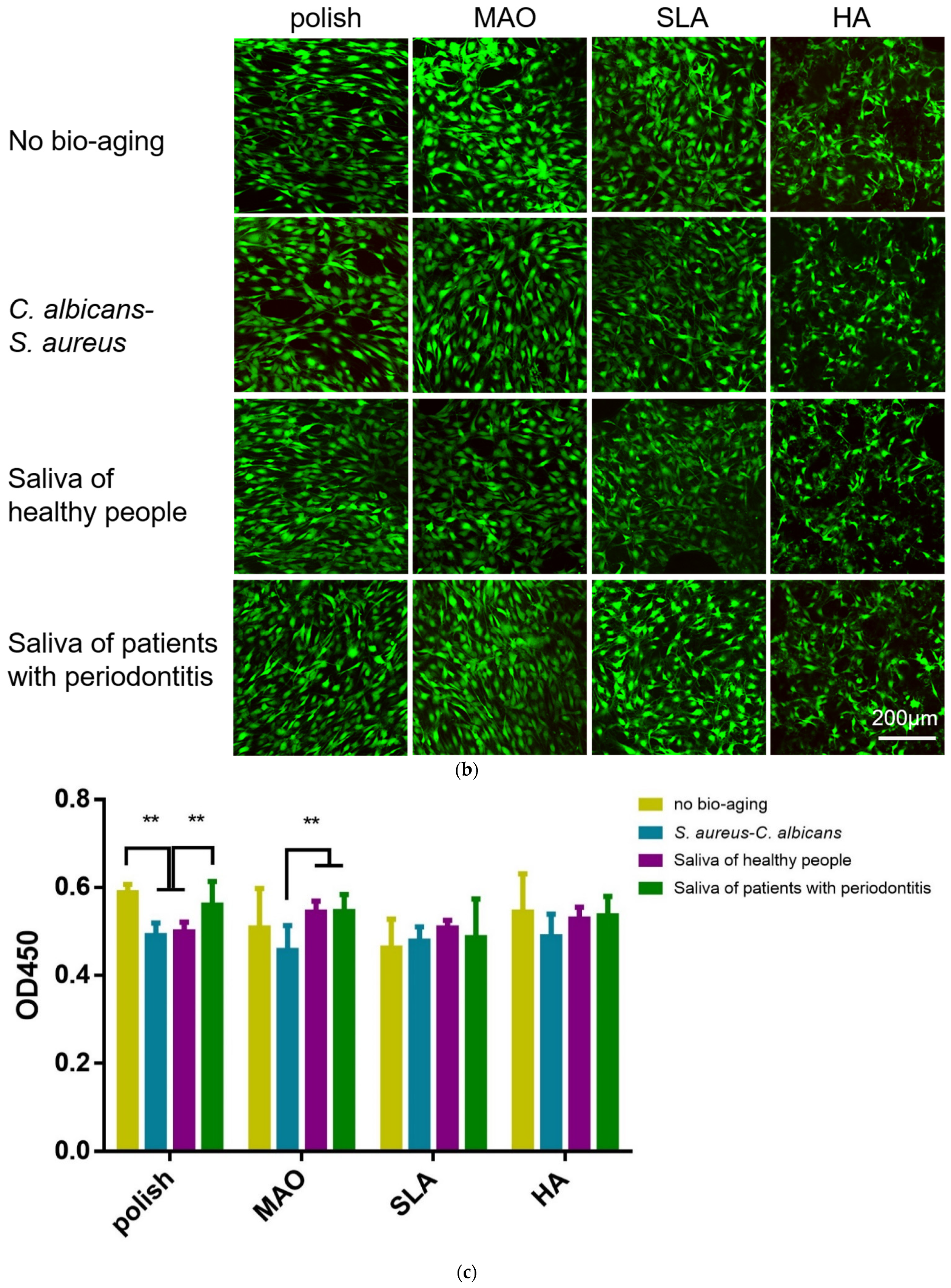
2.3. Adhesion and Proliferation of HGEs and HGFs
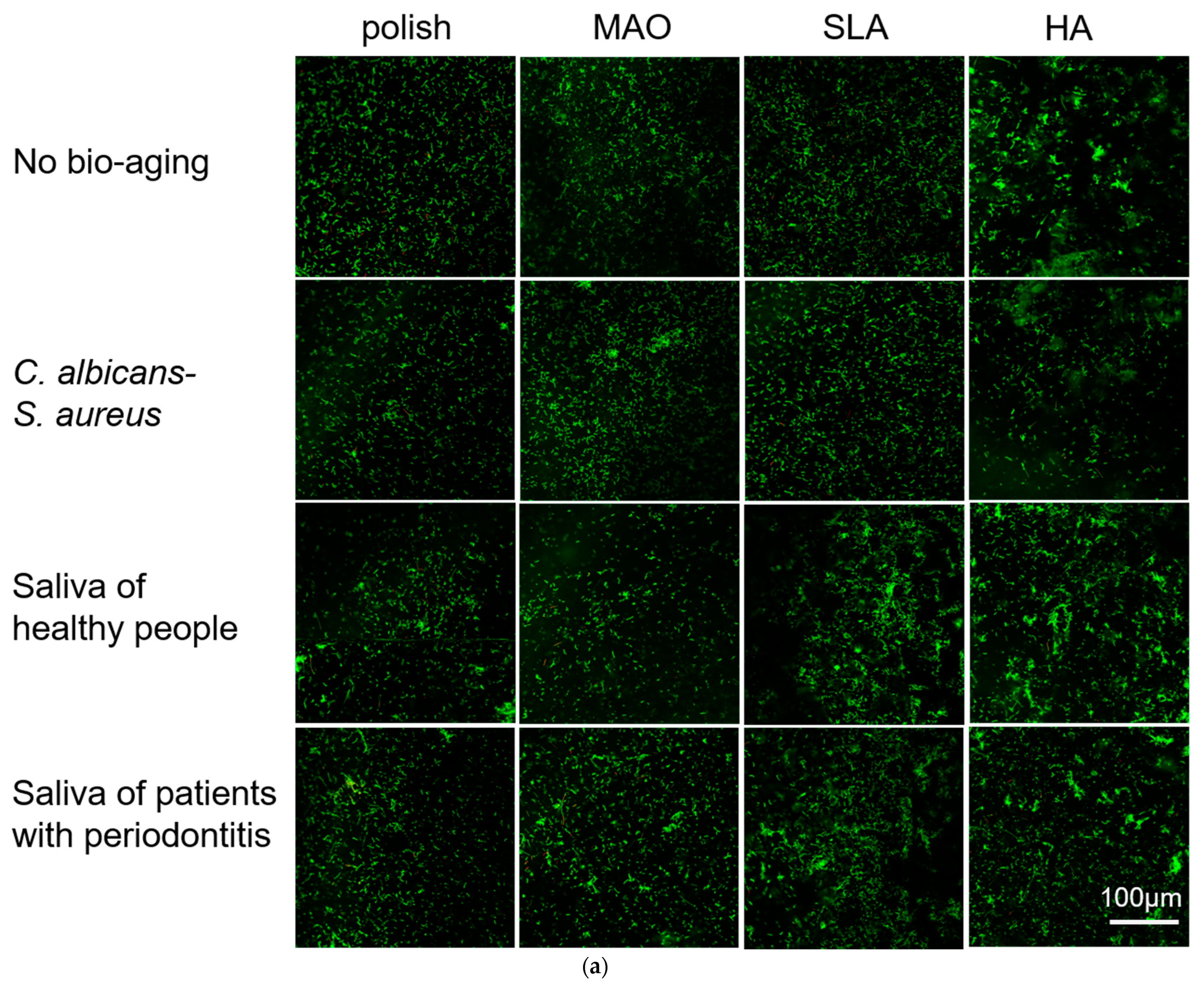
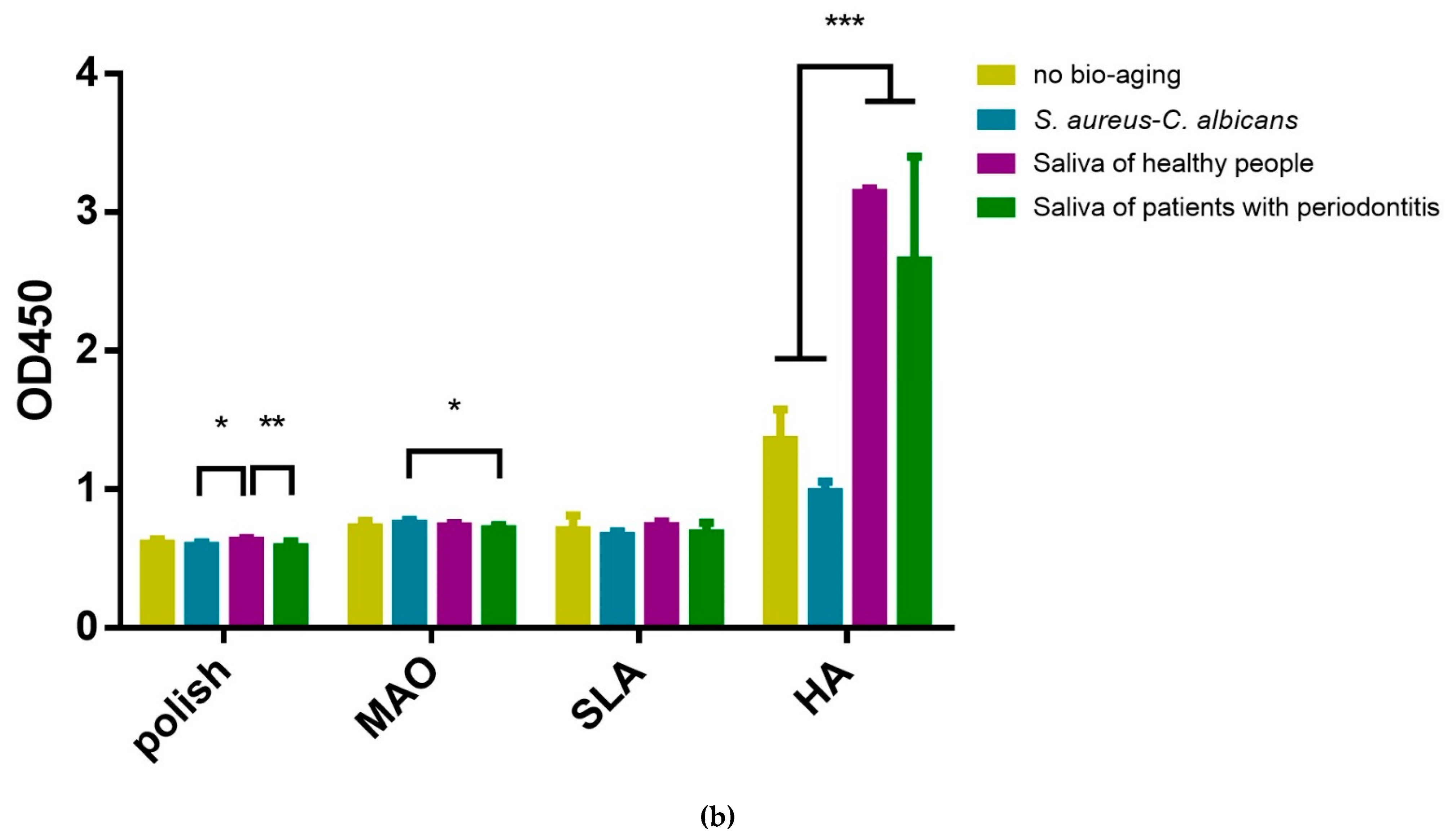
2.4. Adhesion and Proliferation of Multispecies Biofilms
3. Discussion
4. Materials and Methods
4.1. Subject Recruitment and Sampling
4.2. Bacteria, Fungus and Culture Conditions
4.3. Cell Culture
4.4. Bio-Aging with Biofilms
4.5. Surface Morphology Observation
4.6. Surface Chemical Changes
4.7. Water Contact Angle
4.8. Adhesion and Proliferation of HGEs and HGFs on Titanium Plates
4.9. Adhesion and Proliferation of Multispecies Biofilms
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Avila-Ortiz, G.; Gonzalez-Martin, O.; Couso-Queiruga, E.; Wang, H.L. The peri-implant phenotype. J. Periodontol. 2020, 91, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.W. Titanium Alloys for Dental Implants: A Review. Prosthesis 2020, 2, 100–116. [Google Scholar] [CrossRef]
- Zhou, W.; Peng, X.; Zhou, X.; Li, M.; Ren, B.; Cheng, L. Influence of bio-aging on corrosion behavior of different implant materials. Clin. Implant. Dent. Relat. Res. 2019, 21, 1225–1234. [Google Scholar] [CrossRef] [PubMed]
- Att, W.; Hori, N.; Takeuchi, M.; Ouyang, J.; Yang, Y.; Anpo, M.; Ogawa, T. Time-dependent degradation of titanium osteoconductivity: An implication of biological aging of implant materials. Biomaterials 2009, 30, 5352–5363. [Google Scholar] [CrossRef]
- Ye, X.; Tang, G. Effect of coupling asynchronous acoustoelectric effects on the corrosion behavior, microhardness and biocompatibility of biomedical titanium alloy strips. J. Mater. Sci. Mater. Med. 2015, 26, 5371. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.C.; Valderrama, P.; Wilson, T.G.; Palmer, K.; Thomas, A.; Sridhar, S.; Adapalli, A.; Burbano, M.; Wadhwani, C. Titanium Corrosion Mechanisms in the Oral Environment: A Retrieval Study. Materials 2013, 6, 5258–5274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fürst, M.M.; Salvi, G.E.; Lang, N.P.; Persson, G.R. Bacterial colonization immediately after installation on oral titanium implants. Clin. Oral Implant. Res. 2007, 18, 501–508. [Google Scholar] [CrossRef]
- Souza, J.; Henriques, M.; Oliveira, R.; Teughels, W.; Celis, J.-P.; Rocha, L. Do oral biofilms influence the wear and corrosion behavior of titanium? Biofouling 2010, 26, 471–478. [Google Scholar] [CrossRef]
- Shen, J.W.; Chen, Y.; Yang, G.L.; Wang, X.X.; He, F.M.; Wang, H.M. Effects of storage medium and UV photofunctionalization on time-related changes of titanium surface characteristics and biocompatibility. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 932–940. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, S.; Peng, X.; Hu, Y.; Ren, B.; Li, M.; Hao, L.; Feng, M.; Cheng, L.; Zhou, X. Effects of water and microbial-based aging on the performance of three dental restorative materials. J. Mech. Behav. Biomed. Mater. 2018, 80, 42–50. [Google Scholar] [CrossRef]
- Zhou, W.; Peng, X.; Ma, Y.; Hu, Y.; Wu, Y.; Lan, F.; Weir, M.D.; Li, M.; Ren, B.; Oates, T.W.; et al. Two-staged time-dependent materials for the prevention of implant-related infections. Acta Biomater. 2020, 101, 128–140. [Google Scholar] [CrossRef]
- Shemtov-Yona, K.; Rittel, D. On the mechanical integrity of retrieved dental implants. J. Mech. Behav. Biomed. Mater. 2015, 49, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Menini, M.; Delucchi, F.; Bagnasco, F.; Pera, F.; Di Tullio, N.; Pesce, P. Analysis of the subgingival microbiota in implant-supported full-arch rehabilitations. Dent. J. 2020, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.D.; Martins, C.C.; Amaral, S.A.; Vieira, T.R.; Albuquerque, B.N.; Cota LO, M.; Esteves Lima, R.P.; Costa, F.O. Periodontitis as a risk factor for peri-implantitis: Systematic review and meta-analysis of observational studies. J. Dent. 2018, 79, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.H.; Wang, H.L. Breaking the wave of peri-implantitis. Periodontology 2000 2020, 84, 145–160. [Google Scholar] [CrossRef]
- Komatsu, K.; Shiba, T.; Takeuchi, Y.; Watanabe, T.; Koyanagi, T.; Nemoto, T.; Shimogishi, M.; Shibasaki, M.; Katagiri, S.; Kasugai, S.; et al. Discriminating Microbial Community Structure Between Peri-Implantitis and Periodontitis With Integrated Metagenomic, Metatranscriptomic, and Network Analysis. Front. Cell Infect. Microbiol. 2020, 10, 596490. [Google Scholar] [CrossRef]
- Sanz-Martin, I.; Doolittle-Hall, J.; Teles, R.P.; Patel, M.; Belibasakis, G.N.; Hammerle CH, F.; Jung, R.E.; Teles FR, F. Exploring the microbiome of healthy and diseased peri-implant sites using Illumina sequencing. J. Clin. Periodontol. 2017, 44, 1274–1284. [Google Scholar] [CrossRef] [Green Version]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implants: A review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef]
- Botero, J.E.; González, A.M.; Mercado, R.A.; Olave, G.; Contreras, A. Subgingival microbiota in peri-implant mucosa lesions and adjacent teeth in partially edentulous patients. J. Periodontol. 2005, 76, 1490–1495. [Google Scholar] [CrossRef]
- Bürgers, R.; Hahnel, S.; Reichert, T.E.; Rosentritt, M.; Behr, M.; Gerlach, T.; Handel, G.; Gosau, M. Adhesion of Candida albicans to various dental implant surfaces and the influence of salivary pellicle proteins. Acta Biomater. 2010, 6, 2307–2313. [Google Scholar] [CrossRef]
- Kunrath, M.F.; Dahlin, C. The Impact of Early Saliva Interaction on Dental Implants and Biomaterials for Oral Regeneration: An Overview. Int. J. Mol. Sci. 2022, 23, 2024. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.C.M.; Sordi, M.B.; Kanazawa, M.; Ravindran, S.; Henriques, B.; Silva, F.S.; Aparicio, C.; Cooper, L.F. Nano-scale modification of titanium implant surfaces to enhance osseointegration. Acta Biomater. 2019, 94, 112–131. [Google Scholar] [CrossRef] [PubMed]
- Matykina, E.; Arrabal, R.; Mohedano, M.; Pardo, A.; Merino, M.; Rivero, E. Stability of plasma electrolytic oxidation coating on titanium in artificial saliva. J. Mater. Sci. Mater. Med. 2013, 24, 37–51. [Google Scholar] [CrossRef]
- Duarte, L.T.; Bolfarini, C.; Biaggio, S.R.; Rocha-Filho, R.C.; Nascente, P.A. Growth of aluminum-free porous oxide layers on titanium and its alloys Ti-6Al-4V and Ti-6Al-7Nb by micro-arc oxidation. Mater. Sci. Eng. C 2014, 41, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Göncü, Y.; Gecgin, M.; Bakan, F.; Ay, N. Electrophoretic deposition of hydroxyapatite-hexagonal boron nitride composite coatings on Ti substrate. Mater. Sci. Eng. C 2017, 79, 343–353. [Google Scholar] [CrossRef]
- Realpe-Jaramillo, J.; Morales-Morales, J.A.; González-Sánchez, J.A.; Cabanzo, R.; Mejía-Ospino, E.; Rodríguez-Pereira, J. Effect of modification substrate on the microstructure of hydroxyapatite coating. J. Phys. Conf. Ser. 2017, 786, 012024. [Google Scholar] [CrossRef]
- Dwivedi, C.; Pandey, I.; Pandey, H.; Ramteke, P.W.; Pandey, A.C.; Mishra, S.B.; Patil, S. Electrospun nanofibrous scaffold as a potential carrier of antimicrobial therapeutics for diabetic wound healing and tissue regeneration. In Nano-and Microscale Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2017; pp. 147–164. [Google Scholar]
- Belibasakis, G.N.; Bao, K.; Bostanci, N. Transcriptional profiling of human gingival fibroblasts in response to multi-species in vitro subgingival biofilms. Mol. Oral Microbiol. 2014, 29, 174–183. [Google Scholar] [CrossRef] [Green Version]
- Ingendoh-Tsakmakidis, A.; Eberhard, J.; Falk, C.S.; Stiesch, M.; Winkel, A. In Vitro Effects of Streptococcus oralis Biofilm on Peri-Implant Soft Tissue Cells. Cells 2020, 9, 1226. [Google Scholar] [CrossRef]
- Jaafar, A.; Hecker, C.; Arki, P.; Joseph, Y. Sol-Gel Derived Hydroxyapatite Coatings for Titanium Implants: A Review. Bioengineering 2020, 7, 127. [Google Scholar] [CrossRef]
- Surmenev, R.A. A review of plasma-assisted methods for calcium phosphate-based coatings fabrication. Surf. Coat. Technol. 2012, 206, 2035–2056. [Google Scholar] [CrossRef]
- Mohseni, E.; Zalnezhad, E.; Bushroa, A.R. Comparative investigation on the adhesion of hydroxyapatite coating on Ti–6Al–4V implant: A review paper. Int. J. Adhes. Adhes. 2014, 48, 238–257. [Google Scholar] [CrossRef]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral Biofilms: Pathogens, Matrix, and Polymicrobial Interactions in Microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Belibasakis, G.; Manoil, D. Microbial community-driven etiopathogenesis of peri-implantitis. J. Dent. Res. 2021, 100, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.; Patil-Sen, Y.; Junkar, I.; Kulkarni, C.V.; Lorenzetti, M.; Iglic, A. Wettability studies of topologically distinct titanium surfaces. Colloids Surf. B Biointerfaces 2015, 129, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Hernandez, M.; Hannig, M.; Garcia-Perez, V.I.; Olivares-Navarrete, R.; Fecher-Trost, C.; Almaguer-Flores, A. Roughness and wettability of titanium implant surfaces modify the salivary pellicle composition. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1017–1028. [Google Scholar] [CrossRef]
- Ingendoh-Tsakmakidis, A.; Mikolai, C.; Winkel, A.; Szafranski, S.P.; Falk, C.S.; Rossi, A.; Walles, H.; Stiesch, M. Commensal and pathogenic biofilms differently modulate peri-implant oral mucosa in an organotypic model. Cell Microbiol. 2019, 21, e13078. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Yu, W.; Zhang, J.; Han, X.; Wang, J.; Sun, D.; Shi, R.; Zhou, Y.; Zhang, H.; Zhao, J. The antibacterial property of zinc oxide/graphene oxide modified porous polyetheretherketone against S. sanguinis, F. nucleatum and P. gingivalis. Biomed Mater. 2022, 17, 025013. [Google Scholar] [CrossRef]
- Roehling, S.; Astasov-Frauenhoffer, M.; Hauser-Gerspach, I.; Braissant, O.; Woelfler, H.; Waltimo, T.; Kniha, H.; Gahlert, M. In vitro biofilm formation on titanium and zirconia implant surfaces. J. Periodontol. 2017, 88, 298–307. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, X.; Li, J.; Aprecio, R.M.; Zhang, W.; Li, Y. Real-time PCR quantification of six periodontal pathogens in saliva samples from healthy young adults. Clin. Oral Investig. 2015, 19, 937–946. [Google Scholar] [CrossRef]
- Ghensi, P.; Manghi, P.; Zolfo, M.; Armanini, F.; Pasolli, E.; Bolzan, M.; Bertelle, A.; Dell’Acqua, F.; Dellasega, E.; Waldner, R.; et al. Strong oral plaque microbiome signatures for dental implant diseases identified by strain-resolution metagenomics. NPJ Biofilms Microbiomes 2020, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Barão, V.A.; Yoon, C.J.; Mathew, M.T.; Yuan, J.C.; Wu, C.D.; Sukotjo, C. Attachment of Porphyromonas gingivalis to corroded commercially pure titanium and titanium-aluminum-vanadium alloy. J. Periodontol. 2014, 85, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A New Classification Scheme for Periodontal and Peri-Implant Diseases and Conditions–Introduction and Key Changes from the 1999 Classification; Wiley Online Library: Hoboken, NJ, USA, 2018; pp. S1–S8. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S173–S182. [Google Scholar] [CrossRef] [Green Version]
- Abrahamian, L.; Pascual-LaRocca, A.; Barallat, L.; Valles, C.; Herrera, D.; Sanz, M.; Nart, J.; Figuero, E. Intra-and inter-examiner reliability in classifying periodontitis according to the 2018 classification of periodontal diseases. J. Clin. Periodontol. 2022, 49, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.L.; Chan, Y.; Zhuang, L.; Lai, H.C.; Lang, N.P.; Keung Leung, W.; Watt, R.M. Intra-oral single-site comparisons of periodontal and peri-implant microbiota in health and disease. Clin. Oral Implants Res. 2019, 30, 760–776. [Google Scholar] [CrossRef] [PubMed]
- Cieplik, F.; Zaura, E.; Brandt, B.W.; Buijs, M.J.; Buchalla, W.; Crielaard, W.; Laine, M.L.; Deng, D.M.; Exterkate RA, M. Microcosm biofilms cultured from different oral niches in periodontitis patients. J. Oral Microbiol. 2019, 11, 1551596. [Google Scholar] [CrossRef] [PubMed]
- Rigolin, M.S.M.; Barbugli, P.A.; Jorge, J.H.; Reis MR, D.; Adabo, G.L.; Casemiro, L.A.; Martins CH, G.; de Lima, O.J.; Mollo Junior, F.A. Effect of the aging of titanium and zirconia abutment surfaces on the viability, adhesion, and proliferation of cells and the adhesion of microorganisms. J. Prosthet. Dent. 2019, 122, 564.e1–564.e10. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.; Bowen, C.; Rautray, T. Dual response of osteoblast activity and antibacterial properties of polarized strontium substituted hydroxyapatite-Barium strontium titanate composites with controlled strontium substitution. J. Biomed. Mater. Res. A 2021, 109, 2027–2035. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, M.; Shi, Y.; Chen, E.; Shou, Y.; Dai, D.; Xian, W.; Ren, B.; Xiao, S.; Cheng, L. The Bio-Aging of Biofilms on Behalf of Various Oral Status on Different Titanium Implant Materials. Int. J. Mol. Sci. 2023, 24, 332. https://doi.org/10.3390/ijms24010332
Liao M, Shi Y, Chen E, Shou Y, Dai D, Xian W, Ren B, Xiao S, Cheng L. The Bio-Aging of Biofilms on Behalf of Various Oral Status on Different Titanium Implant Materials. International Journal of Molecular Sciences. 2023; 24(1):332. https://doi.org/10.3390/ijms24010332
Chicago/Turabian StyleLiao, Min, Yangyang Shi, Enni Chen, Yuke Shou, Dongyue Dai, Wenpan Xian, Biao Ren, Shimeng Xiao, and Lei Cheng. 2023. "The Bio-Aging of Biofilms on Behalf of Various Oral Status on Different Titanium Implant Materials" International Journal of Molecular Sciences 24, no. 1: 332. https://doi.org/10.3390/ijms24010332
APA StyleLiao, M., Shi, Y., Chen, E., Shou, Y., Dai, D., Xian, W., Ren, B., Xiao, S., & Cheng, L. (2023). The Bio-Aging of Biofilms on Behalf of Various Oral Status on Different Titanium Implant Materials. International Journal of Molecular Sciences, 24(1), 332. https://doi.org/10.3390/ijms24010332





