Galectin-2 in Health and Diseases
Abstract
1. Introduction
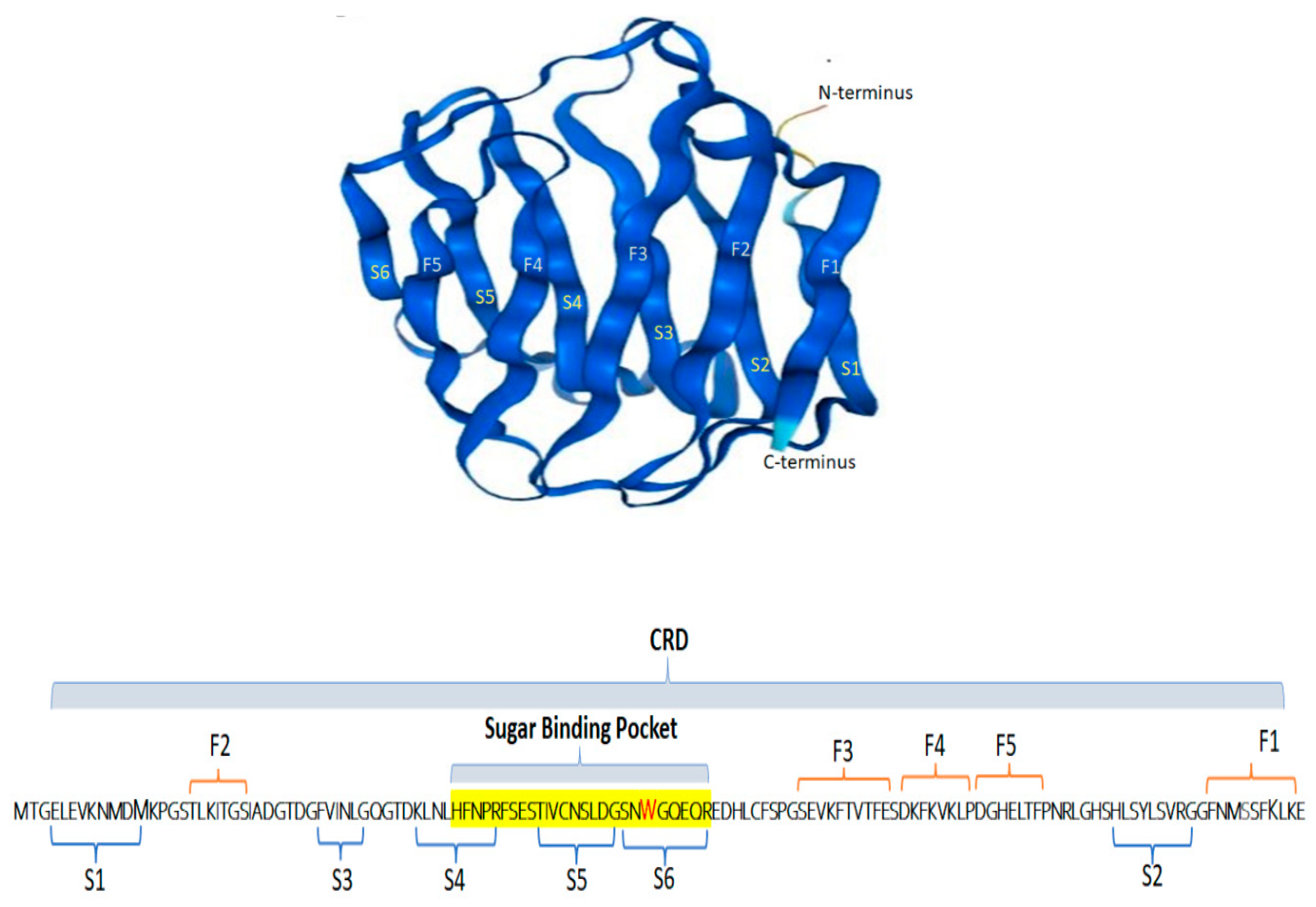
2. Galectin-2 Structure
3. Galectin-2 Binding Ligands
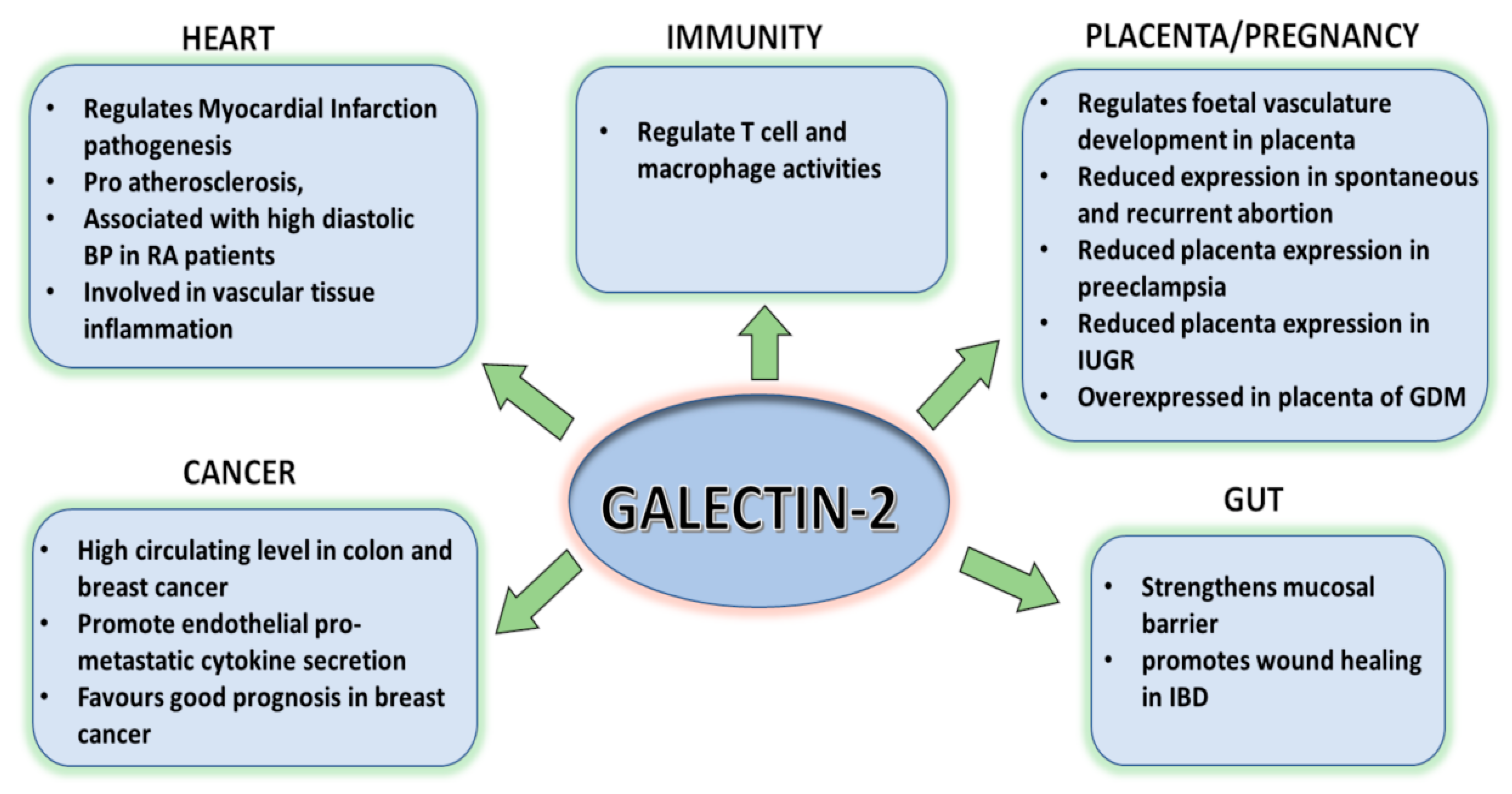
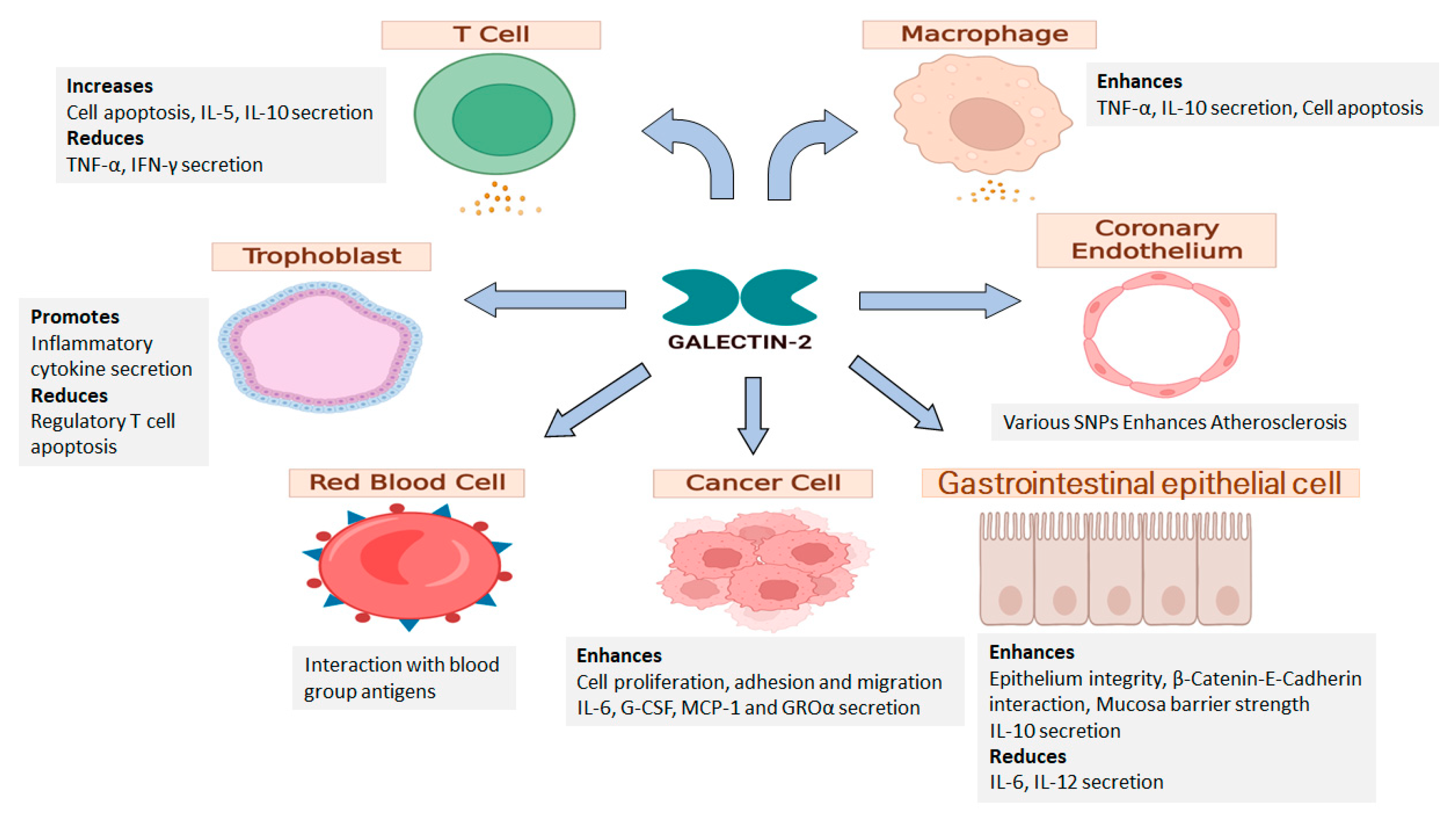
4. Galectin-2 in the Digestive System
5. Galetin-2 in Pregnancy
6. Galectin-2 in Immunity
7. Galectin-2 in the Cardiovascular System
8. GALECTIN-2 in Cancer
9. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a glance. J. Cell Sci. 2018, 131, jcs208884. [Google Scholar] [CrossRef] [PubMed]
- Teichberg, V.I.; Silman, I.; Beitsch, D.D.; Resheff, G. A β D galactoside binding protein from electric organ tissue of Electrophorus electricus. Proc. Natl. Acad. Sci. USA 1975, 72, 1383–1387. [Google Scholar] [CrossRef] [PubMed]
- Nabi, I.R.; Shankar, J.; Dennis, J.W. The galectin lattice at a glance. J. Cell Sci. 2015, 128, 2213–2219. [Google Scholar] [CrossRef] [PubMed]
- Hara, A.; Niwa, M.; Noguchi, K.; Kanayama, T.; Niwa, A.; Matsuo, M.; Hatano, Y.; Tomita, H. Galectin-3 as a Next-Generation Biomarker for Detecting Early Stage of Various Diseases. Biomolecules 2020, 10, 389. [Google Scholar] [CrossRef]
- Gitt, M.A.; Massa, S.M.; Leffler, H.; Barondes, S.H. Isolation and expression of a gene encoding L-14-II, a new human soluble lactose-binding lectin. J. Biol. Chem. 1992, 267, 10601–10606. [Google Scholar] [CrossRef]
- Tamura, M.; Tanaka, T.; Fujii, N.; Tanikawa, T.; Oka, S.; Takeuchi, T.; Hatanaka, T.; Kishimoto, S.; Arata, Y. Potential Interaction between Galectin-2 and MUC5AC in Mouse Gastric Mucus. Biol. Pharm. Bull. 2020, 43, 356–360. [Google Scholar] [CrossRef]
- Charkiewicz, K.; Goscik, J.; Raba, G.; Laudanski, P. Syndecan 4, galectin 2, and death receptor 3 (DR3) as novel proteins in pathophysiology of preeclampsia. J. Matern. Neonatal Med. 2019, 34, 2965–2970. [Google Scholar] [CrossRef]
- Van Der Laan, A.M.; Schirmer, S.H.; De Vries, M.R.; Koning, J.J.; Volger, O.L.; Fledderus, J.O.; Bastiaansen, A.J.; Hollander, M.R.; Baggen, J.M.; Koch, K.T.; et al. Galectin-2 expression is dependent on the rs7291467 polymorphism and acts as an inhibitor of arteriogenesis. Eur. Hear. J. 2011, 33, 1076–1084. [Google Scholar] [CrossRef]
- Yıldırım, C.; Vogel, D.Y.S.; Hollander, M.R.; Baggen, J.M.; Fontijn, R.D.; Nieuwenhuis, S.; Haverkamp, A.; De Vries, M.R.; Quax, P.H.A.; Garcia-Vallejo, J.J.; et al. Galectin-2 Induces a Proinflammatory, Anti-Arteriogenic Phenotype in Monocytes and Macrophages. PLoS ONE 2015, 10, e0124347. [Google Scholar] [CrossRef]
- Li, S.; Yu, Y.; Koehn, C.D.; Zhang, Z.; Su, K. Galectins in the Pathogenesis of Rheumatoid Arthritis. J. Clin. Cell. Immunol. 2013, 4, 1000164. [Google Scholar] [PubMed]
- Panjwani, N. Role of galectins in re-epithelialization of wounds. Ann. Transl. Med. 2014, 2, 89. [Google Scholar] [PubMed]
- Sindrewicz, P.; Lian, L.-Y.; Yu, L.-G. Interaction of the Oncofetal Thomsen–Friedenreich Antigen with Galectins in Cancer Progression and Metastasis. Front. Oncol. 2016, 6, 79. [Google Scholar] [CrossRef]
- Meister, S.; Hahn, L.; Beyer, S.; Mannewitz, M.; Perleberg, C.; Schnell, K.; Anz, D.; Corradini, S.; Schmoeckel, E.; Mayr, D.; et al. Regulatory T Cell Apoptosis during Preeclampsia May Be Prevented by Gal-2. Int. J. Mol. Sci. 2022, 23, 1880. [Google Scholar] [CrossRef] [PubMed]
- Paclik, D.; Lohse, K.; Wiedenmann, B.; Dignass, A.U.; Sturm, A. Galectin-2 and -4, but not Galectin-1, promote intestinal epithelial wound healing in vitro through a TGF-beta-independent mechanism. Inflamm. Bowel Dis. 2008, 14, 1366–1372. [Google Scholar] [CrossRef]
- Lohr, M.; Lensch, M.; André, S.; Kaltner, H.; Siebert, H.-C.; Smetana, K., Jr.; Sinowatz, F.; Gabius, H.-J. Murine homodimeric adhesion/growth-regulatory galectins-1, -2 and -7: Comparative profiling of gene/promoter sequences by database mining, of expression by RT-PCR/immunohistochemistry and of contact sites for carbohydrate ligands by computational chemistr. Folia Biol. 2007, 53, 109–128. [Google Scholar]
- Lobsanov, Y.D.; Gitt, M.A.; Leffler, H.; Barondes, S.H.; Rini, J.M. X-ray crystal structure of the human dimeric S-Lac lectin, L-14-II, in complex with lactose at 2.9-Å resolution. J. Biol. Chem. 1993, 268, 27034–27038. [Google Scholar] [CrossRef]
- Si, Y.; Feng, S.; Gao, J.; Wang, Y.; Zhang, Z.; Meng, Y.; Zhou, Y.; Tai, G.; Su, J. Human galectin-2 interacts with carbohydrates and peptides non-classically: New insight from X-ray crystallography and hemagglutination. Acta Biochim. Biophys. Sin. 2016, 48, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.I.; Stegmayr, J.; Grant, O.C.; Yang, Z.; Nilsson, U.J.; Boos, I.; Carlsson, M.C.; Woods, R.J.; Unverzagt, C.; Leffler, H.; et al. Galectin binding to cells and glycoproteins with genetically modified glycosylation reveals galectin–glycan specificities in a natural context. J. Biol. Chem. 2018, 293, 20249–20262. [Google Scholar] [CrossRef]
- Haudek, K.C.; Patterson, R.J.; Wang, J.L. SR proteins and galectins: What’s in a name? Glycobiology 2010, 20, 1199–1207. [Google Scholar] [CrossRef]
- Sindrewicz, P.; Li, X.; Yates, E.A.; Turnbull, J.E.; Lian, L.-Y.; Yu, L.-G. Intrinsic tryptophan fluorescence spectroscopy reliably determines galectin-ligand interactions. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Sakakura, M.; Tamura, M.; Fujii, N.; Takeuchi, T.; Hatanaka, T.; Kishimoto, S.; Arata, Y.; Takahash, H. Structural mechanisms for the S-nitrosylation-derived protection of mouse galectin-2 from oxidation-induced inactivation revealed by NMR. FEBS J. 2018, 285, 1129–1145. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R. Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim. Biophys. Acta (BBA) Gen. Subj. 1999, 1473, 172–185. [Google Scholar] [CrossRef]
- Bänfer, S.; Jacob, R. Galectins in Intra- and Extracellular Vesicles. Biomolecules 2020, 10, 1232. [Google Scholar] [CrossRef] [PubMed]
- Dvoránková, B.; Lacina, L.; Smetana, K.; Lensch, M.; Manning, J.C.; André, S.; Gabius, H.-J. Human galectin-2: Nuclear presence in vitro and its modulation by quiescence/stress factors. Histol. Histopathol. 2008, 23, 167–178. [Google Scholar]
- Nakahara, S.; Raz, A. Regulation of cancer-related gene expression by galectin-3 and the molecular mechanism of its nuclear import pathway. Cancer Metastasis Rev. 2007, 26, 605–610. [Google Scholar] [CrossRef]
- de Jong, C.G.H.M.; Gabius, H.-J.; Baron, W. The emerging role of galectins in (re)myelination and its potential for developing new approaches to treat multiple sclerosis. Cell. Mol. Life Sci. 2019, 77, 1289–1317. [Google Scholar]
- Kamili, N.A.; Arthur, C.M.; Gerner-Smidt, C.; Tafesse, E.; Blenda, A.; Dias-Baruffi, M.; Stowell, S.R. Key regulators of galectin-glycan interactions. PROTEOMICS 2016, 16, 3111–3125. [Google Scholar] [CrossRef]
- Stowell, S.R.; Arthur, C.M.; Mehta, P.; Slanina, K.A.; Blixt, O.; Leffler, H.; Smith, D.F.; Cummings, R.D. Galectin-1, -2, and -3 Exhibit Differential Recognition of Sialylated Glycans and Blood Group Antigens. J. Biol. Chem. 2008, 283, 10109–10123. [Google Scholar] [CrossRef]
- Sturm, A.; Lensch, M.; André, S.; Kaltner, H.; Wiedenmann, B.; Rosewicz, S.; Dignass, A.U.; Gabius, H.-J. Human Galectin-2: Novel Inducer of T Cell Apoptosis with Distinct Profile of Caspase Activation. J. Immunol. 2004, 173, 3825–3837. [Google Scholar] [CrossRef]
- André, S.; Kaltner, H.; Lensch, M.; Russwurm, R.; Siebert, H.; Fallsehr, C.; Tajkhorshid, E.; Heck, A.J.R.; von Knebel Doeberitz, M.; Gabius, H.-J.; et al. Determination of structural and functional overlap/divergence of five proto-type galectins by analysis of the growth-regulatory interaction with ganglioside GM1 in silico and in vitro on human neuroblastoma cells. Int. J. Cancer 2005, 114, 46–57. [Google Scholar] [CrossRef]
- Barrow, H.; Guo, X.; Wandall, H.H.; Pedersen, J.W.; Fu, B.; Zhao, Q.; Chen, C.; Rhodes, J.M.; Yu, L.-G. Serum Galectin-2, -4, and -8 Are Greatly Increased in Colon and Breast Cancer Patients and Promote Cancer Cell Adhesion to Blood Vascular Endothelium. Clin. Cancer Res. 2011, 17, 7035–7046. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.; Inoue, K.; Sato, H.; Iida, A.; Ohnishi, Y.; Sekine, A.; Sato, H.; Odashiro, K.; Nobuyoshi, M.; Hori, M.; et al. Functional variation in LGALS2 confers risk of myocardial infarction and regulates lymphotoxin-α secretion in vitro. Nature 2004, 429, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Ghosh, A.; Amin, M.N.; Bachvaroff, T.R.; Tasumi, S.; Pasek, M.; Banerjee, A.; Shridhar, S.; Wang, L.-X.; Bianchet, M.A.; et al. Galectin CvGal2 from the Eastern Oyster (Crassostrea virginica) Displays Unique Specificity for ABH Blood Group Oligosaccharides and Differentially Recognizes Sympatric Perkinsus Species. Biochemistry 2015, 54, 4711–4730. [Google Scholar] [CrossRef] [PubMed]
- Tamura, M.; Saito, M.; Yamamoto, K.; Takeuchi, T.; Ohtake, K.; Tateno, H.; Hirabayashi, J.; Kobayashi, J.; Arata, Y. S-nitrosylation of mouse galectin-2 prevents oxidative inactivation by hydrogen peroxide. Biochem. Biophys. Res. Commun. 2015, 457, 712–717. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hirabayashi, J.; Hashidate, T.; Arata, Y.; Nishi, N.; Nakamura, T.; Hirashima, M.; Urashima, T.; Oka, T.; Futai, M.; Muller, W.E.; et al. Oligosaccharide specificity of galectins: A search by frontal affinity chromatography. Biochim. Biophys. Acta Gen. Subj. 2002, 1572, 232–254. [Google Scholar] [CrossRef] [PubMed]
- Saal, I.; Nagy, N.; Lensch, M.; Lohr, M.; Manning, J.C.; Decaestecker, C.; André, S.; Kiss, R.; Salmon, I.; Gabius, H.-J. Human galectin-2: Expression profiling by RT PCR/immunohistochemistry and its introduction as a histochemical tool for ligand localization. Histol. Histopathol. 2005, 20, 1191–1208. [Google Scholar] [PubMed]
- Nio-Kobayashi, J.; Takahashi-Iwanaga, H.; Iwanaga, T. Immunohistochemical Localization of Six Galectin Subtypes in the Mouse Digestive Tract. J. Histochem. Cytochem. 2008, 57, 41–50. [Google Scholar] [CrossRef]
- Oka, T.; Murakami, S.; Arata, Y.; Hirabayashi, J.; Kasai, K.; Wada, Y.; Futai, M. Identification and cloning of rat galectin-2: Expression is predominantly in epithelial cells of the stomach. Arch. Biochem. Biophys. 1999, 361, 195–201. [Google Scholar] [CrossRef]
- Thomsen, M.K.; Hansen, G.H.; Danielsen, E.M. Galectin-2 at the enterocyte brush border of the small intestine. Mol. Membr. Biol. 2009, 26, 347–355. [Google Scholar] [CrossRef]
- Tamura, M.; Sato, D.; Nakajima, M.; Saito, M.; Sasaki, T.; Tanaka, T.; Hatanaka, T.; Takeuchi, T.; Arata, Y. Identification of Galectin-2–Mucin Interaction and Possible Formation of a High Molecular Weight Lattice. Biol. Pharm. Bull. 2017, 40, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Viguier, M.; Advedissian, T.; Delacour, D.; Poirier, F.; Deshayes, F. Galectins in epithelial functions. Tissue Barriers 2014, 2, e29103. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.B.; Dodd, S.; Yu, L.-G.; Subramanian, S. Serum galectins as potential biomarkers of inflammatory bowel diseases. PLoS ONE 2020, 15, e0227306. [Google Scholar] [CrossRef] [PubMed]
- Paclik, D.; Berndt, U.; Guzy, C.; Dankof, A.; Danese, S.; Holzloehner, P.; Rosewicz, S.; Wiedenmann, B.; Wittig, B.M.; Dignass, A.U.; et al. Galectin-2 induces apoptosis of lamina propria T lymphocytes and ameliorates acute and chronic experimental colitis in mice. Klin. Wochenschr. 2007, 86, 1395–1406. [Google Scholar] [CrossRef] [PubMed]
- Krivokuća, M.J.; Vilotić, A.; Nacka-Aleksić, M.; Pirković, A.; Ćujić, D.; Legner, J.; Dekanski, D.; Bojić-Trbojević, Ž. Galectins in Early Pregnancy and Pregnancy-Associated Pathologies. Int. J. Mol. Sci. 2021, 23, 69. [Google Scholar] [CrossRef] [PubMed]
- Hepp, P.; Unverdorben, L.; Hutter, S.; Kuhn, C.; Ditsch, N.; Groß, E.; Mahner, S.; Jeschke, U.; Knabl, J.; Heidegge, H.H. Placental galectin-2 expression in gestational diabetes: A systematic, histological analysis. Int. J. Mol. Sci. 2020, 21, 2404. [Google Scholar] [CrossRef]
- Unverdorben, L.; Haufe, T.; Santoso, L.; Hofmann, S.; Jeschke, U.; Hutter, S. Prototype and Chimera-Type Galectins in Placentas with Spontaneous and Recurrent Miscarriages. Int. J. Mol. Sci. 2016, 17, 644. [Google Scholar] [CrossRef]
- Hutter, S.; Knabl, J.; Andergassen, U.; Hofmann, S.; Kuhn, C.; Mahner, S.; Arck, P.; Jeschke, U. Placental Expression Patterns of Galectin-1, Galectin-2, Galectin-3 and Galectin-13 in Cases of Intrauterine Growth Restriction (IUGR). Int. J. Mol. Sci. 2016, 17, 523. [Google Scholar] [CrossRef]
- Lyall, F.; Robson, S.C.; Bulmer, J.N. Spiral artery remodeling and trophoblast invasion in preeclampsia and fetal growth restriction relationship to clinical outcome. Hypertension 2013, 62, 1046–1054. [Google Scholar] [CrossRef]
- Hutter, S.; Martin, N.; von Schönfeldt, V.; Messner, J.; Kuhn, C.; Hofmann, S.; Andergassen, U.; Knabl, J.; Jeschke, U. Galectin 2 (gal-2) expression is downregulated on protein and mRNA level in placentas of preeclamptic (PE) patients. Placenta 2015, 36, 438–445. [Google Scholar] [CrossRef]
- Ilarregui, J.M.; Bianco, G.A.; Toscano, M.; Rabinovich, G.A. The coming of age of galectins as immunomodulatory agents: Impact of these carbohydrate binding proteins in T cell physiology and chronic inflammatory disorders. Ann. Rheum. Dis. 2005, 64, iv96–iv103. [Google Scholar] [CrossRef]
- Brinchmann, M.F.; Patel, D.M.; Iversen, M.H. The Role of Galectins as Modulators of Metabolism and Inflammation. Mediat. Inflamm. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Paclik, D.; Werner, L.; Guckelberger, O.; Wiedenmann, B.; Sturm, A. Galectins distinctively regulate central monocyte and macrophage function. Cell. Immunol. 2011, 271, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Stowell, S.R.; Karmakar, S.; Stowell, C.J.; Baruffi, M.D.; McEver, R.P.; Cummings, R.D. Human galectin-1, -2, and -4 induce surface exposure of phosphatidylserine in activated human neutrophils but not in activated T cells. Blood 2006, 109, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Tanaka, N.; Arai, T.; Chida, K.; Muramatsu, M.; Sawabe, M. Polymorphisms of LTA, LGALS2, and PSMA6 genes and coronary atherosclerosis: A pathological study of 1503 consecutive autopsy cases. Atherosclerosis 2012, 221, 458–460. [Google Scholar] [CrossRef]
- Sedlacek, K.; Neureuther, K.; Mueller, J.C.; Stark, K.; Fischer, M.; Baessler, A.; Reinhard, W.; Broeckel, U.; Lieb, W.; Erdmann, J.; et al. Lymphotoxin-α and galectin-2 SNPs are not associated with myocardial infarction in two different German populations. Klin. Wochenschr. 2007, 85, 997–1004. [Google Scholar] [CrossRef] [PubMed]
- Mangino, M.; Braund, P.; Singh, R.; Steeds, R.; Thompson, J.R.; Channer, K.; Samani, N.J. LGALS2 functional variant rs7291467 is not associated with susceptibility to myocardial infarction in Caucasians. Atherosclerosis 2007, 194, 112–115. [Google Scholar] [CrossRef]
- Kimura, A.; Takahashi, M.; Choi, B.Y.; Bae, S.W.; Hohta, S.; Sasaoka, T.; Nakahara, K.-I.; Chida, K.; Sawabe, M.; Yasunami, M.; et al. Lack of association between LTA and LGALS2 polymorphisms and myocardial infarction in Japanese and Korean populations. Tissue Antigens 2007, 69, 265–269. [Google Scholar] [CrossRef]
- Li, W.; Xu, J.; Wang, X.; Chen, J.; Zhang, C.; Sun, K.; Hui, R. Lack of association between lymphotoxin-α, galectin-2 polymorphisms and coronary artery disease: A meta-analysis. Atherosclerosis 2010, 208, 433–436. [Google Scholar] [CrossRef]
- Panoulas, V.F.; Douglas, K.M.; Smith, J.P.; Metsios, G.S.; Elisaf, M.S.; Nightingale, P.; Kitas, G.D. Galectin-2(LGALS2)3279C/T Polymorphism may be Independently Associated with Diastolic Blood Pressure in Patients with Rheumatoid Arthritis. Clin. Exp. Hypertens. 2009, 31, 93–104. [Google Scholar] [CrossRef]
- Ozaki, K.; Sato, H.; Inoue, K.; Tsunoda, T.; Sakata, Y.; Mizuno, H.; Lin, T.-H.; Miyamoto, Y.; Aoki, A.; Onouchi, Y.; et al. SNPs in BRAP associated with risk of myocardial infarction in Asian populations. Nat. Genet. 2009, 41, 329–333. [Google Scholar] [CrossRef]
- Tian, J.; Hu, S.; Wang, F.; Yang, X.; Li, Y.; Huang, C. PPARG, AGTR1, CXCL16 and LGALS2 polymorphisms are correlated with the risk for coronary heart disease. Int. J. Clin. Exp. Pathol. 2015, 8, 3138–3143. [Google Scholar] [PubMed]
- Hollander, M.R.; Jansen, M.F.; Hopman, L.; Dolk, E.; van de Ven, P.M.; Knaapen, P.; Horrevoets, A.J.; Lutgens, E.; van Royen, N. Stimulation of Collateral Vessel Growth by Inhibition of Galectin 2 in Mice Using a Single-Domain Llama-Derived Antibody. J. Am. Hear. Assoc. 2019, 8, e012806. [Google Scholar] [CrossRef] [PubMed]
- Kane, J.; Jansen, M.; Hendrix, S.; Bosmans, L.A.; Beckers, L.; van Tiel, C.; Gijbels, M.; Zelcer, N.; de Vries, C.J.; von Hundelshausen, P.; et al. Anti-Galectin-2 Antibody Treatment Reduces Atherosclerotic Plaque Size and Alters Macrophage Polarity. Thromb. Haemost. 2021, 122, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Chetry, M.; Bhandari, A.; Feng, R.; Song, X.; Wang, P.; Lin, J. Overexpression of galectin2 (LGALS2) predicts a better prognosis in human breast cancer. Am. J. Transl. Res. 2022, 14, 2301–2316. [Google Scholar]
- Ji, P.; Gong, Y.; Jin, M.-L.; Wu, H.-L.; Guo, L.-W.; Pei, Y.-C.; Chai, W.-J.; Jiang, Y.-Z.; Liu, Y.; Ma, X.-Y.; et al. In vivo multidimensional CRISPR screens identify Lgals2 as an immunotherapy target in triple-negative breast cancer. Sci. Adv. 2022, 8, eabl8247. [Google Scholar] [CrossRef]
- Takaishi, S.; Wang, T.C. Gene expression profiling in a mouse model of Helicobacter-induced gastric cancer. Cancer Sci. 2007, 98, 284–293. [Google Scholar] [CrossRef]
- Jung, J.-H.; Kim, H.-J.; Yeom, J.; Yoo, C.; Shin, J.; Yoo, J.; Kang, C.S.; Lee, C. Lowered expression of galectin-2 is associated with lymph node metastasis in gastric cancer. J. Gastroenterol. 2011, 47, 37–48. [Google Scholar] [CrossRef]
- Chen, C.; Duckworth, C.A.; Fu, B.; Pritchard, D.M.; Rhodes, J.M.; Yu, L.-G. Circulating galectins -2, -4 and -8 in cancer patients make important contributions to the increased circulation of several cytokines and chemokines that promote angiogenesis and metastasis. Br. J. Cancer 2014, 110, 741–752. [Google Scholar] [CrossRef]
| Glycans | KD Value | Assesment Methods | Reference |
|---|---|---|---|
| Galactose | 35.6 mM | TFS * | [20] |
| Lactose (Galβ1-4GlcNAc) | 1.3 mM | ||
| N-acetyllactosamine (Galβ1-4GlcNAc, LacNAc) | 654.4 µM | ||
| Lactose | 960.5 µM | ITC | |
| N-acetyllactosamine | 554.2 µM | ||
| Galβ1-3GlcNAc | 68 µM | FAC | [35] |
| N-acetyllactosamine | 130 µM | ||
| GM1 | 240 µM | ||
| Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAc | 140 µM | ||
| Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAc | 90 µM | ||
| Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAcβ1-3Galβ1-4GlcNAc | 85 µM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negedu, M.N.; Duckworth, C.A.; Yu, L.-G. Galectin-2 in Health and Diseases. Int. J. Mol. Sci. 2023, 24, 341. https://doi.org/10.3390/ijms24010341
Negedu MN, Duckworth CA, Yu L-G. Galectin-2 in Health and Diseases. International Journal of Molecular Sciences. 2023; 24(1):341. https://doi.org/10.3390/ijms24010341
Chicago/Turabian StyleNegedu, Muhammed N., Carrie A. Duckworth, and Lu-Gang Yu. 2023. "Galectin-2 in Health and Diseases" International Journal of Molecular Sciences 24, no. 1: 341. https://doi.org/10.3390/ijms24010341
APA StyleNegedu, M. N., Duckworth, C. A., & Yu, L.-G. (2023). Galectin-2 in Health and Diseases. International Journal of Molecular Sciences, 24(1), 341. https://doi.org/10.3390/ijms24010341








