Recent Advances in Cardiovascular Diseases Research Using Animal Models and PET Radioisotope Tracers
Abstract
:1. Introduction
2. Principles of Molecular Imaging in Animal Model of CVD
2.1. PET Imaging Overview
2.1.1. PET Modalities
2.1.2. PET Radiocompounds
2.2. Cardiovascular Disease Modelling in Small Animal Models
3. Radiotracer Based Imaging of Cardiovascular Related Processes, Structures, and Conditions of Heart and Cardiovascular System
3.1. Angiogenesis
3.2. Atherosclerosis
3.2.1. Monitoring the Immune Cells within the Atherosclerotic Plaques
3.2.2. Microcalcification Imaging with [18F]-NaF
3.2.3. Arteriosclerosis Monitoring with Gallium-68 Based Radiocompounds
3.3. Myocarditis
3.3.1. Imaging Inflammation of the Cardiac System with [18F]-FDG
3.3.2. Leukocyte Monitoring in Myocarditis
3.3.3. Myocarditis Imaging Based on Translocation Proteins
3.4. Myocardial Infarction
3.4.1. Cardiac Metabolism Imaging Using [18F]-Based Tracers
3.4.2. Monitoring of Fatty Acids Metabolism
3.4.3. Monitoring Remodelling Processes of the Heart Using [68Ga]-Based Tracers
3.4.4. Radioisotopic Monitoring of Cardiomyocytes Apoptosis
3.4.5. Ischemic Heart Disease
3.5. Radioisotopic Imaging of Perfusion
3.5.1. Application of 82Rb as a Sodium-Potassium ATPase Pump Marker
3.5.2. Application of [13N]-Ammonia
3.5.3. Application of [15O]-Water
3.5.4. Application of [11C]-Acetate
3.5.5. Application of Mitochondrial Complex-1 with Novel [18F]-Flurpiridaz
4. Challenges and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jagannathan, R.; Patel, S.A.; Ali, M.K.; Narayan, K.M.V. Global Updates on Cardiovascular Disease Mortality Trends and Attribution of Traditional Risk Factors. Curr. Diab. Rep. 2019, 19, 44. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, H.J.; Benjamin, E.J.; Callaway, C.W.; Carson, A.P.; Cheng, S.; Elkind, M.S.V.; Evenson, K.R.; Ferguson, J.F.; Knutson, K.L.; Lee, C.D.; et al. Heart Disease and Stroke Statistics-2021 Update A Report from the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar]
- Amini, M.; Zayeri, F.; Salehi, M. Trend Analysis of Cardiovascular Disease Mortality, Incidence, and Mortality-to-Incidence Ratio: Results from Global Burden of Disease Study 2017. BMC Public Health 2021, 21, 401. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.; Vasan, R.S. Epidemiology of Cardiovascular Disease in Young Individuals. Nat. Rev. Cardiol. 2018, 15, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Nakano, Y.; Adachi, S.; Murohara, T. Effects of Tobacco Smoking on Cardiovascular Disease. Circ. J. 2019, 83, 1980–1985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Gupte, A.A.; Zhang, A.; Hamilton, D.J. Pet Imaging and Its Application in Cardiovascular Diseases. Methodist DeBakey Cardiovasc. J. 2017, 13, 29–33. [Google Scholar] [CrossRef] [Green Version]
- Vaz, S.C.; Oliveira, F.; Herrmann, K.; Veit-Haibach, P. Nuclear Medicine and Molecular Imaging Advances in the 21st Century. Br. J. Radiol. 2020, 93, 20200095. [Google Scholar] [CrossRef]
- MacRitchie, N.; Noonan, J.; Guzik, T.J.; Maffia, P. Molecular Imaging of Cardiovascular Inflammation. Br. J. Pharmacol. 2021, 178, 4216–4245. [Google Scholar] [CrossRef]
- Hickman, D.L.; Johnson, J.; Vemulapalli, T.H.; Crisler, J.R.; Shepherd, R. Commonly Used Animal Models. In Principles of Animal Research; Elsevier: Amsterdam, The Netherlands, 2017; pp. 117–175. ISBN 978-0-12-802151-4. [Google Scholar]
- Mena, E.; Black, P.C.; Rais-Bahrami, S.; Gorin, M.; Allaf, M.; Choyke, P. Novel PET Imaging Methods for Prostate Cancer. World J. Urol. 2021, 39, 687–699. [Google Scholar] [CrossRef]
- Unterrainer, M.; Eze, C.; Ilhan, H.; Marschner, S.; Roengvoraphoj, O.; Schmidt-Hegemann, N.S.; Walter, F.; Kunz, W.G.; af Rosenschöld, P.M.; Jeraj, R.; et al. Recent Advances of PET Imaging in Clinical Radiation Oncology. Radiat. Oncol. 2020, 15, 88. [Google Scholar] [CrossRef] [Green Version]
- Holzgreve, A.; Albert, N.L.; Galldiks, N.; Suchorska, B. Use of PET Imaging in Neuro-Oncological Surgery. Cancers 2021, 13, 2093. [Google Scholar] [CrossRef] [PubMed]
- On behalf of the Quantitative Cardiac Imaging Study Group; Dewey, M.; Siebes, M.; Kachelrieß, M.; Kofoed, K.F.; Maurovich-Horvat, P.; Nikolaou, K.; Bai, W.; Kofler, A.; Manka, R.; et al. Clinical Quantitative Cardiac Imaging for the Assessment of Myocardial Ischaemia. Nat. Rev. Cardiol. 2020, 17, 427–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pijl, J.P.; Kwee, T.C.; Slart, R.H.J.A.; Glaudemans, A.W.J.M. PET/CT Imaging for Personalized Management of Infectious Diseases. J. Pers. Med. 2021, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Villemain, O.; Baranger, J.; Jalal, Z.; Lam, C.; Calais, J.; Pernot, M.; Cifra, B.; Friedberg, M.K.; Mertens, L. Non-Invasive Imaging Techniques to Assess Myocardial Perfusion. Expert Rev. Med. Devices 2020, 17, 1133–1144. [Google Scholar] [CrossRef] [PubMed]
- Küstner, T.; Schwartz, M.; Martirosian, P.; Gatidis, S.; Seith, F.; Gilliam, C.; Blu, T.; Fayad, H.; Visvikis, D.; Schick, F.; et al. MR-Based Respiratory and Cardiac Motion Correction for PET Imaging. Med. Image Anal. 2017, 42, 129–144. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, X. MRI-Driven PET Image Optimization for Neurological Applications. Front. Neurosci. 2019, 13, 782. [Google Scholar] [CrossRef] [Green Version]
- Ohiduzzaman, M.; Khatun, R.; Reza, S.; Khan, K.A.; Akter, S.; Uddin, M.F.; Ahasan, M.M. Study of Exposure Rates from Various Nuclear Medicine Scan at INMAS, Dhaka. Int. J. Adv. Res. Innov. Ideas Educ. 2019, 5, 12. [Google Scholar]
- Koike, H.; Okumura, T.; Murohara, T.; Katsuno, M. Multidisciplinary Approaches for Transthyretin Amyloidosis. Cardiol. Ther. 2021, 10, 289–311. [Google Scholar] [CrossRef]
- Dulski, K.; Bass, S.D.; Chhokar, J.; Chug, N.; Curceanu, C.; Czerwiński, E.; Dagdar, M.; Gajewski, J.; Gajos, A.; Gorgol, M.; et al. The J-PET Detector—A Tool for Precision Studies of Ortho-Positronium Decays. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2021, 1008, 165452. [Google Scholar] [CrossRef]
- Farag, A.; Thompson, R.T.; Thiessen, J.D.; Biernaski, H.; Prato, F.S.; Théberge, J. Evaluation of 511 KeV Photon Attenuation by a Novel 32-Channel Phased Array Prospectively Designed for Cardiovascular Hybrid PET/MRI Imaging. Eur. J. Hybrid Imaging 2020, 4, 7. [Google Scholar] [CrossRef]
- Song, H.; Kang, I.S.; Kim, K.B.; Park, C.; Baek, M.K.; Lee, S.; Chung, Y.H. Performance Evaluation of an Adjustable Gantry PET (AGPET) for Small Animal PET Imaging. Nucl. Eng. Technol. 2021, 53, 2646–2651. [Google Scholar] [CrossRef]
- Lee, J.S. A Review of Deep-Learning-Based Approaches for Attenuation Correction in Positron Emission Tomography. IEEE Trans. Radiat. Plasma Med. Sci. 2021, 5, 160–184. [Google Scholar] [CrossRef]
- Ladefoged, C.N.; Law, I.; Anazodo, U.; St. Lawrence, K.; Izquierdo-Garcia, D.; Catana, C.; Burgos, N.; Cardoso, M.J.; Ourselin, S.; Hutton, B.; et al. A Multi-Centre Evaluation of Eleven Clinically Feasible Brain PET/MRI Attenuation Correction Techniques Using a Large Cohort of Patients. NeuroImage 2017, 147, 346–359. [Google Scholar] [CrossRef] [Green Version]
- Crișan, G.; Moldovean-Cioroianu, N.S.; Timaru, D.-G.; Andrieș, G.; Căinap, C.; Chiș, V. Radiopharmaceuticals for PET and SPECT Imaging: A Literature Review over the Last Decade. Int. J. Mol. Sci. 2022, 23, 5023. [Google Scholar] [CrossRef] [PubMed]
- Jødal, L.; Le Loirec, C.; Champion, C. Positron range in PET imaging: Non-conventional isotopes. Phys. Med. Biol. 2014, 59, 7419–7434. [Google Scholar] [CrossRef] [PubMed]
- Coenen, H.H.; Gee, A.D.; Adam, M.; Antoni, G.; Cutler, C.S.; Fujibayashi, Y.; Jeong, J.M.; Mach, R.H.; Mindt, T.L.; Pike, V.W.; et al. Consensus Nomenclature Rules for Radiopharmaceutical Chemistry—Setting the Record Straight. Nucl. Med. Biol. 2017, 55, v–xi. [Google Scholar] [CrossRef] [Green Version]
- Cusnir, R.; Leresche, M.; Pilloud, C.; Straub, M. An Investigation of Aspects of Radiochemical Purity of 99mTc-Labelled Human Serum Albumin Nanocolloid. EJNMMI Radiopharm. Chem. 2021, 6, 35. [Google Scholar] [CrossRef]
- Krishnan, A.; Samtani, R.; Dhanantwari, P.; Lee, E.; Yamada, S.; Shiota, K.; Donofrio, M.T.; Leatherbury, L.; Lo, C.W. A Detailed Comparison of Mouse and Human Cardiac Development. Pediatr. Res. 2014, 76, 500–507. [Google Scholar] [CrossRef] [Green Version]
- Janssen, P.M.L.; Biesiadecki, B.J.; Ziolo, M.T.; Davis, J.P. The Need for Speed: Mice, Men, and Myocardial Kinetic Reserve. Circ. Res. 2016, 119, 418–421. [Google Scholar] [CrossRef] [Green Version]
- Bukhari, Q.; Schroeter, A.; Rudin, M. Increasing Isoflurane Dose Reduces Homotopic Correlation and Functional Segregation of Brain Networks in Mice as Revealed by Resting-State FMRI. Sci. Rep. 2018, 8, 10591. [Google Scholar] [CrossRef] [Green Version]
- You, T.; Im, G.H.; Kim, S.-G. Characterization of Brain-Wide Somatosensory BOLD FMRI in Mice under Dexmedetomidine/Isoflurane and Ketamine/Xylazine. Sci. Rep. 2021, 11, 13110. [Google Scholar] [CrossRef] [PubMed]
- Paterek, A.; Kępska, M.; Kołodziejczyk, J.; Leszek, P.; Mackiewicz, U.; Mączewski, M. Acute Heart Rate-Dependent Hemodynamic Function of the Heart in the Post-Myocardial Infarction Rat Model: Change Over Time. Can. J. Cardiol. 2018, 34, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Sessa, F.; Anna, V.; Messina, G.; Cibelli, G.; Monda, V.; Marsala, G.; Ruberto, M.; Biondi, A.; Cascio, O.; Bertozzi, G.; et al. Heart Rate Variability as Predictive Factor for Sudden Cardiac Death. Aging 2018, 10, 166–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constantinesco, A.; Choquet, P.; Goetz, C.; Monassier, L. PET, SPECT, CT, and MRI in Mouse Cardiac Phenotyping: An Overview. Curr. Protoc. Mouse Biol. 2012, 2, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, D.; Xanthos, T.; Dontas, I.; Lelovas, P.; Perrea, D. The Use of Mice and Rats as Animal Models for Cardiopulmonary Resuscitation Research. Lab. Anim. 2008, 42, 265–276. [Google Scholar] [CrossRef]
- Golforoush, P.; Yellon, D.M.; Davidson, S.M. Mouse Models of Atherosclerosis and Their Suitability for the Study of Myocardial Infarction. Basic Res. Cardiol. 2020, 115, 73. [Google Scholar] [CrossRef]
- Zhuang, X.; Feng, Y.; Li, J.; Zhao, F.; Zhang, Y.; Chen, Y. A Longitudinal 18F-Fluorodeoxyglucose (18F-FDG) and 18F-Sodium Fluoride (18F-NaF) Positron Emission Tomography/Computed Tomography (PET/CT) Study in Apolipoprotein E (ApoE) Knockout Rats Fed with a Western Diet. Cardiovasc. Diagn. Ther. 2021, 11, 39–49. [Google Scholar] [CrossRef]
- Shen, S.; Li, H.; Ge, S.; Huang, H.; Zhang, H.; Li, F.; Feng, Y.; Wang, L.; Weng, X.; Lu, Y.; et al. 18 F-fluorodeoxyglucose Positron Emission Tomography for the Detection of Inflammatory Lesions of the Arterial Vessel Walls in Wistar Rats. Exp. Ther. Med. 2021, 21, 370. [Google Scholar] [CrossRef]
- Laitinen, I.; Marjamäki, P.; Haaparanta, M.; Savisto, N.; Laine, V.J.O.; Soini, S.L.; Wilson, I.; Leppänen, P.; Ylä-Herttuala, S.; Roivainen, A.; et al. Non-Specific Binding of [18F]FDG to Calcifications in Atherosclerotic Plaques: Experimental Study of Mouse and Human Arteries. Eur. J. Nucl. Med. Mol. Imaging 2006, 33, 1461–1467. [Google Scholar] [CrossRef]
- Werner, R.A.; Wakabayashi, H.; Bauer, J.; Schütz, C.; Zechmeister, C.; Hayakawa, N.; Javadi, M.S.; Lapa, C.; Jahns, R.; Ergün, S.; et al. Longitudinal 18F-FDG PET Imaging in a Rat Model of Autoimmune Myocarditis. Eur. Heart J.-Cardiovasc. Imaging 2019, 20, 467–474. [Google Scholar] [CrossRef] [Green Version]
- Galvin, J.E.; Hemric, M.E.; Kosanke, S.D.; Factor, S.M.; Quinn, A.; Cunningham, M.W. Induction of myocarditis and valvulitis in lewis rats by different epitopes of cardiac myosin and its implications in rheumatic carditis. Am. J. Pathol. 2002, 160, 297–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Zhu, Y.; Mu, Q.; Luo, H.; Zhi, Y.; Shen, X. Oxymatrine Provides Protection against Coxsackievirus B3-Induced Myocarditis in BALB/c Mice. Antivir. Res. 2017, 141, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Fernández, B.; Durán, A.C.; Fernández, M.C.; Fernández-Gallego, T.; Icardo, J.M.; Sans-Coma, V. The coronary arteries of the C57BL/6 mouse strains: Implications for comparison with mutant models. J. Anat. 2008, 212, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wei, J.; Wang, L.; Zhu, Y.; Li, L.; Olunga, M.A.; Gao, X.M.; Fan, G.W. Cardioprotection against ischemia/reperfusion injury by QiShenYiQi Pill® via ameliorate of multiple mitochondrial dysfunctions. Drug Des. Devel. Ther. 2015, 9, 3051–3066. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werner, R.A.; Hess, A.; Koenig, T.; Diekmann, J.; Derlin, T.; Melk, A.; Thackeray, J.T.; Bauersachs, J.; Bengel, F.M. Molecular Imaging of Inflammation Crosstalk along the Cardio-Renal Axis Following Acute Myocardial Infarction. Theranostics 2021, 11, 7984–7994. [Google Scholar] [CrossRef] [PubMed]
- Kolanowski, T.J.; Wargocka-Matuszewska, W.; Zimna, A.; Cheda, L.; Zyprych-Walczak, J.; Rugowska, A.; Drabik, M.; Fiedorowicz, M.; Krajewski, S.; Steczek, Ł.; et al. Multiparametric Evaluation of Post-MI Small Animal Models Using Metabolic ([18F]FDG) and Perfusion-Based (SYN1) Heart Viability Tracers. Int. J. Mol. Sci. 2021, 22, 12591. [Google Scholar] [CrossRef]
- Park, S.-J.; Kim, R.Y.; Park, B.-W.; Lee, S.; Choi, S.W.; Park, J.-H.; Choi, J.J.; Kim, S.-W.; Jang, J.; Cho, D.-W.; et al. Dual Stem Cell Therapy Synergistically Improves Cardiac Function and Vascular Regeneration Following Myocardial Infarction. Nat. Commun. 2019, 10, 3123. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Huang, M.; Wu, J.; Jiang, Q.; Zheng, X. Exosomes Isolated from the Plasma of Remote Ischemic Conditioning Rats Improved Cardiac Function and Angiogenesis after Myocardial Infarction through Targeting Hsp70. Aging 2020, 12, 3682–3693. [Google Scholar] [CrossRef]
- Wu, X.; Reboll, M.R.; Korf-Klingebiel, M.; Wollert, K.C. Angiogenesis after Acute Myocardial Infarction. Cardiovasc. Res. 2021, 117, 1257–1273. [Google Scholar] [CrossRef]
- Hoeeg, C.; Ringgaard, L.; Christensen, E.; Follin, B.; Bentsen, S.; Ripa, R.S.; Kjaer, A. Flow Cytometric Evaluation of the Ongoing Angiogenic Response in Rat Cardiac Tissue Following Myocardial Infarction. Curr. Protoc. 2021, 1, e40. [Google Scholar] [CrossRef]
- Dissoki, S.; Abourbeh, G.; Salnikov, O.; Mishani, E.; Jacobson, O. PET Molecular Imaging of Angiogenesis with a Multiple Tyrosine Kinase Receptor-Targeted Agent in a Rat Model of Myocardial Infarction. Mol. Imaging Biol. 2015, 17, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Shang, J.; Xie, L.; Hanyu, M.; Zhang, Y.; Yang, Z.; Xu, H.; Wang, L.; Zhang, M.-R. PET Imaging of VEGFR with a Novel 64Cu-Labeled Peptide. ACS Omega 2020, 5, 8508–8514. [Google Scholar] [CrossRef] [PubMed]
- Stacy, M.R.; Paeng, J.C.; Sinusas, A.J. The Role of Molecular Imaging in the Evaluation of Myocardial and Peripheral Angiogenesis. Ann. Nucl. Med. 2015, 29, 217–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mezu-Ndubuisi, O.J.; Maheshwari, A. The Role of Integrins in Inflammation and Angiogenesis. Pediatr. Res. 2021, 89, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.I.; Döring, P.; Gäbel, R.; Vasudevan, P.; Lemcke, H.; Müller, P.; Stenzel, J.; Lindner, T.; Joksch, M.; Kurth, J.; et al. [68Ga]-NODAGA-RGD Positron Emission Tomography (PET) for Assessment of Post Myocardial Infarction Angiogenesis as a Predictor for Left Ventricular Remodeling in Mice after Cardiac Stem Cell Therapy. Cells 2020, 9, 1358. [Google Scholar] [CrossRef]
- Cai, M.; Ren, L.; Yin, X.; Guo, Z.; Li, Y.; He, T.; Tang, Y.; Long, T.; Liu, Y.; Liu, G.; et al. PET Monitoring Angiogenesis of Infarcted Myocardium after Treatment with Vascular Endothelial Growth Factor and Bone Marrow Mesenchymal Stem Cells. Amino Acids 2016, 48, 811–820. [Google Scholar] [CrossRef]
- Saraste, A.; Knuuti, J. PET Imaging in Heart Failure: The Role of New Tracers. Heart Fail. Rev. 2017, 22, 501–511. [Google Scholar] [CrossRef]
- Moyon, A.; Garrigue, P.; Balasse, L.; Fernandez, S.; Brige, P.; Nollet, M.; Hache, G.; Blot-Chabaud, M.; Dignat-George, F.; Guillet, B. Early Prediction of Revascularisation by Angiomotin-Targeting Positron Emission Tomography. Theranostics 2018, 8, 4985–4994. [Google Scholar] [CrossRef]
- Kubena, P.; Arrigo, M.; Parenica, J.; Gayat, E.; Sadoune, M.; Ganovska, E.; Pavlusova, M.; Littnerova, S.; Spinar, J.; Mebazaa, A.; et al. Plasma Levels of Soluble CD146 Reflect the Severity of Pulmonary Congestion Better Than Brain Natriuretic Peptide in Acute Coronary Syndrome. Ann. Lab. Med. 2016, 36, 300–305. [Google Scholar] [CrossRef] [Green Version]
- Moyon, A.; Garrigue, P.; Fernandez, S.; Hubert, F.; Balasse, L.; Brige, P.; Hache, G.; Nail, V.; Blot-Chabaud, M.; Dignat-George, F.; et al. Comparison of a New68ga-Radiolabelled Pet Imaging Agent Scd146 and Rgd Peptide for in Vivo Evaluation of Angiogenesis in Mouse Model of Myocardial Infarction. Cells 2021, 10, 2305. [Google Scholar] [CrossRef]
- Rageh, M.M.; El-Gebaly, R.H.; Maamoun, I. Estimation of Organ Absorbed Doses in Mice from 99MTC-mibi Using Myocardial Perfusion Imaging. Rom. J. Biophys. 2019, 29, 7. [Google Scholar]
- Florea, A.; Mottaghy, F.M.; Bauwens, M. Molecular Imaging of Angiogenesis in Oncology: Current Preclinical and Clinical Status. Int. J. Mol. Sci. 2021, 22, 5544. [Google Scholar] [CrossRef] [PubMed]
- Hayman, L.L. Prevention of Atherosclerotic Cardiovascular Disease in Childhood. Curr. Cardiol. Rep. 2020, 22, 86. [Google Scholar] [CrossRef] [PubMed]
- von Scheidt, M.; Zhao, Y.; Kurt, Z.; Pan, C.; Zeng, L.; Yang, X.; Schunkert, H.; Lusis, A.J. Applications and Limitations of Mouse Models for Understanding Human Atherosclerosis. Cell Metab. 2017, 25, 248–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucerius, J.; Dijkgraaf, I.; Mottaghy, F.M.; Schurgers, L.J. Target Identification for the Diagnosis and Intervention of Vulnerable Atherosclerotic Plaques beyond 18F-Fluorodeoxyglucose Positron Emission Tomography Imaging: Promising Tracers on the Horizon. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 251–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dilsizian, V.; Jadvar, H. Science to Practice: Does FDG Differentiate Morphologically Unstable from Stable Atherosclerotic Plaque? Radiology 2017, 283, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Tavakoli, S.; Short, J.D.; Downs, K.; Nguyen, H.N.; Lai, Y.; Zhang, W.; Jerabek, P.; Goins, B.; Sadeghi, M.M.; Asmis, R. Differential Regulation of Macrophage Glucose Metabolism by Macrophage Colony-Stimulating Factor and Granulocyte-Macrophage Colony-Stimulating Factor: Implications for 18F-FDG PET Imaging of Vessel Wall Inflammation. Radiology 2017, 283, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Creager, M.D.; Hohl, T.; Hutcheson, J.D.; Moss, A.J.; Schlotter, F.; Blaser, M.C.; Park, M.A.; Ho Lee, L.; Singh, S.A.; Alcaide-Corral, C.J.; et al. 18F-Fluoride Signal Amplification Identifies Microcalcifications Associated with Atherosclerotic Plaque Instability in Positron Emission Tomography/Computed Tomography Images. Circ. Cardiovasc. Imaging 2019, 12, e007835. [Google Scholar] [CrossRef] [Green Version]
- Irkle, A.; Vesey, A.T.; Lewis, D.Y.; Skepper, J.N.; Bird, J.L.E.; Dweck, M.R.; Joshi, F.R.; Gallagher, F.A.; Warburton, E.A.; Bennett, M.R.; et al. Identifying Active Vascular Microcalcification by 18F-Sodium Fluoride Positron Emission Tomography. Nat. Commun. 2015, 6, 7495. [Google Scholar] [CrossRef] [Green Version]
- Tzolos, E.; Dweck, M.R. 18F-Sodium Fluoride (18F-NaF) for Imaging Microcalcification Activity in the Cardiovascular System. Arter. Thromb. Vasc. Biol. 2020, 40, 1620–1626. [Google Scholar] [CrossRef] [PubMed]
- Florea, A.; Sigl, J.P.; Morgenroth, A.; Vogg, A.; Sahnoun, S.; Winz, O.H.; Bucerius, J.; Schurgers, L.J.; Mottaghy, F.M. Sodium [18F]Fluoride PET Can Efficiently Monitor In Vivo Atherosclerotic Plaque Calcification Progression and Treatment. Cells 2021, 10, 275. [Google Scholar] [CrossRef] [PubMed]
- Rucher, G.; Cameliere, L.; Fendri, J.; Anfray, A.; Abbas, A.; Kamel, S.; Dupas, Q.; Delcroix, N.; Berger, L.; Manrique, A.; et al. Molecular Imaging of Endothelial Activation and Mineralization in a Mouse Model of Accelerated Atherosclerosis. EJNMMI Res. 2019, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Høilund-Carlsen, P.F.; Piri, R.; Constantinescu, C.; Iversen, K.K.; Werner, T.J.; Sturek, M.; Alavi, A.; Gerke, O. Atherosclerosis Imaging with 18F-Sodium Fluoride PET. Diagnostics 2020, 10, 852. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, S.; Sadeghi, M.M. 18F-Sodium Fluoride Positron Emission Tomography and Plaque Calcification: How Complicated Can It Be? Circ. Cardiovasc. Imaging 2019, 12, e008712. [Google Scholar] [CrossRef] [Green Version]
- Vesey, A.T.; Jenkins, W.S.A.; Irkle, A.; Moss, A.; Sng, G.; Forsythe, R.O.; Clark, T.; Roberts, G.; Fletcher, A.; Lucatelli, C.; et al. 18F-Fluoride and 18F-Fluorodeoxyglucose Positron Emission Tomography After Transient Ischemic Attack or Minor Ischemic Stroke: Case–Control Study. Circ. Cardiovasc. Imaging 2017, 10, e004976. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.; Seok, J.W. An Update on [18f]Fluoride Pet Imaging for Atherosclerotic Disease. J. Lipid Atheroscler. 2020, 9, 349–361. [Google Scholar] [CrossRef]
- Vatsa, R.; Ashwathanarayana, A.A.; Shukla, J.; Singh, S.; Bhusari, P.; Kumar, R.; Singh, H.; Mittal, B.R. Multifarious Ga-68 Labeled PET Radiopharmaceuticals in Imaging Various Malignancies. Indian J. Nucl. Med. 2018, 33, 242–244. [Google Scholar] [CrossRef]
- Lechner, M.; Schartinger, V.H.; Steele, C.D.; Nei, W.L.; Ooft, M.L.; Schreiber, L.M.; Pipinikas, C.P.; Chung, G.T.Y.; Chan, Y.Y.; Wu, F.; et al. Somatostatin Receptor 2 Expression in Nasopharyngeal Cancer Is Induced by Epstein Barr Virus Infection: Impact on Prognosis, Imaging and Therapy. Nat. Commun. 2021, 12, 117. [Google Scholar] [CrossRef]
- Heo, G.S.; Sultan, D.; Liu, Y. Current and Novel Radiopharmaceuticals for Imaging Cardiovascular Inflammation. Q. J. Nucl. Med. Mol. Imaging 2020, 64, 4–20. [Google Scholar] [CrossRef]
- Bartlett, B.; Ludewick, H.P.; Lee, S.; Verma, S.; Francis, R.J.; Dwivedi, G. Imaging Inflammation in Patients and Animals: Focus on PET Imaging the Vulnerable Plaque. Cells 2021, 10, 2573. [Google Scholar] [CrossRef] [PubMed]
- Rinne, P.; Hellberg, S.; Kiugel, M.; Virta, J.; Li, X.G.; Käkelä, M.; Helariutta, K.; Luoto, P.; Liljenbäck, H.; Hakovirta, H.; et al. Comparison of Somatostatin Receptor 2-Targeting PET Tracers in the Detection of Mouse Atherosclerotic Plaques. Mol. Imaging Biol. 2016, 18, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, H.; Silvola, J.M.U.; Autio, A.; Li, X.G.; Liljenbäck, H.; Hellberg, S.; Siitonen, R.; Stahle, M.; Käkelä, M.; Airaksinen, A.J.; et al. Comparison of 68Ga-DOTA-Siglec-9 and 18F-Fluorodeoxyribose-Siglec-9: Inflammation Imaging and Radiation Dosimetry. Contrast Media Mol. Imaging 2017, 2017, 7645070. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.S.; Sia, C.H.; Chan, M.Y.; Lin, W.; Wong, R.C. Coronavirus-Induced Myocarditis: A Meta-Summary of Cases. Heart Lung 2020, 49, 681–685. [Google Scholar] [CrossRef]
- Ammirati, E.; Veronese, G.; Bottiroli, M.; Wang, D.W.; Cipriani, M.; Garascia, A.; Pedrotti, P.; Adler, E.D.; Frigerio, M. Update on Acute Myocarditis. Trends Cardiovasc. Med. 2021, 31, 370–379. [Google Scholar] [CrossRef]
- Chen, W.; Jeudy, J. Assessment of Myocarditis: Cardiac MR, PET/CT, or PET/MR? Curr. Cardiol. Rep. 2019, 21, 76. [Google Scholar] [CrossRef]
- Ammirati, E.; Frigerio, M.; Adler, E.D.; Basso, C.; Birnie, D.H.; Brambatti, M.; Friedrich, M.G.; Klingel, K.; Lehtonen, J.; Moslehi, J.J.; et al. Management of Acute Myocarditis and Chronic Inflammatory Cardiomyopathy: An Expert Consensus Document. Circ. Heart Fail. 2020, 13, 663–687. [Google Scholar] [CrossRef]
- Kircher, M.; Lapa, C. Novel Noninvasive Nuclear Medicine Imaging Techniques for Cardiac Inflammation. Curr. Cardiovasc. Imaging Rep. 2017, 10, 6. [Google Scholar] [CrossRef] [Green Version]
- Wong, H.-L.; Hu, M.; Zhou, C.K.; Lloyd, P.C.; Amend, K.L.; Beachler, D.C.; Secora, A.; McMahill-Walraven, C.N.; Lu, Y.; Wu, Y.; et al. Risk of Myocarditis and Pericarditis after the COVID-19 MRNA Vaccination in the USA: A Cohort Study in Claims Databases. Lancet 2022, 399, 2191–2199. [Google Scholar] [CrossRef]
- Li, C.; Chen, Y.; Zhao, Y.; Lung, D.C.; Ye, Z.; Song, W.; Liu, F.-F.; Cai, J.-P.; Wong, W.-M.; Yip, C.C.-Y.; et al. Intravenous injection of coronavirus disease 2019 (COVID-19) mRNA vaccine can induce acute myopericarditis in mouse model. Clin. Infect. Dis. 2022, 74, 1933–1950. [Google Scholar] [CrossRef]
- Blyszczuk, P. Myocarditis in Humans and in Experimental Animal Models. Front. Cardiovasc. Med. 2019, 6, 64. [Google Scholar] [CrossRef] [Green Version]
- Wargocka-Matuszewska, W.; Fiedorowicz, K.; Rugowska, A.; Bednarowicz, K.; Zimna, A.; Lukasz, C.; Hamankiewicz, P.; Kilian, K.; Fiedorowicz, M.; Drabik, M.; et al. Molecular Imaging of Myogenic Stem/Progenitor Cells with [18F]-FHBG PET/CT System in SCID Mice Model of Post-Infarction Heart. Sci. Rep. 2021, 11, 19825. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, P.; Gaebel, R.; Doering, P.; Mueller, P.; Lemcke, H.; Stenzel, J.; Lindner, T.; Kurth, J.; Steinhoff, G.; Vollmar, B.; et al. 18F-FDG PET-Based Imaging of Myocardial Inflammation Predicts a Functional Outcome Following Transplantation of MESC-Derived Cardiac Induced Cells in a Mouse Model of Myocardial Infarction. Cells 2019, 8, 1613. [Google Scholar] [CrossRef] [PubMed]
- Clément, A.; Boutley, H.; Poussier, S.; Pierson, J.; Lhuillier, M.; Kolodziej, A.; Olivier, J.L.; Karcher, G.; Marie, P.Y.; Maskali, F. A 1-Week Extension of a Ketogenic Diet Provides a Further Decrease in Myocardial 18F-FDG Uptake and a High Detectability of Myocarditis with FDG-PET. J. Nucl. Cardiol. 2020, 27, 612–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahandideh, A.; Uotila, S.; Ståhle, M.; Virta, J.; Li, X.G.; Kytö, V.; Marjamäki, P.; Liljenbäck, H.; Taimen, P.; Oikonen, V.; et al. Folate Receptor b–Targeted PET Imaging of Macrophages in Autoimmune Myocarditis. J. Nucl. Med. 2020, 61, 1643–1649. [Google Scholar] [CrossRef]
- Hotta, M.; Minamimoto, R.; Miwa, K. 11C-Methionine-PET for Differentiating Recurrent Brain Tumor from Radiation Necrosis: Radiomics Approach with Random Forest Classifier. Sci. Rep. 2019, 9, 15666. [Google Scholar] [CrossRef] [Green Version]
- Pessina, F.; Navarria, P.; Clerici, E.; Bellu, L.; Franzini, A.; Milani, D.; Simonelli, M.; Persico, P.; Politi, L.S.; Casarotti, A.; et al. Role of 11C Methionine Positron Emission Tomography (11CMETPET) for Surgery and Radiation Therapy Planning in Newly Diagnosed Glioblastoma Patients Enrolled into a Phase II Clinical Study. J. Clin. Med. 2021, 10, 2313. [Google Scholar] [CrossRef]
- Maya, Y.; Werner, R.A.; Schütz, C.; Wakabayashi, H.; Samnick, S.; Lapa, C.; Zechmeister, C.; Jahns, R.; Jahns, V.; Higuchi, T. 11C-Methionine PET of Myocardial Inflammation in a Rat Model of Experimental Autoimmune Myocarditis. J. Nucl. Med. 2016, 57, 1985–1990. [Google Scholar] [CrossRef] [Green Version]
- Borchert, T.; Beitar, L.; Langer, L.B.N.; Polyak, A.; Wester, H.J.; Ross, T.L.; Hilfiker-Kleiner, D.; Bengel, F.M.; Thackeray, J.T. Dissecting the Target Leukocyte Subpopulations of Clinically Relevant Inflammation Radiopharmaceuticals. J. Nucl. Cardiol. 2021, 28, 1636–1645. [Google Scholar] [CrossRef]
- Tarkin, J.M.; Calcagno, C.; Dweck, M.R.; Evans, N.R.; Chowdhury, M.M.; Gopalan, D.; Newby, D.E.; Fayad, Z.A.; Bennett, M.R.; Rudd, J.H.F. 68Ga-DOTATATE PET Identifies Residual Myocardial Inflammation and Bone Marrow Activation After Myocardial Infarction. J. Am. Coll. Cardiol. 2019, 73, 2489–2491. [Google Scholar] [CrossRef]
- Toner, Y.C.; Ghotbi, A.A.; Naidu, S.; Sakurai, K.; van Leent, M.M.T.; Jordan, S.; Ordikhani, F.; Amadori, L.; Sofias, A.M.; Fisher, E.L.; et al. Systematically Evaluating DOTATATE and FDG as PET Immuno-Imaging Tracers of Cardiovascular Inflammation. Sci. Rep. 2022, 12, 6185. [Google Scholar] [CrossRef] [PubMed]
- Johnbeck, C.B.; Knigge, U.; Loft, A.; Berthelsen, A.K.; Mortensen, J.; Oturai, P.; Langer, S.W.; Elema, D.R.; Kjaer, A. Head-to-Head Comparison of 64Cu-DOTATATE and 68Ga-DOTATOC PET/CT: A Prospective Study of 59 Patients with Neuroendocrine Tumors. J. Nucl. Med. 2017, 58, 451–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glasenapp, A.; Derlin, K.; Gutberlet, M.; Hess, A.; Ross, T.L.; Wester, H.J.; Bengel, F.M.; Thackeray, J.T. Molecular Imaging of Inflammation and Fibrosis in Pressure Overload Heart Failure. Circ. Res. 2021, 129, 369–382. [Google Scholar] [CrossRef]
- Nack, A.; Brendel, M.; Nedelcu, J.; Daerr, M.; Nyamoya, S.; Beyer, C.; Focke, C.; Deussing, M.; Hoornaert, C.; Ponsaerts, P.; et al. Expression of Translocator Protein and [18F]-GE180 Ligand Uptake in Multiple Sclerosis Animal Models. Cells 2019, 8, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thackeray, J.T.; Hupe, H.C.; Wang, Y.; Bankstahl, J.P.; Berding, G.; Ross, T.L.; Bauersachs, J.; Wollert, K.C.; Bengel, F.M. Myocardial Inflammation Predicts Remodeling and Neuroinflammation After Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 71, 263–275. [Google Scholar] [CrossRef]
- Keller, T.; Krzyczmonik, A.; Forsback, S.; Picón, F.R.L.; Kirjavainen, A.K.; Takkinen, J.; Rajander, J.; Cacheux, F.; Damont, A.; Dollé, F.; et al. Radiosynthesis and Preclinical Evaluation of [18F]F-DPA, A Novel Pyrazolo[1,5a]Pyrimidine Acetamide TSPO Radioligand, in Healthy Sprague Dawley Rats. Mol. Imaging Biol. 2017, 19, 736–745. [Google Scholar] [CrossRef] [Green Version]
- Mou, T.; Tian, J.; Tian, Y.; Yun, M.; Li, J.; Dong, W.; Lu, X.; Zhu, Z.; Mi, H.; Zhang, X.; et al. Automated Synthesis and Preliminary Evaluation of [18F]FDPA for Cardiac Inflammation Imaging in Rats after Myocardial Infarction. Sci. Rep. 2020, 10, 18685. [Google Scholar] [CrossRef]
- Kim, G.R.; Paeng, J.C.; Jung, J.H.; Moon, B.S.; Lopalco, A.; Denora, N.; Lee, B.C.; Kim, S.E. Assessment of TSPO in a Rat Experimental Autoimmune Myocarditis Model: A Comparison Study between [18F] Fluoromethyl-PBR28 and [18F]CB251. Int. J. Mol. Sci. 2018, 19, 276. [Google Scholar] [CrossRef] [Green Version]
- MacAskill, M.G.; Wimberley, C.; Morgan, T.E.F.; Alcaide-Corral, C.J.; Newby, D.E.; Lucatelli, C.; Sutherland, A.; Pimlott, S.L.; Tavares, A.A.S. Modelling [18F]LW223 PET Data Using Simplified Imaging Protocols for Quantification of TSPO Expression in the Rat Heart and Brain. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 137–145. [Google Scholar] [CrossRef]
- MacAskill, M.G.; Stadulyte, A.; Williams, L.; Morgan, T.E.F.; Sloan, N.L.; Alcaide-Corral, C.J.; Walton, T.; Wimberley, C.; McKenzie, C.-A.; Spath, N.; et al. Quantification of Macrophage-Driven Inflammation During Myocardial Infarction with 18 F-LW223, a Novel TSPO Radiotracer with Binding Independent of the Rs6971 Human Polymorphism. J. Nucl. Med. 2021, 62, 536–544. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Fourth Universal Definition of Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef] [PubMed]
- Corrado, P.A.; Barton, G.P.; Razalan-Krause, F.C.; François, C.J.; Chesler, N.C.; Wieben, O.; Eldridge, M.; McMillan, A.B.; Goss, K.N. Dynamic FDG Pet Imaging to Probe for Cardiac Metabolic Remodeling in Adults Born Premature. J. Clin. Med. 2021, 10, 1301. [Google Scholar] [CrossRef] [PubMed]
- Zuurbier, C.J.; Bertrand, L.; Beauloye, C.R.; Andreadou, I.; Ruiz-Meana, M.; Jespersen, N.R.; Kula-Alwar, D.; Prag, H.A.; Eric Botker, H.; Dambrova, M.; et al. Cardiac Metabolism as a Driver and Therapeutic Target of Myocardial Infarction. J. Cell. Mol. Med. 2020, 24, 5937–5954. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Rahmim, A.; Gunn, R.N. PET Parametric Imaging: Past, Present, and Future. IEEE Trans. Radiat. Plasma Med. Sci. 2020, 4, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Weinberger, T.; Messerer, D.; Zacherl, M.J.; Schulz, C.; Massberg, S.; Bartenstein, P.; Lehner, S.; Boening, G.; Todica, A. Comparison of Transient and Permanent LAD Ligation in Mice Using 18F-FDG PET Imaging. Ann. Nucl. Med. 2022, 36, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Zacherl, M.J.; Weckbach, L.; Paintmayer, L.; Weinberger, T.; Stark, K.; Massberg, S.; Bartenstein, P.; Lehner, S.; Schulz, C.; et al. Cardiac 18F-FDG Positron Emission Tomography: An Accurate Tool to Monitor in Vivo Metabolic and Functional Alterations in Murine Myocardial Infarction. Front. Cardiovasc. Med. 2021, 8, 508. [Google Scholar] [CrossRef] [PubMed]
- Lauro, C.; Limatola, C. Metabolic Reprograming of Microglia in the Regulation of the Innate Inflammatory Response. Front. Immunol. 2020, 11, 493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangatchew, F.; Vester-Glowinski, P.; Rasmussen, B.S.; Haastrup, E.; Munthe-Fog, L.; Talman, M.-L.; Bonde, C.; Drzewiecki, K.T.; Fischer-Nielsen, A.; Holmgaard, R. Mesenchymal Stem Cell Therapy of Acute Thermal Burns: A Systematic Review of the Effect on Inflammation and Wound Healing. Burns 2021, 47, 270–294. [Google Scholar] [CrossRef]
- Glatz, J.F.C.; Nabben, M.; Young, M.E.; Schulze, P.C.; Taegtmeyer, H.; Luiken, J.J.F.P. Re-Balancing Cellular Energy Substrate Metabolism to Mend the Failing Heart. Biochim. Biophys. Acta—Mol. Basis Dis. 2020, 1866, 165579. [Google Scholar] [CrossRef]
- Merritt, J.L., II; Norris, M.; Kanungo, S. Fatty Acid Oxidation Disorders. Ann. Transl. Med. 2018, 6, 473. [Google Scholar] [CrossRef]
- Lv, Z.-H.; Ma, P.; Luo, W.; Xiong, H.; Han, L.; Li, S.-W.; Zhou, X.; Tu, J.-C. Association between Serum Free Fatty Acid Levels and Possible Related Factors in Patients with Type 2 Diabetes Mellitus and Acute Myocardial Infarction. BMC Cardiovasc. Disord. 2014, 14, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kan, Y.; Wang, H.; Lu, J.; Lin, Z.; Lin, J.; Gong, P. Significance of Plasma Free Fatty Acid Level for Assessing and Diagnosing Acute Myocardial Infarction. Biomark. Med. 2020, 14, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, W.; Wu, H.; Liu, G. Advanced Tracers in PET Imaging of Cardiovascular Disease. BioMed Res. Int. 2014, 2014, 504532. [Google Scholar] [CrossRef]
- Goud, N.S.; Bhattacharya, A.; Joshi, R.K.; Nagaraj, C.; Bharath, R.D.; Kumar, P. Carbon-11: Radiochemistry and Target-Based PET Molecular Imaging Applications in Oncology, Cardiology, and Neurology. J. Med. Chem. 2021, 64, 1223–1259. [Google Scholar] [CrossRef] [PubMed]
- Caribé, P.R.R.V.; Vandenberghe, S.; Diogo, A.; Pérez-Benito, D.; Efthimiou, N.; Thyssen, C.; D’Asseler, Y.; Koole, M. Monte Carlo Simulations of the GE Signa PET/MR for Different Radioisotopes. Front. Physiol. 2020, 11, 525575. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, T.; Zhang, X.; Zhong, M.; Walker, N.N.; He, J.; Berr, S.S.; Keller, S.R.; Kundu, B.K. Determination of Fatty Acid Metabolism with Dynamic [11 C]Palmitate Positron Emission Tomography of Mouse Heart In Vivo. Mol. Imaging 2015, 14, 516–523. [Google Scholar] [CrossRef] [Green Version]
- Luyten, K.; Schoenberger, M. Molecular Imaging of Cardiac Metabolism, Innervation, and Conduction. EMJ Cardiol. 2017, 5, 70–78. [Google Scholar] [CrossRef]
- Wu, X.; Wang, P.; Liu, R.; Zeng, H.; Chao, F.; Liu, H.; Xu, C.; Hou, H.; Yao, Q. Development of 11C-Labeled ω-Sulfhydryl Fatty Acid Tracer for Myocardial Imaging with PET. Eur. J. Med. Chem. 2018, 143, 1657–1666. [Google Scholar] [CrossRef]
- Bradley, J.M.; Spaletra, P.; Li, Z.; Sharp, T.E.; Goodchild, T.T.; Corral, L.G.; Fung, L.; Chan, K.W.H.; Sullivan, R.W.; Swindlehurst, C.A.; et al. A Novel Fibroblast Activation Inhibitor Attenuates Left Ventricular Remodeling and Preserves Cardiac Function in Heart Failure. Am. J. Physiol.-Heart Circ. Physiol. 2018, 315, H563–H570. [Google Scholar] [CrossRef] [Green Version]
- Heckmann, M.B.; Reinhardt, F.; Finke, D.; Katus, H.A.; Haberkorn, U.; Leuschner, F.; Lehmann, L.H. Relationship Between Cardiac Fibroblast Activation Protein Activity by Positron Emission Tomography and Cardiovascular Disease. Circ. Cardiovasc. Imaging 2020, 13, e010628. [Google Scholar] [CrossRef]
- Altmann, A.; Haberkorn, U.; Siveke, J. The Latest Developments in Imaging of Fibroblast Activation Protein. J. Nucl. Med. 2021, 62, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.E.; Berezin, A.A. Adverse Cardiac Remodelling after Acute Myocardial Infarction: Old and New Biomarkers. Dis. Mrk. 2020, 2020, 1215802. [Google Scholar] [CrossRef] [PubMed]
- Glasenapp, A.; Hess, A.; Thackeray, J.T. Molecular Imaging in Nuclear Cardiology: Pathways to Individual Precision Medicine. J. Nucl. Cardiol. 2020, 27, 2195–2201. [Google Scholar] [CrossRef]
- Varasteh, Z.; Mohanta, S.; Robu, S.; Braeuer, M.; Li, Y.; Omidvari, N.; Topping, G.; Sun, T.; Nekolla, S.G.; Richter, A.; et al. Molecular Imaging of Fibroblast Activity after Myocardial Infarction Using a 68Ga-Labeled Fibroblast Activation Protein Inhibitor, FAPI-04. J. Nucl. Med. 2019, 60, 1743–1749. [Google Scholar] [CrossRef] [PubMed]
- Langer, L.B.N.; Hess, A.; Korkmaz, Z.; Tillmanns, J.; Reffert, L.M.; Bankstahl, J.P.; Bengel, F.M.; Thackeray, J.T.; Ross, T.L. Molecular Imaging of Fibroblast Activation Protein after Myocardial Infarction Using the Novel Radiotracer [68Ga]MHLL1. Theranostics 2021, 11, 7755–7766. [Google Scholar] [CrossRef] [PubMed]
- Teringova, E.; Tousek, P. Apoptosis in Ischemic Heart Disease. J. Transl. Med. 2017, 15, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, M.; Olivier, J.; Lindner, S.; Zacherl, M.J.; Massberg, S.; Bartenstein, P.; Ziegler, S.; Brendel, M.; Lehner, S.; Boening, G.; et al. Detection of Cardiac Apoptosis by [18F]ML-10 in a Mouse Model of Permanent LAD Ligation. Mol. Imaging Biol. 2022, 24, 666–674. [Google Scholar] [CrossRef]
- Ma, H.; Liu, S.; Xiong, Y.; Zhang, Z.; Sun, A.; Su, S.; Liang, H.; Yuan, G.; Tang, G. PET Imaging of Cardiomyocyte Apoptosis in a Rat Myocardial Infarction Model. Apoptosis 2018, 23, 396–407. [Google Scholar] [CrossRef]
- Sun, T.; Wei, L.; Tian, H.; Zhan, W.; Ma, H.; Nie, D.; Wang, S.; Chen, X.; Tang, G. Novel PET/CT Tracers for Targeted Imaging of Membrane Receptors to Evaluate Cardiomyocyte Apoptosis and Tissue Repair Process in a Rat Model of Myocardial Infarction. Apoptosis 2021, 26, 460–473. [Google Scholar] [CrossRef]
- Vestergaard, M.; Berglund, N.A.; Hsu, P.-C.; Song, C.; Koldsø, H.; Schiøtt, B.; Sansom, M.S.P. Structure and Dynamics of Cinnamycin–Lipid Complexes: Mechanisms of Selectivity for Phosphatidylethanolamine Lipids. ACS Omega 2019, 4, 18889–18899. [Google Scholar] [CrossRef]
- Martins-Marques, T.; Hausenloy, D.J.; Sluijter, J.P.G.; Leybaert, L.; Girao, H. Intercellular Communication in the Heart: Therapeutic Opportunities for Cardiac Ischemia. Trends Mol. Med. 2021, 27, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Miller, E.J.; Sadeghi, M.M. PET-Based Imaging of Ischemic Heart Disease. PET Clin. 2019, 14, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Chirumamilla, A.; Travin, M.I. Cardiac Applications of 123I-MIBG Imaging. Semin. Nucl. Med. 2011, 41, 374–387. [Google Scholar] [CrossRef] [PubMed]
- Ismailani, U.S.; Buchler, A.; Farber, G.; Pekošak, A.; Farber, E.; MacMullin, N.; Suuronen, E.J.; Vasdev, N.; Beanlands, R.S.B.; Rotstein, B.H. Cardiac Sympathetic PET Imaging with Meta-[18F]Fluorobenzylguanidine Is Sensitive to Uptake-1 in Rats. ACS Chem. Neurosci. 2021, 12, 4350–4360. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.K.; Moon, B.S.; Kim, B.S.; Kim, M.H.; Lee, Y.J.; Jung, J.H.; Lee, K.C.; Seo, Y.; Kim, W.; Lim, S.M.; et al. Feasibility of Myocardial PET Imaging Using a Benzylguanidine Analog: Meta-(3-[18F]Fluoropropyl)Benzylguanidine ([18F]MFPBG). Nucl. Med. Biol. 2018, 61, 63–70. [Google Scholar] [CrossRef]
- Klein, R.; Celiker-Guler, E.; Rotstein, B.H.; deKemp, R.A. PET and SPECT Tracers for Myocardial Perfusion Imaging. Semin. Nucl. Med. 2020, 50, 208–218. [Google Scholar] [CrossRef]
- Sasidharan, S.; Bovendeerd, P.; Huyghe, J. Failure of Myocardial Tissue: Simulation of Blood Perfusion. In Proceedings of the 14th WCCM-ECCOMAS Congress; CIMNE, Paris, France, 11–15 January 2021. [Google Scholar]
- Davidson, C.Q.; Phenix, C.P.; Tai, T.C.; Khaper, N.; Lees, S.J. Searching for Novel PET Radiotracers: Imaging Cardiac Perfusion, Metabolism and Inflammation. Am. J. Nucl. Med. Mol. Imaging 2018, 8, 200–227. [Google Scholar]
- Sciagra, R.; Lubberink, M.; Hyafil, F.; Saraste, A.; Slart, R.; Agostini, D.; Nappi, C.; Georgoulias, P.; Bucerius, J.; Rischpler, C.; et al. EANM procedural guidelines for PET/CT quantitative myocardial perfusion imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1040–1069. [Google Scholar] [CrossRef]
- Jochumsen, M.R.; Sörensen, J.; Pedersen, B.G.; Nyengaard, J.R.; Krag, S.R.P.; Frøkiær, J.; Borre, M.; Bouchelouche, K.; Tolbod, L.P. Tumour Blood Flow for Prediction of Human Prostate Cancer Aggressiveness: A Study with Rubidium-82 PET, MRI and Na+/K+-ATPase-Density. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 532–542. [Google Scholar] [CrossRef]
- Clemmensen, A.E.; Ghotbi, A.A.; Bodholdt, R.P.; Hag, A.M.F.; Hasbak, P.; Ripa, R.S.; Kjaer, A. Perfusion Imaging Using Rubidium-82 (82Rb) PET in Rats with Myocardial Infarction: First Small Animal Cardiac 82Rb-PET. J. Nucl. Cardiol. 2017, 24, 750–752. [Google Scholar] [CrossRef] [Green Version]
- Ghotbi, A.A.; Clemmensen, A.; Kyhl, K.; Follin, B.; Hasbak, P.; Engstrøm, T.; Ripa, R.S.; Kjaer, A. Rubidium-82 PET Imaging Is Feasible in a Rat Myocardial Infarction Model. J. Nucl. Cardiol. 2019, 26, 798–809. [Google Scholar] [CrossRef] [Green Version]
- Bentsen, S.; Bang, L.E.; Hasbak, P.; Kjaer, A.; Ripa, R.S. Amiodarone Attenuates Cardiac Rubidium-82 in Consecutive PET/CT Scans in a Rodent Model. J. Nucl. Cardiol. 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.; Bentsen, S.; Clemmensen, A.; Jensen, J.K.; Madsen, J.; Rossing, J.; Laier, A.; Hasbak, P.; Kjaer, A.; Ripa, R.S. Feasibility of Positron Range Correction in 82-Rubidium Cardiac PET/CT. EJNMMI Phys. 2022, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Dilsizian, V.; Bacharach, S.L.; Beanlands, R.S.; Bergmann, S.R.; Delbeke, D.; Dorbala, S.; Gropler, R.J.; Knuuti, J.; Schelbert, H.R.; Travin, M.I. ASNC Imaging Guidelines/SNMMI Procedure Standard for Positron Emission Tomography (PET) Nuclear Cardiology Procedures. J. Nucl. Cardiol. 2016, 23, 1187–1226. [Google Scholar] [CrossRef] [PubMed]
- Yokell, D.L.; Rice, P.A.; Neelamegam, R.; El Fakhri, G. Development, Validation and Regulatory Acceptance of Improved Purification and Simplified Quality Control of [13N] Ammonia. EJNMMI Radiopharm. Chem. 2020, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Carvajal-Juarez, I.; Monroy-Gonzalez, A.; Espinola-Zavaleta, N.; Meave-Gonzalez, A.; Alexanderson-Rosas, E. PET/CT with 13N-Ammonia: Characteristics and Utility in Coronary Artery Disease. Ann. Nucl. Cardiol. 2019, 5, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Hess, A.; Nekolla, S.G.; Meier, M.; Bengel, F.M.; Thackeray, J.T. Accuracy of Cardiac Functional Parameters Measured from Gated Radionuclide Myocardial Perfusion Imaging in Mice. J. Nucl. Cardiol. 2020, 27, 1317–1327. [Google Scholar] [CrossRef]
- Stegger, L.; Heijman, E.; Schäfers, K.P.; Nicolay, K.; Schäfers, M.A.; Strijkers, G.J. Quantification of Left Ventricular Volumes and Ejection Fraction in Mice Using PET, Compared with MRI. J. Nucl. Med. 2009, 50, 132–138. [Google Scholar] [CrossRef] [Green Version]
- Guehl, N.J.; Pelletier-Galarneau, M.; Wooten, D.W.; Guerrero, J.L.; Kas, A.; Normandin, M.D.; Fakhri, G.E.; Alpert, N.M. Preclinical Validation of a Single-Scan Rest/Stress Imaging Technique for 13N-Ammonia Positron Emission Tomography Cardiac Perfusion Studies. Circ. Cardiovasc. Imaging 2020, 13, e009407. [Google Scholar] [CrossRef]
- Fathala, A.; Aboulkheir, M.; Shoukri, M.M.; Alsergani, H. Diagnostic Accuracy of 13N-Ammonia Myocardial Perfusion Imaging with PET-CT in the Detection of Coronary Artery Disease. Cardiovasc. Diagn. 2019, 9, 35–42. [Google Scholar] [CrossRef]
- Bentourkia, M.; Croteau, E.; Langlois, R.; Aliaga, A.; Cadorette, J.; Benard, F.; Lesur, O.; Lecomte, R. Cardiac Studies in Rats with /Sup 11/C-Acetate and PET: A Comparison with /Sup 13/N-Ammonia. IEEE Trans. Nucl. Sci. 2002, 49, 2322–2327. [Google Scholar] [CrossRef]
- Nekolla, S.G.; Reder, S.; Saraste, A.; Higuchi, T.; Dzewas, G.; Preissel, A.; Huisman, M.; Poethko, T.; Schuster, T.; Yu, M.; et al. Evaluation of the Novel Myocardial Perfusion Positron-Emission Tomography Tracer 18F-BMS-747158-02: Comparison to 13 N-Ammonia and Validation with Microspheres in a Pig Model. Circulation 2009, 119, 2333–2342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.-Y.; Kim, H.S.; Jang, H.Y.; Kim, J.H.; Bom, H.-S.; Min, J.-J. Comparison of the Cardiac MicroPET Images Obtained Using [18F]FPTP and [13N]NH3 in Rat Myocardial Infarction Models. ACS Med. Chem. Lett. 2014, 5, 1124–1128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Mpharm, S.L.; Liu, T.W.; Zhang, J.M.; Chen, Y.; Li, J.M.; Xu, W.G. Preliminary and Comparative Experiment Study Between 18F-Flurpiridaz and 13N-NH3·H2O Myocardial Perfusion Imaging With PET/CT in Miniature Pigs. Mol. Imaging 2020, 19, 1536012120947506. [Google Scholar] [CrossRef] [PubMed]
- Grönman, M.; Tarkia, M.; Stark, C.; Vähäsilta, T.; Kiviniemi, T.; Lubberink, M.; Halonen, P.; Kuivanen, A.; Saunavaara, V.; Tolvanen, T.; et al. Assessment of Myocardial Viability with [15O]Water PET: A Validation Study in Experimental Myocardial Infarction. J. Nucl. Cardiol. 2021, 28, 1271–1280. [Google Scholar] [CrossRef] [Green Version]
- Williams, M.C.; Mirsadraee, S.; Dweck, M.R.; Weir, N.W.; Fletcher, A.; Lucatelli, C.; MacGillivray, T.; Golay, S.K.; Cruden, N.L.; Henriksen, P.A.; et al. Computed Tomography Myocardial Perfusion vs 15O-Water Positron Emission Tomography and Fractional Flow Reserve. Eur. Radiol. 2017, 27, 1114–1124. [Google Scholar] [CrossRef] [Green Version]
- Grönman, M.; Tarkia, M.; Kiviniemi, T.; Halonen, P.; Kuivanen, A.; Savunen, T.; Tolvanen, T.; Teuho, J.; Käkelä, M.; Metsälä, O.; et al. Imaging of Avβ3 Integrin Expression in Experimental Myocardial Ischemia with [68Ga]NODAGA-RGD Positron Emission Tomography. J. Transl. Med. 2017, 15, 144. [Google Scholar] [CrossRef] [Green Version]
- Kudomi, N.; Sipilä, H.; Autio, A.; Oikonen, V.; Liljenbäck, H.; Tarkia, M.; Laivola, J.; Johansson, J.; Teräs, M.; Roivainen, A. Cross-Validation of Input Functions Obtained by H2 15O PET Imaging of Rat Heart and a Blood Flow-through Detector. Mol. Imaging Biol. 2012, 14, 509–516. [Google Scholar] [CrossRef]
- Manabe, O.; Naya, M.; Tamaki, N. Feasibility of PET for the Management of Coronary Artery Disease: Comparison between CFR and FFR. J. Cardiol. 2017, 70, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Herrero, P.; Kim, J.; Sharp, T.L.; Engelbach, J.A.; Lewis, J.S.; Gropler, R.J.; Welch, M.J. Assessment of Myocardial Blood Flow Using 15O-Water and 1-11C-Acetate in Rats with Small-Animal PET. J. Nucl. Med. 2006, 47, 477–485. [Google Scholar]
- Magnusson, P.; Nordström, J.; Harms, H.J.; Lubberink, M.; Gadler, F.; Sörensen, J.; Mörner, S. Positron Emission Tomography (15O-Water, 11C-Acetate, 11C-HED) Risk Markers and Nonsustained Ventricular Tachycardia in Hypertrophic Cardiomyopathy. IJC Heart Vasc. 2020, 26, 100452. [Google Scholar] [CrossRef] [PubMed]
- Grassi, I.; Nanni, C.; Allegri, V.; Morigi, J.J.; Montini, G.C.; Castellucci, P.; Fanti, S. The Clinical Use of PET with 11C-Acetate. Am. J. Nucl. Med. Mol. Imaging 2012, 2, 33. [Google Scholar] [PubMed]
- Murashige, D.; Jang, C.; Neinast, M.; Edwards, J.J.; Cowan, A.; Hyman, M.C.; Rabinowitz, J.D.; Frankel, D.S.; Arany, Z. Comprehensive Quantification of Fuel Use by the Failing and Nonfailing Human Heart. Science 2020, 370, 364–368. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, A.C.; Evans, R.; Silvola, J.M.U.; Miller, I.S.; Conroy, E.; Hector, S.; Cary, M.; Murray, D.W.; Jarzabek, M.A.; Maratha, A.; et al. A Novel Positron Emission Tomography (PET) Approach to Monitor Cardiac Metabolic Pathway Remodeling in Response to Sunitinib Malate. PLoS ONE 2017, 12, e0169964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croteau, E.; Renaud, J.M.; McDonald, M.; Klein, R.; DaSilva, J.N.; Beanlands, R.S.B.; DeKemp, R.A. Test–Retest Repeatability of Myocardial Blood Flow and Infarct Size Using 11C-Acetate Micro-PET Imaging in Mice. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1589–1600. [Google Scholar] [CrossRef]
- Werner, R.A.; Chen, X.; Rowe, S.P.; Lapa, C.; Javadi, M.S.; Higuchi, T. Moving into the next Era of PET Myocardial Perfusion Imaging: Introduction of Novel 18F-Labeled Tracers. Int. J. Cardiovasc. Imaging 2019, 35, 569–577. [Google Scholar] [CrossRef] [Green Version]
- Maddahi, J.; Lazewatsky, J.; Udelson, J.E.; Berman, D.S.; Beanlands, R.S.B.; Heller, G.V.; Bateman, T.M.; Knuuti, J.; Orlandi, C. Phase-III Clinical Trial of Fluorine-18 Flurpiridaz Positron Emission Tomography for Evaluation of Coronary Artery Disease. J. Am. Coll. Cardiol. 2020, 76, 391–401. [Google Scholar] [CrossRef]
- Ahmed, H.; Haider, A.; Gisler, L.; Schibli, R.; Gebhard, C.; Ametamey, S.M. [18F]Flurpiridaz: Facile and Improved Precursor Synthesis for This Next-Generation Cardiac Positron Emission Tomography Imaging Agent. ChemMedChem 2020, 15, 1040–1043. [Google Scholar] [CrossRef]
- Saraste, A.; Ståhle, M.; Roivainen, A. Evaluation of Cardiac Function by Nuclear Imaging in Preclinical Studies. J. Nucl. Cardiol. 2020, 27, 1328–1330. [Google Scholar] [CrossRef] [Green Version]
- Bengs, S.; Warnock, G.I.; Portmann, A.; Mikail, N.; Rossi, A.; Ahmed, H.; Etter, D.; Treyer, V.; Gisler, L.; Pfister, S.K.; et al. Rest/Stress Myocardial Perfusion Imaging by Positron Emission Tomography with 18F-Flurpiridaz: A Feasibility Study in Mice. J. Nucl. Cardiol. 2022, 1–12. [Google Scholar] [CrossRef]
- Kaseda, K. Recent and Current Advances in FDG-PET Imaging within the Field of Clinical Oncology in NSCLC: A Review of the Literature. Diagnostics 2020, 10, 561. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, Z. The Risk Prediction of Alzheimer’s Disease Based on the Deep Learning Model of Brain 18F-FDG Positron Emission Tomography. Saudi J. Biol. Sci. 2020, 27, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Derlin, T.; Grünwald, V.; Steinbach, J.; Wester, H.-J.; Ross, T.L. Molecular Imaging in Oncology Using Positron Emission Tomography. Dtsch. Ärzteblatt Int. 2018, 115, 175–181. [Google Scholar] [CrossRef] [Green Version]
- Im, H.-J.; Bradshaw, T.; Solaiyappan, M.; Cho, S.Y. Current Methods to Define Metabolic Tumor Volume in Positron Emission Tomography: Which One Is Better? Nucl. Med. Mol. Imaging 2018, 52, 5–15. [Google Scholar] [CrossRef]
- Minamimoto, R. Series of Myocardial FDG Uptake Requiring Considerations of Myocardial Abnormalities in FDG-PET/CT. Jpn. J. Radiol. 2021, 39, 540–557. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Klein, R.; Lewin, H.C.; Beanlands, R.S.B.; deKemp, R.A. Rubidium-82 Generator Yield and Efficiency for PET Perfusion Imaging: Comparison of Two Clinical Systems. J. Nucl. Cardiol. 2020, 27, 1728–1738. [Google Scholar] [CrossRef]
- Boursier, C.; Duval, X.; Bourdon, A.; Imbert, L.; Mahida, B.; Chevalier, E.; Claudin, M.; Hoen, B.; Goehringer, F.; Selton-Suty, C.; et al. ECG-Gated Cardiac FDG PET Acquisitions Significantly Improve Detectability of Infective Endocarditis. JACC Cardiovasc. Imaging 2020, 13, 2691–2693. [Google Scholar] [CrossRef]
- Qin, X.; Wang, S.; Liu, X.; Duan, J.; Cheng, K.; Mu, Z.; Jia, J.; Wei, Y.; Yuan, S. Diagnostic Value of 18 F-NOTA-FAPI PET/CT in A Rat Model of Radiation-Induced Lung Damage. Front. Oncol. 2022, 12, 879281. [Google Scholar] [CrossRef]
- Fu, L.; Huang, S.; Wu, H.; Dong, Y.; Xie, F.; Wu, R.; Zhou, K.; Tang, G.; Zhou, W. Superiority of [68Ga]Ga-FAPI-04/[18F]FAPI-42 PET/CT to [18F]FDG PET/CT in Delineating the Primary Tumor and Peritoneal Metastasis in Initial Gastric Cancer. Eur. Radiol. 2022, 32, 6281–6290. [Google Scholar] [CrossRef]
- Hu, K.; Wang, L.; Wu, H.; Huang, S.; Tian, Y.; Wang, Q.; Xiao, C.; Han, Y.; Tang, G. [18F]FAPI-42 PET Imaging in Cancer Patients: Optimal Acquisition Time, Biodistribution, and Comparison with [68Ga]Ga-FAPI-04. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2833–2843. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, X.; Shen, T.; Yao, Y.; Chen, M.; Li, Z.; Li, X.; Shen, J.; Kou, Y.; Chen, S.; et al. FAPI-04 PET/CT Using [18F]AlF Labeling Strategy: Automatic Synthesis, Quality Control, and In Vivo Assessment in Patient. Front. Oncol. 2021, 11, 649148. [Google Scholar] [CrossRef] [PubMed]
- Bahit, M.C.; Kochar, A.; Granger, C.B. Post-Myocardial Infarction Heart Failure. JACC Heart Fail. 2018, 6, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Paulsen, M.J.; Hironaka, C.E.; Shin, H.S.; Farry, J.M.; Thakore, A.D.; Jung, J.; Lucian, H.J.; Eskandari, A.; Anilkumar, S.; et al. Natural Heart Regeneration in a Neonatal Rat Myocardial Infarction Model. Cells 2020, 9, 229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foglio, E.; Puddighinu, G.; Germani, A.; Russo, M.A.; Limana, F. HMGB1 Inhibits Apoptosis Following MI and Induces Autophagy via MTORC1 Inhibition: Hmgb1 Autophagy and Apoptosis in the Infarcted Heart. J. Cell. Physiol. 2017, 232, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Slart, R.H.J.A.; Glaudemans, A.W.J.M.; Gheysens, O.; Lubberink, M.; Kero, T.; Dweck, M.R.; Habib, G.; Gaemperli, O.; Saraste, A.; Gimelli, A.; et al. Procedural recommendations of cardiac PET/CT imaging: Standardization in inflammatory-, infective-, infiltrative-, and innervation (4Is)-related cardiovascular diseases: A joint collaboration of the EACVI and the EANM. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1016–1039. [Google Scholar] [CrossRef] [PubMed]
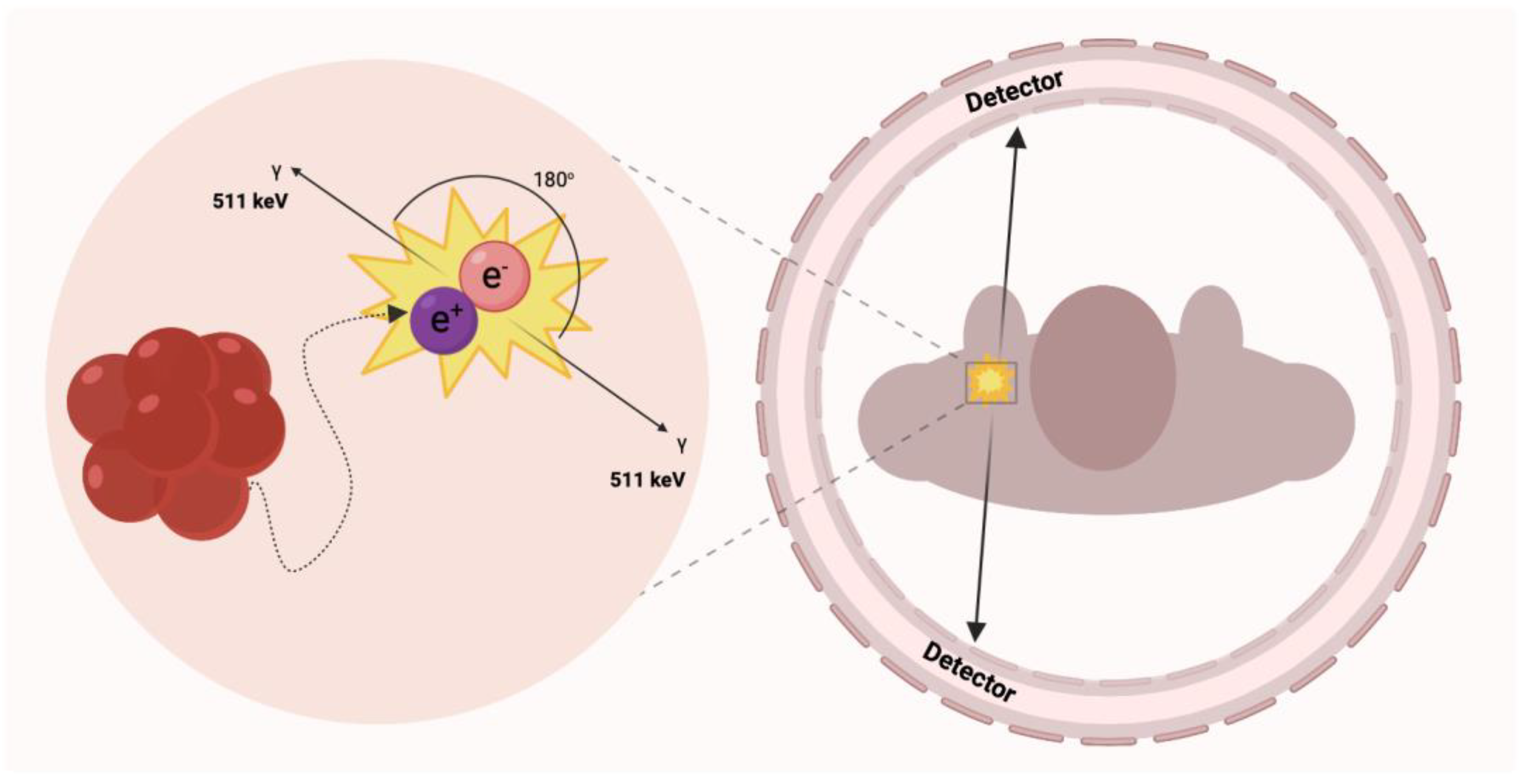


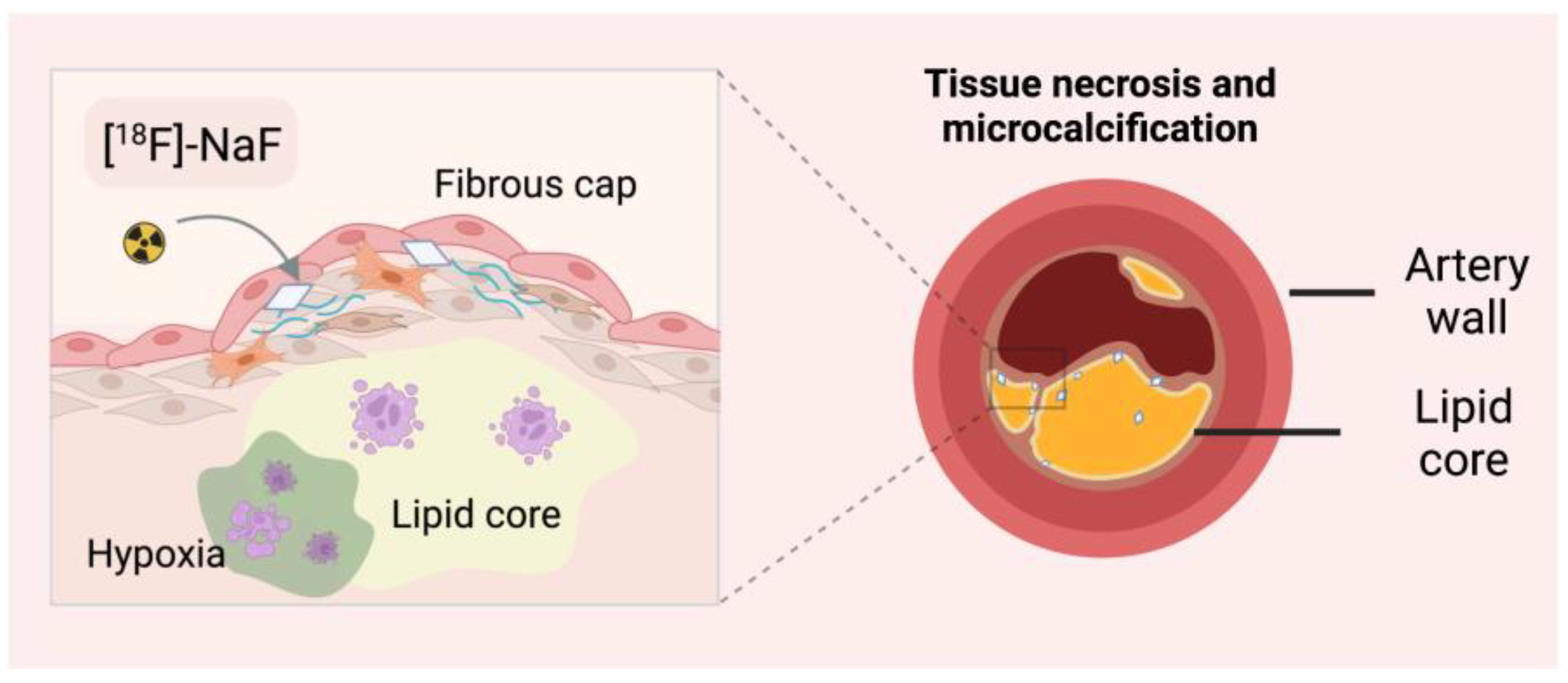
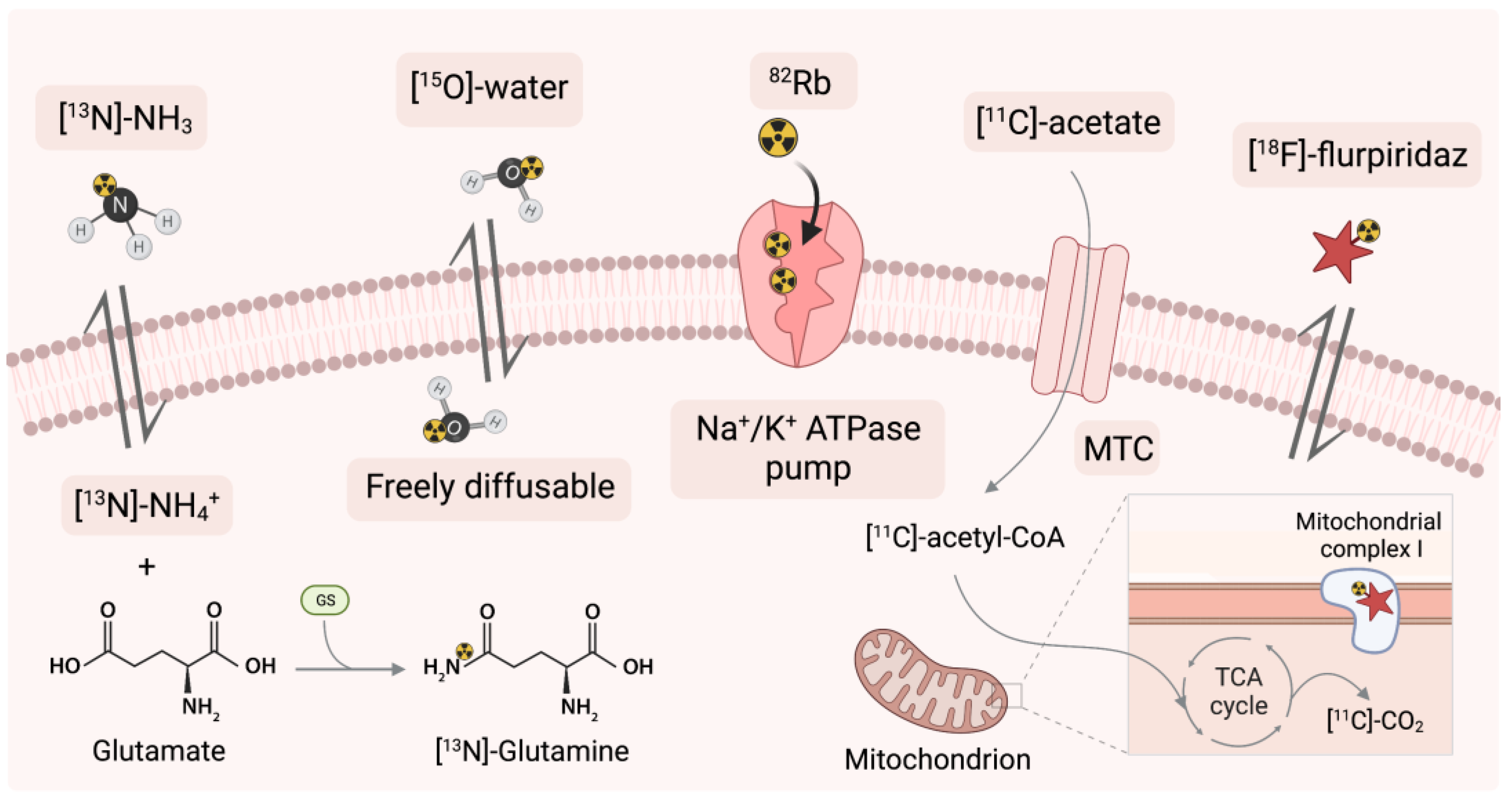
| Radionuclide | Half-Life (Min) | Decay | Production | Reaction | Positron Range/mm |
|---|---|---|---|---|---|
| 11C | 20.3 | β+ | Cyclotron | 10B(d,n)11C | 1.27 |
| 14N(p,α)11C | |||||
| 13N | 9.97 | β+ | Cyclotron | 12C(d,n)13N | |
| 16O(p,α)13N | 1.73 | ||||
| 13C(p,n)13N | |||||
| 15O | 2.04 | β+ | Cyclotron | 14N(d,n)15O | 2.96 |
| 15N(p,n)15O | |||||
| 18F | 110 | EC, β+ | Cyclotron | 18O(p,n)18F | 0.66 |
| 64Cu | 762 | EC, β−, β+ | Cyclotron | 63Cu(n,γ)64Cu | |
| 64Zn(n,p)64Cu | 0.69 | ||||
| 64Ni(p,n)64Cu | |||||
| 68Ga | 68.3 | EC, β⁺ | Generator | 68Ge/68Ga | 3.56 |
| Cyclotron | 68Zn(p,n)68Ga | ||||
| 82Rb | 1.27 | EC, β+ | Generator | 82Sr/82Rb | 7.49 |
| 124I | 6013 | EC, β+ | Cyclotron | 124Te(p,n)124I | 1.70 |
| Medical Condition | Strain | Phenotype Occurrence or Induction | Reference |
|---|---|---|---|
| ApoE −/− mice | display delayed lipoprotein clearance and develop dyslipoproteinemia, hypercholesterolemia and atherosclerotic lesions even when fed normal chow | [37,38] | |
| LDRL −/− mice | fed normal chow develop atherosclerosis gradually, can be accelerated on a high-fat diet, hyperlipidemia with human-like profile | [37,39] | |
| OPG −/− mice | exhibit an unexpected increase in vascular calcification in the aorta and renal arteries | [40] | |
| Lewis rats | immunisation with porcine cardiac myosin fraction | [41,42] | |
| C3H mice (Harlan) | viral myocarditis with Coxsackie virus B3 (CVB3) | [43] | |
| Myocardial infarction and perfusion | C57BL/6J mice | coronary artery ligation | [44] |
| Ischemia-reperfusion | Sprague Dawley rats | development of an ischemia-reperfusion injury, temporal coronary artery ligation for 30 min followed by a 120 min reperfusion | [45,46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wargocka-Matuszewska, W.; Uhrynowski, W.; Rozwadowska, N.; Rogulski, Z. Recent Advances in Cardiovascular Diseases Research Using Animal Models and PET Radioisotope Tracers. Int. J. Mol. Sci. 2023, 24, 353. https://doi.org/10.3390/ijms24010353
Wargocka-Matuszewska W, Uhrynowski W, Rozwadowska N, Rogulski Z. Recent Advances in Cardiovascular Diseases Research Using Animal Models and PET Radioisotope Tracers. International Journal of Molecular Sciences. 2023; 24(1):353. https://doi.org/10.3390/ijms24010353
Chicago/Turabian StyleWargocka-Matuszewska, Weronika, Witold Uhrynowski, Natalia Rozwadowska, and Zbigniew Rogulski. 2023. "Recent Advances in Cardiovascular Diseases Research Using Animal Models and PET Radioisotope Tracers" International Journal of Molecular Sciences 24, no. 1: 353. https://doi.org/10.3390/ijms24010353
APA StyleWargocka-Matuszewska, W., Uhrynowski, W., Rozwadowska, N., & Rogulski, Z. (2023). Recent Advances in Cardiovascular Diseases Research Using Animal Models and PET Radioisotope Tracers. International Journal of Molecular Sciences, 24(1), 353. https://doi.org/10.3390/ijms24010353





