Circadian Rhythm Disorders Aggravate Periodontitis by Modulating BMAL1
Abstract
:1. Introduction
2. Results
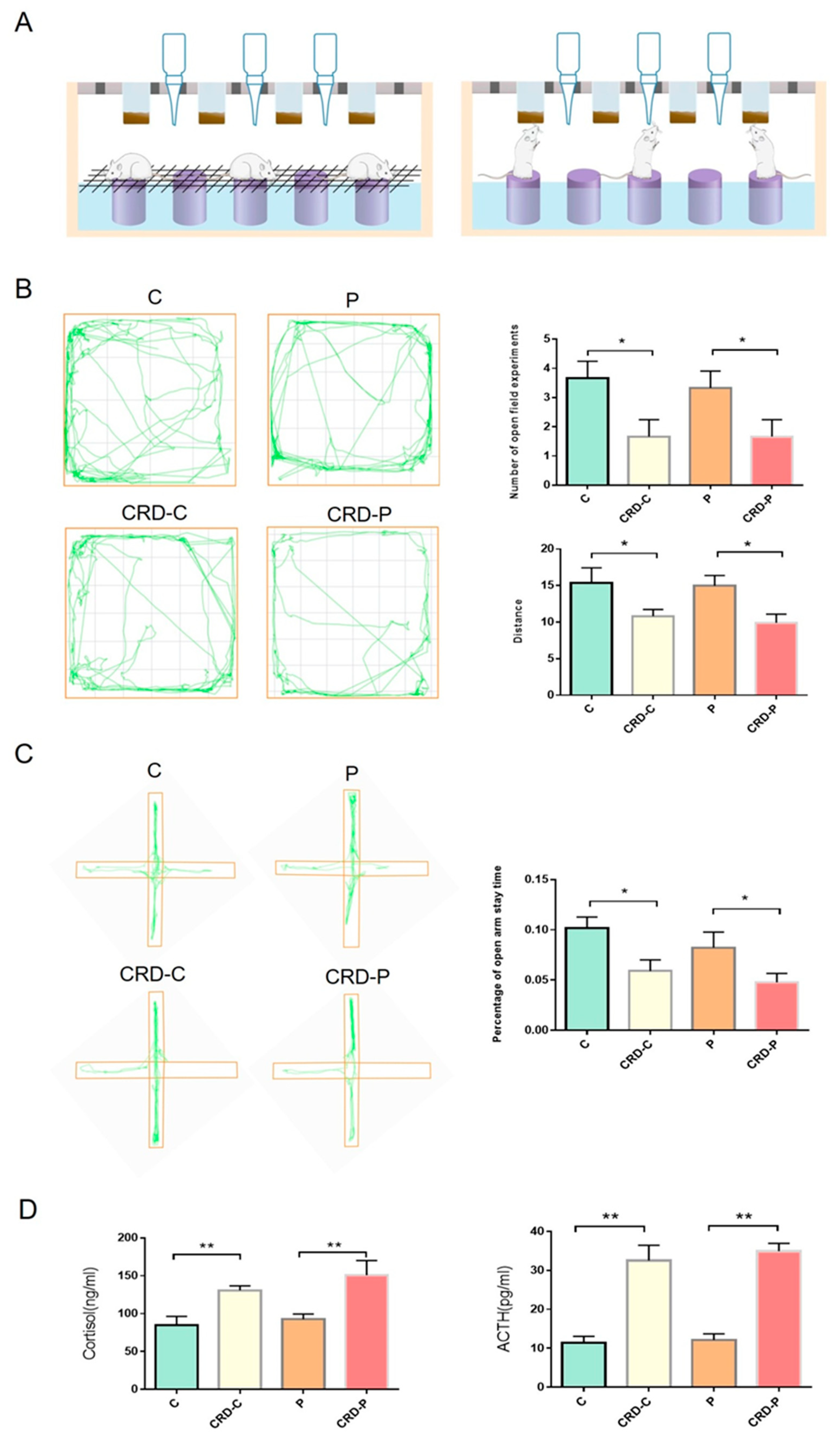
2.1. CRD Model Was Constructed in Rats
2.2. CRD Promotes the Progression of Periodontitis
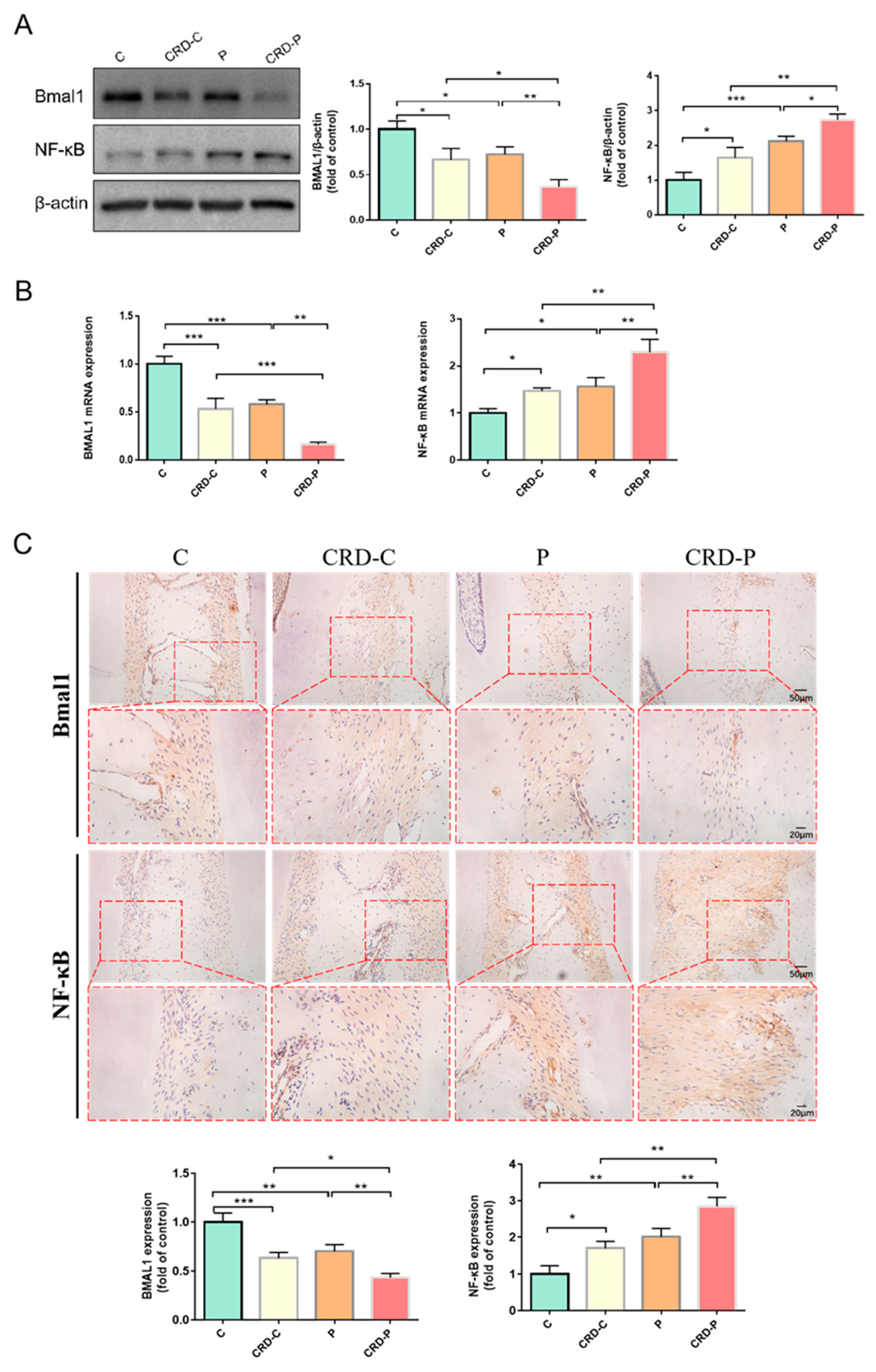
2.3. CRD Promotes the Progression of Periodontitis by Regulating BMAL1
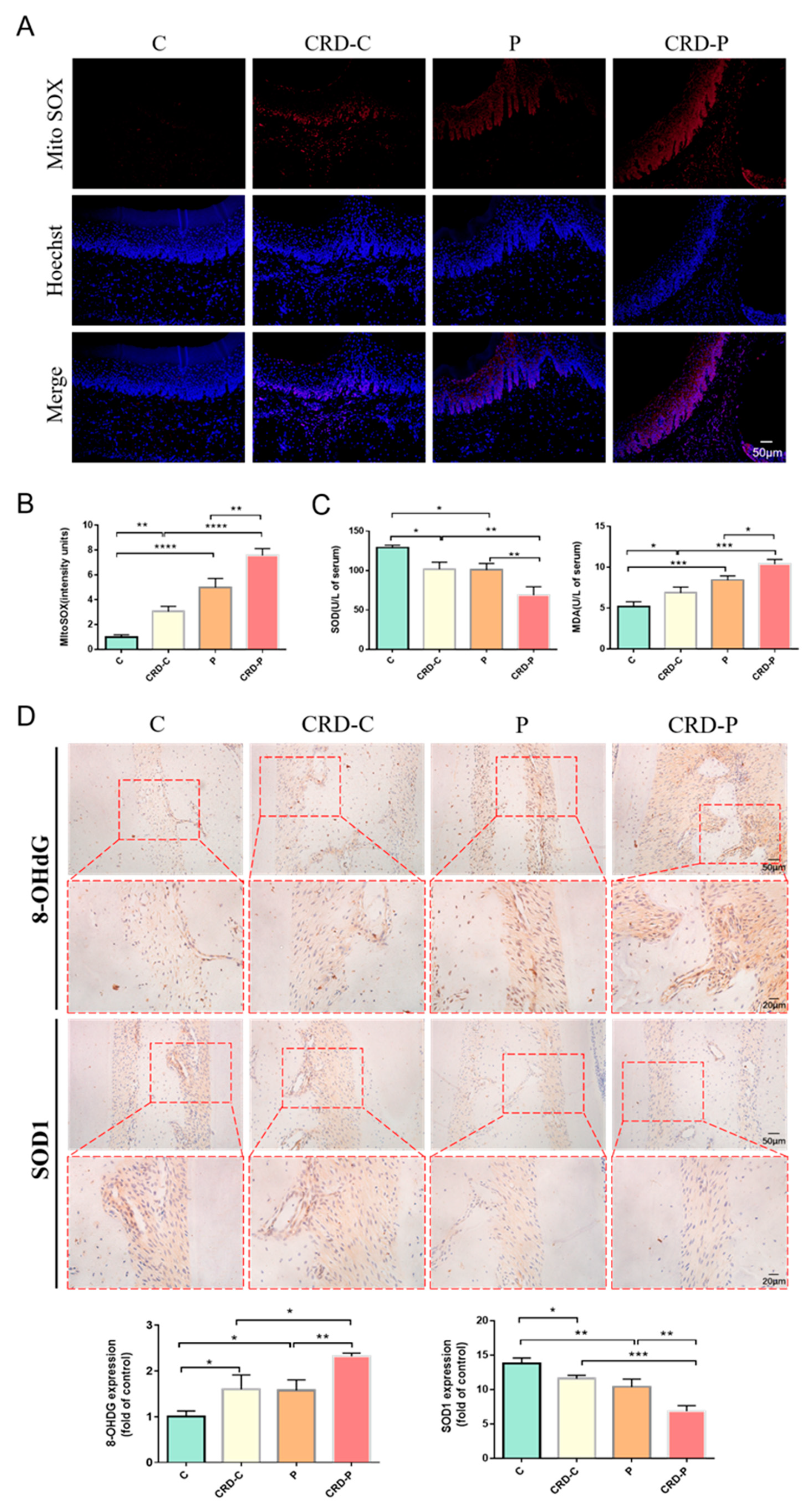
2.4. CRD Exacerbates the Redox State of Periodontal Tissues
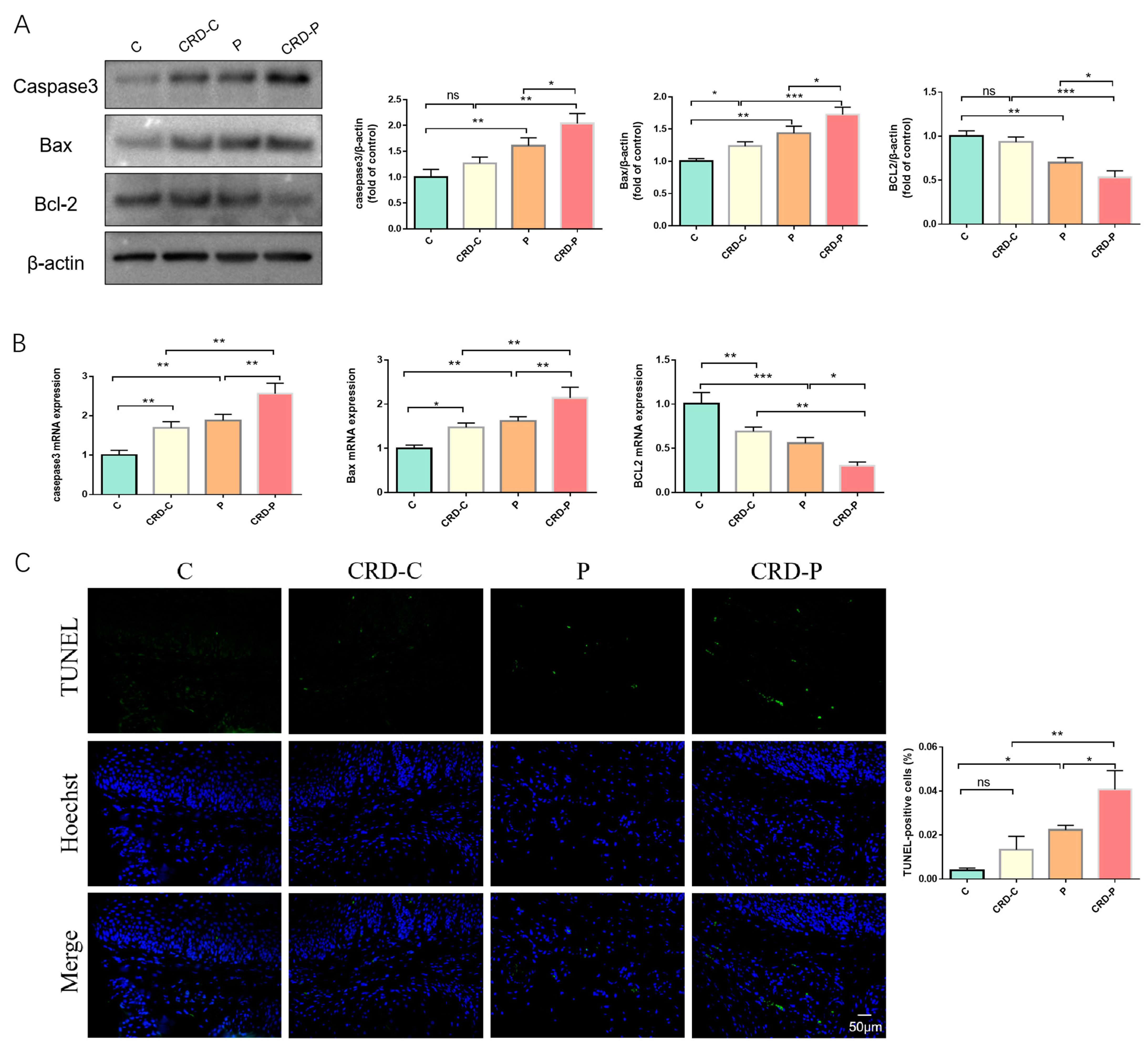
2.5. Disturbed Circadian Rhythms Exacerbate Apoptosis in Periodontal Tissues
3. Discussion
4. Materials and Methods
4.1. Animal
4.2. Enzyme-Linked Immunosorbent Assay (Elisa)
4.3. Periodontal Clinical Index Examination
4.4. Micro-Computed Tomography (Micro-CT) Analysis
4.5. Histopathological Staining
4.6. Detection of Oxidative Stress Levels
4.7. Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Staining
4.8. Mito SOX Staining
4.9. Immunohistochemical Analysis
4.10. qRT-PCR
4.11. Western Blot Analysis
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohawk, J.A.; Takahashi, J.S. Cell autonomy and synchrony of suprachiasmatic nucleus circadian oscillators. Trends Neurosci. 2011, 34, 349–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bass, J.; Takahashi, J.S. Circadian integration of metabolism and energetics. Science 2010, 330, 1349–1354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, D.; Chen, J.; Wang, J.; Yao, J.; Huang, Y.; Zhang, G.; Bao, Z. Circadian Clock Genes in the Metabolism of Non-alcoholic Fatty Liver Disease. Front. Physiol. 2019, 10, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheiermann, C.; Gibbs, J.; Ince, L.; Loudon, A. Clocking into immunity. Nat. Rev. Immunol. 2018, 18, 423–437. [Google Scholar] [CrossRef]
- Voigt, R.M.; Summa, K.C.; Forsyth, C.B.; Green, S.J.; Engen, P.; Naqib, A.; Vitaterna, M.H.; Turek, F.W.; Keshavarzian, A. The Circadian Clock Mutation Promotes Intestinal Dysbiosis. Alcohol. Clin. Exp. Res. 2016, 40, 335–347. [Google Scholar] [CrossRef] [Green Version]
- Carra, M.C.; Schmitt, A.; Thomas, F.; Danchin, N.; Pannier, B.; Bouchard, P. Sleep disorders and oral health: A cross-sectional study. Clin. Oral. Investig. 2017, 21, 975–983. [Google Scholar] [CrossRef]
- Wang, L.; Ren, B.; Hui, Y.; Chu, C.; Zhao, Z.; Zhang, Y.; Zhao, B.; Shi, R.; Ren, J.; Dai, X.; et al. Methionine Restriction Regulates Cognitive Function in High-Fat Diet-Fed Mice: Roles of Diurnal Rhythms of SCFAs Producing- and Inflammation-Related Microbes. Mol. Nutr. Food Res. 2020, 64, e2000190. [Google Scholar] [CrossRef]
- Baron, K.G.; Reid, K.J. Circadian misalignment and health. Int. Rev. Psychiatry 2014, 26, 139–154. [Google Scholar] [CrossRef] [Green Version]
- Morris, C.J.; Yang, J.N.; Scheer, F. The impact of the circadian timing system on cardiovascular and metabolic function. Prog. Brain Res. 2012, 199, 337–358. [Google Scholar]
- Alqaderi, H.; Tavares, M.; Hartman, M.; Goodson, J.M. Effect of Sleep and Salivary Glucose on Gingivitis in Children. J. Dent. Res. 2016, 95, 1387–1393. [Google Scholar] [CrossRef]
- Alqaderi, H.; Goodson, J.M.; Agaku, I. Association between sleep and severe periodontitis in a nationally representative adult US population. J. Periodontol. 2020, 91, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.R.; Hayashi, S.; Chen, W.; Sano, M.; Machida, M.; Shigeyoshi, Y.; Iino, M.; Hashimoto, S. System-level identification of transcriptional circuits underlying mammalian circadian clocks. Nat. Genet. 2005, 37, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Patke, A.; Young, M.W.; Axelrod, S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 2020, 21, 67–84. [Google Scholar] [CrossRef] [PubMed]
- Welsh, D.K.; Takahashi, J.S.; Kay, S.A. Suprachiasmatic nucleus: Cell autonomy and network properties. Annu. Rev. Physiol. 2010, 72, 551–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunlap, J.C. Molecular bases for circadian clocks. Cell 1999, 96, 271–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kubra, S.; Zhang, H.; Si, Y.; Gao, X.; Wang, T.; Pan, L.; Li, L.; Zhong, N.; Fu, J.; Zhang, B.; et al. REGγ regulates circadian clock by modulating BMAL1 protein stability. Cell Death Discov. 2021, 7, 335. [Google Scholar] [CrossRef] [PubMed]
- Bunger, M.K.; Wilsbacher, L.D.; Moran, S.M.; Clendenin, C.; Radcliffe, L.A.; Hogenesch, J.B.; Simon, M.C.; Takahashi, J.S.; Bradfield, C.A. Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 2000, 103, 1009–1017. [Google Scholar] [CrossRef] [Green Version]
- Sulli, G.; Rommel, A.; Wang, X.; Kolar, M.J.; Puca, F.; Saghatelian, A.; Plikus, M.V.; Verma, I.M.; Panda, S. Pharmacological activation of REV-ERBs is lethal in cancer and oncogene-induced senescence. Nature 2018, 553, 351–355. [Google Scholar] [CrossRef]
- Wada, T.; Ichihashi, Y.; Suzuki, E.; Kosuge, Y.; Ishige, K.; Uchiyama, T.; Makishima, M.; Nakao, R.; Oishi, K.; Shimba, S. Deletion of Bmal1 Prevents Diet-Induced Ectopic Fat Accumulation by Controlling Oxidative Capacity in the Skeletal Muscle. Int. J. Mol. Sci. 2018, 19, 2813. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Liu, Y.; Cheng, Q.; Shen, Y.; Yuan, Y.; Jiang, X.; Li, X.; Guo, D.; Jiang, J.; Lin, C. Bmal1 Downregulation Worsens Critical Limb Ischemia by Promoting Inflammation and Impairing Angiogenesis. Front. Cardiovasc. Med. 2021, 8, 712903. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, M.; Xu, L.; Cheng, J.; Shen, J.; Yang, T.; Zhang, L. Bmal1 Regulates Macrophage Polarize Through Glycolytic Pathway in Alcoholic Liver Disease. Front. Pharmacol. 2021, 12, 640521. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Tang, Q.; Xie, M.; Zhou, X.; Long, Y.; Xie, Y.; Guo, F.; Chen, L. Circadian BMAL1 regulates mandibular condyle development by hedgehog pathway. Cell Prolif. 2020, 53, e12727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stokes, K.; Nunes, M.; Trombley, C.; Flôres, D.; Wu, G.; Taleb, Z.; Alkhateeb, A.; Banskota, S.; Harris, C.; Love, O.P.; et al. The Circadian Clock Gene, Bmal1, Regulates Intestinal Stem Cell Signaling and Represses Tumor Initiation. Cell. Mol. Gastroenterol. Hepatol. 2021, 12, 1847–1872.e0. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Cao, Q.; Gao, W.; Li, B.Y.; Zeng, C.; Xia, Z.; Zhao, B. Melatonin ameliorates cerebral ischemia-reperfusion injury in diabetic mice by enhancing autophagy via the SIRT1-BMAL1 pathway. FASEB J. 2021, 35, e22040. [Google Scholar] [CrossRef] [PubMed]
- Kalsbeek, A.; Van Der Spek, R.; Lei, J.; Endert, E.; Buijs, R.M.; Fliers, E. Circadian rhythms in the hypothalamo-pituitary-adrenal (HPA) axis. Mol. Cell. Endocrinol. 2012, 349, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Nader, N.; Chrousos, G.P.; Kino, T. Interactions of the circadian CLOCK system and the HPA axis. Trends. Endocrinol. Metab. 2010, 21, 277–286. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Xiao, W.; Jiang, Y.; Zou, L.; Chen, F.; Xiao, W.; Zhang, X.; Cao, Y.; Xu, L.; Zhu, Y. Bmal1 Regulates the Redox Rhythm of HSPB1, and Homooxidized HSPB1 Attenuates the Oxidative Stress Injury of Cardiomyocytes. Oxid. Med. Cell. Longev. 2021, 2021, 5542815. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Ding, H.; Zhao, J.; Liu, H.; Wang, J.; Lu, W. BMAL1 knockdown promoted apoptosis and reduced testosterone secretion in TM3 Leydig cell line. Gene 2020, 747, 144672. [Google Scholar] [CrossRef]
- Liang, S.; Hu, J.; Zhang, A.; Li, F.; Li, X. miR-155 induces endothelial cell apoptosis and inflammatory response in atherosclerosis by regulating Bmal1. Exp. Ther. Med. 2020, 20, 128. [Google Scholar] [CrossRef]
- Mohawk, J.A.; Green, C.B.; Takahashi, J.S. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Mchill, A.W.; Wright, K.P., Jr. Role of sleep and circadian disruption on energy expenditure and in metabolic predisposition to human obesity and metabolic disease. Obes. Rev. 2017, 18 (Suppl. S1), 15–24. [Google Scholar] [CrossRef] [PubMed]
- Kadono, M.; Nakanishi, N.; Yamazaki, M.; Hasegawa, G.; Nakamura, N.; Fukui, M. Various patterns of disrupted daily rest-activity rhythmicity associated with diabetes. J. Sleep Res. 2016, 25, 426–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamaldo, C.E.; Chung, Y.; Kang, Y.M.; Salas, R.M. Tick-tock-tick-tock: The impact of circadian rhythm disorders on cardiovascular health and wellness. J. Am. Soc. Hypertens. 2014, 8, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Dickerman, B.A.; Markt, S.C.; Koskenvuo, M.; Hublin, C.; Pukkala, E.; Mucci, L.A.; Kaprio, J. Sleep disruption, chronotype, shift work, and prostate cancer risk and mortality: A 30-year prospective cohort study of Finnish twins. Cancer Causes Control 2016, 27, 1361–1370. [Google Scholar] [CrossRef] [Green Version]
- Arble, D.M.; Ramsey, K.M.; Bass, J.; Turek, F.W. Circadian disruption and metabolic disease: Findings from animal models. Best Pract. Res. Clin. Endocrinol. Metab. 2010, 24, 785–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rijo-Ferreira, F.; Takahashi, J.S. Genomics of circadian rhythms in health and disease. Genome Med. 2019, 11, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, E.; Ono, B.; Souza, J.C. Sleep and immunity in times of COVID-19. Rev. Assoc. Med. Bras. 2020, 66 (Suppl. S2), 143–147. [Google Scholar] [CrossRef]
- Shen, Y.; Endale, M.; Wang, W.; Morris, A.R.; Francey, L.J.; Harold, R.L.; Hammers, D.W.; Huo, Z.; Partch, C.L.; Hogenesch, J.B.; et al. NF-κB modifies the mammalian circadian clock through interaction with the core clock protein BMAL1. PLoS Genet. 2021, 17, e1009933. [Google Scholar] [CrossRef]
- Scheiermann, C.; Kunisaki, Y.; Frenette, P.S. Circadian control of the immune system. Nat. Rev. Immunol. 2013, 13, 190–198. [Google Scholar] [CrossRef] [Green Version]
- Narasimamurthy, R.; Hatori, M.; Nayak, S.K.; Panda, S.; Verma, I.M. Circadian clock protein cryptochrome regulates the expression of proinflammatory cytokines. Proc. Natl. Acad. Sci. USA 2012, 109, 12662–12667. [Google Scholar] [CrossRef] [PubMed]
- Janjić, K.; Kurzmann, C.; Moritz, A.; Agis, H. Expression of circadian core clock genes in fibroblasts of human gingiva and periodontal ligament is modulated by L-Mimosine and hypoxia in monolayer and spheroid cultures. Arch. Oral Biol. 2017, 79, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J.E.; Blaikley, J.; Beesley, S.; Matthews, L.; Simpson, K.D.; Boyce, S.H.; Farrow, S.N.; Else, K.J.; Singh, D.; Ray, D.W.; et al. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc. Natl. Acad. Sci. USA 2012, 109, 582–587. [Google Scholar] [CrossRef] [Green Version]
- Yeung, F.; Hoberg, J.E.; Ramsey, C.S.; Keller, M.D.; Jones, D.R.; Frye, R.A.; Mayo, M.W. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004, 23, 2369–2380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sehirli, A.; Chukwunyere, U.; Aksoy, U.; Sayiner, S.; Abacioglu, N. The circadian clock gene Bmal1: Role in COVID-19 and periodontitis. Chronobiol. Int. 2021, 38, 779–784. [Google Scholar] [CrossRef]
- Kondratov, R.V.; Kondratova, A.A.; Gorbacheva, V.Y.; Vykhovanets, O.V.; Antoch, M.P. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006, 20, 1868–1873. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.W.; Wei, S.Z.; Huang, G.D.; Liu, L.B.; Gu, C.; Shen, Y.; Wang, X.H.; Xia, S.T.; Xie, A.M.; Hu, L.F.; et al. BMAL1 regulation of microglia-mediated neuroinflammation in MPTP-induced Parkinson’s disease mouse model. FASEB J. 2020, 34, 6570–6581. [Google Scholar] [CrossRef]
- Machado, R.B.; Hipólide, D.C.; Benedito-Silva, A.A.; Tufik, S. Sleep deprivation induced by the modified multiple platform technique: Quantification of sleep loss and recovery. Brain Res. 2004, 1004, 45–51. [Google Scholar] [CrossRef]
- Chen, G.; Zhao, H.; Ma, S.; Chen, L.; Wu, G.; Zhu, Y.; Zhu, J.; Ma, C.; Zhao, H. Circadian Rhythm Protein Bmal1 Modulates Cartilage Gene Expression in Temporomandibular Joint Osteoarthritis via the MAPK/ERK Pathway. Front. Pharmacol. 2020, 11, 527744. [Google Scholar] [CrossRef]
- National Research Council Committee on Guidelines for the Use of Animals in Neuroscience and Behavioral Research. The National Academies Collection: Reports Funded by National Institutes of Health, Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research; National Academy of Sciences; National Academies Press: Washington, DC, USA, 2003. [Google Scholar]
- Hu, Y.; Zhang, X.; Zhang, J.; Xia, X.; Li, H.; Qiu, C.; Liao, Y.; Chen, H.; He, Z.; Song, Z. Activated STAT3 signaling pathway by ligature-induced periodontitis could contribute to neuroinflammation and cognitive impairment in rats. J. Neuroinflamm. 2021, 18, 80. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, X.; Li, Y.; Xia, B.; Yu, W. The role of TLR4/MyD88/NF-κB pathway in periodontitis-induced liver inflammation of rats. Oral Dis. 2021, 27, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.C.; Ding, X.; Liu, X.M.; Cao, N.B.; Deng, Y.; Hou, Y.B.; Yu, W.X. Resveratrol protects renal damages induced by periodontitis via preventing mitochondrial dysfunction in rats. Oral Dis. 2022. [Google Scholar] [CrossRef] [PubMed]

| Gene | Gene Accession No. | Primer Sequence (5′–3′) |
|---|---|---|
| BMAL1 | NM_024362.2 | F: GCTTTGAGGTGACCAGCAAGTACA |
| R: AAGGGCTCCAAGGTCCACAG | ||
| NF-κB | NM_199267.2 | F: CGACGTATTGCTGTGCCTTC |
| R: TTGAGATCTGCCCAGGTGGTA | ||
| Bax | NM_017059.2 | F: CCACCAAGAAGCTGAGCGA |
| R: GCTGCCACACGGAAGAAGA | ||
| Bcl-2 | NM_016993.2 | F: TTGAGTTCGGTGGGGTCATG |
| R: GATCCAGGTGTGCAGATGCC | ||
| Caspase3 | NM_012922.2 | F: AGCCGAAACTCTTCATCATTCA |
| R: CCATATCATCGTCAGTTCCACT | ||
| β-actin | NM_031144.3 | F: GGAGATTACTGCCCTGGCTCCTA |
| R: GACTCATCGTACTCCTGCTTGCTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Cao, N.; Liu, X.; Deng, Y.; Xin, Y.; Fu, R.; Xin, X.; Hou, Y.; Yu, W. Circadian Rhythm Disorders Aggravate Periodontitis by Modulating BMAL1. Int. J. Mol. Sci. 2023, 24, 374. https://doi.org/10.3390/ijms24010374
Liu X, Cao N, Liu X, Deng Y, Xin Y, Fu R, Xin X, Hou Y, Yu W. Circadian Rhythm Disorders Aggravate Periodontitis by Modulating BMAL1. International Journal of Molecular Sciences. 2023; 24(1):374. https://doi.org/10.3390/ijms24010374
Chicago/Turabian StyleLiu, Xiaomeng, Niuben Cao, Xinchan Liu, Yu Deng, Yu Xin, Ruobing Fu, Xirui Xin, Yubo Hou, and Weixian Yu. 2023. "Circadian Rhythm Disorders Aggravate Periodontitis by Modulating BMAL1" International Journal of Molecular Sciences 24, no. 1: 374. https://doi.org/10.3390/ijms24010374





