SLC38A4 Amino Acid Transporter Expression Is Significantly Lower in Early Preterm Intrauterine Growth Restriction Complicated Placentas
Abstract
:1. Introduction
2. Results
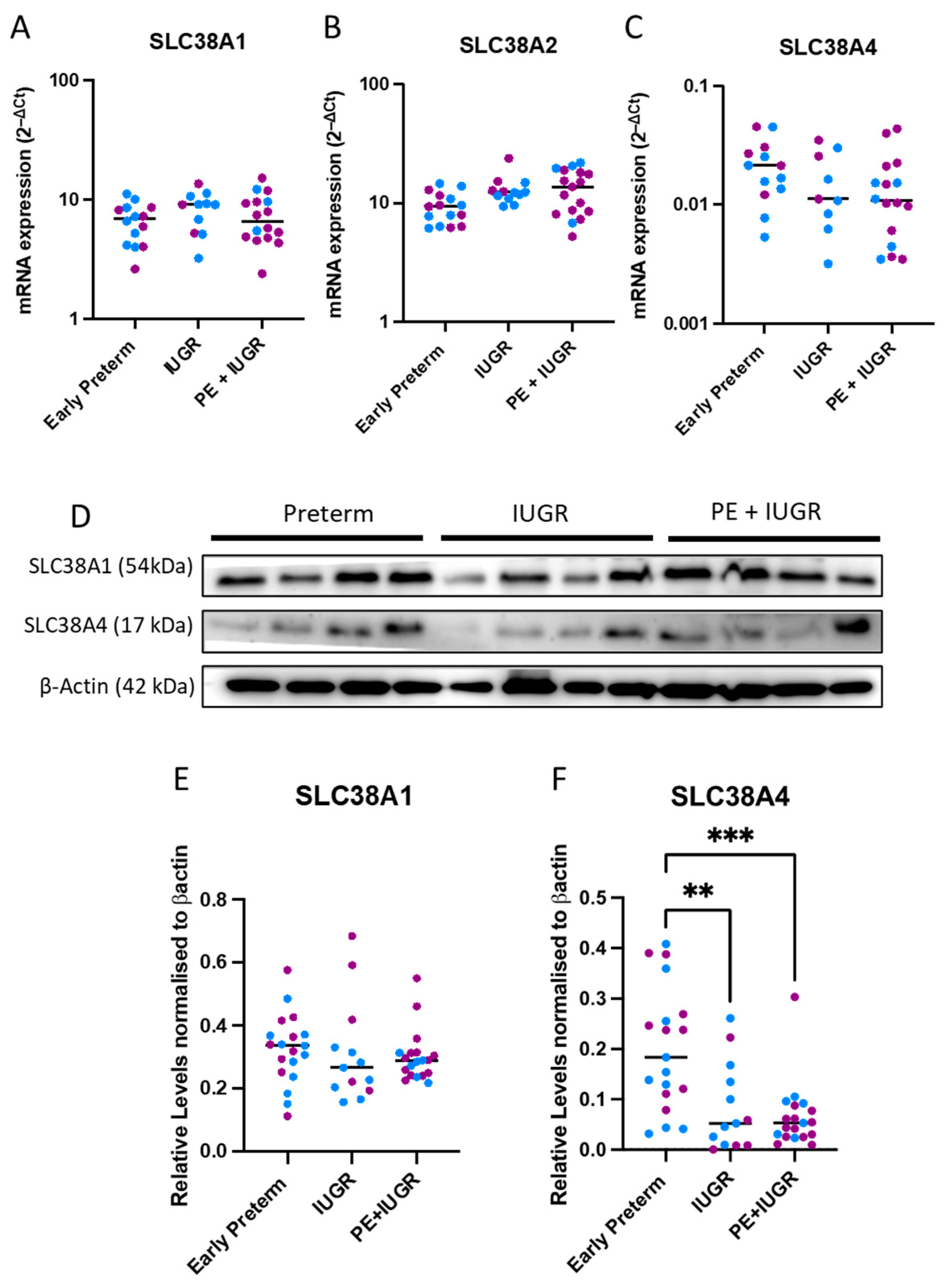
2.1. System A Sodium-Coupled Transporter Expression in Placentas from Pregnancies Complicated by Early Preterm IUGR and Preeclampsia
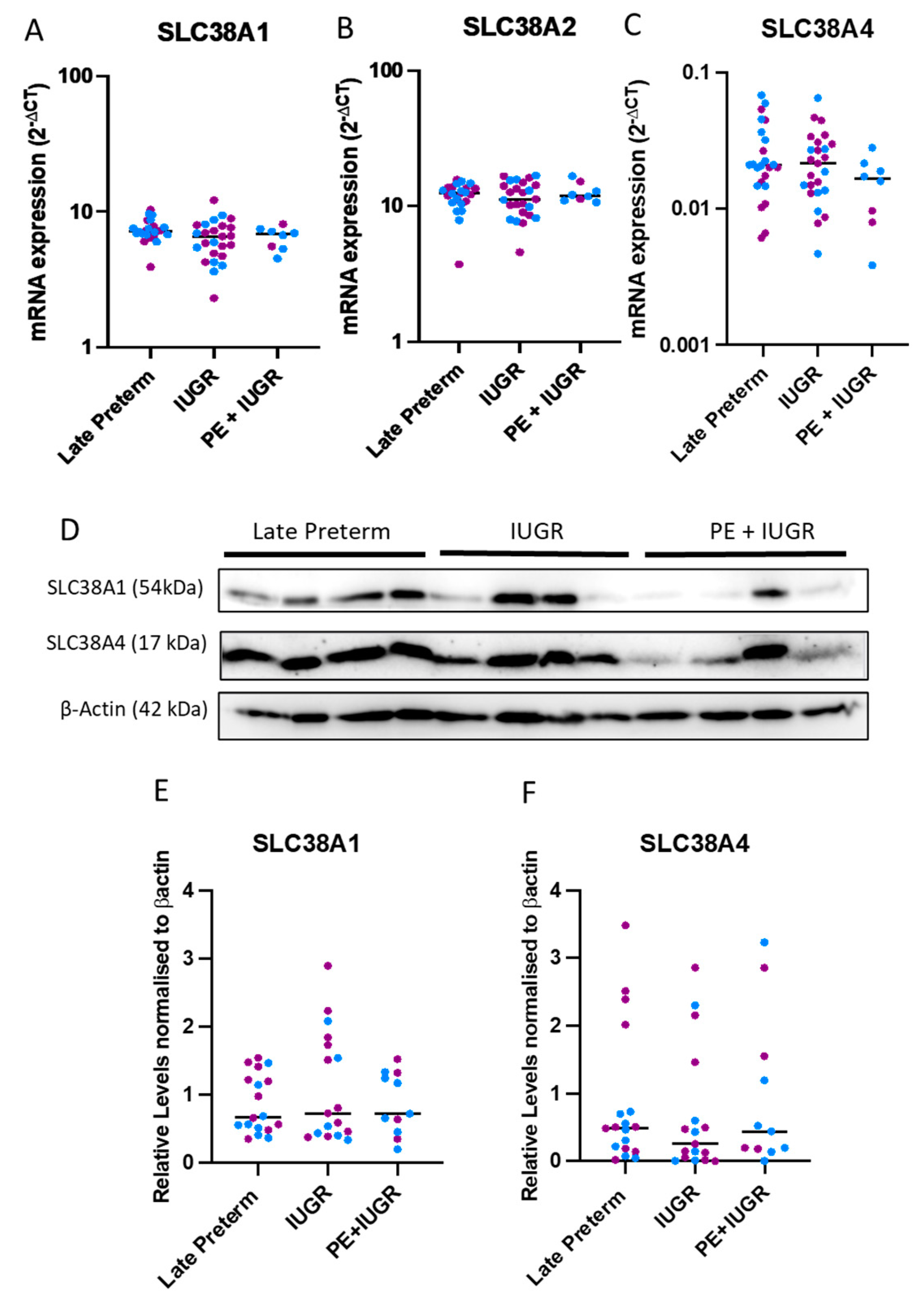
2.2. System A Sodium-Coupled Transporter Expression in Placentas from Pregnancies Complicated by Late Preterm IUGR and Preeclampsia
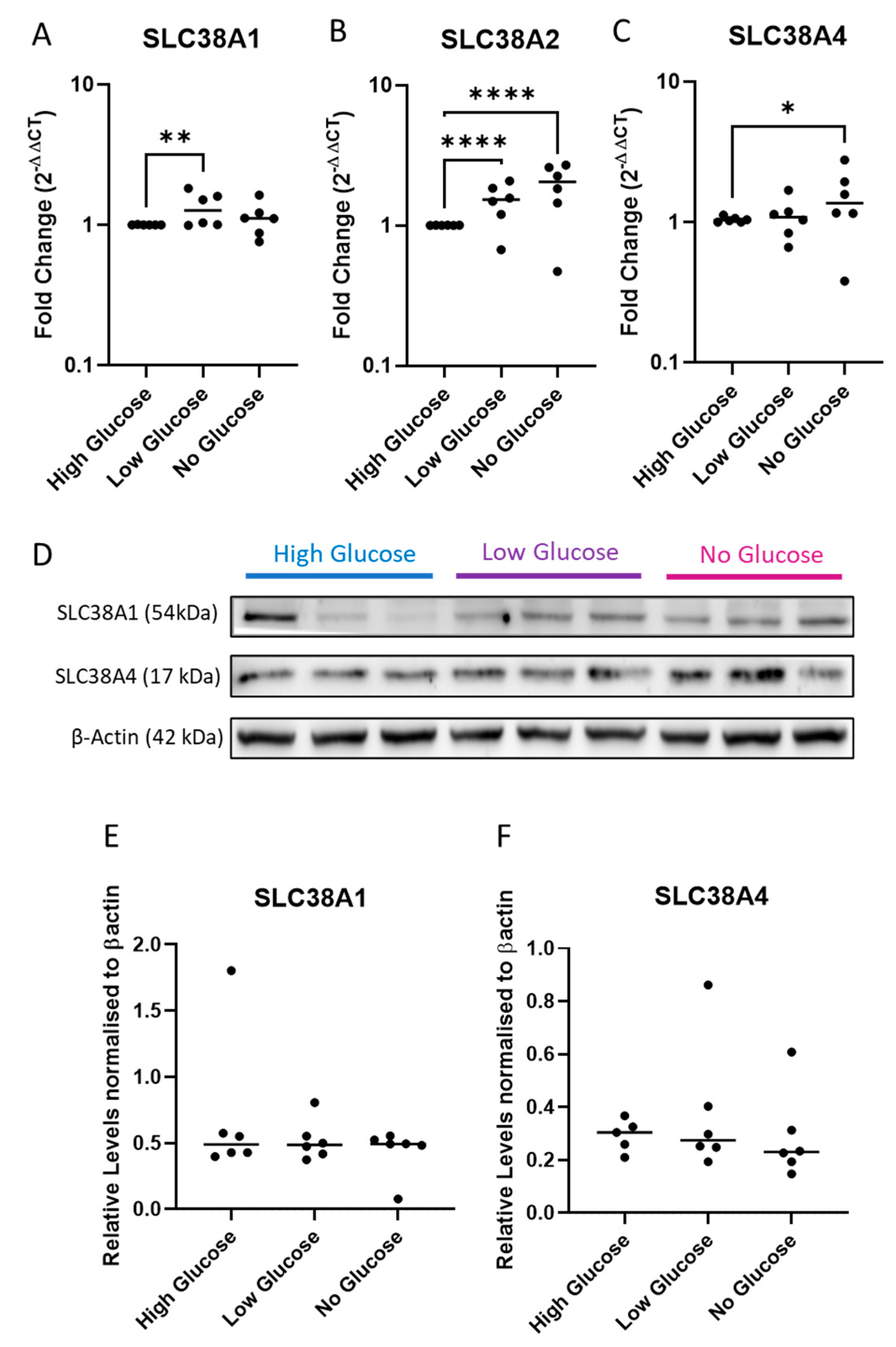
2.3. Expression of System A Sodium-Coupled Transporters in Trophoblast Cells under IUGR Relevant Stress Conditions
3. Discussion
4. Materials and Methods
4.1. Patient Samples
4.2. Isolating and Treating Primary Human Cytotrophoblast Cells and Placental Explants
4.3. Quantitative Polymerase Chain Reaction (qPCR)
4.4. Western Blots
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Malhotra, A.; Allison, B.J.; Castillo-Melendez, M.; Jenkin, G.; Polglase, G.R.; Miller, S.L. Neonatal Morbidities of Fetal Growth Restriction: Pathophysiology and Impact. Front. Endocrinol. 2019, 10, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaccioliy, F.; Huang, C.C.; Wang, C.; Bevilacqua, E.; Franchi-Gazzola, R.; Gazzola, G.C.; Bussolati, O.; Snider, M.D.; Hatzoglou, M. Amino Acid Starvation Induces the SNAT2 Neutral Amino Acid Transporter by a Mechanism That Involves Eukaryotic Initiation Factor 2α Phosphorylation and cap-independent Translation. J. Biol. Chem. 2006, 281, 17929–17940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacKenzie, B.; Erickson, J.D. Sodium-coupled neutral amino acid (System N/A) transporters of the SLC38 gene family. Pflügers Archiv. 2004, 447, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Matoba, S.; Nakamuta, S.; Miura, K.; Hirose, M.; Shiura, H.; Kohda, T.; Nakamuta, N.; Ogura, A. Paternal knockout of Slc38a4/SNAT4 causes placental hypoplasia associated with intrauterine growth restriction in mice. Proc. Natl. Acad. Sci. USA 2019, 116, 21047–21053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glazier, J.D.; Cetin, I.; Perugino, G.; Ronzoni, S.; Grey, A.M.; Mahendran, D.; Marconi, A.M.; Pardi, G.; Sibley, C.P. Association between the Activity of the System A Amino Acid Transporter in the Microvillous Plasma Membrane of the Human Placenta and Severity of Fetal Compromise in Intrauterine Growth Restriction. Pediatr. Res. 1997, 42, 514–519. [Google Scholar] [CrossRef]
- Cetin, I.; Ronzoni, S.; Marconi, A.M.; Perugino, G.; Corbetta, C.; Battaglia, F.C.; Pardi, G. Maternal concentrations and fetal-maternal concentration differences of plasma amino acids in normal and intrauterine growth-restricted pregnancies. Am. J. Obstet. Gynecol. 1996, 174, 1575–1583. [Google Scholar] [CrossRef]
- Hoffmann, T.M.; Cwiklinski, E.; Shah, D.S.; Stretton, C.; Hyde, R.; Taylor, P.M.; Hundal, H.S. Effects of Sodium and Amino Acid Substrate Availability upon the Expression and Stability of the SNAT2 (SLC38A2) Amino Acid Transporter. Front. Pharmacol. 2018, 9, 63. [Google Scholar] [CrossRef] [Green Version]
- Gaccioli, F.; Lager, S. Placental Nutrient Transport and Intrauterine Growth Restriction. Front. Physiol. 2016, 7, 40. [Google Scholar] [CrossRef] [Green Version]
- Coan, P.M.; Angiolini, E.; Sandovici, I.; Burton, G.J.; Constancia, M.; Fowden, A.L. Adaptations in placental nutrient transfer capacity to meet fetal growth demands depend on placental size in mice. J. Physiol. 2008, 586, 4567–4576. [Google Scholar] [CrossRef]
- Rosario, F.J.; Kramer, A.; Li, C.; Galan, H.L.; Powell, T.L.; Nathanielsz, P.W.; Jansson, T. Reduction of In Vivo Placental Amino Acid Transport Precedes the Development of Intrauterine Growth Restriction in the Non-Human Primate. Nutrients 2021, 13, 2892. [Google Scholar] [CrossRef]
- Jansson, N.; Pettersson, J.; Haafiz, A.; Ericsson, A.; Palmberg, I.; Tranberg, M.; Ganapathy, V.; Powell, T.L.; Jansson, T. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J. Physiol. 2006, 576, 935–946. [Google Scholar] [PubMed]
- Roos, S.; Jansson, N.; Palmberg, I.; Säljö, K.; Powell, T.; Jansson, T. Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growth. J. Physiol. 2007, 582, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Shibata, E.; Hubel, C.; Powers, R.; von Versen-Hoeynck, F.; Gammill, H.; Rajakumar, A.; Roberts, J. Placental System A Amino Acid Transport is Reduced in Pregnancies With Small For Gestational Age (SGA) Infants but Not in Preeclampsia with SGA Infants. Placenta 2008, 29, 879–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cutland, C.L.; Lackritz, E.M.; Mallett-Moore, T.; Bardají, A.; Chandrasekaran, R.; Lahariya, C.; Nisar, M.I.; Tapia, M.D.; Pathirana, J.; Kochhar, S.; et al. Low birth weight: Case definition & guidelines for data collection, analysis, and presentation of maternal immunization safety data. Vaccine 2017, 35, 6492–6500. [Google Scholar] [CrossRef]
- Sharma, D.; Shastri, S.; Sharma, P. Intrauterine Growth Restriction: Antenatal and Postnatal Aspects. Clin. Med. Insights Pediatr. 2016, 10, 67–83. [Google Scholar] [CrossRef] [Green Version]
- Norberg, S.; Powell, T.L.; Jansson, T. Intrauterine Growth Restriction Is Associated with a Reduced Activity of Placental Taurine Transporters. Pediatr. Res. 1998, 44, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Desforges, M.; Lacey, H.A.; Glazier, J.D.; Greenwood, S.L.; Mynett, K.J.; Speake, P.F.; Sibley, C.P. SNAT4 isoform of system A amino acid transporter is expressed in human placenta. Am. J. Physiol. Physiol. 2006, 290, C305–C312. [Google Scholar] [CrossRef] [Green Version]
- Vaughan, O.R.; Maksym, K.; Silva, E.; Barentsen, K.; Anthony, R.V.; Brown, T.L.; Hillman, S.L.; Spencer, R.; David, A.L.; Rosario, F.J.; et al. Placenta-specific Slc38a2/SNAT2 knockdown causes fetal growth restriction in mice. Clin. Sci. 2021, 135, 2049–2066. [Google Scholar] [CrossRef]
- Marconi, A.M.; Paolini, C.; Buscaglia, M.; Zerbe, G.; Battaglia, F.C.; Pardi, G. The impact of gestational age and fetal growth on the maternal-fetal glucose concentration difference. Obstet. Gynecol. 1996, 87, 937–942. [Google Scholar] [CrossRef] [PubMed]
- Economides, D.; Nicolaides, K. Blood glucose and oxygen tension levels in small-for-gestational-age fetuses. Am. J. Obstet. Gynecol. 1989, 160, 385–389. [Google Scholar] [CrossRef]
- Wesolowski, S.R.; Hay, W.W., Jr. Role of placental insufficiency and intrauterine growth restriction on the activation of fetal hepatic glucose production. Mol. Cell Endocrinol. 2016, 435, 61–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hundal, H.S.; Bilan, P.J.; Tsakiridis, T.; Marette, A.; Klip, A. Structural disruption of the trans-Golgi network does not interfere with the acute stimulation of glucose and amino acid uptake by insulin-like growth factor I in muscle cells. Biochem. J. 1994, 297, 289–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hajduch, E.; Alessi, D.R.; Hemmings, B.A.; Hundal, H.S. Constitutive activation of protein kinase B alpha by membrane targeting promotes glucose and system A amino acid transport, protein synthesis, and inactivation of glycogen synthase kinase 3 in L6 muscle cells. Diabetes 1998, 47, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Menchini, R.J.; Chaudhry, F.A. Multifaceted regulation of the system A transporter Slc38a2 suggests nanoscale regulation of amino acid metabolism and cellular signaling. Neuropharmacology 2019, 161, 107789. [Google Scholar] [CrossRef]
- Ortiz, V.; Alemán, G.; Escamilla-Del-Arenal, M.; Recillas-Targa, F.; Torres, N.; Tovar, A.R. Promoter characterization and role of CRE in the basal transcription of the rat SNAT2 gene. Am. J. Physiol. Metab. 2011, 300, E1092–E1102. [Google Scholar] [CrossRef] [Green Version]
- Horie, T.; Fukasawa, K.; Iezaki, T.; Park, G.; Onishi, Y.; Ozaki, K.; Kanayama, T.; Hiraiwa, M.; Kitaguchi, Y.; Kaneda, K.; et al. Hypoxic Stress Upregulates the Expression of Slc38a1 in Brown Adipocytes via Hypoxia-Inducible Factor-1α. Pharmacology 2017, 101, 64–71. [Google Scholar] [CrossRef]
- Morotti, M.; Bridges, E.; Valli, A.; Choudhry, H.; Sheldon, H.; Wigfield, S.; Gray, N.; Zois, C.E.; Grimm, F.; Jones, D.; et al. Hypoxia-induced switch in SNAT2/SLC38A2 regulation generates endocrine resistance in breast cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 12452–12461. [Google Scholar] [CrossRef] [Green Version]
- Nelson, D.M.; Smith, S.D.; Furesz, T.C.; Sadovsky, Y.; Ganapathy, V.; Parvin, C.A.; Smith, C.H. Hypoxia reduces expression and function of system A amino acid transporters in cultured term human trophoblasts. Am. J. Physiol. Physiol. 2003, 284, C310–C315. [Google Scholar] [CrossRef] [Green Version]
- Jones, H.N.; Ashworth, C.J.; Page, K.R.; McArdle, H.J. Expression and adaptive regulation of amino acid transport system A in a placental cell line under amino acid restriction. J. Reprod. 2006, 131, 951–960. [Google Scholar] [CrossRef] [Green Version]
- Dobbins, T.A.; Sullivan, E.A.; Roberts, C.L.; Simpson, J.M. Australian national birthweight percentiles by sex and gestational age, 1998–2007. Med. J. Aust. 2012, 197, 291–294. [Google Scholar] [CrossRef]
- Roberts, J.M.; August, P.A.; Bakris, G.; Barton, J.R.; Bernstein, I.M.; Druzin, M.; Gaiser, R.R.; Granger, J.P.; Jeyabalan, A.; Johnson, D.D.; et al. Hypertension in Pregnancy: Executive Summary. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar]
- Brownfoot, F.; Hannan, N.; Onda, K.; Tong, S.; Kaitu’U-Lino, T. Soluble endoglin production is upregulated by oxysterols but not quenched by pravastatin in primary placental and endothelial cells. Placenta 2014, 35, 724–731. [Google Scholar] [CrossRef] [PubMed]

| Patient Characteristics | Preterm (n = 20) | IUGR (n = 13) | IUGR + PE (n = 22) |
|---|---|---|---|
| Maternal age (years) | 32 (25–37) | 30.5 (24.3–36) | 31 (25–32) |
| Body–mass index (kg/m2) | 29.2 (25.3–35.2) | 23.6 (18.6–30.5) | 28 (25–35.5) |
| Smoking during pregnancy (%) | 4 (27%) | 5 (42%) | 1 (5.9%) |
| Diabetes during pregnancy (%) | 3 (21%) | 3 (25%) | 1 (5.9%) |
| Gestational age at delivery (weeks) | 30 (29–32) | 31.5 (30.1–32.8) | 30.1 (26.9–31.3) |
| Birthweight (grams) | 1585 (1237–2000) | 993.5 (861–1257.3) | 911 (557.5–1173) |
| Fetal Sex (Female/Male) | 9/11 | 8/5 | 14/8 |
| Patient Characteristics | Preterm (n = 24) | IUGR (n = 25) | IUGR + PE (n = 11) |
|---|---|---|---|
| Maternal age (years) | 30.5 (28–34.8) | 32.24 (30–35.5) | 35.5 (27.3–36) |
| Body-mass index (kg/m2) | 24 (21–27) | 26.05 (22.2–28) | 23.5 (20.5–25.7) |
| Smoking during pregnancy (%) | 1 (4.2%) | 2 (6.9%) | 0 (0%) |
| Diabetes during pregnancy (%) | 2 (8.3%) | 6 (21%) | 3 (38%) |
| Gestational age at delivery, weeks | 34.9 (34.6–35.9) | 35.52 (34.4–37.4) | 36.1 (34.5–36.3) |
| Birthweight, grams | 2605 (2239.8–2810) | 1932.68 (1672.5–2240) | 1958 (1774.5–2085) |
| Fetal Sex (Female/Male) | 11/13 | 16/9 | 4/7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadife, E.; Harper, A.; De Alwis, N.; Chien, K.; Hannan, N.; Brownfoot, F.C. SLC38A4 Amino Acid Transporter Expression Is Significantly Lower in Early Preterm Intrauterine Growth Restriction Complicated Placentas. Int. J. Mol. Sci. 2023, 24, 403. https://doi.org/10.3390/ijms24010403
Kadife E, Harper A, De Alwis N, Chien K, Hannan N, Brownfoot FC. SLC38A4 Amino Acid Transporter Expression Is Significantly Lower in Early Preterm Intrauterine Growth Restriction Complicated Placentas. International Journal of Molecular Sciences. 2023; 24(1):403. https://doi.org/10.3390/ijms24010403
Chicago/Turabian StyleKadife, Elif, Alesia Harper, Natasha De Alwis, Keegan Chien, Natalie Hannan, and Fiona C. Brownfoot. 2023. "SLC38A4 Amino Acid Transporter Expression Is Significantly Lower in Early Preterm Intrauterine Growth Restriction Complicated Placentas" International Journal of Molecular Sciences 24, no. 1: 403. https://doi.org/10.3390/ijms24010403
APA StyleKadife, E., Harper, A., De Alwis, N., Chien, K., Hannan, N., & Brownfoot, F. C. (2023). SLC38A4 Amino Acid Transporter Expression Is Significantly Lower in Early Preterm Intrauterine Growth Restriction Complicated Placentas. International Journal of Molecular Sciences, 24(1), 403. https://doi.org/10.3390/ijms24010403






