Molecular Variation and Genomic Function of Citrus Vein Enation Virus
Abstract
1. Introduction
2. Results
2.1. Small RNA Deep Sequencing and CVEV-DT1 Identification
2.2. Characterization of CVEV-DT1-Derived siRNAs
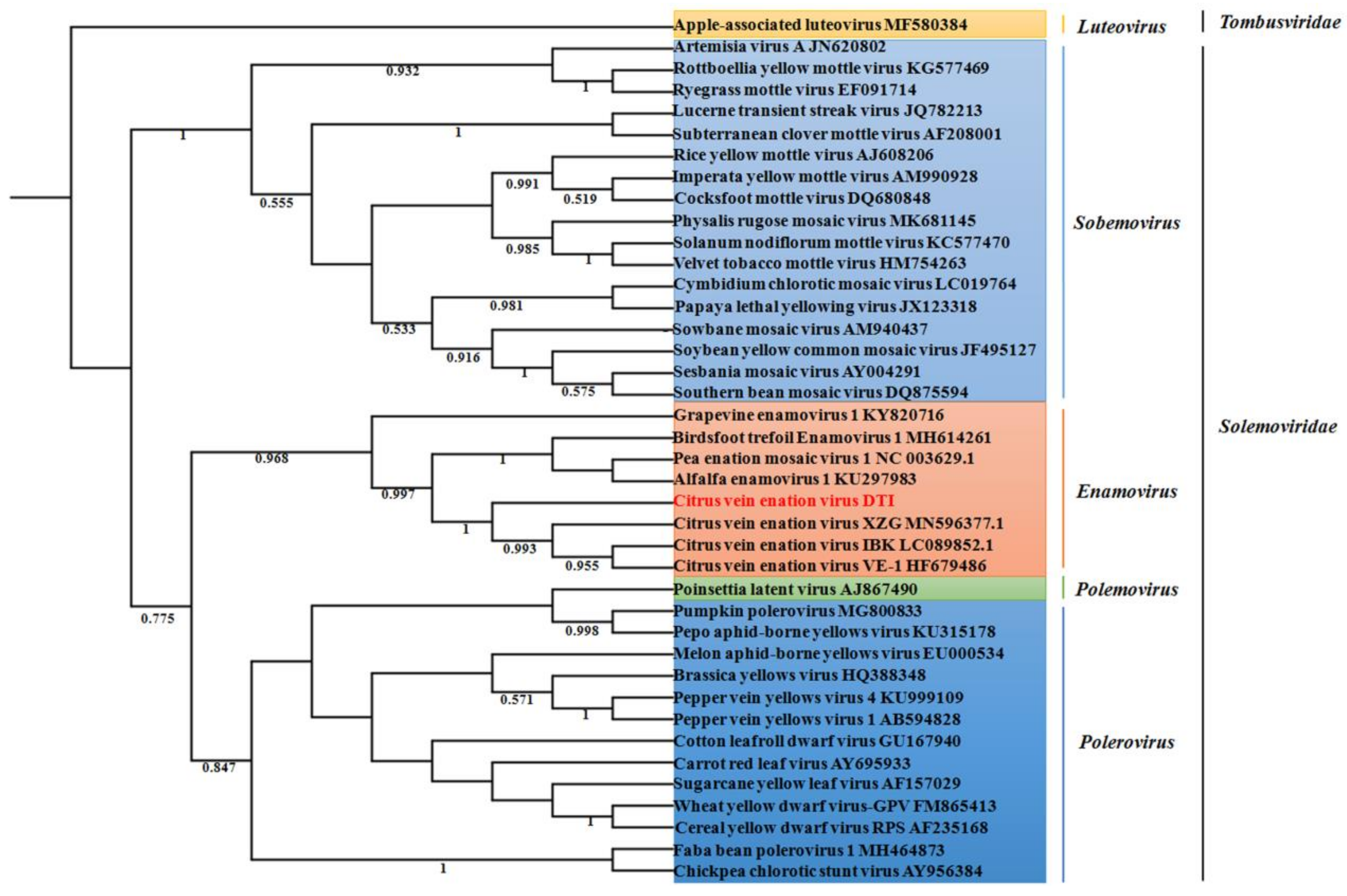
2.3. Phylogenetic Analysis of CVEV-DT1
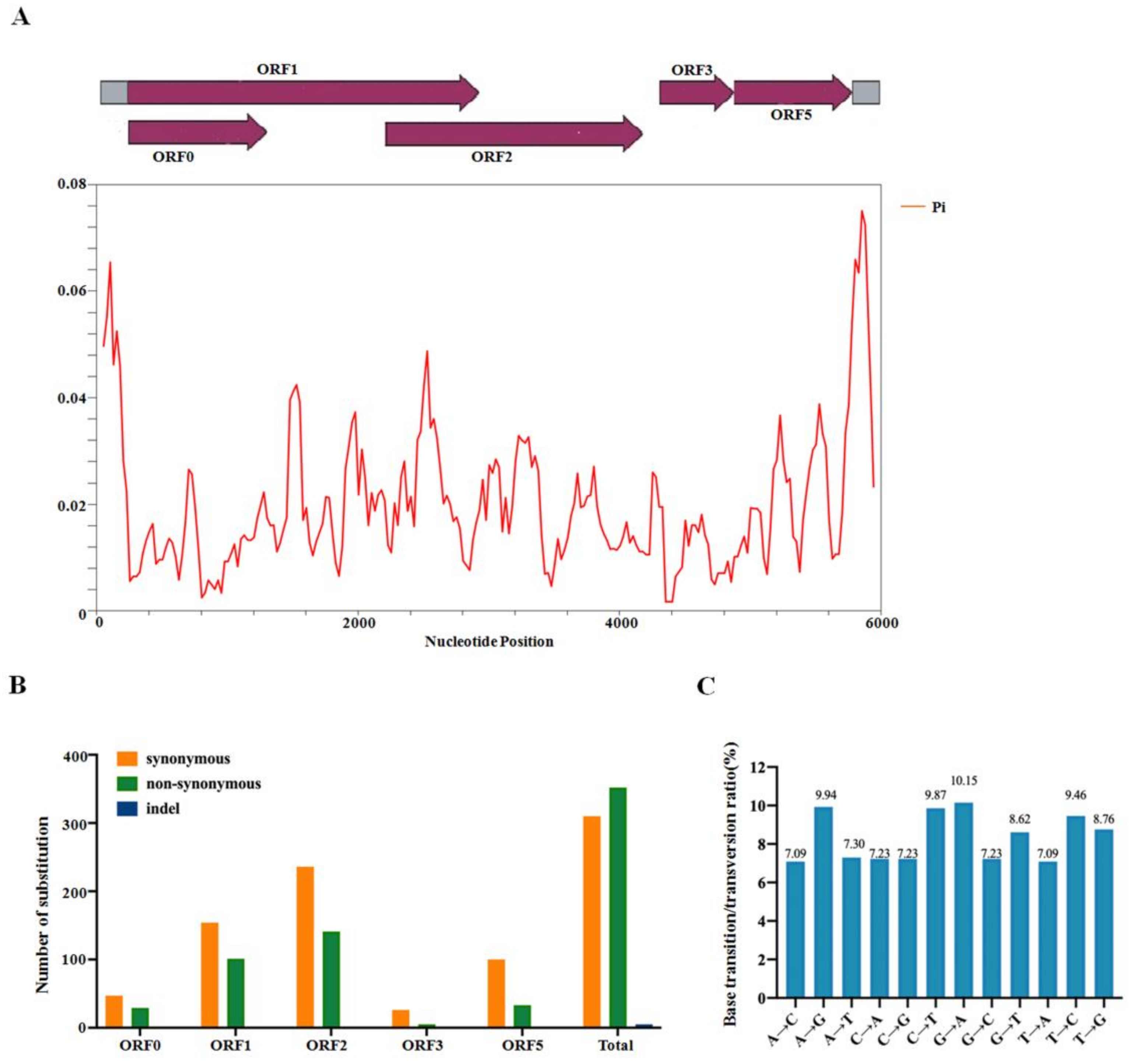
2.4. Population Variation Rate and Evolutionary Analysis of CVEV
2.5. Recombination Analysis of CVEV
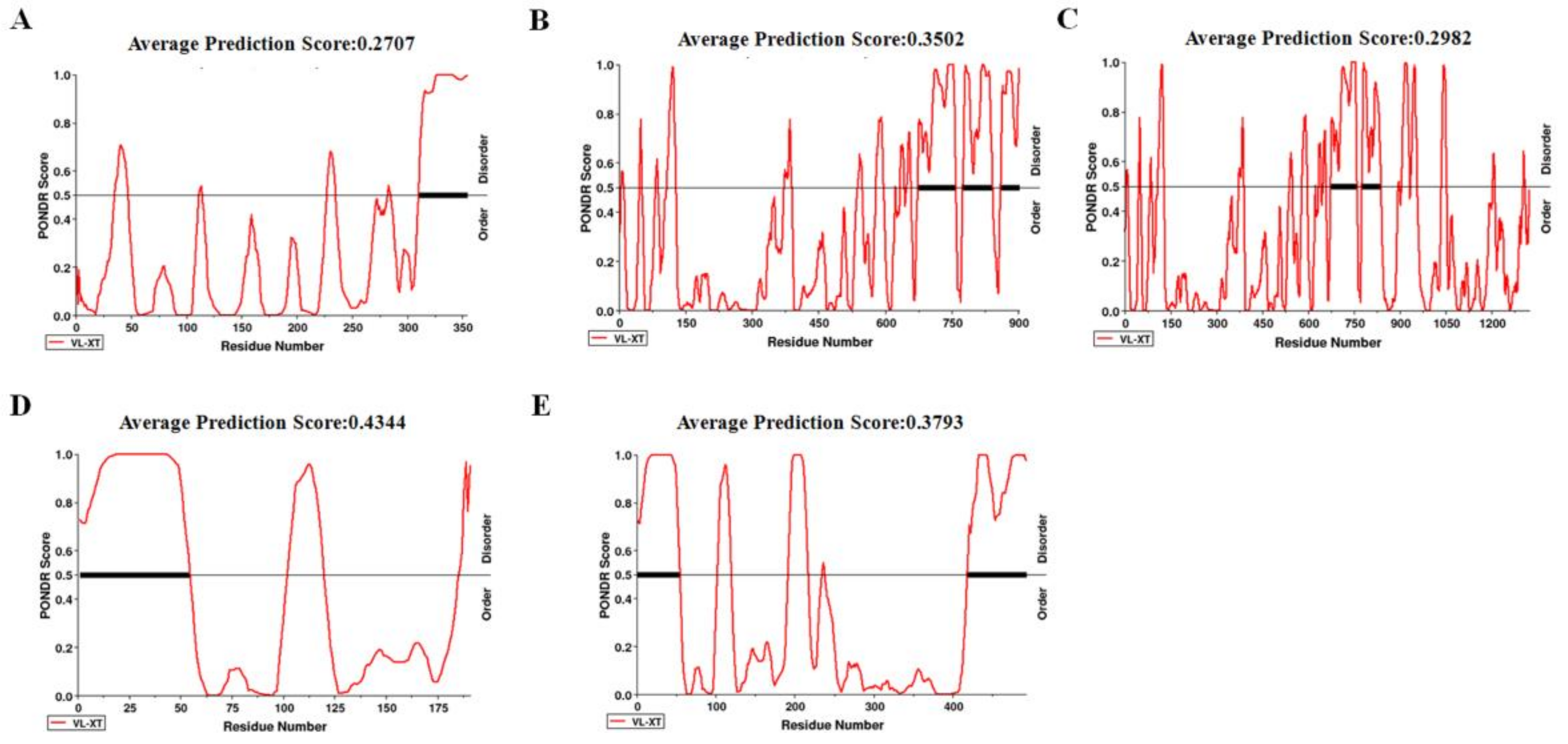
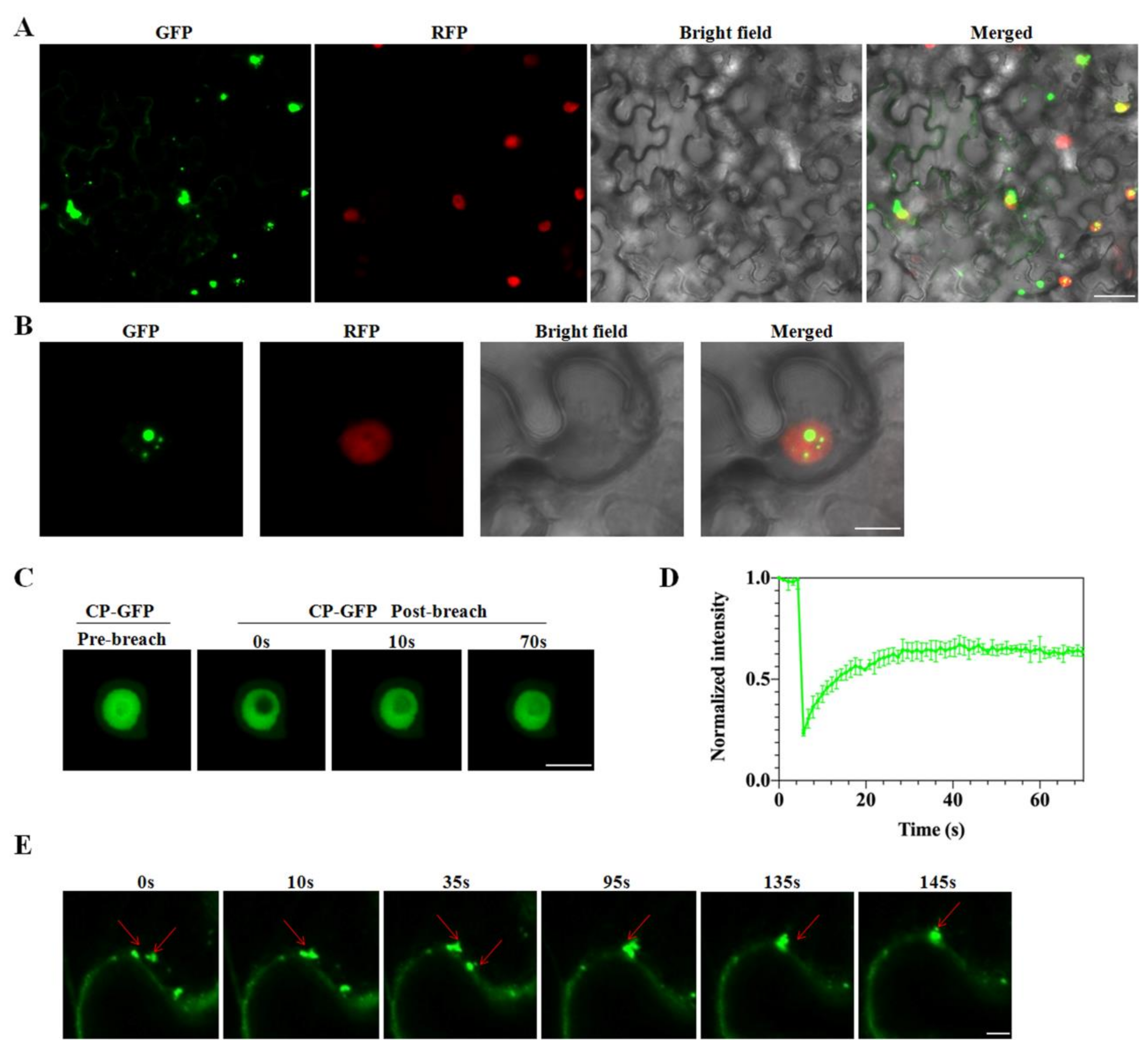
2.6. Disorder across CP of CVEV-DT1
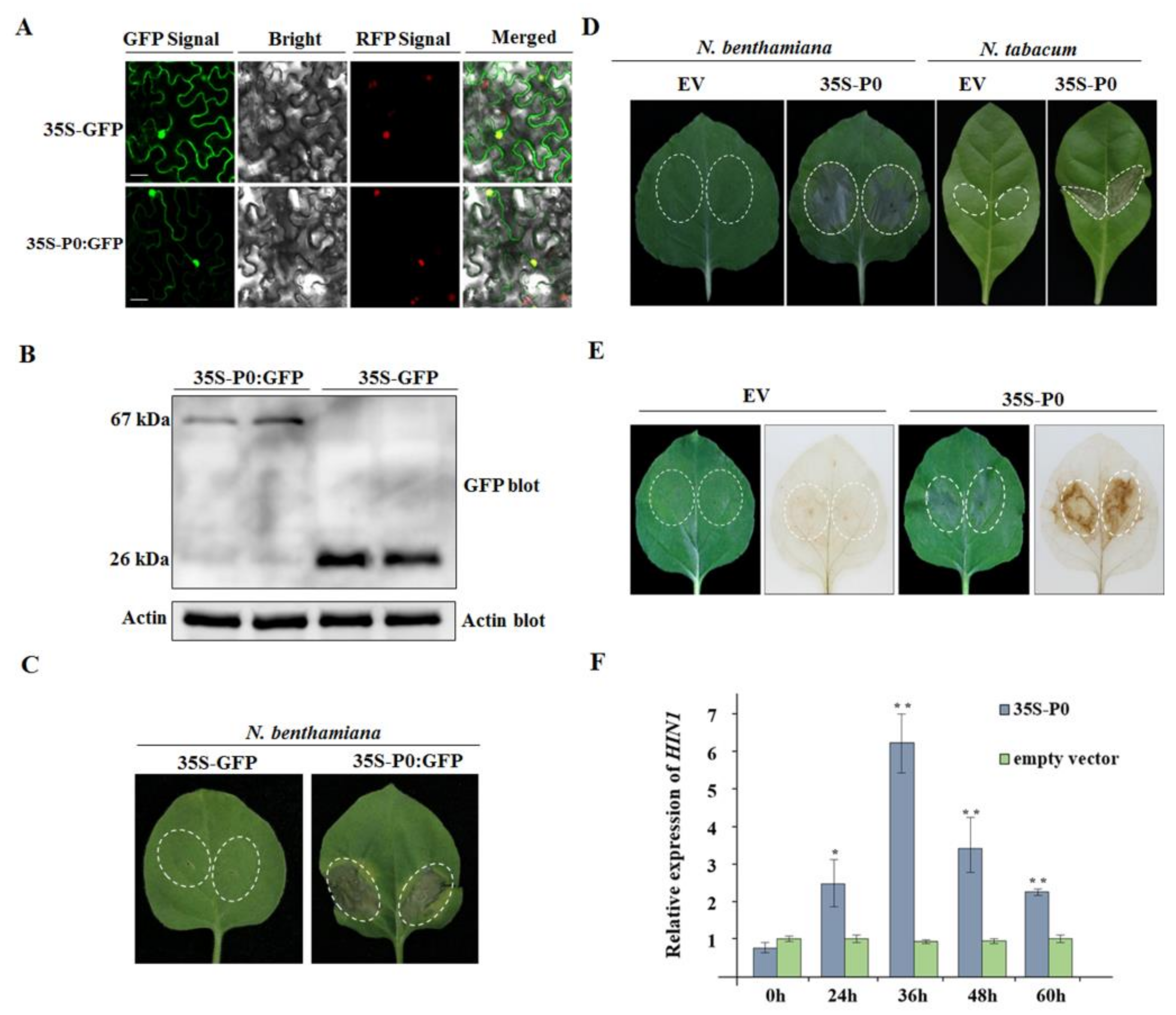
2.7. CVEV-DT1 P0 as an Inducer of Hypersensitive-Response-like Cell Death
2.8. CVEV-DT1 P0 Elicits Cell Death in a PVX Expression Assay
2.9. CVEV-DT1 P0 Is a Weak Suppressor of PTGS
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Small RNA Deep Sequencing and Data Processing
4.3. RT-PCR Validation, Full-Length Genome Amplification, and Sequencing
4.4. Phylogenetic Analysis
4.5. Diversity and Variation Analysis
4.6. Genomic Sequence Analysis
4.7. Prediction of IDR
4.8. Fluorescence Recovery after Photobleaching (FRAP) Assay
4.9. Vector Construction and Plant Inoculation
4.10. Subcellular Localization
4.11. PTGS Assay
4.12. H2O2 Detection in Plants
4.13. Protein Extraction and Western Blotting
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Somera, M.; Fargette, D.; Hebrard, E.; Sarmiento, C.; Consortium, I.R. ICTV Virus Taxonomy Profile: Solemoviridae. J. Gen. Virol. 2021, 102, 001707. [Google Scholar] [PubMed]
- LaTourrette, K.; Holste, N.M.; Garcia-Ruiz, H. Polerovirus genomic variation. Virus Evol. 2021, 7, veab102. [Google Scholar] [CrossRef] [PubMed]
- Vives, M.C.; Velazquez, K.; Antonio Pina, J.; Moreno, P.; Guerri, J.; Navarro, L. Identification of a new enamovirus associated with citrus vein enation disease by deep sequencing of small RNAs. Phytopathology 2013, 103, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Laird, E.F.; Weathers, L.G. Aphis gossypii, a vector of citrus vein-enation virus. Plant Dis. Rep. 1961, 45, 877. [Google Scholar]
- Hermoso De Mendoza, A.; Pina, J.A.; Ballester-Olmos, J.F.; Navarro, L. Persistent transmission of citrus vein enation virus by Aphis gossypii and Myzus persicae. In Twelfth Conference of the International Organization of Citrus Virologists; Moreno, P., da Graça, J.V., Timmer, L.W., Eds.; IOCV: Riverside, CA, USA, 1993; pp. 361–363. [Google Scholar]
- Wallace, J.M.; Deake, R. A virus-induced vein enation in citrus. Citrus Leaves 1953, 33, 22–24. [Google Scholar]
- Mcclean, A.P.D. Citrus-vein enation. S. Afr. J. Sci. 1954, 50, 147–151. [Google Scholar]
- Wallace, J.M.; Drake, R.J. Further studies on woody gall virus disease of citrus. Phytopathology 1962, 52, 756. [Google Scholar]
- Wallace, J.M.; Drake, R.J. Induction of woody galls by wounding of citrus infected with vein-enation virus. Plant Dis. Rep. 1961, 45, 682. [Google Scholar]
- Minutolo, M.; Cinque, M.; Altamura, G.; Di Serio, F.; Alioto, D.; Navarro, B. Identification, full-length genome sequencing, and field survey of citrus vein enation virus in Italy. Phytopathol. Mediterr. 2021, 60, 293–301. [Google Scholar] [CrossRef]
- Nakazono-Nagaoka, E.; Fujikawa, T.; Iwanami, T. Nucleotide sequences of Japanese isolates of citrus vein enation virus. Arch. Virol. 2017, 162, 879–883. [Google Scholar] [CrossRef]
- Yang, H.J.; Oh, J.; Lee, H.K.; Lee, D.S.; Kim, S.Y.; Kim, M.H.; Park, C.Y.; Kim, H.; Lee, S.I.; Jeong, R.D.; et al. First report of citrus vein enation virus in Satsuma Mandarin (Citrus unshiu) in Korea. Plant Dis. 2019, 103, 2703–2704. [Google Scholar] [CrossRef]
- Yang, H.J.; Choi, E.J.; Lee, S.H.; Woo, J.H.; Lim, S.; Lee, S.J. Complete genome sequence of citrus vein enation virus identified from a Korean Yuja Tree. Microbiol. Resour. Announc. 2022, 11, e00424-22. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.J.; Song, Z.; Cao, M.J.; Chen, H.M.; Li, Z.A.; Zhou, C.Y. The complete genome sequence of citrus vein enation virus from China. J. Integr. Agric. 2015, 14, 598–601. [Google Scholar] [CrossRef]
- Wu, J.X.; Liu, Q.Y.; Qiu, Y.J.; Zhang, S.; Li, Z.A.; Li, R.H.; Zhou, C.Y.; Cao, M.J. First report of citrus vein enation virus from citrus cultivar Huangguogan in Sichuan Province, China. Plant Dis. 2019, 103, 2701. [Google Scholar] [CrossRef]
- Rodriguez-Negrete, E.A.; Carrillo-Tripp, J.; Rivera-Bustamante, R.F. RNA silencing against geminivirus: Complementary action of posttranscriptional gene silencing and transcriptional gene silencing in host recovery. J. Virol. 2009, 83, 1332–1340. [Google Scholar] [CrossRef]
- Yang, X.L.; Xie, Y.; Raja, P.; Li, S.Z.; Wolf, J.N.; Shen, Q.T.; Bisaro, D.M.; Zhou, X.P. Suppression of methylation-mediated transcriptional gene silencing by beta C1-SAHH protein interaction during geminivirus-betasatellite infection. PLoS Pathog. 2011, 7, e1002329. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.Q.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Li, Y.Y.; Sun, Q.; Zhao, T.Y.; Xiang, H.Y.; Zhang, X.Y.; Wu, Z.Y.; Zhou, C.J.; Zhang, X.; Wang, Y.; Zhang, Y.L.; et al. Interaction between brassica yellows virus silencing suppressor P0 and plant SKP1 facilitates stability of P0 in vivo against degradation by proteasome and autophagy pathways. New Phytol. 2019, 222, 1458–1473. [Google Scholar] [CrossRef]
- Delfosse, V.C.; Barrios Baron, M.P.; Distefano, A.J. What we know about poleroviruses: Advances in understanding the functions of polerovirus proteins. Plant Pathol. 2021, 70, 1047–1061. [Google Scholar] [CrossRef]
- Fusaro, A.F.; Correa, R.L.; Nakasugi, K.; Jackson, C.; Kawchuk, L.; Vaslin, M.F.S.; Waterhouse, P.M. The enamovirus P0 protein is a silencing suppressor which inhibits local and systemic RNA silencing through AGO1 degradation. Virology 2012, 426, 178–187. [Google Scholar] [CrossRef]
- Chen, S.; Jiang, G.Z.; Wu, J.X.; Liu, Y.; Qian, Y.J.; Zhou, X.P. Characterization of a novel polerovirus infecting maize in China. Viruses 2016, 8, 120. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, C.; Zheng, Y.; Li, R.; Fei, Z.; Ling, K.S. Small RNA deep sequencing revealed that mixed infection of known and unknown viruses were common in field collected vegetable samples. Phytopathology 2015, 105, 106–107. [Google Scholar]
- Asiimwe, T.; Stewart, L.R.; Willie, K.; Massawe, D.P.; Kamatenesi, J.; Redinbaugh, M.G. Maize lethal necrosis viruses and other maize viruses in Rwanda. Plant Pathol. 2020, 69, 585–597. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Wang, Y.J.; Duan, Y.; Ma, Z.M.; Bin, Y.; Zhou, C.Y.; Song, Z. Construction of genome-length cDNA of citrus vein enation virus and identification of its infectivity. Sci. Agric. Sin. 2020, 53, 3707–3715. [Google Scholar]
- Sun, S.R.; Chen, J.S.; He, E.Q.; Huang, M.T.; Fu, H.Y.; Lu, J.J.; Gao, S.J. Genetic variability and molecular evolution of maize yellow mosaic virus populations from different geographic origins. Plant Dis. 2021, 105, 896–903. [Google Scholar] [CrossRef]
- Saleem, A.; Ali, Z.; Yeh, S.D.; Saeed, W.; Imdad, A.B.; Akbar, M.F.; Goodman, R.E.; Naseem, S. Genetic variability and evolutionary dynamics of atypical papaya ringspot virus infecting papaya. PLoS ONE 2021, 16, e0258298. [Google Scholar] [CrossRef]
- Yang, X.; Chen, B.; Zhang, T.; Li, Z.B.; Xu, C.H.; Zhou, G.H. Geographic distribution and genetic diversity of rice stripe mosaic virus in Southern China. Front. Microbiol. 2018, 9, 3068. [Google Scholar] [CrossRef]
- Lu, L.N.; Wu, S.L.; Jiang, J.; Liang, J.T.; Zhou, X.P.; Wu, J.X. Whole genome deep sequencing revealed host impact on population structure, variation and evolution of rice stripe virus. Virology 2018, 524, 32–44. [Google Scholar] [CrossRef]
- Duffy, S.; Shackelton, L.A.; Holmes, E.C. Rates of evolutionary change in viruses: Patterns and determinants. Nat. Rev. Genet. 2008, 9, 267–276. [Google Scholar] [CrossRef]
- Lefeuvre, P.; Martin, D.P.; Elena, S.F.; Shepherd, D.N.; Roumagnac, P.; Varsani, A. Evolution and ecology of plant viruses. Nat. Rev. Microbiol. 2019, 17, 632–644. [Google Scholar] [CrossRef]
- Ramos-Sobrinho, R.; Adegbola, R.O.; Lawrence, K.; Schrimsher, D.W.; Isakeit, T.; Alabi, O.J.; Brown, J.K. Cotton leafroll dwarf virus US Genomes comprise divergent subpopulations and harbor extensive variability. Viruses 2021, 13, 2230. [Google Scholar] [CrossRef] [PubMed]
- Sztuba-Solinska, J.; Urbanowicz, A.; Figlerowicz, M.; Bujarski, J.J. RNA-RNA recombination in plant virus replication and evolution. Annu. Rev. Phytopathol. 2011, 49, 415–443. [Google Scholar] [CrossRef] [PubMed]
- Kwak, H.R.; Lee, H.J.; Kim, E.A.; Seo, J.K.; Kim, C.S.; Lee, S.G.; Kim, J.S.; Choi, H.S.; Kim, M. Complete genome sequences and evolutionary analysis of cucurbit aphid-borne yellows virus isolates from melon in Korea. Plant Pathol. J. 2018, 34, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Dombrovsky, A.; Glanz, E.; Lachman, O.; Sela, N.; Doron-Faigenboim, A.; Antignus, Y. The Complete genomic sequence of pepper yellow leaf curl virus (PYLCV) and its implications for our understanding of evolution dynamics in the genus Polerovirus. PLoS ONE 2013, 8, e70722. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Li, H.; Schneider, W.L.; Sherman, D.J.; Gray, S.; Smith, D.; Roossinck, M.J. Analysis of genetic bottlenecks during horizontal transmission of cucumber mosaic virus. J. Virol. 2006, 80, 8345–8350. [Google Scholar] [CrossRef]
- Banani, S.F.; Lee, H.O.; Hyman, A.A.; Rosen, M.K. Biomolecular condensates: Organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 2017, 18, 285–298. [Google Scholar] [CrossRef]
- Brocca, S.; Grandori, R.; Longhi, S.; Uversky, V. Liquid-liquid phase separation by intrinsically disordered protein regions of viruses: Roles in viral life cycle and control of virus-host interactions. Int. J. Mol. Sci. 2020, 21, 9045. [Google Scholar] [CrossRef] [PubMed]
- Sagan, S.M.; Weber, S.C. Let’s phase it: Viruses are master architects of biomolecular condensates. Trends Biochem. Sci. 2022, in press. [Google Scholar] [CrossRef]
- Zhou, Y.; Su, J.M.; Samuel, C.E.; Ma, D. Measles virus forms inclusion bodies with properties of liquid organelles. J. Virol. 2019, 93, e00948-19. [Google Scholar] [CrossRef]
- Nikolic, J.; Le Bars, R.; Lama, Z.; Scrima, N.; Lagaudriere-Gesbert, C.; Gaudin, Y.; Blondel, D. Negri bodies are viral factories with properties of liquid organelles. Nat. Commun. 2017, 8, 58. [Google Scholar] [CrossRef]
- Fang, X.D.; Gao, Q.; Zang, Y.; Qiao, J.H.; Gao, D.M.; Xu, W.Y.; Wang, Y.; Li, D.; Wang, X.B. Host casein kinase 1-mediated phosphorylation modulates phase separation of a rhabdovirus phosphoprotein and virus infection. eLife 2022, 11, e74884. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.L.; Garrison, D.J.; May, J.P. Phase separation of a plant virus movement protein and cellular factors support virus-host interactions. PLoS Pathog. 2021, 17, e1009622. [Google Scholar] [CrossRef] [PubMed]
- Jack, A.; Ferro, L.S.; Trnka, M.J.; Wehri, E.; Nadgir, A.; Nguyenla, X.; Fox, D.; Costa, K.; Stanley, S.; Schaletzky, J.; et al. SARS-CoV-2 nucleocapsid protein forms condensates with viral genomic RNA. PLoS Biol. 2021, 19, e3001425. [Google Scholar] [CrossRef] [PubMed]
- Somasekharan, S.P.; Gleave, M. SARS-CoV-2 nucleocapsid protein interacts with immunoregulators and stress granules and phase separates to form liquid droplets. Febs Lett. 2021, 595, 2872–2896. [Google Scholar] [CrossRef] [PubMed]
- Ambroggio, E.E.; Costa Navarro, G.S.; Perez Socas, L.B.; Bagatolli, L.A.; Gamarnik, A.V. Dengue and Zika virus capsid proteins bind to membranes and self-assemble into liquid droplets with nucleic acids. J. Biol. Chem. 2021, 297, 101059. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Hallwass, M.; De Oliveira, A.S.; Dianese, E.D.; Lohuis, D.; Boiteux, L.S.; Inoue-Nagata, A.K.; Resende, R.O.; Kormelink, R. The tomato spotted wilt virus cell-to-cell movement protein (NSM) triggers a hypersensitive response in Sw-5-containing resistant tomato lines and in Nicotiana benthamiana transformed with the functional Sw-5b resistance gene copy. Mol. Plant Pathol. 2014, 15, 871–880. [Google Scholar] [CrossRef]
- Kim, B.M.; Suehiro, N.; Natsuaki, T.; Inukai, T.; Masuta, C. The P3 protein of turnip mosaic virus can alone induce hypersensitive response-like cell death in Arabidopsis thaliana carrying TuNI. Mol. Plant-Microbe Interact. 2010, 23, 144–152. [Google Scholar] [CrossRef]
- Wang, K.D.; Empleo, R.; Nguyen, T.T.V.; Moffett, P.; Sacco, M.A. Elicitation of hypersensitive responses in Nicotiana glutinosa by the suppressor of RNA silencing protein P0 from poleroviruses. Mol. Plant Pathol. 2015, 16, 435–448. [Google Scholar] [CrossRef]
- Bortolamiol, D.; Pazhouhandeh, M.; Marrocco, K.; Genschik, P.; Ziegler-Graff, V. The polerovirus F box protein P0 targets ARGONAUTE1 to suppress RNA silencing. Curr. Biol. 2007, 17, 1615–1621. [Google Scholar] [CrossRef]
- Hendelman, A.; Kravchik, M.; Stav, R.; Zik, M.; Lugassi, N.; Arazi, T. The developmental outcomes of P0-mediated ARGONAUTE destabilization in tomato. Planta 2013, 237, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Michaeli, S.; Clavel, M.; Lechner, E.; Viotti, C.; Wu, J.; Dubois, M.; Hacquard, T.; Derrien, B.; Izquierdo, E.; Lecorbeiller, M.; et al. The viral F-box protein P0 induces an ER-derived autophagy degradation pathway for the clearance of membrane-bound AGO1. Proc. Natl. Acad. Sci. USA 2019, 116, 22872–22883. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.F.; Wu, J.X.; Lu, L.N.; Xu, Y.; Zhou, X.P. Interaction between rice stripe virus disease-specific protein and host PsbP enhances virus symptoms. Mol. Plant 2014, 7, 691–708. [Google Scholar] [CrossRef]
- Sun, S.W.; Hu, Y.; Jiang, G.Z.; Tian, Y.M.; Ding, M.; Yu, C.; Zhou, X.P.; Qian, Y.J. Molecular characterization and genomic function of grapevine geminivirus A. Front. Microbiol. 2020, 11, 2121. [Google Scholar] [CrossRef] [PubMed]




| Genomic Region | Amino Acids (aa) | Nucleotide (nt) | Mutations | ||||
|---|---|---|---|---|---|---|---|
| Length (aa) | ID (%) | InDels | Length (nt) | ID (%) | Syn | Non | |
| ORF0(P0) | 354 | 99.25% | None | 1065 | 99.34% | 47 | 29 |
| ORF1(P1) | 902 | 98.86% | None | 2709 | 98.95% | 154 | 101 |
| ORF2(RdRP) | 1323 | 98.84% | None | 3972 | 98.46% | 190 | 187 |
| ORF3(CP) | 191 | 99.84% | None | 576 | 99.47% | 26 | 5 |
| ORF5 | 494 | 99.25% | None | 1485 | 99.01% | 100 | 33 |
| ORF | FEL | SLAC | FUBAR | dn/ds | |||
|---|---|---|---|---|---|---|---|
| PS | NS | PS | NS | PS | NS | ||
| 0 | 3 | 13 | 0 | 4 | 7 | 12 | 0.3770 |
| 1 | 5 | 72 | 1 | 33 | 12 | 69 | 0.2963 |
| 2 | 11 | 88 | 0 | 44 | 20 | 94 | 0.3289 |
| 3 | 0 | 14 | 0 | 2 | 0 | 12 | 0.0954 |
| 5 | 3 | 41 | 0 | 20 | 4 | 36 | 0.1760 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, R.; Huang, Q.; Hu, T.; Yu, F.; Hu, H.; Wang, Y.; Zhou, X.; Qian, Y. Molecular Variation and Genomic Function of Citrus Vein Enation Virus. Int. J. Mol. Sci. 2023, 24, 412. https://doi.org/10.3390/ijms24010412
Dou R, Huang Q, Hu T, Yu F, Hu H, Wang Y, Zhou X, Qian Y. Molecular Variation and Genomic Function of Citrus Vein Enation Virus. International Journal of Molecular Sciences. 2023; 24(1):412. https://doi.org/10.3390/ijms24010412
Chicago/Turabian StyleDou, Runqiu, Qingqing Huang, Tao Hu, Fengzhe Yu, Hongxia Hu, Yaqin Wang, Xueping Zhou, and Yajuan Qian. 2023. "Molecular Variation and Genomic Function of Citrus Vein Enation Virus" International Journal of Molecular Sciences 24, no. 1: 412. https://doi.org/10.3390/ijms24010412
APA StyleDou, R., Huang, Q., Hu, T., Yu, F., Hu, H., Wang, Y., Zhou, X., & Qian, Y. (2023). Molecular Variation and Genomic Function of Citrus Vein Enation Virus. International Journal of Molecular Sciences, 24(1), 412. https://doi.org/10.3390/ijms24010412






