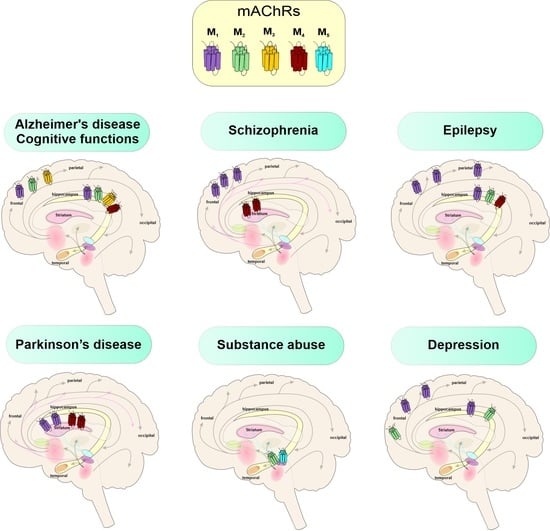
Modulation of Muscarinic Signalling in the Central Nervous System by Steroid Hormones and Neurosteroids
Abstract
1. Introduction
2. Neurosteroids and Neuroactive Steroids in CNS
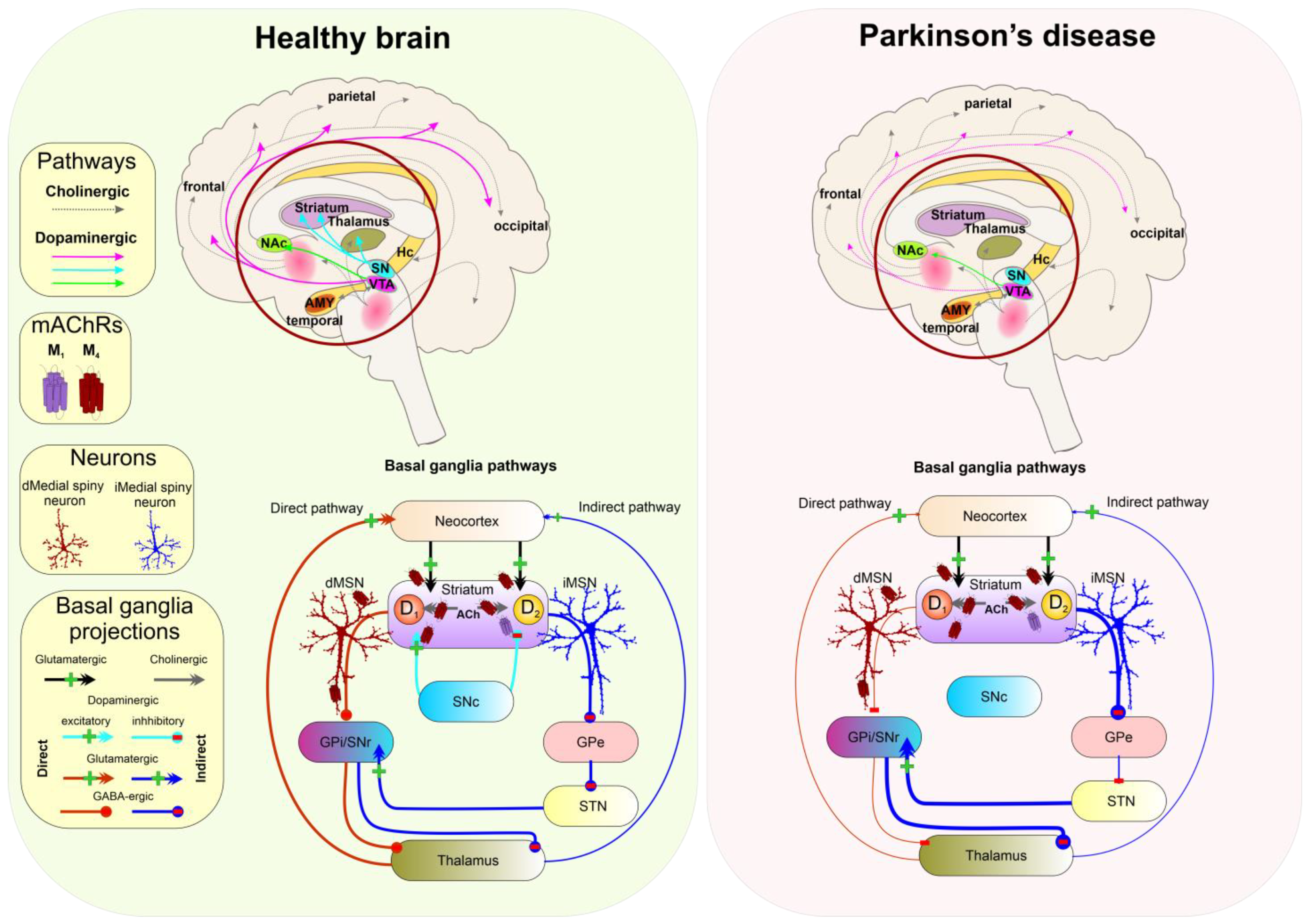
3. Muscarinic Receptors in CNS
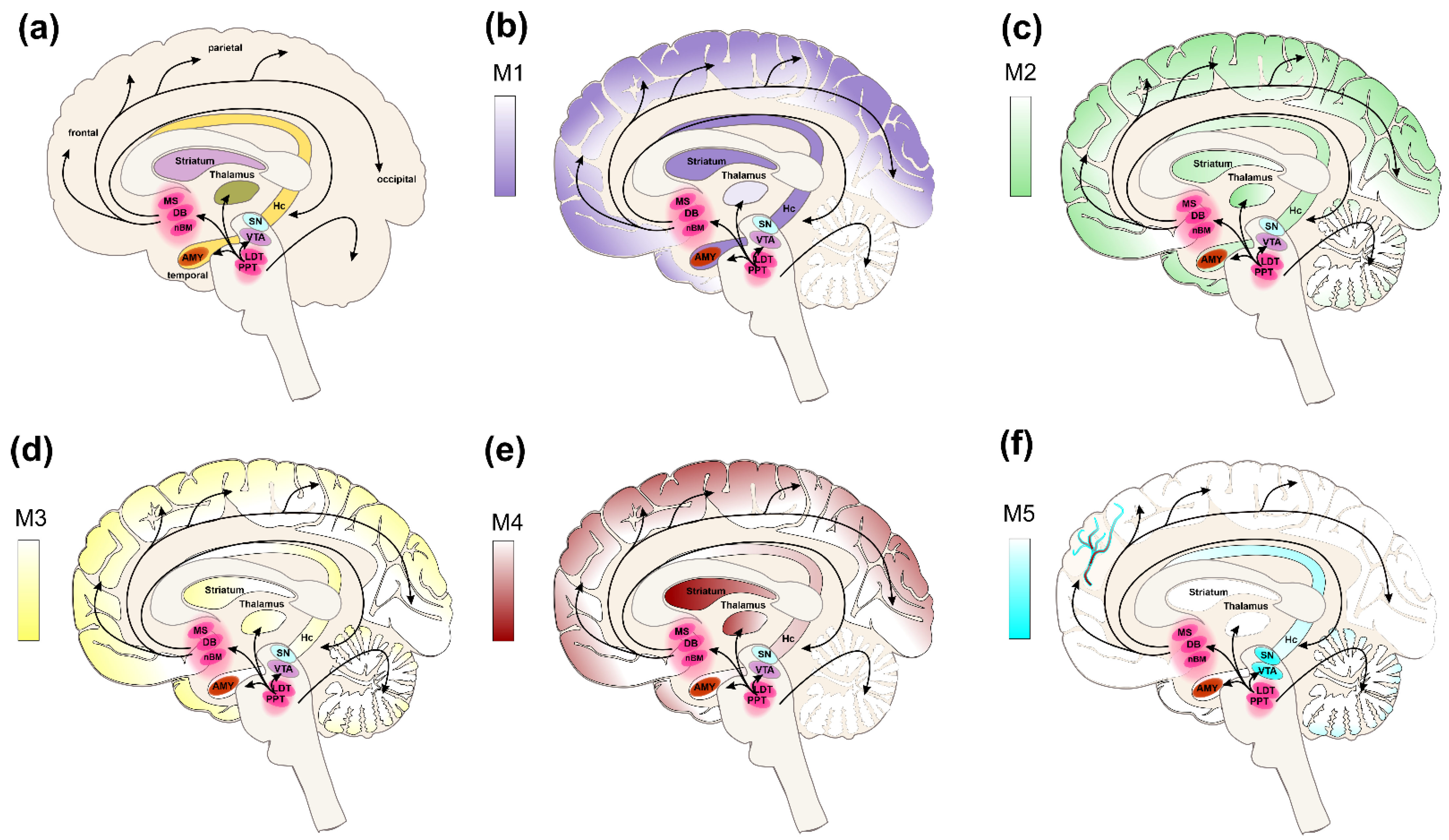
4. Genomic Effects of Steroids on mAChRs in CNS
5. Neurosteroids and Muscarinic Receptors in CNS
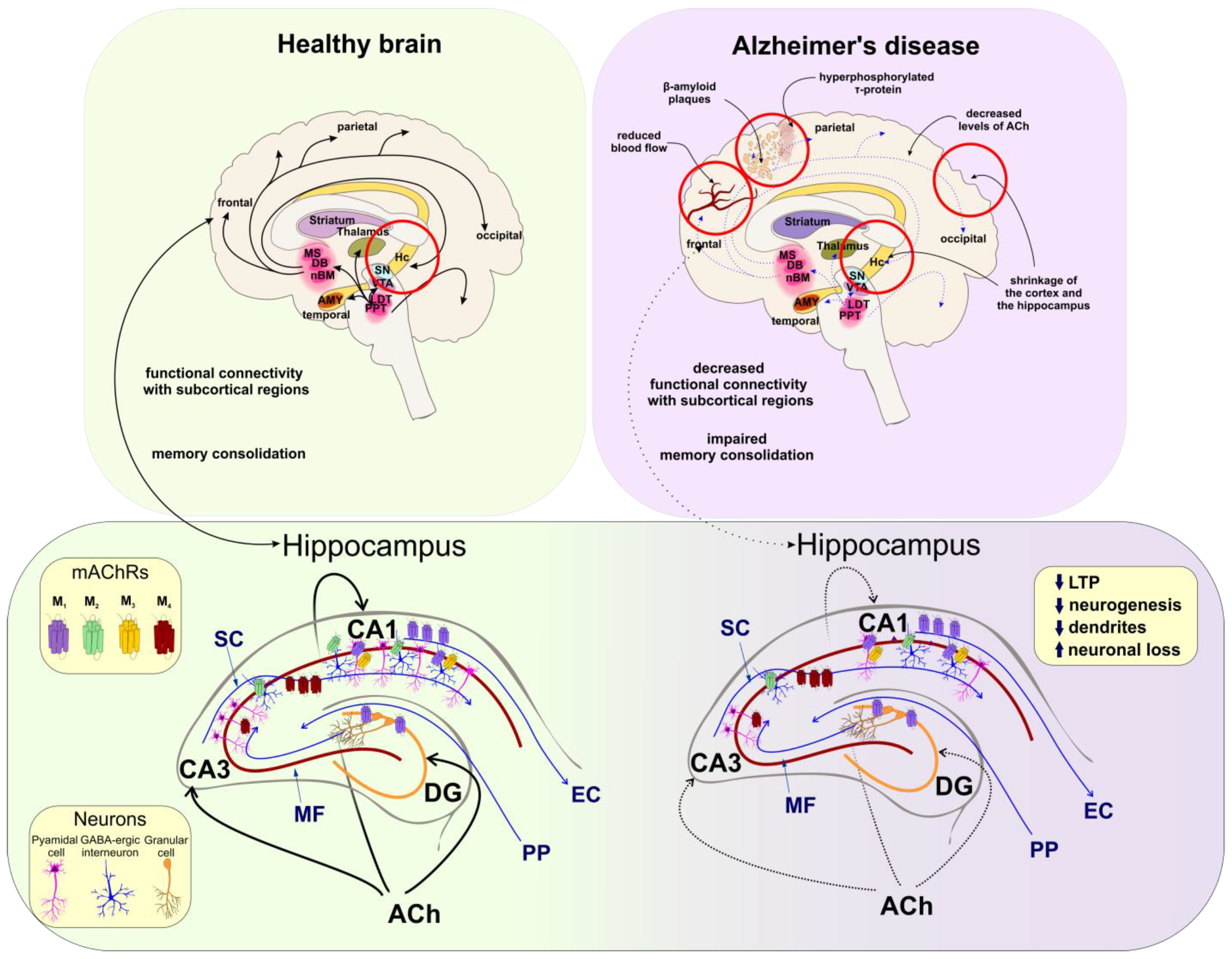
5.1. Cognitive Functions
5.2. Alzheimer’s Disease
| M1 | M2 | |
|---|---|---|
| 17-β estradiol (mechanism independent from mAChRs) ↑ ACh levels ↑ LTP ↑ Learning acquisition phase ↑ Memory consolidation | ↑ ACh potency ↓ Activation of GABA-ergic neurons (Hc) ↑ Excitability of pyramidal neurons (Hc) ↑ Activation of NMDARs ↑ LTP ↑ Learning acquisition phase ↑ Memory consolidation | |
| Progesterone | ↑ ACh efficacy ↑ LTP ↑ Learning acquisition phase ↑ Memory consolidation | ↓ ACh efficacy ↑ Activation of GABA-ergic neurons (Hc) ↓ Excitability of pyramidal neurons (Hc) ↓ Activation of NMDARs ↓ Learning acquisition phase |
| Corticosterone | ↑ ACh potency ↓ Activation of GABA-ergic neurons (Hc) ↑ Excitability of pyramidal neurons (Hc) ↑ Learning acquisition phase |
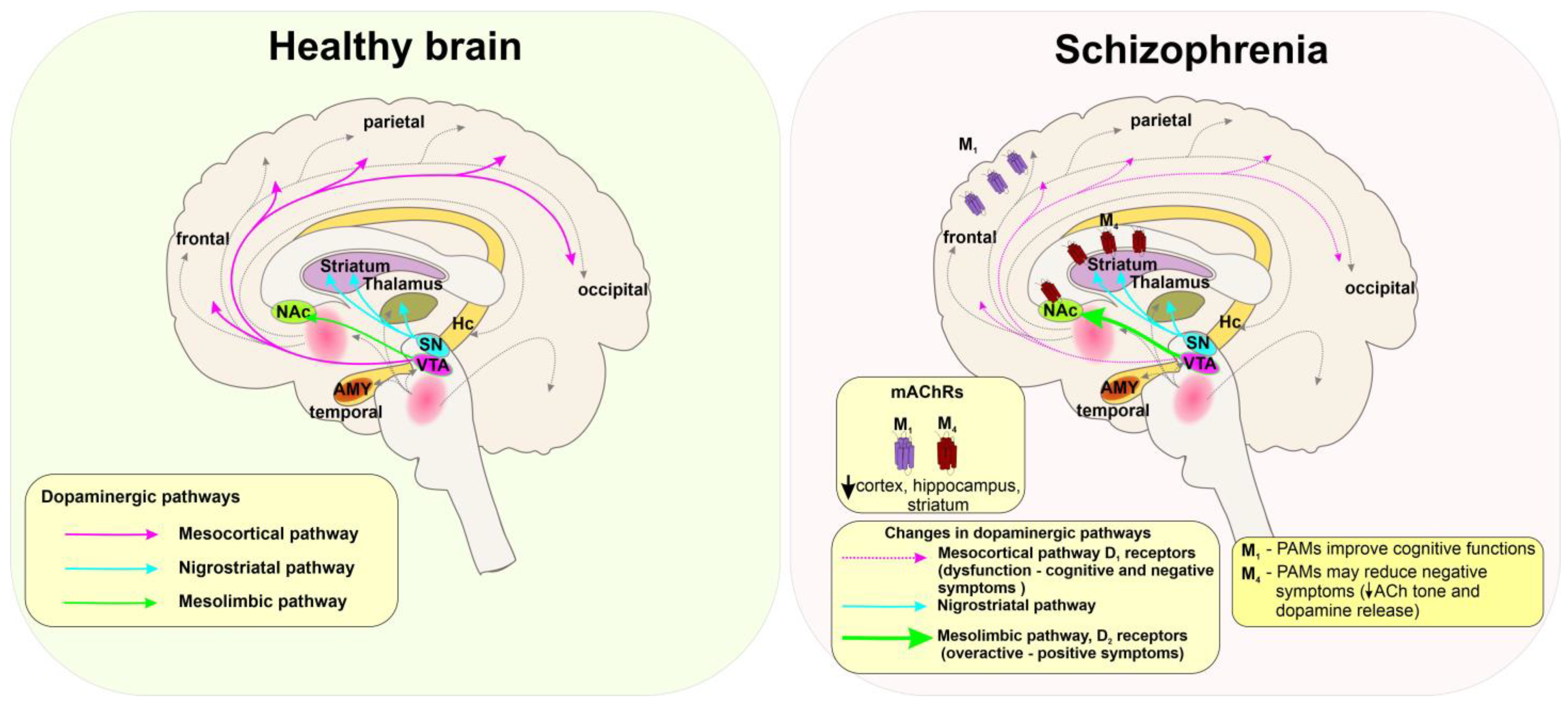
5.3. Schizophrenia
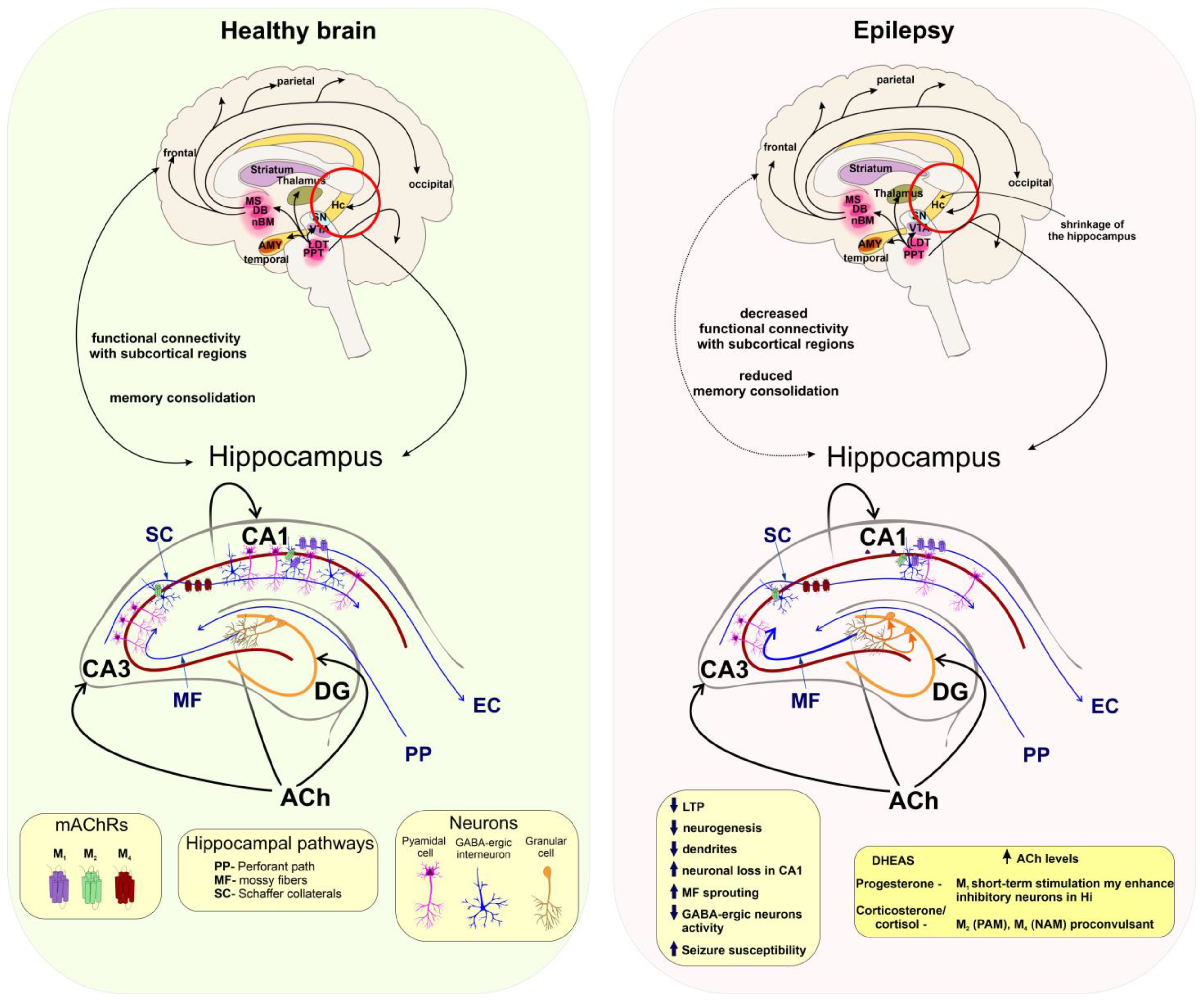
5.4. Seizures and Epilepsy
5.5. Parkinson’s Disease
5.6. Substance Abuse
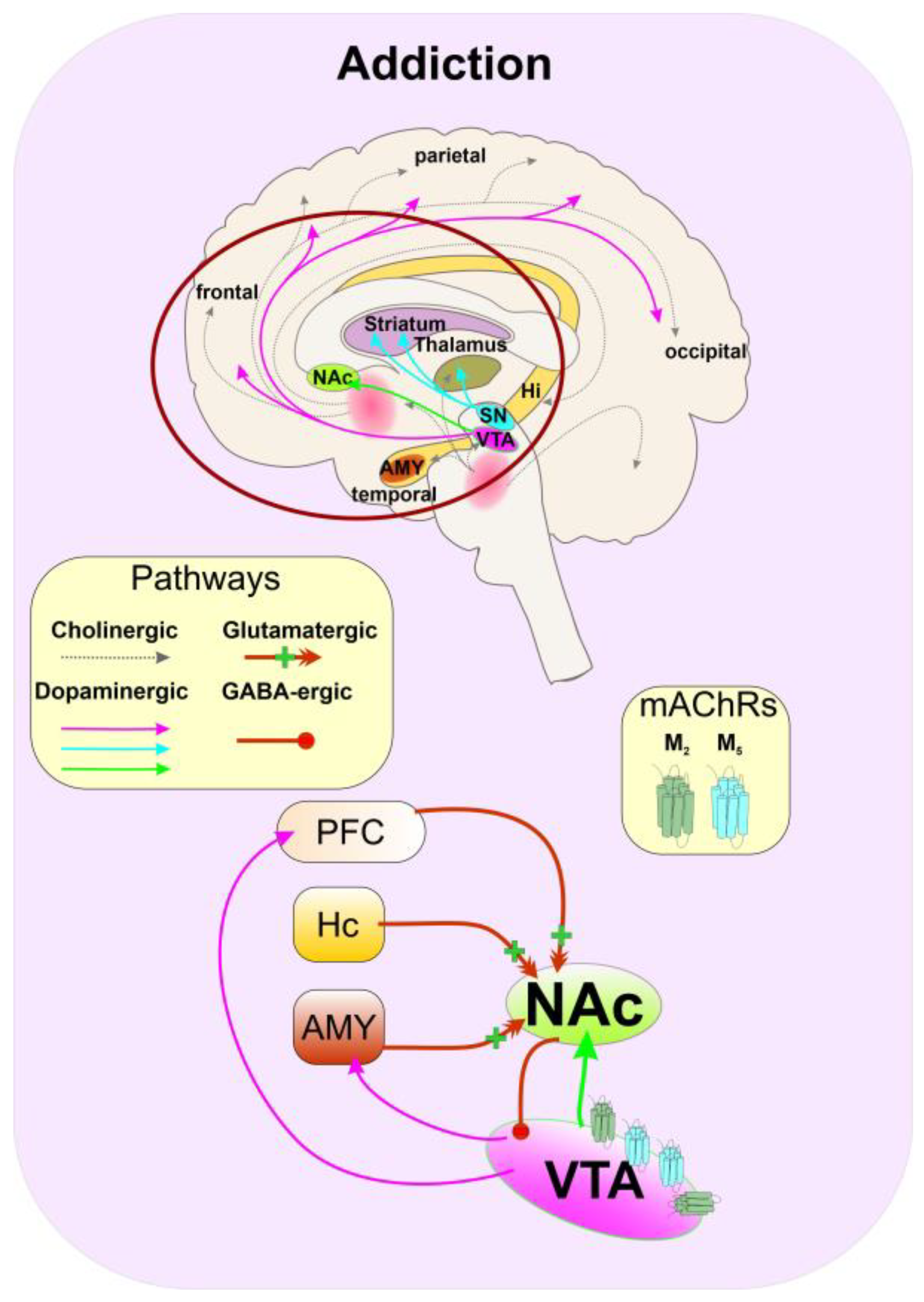
5.7. Depression
6. Conclusions
7. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lambert, J.J.; Cooper, M.A.; Simmons, R.D.J.; Weir, C.J.; Belelli, D. Neurosteroids: Endogenous Allosteric Modulators of GABA(A) Receptors. Psychoneuroendocrinology 2009, 34 (Suppl. S1), S48–S58. [Google Scholar] [CrossRef] [PubMed]
- Sedlácek, M.; Korínek, M.; Petrovic, M.; Cais, O.; Adamusová, E.; Chodounská, H.; Vyklický, L. Neurosteroid Modulation of Ionotropic Glutamate Receptors and Excitatory Synaptic Transmission. Physiol. Res. 2008, 57 (Suppl. S3), S49–S57. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.; Sørensen, G.; Dencker, D. Physiological Roles of CNS Muscarinic Receptors Gained from Knockout Mice. Neuropharmacology 2018, 136, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Haga, K.; Kruse, A.C.; Asada, H.; Yurugi-Kobayashi, T.; Shiroishi, M.; Zhang, C.; Weis, W.I.; Okada, T.; Kobilka, B.K.; Haga, T.; et al. Structure of the Human M2 Muscarinic Acetylcholine Receptor Bound to an Antagonist. Nature 2012, 482, 547–551. [Google Scholar] [CrossRef] [PubMed]
- Kruse, A.C.; Hu, J.; Pan, A.C.; Arlow, D.H.; Rosenbaum, D.M.; Rosemond, E.; Green, H.F.; Liu, T.; Chae, P.S.; Dror, R.O.; et al. Structure and Dynamics of the M3 Muscarinic Acetylcholine Receptor. Nature 2012, 482, 552–556. [Google Scholar] [CrossRef]
- Thal, D.M.; Sun, B.; Feng, D.; Nawaratne, V.; Leach, K.; Felder, C.C.; Bures, M.G.; Evans, D.A.; Weis, W.I.; Bachhawat, P.; et al. Crystal Structures of the M1 and M4 Muscarinic Acetylcholine Receptors. Nature 2016, 531, 335–340. [Google Scholar] [CrossRef]
- Vuckovic, Z.; Gentry, P.R.; Berizzi, A.E.; Hirata, K.; Varghese, S.; Thompson, G.; van der Westhuizen, E.T.; Burger, W.A.C.; Rahmani, R.; Valant, C.; et al. Crystal Structure of the M5 Muscarinic Acetylcholine Receptor. Proc. Natl. Acad. Sci. USA 2019, 116, 26001–26007. [Google Scholar] [CrossRef]
- Jakubík, J.; Randáková, A.; Chetverikov, N.; El-Fakahany, E.E.; Doležal, V. The Operational Model of Allosteric Modulation of Pharmacological Agonism. Sci. Rep. 2020, 10, 14421. [Google Scholar] [CrossRef]
- Wootten, D.; Christopoulos, A.; Sexton, P.M. Emerging Paradigms in GPCR Allostery: Implications for Drug Discovery. Nat. Rev. Drug Discov. 2013, 12, 630–644. [Google Scholar] [CrossRef]
- Jakubik, J.; El-Fakahany, E.E. Current Advances in Allosteric Modulation of Muscarinic Receptors. Biomolecules 2020, 10, 325. [Google Scholar] [CrossRef]
- Jakubík, J.; El-Fakahany, E.E. Allosteric Modulation of GPCRs of Class A by Cholesterol. Int. J. Mol. Sci. 2021, 22, 1953. [Google Scholar] [CrossRef]
- Szczurowska, E.; Szánti-Pintér, E.; Randáková, A.; Jakubík, J.; Kudova, E. Allosteric Modulation of Muscarinic Receptors by Cholesterol, Neurosteroids and Neuroactive Steroids. Int. J. Mol. Sci. 2022, 23, 13075. [Google Scholar] [CrossRef]
- Dolejší, E.; Szánti-Pintér, E.; Chetverikov, N.; Nelic, D.; Randáková, A.; Doležal, V.; Kudová, E.; Jakubík, J. Neurosteroids and Steroid Hormones Are Allosteric Modulators of Muscarinic Receptors. Neuropharmacology 2021, 199, 108798. [Google Scholar] [CrossRef]
- Dolejší, E.; Chetverikov, N.; Szánti-Pintér, E.; Nelic, D.; Randáková, A.; Doležal, V.; El-Fakahany, E.E.; Kudová, E.; Jakubík, J. Neuroactive Steroids, WIN-Compounds and Cholesterol Share a Common Binding Site on Muscarinic Acetylcholine Receptors. Biochem. Pharmacol. 2021, 192, 114699. [Google Scholar] [CrossRef]
- Reddy, D.S. Neurosteroids: Endogenous Role in the Human Brain and Therapeutic Potentials. Prog. Brain Res. 2010, 186, 113–137. [Google Scholar] [CrossRef]
- Baulieu, E.E.; Robel, P. Neurosteroids: A New Brain Function? J. Steroid Biochem. Mol. Biol. 1990, 37, 395–403. [Google Scholar] [CrossRef]
- Jo, D.H.; Abdallah, M.A.; Young, J.; Baulieu, E.E.; Robel, P. Pregnenolone, Dehydroepiandrosterone, and Their Sulfate and Fatty Acid Esters in the Rat Brain. Steroids 1989, 54, 287–297. [Google Scholar] [CrossRef]
- Levey, A.I.; Kitt, C.A.; Simonds, W.F.; Price, D.L.; Brann, M.R. Identification and Localization of Muscarinic Acetylcholine Receptor Proteins in Brain with Subtype-Specific Antibodies. J. Neurosci. 1991, 11, 3218–3226. [Google Scholar] [CrossRef]
- Abrams, P.; Andersson, K.-E.; Buccafusco, J.J.; Chapple, C.; de Groat, W.C.; Fryer, A.D.; Kay, G.; Laties, A.; Nathanson, N.M.; Pasricha, P.J.; et al. Muscarinic Receptors: Their Distribution and Function in Body Systems, and the Implications for Treating Overactive Bladder. Br. J. Pharmacol. 2006, 148, 565–578. [Google Scholar] [CrossRef]
- Bubser, M.; Byun, N.; Wood, M.R.; Jones, C.K. Muscarinic Receptor Pharmacology and Circuitry for the Modulation of Cognition. Handb. Exp. Pharmacol. 2012, 208, 121–166. [Google Scholar] [CrossRef]
- Crook, J.M.; Tomaskovic-Crook, E.; Copolov, D.L.; Dean, B. Low Muscarinic Receptor Binding in Prefrontal Cortex from Subjects with Schizophrenia: A Study of Brodmann’s Areas 8, 9, 10, and 46 and the Effects of Neuroleptic Drug Treatment. Am. J. Psychiatry 2001, 158, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Tsang, S.W.Y.; Lai, M.K.P.; Kirvell, S.; Francis, P.T.; Esiri, M.M.; Hope, T.; Chen, C.P.L.-H.; Wong, P.T.-H. Impaired Coupling of Muscarinic M1 Receptors to G-Proteins in the Neocortex Is Associated with Severity of Dementia in Alzheimer’s Disease. Neurobiol Aging 2006, 27, 1216–1223. [Google Scholar] [CrossRef] [PubMed]
- Potter, P.E.; Rauschkolb, P.K.; Pandya, Y.; Sue, L.I.; Sabbagh, M.N.; Walker, D.G.; Beach, T.G. Pre- and Post-Synaptic Cortical Cholinergic Deficits Are Proportional to Amyloid Plaque Presence and Density at Preclinical Stages of Alzheimer’s Disease. Acta Neuropathol. 2011, 122, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Scarr, E. Muscarinic Receptors: Their Roles in Disorders of the Central Nervous System and Potential as Therapeutic Targets. CNS Neurosci. Ther. 2012, 18, 369–379. [Google Scholar] [CrossRef] [PubMed]
- Moran, S.P.; Maksymetz, J.; Conn, P.J. Targeting Muscarinic Acetylcholine Receptors for the Treatment of Psychiatric and Neurological Disorders. Trends Pharmacol. Sci. 2019, 40, 1006–1020. [Google Scholar] [CrossRef]
- Westfall, T.C. Cholinergic Neurotransmission in the Autonomic and Somatic Motor Nervous System. In Encyclopedia of Neuroscience; Squire, L.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 827–834. ISBN 9780080450469. [Google Scholar]
- Saygı Bacanak, M.; Aydın, B.; Cabadak, H.; Nurten, A.; Gören, M.Z.; Enginar, N. Contribution of M1 and M2 Muscarinic Receptor Subtypes to Convulsions in Fasted Mice Treated with Scopolamine and given Food. Behav. Brain Res. 2019, 364, 423–430. [Google Scholar] [CrossRef]
- Reddy, D.S. Role of Hormones and Neurosteroids in Epileptogenesis. Front. Cell Neurosci. 2013, 7, 115. [Google Scholar] [CrossRef]
- Tata, A.M. Muscarinic Acetylcholine Receptors: New Potential Therapeutic Targets in Antinociception and in Cancer Therapy. Recent Pat. CNS Drug. Discov. 2008, 3, 94–103. [Google Scholar] [CrossRef]
- Comings, D.E.; Wu, S.; Rostamkhani, M.; McGue, M.; Iacono, W.G.; MacMurray, J.P. Association of the Muscarinic Cholinergic 2 Receptor (CHRM2) Gene with Major Depression in Women. Am. J. Med. Genet. 2002, 114, 527–529. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Agnati, L.F.; Fuxe, K.; Ciruela, F. Muscarinic Acetylcholine Receptor-Interacting Proteins (MAChRIPs): Targeting the Receptorsome. Curr. Drug Targets 2012, 13, 53–71. [Google Scholar] [CrossRef]
- Pancani, T.; Bolarinwa, C.; Smith, Y.; Lindsley, C.W.; Conn, P.J.; Xiang, Z. M4 MAChR-Mediated Modulation of Glutamatergic Transmission at Corticostriatal Synapses. ACS Chem. Neurosci. 2014, 5, 318–324. [Google Scholar] [CrossRef]
- Fiorino, D.F.; Garcia-Guzman, M. Muscarinic Pain Pharmacology: Realizing the Promise of Novel Analgesics by Overcoming Old Challenges. Handb. Exp. Pharmacol. 2012, 208, 191–221. [Google Scholar] [CrossRef]
- Moehle, M.S.; Conn, P.J. Roles of the M4 Acetylcholine Receptor in the Basal Ganglia and the Treatment of Movement Disorders. Mov. Disord. 2019, 34, 1089–1099. [Google Scholar] [CrossRef]
- Riljak, V.; Janisova, K.; Myslivecek, J. Lack of M4 Muscarinic Receptors in the Striatum, Thalamus and Intergeniculate Leaflet Alters the Biological Rhythm of Locomotor Activity in Mice. Brain Struct. Funct. 2020, 225, 1615–1629. [Google Scholar] [CrossRef]
- Dean, B.; Scarr, E. Muscarinic M1 and M4 Receptors: Hypothesis Driven Drug Development for Schizophrenia. Psychiatry Res. 2020, 288, 112989. [Google Scholar] [CrossRef]
- Crook, J.M.; Tomaskovic-Crook, E.; Copolov, D.L.; Dean, B. Decreased Muscarinic Receptor Binding in Subjects with Schizophrenia: A Study of the Human Hippocampal Formation. Biol. Psychiatry 2000, 48, 381–388. [Google Scholar] [CrossRef]
- Raedler, T.J.; Bymaster, F.P.; Tandon, R.; Copolov, D.; Dean, B. Towards a Muscarinic Hypothesis of Schizophrenia. Mol. Psychiatry 2007, 12, 232–246. [Google Scholar] [CrossRef]
- Tozzi, A.; de Iure, A.; Tantucci, M.; Durante, V.; Quiroga-Varela, A.; Giampà, C.; Di Mauro, M.; Mazzocchetti, P.; Costa, C.; Di Filippo, M.; et al. Endogenous 17β-Estradiol Is Required for Activity-Dependent Long-Term Potentiation in the Striatum: Interaction with the Dopaminergic System. Front. Cell Neurosci. 2015, 9, 1–14. [Google Scholar] [CrossRef]
- De Angelis, F.; Tata, A.M. Analgesic Effects Mediated by Muscarinic Receptors: Mechanisms and Pharmacological Approaches. Cent. Nerv. Syst. Agents Med. Chem. 2016, 16, 218–226. [Google Scholar] [CrossRef]
- Piggott, M.; Owens, J.; O’Brien, J.; Paling, S.; Wyper, D.; Fenwick, J.; Johnson, M.; Perry, R.; Perry, E. Comparative Distribution of Binding of the Muscarinic Receptor Ligands Pirenzepine, AF-DX 384, (R,R)-I-QNB and (R,S)-I-QNB to Human Brain. J. Chem. Neuroanat. 2002, 24, 211–223. [Google Scholar] [CrossRef]
- Yamada, M.; Lamping, K.G.; Duttaroy, A.; Zhang, W.; Cui, Y.; Bymaster, F.P.; McKinzie, D.L.; Felder, C.C.; Deng, C.X.; Faraci, F.M.; et al. Cholinergic Dilation of Cerebral Blood Vessels Is Abolished in M5 Muscarinic Acetylcholine Receptor Knockout Mice. Proc. Natl. Acad. Sci. USA 2001, 98, 14096–14101. [Google Scholar] [CrossRef] [PubMed]
- Bender, A.M.; Garrison, A.T.; Lindsley, C.W. The Muscarinic Acetylcholine Receptor M5: Therapeutic Implications and Allosteric Modulation. ACS Chem. Neurosci. 2019, 10, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Gould, R.W.; Gunter, B.W.; Bubser, M.; Matthews, R.T.; Teal, L.B.; Ragland, M.G.; Bridges, T.M.; Garrison, A.T.; Winder, D.G.; Lindsley, C.W.; et al. Acute Negative Allosteric Modulation of M5 Muscarinic Acetylcholine Receptors Inhibits Oxycodone Self-Administration and Cue-Induced Reactivity with No Effect on Antinociception. ACS Chem. Neurosci. 2019, 10, 3740–3750. [Google Scholar] [CrossRef] [PubMed]
- Ballinger, E.C.; Ananth, M.; Talmage, D.A.; Role, L.W. Basal Forebrain Cholinergic Circuits and Signaling in Cognition and Cognitive Decline. Neuron 2016, 91, 1199–1218. [Google Scholar] [CrossRef] [PubMed]
- Bacanak, M.S. Muscarinic M1 and M2 Receptors, Fasting and Seizure Development in Animals. Clin. Exp. Health Sci. 2018, 8, 308–313. [Google Scholar]
- Zhang, W.; Yamada, M.; Gomeza, J.; Basile, A.S.; Wess, J. Multiple Muscarinic Acetylcholine Receptor Subtypes Modulate Striatal Dopamine Release, as Studied with M1-M5 Muscarinic Receptor Knock-out Mice. J. Neurosci. 2002, 22, 6347–6352. [Google Scholar] [CrossRef]
- Lösel, R.; Wehling, M. Nongenomic Actions of Steroid Hormones. Nat. Rev. Mol. Cell Biol. 2003, 4, 46–56. [Google Scholar] [CrossRef]
- Bixo, M.; Andersson, A.; Winblad, B.; Purdy, R.H.; Bäckström, T. Progesterone, 5α-Pregnane-3,20-Dione and 3α-Hydroxy-5α-Pregnane-20-One in Specific Regions of the Human Female Brain in Different Endocrine States. Brain Res. 1997, 764, 173–178. [Google Scholar] [CrossRef]
- Janowsky, J.S. The Role of Ovarian Hormones in Preserving Cognition in Aging. Curr. Psychiatry Rep. 2002, 4, 467–473. [Google Scholar] [CrossRef]
- Gibbs, R.B. Estrogen Therapy and Cognition: A Review of the Cholinergic Hypothesis. Endocr. Rev. 2010, 31, 224–253. [Google Scholar] [CrossRef]
- Guennoun, R. Progesterone in the Brain: Hormone, Neurosteroid and Neuroprotectant. Int. J. Mol. Sci. 2020, 21, 5271. [Google Scholar] [CrossRef]
- Caruso, D.; Pesaresi, M.; Abbiati, F.; Calabrese, D.; Giatti, S.; Garcia-Segura, L.M.; Melcangi, R.C. Comparison of Plasma and Cerebrospinal Fluid Levels of Neuroactive Steroids with Their Brain, Spinal Cord and Peripheral Nerve Levels in Male and Female Rats. Psychoneuroendocrinology 2013, 38, 2278–2290. [Google Scholar] [CrossRef]
- Nordberg, A.; Alafuzoff, I.; Winblad, B. Nicotinic and Muscarinic Subtypes in the Human Brain: Changes with Aging and Dementia. J. Neurosci. Res. 1992, 31, 103–111. [Google Scholar] [CrossRef]
- Davies, P.; Verth, A.H. Regional Distribution of Muscarinic Acetylcholine Receptor in Normal and Alzheimer’s-Type Dementia Brains. Brain Res. 1977, 138, 385–392. [Google Scholar] [CrossRef]
- Lee, K.S.; Frey, K.A.; Koeppe, R.A.; Buck, A.; Mulholland, G.K.; Kuhl, D.E. In Vivo Quantification of Cerebral Muscarinic Receptors in Normal Human Aging Using Positron Emission Tomography and [11C]Tropanyl Benzilate. J. Cereb. Blood Flow Metab. 1996, 16, 303–310. [Google Scholar] [CrossRef]
- Zubieta, J.K.; Koeppe, R.A.; Frey, K.A.; Kilbourn, M.R.; Mangner, T.J.; Foster, N.L.; Kuhl, D.E. Assessment of Muscarinic Receptor Concentrations in Aging and Alzheimer Disease with [11C]NMPB and PET. Synapse 2001, 39, 275–287. [Google Scholar] [CrossRef]
- Dewey, S.L.; Volkow, N.D.; Logan, J.; MacGregor, R.R.; Fowler, J.S.; Schlyer, D.J.; Bendriem, B. Age-Related Decreases in Muscarinic Cholinergic Receptor Binding in the Human Brain Measured with Positron Emission Tomography (PET). J. Neurosci. Res. 1990, 27, 569–575. [Google Scholar] [CrossRef]
- Suhara, T.; Inoue, O.; Kobayashi, K.; Suzuki, K.; Tateno, Y. Age-Related Changes in Human Muscarinic Acetylcholine Receptors Measured by Positron Emission Tomography. Neurosci. Lett. 1993, 149, 225–228. [Google Scholar] [CrossRef]
- Podruchny, T.A.; Connolly, C.; Bokde, A.; Herscovitch, P.; Eckelman, W.C.; Kiesewetter, D.O.; Sunderland, T.; Carson, R.E.; Cohen, R.M. In Vivo Muscarinic 2 Receptor Imaging in Cognitively Normal Young and Older Volunteers. Synapse 2003, 48, 39–44. [Google Scholar] [CrossRef]
- Norbury, R.; Travis, M.J.; Erlandsson, K.; Waddington, W.; Owens, J.; Pimlott, S.; Ell, P.J.; Murphy, D.G.M. In Vivo Imaging of Muscarinic Receptors in the Aging Female Brain with (R,R)[123I]-I-QNB and Single Photon Emission Tomography. Exp. Gerontol. 2005, 40, 137–145. [Google Scholar] [CrossRef]
- Gibbs, R.B. Estrogen Replacement Enhances Acquisition of a Spatial Memory Task and Reduces Deficits Associated with Hippocampal Muscarinic Receptor Inhibition. Horm. Behav. 1999, 36, 222–233. [Google Scholar] [CrossRef] [PubMed]
- El-Bakri, N.K.; Adem, A.; Suliman, I.A.; Mulugeta, E.; Karlsson, E.; Lindgren, J.U.; Winblad, B.; Islam, A. Estrogen and Progesterone Treatment: Effects on Muscarinic M(4) Receptor Subtype in the Rat Brain. Brain Res. 2002, 948, 131–137. [Google Scholar] [CrossRef] [PubMed]
- van Huizen, F.; March, D.; Cynader, M.S.; Shaw, C. Muscarinic Receptor Characteristics and Regulation in Rat Cerebral Cortex: Changes during Development, Aging and the Oestrous Cycle. Eur. J. Neurosci. 1994, 6, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Vaucher, E.; Reymond, I.; Najaffe, R.; Kar, S.; Quirion, R.; Miller, M.M.; Franklin, K.B.J. Estrogen Effects on Object Memory and Cholinergic Receptors in Young and Old Female Mice. Neurobiol. Aging 2002, 23, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Bartholomeusz, C.F.; Wesnes, K.A.; Kulkarni, J.; Vitetta, L.; Croft, R.J.; Nathan, P.J. Estradiol Treatment and Its Interaction with the Cholinergic System: Effects on Cognitive Function in Healthy Young Women. Horm. Behav. 2008, 54, 684–693. [Google Scholar] [CrossRef]
- Rainbow, T.C.; Degroff, V.; Luine, V.N.; McEwen, B.S. Estradiol 17β Increases the Number of Muscarinic Receptors in Hypothalamic Nuclei. Brain Res. 1980, 198, 239–243. [Google Scholar] [CrossRef]
- Dohanich, G.P.; Witcher, J.A.; Weaver, D.R.; Clemens, L.G. Alteration of Muscarinic Binding in Specific Brain Areas Following Estrogen Treatment. Brain Res. 1982, 241, 347–350. [Google Scholar] [CrossRef]
- Cardoso, C.C.; Pereira, R.T.S.; Koyama, C.A.; Porto, C.S.; Abdalla, F.M.F. Effects of Estrogen on Muscarinic Acetylcholine Receptors in the Rat Hippocampus. Neuroendocrinology 2004, 80, 379–386. [Google Scholar] [CrossRef]
- Cardoso, C.C.; Ricardo, V.P.; Frussa-Filho, R.; Porto, C.S.; Abdalla, F.M.F. Effects of 17ß-Estradiol on Expression of Muscarinic Acetylcholine Receptor Subtypes and Estrogen Receptor Alpha in Rat Hippocampus. Eur. J. Pharmacol. 2010, 634, 192–200. [Google Scholar] [CrossRef]
- dos Santos Pereira, R.T.; Porto, C.S.; Godinho, R.O.; Abdalla, F.M.F. Effects of Estrogen on Intracellular Signaling Pathways Linked to Activation of Muscarinic Acetylcholine Receptors and on Acetylcholinesterase Activity in Rat Hippocampus. Biochem. Pharmacol. 2008, 75, 1827–1834. [Google Scholar] [CrossRef]
- Ch’ng, S.S.; Walker, A.J.; McCarthy, M.; Le, T.-K.; Thomas, N.; Gibbons, A.; Udawela, M.; Kusljic, S.; Dean, B.; Gogos, A. The Impact of Removal of Ovarian Hormones on Cholinergic Muscarinic Receptors: Examining Prepulse Inhibition and Receptor Binding. Brain Sci. 2020, 10, 106. [Google Scholar] [CrossRef]
- Aguirre, C.; Jayaraman, A.; Pike, C.; Baudry, M. Progesterone Inhibits Estrogen-Mediated Neuroprotection against Excitotoxicity by down-Regulating Estrogen Receptor-β. J. Neurochem. 2010, 115, 1277–1287. [Google Scholar] [CrossRef]
- Norbury, R.; Travis, M.J.; Erlandsson, K.; Waddington, W.; Ell, P.J.; Murphy, D.G.M. Estrogen Therapy and Brain Muscarinic Receptor Density in Healthy Females: A SPET Study. Horm. Behav. 2007, 51, 249–257. [Google Scholar] [CrossRef]
- Hösli, E.; Hösli, L. Cellular Localization of Estrogen Receptors on Neurones in Various Regions of Cultured Rat CNS: Coexistence with Cholinergic and Galanin Receptors. Int. J. Dev. Neurosci. 1999, 17, 317–330. [Google Scholar] [CrossRef]
- Hammond, R.; Nelson, D.; Gibbs, R.B. GPR30 Co-Localizes with Cholinergic Neurons in the Basal Forebrain and Enhances Potassium-Stimulated Acetylcholine Release in the Hippocampus. Psychoneuroendocrinology 2011, 36, 182–192. [Google Scholar] [CrossRef]
- Toran-Allerand, C.D.; Miranda, R.C.; Bentham, W.D.; Sohrabji, F.; Brown, T.J.; Hochberg, R.B.; MacLusky, N.J. Estrogen Receptors Colocalize with Low-Affinity Nerve Growth Factor Receptors in Cholinergic Neurons of the Basal Forebrain. Proc. Natl. Acad. Sci. USA 1992, 89, 4668–4672. [Google Scholar] [CrossRef]
- Russell, J.K.; Jones, C.K.; Newhouse, P.A. The Role of Estrogen in Brain and Cognitive Aging. Neurotherapeutics 2019, 16, 649–665. [Google Scholar] [CrossRef]
- Bredfeldt, T.G.; Greathouse, K.L.; Safe, S.H.; Hung, M.-C.; Bedford, M.T.; Walker, C.L. Xenoestrogen-Induced Regulation of EZH2 and Histone Methylation via Estrogen Receptor Signaling to PI3K/AKT. Mol. Endocrinol. 2010, 24, 993–1006. [Google Scholar] [CrossRef]
- Wilkenfeld, S.R.; Lin, C.; Frigo, D.E. Communication between Genomic and Non-Genomic Signaling Events Coordinate Steroid Hormone Actions. Steroids 2018, 133, 2–7. [Google Scholar] [CrossRef]
- Finkelstein, Y.; Koffler, B.; Rabey, J.M.; Gilad, G.M. Dynamics of Cholinergic Synaptic Mechanisms in Rat Hippocampus after Stress. Brain Res. 1985, 343, 314–319. [Google Scholar] [CrossRef]
- Myslivecek, J.; Kvetnanský, R. The Effects of Stress on Muscarinic Receptors. Heterologous Receptor Regulation: Yes or No? Auton. Autacoid Pharmacol. 2006, 26, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Sarter, M.; Hasselmo, M.E.; Bruno, J.P.; Givens, B. Unraveling the Attentional Functions of Cortical Cholinergic Inputs: Interactions between Signal-Driven and Cognitive Modulation of Signal Detection. Brain Res. Rev. 2005, 48, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Solari, N.; Hangya, B. Cholinergic Modulation of Spatial Learning, Memory and Navigation. Eur. J. Neurosci. 2018, 48, 2199–2230. [Google Scholar] [CrossRef] [PubMed]
- Blokland, A.; Sambeth, A.; Prickaerts, J.; Riedel, W.J. Why an M1 Antagonist Could Be a More Selective Model for Memory Impairment than Scopolamine. Front. Neurol. 2016, 7, 167. [Google Scholar] [CrossRef] [PubMed]
- Chintoh, A.; Fulton, J.; Koziel, N.; Aziz, M.; Sud, M.; Yeomans, J.S. Role of Cholinergic Receptors in Locomotion Induced by Scopolamine and Oxotremorine-M. Pharmacol. Biochem. Behav. 2003, 76, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Bartolomeo, A.C.; Morris, H.; Buccafusco, J.J.; Kille, N.; Rosenzweig-Lipson, S.; Husbands, M.G.; Sabb, A.L.; Abou-Gharbia, M.; Moyer, J.A.; Boast, C.A. The Preclinical Pharmacological Profile of WAY-132983, a Potent M1 Preferring Agonist. J. Pharmacol. Exp. Ther. 2000, 292, 584–596. [Google Scholar]
- Bakker, C.; Tasker, T.; Liptrot, J.; Hart, E.P.; Klaassen, E.S.; Doll, R.J.; Brown, G.A.; Brown, A.; Congreve, M.; Weir, M.; et al. Safety, Pharmacokinetics and Exploratory pro-Cognitive Effects of HTL0018318, a Selective M1 Receptor Agonist, in Healthy Younger Adult and Elderly Subjects: A Multiple Ascending Dose Study. Alzheimers. Res. Ther. 2021, 13, 87. [Google Scholar] [CrossRef]
- Long, N.M.; Kuhl, B.A.; Chun, M.M. Memory and Attention. In Stevens’ Handbook of Experimental Psychology and Cognitive Neuroscience; Wiley: New York, NY, USA, 2018; pp. 1–37. [Google Scholar]
- Chun, M.M.; Johnson, M.K. Memory: Enduring Traces of Perceptual and Reflective Attention. Neuron 2011, 72, 520–535. [Google Scholar] [CrossRef]
- Bichot, N.P.; Heard, M.T.; DeGennaro, E.M.; Desimone, R. A Source for Feature-Based Attention in the Prefrontal Cortex. Neuron 2015, 88, 832–844. [Google Scholar] [CrossRef]
- Thiele, A.; Bellgrove, M.A. Neuromodulation of Attention. Neuron 2018, 97, 769–785. [Google Scholar] [CrossRef]
- Levey, A.I. Muscarinic Acetylcholine Receptor Expression in Memory Circuits: Implications for Treatment of Alzheimer Disease. Proc. Natl. Acad. Sci. USA 1996, 93, 13541–13546. [Google Scholar] [CrossRef]
- Volpicelli, L.A.; Levey, A.I. Muscarinic Acetylcholine Receptor Subtypes in Cerebral Cortex and Hippocampus. Prog. Brain Res. 2004, 145, 59–66. [Google Scholar] [CrossRef]
- Eglen, R.M. Overview of Muscarinic Receptor Subtypes. In Handb Exp Pharmacol; Fryer, A.D., Arthur, C., Nathanson, N.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 3–28. ISBN 0171-2004. [Google Scholar]
- Jiang, S.; Li, Y.; Zhang, C.; Zhao, Y.; Bu, G.; Xu, H.; Zhang, Y.-W. M1 Muscarinic Acetylcholine Receptor in Alzheimer’s Disease. Neurosci. Bull. 2014, 30, 295–307. [Google Scholar] [CrossRef]
- Buchanan, K.A.; Petrovic, M.M.; Chamberlain, S.E.L.; Marrion, N.V.; Mellor, J.R. Facilitation of Long-Term Potentiation by Muscarinic M(1) Receptors Is Mediated by Inhibition of SK Channels. Neuron 2010, 68, 948–963. [Google Scholar] [CrossRef]
- Kim, W.B.; Cho, J.-H. Encoding of Contextual Fear Memory in Hippocampal-Amygdala Circuit. Nat. Commun. 2020, 11, 1382. [Google Scholar] [CrossRef]
- Poulin, B.; Butcher, A.; McWilliams, P.; Bourgognon, J.-M.; Pawlak, R.; Kong, K.C.; Bottrill, A.; Mistry, S.; Wess, J.; Rosethorne, E.M.; et al. The M3-Muscarinic Receptor Regulates Learning and Memory in a Receptor Phosphorylation/Arrestin-Dependent Manner. Proc. Natl. Acad. Sci. USA 2010, 107, 9440–9445. [Google Scholar] [CrossRef]
- Ratner, M.H.; Kumaresan, V.; Farb, D.H. Neurosteroid Actions in Memory and Neurologic/Neuropsychiatric Disorders. Front. Endocrinol. (Lausanne) 2019, 10, 169. [Google Scholar] [CrossRef]
- Daniel, J.M.; Hulst, J.L.; Berbling, J.L. Estradiol Replacement Enhances Working Memory in Middle-Aged Rats When Initiated Immediately after Ovariectomy But Not after a Long-Term Period of Ovarian Hormone Deprivation. Endocrinology 2006, 147, 607–614. [Google Scholar] [CrossRef]
- Pallarés, M.; Darnaudéry, M.; Day, J.; Le Moal, M.; Mayo, W. The Neurosteroid Pregnenolone Sulfate Infused into the Nucleus Basalis Increases Both Acetylcholine Release in the Frontal Cortex or Amygdala and Spatial Memory. Neuroscience 1998, 87, 551–558. [Google Scholar] [CrossRef]
- Darnaudéry, M.; Koehl, M.; Piazza, P.V.; Le Moal, M.; Mayo, W. Pregnenolone Sulfate Increases Hippocampal Acetylcholine Release and Spatial Recognition. Brain Res. 2000, 852, 173–179. [Google Scholar] [CrossRef]
- Luine, V.N.; Khylchevskaya, R.I.; McEwen, B.S. Effect of Gonadal Steroids on Activities of Monoamine Oxidase and Choline Acetylase in Rat Brain. Brain Res. 1975, 86, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Meyer, E.M.; Millard, W.J.; Simpkins, J.W. Ovarian Steroid Deprivation Results in a Reversible Learning Impairment and Compromised Cholinergic Function in Female Sprague-Dawley Rats. Brain Res. 1994, 644, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Fader, A.J.; Johnson, P.E.; Dohanich, G.P. Estrogen Improves Working but Not Reference Memory and Prevents Amnestic Effects of Scopolamine of a Radial-Arm Maze. Pharmacol. Biochem. Behav. 1999, 62, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, F.; Miyasaka, N.; Kubota, T.; Aso, T. Estrogen and Progesterone Improve Scopolamine-Induced Impairment of Spatial Memory. J. Med. Dent. Sci. 2004, 51, 89–98. [Google Scholar] [PubMed]
- Daniel, J.M.; Dohanich, G.P. Acetylcholine Mediates the Estrogen-Induced Increase in NMDA Receptor Binding in CA1 of the Hippocampus and the Associated Improvement in Working Memory. J. Neurosci. 2001, 21, 6949–6956. [Google Scholar] [CrossRef]
- Steffensen, S.C.; Jones, M.D.; Hales, K.; Allison, D.W. Dehydroepiandrosterone Sulfate and Estrone Sulfate Reduce GABA-Recurrent Inhibition in the Hippocampus via Muscarinic Acetylcholine Receptors. Hippocampus 2006, 16, 1080–1090. [Google Scholar] [CrossRef]
- Frick, K.M.; Kim, J. Mechanisms Underlying the Rapid Effects of Estradiol and Progesterone on Hippocampal Memory Consolidation in Female Rodents. Horm. Behav. 2018, 104, 100–110. [Google Scholar] [CrossRef]
- Barros, L.A.; Tufik, S.; Andersen, M.L. The Role of Progesterone in Memory: An Overview of Three Decades. Neurosci. Biobehav. Rev. 2015, 49, 193–204. [Google Scholar] [CrossRef]
- Ouanes, S.; Popp, J. High Cortisol and the Risk of Dementia and Alzheimer’s Disease: A Review of the Literature. Front. Aging Neurosci. 2019, 11, 43. [Google Scholar] [CrossRef]
- de Quervain, D.; Schwabe, L.; Roozendaal, B. Stress, Glucocorticoids and Memory: Implications for Treating Fear-Related Disorders. Nat. Rev. Neurosci. 2017, 18, 7–19. [Google Scholar] [CrossRef]
- Schwabe, L.; Joëls, M.; Roozendaal, B.; Wolf, O.T.; Oitzl, M.S. Stress Effects on Memory: An Update and Integration. Neurosci. Biobehav. Rev. 2012, 36, 1740–1749. [Google Scholar] [CrossRef]
- Sánchez-Resendis, O.; Medina, A.C.; Serafín, N.; Prado-Alcalá, R.A.; Roozendaal, B.; Quirarte, G.L. Glucocorticoid-Cholinergic Interactions in the Dorsal Striatum in Memory Consolidation of Inhibitory Avoidance Training. Front. Behav. Neurosci. 2012, 6, 33. [Google Scholar] [CrossRef]
- Power, A.E.; Roozendaal, B.; McGaugh, J.L. Glucocorticoid Enhancement of Memory Consolidation in the Rat Is Blocked by Muscarinic Receptor Antagonism in the Basolateral Amygdala. Eur. J. Neurosci. 2000, 12, 3481–3487. [Google Scholar] [CrossRef]
- Kelemen, E.; Bahrendt, M.; Born, J.; Inostroza, M. Hippocampal Corticosterone Impairs Memory Consolidation during Sleep but Improves Consolidation in the Wake State. Hippocampus 2014, 24, 510–515. [Google Scholar] [CrossRef]
- Aguilera, G. HPA Axis Responsiveness to Stress: Implications for Healthy Aging. Exp. Gerontol. 2011, 46, 90–95. [Google Scholar] [CrossRef]
- Mizoguchi, K.; Shoji, H.; Ikeda, R.; Tanaka, Y.; Maruyama, W.; Tabira, T. Suppression of Glucocorticoid Secretion Enhances Cholinergic Transmission in Rat Hippocampus. Brain Res. Bull. 2008, 76, 612–615. [Google Scholar] [CrossRef]
- Hemrick-Luecke, S.K.; Bymaster, F.P.; Evans, D.C.; Wess, J.; Felder, C.C. Muscarinic Agonist-Mediated Increases in Serum Corticosterone Levels Are Abolished in m(2) Muscarinic Acetylcholine Receptor Knockout Mice. J. Pharmacol. Exp. Ther. 2002, 303, 99–103. [Google Scholar] [CrossRef]
- Meziane, H.; Mathis, C.; Ungerer, A.; Paul, S.M. The Neurosteroid Pregnenolone Sulfate Reduces Learning Deficits Induced by Scopolamine and Has Promnestic Effects in Mice Performing an Appetitive Learning Task. Psychopharmacology 1996, 126, 323–330. [Google Scholar] [CrossRef]
- Urani, A.; Privat, A.; Maurice, T. The Modulation by Neurosteroids of the Scopolamine-Induced Learning Impairment in Mice Involves an Interaction with Sigma1 (Σ1) Receptors. Brain Res. 1998, 799, 64–77. [Google Scholar] [CrossRef]
- Johnson, D.A.; Wu, T.H.; Li, P.K.; Maher, T.J. The Effect of Steroid Sulfatase Inhibition on Learning and Spatial Memory. Brain Res. 2000, 865, 286–290. [Google Scholar] [CrossRef]
- Rhodes, M.E.; Li, P.K.; Burke, A.M.; Johnson, D.A. Enhanced Plasma DHEAS, Brain Acetylcholine and Memory Mediated by Steroid Sulfatase Inhibition. Brain Res. 1997, 773, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.A.; Higgins, G.A. Alzheimer’s Disease: The Amyloid Cascade Hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-F.; Xu, T.-H.; Yan, Y.; Zhou, Y.-R.; Jiang, Y.; Melcher, K.; Xu, H.E. Amyloid Beta: Structure, Biology and Structure-Based Therapeutic Development. Acta Pharmacol. Sin. 2017, 38, 1205–1235. [Google Scholar] [CrossRef] [PubMed]
- Rudajev, V.; Novotny, J. Cholesterol as a Key Player in Amyloid β-Mediated Toxicity in Alzheimer’s Disease. Front. Mol. Neurosci. 2022, 15, 937056. [Google Scholar] [CrossRef]
- Nitsch, R.M.; Slack, B.E.; Wurtman, R.J.; Growdon, J.H. Release of Alzheimer Amyloid Precursor Derivatives Stimulated by Activation of Muscarinic Acetylcholine Receptors. Science 1992, 258, 304–307. [Google Scholar] [CrossRef]
- Hock, C.; Maddalena, A.; Raschig, A.; Müller-Spahn, F.; Eschweiler, G.; Hager, K.; Heuser, I.; Hampel, H.; Müller-Thomsen, T.; Oertel, W.; et al. Treatment with the Selective Muscarinic M1 Agonist Talsaclidine Decreases Cerebrospinal Fluid Levels of A Beta 42 in Patients with Alzheimer’s Disease. Amyloid 2003, 10, 1–6. [Google Scholar] [CrossRef]
- Cisse, M.; Braun, U.; Leitges, M.; Fisher, A.; Pages, G.; Checler, F.; Vincent, B. ERK1-Independent α-Secretase Cut of β-Amyloid Precursor Protein via M1 Muscarinic Receptors and PKCα/ε. Mol. Cell Neurosci. 2011, 47, 223–232. [Google Scholar] [CrossRef]
- Giacobini, E.; Cuello, A.C.; Fisher, A. Reimagining Cholinergic Therapy for Alzheimer’s Disease. Brain 2022, 145, 2250–2275. [Google Scholar] [CrossRef]
- Caccamo, A.; Oddo, S.; Billings, L.M.; Green, K.N.; Martinez-Coria, H.; Fisher, A.; LaFerla, F.M. M1 Receptors Play a Central Role in Modulating AD-like Pathology in Transgenic Mice. Neuron 2006, 49, 671–682. [Google Scholar] [CrossRef]
- Fisher, A.; Bezprozvanny, I.; Wu, L.; Ryskamp, D.A.; Bar-Ner, N.; Natan, N.; Brandeis, R.; Elkon, H.; Nahum, V.; Gershonov, E.; et al. AF710B, a Novel M1/Σ1 Agonist with Therapeutic Efficacy in Animal Models of Alzheimer’s Disease. Neurodegener. Dis. 2016, 16, 95–110. [Google Scholar] [CrossRef]
- Dwomoh, L.; Tejeda, G.S.; Tobin, A.B. Targeting the M1 Muscarinic Acetylcholine Receptor in Alzheimer’s Disease. Neuronal Signal. 2022, 6, NS20210004. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.-M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The Cholinergic System in the Pathophysiology and Treatment of Alzheimer’s Disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef]
- Newhouse, P.; Dumas, J. Estrogen-Cholinergic Interactions: Implications for Cognitive Aging. Horm. Behav. 2015, 74, 173–185. [Google Scholar] [CrossRef]
- Fernandez, J.W.; Grizzell, J.A.; Wecker, L. The Role of Estrogen Receptor β and Nicotinic Cholinergic Receptors in Postpartum Depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 40, 199–206. [Google Scholar] [CrossRef]
- Bettio, L.E.B.; Rajendran, L.; Gil-Mohapel, J. The Effects of Aging in the Hippocampus and Cognitive Decline. Neurosci. Biobehav. Rev. 2017, 79, 66–86. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Kahn, R.S.; Sommer, I.E.; Murray, R.M.; Meyer-Lindenberg, A.; Weinberger, D.R.; Cannon, T.D.; O’Donovan, M.; Correll, C.U.; Kane, J.M.; van Os, J.; et al. Schizophrenia. Nat. Rev. Dis. Prim. 2015, 1, 15067. [Google Scholar] [CrossRef]
- Shen, L.-H.; Liao, M.-H.; Tseng, Y.-C. Recent Advances in Imaging of Dopaminergic Neurons for Evaluation of Neuropsychiatric Disorders. J. Biomed. Biotechnol. 2012, 2012, 259349. [Google Scholar] [CrossRef]
- O’Donnell, P.; Grace, A.A. Dysfunctions in Multiple Interrelated Systems as the Neurobiological Bases of Schizophrenic Symptom Clusters. Schizophr. Bull. 1998, 24, 267–283. [Google Scholar] [CrossRef]
- Sur, C.; Mallorga, P.J.; Wittmann, M.; Jacobson, M.A.; Pascarella, D.; Williams, J.B.; Brandish, P.E.; Pettibone, D.J.; Scolnick, E.M.; Conn, P.J. N-Desmethylclozapine, an Allosteric Agonist at Muscarinic 1 Receptor, Potentiates N-Methyl-D-Aspartate Receptor Activity. Proc. Natl. Acad. Sci. USA 2003, 100, 13674–13679. [Google Scholar] [CrossRef]
- Miller, A.D.; Blaha, C.D. Midbrain Muscarinic Receptor Mechanisms Underlying Regulation of Mesoaccumbens and Nigrostriatal Dopaminergic Transmission in the Rat. Eur. J. Neurosci. 2005, 21, 1837–1846. [Google Scholar] [CrossRef] [PubMed]
- Scarr, E.; Dean, B. Muscarinic Receptors: Do They Have a Role in the Pathology and Treatment of Schizophrenia? J. Neurochem. 2008, 107, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Mancama, D.; Arranz, M.J.; Landau, S.; Kerwin, R. Reduced Expression of the Muscarinic 1 Receptor Cortical Subtype in Schizophrenia. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2003, 119B, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Shannon, H.E.; Rasmussen, K.; Bymaster, F.P.; Hart, J.C.; Peters, S.C.; Swedberg, M.D.; Jeppesen, L.; Sheardown, M.J.; Sauerberg, P.; Fink-Jensen, A. Xanomeline, an M(1)/M(4) Preferring Muscarinic Cholinergic Receptor Agonist, Produces Antipsychotic-like Activity in Rats and Mice. Schizophr. Res. 2000, 42, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Bymaster, F.P.; Felder, C.; Ahmed, S.; McKinzie, D. Muscarinic Receptors as a Target for Drugs Treating Schizophrenia. Curr Drug Targets CNS Neurol Disord 2002, 1, 163–181. [Google Scholar] [CrossRef]
- Bodick, N.C.; Offen, W.W.; Levey, A.I.; Cutler, N.R.; Gauthier, S.G.; Satlin, A.; Shannon, H.E.; Tollefson, G.D.; Rasmussen, K.; Bymaster, F.P.; et al. Effects of Xanomeline, a Selective Muscarinic Receptor Agonist, on Cognitive Function and Behavioral Symptoms in Alzheimer Disease. Arch. Neurol. 1997, 54, 465–473. [Google Scholar] [CrossRef]
- Shekhar, A.; Potter, W.Z.; Lightfoot, J.; Lienemann, J.; Dubé, S.; Mallinckrodt, C.; Bymaster, F.P.; McKinzie, D.L.; Felder, C.C. Selective Muscarinic Receptor Agonist Xanomeline as a Novel Treatment Approach for Schizophrenia. Am. J. Psychiatry 2008, 165, 1033–1039. [Google Scholar] [CrossRef]
- Sako, Y.; Kurimoto, E.; Mandai, T.; Suzuki, A.; Tanaka, M.; Suzuki, M.; Shimizu, Y.; Yamada, M.; Kimura, H. TAK-071, a Novel M1 Positive Allosteric Modulator with Low Cooperativity, Improves Cognitive Function in Rodents with Few Cholinergic Side Effects. Neuropsychopharmacology 2019, 44, 950–960. [Google Scholar] [CrossRef]
- Gogos, A.; Sbisa, A.M.; Sun, J.; Gibbons, A.; Udawela, M.; Dean, B. A Role for Estrogen in Schizophrenia: Clinical and Preclinical Findings. Int. J. Endocrinol. 2015, 2015, 1–16. [Google Scholar] [CrossRef]
- Marx, C.E.; Bradford, D.W.; Hamer, R.M.; Naylor, J.C.; Allen, T.B.; Lieberman, J.A.; Strauss, J.L.; Kilts, J.D. Pregnenolone as a Novel Therapeutic Candidate in Schizophrenia: Emerging Preclinical and Clinical Evidence. Neuroscience 2011, 191, 78–90. [Google Scholar] [CrossRef]
- Ahmed Juvale, I.I.; Che Has, A.T. The Evolution of the Pilocarpine Animal Model of Status Epilepticus. Heliyon 2020, 6, e04557. [Google Scholar] [CrossRef]
- Williamson, J.; Singh, T.; Kapur, J. Neurobiology of Organophosphate-Induced Seizures. Epilepsy Behav. 2019, 101, 106426. [Google Scholar] [CrossRef]
- Duysen, E.G.; Stribley, J.A.; Fry, D.L.; Hinrichs, S.H.; Lockridge, O. Rescue of the Acetylcholinesterase Knockout Mouse by Feeding a Liquid Diet; Phenotype of the Adult Acetylcholinesterase Deficient Mouse. Brain Res. Dev. Brain Res. 2002, 137, 43–54. [Google Scholar] [CrossRef]
- Farar, V.; Mohr, F.; Legrand, M.; Lamotte d’Incamps, B.; Cendelin, J.; Leroy, J.; Abitbol, M.; Bernard, V.; Baud, F.; Fournet, V.; et al. Near-Complete Adaptation of the PRiMA Knockout to the Lack of Central Acetylcholinesterase. J. Neurochem. 2012, 122, 1065–1080. [Google Scholar] [CrossRef]
- Miller, S.L.; Aroniadou-Anderjaska, V.; Pidoplichko, V.I.; Figueiredo, T.H.; Apland, J.P.; Krishnan, J.K.S.; Braga, M.F.M. The M1 Muscarinic Receptor Antagonist VU0255035 Delays the Development of Status Epilepticus after Organophosphate Exposure and Prevents Hyperexcitability in the Basolateral Amygdala. J. Pharmacol. Exp. Ther. 2017, 360, 23–32. [Google Scholar] [CrossRef]
- Palomero-Gallagher, N.; Schleicher, A.; Bidmon, H.-J.; Pannek, H.-W.; Hans, V.; Gorji, A.; Speckmann, E.-J.; Zilles, K. Multireceptor Analysis in Human Neocortex Reveals Complex Alterations of Receptor Ligand Binding in Focal Epilepsies. Epilepsia 2012, 53, 1987–1997. [Google Scholar] [CrossRef]
- Akyüz, E.; Doğanyiğit, Z.; Paudel, Y.N.; Kaymak, E.; Yilmaz, S.; Uner, A.; Shaikh, M.F. Increased ACh-Associated Immunoreactivity in Autonomic Centers in PTZ Kindling Model of Epilepsy. Biomedicines 2020, 8, 113. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, B.; Wang, Y.; Chen, Z. Cholinergic Signaling, Neural Excitability, and Epilepsy. Molecules 2021, 26, 2258. [Google Scholar] [CrossRef]
- Reddy, D.S.; Estes, W.A. Clinical Potential of Neurosteroids for CNS Disorders. Trends Pharmacol. Sci. 2016, 37, 543–561. [Google Scholar] [CrossRef]
- Rhodes, M.E.; Li, P.K.; Flood, J.F.; Johnson, D.A. Enhancement of Hippocampal Acetylcholine Release by the Neurosteroid Dehydroepiandrosterone Sulfate: An in Vivo Microdialysis Study. Brain Res. 1996, 733, 284–286. [Google Scholar] [CrossRef]
- Osborne, D.M.; Frye, C.A. Estrogen Increases Latencies to Seizures and Levels of 5alpha-Pregnan-3alpha-Ol-20-One in Hippocampus of Wild-Type, but Not 5alpha-Reductase Knockout, Mice. Epilepsy Behav. 2009, 16, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.S. Neuroendocrine Aspects of Catamenial Epilepsy. Horm. Behav. 2013, 63, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Thorn, C.A.; Popiolek, M.; Stark, E.; Edgerton, J.R. Effects of M1 and M4 Activation on Excitatory Synaptic Transmission in CA1. Hippocampus 2017, 27, 794–810. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Gulledge, A.T. M1 and M4 Receptors Modulate Hippocampal Pyramidal Neurons. J. Neurophysiol. 2011, 105, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Righes Marafiga, J.; Vendramin Pasquetti, M.; Calcagnotto, M.E. GABAergic Interneurons in Epilepsy: More than a Simple Change in Inhibition. Epilepsy Behav. 2021, 121, 106935. [Google Scholar] [CrossRef]
- Jones, N.C.; Lee, H.E.; Yang, M.; Rees, S.M.; Morris, M.J.; O’Brien, T.J.; Salzberg, M.R. Repeatedly Stressed Rats Have Enhanced Vulnerability to Amygdala Kindling Epileptogenesis. Psychoneuroendocrinology 2013, 38, 263–270. [Google Scholar] [CrossRef]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.-E.; Lang, A.E. Parkinson Disease. Nat. Rev. Dis. Prim. 2017, 3, 17013. [Google Scholar] [CrossRef]
- McGregor, M.M.; Nelson, A.B. Circuit Mechanisms of Parkinson’s Disease. Neuron 2019, 101, 1042–1056. [Google Scholar] [CrossRef]
- Acharya, S.; Kim, K.-M. Roles of the Functional Interaction between Brain Cholinergic and Dopaminergic Systems in the Pathogenesis and Treatment of Schizophrenia and Parkinson’s Disease. Int. J. Mol. Sci. 2021, 22, 4299. [Google Scholar] [CrossRef]
- Moehle, M.S.; Pancani, T.; Byun, N.; Yohn, S.E.; Wilson, G.H.; Dickerson, J.W.; Remke, D.H.; Xiang, Z.; Niswender, C.M.; Wess, J.; et al. Cholinergic Projections to the Substantia Nigra Pars Reticulata Inhibit Dopamine Modulation of Basal Ganglia through the M4 Muscarinic Receptor. Neuron 2017, 96, 1358–1372.e4. [Google Scholar] [CrossRef]
- Foster, D.J.; Wilson, J.M.; Remke, D.H.; Mahmood, M.S.; Uddin, M.J.; Wess, J.; Patel, S.; Marnett, L.J.; Niswender, C.M.; Jones, C.K.; et al. Antipsychotic-like Effects of M 4 Positive Allosteric Modulators Are Mediated by CB 2 Receptor-Dependent Inhibition of Dopamine Release. Neuron 2016, 91, 1244–1252. [Google Scholar] [CrossRef]
- Eskow Jaunarajs, K.L.; Bonsi, P.; Chesselet, M.F.; Standaert, D.G.; Pisani, A. Striatal Cholinergic Dysfunction as a Unifying Theme in the Pathophysiology of Dystonia. Prog. Neurobiol. 2015, 127–128, 91–107. [Google Scholar] [CrossRef]
- Sawada, H.; Ibi, M.; Kihara, T.; Honda, K.; Nakamizo, T.; Kanki, R.; Nakanishi, M.; Sakka, N.; Akaike, A.; Shimohama, S. Estradiol Protects Dopaminergic Neurons in a MPP+Parkinson’s Disease Model. Neuropharmacology 2002, 42, 1056–1064. [Google Scholar] [CrossRef]
- Borowicz-Reutt, K. Neurosteroids and Their Neuroprotective Actions. In Proceedings of the Health Science International Conference (HSIC 2017), 4–5 October 2017, Malang, Indonesia; Atlantis Press: Paris, France, 2017; Volume 2. [Google Scholar]
- Nestler, E.J. Role of the Brain’s Reward Circuitry in Depression. In International Review of Neurobiology; Academic Press Inc.: Cambridge, MA, USA, 2015; Volume 124, pp. 151–170. ISBN 9780128015834. [Google Scholar]
- Cooper, S.; Robison, A.J.; Mazei-Robison, M.S. Reward Circuitry in Addiction. Neurotherapeutics 2017, 14, 687–697. [Google Scholar] [CrossRef]
- Moran-Santa Maria, M.M.; Flanagan, J.; Brady, K. Ovarian Hormones and Drug Abuse. Curr. Psychiatry Rep. 2014, 16, 511. [Google Scholar] [CrossRef]
- Fox, H.C.; Sofuoglu, M.; Morgan, P.T.; Tuit, K.L.; Sinha, R. The Effects of Exogenous Progesterone on Drug Craving and Stress Arousal in Cocaine Dependence: Impact of Gender and Cue Type. Psychoneuroendocrinology 2013, 38, 1532–1544. [Google Scholar] [CrossRef]
- Lynch, W.J.; Roth, M.E.; Mickelberg, J.L.; Carroll, M.E. Role of Estrogen in the Acquisition of Intravenously Self-Administered Cocaine in Female Rats. Pharmacol. Biochem. Behav. 2001, 68, 641–646. [Google Scholar] [CrossRef]
- Everitt, B.J.; Robbins, T.W. Neural Systems of Reinforcement for Drug Addiction: From Actions to Habits to Compulsion. Nat. Neurosci. 2005, 8, 1481–1489. [Google Scholar] [CrossRef]
- Gunter, B.W.; Gould, R.W.; Bubser, M.; McGowan, K.M.; Lindsley, C.W.; Jones, C.K. Selective Inhibition of M5 Muscarinic Acetylcholine Receptors Attenuates Cocaine Self-Administration in Rats. Addict. Biol. 2018, 23, 1106–1116. [Google Scholar] [CrossRef]
- Gentry, P.R.; Kokubo, M.; Bridges, T.M.; Kett, N.R.; Harp, J.M.; Cho, H.P.; Smith, E.; Chase, P.; Hodder, P.S.; Niswender, C.M.; et al. Discovery of the First M5-Selective and CNS Penetrant Negative Allosteric Modulator (NAM) of a Muscarinic Acetylcholine Receptor: (S)-9b-(4-Chlorophenyl)-1-(3,4-Difluorobenzoyl)-2,3-Dihydro-1H-Imidazo[2,1-a]Isoindol-5(9bH)-One (ML375). J. Med. Chem. 2013, 56, 9351–9355. [Google Scholar] [CrossRef]
- Wohleb, E.S.; Wu, M.; Gerhard, D.M.; Taylor, S.R.; Picciotto, M.R.; Alreja, M.; Duman, R.S. GABA Interneurons Mediate the Rapid Antidepressant-like Effects of Scopolamine. J. Clin. Investig. 2016, 126, 2482–2494. [Google Scholar] [CrossRef] [PubMed]
- Dagytė, G.; Den Boer, J.A.; Trentani, A. The Cholinergic System and Depression. Behav. Brain Res. 2011, 221, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Gibbons, A.S.; Scarr, E.; McLean, C.; Sundram, S.; Dean, B. Decreased Muscarinic Receptor Binding in the Frontal Cortex of Bipolar Disorder and Major Depressive Disorder Subjects. J. Affect. Disord. 2009, 116, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Gillin, J.C.; Sutton, L.; Ruiz, C.; Darko, D.; Golshan, S.; Risch, S.C.; Janowsky, D. The Effects of Scopolamine on Sleep and Mood in Depressed Patients with a History of Alcoholism and a Normal Comparison Group. Biol. Psychiatry 1991, 30, 157–169. [Google Scholar] [CrossRef]
- Witkin, J.M.; Overshiner, C.; Li, X.; Catlow, J.T.; Wishart, G.N.; Schober, D.A.; Heinz, B.A.; Nikolayev, A.; Tolstikov, V.V.; Anderson, W.H.; et al. M1 and M2 Muscarinic Receptor Subtypes Regulate Antidepressant-like Effects of the Rapidly Acting Antidepressant Scopolamine. J. Pharmacol. Exp. Ther. 2014, 351, 448–456. [Google Scholar] [CrossRef]
- Navarria, A.; Wohleb, E.S.; Voleti, B.; Ota, K.T.; Dutheil, S.; Lepack, A.E.; Dwyer, J.M.; Fuchikami, M.; Becker, A.; Drago, F.; et al. Rapid Antidepressant Actions of Scopolamine: Role of Medial Prefrontal Cortex and M1-Subtype Muscarinic Acetylcholine Receptors. Neurobiol. Dis. 2015, 82, 254–261. [Google Scholar] [CrossRef]
- Standeven, L.R.; McEvoy, K.O.; Osborne, L.M. Progesterone, Reproduction, and Psychiatric Illness. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 69, 108–126. [Google Scholar] [CrossRef]
- Furey, M.L.; Khanna, A.; Hoffman, E.M.; Drevets, W.C. Scopolamine Produces Larger Antidepressant and Antianxiety Effects in Women than in Men. Neuropsychopharmacology 2010, 35, 2479–2488. [Google Scholar] [CrossRef]
- Andréen, L.; Sundström-Poromaa, I.; Bixo, M.; Nyberg, S.; Bäckström, T. Allopregnanolone Concentration and Mood--a Bimodal Association in Postmenopausal Women Treated with Oral Progesterone. Psychopharmacology 2006, 187, 209–221. [Google Scholar] [CrossRef]
- Wharton, W.; Gleason, C.E.; Olson, S.R.M.S.; Carlsson, C.M.; Asthana, S. Neurobiological Underpinnings of the Estrogen—Mood Relationship. Curr. Psychiatry Rev. 2012, 8, 247–256. [Google Scholar] [CrossRef]
| Response | Expression | Activation | Inhibition | Implications in CNS Disorders | |
|---|---|---|---|---|---|
| M1 | ↑ PLC, IP3, DAG, Ca2+ and PKC; depolarization and excitation (EPSPs) | neocortex, hippocampus, striatum [18,19] | ↑ neuronal depolarization [31], ↑ learning and memory [20], ↓ dopamine release, locomotion, ↑ wakefulness, ↓ delta sleep, ↑ seizure activity [27] | ↓ REM sleep [46] ↓ dopamine release | AD [22,23], schizophrenia [24,25], PD [39], cognitive dysfunction [24,25,38], seizures/epilepsy [27,28] |
| M2 | ↓ cAMP, ↑ GIRKs, ↓ VGCCs, neuronal hyperpolarization | hippocampus, cortex, olfactory bulb, basal forebrain, thalamus, cerebellum [19,41] | ↓ neurotransmitter release, tremor, hypothermia, analgesia [26] | ↑ locomotor activity, ↑ vasomotor centre, ↓ pituitary, ↓ food intake, ↓ growth hormone, ↓ prolactin, [26] | pain management [29], depression [30] |
| M3 | ↑ PLC, IP3, DAG, Ca2+ and PKC; depolarization and excitation (EPSPs) | cortex, basal ganglia, cerebellum [19,41] | ↑ learning, memory [31] | disinhibition of dopamine release [47] | |
| M4 | ↓ adenylyl cyclase, ↓cAMP, ↑ GIRKs, ↓ VGCCs, hyperpolarization | striatum, neocortexhippocampus basal ganglia [19,24,32] | ↓ neurotransmitter release, analgesia, ↓ dopamine release [34], anti-psychotic effects [36] | schizophrenia [21,24,25,37,38], PD [34,39], pain management [40] | |
| M5 | ↑ PLC, IP3, DAG, Ca2+ and PKC; depolarization and excitation (EPSPs); ↑ PLD2 | VTA, SN, brain microvasculature, cerebellum [19,24,31,41] | ↑ cerebral vasodilation, ↑ dopamine release [26,27]; ↑ drug-seeking behaviour and reward [25] | ↓ cerebral vasodilation [27] ↓ drug-seeking behaviour and reward [25] | AD [42,43], schizophrenia [25], substance abuse [43,44] |
| Treatment | Brain Area | Subtypes | Methods | Post-Ovariectomy Time | Duration of Hormone Treatment | Effect | Ref. |
|---|---|---|---|---|---|---|---|
| proestrus stage (high 17β-estradiol level) | rat cerebral cortex | all | [3H]NMS binding | ↑ | [64] | ||
| diestrus stage (low 17β-estradiol level) | ↓ | ||||||
| EB and PROG replacement after ovariectomy | 7 days | ↑ | |||||
| EB replacement after ovariectomy | rat medial basal hypothalamus | all | [3H]QNB binding | 2 days | ↑ | [67] | |
| EB replacement after ovariectomy | rat medial basal hypothalamus | all | [3H]QNB binding | 14 days | ↑ | [68] | |
| rat medial preoptic area | ↓ | ||||||
| 17β-estradiol deprivation (ovariectomy) | rat hippocampus, frontal cortex, hypothalamus | M4 | autoradiography | 10 days | ↑ | [63] | |
| 17β-estradiol replacement | 2 days | 10 weeks | ↓ | ||||
| progesterone | ↑ | ||||||
| 17β-estradiol deprivation (ovariectomy) | rat hippocampus | all | [3H]QNB binding | 2, 10, 15 days | ↑ | [69] | |
| EB replacement | 15 days | 7 days | 0 | ||||
| immediate treatment | 21 days | ↓ | |||||
| 17β-estradiol deprivation (ovariectomy) | rat hippocampus | M1–M5 | immuno-precipitation | 15 days | ↑ | [70] | |
| EB replacement | 15 days | 7 days | 0 | ||||
| immediate treatment | 21 days | ↓ | |||||
| 17β-estradiol deprivation (ovariectomy) | [3H]QNB binding | 15 days | ↑ | ||||
| EB replacement | 15 days | 7 days | 0 | ||||
| immediate treatment | 21 days | ↓ | |||||
| 17β-estradiol deprivation (ovariectomy) | rat amygdala, caudate putamen, dorsal hippocampus, motor cortex, retrosplenial cortex, ventromedial hypothalamus | M1, M2/M4, M3 | autoradiography | 10 days | 0 | [72] | |
| long-term estrogen therapy | human left striatum, hippocampus, lateral frontal cortex, thalamus | M1 and M4 | (SPET) | ↑ | [74] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczurowska, E.; Szánti-Pintér, E.; Chetverikov, N.; Randáková, A.; Kudová, E.; Jakubík, J. Modulation of Muscarinic Signalling in the Central Nervous System by Steroid Hormones and Neurosteroids. Int. J. Mol. Sci. 2023, 24, 507. https://doi.org/10.3390/ijms24010507
Szczurowska E, Szánti-Pintér E, Chetverikov N, Randáková A, Kudová E, Jakubík J. Modulation of Muscarinic Signalling in the Central Nervous System by Steroid Hormones and Neurosteroids. International Journal of Molecular Sciences. 2023; 24(1):507. https://doi.org/10.3390/ijms24010507
Chicago/Turabian StyleSzczurowska, Ewa, Eszter Szánti-Pintér, Nikolai Chetverikov, Alena Randáková, Eva Kudová, and Jan Jakubík. 2023. "Modulation of Muscarinic Signalling in the Central Nervous System by Steroid Hormones and Neurosteroids" International Journal of Molecular Sciences 24, no. 1: 507. https://doi.org/10.3390/ijms24010507
APA StyleSzczurowska, E., Szánti-Pintér, E., Chetverikov, N., Randáková, A., Kudová, E., & Jakubík, J. (2023). Modulation of Muscarinic Signalling in the Central Nervous System by Steroid Hormones and Neurosteroids. International Journal of Molecular Sciences, 24(1), 507. https://doi.org/10.3390/ijms24010507