Influence of the Peripheral Nervous System on Murine Osteoporotic Fracture Healing and Fracture-Induced Hyperalgesia
Abstract
:1. Introduction
2. Results
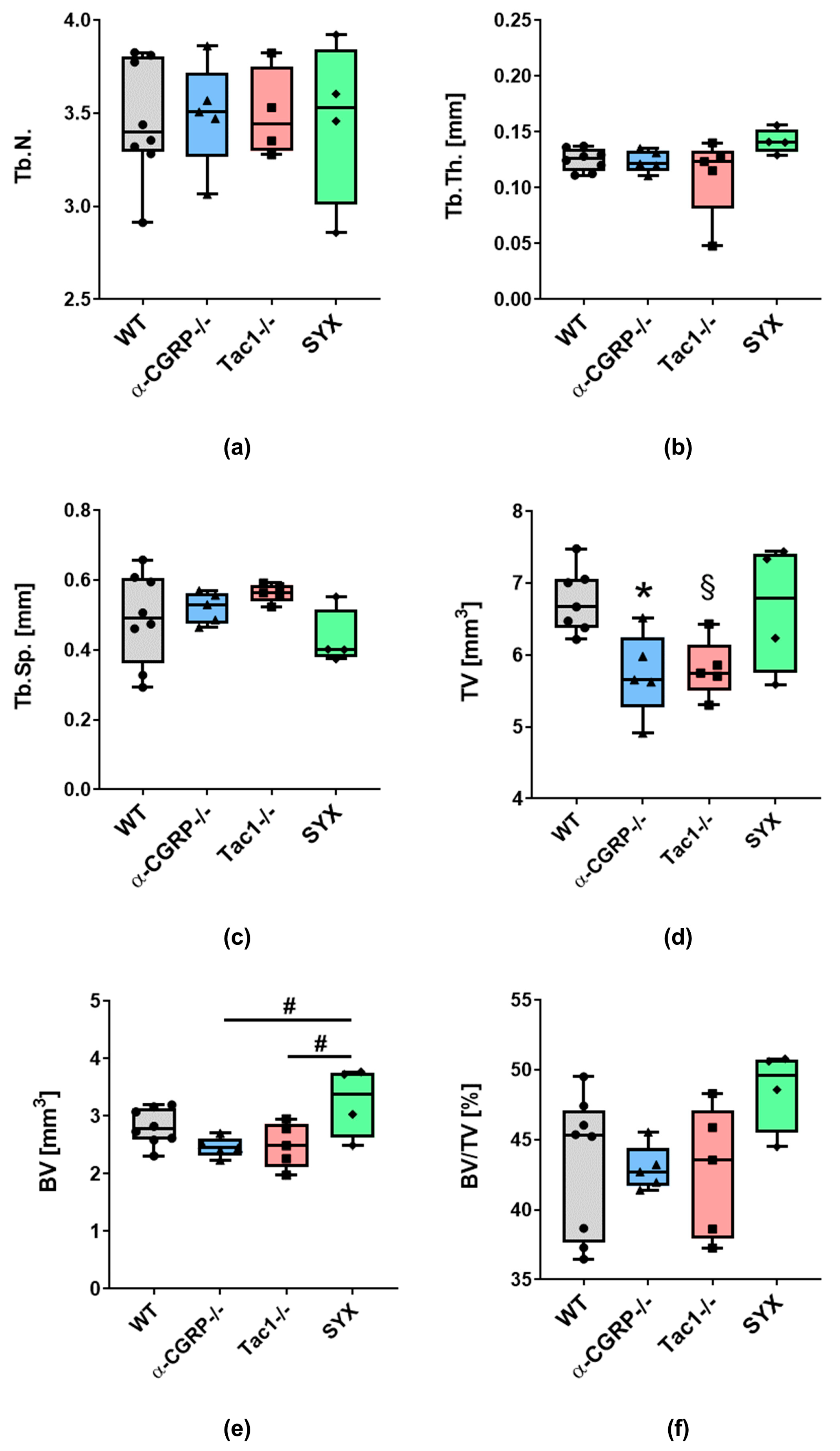
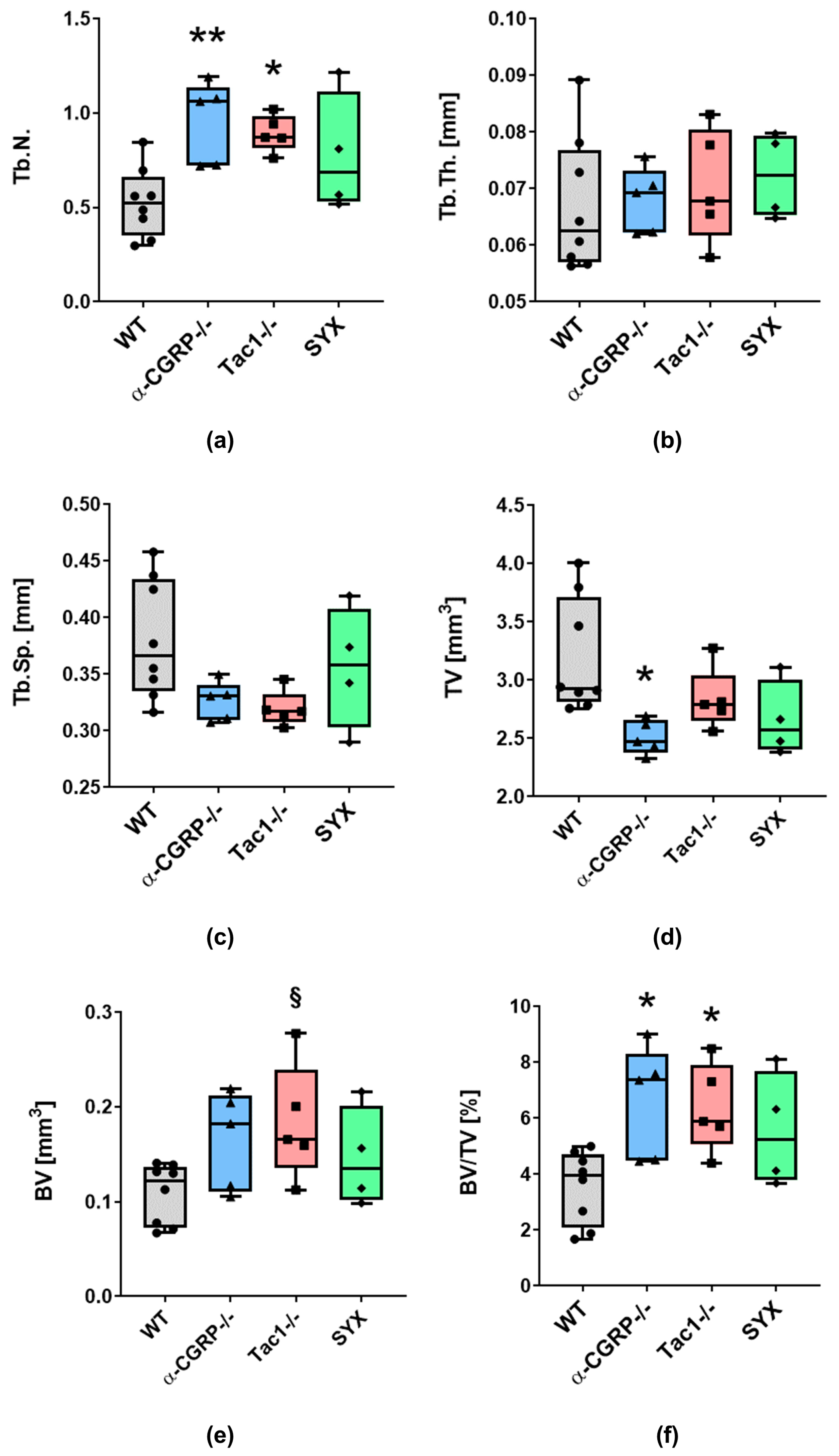
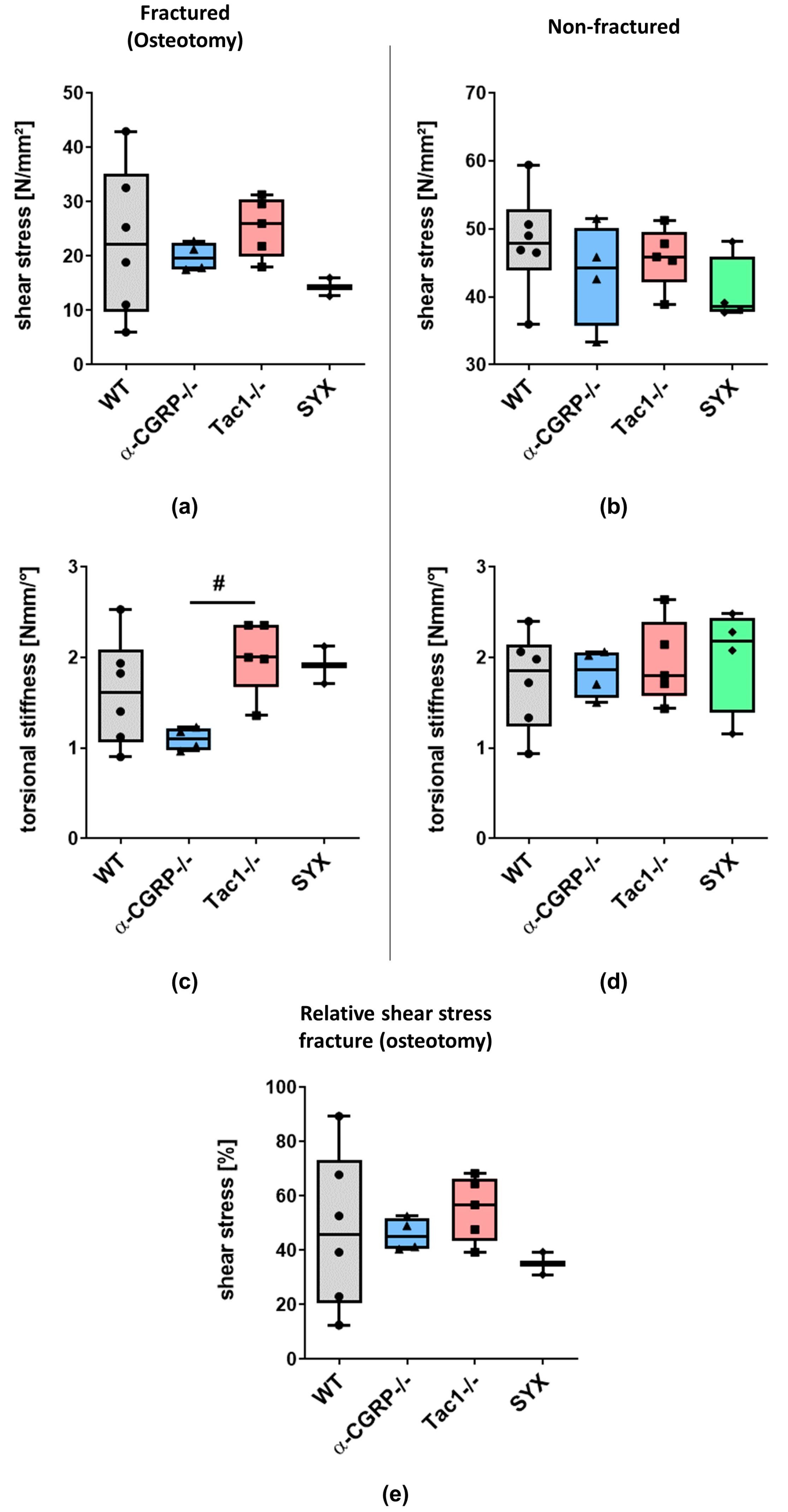
2.1. µCT Analysis and Biomechanical Tests
2.1.1. µCT Analysis
2.1.2. Biomechanical Tests
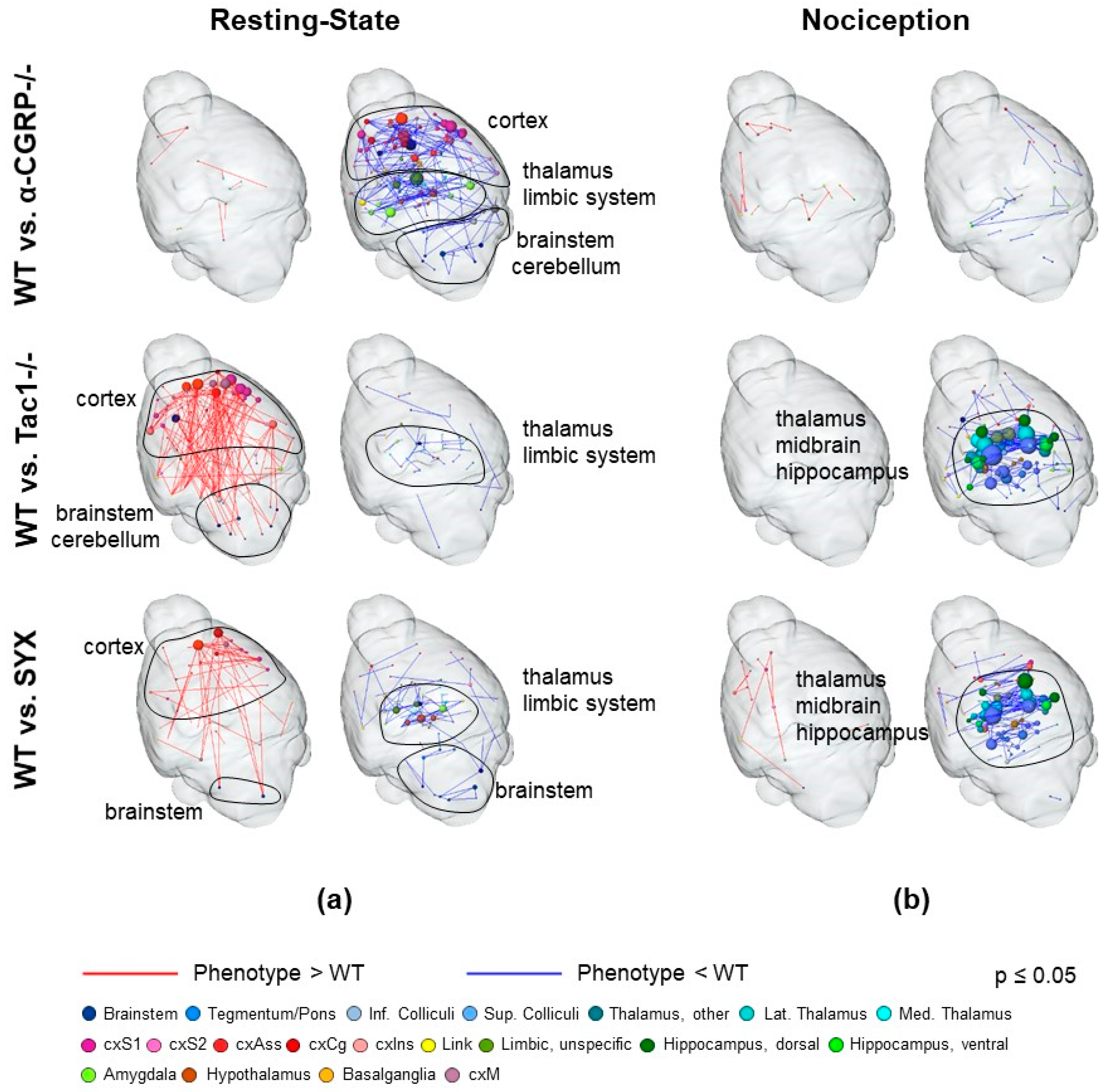
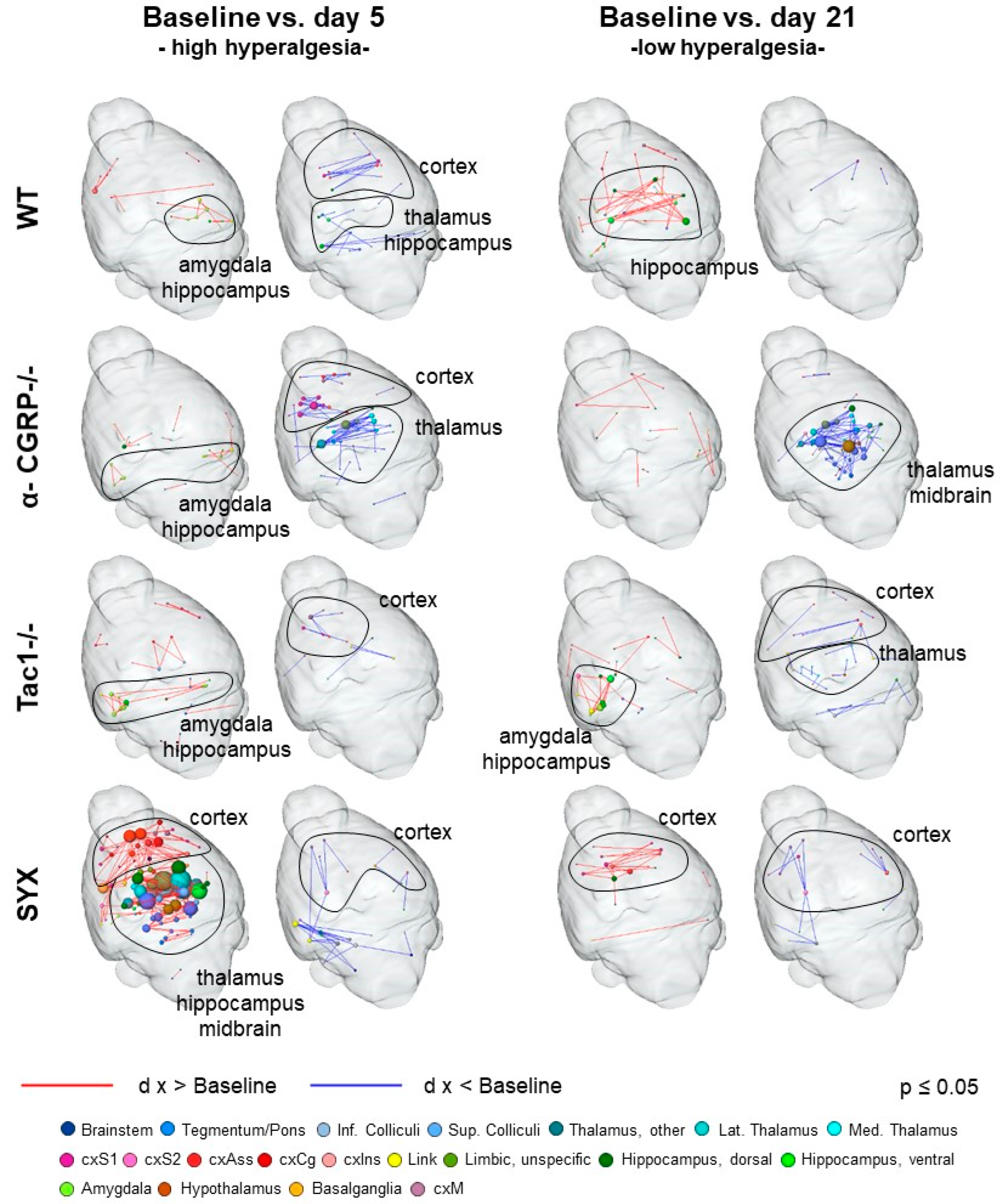
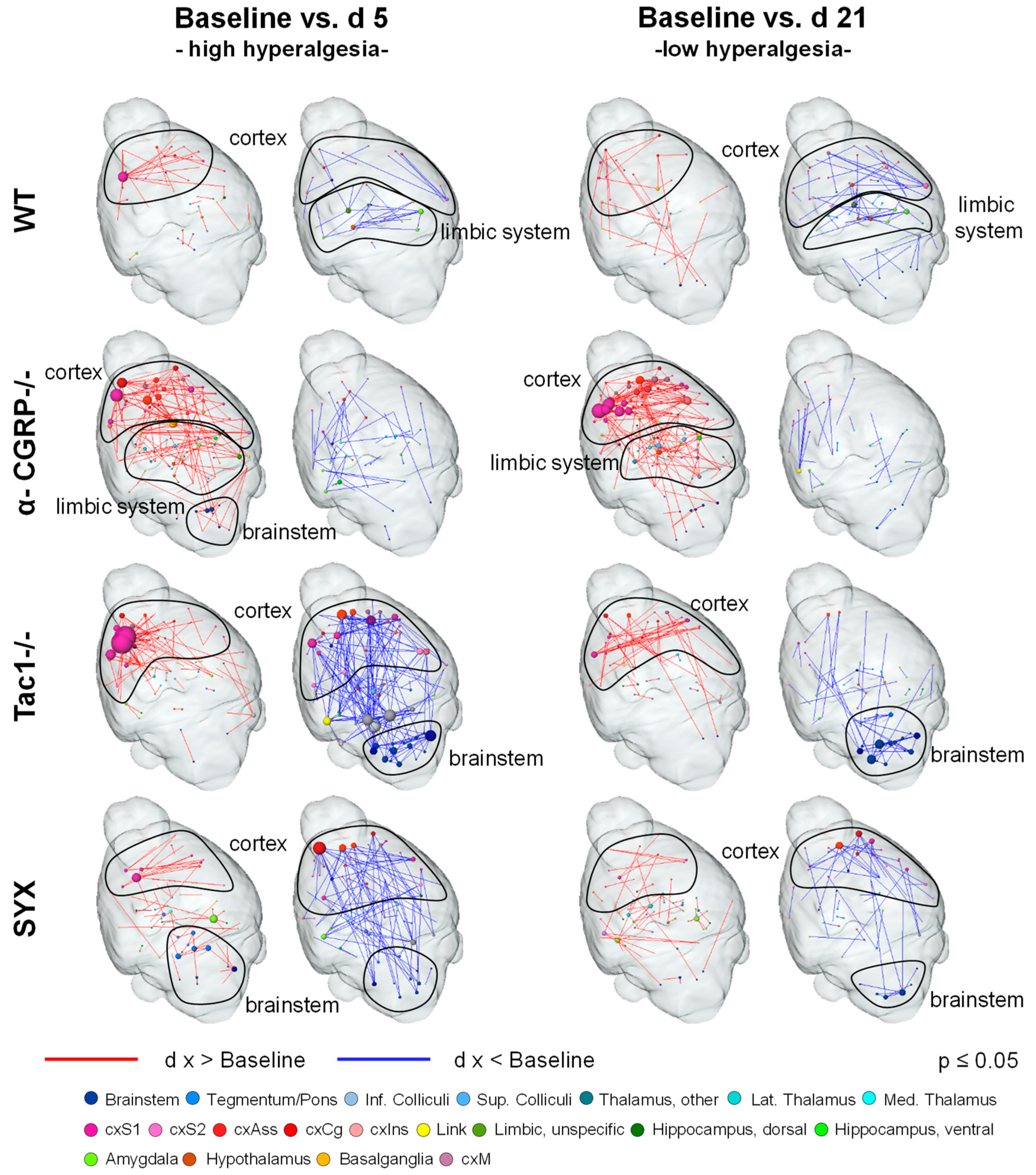
2.2. fMRI Analysis
2.2.1. Characterization of Neurotransmitter-Deficient Mouse Models
2.2.2. Nociceptive Functional Connectivity
2.2.3. Resting-State Functional Connectivity
3. Discussion
3.1. Opposite Effects of Sensory and Sympathetic Signaling on Bony Callus Microarchitecture in the Remodeling Phase
3.2. Impaired Bridging at the Fracture Site in WT and SYX Mice after OVX
3.3. Depletion of Sensory and Sympathetic Neurotransmitter Signaling Modulates Interaction of Brain Structures
3.4. Bone Fracture Evokes Longitudinal Changes in Nociceptive Processing
3.5. Bone Fracture Induces Recognizable Changes on Resting-State Functional Connectivity
3.6. Changes in Neurotransmitter Abundancy Does Not Linearly Account for the Modulatory Effect on Brain Functional Connectivity
3.7. Summary
4. Materials and Methods
4.1. Animals
4.1.1. Animal Description
4.1.2. Mouse Models
4.1.3. Animal Housing
4.1.4. Group Assignment and Dropout
4.2. Protocols and Analysis Overview
4.2.1. Study Design and fMRI Paradigm
4.2.2. Surgical Procedures
4.2.3. µCT Protocols and Analysis
4.2.4. Biomechanical Analysis
4.2.5. FMRI Preparation, Protocols and Stimulation Paradigm
4.3. Data Analysis, Statistics and Blinding
4.3.1. µCT and Biomechanical Tests
4.3.2. Stimulus-Driven fMRI
4.3.3. Resting-State fMRI
4.3.4. Graph Theoretical Visualization
4.3.5. Gene Expression Network Correlation Analysis
4.3.6. MRI Statistics
4.3.7. Randomization and Blinding
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalu, D.N.; Chen, C. Ovariectomized murine model of postmenopausal calcium malabsorption. J. Bone Miner. Res. 1999, 14, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Klinck, J.; Boyd, S.K. The magnitude and rate of bone loss in ovariectomized mice differs among inbred strains as determined by longitudinal in vivo micro-computed tomography. Calcif. Tissue Int. 2008, 83, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Wang, G.; Qiao, L.; Ge, Q.; Chen, D.; Xu, Z.; Shi, D.; Dai, J.; Qin, J.; Teng, H.; et al. Age-dependent variations of cancellous bone in response to ovariectomy in C57BL/6J mice. Exp. Ther. Med. 2018, 15, 3623–3632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmer, A.; Zimmer, A.M.; Baffi, J.; Usdin, T.; Reynolds, K.; König, M.; Palkovits, M.; Mezey, E. Hypoalgesia in mice with a targeted deletion of the tachykinin 1 gene. Proc. Natl. Acad. Sci. USA 1998, 95, 2630–2635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.T.; Son, Y.J.; Lee, J.; Jetton, T.L.; Shiota, M.; Moscoso, L.; Niswender, K.D.; Loewy, A.D.; Magnuson, M.A.; Sanes, J.R.; et al. Mice lacking alpha-calcitonin gene-related peptide exhibit normal cardiovascular regulation and neuromuscular development. Mol. Cell. Neurosci. 1999, 14, 99–120. [Google Scholar] [CrossRef] [PubMed]
- Härle, P.; Möbius, D.; Carr, D.J.J.; Schölmerich, J.; Straub, R.H. An opposing time-dependent immune-modulating effect of the sympathetic nervous system conferred by altering the cytokine profile in the local lymph nodes and spleen of mice with type II collagen-induced arthritis. Arthritis Rheum. 2005, 52, 1305–1313. [Google Scholar] [CrossRef]
- Schlereth, T.; Birklein, F. The sympathetic nervous system and pain. Neuromol. Med. 2008, 10, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Monazzah, S.; Bigham-Sadegh, A. Bone injury and fracture healing biology. Biomed. Environ. Sci. 2015, 28, 57–71. [Google Scholar] [CrossRef]
- Harwood, P.J.; Newman, J.B.; Michael, A.L. (ii) An update on fracture healing and non-union. Orthop. Trauma 2010, 24, 9–23. [Google Scholar] [CrossRef]
- Catalano, A.; Martino, G.; Morabito, N.; Scarcella, C.; Gaudio, A.; Basile, G.; Lasco, A. Pain in Osteoporosis: From Pathophysiology to Therapeutic Approach. Drugs Aging 2017, 34, 755–765. [Google Scholar] [CrossRef]
- Harrison, S.; Geppetti, P. Substance p. Int. J. Biochem. Cell Biol. 2001, 33, 555–576. [Google Scholar] [CrossRef]
- Niedermair, T.; Straub, R.H.; Brochhausen, C.; Grässel, S. Impact of the Sensory and Sympathetic Nervous System on Fracture Healing in Ovariectomized Mice. Int. J. Mol. Sci. 2020, 21, 405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zieglgänsberger, W. Substance P and pain chronicity. Cell Tissue Res. 2019, 375, 227–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lerner, U.H.; Persson, E. Osteotropic effects by the neuropeptides calcitonin gene-related peptide, substance P and vasoactive intestinal peptide. J. Musculoskelet. Neuronal Interact. 2008, 8, 154–165. [Google Scholar] [PubMed]
- Gutierrez, S.; Alvarado-Vázquez, P.A.; Eisenach, J.C.; Romero-Sandoval, E.A.; Boada, M.D. Tachykinins modulate nociceptive responsiveness and sensitization: In vivo electrical characterization of primary sensory neurons in tachykinin knockout (Tac1 KO) mice. Mol. Pain 2019, 15, 1744806919845750. [Google Scholar] [CrossRef] [Green Version]
- DeVane, C.L. Substance P: A New Era, a New Role. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2001, 21, 1061–1069. [Google Scholar] [CrossRef]
- Niedermair, T.; Kuhn, V.; Doranehgard, F.; Stange, R.; Wieskötter, B.; Beckmann, J.; Salmen, P.; Springorum, H.-R.; Straub, R.H.; Zimmer, A.; et al. Absence of substance P and the sympathetic nervous system impact on bone structure and chondrocyte differentiation in an adult model of endochondral ossification. Matrix Biol. 2014, 38, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Grässel, S.G. The role of peripheral nerve fibers and their neurotransmitters in cartilage and bone physiology and pathophysiology. Arthritis Res. Ther. 2014, 16, 485. [Google Scholar] [CrossRef] [Green Version]
- Imai, S.; Matsusue, Y. Neuronal regulation of bone metabolism and anabolism: Calcitonin gene-related peptide-, substance P-, and tyrosine hydroxylase-containing nerves and the bone. Microsc. Res. Tech. 2002, 58, 61–69. [Google Scholar] [CrossRef]
- Irie, K.; Hara-Irie, F.; Ozawa, H.; Yajima, T. Calcitonin gene-related peptide (CGRP)-containing nerve fibers in bone tissue and their involvement in bone remodeling. Microsc. Res. Tech. 2002, 58, 85–90. [Google Scholar] [CrossRef]
- Schou, W.S.; Ashina, S.; Amin, F.M.; Goadsby, P.J.; Ashina, M. Calcitonin gene-related peptide and pain: A systematic review. J. Headache Pain 2017, 18, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schinke, T.; Liese, S.; Priemel, M.; Haberland, M.; Schilling, A.F.; Catala-Lehnen, P.; Blicharski, D.; Rueger, J.M.; Gagel, R.F.; Emeson, R.B.; et al. Decreased bone formation and osteopenia in mice lacking alpha-calcitonin gene-related peptide. J. Bone Miner. Res. 2004, 19, 2049–2056. [Google Scholar] [CrossRef] [PubMed]
- Niedermair, T.; Schirner, S.; Lasheras, M.G.; Straub, R.H.; Grässel, S. Absence of α-calcitonin gene-related peptide modulates bone remodeling properties of murine osteoblasts and osteoclasts in an age-dependent way. Mech. Ageing Dev. 2020, 189, 111265. [Google Scholar] [CrossRef] [PubMed]
- Pertovaara, A. The noradrenergic pain regulation system: A potential target for pain therapy. Eur. J. Pharmacol. 2013, 716, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Reference Atlas: Allen Brain Atlas: Mouse Brain. Available online: https://mouse.brain-map.org/static/atlas (accessed on 3 January 2022).
- ISH Data: Allen Brain Atlas: Mouse Brain. Available online: https://mouse.brain-map.org/ (accessed on 3 January 2022).
- Sophocleous, A.; Idris, A.I. Rodent models of osteoporosis. Bonekey Rep. 2014, 3, 614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouxsein, M.L.; Myers, K.S.; Shultz, K.L.; Donahue, L.R.; Rosen, C.J.; Beamer, W.G. Ovariectomy-Induced Bone Loss Varies Among Inbred Strains of Mice. J. Bone Miner. Res. 2005, 20, 1085–1092. [Google Scholar] [CrossRef]
- Straub, R.H.; Wiest, R.; Strauch, U.G.; Härle, P.; Schölmerich, J. The role of the sympathetic nervous system in intestinal inflammation. Gut 2006, 55, 1640. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Liu, Y.; Yang, Z.; Wu, T.; Lo, H.T.; Xu, J.; Zhang, J.; Lin, W.; Zhang, J.; Feng, L.; et al. Vasoactive Intestinal Peptide Promotes Fracture Healing in Sympathectomized Mice. Calcif. Tissue Int. 2021, 109, 55–65. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Devlin, M.J.; Glatt, V.; Dhillon, H.; Pierroz, D.D.; Ferrari, S.L. Mice lacking beta-adrenergic receptors have increased bone mass but are not protected from deleterious skeletal effects of ovariectomy. Endocrinology 2009, 150, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Ding, W.-G.; Zhang, Z.-M.; Zhang, Y.-H.; Jiang, S.-D.; Jiang, L.-S.; Dai, L.-Y. Changes of substance P during fracture healing in ovariectomized mice. Regul. Pept. 2010, 159, 28–34. [Google Scholar] [CrossRef]
- Zheng, X.-F.; Li, B.; Zhang, Y.-H.; Yang, Y.-H.; Meng, X.-Y.; Jiang, S.-D.; Jiang, L.-S. Blockade of substance P receptor attenuates osteoporotic pain, but not bone loss, in ovariectomized mice. Menopause 2013, 20, 1074. [Google Scholar] [CrossRef]
- Zheng, X.-F.; Zhao, E.-D.; He, J.-Y.; Zhang, Y.-H.; Jiang, S.-D.; Jiang, L.-S. Inhibition of substance P signaling aggravates the bone loss in ovariectomy-induced osteoporosis. Prog. Biophys. Mol. Biol. 2016, 122, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Maggi, C.A.; Patacchini, R.; Rovero, P.; Giachetti, A. Tachykinin receptors and tachykinin receptor antagonists. J. Auton. Pharmacol. 1993, 13, 23–93. [Google Scholar] [CrossRef] [PubMed]
- Sandweiss, A.J.; Vanderah, T.W. The pharmacology of neurokinin receptors in addiction: Prospects for therapy. Subst. Abus. Rehabil. 2015, 6, 93–102. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.; Li, F.; Han, Y.; Li, Z.; Xiao, J. Neuropeptides are associated with pain threshold and bone microstructure in ovariectomized rats. Neuropeptides 2020, 81, 101995. [Google Scholar] [CrossRef] [PubMed]
- Appelt, J.; Baranowsky, A.; Jahn, D.; Yorgan, T.; Köhli, P.; Otto, E.; Farahani, S.K.; Graef, F.; Fuchs, M.; Herrera, A.; et al. The neuropeptide calcitonin gene-related peptide alpha is essential for bone healing. EBioMedicine 2020, 59, 102970. [Google Scholar] [CrossRef]
- Strong, J.A.; Zhang, J.-M.; Schaible, H.-G. The Sympathetic Nervous System and Pain. In The Oxford Handbook of the Neurobiology of Pain; Oxford University Press: Oxford, UK, 2020. [Google Scholar]
- Greco, R.; Tassorelli, C.; Sandrini, G.; Di, B.P.; Buscone, S.; Nappi, G. Role of calcitonin gene-related peptide and substance P in different models of pain. Cephalalgia Int. J. Headache 2008, 28, 114–126. [Google Scholar] [CrossRef]
- Hunyady, Á.; Hajna, Z.; Gubányi, T.; Scheich, B.; Kemény, Á.; Gaszner, B.; Borbély, É.; Helyes, Z. Hemokinin-1 is an important mediator of pain in mouse models of neuropathic and inflammatory mechanisms. Brain Res. Bull. 2019, 147, 165–173. [Google Scholar] [CrossRef]
- Pilowsky, P.; Minson, J.; Hodgson, A.; Howe, P.; Chalmers, J. Does substance P coexist with adrenaline in neurones of the rostral ventrolateral medulla in the rat? Neurosci. Lett. 1986, 71, 293–298. [Google Scholar] [CrossRef]
- Altier, N.; Stewart, J. Intra-VTA infusions of the substance P analogue, DiMe-C7, and intra-accumbens infusions of amphetamine induce analgesia in the formalin test for tonic pain. Brain Res. 1993, 628, 279–285. [Google Scholar] [CrossRef]
- Lassen, L.H.; Jacobsen, V.B.; Haderslev, P.A.; Sperling, B.; Iversen, H.K.; Olesen, J.; Tfelt-Hansen, P. Involvement of calcitonin gene-related peptide in migraine: Regional cerebral blood flow and blood flow velocity in migraine patients. J. Headache Pain 2008, 9, 151–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, K.A.; Birk, S.; Lassen, L.H.; Kruuse, C.; Jonassen, O.; Lesko, L.; Olesen, J. The CGRP-antagonist, BIBN4096BS does not affect cerebral or systemic haemodynamics in healthy volunteers. Cephalalgia Int. J. Headache 2005, 25, 139–147. [Google Scholar] [CrossRef] [PubMed]
- May, A.; Goadsby, P.J. Substance P receptor antagonists in the therapy of migraine. Expert Opin. Investig. Drugs 2001, 10, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Charles, A.; Pozo-Rosich, P. Targeting calcitonin gene-related peptide: A new era in migraine therapy. Lancet 2019, 394, 1765–1774. [Google Scholar] [CrossRef]
- Haubensak, W.; Kunwar, P.S.; Cai, H.; Ciocchi, S.; Wall, N.R.; Ponnusamy, R.; Biag, J.; Dong, H.-W.; Deisseroth, K.; Callaway, E.M.; et al. Genetic dissection of an amygdala microcircuit that gates conditioned fear. Nature 2010, 468, 270–276. [Google Scholar] [CrossRef] [Green Version]
- Ehrlich, I.; Humeau, Y.; Grenier, F.; Ciocchi, S.; Herry, C.; Lüthi, A. Amygdala inhibitory circuits and the control of fear memory. Neuron 2009, 62, 757–771. [Google Scholar] [CrossRef] [Green Version]
- Hua, T.; Chen, B.; Lu, D.; Sakurai, K.; Zhao, S.; Han, B.-X.; Kim, J.; Yin, L.; Chen, Y.; Lu, J.; et al. General anesthetics activate a potent central pain-suppression circuit in the amygdala. Nat. Neurosci. 2020, 23, 854–868. [Google Scholar] [CrossRef]
- Wank, I.; Pliota, P.; Badurek, S.; Kraitsy, K.; Kaczanowska, J.; Griessner, J.; Kreitz, S.; Hess, A.; Haubensak, W. Central amygdala circuitry modulates nociceptive processing through differential hierarchical interaction with affective network dynamics. Commun. Biol. 2021, 4, 732. [Google Scholar] [CrossRef]
- Ghosh, S.; Laxmi, T.R.; Chattarji, S. Functional connectivity from the amygdala to the hippocampus grows stronger after stress. J. Neurosci. 2013, 33, 7234–7244. [Google Scholar] [CrossRef] [Green Version]
- Ebner, K.; Muigg, P.; Singewald, G.; Singewald, N. Substance P in stress and anxiety: NK-1 receptor antagonism interacts with key brain areas of the stress circuitry. Ann. N. Y. Acad. Sci. 2008, 1144, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Avegno, E.M.; Lobell, T.D.; Itoga, C.A.; Baynes, B.B.; Whitaker, A.M.; Weera, M.M.; Edwards, S.; Middleton, J.W.; Gilpin, N.W. Central Amygdala Circuits Mediate Hyperalgesia in Alcohol-Dependent Rats. J. Neurosci. 2018, 38, 7761–7773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanegas, H.; Schaible, H.-G. Descending control of persistent pain: Inhibitory or facilitatory? Brain Res. Brain Res. Rev. 2004, 46, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.M.; Huang, S.M.; Strangman, N.M.; Tsou, K.; Sañudo-Peña, M.C. Pain modulation by release of the endogenous cannabinoid anandamide. Proc. Natl. Acad. Sci. USA 1999, 96, 12198–12203. [Google Scholar] [CrossRef] [Green Version]
- Hosobuchi, Y.; Adams, J.E.; Linchitz, R. Pain relief by electrical stimulation of the central gray matter in humans and its reversal by naloxone. Science 1977, 197, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Damoiseaux, J.S.; Rombouts, S.; Barkhof, F.; Scheltens, P.; Stam, C.J.; Smith, S.M.; Beckmann, C.F. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. USA 2006, 103, 13848–13853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grandjean, J.; Canella, C.; Anckaerts, C.; Ayrancı, G.; Bougacha, S.; Bienert, T.; Buehlmann, D.; Coletta, L.; Gallino, D.; Gass, N.; et al. Common functional networks in the mouse brain revealed by multi-centre resting-state fMRI analysis. Neuroimage 2020, 205, 116278. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, J.; Schroeter, A.; Batata, I.; Rudin, M. Optimization of anesthesia protocol for resting-state fMRI in mice based on differential effects of anesthetics on functional connectivity patterns. Neuroimage 2014, 102 Pt 2, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Lenz, C.; Rebel, A.; van Ackern, K.; Kuschinsky, W.; Waschke, K.F. Local cerebral blood flow, local cerebral glucose utilization, and flow-metabolism coupling during sevoflurane versus isoflurane anesthesia in rats. Anesthesiology 1998, 89, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- Masamoto, K.; Fukuda, M.; Vazquez, A.; Kim, S.-G. Dose-dependent effect of isoflurane on neurovascular coupling in rat cerebral cortex. Eur. J. Neurosci. 2009, 30, 242–250. [Google Scholar] [CrossRef] [Green Version]
- Jonckers, E.; Delgado y Palacios, R.; Shah, D.; Guglielmetti, C.; Verhoye, M.; van der Linden, A. Different anesthesia regimes modulate the functional connectivity outcome in mice. Magn. Reson. Med. 2014, 72, 1103–1112. [Google Scholar] [CrossRef]
- Canario, E.; Chen, D.; Biswal, B. A review of resting-state fMRI and its use to examine psychiatric disorders. Psychoradiology 2021, 1, 42–53. [Google Scholar] [CrossRef]
- Hohenfeld, C.; Werner, C.J.; Reetz, K. Resting-state connectivity in neurodegenerative disorders: Is there potential for an imaging biomarker? NeuroImage Clin. 2018, 18, 849–870. [Google Scholar] [CrossRef]
- Kreitz, S.; Zambon, A.; Ronovsky, M.; Budinsky, L.; Helbich, T.H.; Sideromenos, S.; Ivan, C.; Konerth, L.; Wank, I.; Berger, A.; et al. Maternal immune activation during pregnancy impacts on brain structure and function in the adult offspring. Brain Behav. Immun. 2020, 83, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Loggia, M.L.; Cahalan, C.M.; Harris, R.E.; Beissner, F.; Garcia, R.G.; Kim, H.; Wasan, A.D.; Edwards, R.R.; Napadow, V. The somatosensory link in fibromyalgia: Functional connectivity of the primary somatosensory cortex is altered by sustained pain and is associated with clinical/autonomic dysfunction. Arthritis Rheumatol. 2015, 67, 1395–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Loggia, M.L.; Edwards, R.R.; Wasan, A.D.; Gollub, R.L.; Napadow, V. Sustained deep-tissue pain alters functional brain connectivity. Pain 2013, 154, 1343–1351. [Google Scholar] [CrossRef] [Green Version]
- Bolwerk, A.; Seifert, F.; Maihöfner, C. Altered Resting-State Functional Connectivity in Complex Regional Pain Syndrome. J. Pain 2013, 14, 1107–1115.e8. [Google Scholar] [CrossRef]
- Agcaoglu, O.; Miller, R.; Mayer, A.R.; Hugdahl, K.; Calhoun, V.D. Lateralization of resting state networks and relationship to age and gender. Neuroimage 2015, 104, 310–325. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Zhang, Z.; Liu, J.; Qin, L.; Pang, X.; Zheng, J. Disruption and lateralization of cerebellar-cerebral functional networks in right temporal lobe epilepsy: A resting-state fMRI study. Epilepsy Behav. 2019, 96, 80–86. [Google Scholar] [CrossRef]
- Lee, K.; Khoo, H.M.; Lina, J.-M.; Dubeau, F.; Gotman, J.; Grova, C. Disruption, emergence and lateralization of brain network hubs in mesial temporal lobe epilepsy. NeuroImage Clin. 2018, 20, 71–84. [Google Scholar] [CrossRef]
- Lv, H.; Zhao, P.; Liu, Z.; Liu, X.; Ding, H.; Liu, L.; Wang, G.; Xie, J.; Zeng, R.; Chen, Y.; et al. Lateralization effects on functional connectivity of the auditory network in patients with unilateral pulsatile tinnitus as detected by functional MRI. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 81, 228–235. [Google Scholar] [CrossRef]
- Ke, J.; Yu, Y.; Zhang, X.; Su, Y.; Wang, X.; Hu, S.; Dai, H.; Hu, C.; Zhao, H.; Dai, L. Functional Alterations in the Posterior Insula and Cerebellum in Migraine Without Aura: A Resting-State MRI Study. Front. Behav. Neurosci. 2020, 14, 567588. [Google Scholar] [CrossRef] [PubMed]
- Valet, M.; Sprenger, T.; Boecker, H.; Willoch, F.; Rummeny, E.; Conrad, B.; Erhard, P.; Tolle, T.R. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain--an fMRI analysis. Pain 2004, 109, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Jensen, K.; Loiotile, R.; Cheetham, A.; Wey, H.-Y.; Tan, Y.; Rosen, B.; Smoller, J.W.; Kaptchuk, T.J.; Gollub, R.L. Functional connectivity of the frontoparietal network predicts cognitive modulation of pain. Pain 2013, 154, 459–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flodin, P.; Martinsen, S.; Altawil, R.; Waldheim, E.; Lampa, J.; Kosek, E.; Fransson, P. Intrinsic Brain Connectivity in Chronic Pain: A Resting-State fMRI Study in Patients with Rheumatoid Arthritis. Front. Hum. Neurosci. 2016, 10, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millan, M.J. Descending control of pain. Prog. Neurobiol. 2002, 66, 355–474. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. Pain-autonomic interactions: A selective review. Clin. Auton. Res. 2001, 11, 343–349. [Google Scholar] [CrossRef]
- Sachs, C.; Jonsson, G. Mechanisms of action of 6-hydroxydopamine. Biochem. Pharmacol. 1975, 24, 1–8. [Google Scholar] [CrossRef]
- Mitchell, S.A.T.; Majuta, L.A.; Mantyh, P.W. New Insights in Understanding and Treating Bone Fracture Pain. Curr. Osteoporos. Rep. 2018, 16, 325–332. [Google Scholar] [CrossRef]
- Röntgen, V.; Blakytny, R.; Matthys, R.; Landauer, M.; Wehner, T.; Göckelmann, M.; Jermendy, P.; Amling, M.; Schinke, T.; Claes, L.; et al. Fracture healing in mice under controlled rigid and flexible conditions using an adjustable external fixator. J. Orthop. Res. 2010, 28, 1456–1462. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: Cambridge, MA, USA, 2006; ISBN 9780080475158. [Google Scholar]
- Fox, M.D.; Zhang, D.; Snyder, A.Z.; Raichle, M.E. The global signal and observed anticorrelated resting state brain networks. J. Neurophysiol. 2009, 101, 3270–3283. [Google Scholar] [CrossRef]
- Kreitz, S.; de Celis Alonso, B.; Uder, M.; Hess, A. A New Analysis of Resting State Connectivity and Graph Theory Reveals Distinctive Short-Term Modulations due to Whisker Stimulation in Rats. Front. Neurosci. 2018, 12, 334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lein, E.S.; Hawrylycz, M.J.; Ao, N.; Ayres, M.; Bensinger, A.; Bernard, A.; Boe, A.F.; Boguski, M.S.; Brockway, K.S.; Byrnes, E.J.; et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature 2007, 445, 168–176. [Google Scholar] [CrossRef] [PubMed]

| Resting State | Neurotransmitter | WT vs. α-CGRP−/− | WT vs. Tac1−/− | WT vs. SYX |
| Noradrenaline | ↓: Bs, cxAss, cxS | ↑: cxAss, cxS, Hc | ↑: cxAss, cxS ↓: Bs | |
| Substance P | ↓: Bs, cxAss, Hy, Am, Bg | ↑: cxAss ↓: Am | ↑: cxAss, Am, Bg ↓: Bs, Hy, Am | |
| α-CGRP | ↓: Hy, Bg | |||
| Nociception | Neurotransmitter | WT vs. α-CGRP−/− | WT vs. Tac1−/− | WT vs. SYX |
| Noradrenaline | ↓: Th, Hc | ↓: Th, Hc | ||
| Substance P | ↓: Bs, Hy, Am | ↓: Bs, Hy, Am | ||
| α-CGRP |
| Baseline vs. D5 | Neurotransmitter | WT | α-CGRP−/− | Tac1−/− | SYX |
| Noradrenaline | ↓: cxS, Hc | ↑: Hc ↓: Th, cxAss, cxS | ↑: cxS (right) ↓: cxS (left) | ↑: Bs, Th, cxAss, cxS, Hc | |
| Substance P | ↑: Am | ↑: Am | ↑: Bs, Am | ↑: Bs, cxAss, Am, Bg | |
| α-CGRP | |||||
| Baseline vs. D21 | Neurotransmitter | WT | α-CGRP−/− | Tac1−/− | SYX |
| Noradrenaline | ↑: cxS, Hc | ↑: cxS ↓: Bs, Th, Hc | ↑: Hc | ↑: cxS, Hc | |
| Substance P | ↑: cxAss, Hc, PAG | ↓: Bs, PAG | ↑: Bs, Am, Hc | ↑: Hc | |
| α-CGRP |
| Baseline vs. D5 | Neurotransmitter | WT | α-CGRP−/− | Tac1−/− | SYX |
| Noradrenaline | ↑: cxAss, cxS | ↑: Bs, Th, cxAss, cxS, Hc | ↑: cxAss (left) cxS (left) ↓: Bs, Th, cxAss (right), cxS (right), Hc | ↑: Bs, Th, cxAss, cxS, Hc | |
| Substance P | ↓: Am, Hy | ↑: cxAss, Am, Hy, Bg ↓: Am | ↓: cxAss, Bs, Am, Hy | ↑: Bs, cxAss, Am, Hy, Bg | |
| α-CGRP | ↓: Cb | ↑: Cb | |||
| Baseline vs. D21 | Neurotransmitter | WT | α-CGRP−/− | Tac1−/− | SYX |
| Noradrenaline | ↑: Bs, cxS | ↑: Bs, Th, cxAss, cxS, Hc | ↑: cxAss, cxS ↓: Bs, Hc | ↑: Bs ↓: cxAss, cxS | |
| Substance P | ↑: Bs, cxAss, Am, Bg ↓: Am | ↑: Bs, cxAss, Am, Hy, Bg | ↑: cxAss, Bg ↓: Bs, Am, Hy | ↑: Am ↓: Bs, cxAss, Hy, Bg | |
| α-CGRP | ↓: Cb | ↑: Cb | ↓: Cb |
| Treatment Duration | −2 Days Before Fracture | 5 Days Post Fracture | 21 Days Post Fracture | |
|---|---|---|---|---|
| Mouse Models | ||||
| Wildtype (WT) | 13 | 13 | 5 (+3 of cohort 2 for biomechanical tests) | |
| alpha-CGRP-knockout (α-CGRP−/−) | 9 | 9 | 5 | |
| Tachykinin-1-knockout (Tac1−/−) | 9 | 9 | 5 | |
| Sympathectomized WT (SYX) | 8 (−1) | 8 (−1) | 4 (−1) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wank, I.; Niedermair, T.; Kronenberg, D.; Stange, R.; Brochhausen, C.; Hess, A.; Grässel, S. Influence of the Peripheral Nervous System on Murine Osteoporotic Fracture Healing and Fracture-Induced Hyperalgesia. Int. J. Mol. Sci. 2023, 24, 510. https://doi.org/10.3390/ijms24010510
Wank I, Niedermair T, Kronenberg D, Stange R, Brochhausen C, Hess A, Grässel S. Influence of the Peripheral Nervous System on Murine Osteoporotic Fracture Healing and Fracture-Induced Hyperalgesia. International Journal of Molecular Sciences. 2023; 24(1):510. https://doi.org/10.3390/ijms24010510
Chicago/Turabian StyleWank, Isabel, Tanja Niedermair, Daniel Kronenberg, Richard Stange, Christoph Brochhausen, Andreas Hess, and Susanne Grässel. 2023. "Influence of the Peripheral Nervous System on Murine Osteoporotic Fracture Healing and Fracture-Induced Hyperalgesia" International Journal of Molecular Sciences 24, no. 1: 510. https://doi.org/10.3390/ijms24010510
APA StyleWank, I., Niedermair, T., Kronenberg, D., Stange, R., Brochhausen, C., Hess, A., & Grässel, S. (2023). Influence of the Peripheral Nervous System on Murine Osteoporotic Fracture Healing and Fracture-Induced Hyperalgesia. International Journal of Molecular Sciences, 24(1), 510. https://doi.org/10.3390/ijms24010510






