Dosimetry Effects Due to the Presence of Fe Nanoparticles for Potential Combination of Hyperthermic Cancer Treatment with MRI-Based Image-Guided Radiotherapy
Abstract
1. Introduction
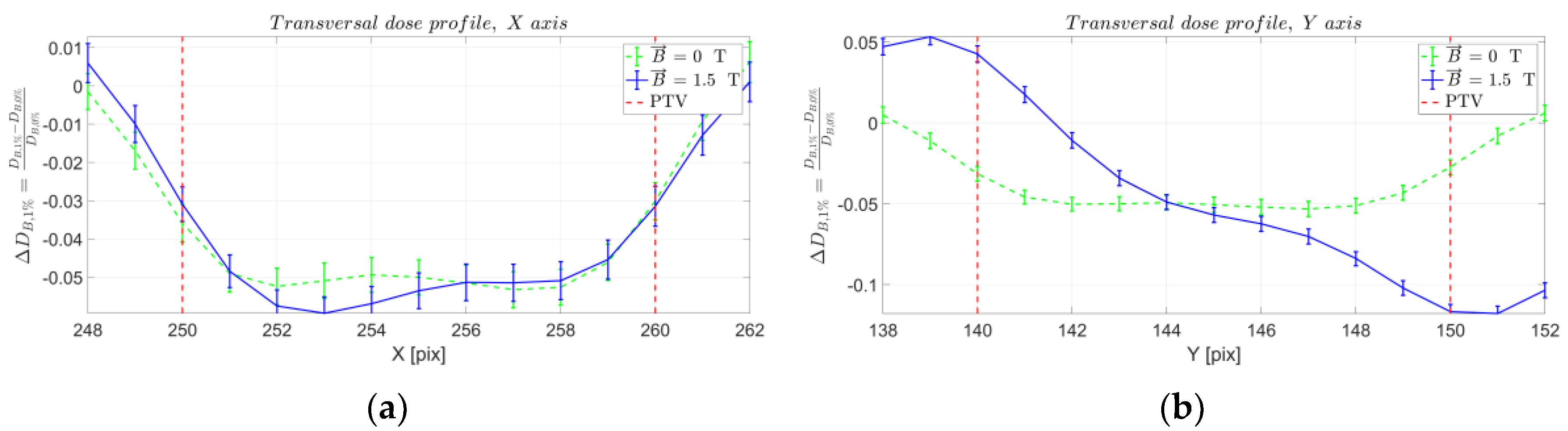
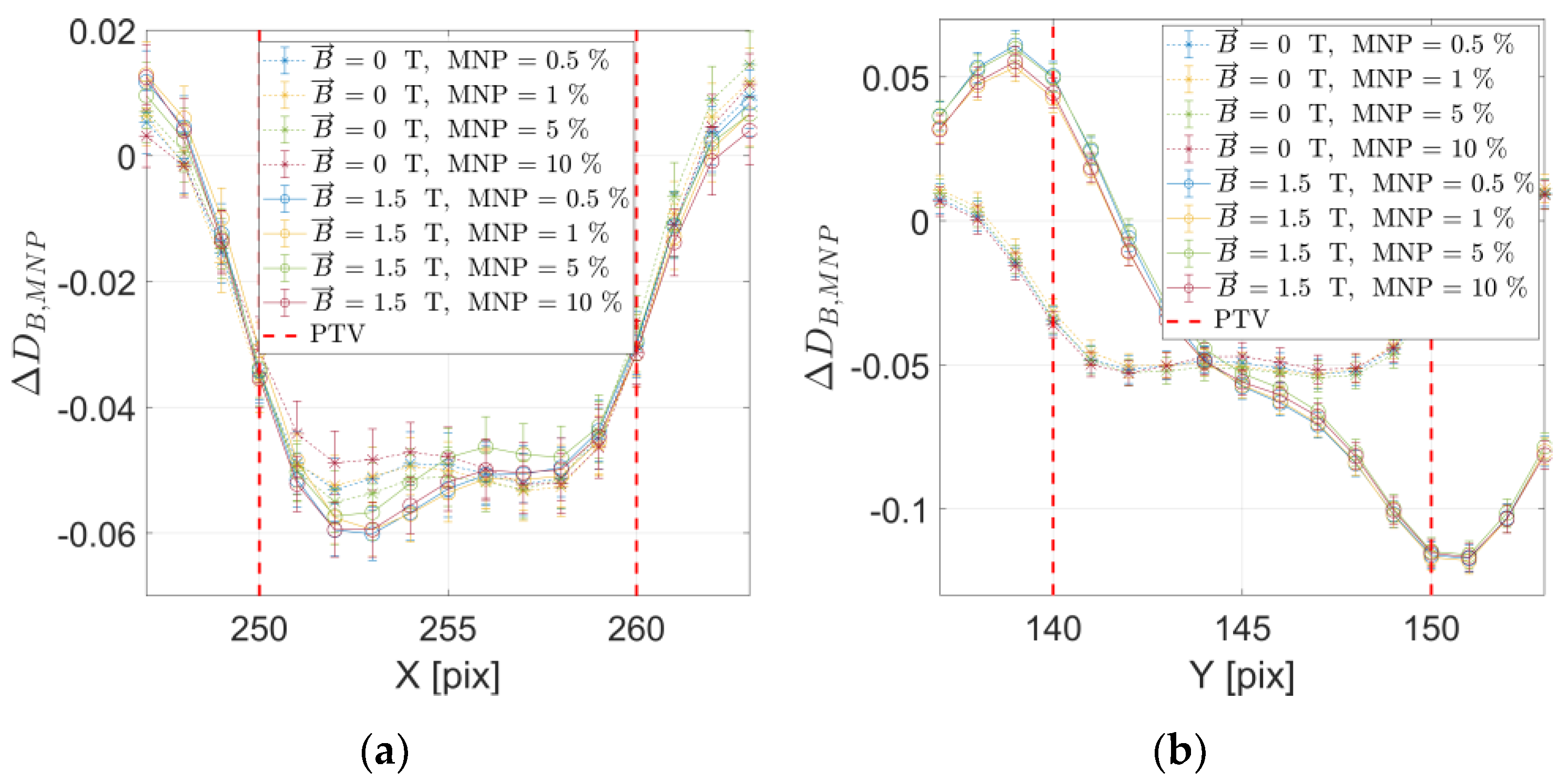
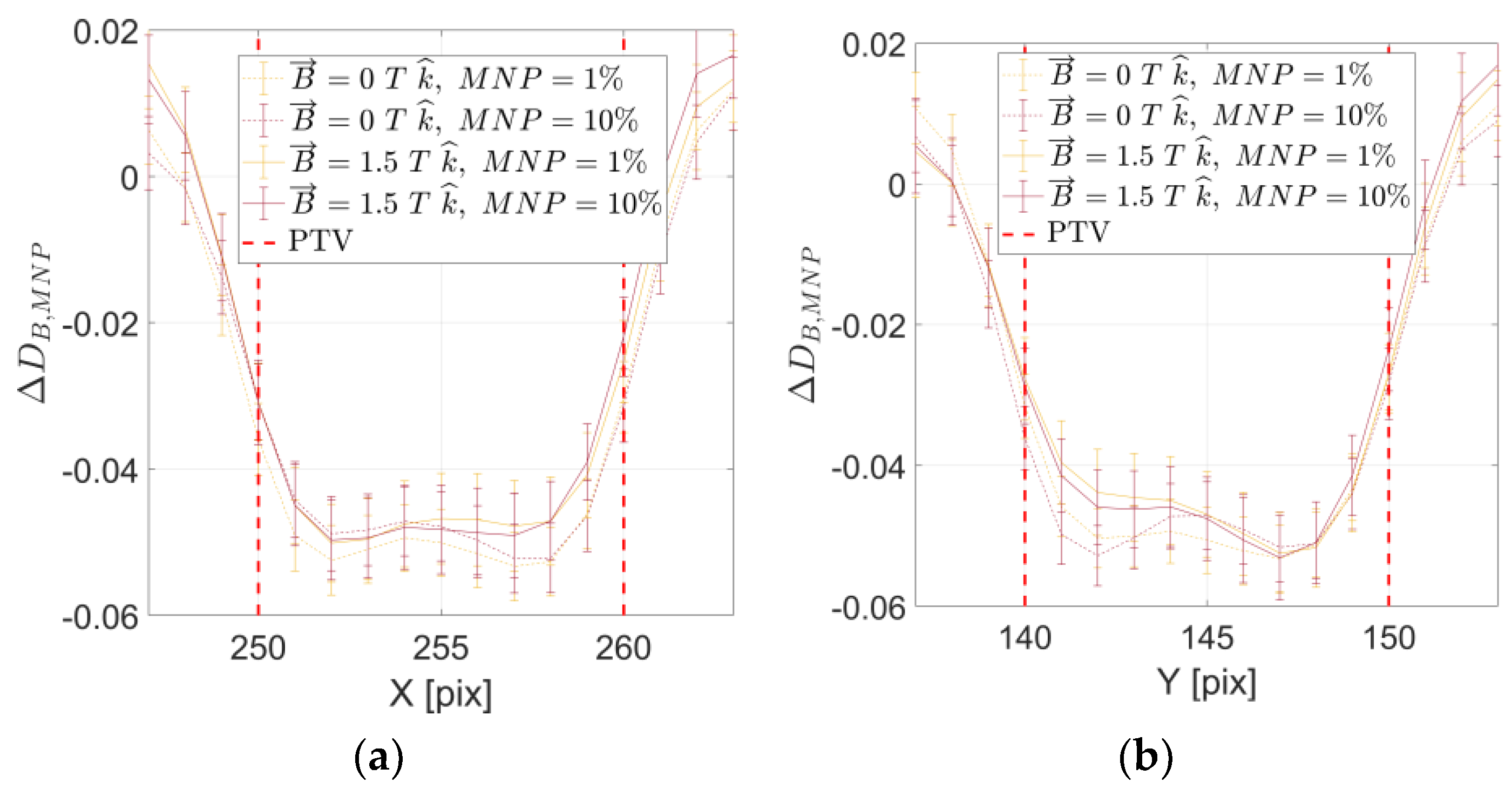
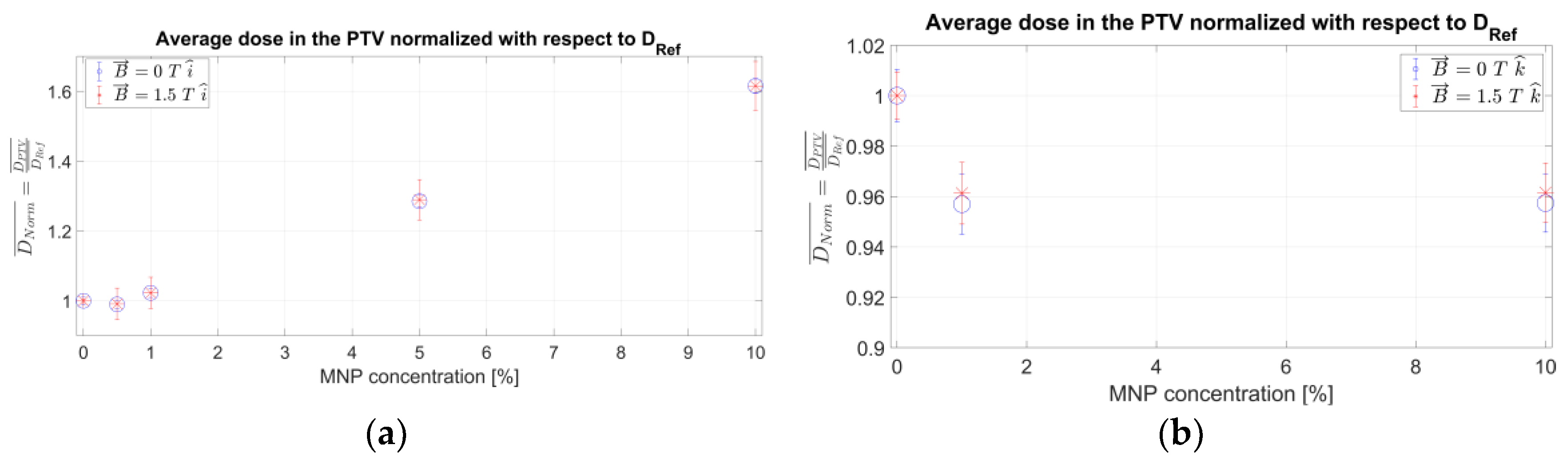
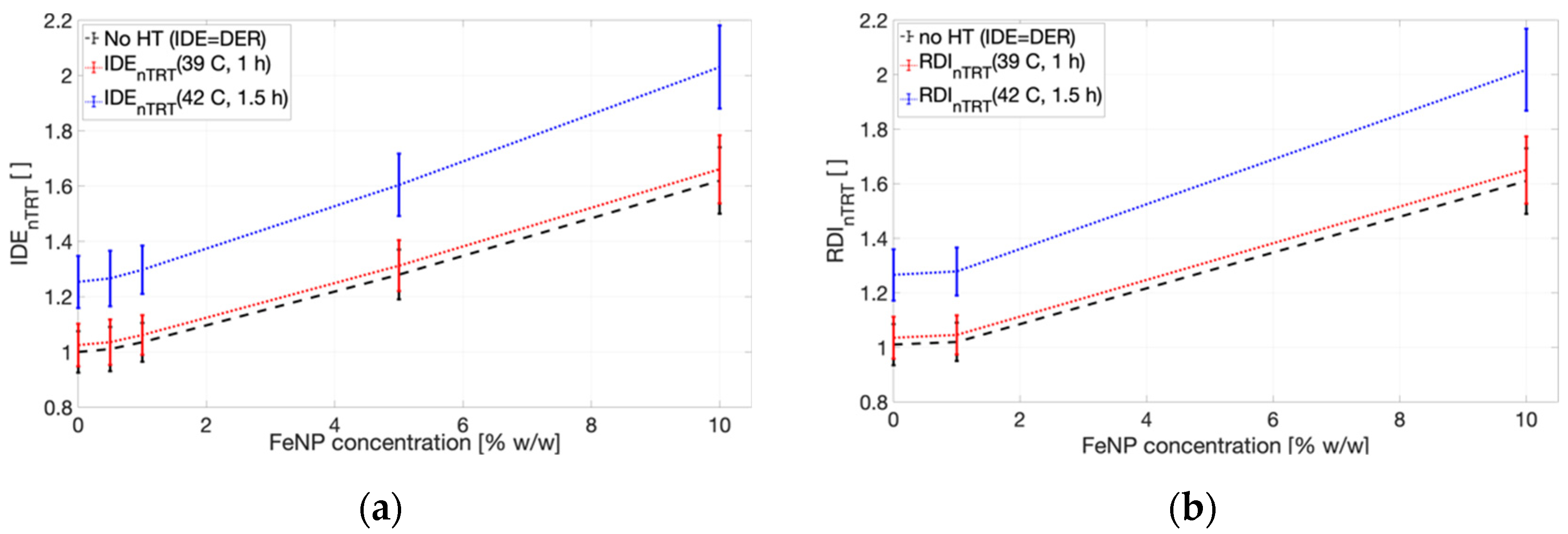
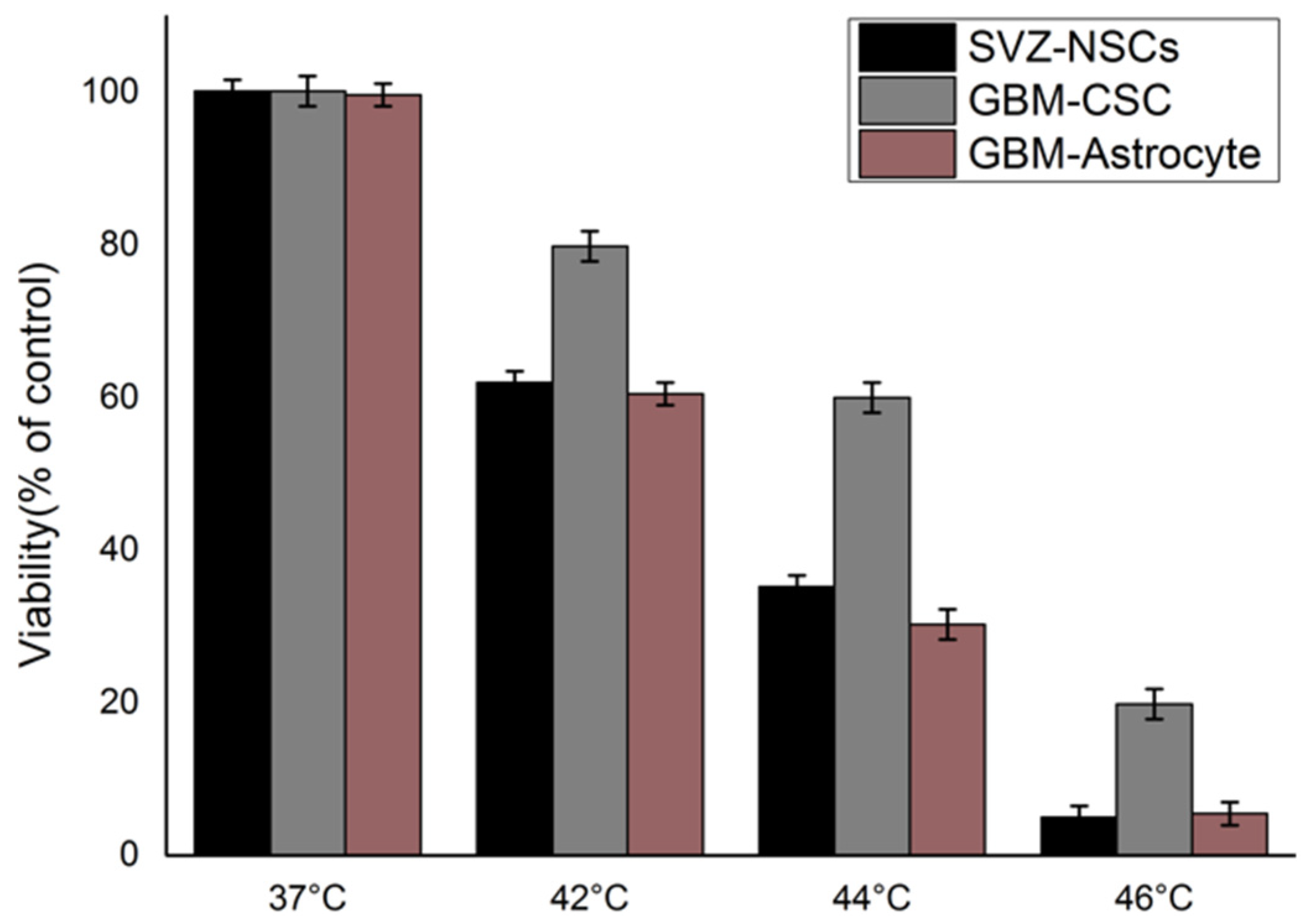
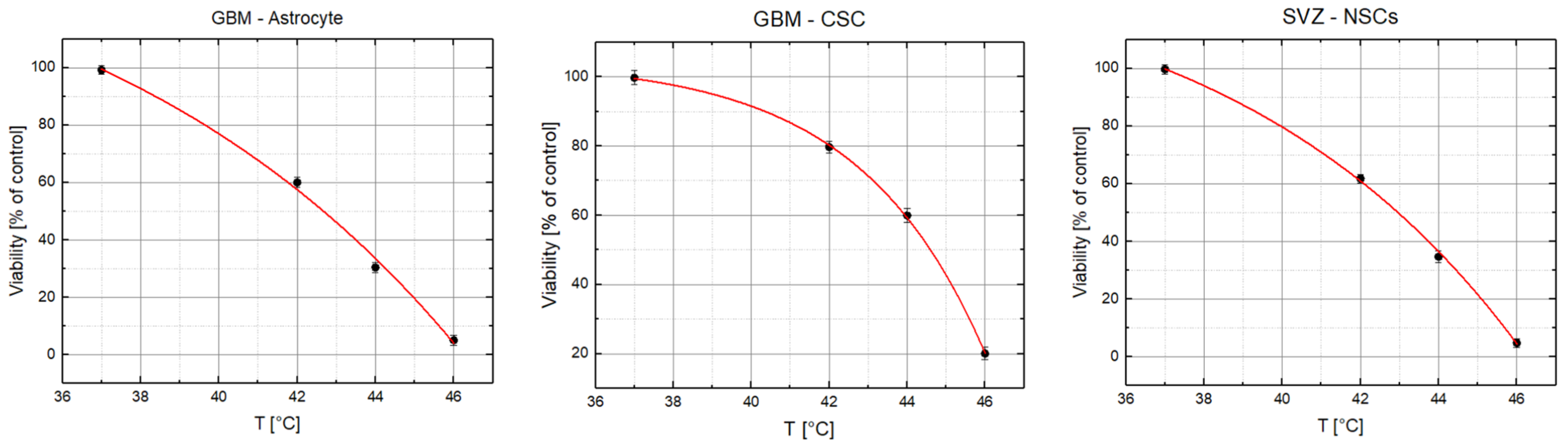
2. Results
3. Discussion
4. Materials and Methods
4.1. Dosimetry Effects Due to FeNPs Characterized by Monte Carlo Simulations
4.1.1. Monte Carlo Model Accounting for External Magnetic Field
4.1.2. Simulation of Realistic Patient-Specific Setup
4.2. Thermal Radiosentization Model
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neuberger, T.; Schöpf, B.; Hofmann, H.; Hofmann, M.; von Rechenberg, B. Superparamagnetic nanoparticles for biomedical applications: Possibilities and limitations of a new drug delivery system. J. Magn. Magn. Mater. 2005, 293, 483–496. [Google Scholar] [CrossRef]
- Berry, C.C. Possible exploitation of magnetic nanoparticle–cell interaction for biomedical applications. J. Mater. Chem. 2005, 15, 543–547. [Google Scholar] [CrossRef]
- Tran, N.; Webster, T.J. Magnetic nanoparticles: Biomedical applications and challenges. J. Mater. Chem. 2010, 40, 8760–8767. [Google Scholar] [CrossRef]
- Yaseen Ahmad, M.; Yue, H.; Tegafaw, T.; Liu, S.; Long, D.S.; Lee, G.H.; Nam, S.-W.; Chang, Y. Functionalized Lanthanide Oxide Nanoparticles for Tumor Targeting, Medical Imaging, and Therapy. Pharmaceutics 2021, 13, 1890. [Google Scholar] [CrossRef] [PubMed]
- Nedyalkova, M.; Donkova, B.; Romanova, J.; Tzvetkov, G.; Madurga, S.; Simeonov, V. Iron oxide nanoparticles—In vivo/in vitro biomedical applications and in silico studies. Adv. Colloid Interface Sci. 2017, 249, 192–212. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2015, 99, 28–51. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Shen, Z.; Yu, L.; Wei, M.; Huilin, Y. Manipulating nanoparticle transport within blood flow through external forces: An exemplar of mechanics in nanomedicine. Proc. R. Soc. A Math. Phys. Eng. Sci. 2018, 474, 20170845. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.; Goetsch, L.; Dumontet, C.; Corvaïa, N. Strategies and challenges for the next generation of antibody-drug conjugates. Nat. Rev. Drug Discov. 2017, 16, 315–337. [Google Scholar] [CrossRef]
- Dormann, J.L.; Fiorani, D. Magnetic Properties of Fine Particles, 1st ed.; ElSevier: Amsterdam, The Netherlands, 1992; ISBN 9780444597410. [Google Scholar]
- Debaisieux, S.; Rayne, F.; Yezid, H.; Beaumelle, B. The Ins and Outs of HIV-1 Tat. Traffic 2011, 13, 355–363. [Google Scholar] [CrossRef]
- Kodama, R.H. Magnetic nanoparticles. J. Magn. Magn. Mater. 1999, 200, 359–372. [Google Scholar] [CrossRef]
- Comes Franchini, M.; Baldi, G.; Bonacchi, D.; Gentili, D.; Giudetti, G.; Lascialfari, A.; Corti, M.; Marmorato, P.; Ponti, J.; Micotti, E.; et al. Bovine Serum Albumin-Based Magnetic Nanocarrier for MRI Diagnosis and Hyperthermic Therapy: A Potential Theranostic Approach Against Cancer. Small 2010, 6, 366–370. [Google Scholar] [CrossRef]
- Joseph, C.; Daniels, A.; Singh, S.; Singh, M. Histidine-Tagged Folate-Targeted Gold Nanoparticles for Enhanced Transgene Expression in Breast Cancer Cells In Vitro. Pharmaceutics 2022, 4, 53. [Google Scholar] [CrossRef] [PubMed]
- Miele, D.; Xia, X.; Catenacci, L.; Sorrenti, M.; Rossi, S.; Sandri, S.; Ferrari, F.; Rossi, J.J.; Bonferoni, M.C. Chitosan Oleate Coated PLGA Nanoparticles as siRNA Drug Delivery System. Pharmaceutics 2021, 13, 1716. [Google Scholar] [CrossRef] [PubMed]
- Mody, V.V.; Cox, A.; Shah, S.; Singh, A.; Bevins, W.; Parihar, H. Magnetic nanoparticle drug delivery systems for targeting tumor. Appl. Nanosci. 2014, 4, 385–392. [Google Scholar] [CrossRef]
- McBain, S.C.; Yiu, H.H.; Dobson, J. Magnetic nanoparticles for gene and drug delivery. Int. J. Nanomed. 2008, 3, 169–180. [Google Scholar] [CrossRef]
- Chertok, B.; David, A.E.; Yang, V.C. Polyethyleneimine-modified iron oxide nanoparticles for brain tumor drug delivery using magnetic targeting and intra-carotid administration. Biomaterials 2010, 31, 6317–6324. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, J. Nanoparticulate systems for brain delivery of drugs. Adv. Drug Deliv. Rev. 2001, 47, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Lockman, P.R.; Mumper, R.J.; Khan, M.A.; Allen, D.D. Nanoparticle Technology for Drug Delivery Across the Blood-Brain Barrier. Drug Dev. Ind. Pharm. 2002, 28, 1–13. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurences and Uses, 2nd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003; ISBN 9783527302741. [Google Scholar]
- Bañobre-López, M.; Teijeiro, A.; Rivas, J. Magnetic nanoparticle-based hyperthermia for cancer treatment. Rep. Pract. Oncol. Radiother. 2013, 18, 397–400. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, L.; Cheng, R.; Mao, L.; Arnold, R.D.; Howerth, E.W.; Chen, Z.G.; Platt, S. Magnetic Nanoparticle-Based Hyperthermia for Head & Neck Cancer in Mouse Models. Theranostics 2012, 2, 113–121. [Google Scholar] [CrossRef]
- Aggarwal, P.; Hall, J.B.; McLeland, C.B.; Dobrovolskaia, M.A.; McNeil, S.E. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv. Drug Deliv. Rev. 2009, 61, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Dobrovolskaia, M.A. Immunological effects of iron oxide nanoparticles and iron-based complex drug formulations: Therapeutic benefits, toxicity, mechanistic insights, and translational considerations. Nanomedicine 2018, 14, 977–990. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Zhu, S.; Qin, K.; Fan, K.; An, L. Macrophages Loaded with Fe Nanoparticles for Enhanced Photothermal Ablation of Tumors. J. Funct. Biomater. 2022, 13, 94. [Google Scholar] [CrossRef] [PubMed]
- Azarmi, S.; Roa, W.H.; Löbenberg, R. Targeted delivery of nanoparticles for the treatment of lung diseases. Adv. Drug Deliv. Rev. 2008, 60, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kohler, N.; Zhang, M. Surface modification of superparamagnetic magnetite nanoparticles and their intracellular uptake. Biomaterials 2002, 23, 1553–1561. [Google Scholar] [CrossRef]
- Hong, R.Y.; Feng, B.; Chen, L.L.; Liu, G.H.; Li, H.Z.; Zheng, Y.Z.; Wei, D.G. Synthesis, characterization and MRI application of dextran-coated Fe3O4 magnetic nanoparticles. Biochem. Eng. J. 2008, 42, 290–300. [Google Scholar] [CrossRef]
- Enpuku, K.; Minotani, T.; Gima, T.; Kuroki, Y.; Itoh, Y.; Yamashita, M.; Katakura, Y.; Kuhara, S. Detection of Magnetic Nanoparticles with Superconducting Quantum Interference Device (SQUID) Magnetometer and Application to Immunoassays. Jpn. J. Appl. Phys. 1999, 38. [Google Scholar] [CrossRef]
- Harivardhan Reddy, L.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic Nanoparticles: Design and Characterization, Toxicity and Biocompatibility, Pharmaceutical and Biomedical Applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef]
- Jain, T.K.; Reddy, M.K.; Morales, M.A.; Leslie-Pelecky, D.L.; Labhasetwar, V. Biodistribution, Clearance, and Biocompatibility of Iron Oxide Magnetic Nanoparticles in Rats. Mol. Pharm. 2008, 5, 316–327. [Google Scholar] [CrossRef]
- Park, S.-J.; Kim, S.; Lee, S.; Khim, Z.G.; Char, K.; Hyeon, T. Synthesis and Magnetic Studies of Uniform Iron Nanorods and Nanospheres. J. Am. Chem. Soc. 2000, 122, 8581–8582. [Google Scholar] [CrossRef]
- Vázquez, M.; Luna, C.; Morales, M.-P.; Sanz, R.; Serna, C.J.; Mijangos, C. Magnetic nanoparticles: Synthesis, ordering and properties. Phys. B Condens. Matter 2004, 354, 71–79. [Google Scholar] [CrossRef]
- Shearwood, C.; Blundell, S.J.; Baird, M.J.; Bland, J.A.C.; Gester, M.; Ahmed, H.; Hughes, H.P. Magnetoresistance and magnetization in submicron ferromagnetic gratings. J. Appl. Phys. 1994, 75. [Google Scholar] [CrossRef]
- Pollard, J.M.; Wen, Z.; Sadagopan, R.; Wang, J.; Ibbott, G.S. The future of image-guided radiotherapy will be MR guided. Br. J. Radiol. 2017, 90, 20160667. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Budgell, G.; MacKay, R.; Falk, S.; Faivre-Finn, C.; Dubec, M.; van Herk, M.; McWilliam, A. The Future of Image-guided Radiotherapy. Clin. Oncol. 2017, 28, 662–666. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boda-Heggemann, J.; Lohr, F.; Wenz, F.; Flentje, M.; Guckenberger, M. kV Cone-Beam CT-Based IGRT: A clinical review. Strahlenther. Onkol. 2011, 187, 284e291. [Google Scholar] [CrossRef]
- Chang, J.Y.; Senan, S.; Paul, M.A.; Mehran, R.J.; Louie, A.V.; Balter, P.; Groen, H.J.M.; McRae, S.E.; Widder, J.; Feng, L.; et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: A pooled analysis of two randomised trials. Lancet Oncol. 2015, 16, 630e637. [Google Scholar] [CrossRef] [PubMed]
- De Deene, Y.; Wheatley, M.; Dong, B.; Roberts, N.; Jelen, U.; Waddington, D.; Liney, G. Towards real-time 4D radiation dosimetry on an MRI-Linac. Phys. Med. Biol. 2020, 65. [Google Scholar] [CrossRef]
- Kirkby, C.; Stanescu, T.; Rathee, S.; Carlone, M.; Murray, B.; Fallone, B.G. Patient dosimetry for hybrid MRI-radiotherapy systems. Med. Phys. 2008, 35, 1019–1027. [Google Scholar] [CrossRef]
- Jelen, U.; Begg, J. Dosimetry needs for MRI-linacs. J. Phys. Conf. Ser. 2019, 1305, 012010. [Google Scholar] [CrossRef]
- van Asselen, B.; Woodings, S.J.; Hackett, S.L.; van Soest, T.L.; Kok, J.G.; Raaymakers, B.W.; Wolthaus, J.W. A formalism for reference dosimetry in photon beams in the presence of a magnetic field. Phys. Med. Biol. 2018, 63, 125008. [Google Scholar] [CrossRef]
- Raaymakers, B.W.; Lagendijk, J.J.; Overweg, J.; Kok, J.G.; Raaijmakers, A.J.; Kerkhof, E.M.; Van Der Put, R.W.; Meijsing, I.; Crijns, S.P.; Benedosso, F.; et al. Integrating a 1.5 T MRI scanner with a 6 MV accelerator: Proof of concept. Phys. Med. Biol. 2009, 54, N229eN237. [Google Scholar] [CrossRef] [PubMed]
- Trachsel, M.; Pojtinger, S.; Meier, M.; Schrader, M.; Kapsch, R.-P.; Kottler, C. Chemical radiation dosimetry in magnetic fields: Characterization of a Fricke-type chemical detector in 6 MV photon beams and magnetic fields up to 1.42 T. Phys. Med. Biol. 2020, 65, 065005. [Google Scholar] [CrossRef] [PubMed]
- Shokrollahi, H. Contrast agents for MRI. Mater. Sci. Eng. C 2013, 33, 4485–4497. [Google Scholar] [CrossRef]
- Caspani, S.; Magalhães, R.; Araújo, J.P.; Tavares Sousa, C. Review. Magnetic Nanomaterials as Contrast Agents for MRI. Materials 2020, 13, 2586. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, O.; Lincoln, J.D.; Melong, N.; Orr, B.N.; Fernandez, N.R.; Borsavage, J.; Berman, J.N.; Robar, J.; Ha, M.N. Radiation dose enhancement using gold nanoparticles with a diamond linear accelerator target: A multiple cell type analysis. Sci. Rep. 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Detappe, A.; Kunjachan, S.; Rottmann, J.; Robar, J.; Tsiamas, P.; Korideck, H.; Tillement, O.; Berbeco, R. AGuIX nanoparticles as a promising platform for image-guided radiation therapy. Cancer Nanotechnol. 2015, 6. [Google Scholar] [CrossRef]
- Gleich, B.; Weizenecker, J. Tomographic imaging using the nonlinear response of magnetic particles. Nature 2005, 435, 1214–1217. [Google Scholar] [CrossRef]
- Yue, R.; Zhang, C.; Xu, L.; Wang, Y.; Guan, G.; Lei, L.; Zhang, X.; Song, G. Dual key co-activated nanoplatform for switchable MRI monitoring accurate ferroptosis-based synergistic therapy. Chem 2022, 8, 1956–1981. [Google Scholar] [CrossRef]
- Macchione, M.; Lechón Páez, L.S.; Strumia, M.; Valente, M.; Mattea, F. Chemical Overview of Gel Dosimetry Systems: A Comprehensive Review. Gels 2022, 8, 663. [Google Scholar] [CrossRef]
- Gray, T.; Bassiri, N.; David, S.; Patel, D.Y.; Stathakis, S.; Kirby, N.; Mayer, K.M. A detailed experimental and Monte Carlo analysis of gold nanoparticle dose enhancement using 6 MV and 18 MV external beam energies in a macroscopic scale. Appl. Radiat. Isot. 2021, 171. [Google Scholar] [CrossRef]
- Wolfel, A.; Chacón, D.; Romero, M.; Valente, M.; Mattea, F. Synthesis of a metal chelating monomer for radiation polymer dosimetry. Radiat. Phys. Chem. 2021, 180, 109295. [Google Scholar] [CrossRef]
- Santibáñez, M.; Saavedra, R.; Vedelago, J.; Malano, F.; Valente, M. Optimized EDXRF system for simultaneous detection of gold and silver nanoparticles in tumor phantom. Radiat. Phys. Chem. 2019, 165, 108415. [Google Scholar] [CrossRef]
- Malano, F.; Mattea, F.; Geser, F.; Pérez, P.; Barraco, D.; Santibáñez, M.; Figueroa, R.; Valente, M. Assessment of FLUKA, PENELOPE and MCNP6 Monte Carlo codes for estimating gold fluorescence applied to the detection of gold-infused tumoral volumes. Appl. Radiat. Isot. 2019, 151, 280–288. [Google Scholar] [CrossRef]
- Vedelago, J.; Obando, D.C.; Malano, F.; Conejeros, R.; Figueroa, R.; Garcia, D.; González, G.; Romero, M.; Santibañez, M.; Strumia, M.; et al. Fricke and polymer gel 2D dosimetry validation using Monte Carlo simulation. Radiat. Meas. 2016, 91, 54–64. [Google Scholar] [CrossRef]
- Casanelli, B.; Santibáñez, M.; Valente, M. Particle size effect on fluorescence emission for Au-infused soft tissues. Radiat. Phys. Chem. 2020, 167, 108302. [Google Scholar] [CrossRef]
- Vedelago, J.; Mattea, F.; Valente, M. Integration of Fricke gel dosimetry with Ag nanoparticles for experimental dose enhancement determination in theranostics. Appl. Radiat. Isot. 2018, 141, 182–186. [Google Scholar] [CrossRef]
- Bouchard, H.; Bielajew, A. Lorentz force correction to the Boltzmann radiation transport equation and its implications for Monte Carlo algorithms. Phys. Med. Biol. 2015, 60. [Google Scholar] [CrossRef]
- Bielajew, A. Chapter on Electron Transport in Electric and Magnetic Fields Fundamentals of the Monte Carlo Method for Neutral and Charged Particle Transport; University of Michigan Press: Ann Arbor, MI, USA, 2001. [Google Scholar]
- Gubin, S.P. Magnetic Nanoparticles; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2009; ISBN 978-3-527-40790-3. [Google Scholar]
- Jordan, A.; Scholz, R.; Wust, P.; Schirra, H.; Schiestel, T.; Schmidt, H.; Felix, R. Endocytosis of dextran and silan-coated magnetite nanoparticles and the effect of intracellular hyperthermia on human mammary carcinoma cells in vitro. J. Magn. Magn. Mater. 1999, 194, 185–196. [Google Scholar] [CrossRef]
- Kolosnjaj-Tabi, J.; Di Corato, R.; Lartigue, L.; Marangon, I.; Guardia, P.; Silva, A.K.; Luciani, N.; Clément, O.; Flaud, P.; Singh, J.V.; et al. Heat-generating iron oxide nanocubes: Subtle “destructurators” of the tumoral microenvironment. ACS Nano 2014, 8, 4268–4283. [Google Scholar] [CrossRef]
- Mukherjee, S.; Liang, L.; Veiseh, O. Review. Recent Advancements of Magnetic Nanomaterials in Cancer Therapy. Pharmaceutics 2020, 12, 147. [Google Scholar] [CrossRef]
- Lin, Y.; Paganetti, H.; McMahon, S.J.; Schuemann, J. Gold nanoparticle induced vasculature damage in radiotherapy: Comparing protons, megavoltage photons, and kilovoltage photons. Med. Phys. 2015, 42, 5890–5902. [Google Scholar] [CrossRef]
- Montinola, D.; McNamara, A.L.; Kuncic, Z.; Byrne, H.L. Investigation of Micron-Scale Radiotherapy Dose Deposition in the Lung: Effect of Magnetic Field and Nanoparticles—A Monte Carlo Simulation. Front. Phys. 2022, 17, 835016. [Google Scholar] [CrossRef]
- Valente, M.; Chacón, D.; Mattea, F.; Meilij, R.; Pérez, P.; Romero, M.; Scarinci, I.; Vedelago, J.; Vitullo, F.; Wolfel, A. Linear energy transfer characterization of five gel dosimeter formulations for electron and proton therapeutic beams. Appl. Radiat. Isot. 2021, 178. [Google Scholar] [CrossRef]
- Tsiamas, P.; Liu, B.; Cifter, F.; Ngwa, W.F.; Berbeco, R.I.; Kappas, C.; Theodorou, K.; Marcus, K.; Makrigiorgos, M.G.; Sajo, E.; et al. Impact of beam quality on megavoltage radiotherapy treatment techniques utilizing gold nanoparticles for dose enhancement. Phys. Med. Biol. 2013, 58. [Google Scholar] [CrossRef]
- Overgaard, J. Formula to Estimate the Thermal Enhancement Ratio of a Single Simultaneous Hyperthermia and Radiation Treatment. Acta Radiol. Oncol. 1984, 23. [Google Scholar] [CrossRef]
- De Mendoza, A.M.; Michlíková, S.; Berger, J.; Karschau, J.; Kunz-Schughart, L.A.; McLeod, D.D. Mathematical model for the thermal enhancement of radiation response: Thermodynamic approach. Sci. Rep. 2021, 11, 5503. [Google Scholar] [CrossRef]
- Agostinelli, S.; Allison, J.; Amako, K.; Apostolakis, J.; Araujo, H.; Arce, P.; Asai, M.; Axen, D.; Banerjee, S.; Barrand, G.; et al. The GEANT4 collaboration. GEANT4-a simulation toolkit. Nucl. Instrum. Methods Phys. Res. A 2003, 506, 250–303. [Google Scholar] [CrossRef]
- Salvat, F.; Fernández-Varea, J.M.; Sempau, J. PENELOPE-2008: A Code System for Monte Carlo Simulation of Electron and Photon Transport Technical Report, Workshop Proc. Barcelona, Spain 30 June–3 July 2008; Nuclear Energy Agency no. 6416; OECD: Paris, France, 2008; ISBN 978-92-64-99066-1. [Google Scholar]
- Kawrakow, I. Accurate condensed history Monte Carlo simulation of electron transport. I.EGSnrc, the newEGS4version. Med. Phys. 2000, 27, 485–498. [Google Scholar] [CrossRef]
- Mashnik, S.G.; Bull, J.S.; Hughes, H.G.; Prael, R.E.; Sierk, A.J. Current status of MCNP6 as a simulation tool useful for space and accelerator applications. AIP Conf. Proc. 2013. [Google Scholar] [CrossRef]
- Battistoni, G.; Cerutti, F.; Fassò, A.; Ferrari, A.; Muraro, S.; Ranft, J.; Roesler, S.; Sala, P.R. The FLUKA code: Description and benchmarking. AIP Conf. Proc. 2007. [Google Scholar] [CrossRef]
- Ferrari, A.; Sala, P.R.; Fassò, A.; Ranft, J. FLUKA: A Multi-Particle Transport Code; CERN-2005-10, INFN/TC\_05/11, SLACR-773; CERN: Geneva, Switzerland, 2005. [Google Scholar]
- Mariotti, V.; Gayol, A.; Pianoschi, T.; Mattea, F.; Vedelago, J.; Pérez, P.; Valente, M.; Alva-Sánchez, M. Radiotherapy dosimetry parameters intercomparison among eight gel dosimeters by Monte Carlo simulation. Radiat. Phys. Chem. 2022, 190, 109782. [Google Scholar] [CrossRef]
- Ay, M.R.; Shahriari, M.; Sarkar, S.; Adib, M.; Zaidi, H. Monte Carlo simulation of x-ray spectra in diagnostic radiology and mammography using MCNP4C. Phys. Med. Biol. 2004, 49, 4897–4917. [Google Scholar] [CrossRef]
- Zaidi, H. Relevance of accurate Monte Carlo modeling in nuclear medical imaging. Med. Phys. 1999, 26, 574–608. [Google Scholar] [CrossRef]
- Pérez, P.; Valente, M. DOSIS: An integrated computational tool for patient-specific dosimetry in nuclear medicine by Monte Carlo and dose point kernel approaches. Appl. Radiat. Isot. 2019, 150, 135–140. [Google Scholar] [CrossRef]
- Wang, X.; Pan, H.; Cheng, Q.; Wang, X.; Xu, W. Dosimetric Deviations of Bragg-Peak Position Shifts in Uniform Magnetic Fields for Magnetic Resonance Imaging-Guiding Proton Radiotherapy: A Monte Carlo Study. Front. Public Health 2021. [Google Scholar] [CrossRef]
- Botta, F.; Mairani, A.; Battistoni, G.; Cremonesi, M.; Di Dia, A.; Fassò, A.; Ferrari, A.; Paganelli, G.; Pedroli, G.; Valente, M. Calculation of electron and isotopes dose point kernels with fluka Monte Carlo code for dosimetry in nuclear medicine therapy. Med. Phys. 2011, 38, 3944–3954. [Google Scholar] [CrossRef]
- Figueroa, R.G.; Rojas, L.; Valente, M. Trajectory control of electron beams using high intensity permanent magnests for linac-adaptable convergent beam radiotherapy. Appl. Radiat. Isot. 2019, 151, 13–18. [Google Scholar] [CrossRef]
- Gayol, A. Estudio y Caracterización Dosimétrica Del Efecto de Campos Magnéticos Intensos en Técnicas Modernas de Radioterapia Oncológica. Master’s Thesis, Universidad Nacional de Córdoba, Córdoba, Argentina, 2021. [Google Scholar]
- Chow, J.C. Photon and electron interactions with gold nanoparticles: A Monte Carlo study on gold nanoparticle-enhanced radiotherapy. Nanobiomat. Med. Imag. 2016, 8, 45–70. [Google Scholar] [CrossRef]
- Mattea, F.; Vedelago, J.; Malano, F.; Gomez, C.; Strumia, M.C.; Valente, M. Silver nanoparticles in X-ray biomedical applications. Radiat. Phys. Chem. 2017, 136, 442–450. [Google Scholar] [CrossRef]
- de Pooter, J.; Billas, I.; de Prez, L.; Duane, S.; Kapsch, R.-P.; Karger, C.P.; van Asselen, B.; Wolthaus, J. Reference dosimetry in MRI-linacs: Evaluation of available protocols and data to establish a Code of Practice. Phys. Med. Biol. 2021, 66, 05TR02. [Google Scholar] [CrossRef]
- Botta, F.; Mairani, A.; Hobbs, R.F.; Vergara Gil, A.; Pacilio, M.; Parodi, K.; Cremonesi, M.; Pérez, M.A.C.; Di Dia, A.; Ferrari, M.; et al. Use of the FLUKA Monte Carlo code for 3D patient-specific dosimetry on PET-CT and SPECT-CT images. Phys. Med. Biol. 2013, 58, 8099–8120. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, R.; Leiva, J.; Moncada, R.; Rojas, L.; Santibáñez, M.; Valente, M.; Velásquez, J.; Young, H.; Zelada, G.; Yáñez, R.; et al. Theory, simulation and experiments for precise deflection control of radiotherapy electron beams. Appl. Radiat. Isot. 2018, 141, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Capote, R. INDC International Nuclear Data Committee. In Phase-Space Database for External Beam Radiotherapy; IAEA: Vienna, Austria, 2005. [Google Scholar]
- Scott, R.S.; Johnson, R.J.; Kowal, K.; Krishnamsetty, R.; Story, K.; Clay, L.; Lawerne, C. Hyperthermia in combination with radiotherapy: A review of five years experience in the treatment of superficial tumors. Int. J. Radiat. Oncol. Biol. Phys. 1983, 9, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Bodgi, L.; Canet, A.; Pujo-Menjouet, L.; Lesne, A.; Victor, J.-M.; Foray, N. Mathematical models of radiation action on living cells: From the target theory to the modern approaches. A historical and critical review. J. Theor. Biol. 2016, 394, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Callen, H.B. Thermodynamics and an Introduction to Thermostatistics, 2nd ed.; J. Wiley & Sons: New York, NY, USA, 1991. [Google Scholar]
- Reichl, L. A Modern Course in Statistical Physics, 4th ed.; Wiley-VCH: New York, NY, USA, 2016. [Google Scholar]
- Mahmoudi, K.; Bouras, A.; Bozec, D.; Ivkov, R.; Hadjipanayis, C. Magnetic hyperthermia therapy for the treatment of glioblastoma: A review of the therapy’s history, efficacy and application in humans. Int. J. Hyperth. 2018, 34, 1316–1328. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Abbasiazam, A.; Oraee-Yazdani, S.; Lenzer, J. Response of human glioblastoma cells to hyperthermia: Cellular apoptosis and molecular events. Tissue Cell 2022, 75, 101751. [Google Scholar] [CrossRef] [PubMed]
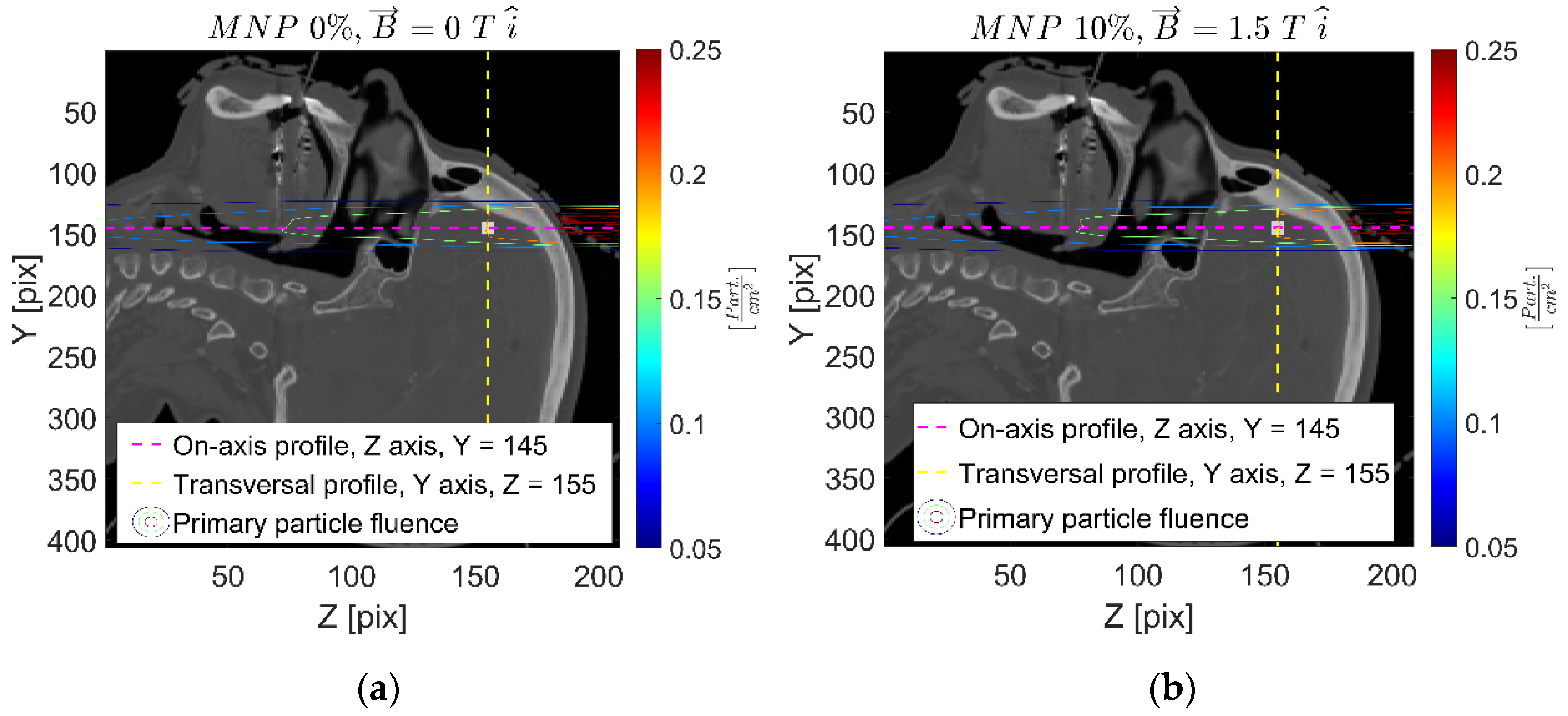
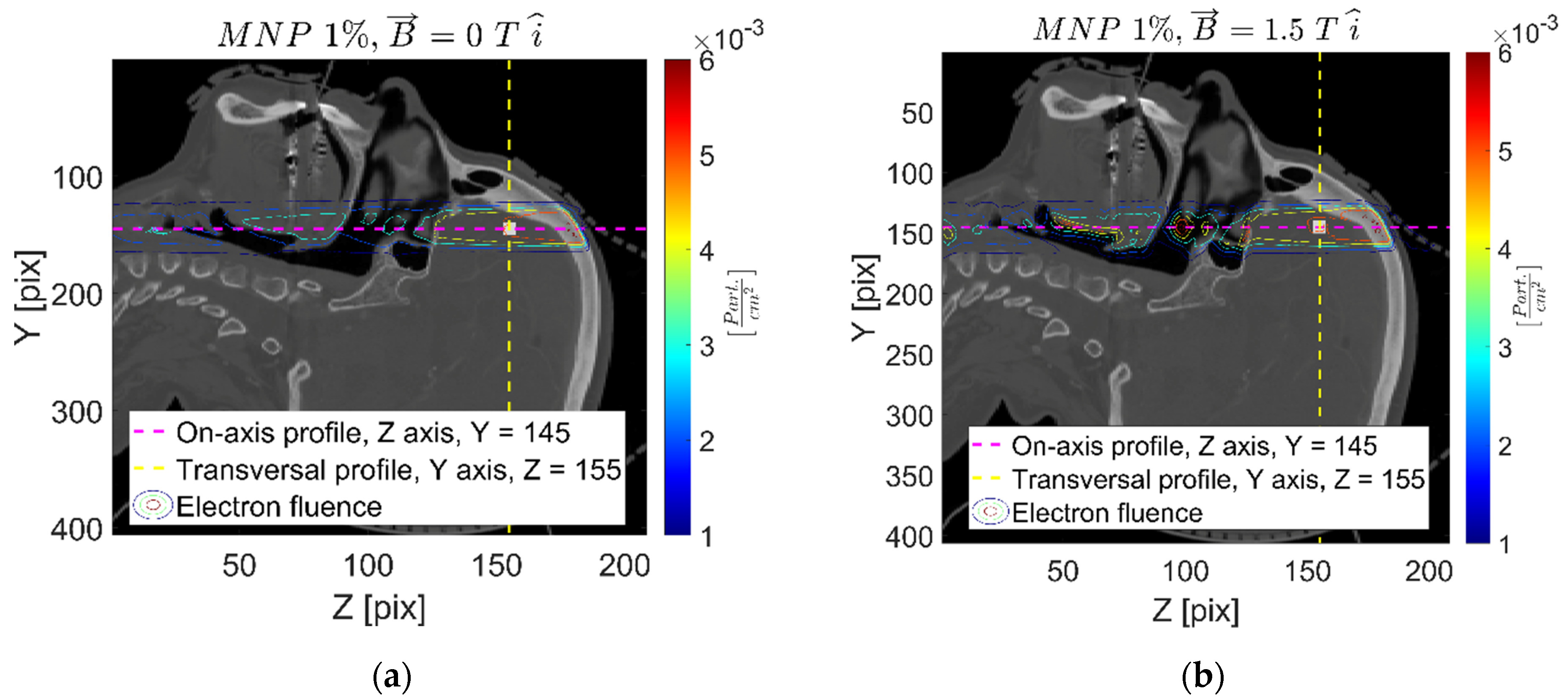
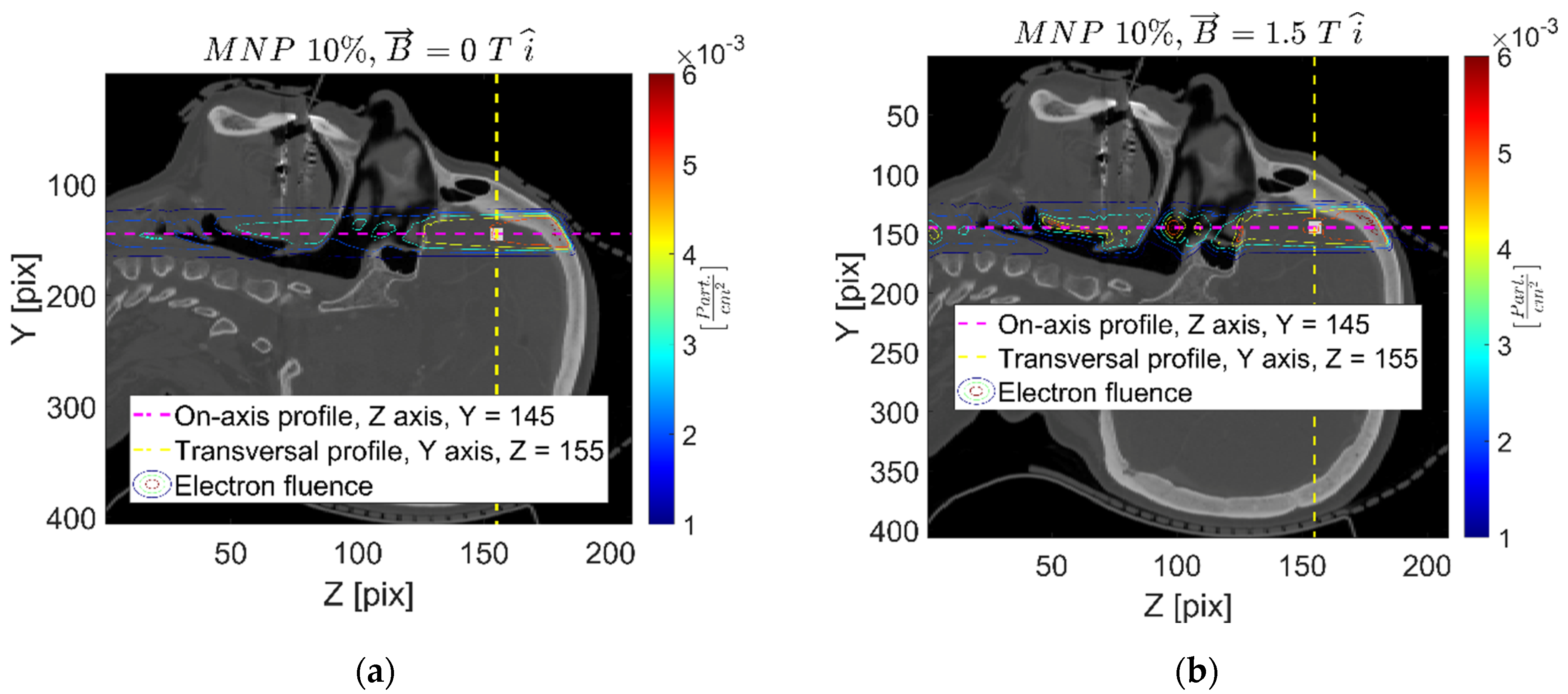
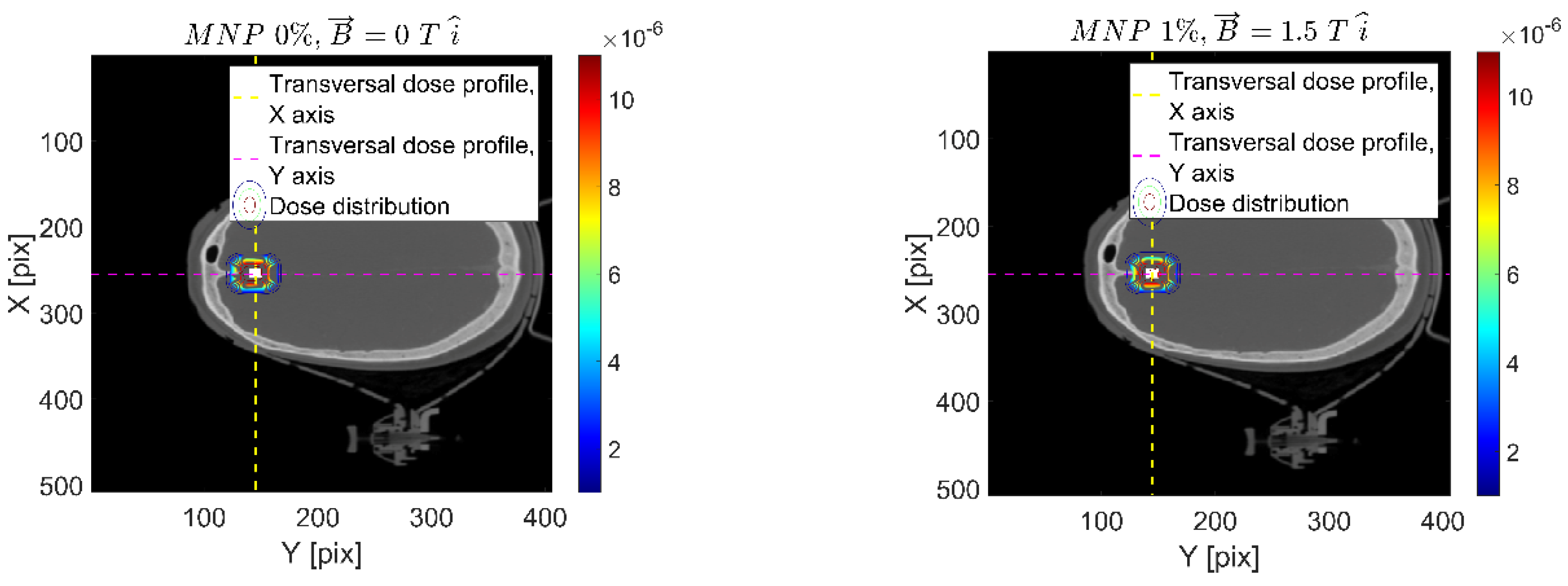




| Setup | FeNP Mass Concentration [%] | Magnetic Field [T] |
|---|---|---|
| Setup 0-0 (Reference) | 0 | (0,0,0) |
| Setup 0-1.5x | 0 | (1.5,0,0) |
| Setup 0-1.5z | 0 | (0,0,1.5) |
| Setup 0.5-0 | 0.5 | (0,0,0) |
| Setup 0.5-1.5x | 0.5 | (1.5,0,0) |
| Setup 1-0 | 1 | (0,0,0) |
| Setup 1-1.5x | 1 | (1.5,0,0) |
| Setup 1-1.5z | 1 | (0,0,1.5) |
| Setup 5-0 | 5 | (0,0,0) |
| Setup 5-1.5x | 5 | (1.5,0,0) |
| Setup 10-0 | 10 | (0,0,0) |
| Setup 10-1.5x | 10 | (1.5,0,0) |
| Setup 10-1.5z | 10 | (0,0,1.5) |
| SVZ-NSC | GBM-CSC | GBM-Astrocyte |
|---|---|---|
| 0.99944 | 0.99978 | 0.99698 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gayol, A.; Malano, F.; Ribo Montenovo, C.; Pérez, P.; Valente, M. Dosimetry Effects Due to the Presence of Fe Nanoparticles for Potential Combination of Hyperthermic Cancer Treatment with MRI-Based Image-Guided Radiotherapy. Int. J. Mol. Sci. 2023, 24, 514. https://doi.org/10.3390/ijms24010514
Gayol A, Malano F, Ribo Montenovo C, Pérez P, Valente M. Dosimetry Effects Due to the Presence of Fe Nanoparticles for Potential Combination of Hyperthermic Cancer Treatment with MRI-Based Image-Guided Radiotherapy. International Journal of Molecular Sciences. 2023; 24(1):514. https://doi.org/10.3390/ijms24010514
Chicago/Turabian StyleGayol, Amiel, Francisco Malano, Clara Ribo Montenovo, Pedro Pérez, and Mauro Valente. 2023. "Dosimetry Effects Due to the Presence of Fe Nanoparticles for Potential Combination of Hyperthermic Cancer Treatment with MRI-Based Image-Guided Radiotherapy" International Journal of Molecular Sciences 24, no. 1: 514. https://doi.org/10.3390/ijms24010514
APA StyleGayol, A., Malano, F., Ribo Montenovo, C., Pérez, P., & Valente, M. (2023). Dosimetry Effects Due to the Presence of Fe Nanoparticles for Potential Combination of Hyperthermic Cancer Treatment with MRI-Based Image-Guided Radiotherapy. International Journal of Molecular Sciences, 24(1), 514. https://doi.org/10.3390/ijms24010514






