Gut Microbiota Alterations and Primary Glomerulonephritis in Children: A Review
Abstract
:1. Introduction
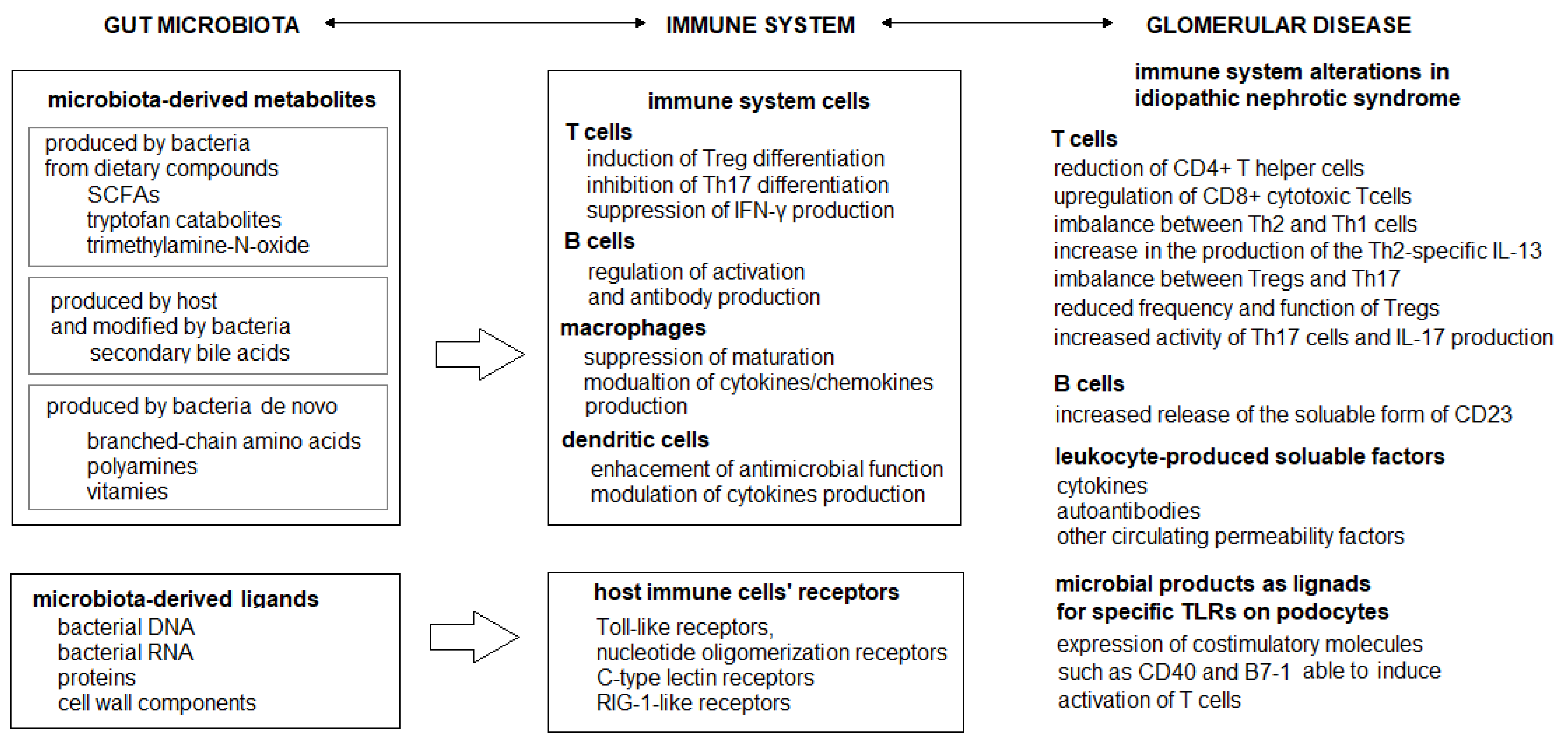
2. Dysbiosis and Immunity
3. Search Strategy and Data Sources
4. Idiopathic Nephrotic Syndrome
5. Membranous Nephropathy
6. Immunoglobulin A Nephropathy
7. Therapeutical Gut Microbiota Modifications
7.1. Probiotics
7.2. Dietary Interventions
7.3. Fecal Microbiota Transplantation
8. Conclusions and Directions for Further Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, C.; Zhu, H.; Yao, Y.; Zeng, R. Gut Dysbiosis and Kidney Diseases. Front. Med. 2022, 9, 829349. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-G.; Yang, J.; Jo, S.-K. Intestinal microbiota and kidney diseases. Kidney Res. Clin. Pract. 2021, 40, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Bai, M.; Zhao, J.; Wang, D.; Ning, X.; Sun, S. A Comparative Study of the Gut Microbiota Associated With Immunoglobulin a Nephropathy and Membranous Nephropathy. Front. Cell. Infect. Microbiol. 2020, 10, 557368. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Tsuji, S.; Kimata, T. Role of gut microbiota in idiopathic nephrotic syndrome in children. Med. Hypotheses 2017, 108, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Luo, D.; Lin, Z.; Zhou, W.; Rao, J.; Li, Y.; Wu, J.; Peng, H.; Lou, T. Dysbiosis of gut microbiota in adult idiopathic membranous nephropathy with nephrotic syndrome. Microb. Pathog. 2020, 147, 104359. [Google Scholar] [CrossRef]
- Haniuda, K.; Gommerman, J.L.; Reich, H.N. The microbiome and IgA nephropathy. Semin. Immunopathol. 2021, 43, 649–656. [Google Scholar] [CrossRef]
- He, H.; Lin, M.; You, L.; Chen, T.; Liang, Z.; Li, D.; Xie, C.; Xiao, G.; Ye, P.; Kong, Y.; et al. Gut Microbiota Profile in Adult Patients with Idiopathic Nephrotic Syndrome. BioMed Res. Int. 2021, 2021, 8854969. [Google Scholar] [CrossRef]
- Derrien, M.; Alvarez, A.-S.; de Vos, W.M. The Gut Microbiota in the First Decade of Life. Trends Microbiol. 2019, 27, 997–1010. [Google Scholar] [CrossRef] [Green Version]
- Vandenplas, Y.; Carnielli, V.P.; Ksiazyk, J.; Luna, M.S.; Migacheva, N.; Mosselmans, J.M.; Picaud, J.C.; Possner, M.; Singhal, A.; Wabitsch, M. Factors affecting early-life intestinal microbiota development. Nutrition 2020, 78, 110812. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, J.; Hu, H.; Xu, C.; Yin, J.; Liu, M.; Zhang, L.; Duan, Y.; Huang, Y. Development of gut microbiota along with its metabolites of preschool children. BMC Pediatr. 2022, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.J.; Ajami, N.J.; O’Brien, J.L.; Hutchinson, D.S.; Smith, D.P.; Wong, M.C.; Ross, M.C.; Lloyd, R.E.; Doddapaneni, H.; Metcalf, G.A.; et al. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 2018, 562, 583–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomaa, E.Z. Human gut microbiota/microbiome in health and diseases: A review. Antonie Leeuwenhoek 2020, 113, 2019–2040. [Google Scholar] [CrossRef]
- Yang, W.; Cong, Y. Gut microbiota-derived metabolites in the regulation of host immune responses and immune-related inflammatory diseases. Cell. Mol. Immunol. 2021, 18, 866–877. [Google Scholar] [CrossRef]
- Monteiro, R.C.; Berthelot, L. Role of gut–kidney axis in renal diseases and IgA nephropathy. Curr. Opin. Gastroenterol. 2021, 37, 565–571. [Google Scholar] [CrossRef]
- Kim, C.H. Immune regulation by microbiome metabolites. Immunology 2018, 154, 220–229. [Google Scholar] [CrossRef]
- Colucci, M.; Corpetti, G.; Emma, F.; Vivarelli, M. Immunology of idiopathic nephrotic syndrome. Pediatr. Nephrol. 2018, 33, 573–584. [Google Scholar] [CrossRef]
- Noone, D.G.; Iijima, K.; Parekh, R. Idiopathic nephrotic syndrome in children. Lancet 2018, 392, 61–74. [Google Scholar] [CrossRef]
- Vivarelli, M.; Massella, L.; Ruggiero, B.; Emma, F. Minimal Change Disease. Clin. J. Am. Soc. Nephrol. 2017, 12, 332–345. [Google Scholar] [CrossRef]
- Chen, J.; Qiao, X.-H.; Mao, J.-H. Immunopathogenesis of idiopathic nephrotic syndrome in children: Two sides of the coin. World J. Pediatr. 2021, 17, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, S.; Kaneko, K. The long and winding road to the etiology of idiopathic nephrotic syndrome in children: Focusing on abnormalities in the gut microbiota. Pediatr. Int. 2021, 63, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; Van Der Veeken, J.; DeRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 2013, 504, 451–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Xiao, Y.; Zhu, Z.; Li, B.; Greene, M.I. Immune regulation by histone deacetylases: A focus on the alteration of FOXP3 activity. Immunol. Cell Biol. 2012, 90, 95–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuji, S.; Suruda, C.; Hashiyada, M.; Kimata, T.; Yamanouchi, S.; Kitao, T.; Kino, J.; Akane, A.; Kaneko, K. Gut Microbiota Dysbiosis in Children with Relapsing Idiopathic Nephrotic Syndrome. Am. J. Nephrol. 2018, 47, 164–170. [Google Scholar] [CrossRef]
- Tsuji, S.; Akagawa, S.; Akagawa, Y.; Yamaguchi, T.; Kino, J.; Yamanouchi, S.; Kimata, T.; Hashiyada, M.; Akane, A.; Kaneko, K. Idiopathic nephrotic syndrome in children: Role of regulatory T cells and gut microbiota. Pediatr. Res. 2021, 89, 1185–1191. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Tsuji, S.; Akagawa, S.; Akagawa, Y.; Kino, J.; Yamanouchi, S.; Kimata, T.; Hashiyada, M.; Akane, A.; Kaneko, K. Clinical Significance of Probiotics for Children with Idiopathic Nephrotic Syndrome. Nutrients 2021, 13, 365. [Google Scholar] [CrossRef]
- Kim, B.-R.; Shin, J.; Guevarra, R.B.; Lee, J.H.; Kim, D.W.; Seol, K.-H.; Lee, J.-H.; Kim, H.B.; Isaacson, R.E. Deciphering Diversity Indices for a Better Understanding of Microbial Communities. J. Microbiol. Biotechnol. 2017, 27, 2089–2093. [Google Scholar] [CrossRef] [Green Version]
- Kang, Y.; Feng, D.; Law, H.K.-W.; Qu, W.; Wu, Y.; Zhu, G.-H.; Huang, W.-Y. Compositional alterations of gut microbiota in children with primary nephrotic syndrome after initial therapy. BMC Nephrol. 2019, 20, 434. [Google Scholar] [CrossRef]
- Qiu, T.; Yi, X.; Xu, L.; Wang, L.; Hu, X.; Li, X. The correlation between gut microbiota dysbiosis and primary nephrotic syndrome in children. Acta Med. Mediterr. 2020, 36, 971–976. [Google Scholar] [CrossRef]
- Szlachciński, R.; Szlachcińska, A.; Szlachciński, Ł.; Borycz-Stevens, I.; Almeer, F.; Tkaczyk, M. Intestinal microbiota in nephrotic children treated with immunosuppressive agents. Pediatr. Polska 2020, 95, 6–13. [Google Scholar] [CrossRef]
- McDonnell, L.; Gilkes, A.; Ashworth, M.; Rowland, V.; Harries, T.H.; Armstrong, D.; White, P. Association between antibiotics and gut microbiome dysbiosis in children: Systematic review and meta-analysis. Gut Microbes 2021, 13, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Couser, W.G. Primary Membranous Nephropathy. Clin. J. Am. Soc. Nephrol. 2017, 12, 983–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safar-Boueri, L.; Piya, A.; Beck, L.H., Jr.; Ayalon, R. Membranous nephropathy: Diagnosis, treatment, and monitoring in the post-PLA2R era. Pediatr. Nephrol. 2021, 36, 19–30. [Google Scholar] [CrossRef]
- Li, M.; Wei, L.; Sun, J.; Zhu, Q.; Yang, H.; Zhang, Y.; Zhang, C.; Xi, L.; Zhao, R.; Du, X. Association of gut microbiota with idiopathic membranous nephropathy. BMC Nephrol. 2022, 23, 164. [Google Scholar] [CrossRef]
- Rodrigues, J.C.; Haas, M.; Reich, H.N. IgA Nephropathy. Clin. J. Am. Soc. Nephrol. 2017, 12, 677–686. [Google Scholar] [CrossRef] [Green Version]
- Aron, A.W. Is There a Role for More Intense Immunosuppression in IgA Nephropathy? Kidney360 2022, 3, 410–412. [Google Scholar] [CrossRef]
- Chai, L.; Luo, Q.; Cai, K.; Wang, K.; Xu, B. Reduced fecal short-chain fatty acids levels and the relationship with gut microbiota in IgA nephropathy. BMC Nephrol. 2021, 22, 209. [Google Scholar] [CrossRef]
- Sallustio, F.; Curci, C.; Chaoul, N.; Fontò, G.; Lauriero, G.; Picerno, A.; Divella, C.; Di Leo, V.; De Angelis, M.; Ben Mkaddem, S.; et al. High levels of gut-homing immunoglobulin A+ B lymphocytes support the pathogenic role of intestinal mucosal hyperresponsiveness in immunoglobulin A nephropathy patients. Nephrol. Dial. Transplant. 2021, 36, 452–464. [Google Scholar] [CrossRef]
- Liu, Y.; Alookaran, J.J.; Rhoads, J.M. Probiotics in Autoimmune and Inflammatory Disorders. Nutrients 2018, 10, 1537. [Google Scholar] [CrossRef]
- Johnson, C.L.; Versalovic, J. The Human Microbiome and Its Potential Importance to Pediatrics. Pediatrics 2012, 129, 950–960. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Sáez, M.J.; Uffing, A.; Leon, J.; Murakami, N.; Watanabe, A.; Borges, T.J.; Sabbisetti, V.S.; Cureton, P.; Kenyon, V.; Keating, L.; et al. Immunological Impact of a Gluten-Free Dairy-Free Diet in Children With Kidney Disease: A Feasibility Study. Front. Immunol. 2021, 12, 624821. [Google Scholar] [CrossRef]
- Fortes, P.M.; Filho, R.V.T.; De Azevêdo, L.H.S.; Queiroz, V.C.J.; Da Costa, P.S.S. Inflammatory cytokines and lipid profile in children and adolescents with nephrotic syndrome receiving L. Plantarum: A randomized, controlled feasibility trial. Rev. Assoc. Med. Bras. 2021, 66, 1487–1492. [Google Scholar] [CrossRef]
- Bibbò, S.; Ianiro, G.; Giorgio, V.; Scaldaferri, F.; Masucci, L.; Gasbarrini, A.; Cammarota, G. The role of diet on gut microbiota composition. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4742–4749. [Google Scholar]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef] [Green Version]
- Uy, N.; Graf, L.; Lemley, K.V.; Kaskel, F. Effects of gluten-free, dairy-free diet on childhood nephrotic syndrome and gut microbiota. Pediatr. Res. 2015, 77, 252–255. [Google Scholar] [CrossRef] [Green Version]
- Leon, J.; Pérez-Sáez, M.J.; Uffing, A.; Murakami, N.; Watanabe, A.; Cureton, P.; Kenyon, V.; Keating, L.; Yee, K.; Satiro, C.A.F.; et al. Effect of Combined Gluten-Free, Dairy-Free Diet in Children With Steroid-Resistant Nephrotic Syndrome: An Open Pilot Trial. Kidney Int. Rep. 2018, 3, 851–860. [Google Scholar] [CrossRef] [Green Version]
- Lemley, K.V.; Faul, C.; Schramm, K.; Meyers, K.; Kaskel, F.; Dell, K.M.; Gipson, D.S.; Gibson, K.; Trachtman, H. The Effect of a Gluten-Free Diet in Children With Difficult-to-Manage Nephrotic Syndrome. Pediatrics 2016, 138, e20154528. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.-W.; Kuo, C.-H.; Kuo, F.-C.; Wang, Y.-K.; Hsu, W.-H.; Yu, F.-J.; Hu, H.-M.; Hsu, P.-I.; Wang, J.-Y.; Wu, D.-C. Fecal microbiota transplantation: Review and update. J. Formos. Med. Assoc. 2019, 118 (Suppl. S1), S23–S31. [Google Scholar] [CrossRef]
- Gurram, B.; Sue, P. Fecal microbiota transplantation in children: Current concepts. Curr. Opin. Pediatr. 2019, 31, 623–629. [Google Scholar] [CrossRef]
- Chen, C.-C.; Chiu, C.-H. Current and future applications of fecal microbiota transplantation for children. Biomed. J. 2022, 45, 11–18. [Google Scholar] [CrossRef]
- Antushevich, H. Fecal microbiota transplantation in disease therapy. Clin. Chim. Acta 2020, 503, 90–98. [Google Scholar] [CrossRef]
- Zhao, J.; Bai, M.; Yang, X.; Wang, Y.; Li, R.; Sun, S. Alleviation of refractory IgA nephropathy by intensive fecal microbiota transplantation: The first case reports. Ren. Fail. 2021, 43, 928–933. [Google Scholar] [CrossRef]
- Zhou, G.; Zeng, J.; Peng, L.; Wang, L.; Zheng, W.; Wu, D.; Yang, Y. Fecal microbiota transplantation for membranous nephropathy. CEN Case Rep. 2021, 10, 261–264. [Google Scholar] [CrossRef]
- Stanford, J.; Charlton, K.; Stefoska-Needham, A.; Ibrahim, R.; Lambert, K. The gut microbiota profile of adults with kidney disease and kidney stones: A systematic review of the literature. BMC Nephrol. 2020, 21, 215. [Google Scholar] [CrossRef]
- Crespo-Salgado, J.; Stewart, T.; Aviles, D.H. Does Dysbiosis in Intestinal Microbiome Plays a Role in Children with Relapsing Idiopathic Nephrotic Syndrome? Am. J. Nephrol. 2018, 47, 162–163. [Google Scholar] [CrossRef] [Green Version]
- Weersma, R.K.; Zhernakova, A.; Fu, J. Interaction between drugs and the gut microbiome. Gut 2020, 69, 1510–1519. [Google Scholar] [CrossRef]
- Le Bastard, Q.; Al-Ghalith, G.A.; Grégoire, M.; Chapelet, G.; Javaudin, F.; Dailly, E.; Batard, E.; Knights, D.; Montassier, E. Systematic review: Human gut dysbiosis induced by non-antibiotic prescription medications. Aliment. Pharmacol. Ther. 2018, 47, 332–345. [Google Scholar] [CrossRef] [Green Version]
- Almeida, A.; Mitchell, A.L.; Boland, M.; Forster, S.C.; Gloor, G.B.; Tarkowska, A.; Lawley, T.D.; Finn, R.D. A new genomic blueprint of the human gut microbiota. Nature 2019, 568, 499–504. [Google Scholar] [CrossRef] [Green Version]
- Pasolli, E.; Asnicar, F.; Manara, S.; Zolfo, M.; Karcher, N.; Armanini, F.; Beghini, F.; Manghi, P.; Tett, A.; Ghensi, P.; et al. Extensive Unexplored Human Microbiome Diversity Revealed by Over 150,000 Genomes from Metagenomes Spanning Age, Geography, and Lifestyle. Cell 2019, 176, 649–662.e20. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Wu, W.; Zheng, H.-M.; Li, P.; McDonald, D.; Sheng, H.-F.; Chen, M.-X.; Chen, Z.-H.; Ji, G.-Y.; Zheng, Z.-D.; et al. Regional variation limits applications of healthy gut microbiome reference ranges and disease models. Nat. Med. 2018, 24, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rodriguez, E.; Southard, C.T.; Kiryluk, K. GWAS-Based Discoveries in IgA Nephropathy, Membranous Nephropathy, and Steroid-Sensitive Nephrotic Syndrome. Clin. J. Am. Soc. Nephrol. 2021, 16, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Kurilshikov, A.; Medina-Gomez, C.; Bacigalupe, R.; Radjabzadeh, D.; Wang, J.; Demirkan, A.; Le Roy, C.I.; Garay, J.A.R.; Finnicum, C.T.; Liu, X.; et al. Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat. Genet. 2021, 53, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Fu, Y.; Sun, T.-Y.; Jiang, Z.; Miao, Z.; Shuai, M.; Gou, W.; Ling, C.-W.; Yang, J.; Wang, J.; et al. The interplay between host genetics and the gut microbiome reveals common and distinct microbiome features for complex human diseases. Microbiome 2020, 8, 145. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Butcher, J.; Stintzi, A.; Figeys, D. Advancing functional and translational microbiome research using meta-omics approaches. Microbiome 2019, 7, 154. [Google Scholar] [CrossRef]
| Study | Country | Study Group | Healthy Controls | Microbiota Assessment Methods and Samples Collection Time | Results |
|---|---|---|---|---|---|
| Tsuji et al. [25] | Japan | children with INS N =12 (male: female = 7:5) divided into relapsing group (R) = 8; median age 3.0 years non-relapsing group (NR) = 4; median age 4.3 years | N = 11 (male: female = 5:6) median age 5.1 years | Fecal samples collection and 16S rRNA sequencing Fecal butyric acid measured using high-performance liquid chromatography Fecal samples from INS patients obtained before starting therapy | Lower proportion of butyric acid-producing bacteria and fecal butyric acid quantities in R group than in HC (p = 0.0013 and p = 0.042 respectively). |
| Tsuji et al. [26] | Japan | children with INS N = 25 (male: female = 20:5) median age 4.0 years divided into non-relapsing group (NR) = 8, median age 5.4 years, frequently relapsing group (FR) = 17, median age 3.5 years | N = 20 (male: female = ns) median age 4.0 years | Fecal samples collection and 16S rRNA sequencing Fecal samples from INS patients obtained before starting therapy | Different distribution of bacteria in FR group compared to HC and NR group. Reduced proportion of butyric acid-producing bacteria in the FR group compared to HC (p = 0.0031). |
| Yamaguchi et al. [27] | Japan | children with INS N = 20 (male: female = 15:5) median age 5.3 years | N = 21 (male: female = ns) median age 4.0 years | Fecal samples collection and 16S rRNA sequencing Fecal samples from INS patients obtained at the onset of INS | The percentage of butyrate-producing bacteria was significantly lower in study group at INS onset compared with HC (p = 0.024). |
| Tingting et al. [30] | China | children with INS N = 29 (male: female =18:11) mean age 7.23 ± 2.06 years | N = 15 (male: female = 10:5) mean age 7.31 ± 2.12 years | Fecal samples collection and 16S rRNA sequencing Fecal samples form INS patients obtained before treatment or remission after treatment | Decreased counts of Lactobacillus, Bifidobacteria, and E.coli before treatment in study group compared to HC (p < 0.05). Decreased Bifidobacteria and E.coli ratio at INS onset. |
| Szlachciński et al. [31] | Poland | children with INS N = 44 (male: female = 26:18) median age ns divided according to treatment protocols: - group A (CsA ± GCS) N = 18 (male: female = 11:7) age 2–14 years - group B (GCS) N = 17 (male: female = 9:8) age 2–17 years - group C (CYC + GCS) N= 9 (male: female = 6:3) age 3–12 years | N = 20 (male: female = 13:7) age 2–15 years | Fecal samples collection and culture (KyberStatus and KyberMyk test) Fecal samples form INS patients obtained once during the therapy | Lower total number of bacterial colonies in group A (p < 0.001) and group B (p = 0.04) compared to HC. Lower number of Bifidobacterium colonies in group C compared to HC (p = 0.01). Higher amount of Candida sp. colonies in group A compared to HC (p = 0.01). |
| Study | Country | Study Group | Microbiota Assessment Methods and Samples Collection Time | Results |
|---|---|---|---|---|
| Kang et al. [28] | China | children with INS N = 20 (male: female = 15:5) mean age 3.5 ± 2.1 years | Fecal samples collection and 16S rRNA sequencing Fecal samples collected before and after 4-week initial therapy | The richness and diversity of gut microbiota were similar before treatment and after 4-week initial therapy and achieved complete remission. The abundance SCFA-producing bacteria including Romboutsia, Stomatobaculum, and Cloacibacillus increased after initial therapy (p < 0.05). |
| Tingting et al. [30] | China | children with INS N = 29 (male: female = 18:11) mean age 7.23 ± 2.06 years | Fecal samples collection and 16S rRNA sequencing Fecal samples form INS patients obtained before treatment or remission after treatment | Counts of Lactobacillus, Bifidobacteria, and E.coli recovered after treatment but did not reach the normal level. Bifidobacteria and E.coli ratio increased after treatment (p < 0.05). |
| Szlachciński et al. [31] | Poland | children with INS N = 44 (male: female = 26:18) median age ns divided into groups according to treatment protocols: - group A (CsA ± GCS) N = 18 (male: female = 11:7) age 2–14 years - group B (GCS) N = 17 (male: female = 9:8) age 2–17 years - group C (CYC + GCS) N = 9 (male: female = 6:3) age 3–12 years | Fecal samples collection and culture (KyberStatus and KyberMyk test) Fecal samples form INS patients obtained once during the therapy | Lower total number of bacterial colonies in group A compared to group B (p = 0.007) and C (p = 0.04). |
| Study | Country | Study Group | Microbiota Assessment Methods and Sample Collection Time | Intervention | Results |
|---|---|---|---|---|---|
| Yamaguchi et al. [27] | Japan | children with INS N = 20 (male: female = 15:5) median age 5.3 years Probiotic-treated group: N = 10, median age 6.4 years Non-probiotic-treated group: N = 10, median age 4.7 years | Fecal sample collection and 16S rRNA sequencing Fecal samples from INS patients obtained at the onset of INS and during treatment with probiotics | Oral administration of butyrate-producing bacteria (C.butyricum MIYAIRI) started at the end of the 8-week steroid administration dosing 3 g/day median period of intervention 25 months (range from 7 to 46 months) | The percentage of butyrate-producing bacteria increased after probiotic treatment (p = 0.017). Probiotic-treated patients experienced fewer INS relapses per year compared with non-probiotic-treated patients (p = 0.016). |
| Perez-Saez et al. [42] | USA | children with steroid-resistant nephrotic syndrome (SRNS) N = 16 (male: female =8:8) mean age 7.0 ± 5.3 years | Fecal sample collection and 16S rRNA sequencing Fecal samples collected at baseline and after the intervention (day 54) | A 4-week summer camp implementing a strict gluten-free and dairy-free diet (GF/DF diet) | Increased fraction of Bacteroides, Lachnospira, and Faecalibacterium after the intervention. GF/DF diet promoted a favorable microbiome modification with potential immune-regulatory phenotype. Overall, 2 out of 16 participants achieved complete remission in proteinuria after 4 weeks on GF/DF diet. Both participants experienced recurrence in proteinuria after returning to unrestricted diet, after which they immediately went back to a GF/DF diet, achieving again a sustained remission in proteinuria. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawalec, A.; Kiliś-Pstrusińska, K. Gut Microbiota Alterations and Primary Glomerulonephritis in Children: A Review. Int. J. Mol. Sci. 2023, 24, 574. https://doi.org/10.3390/ijms24010574
Kawalec A, Kiliś-Pstrusińska K. Gut Microbiota Alterations and Primary Glomerulonephritis in Children: A Review. International Journal of Molecular Sciences. 2023; 24(1):574. https://doi.org/10.3390/ijms24010574
Chicago/Turabian StyleKawalec, Anna, and Katarzyna Kiliś-Pstrusińska. 2023. "Gut Microbiota Alterations and Primary Glomerulonephritis in Children: A Review" International Journal of Molecular Sciences 24, no. 1: 574. https://doi.org/10.3390/ijms24010574
APA StyleKawalec, A., & Kiliś-Pstrusińska, K. (2023). Gut Microbiota Alterations and Primary Glomerulonephritis in Children: A Review. International Journal of Molecular Sciences, 24(1), 574. https://doi.org/10.3390/ijms24010574






