Intra-Articular Mesenchymal Stem Cell Injection for Knee Osteoarthritis: Mechanisms and Clinical Evidence
Abstract
:1. Introduction
2. Arrangement of the Review and Literature Search Strategy
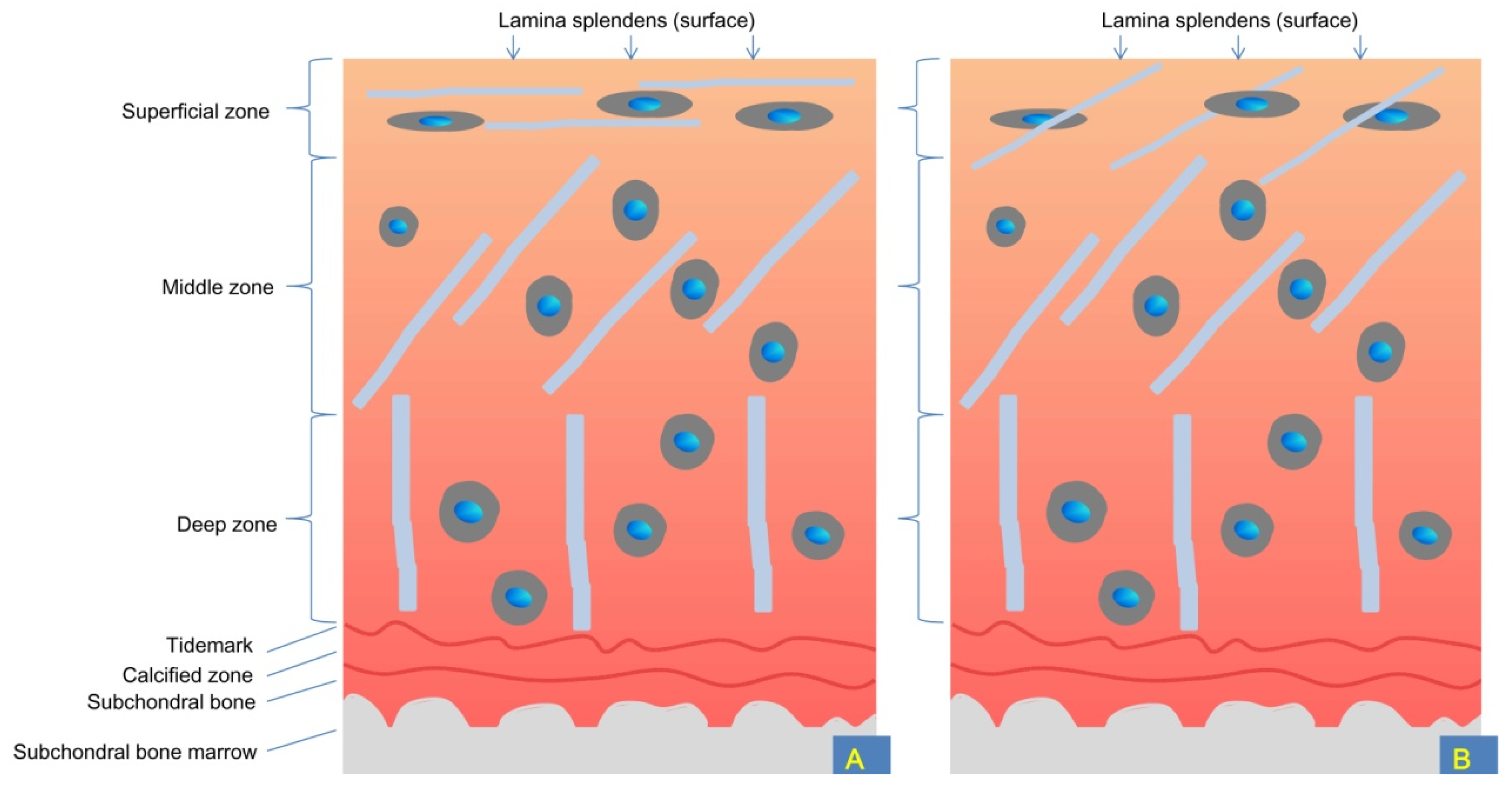
3. Anatomical, Biochemical, and Biomechanical Properties of Knee Cartilage
4. Studies Comparing MSCs with Other Substances
| Cell Type | Cell Dosage | Cell Passage | Combined Interventions | Control with Non-MSC Agents | Knee OA Grading | Injection Time | Follow Up | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Allogenic PDMSCs | 0.5–0.6 × 108 | 12 | No | Normal saline | 1 | 24 weeks | Range of motion improvement and pain reduction until 8 weeks. Chondral thickness improved at 24 weeks, and anterior cruciate ligament healing may be observed, but no meniscus repair was detected by MR arthrography. | [49] | |
| Allogeneic BMMSCs | 40 × 106 | 3 | No | Hyaluronic acid alone | KL II–IV | 1 | 12 months | Better functional improvement and cartilage quality improvements by MRI in the MSCs group. | [50] |
| Allogenic UCMSCs | 20 × 106 | 5 | Avoid physical activity for 48 h after the procedure. | Hyaluronic acid (0 + 6 months) | KL II–III | 1 or 2 (0 + 6 months) | 12 months | Pain reduction and function improvement were only observed in the repeated MSC injection group. | [51] |
| Cell Type | Cell Dosage | Cell Passage | Combined Interventions | Control with Non-MSC Agents | Knee OA Grading | Injection Time | Follow Up | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Autologous BMAC | 34,400 MSCs + 4,620,000 HSCs | 0 | Platelet-poor plasma (to increase injection volume). No brace and physical therapy provided. | Saline injection into the other knee with OA | KL < IV | 1 | 6 months | Similar relief of pain in BMAC- and saline-treated arthritic knees. | [47] |
| Autologous BMMSCs | 100 × 106 | Unavailable | PRP (3 times) | PRP (3 times) alone | KL II–IV | 1 | 12 months | Only the MSCs + PRP had pain reduction and functional improvement. | [54] |
| Autologous BMMSCs | 40 × 106 | ≤2 | Drugs, hydrotherapy, heat, and ultrasound or acupuncture were prohibited. | MSCs + PRP | KL II–IV | 1 | 12 months | Both groups had improvements, but MSCs + PRP induced better effects. | [58] |
| Autologous BMMSCs | 40 × 106 | ≤2 | PRP | Corticosteroid | KL I–IV | 1 | 12 months | MSCs and MSCs + PRP groups showed the highest percentage of improvement compared with the corticosteroid group. | [59] |
| Autologous BMMSCs | 2740–7540 × 20 | 0 | Instructions for immediate full weight-bearing. Physical therapy was considered unnecessary. | Implantation in the subchondral bone of the medial femur and tibia | KL I–IV | 1 | 15 years | Both groups resulted in pain relief, but time conversion to total knee arthroplasty was longer in those receiving subchondral MSC injections. | [60] |
| Cell Type | Cell Dosage | Cell Passage | Combined Interventions | Control with Non-MSC Agents | Knee OA Grading | Injection Time | Follow Up | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Autologous ADMSCs | 1 × 108 | Unavailable | No | Normal saline | KL II–IV | 1 | 6 months | Pain reduction and functional improvement only observed in the MSCs group. Worse cartilage defect by MRI only in the control group. | [48] |
| Autologous ADMSCs | 5 × 107 (0 + 3rd week) | Unavailable | Rest for 24 h following each injection. | Hyaluronic acid (1/week for 4 weeks) | KL I–IV | Higher increase in articular cartilage volume by MRI in the MSCs group. | [52] | ||
| Autologous ADMSCs | 8 × 106 | Unavailable | Avoid weight-bearing motions on the affected knee, such as standing for prolonged periods, jogging, and lifting heavy objects during the first 3 days. | Hyaluronic acid | KL I–IV | 1 | 12 months | Greater improvements observed in the MSCs group. | [53] |
| Autologous ADMSCs | 100 × 106 (single injection)/ 100 × 106 (baseline + 6 month) | 2 | None for the control group. The MSCs group remained non-weight-bearing and used crutches for 4 weeks. A range of motion and quadriceps exercises were also provided. | Conventional conservative management only | KL II–III | 1 or 2 | 12 months | Better functional improvement and pain reduction were observed in the MSCs group. | [61] |
5. Can MSC Injection Induce Cartilage Regeneration?
| Cell Type | Cell Dosage | Cell Passage | Combined Interventions | Control with Non-MSC Agents | Knee OA Grading | Injection Time | Follow Up | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Autologous ADMSCs | 1.2–2.3 × 106 | 0 | Arthroscopic debridement + PRP. Rehabilitation programs were available. | Only arthroscopic debridement + PRP | KL 3.3 ± 0.8 (MSCs group) or 2.7 ± 0.7 (control) | 1 (PRP multiple times) | 12–18 months | Better symptom relief in the MSCs group. Good results obtained only in young patients and those with early cartilage degeneration. | [9] |
| Autologous BMMSCs | 10 × 106 | 1 | Arthroscopic microfracture + hyaluronic acid injection three times. Individualized rehabilitation programs were available. | MSC implantation beneath a sutured periosteal patch over the cartilage defect. | ≥1 symptomatic full-thickness chondral lesion | 1 | 24 months | Both groups had improvements. | [65] |
| Autologous MSCs (from stromal vascular fraction) | 4.11 × 106 | 0 | HTO + PRP | HTO + PRP | KL III or lower | 1 | 14–24 months | HTO + MSCs + PRP resulted in good regenerated fibrocartilage (by arthroscopy) and better pain reduction than HTO + PRP only. | [68] |
| Autologous ADMSCs | 3.19–4.65 × 106 | Unavailable | PRP | Implantation vs. injection | KL 1–2; an isolated full-thickness articular cartilage lesion 3.2–9.4 cm2 | 1 | 24–42 months | MSC implantation resulted in better clinical and second-look arthroscopic outcomes than an MSC injection. | [69] |
| Autologous BMMSCs | 14.6 × 106 | 1 | Hyaluronic acid (3 weeks after HTO + microfracture) | Hyaluronic acid alone (3 weeks after HTO) | Medial OA, KL grading unavailable | 1 | 2 years | Better symptom improvement and cartilage repair (by MRI) were observed in the MSCs group. | [71] |
6. Do More MSCs Lead to Better Effects?
| Cell Type | Cell Dosage | Cell Passage | Combined Interventions | Control with Non-MSC Agents | Knee OA Grading | Injection Time | Follow Up | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Allogenic BMMSCs | 50 × 106/ 150 × 106 | Unavailable | Hyaluronic acid, human serum albumin (1.2%), and plasma-lyte a. Avoid strenuous activities or prolonged weight-bearing for 48 h and running and/or repetitive-impact activity for 6 weeks post-injection. | Hyaluronic acid alone | 7–10 days after partial medial meniscectomy | 1 | 2 years | Increased meniscal volume by MRI and pain reduction only in the MSC group (and better in the low-dose group). | [41] |
| Allogenic ADMSCs | 3.9 ×106/ 6.7 ×106 | Unavailable | None | Placebo | KL 1–3 | 1 | 12 months | Lateral tibial cartilage volume increase by MRI only observed in the low-dose group. | [67] |
| Allogeneic BMMSCs | 25 × 106/ 50 × 106/ 75 × 106/ 150 × 106 | Unavailable | Hyaluronic acid | Plasma-lyte a | KL II–III | 1 | 12 months | The trend of pain reduction only observed in the 25 × 106 dose group (but statistically insignificant). Predominant adverse events observed in the higher-dose groups. No MRI improvements. | [72] |
| Allogeneic ADMSCs | 10 × 106/ 20 × 106/ 50 × 106 | Unavailable | Rest for 24 h following each injection | None | KL II–IV | 2 (0 + 3 weeks) | 48 weeks | The low-dose group had better pain reduction and function improvements. MRI assessments showed slight improvements in the low-dose group. | [73] |
| Cell Type | Cell Dosage | Cell Passage | Combined Interventions | Control with Non-MSC Agents | Knee OA Grading | Injection Time | Follow Up | Outcome | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Autologous BMAC | ≤400 × 106/ >400 × 106 | Unavailable | PRP + platelet lysate. After injection, using a knee orthosis and following a weight-bearing protocol. | None | KL I–IV (>50% in early stage, that is, KL I) | 1 | 12 months | Pain reduction and better function observed in both groups. Greater pain reduction occurred in the high-dose group. | [42] |
| Autologous ADMSC | 1.0 × 107/ 5.0 × 107/ 10 × 107 | Unavailable | None | None | KL II–IV | 1 | 6 months | Better knee function and pain reduction and reduced cartilage defects by regeneration of hyaline-like cartilage (observed by arthroscopy and MRI) only in the highest dose group. | [70] |
| Autologous ADMSC | 2 × 106/ 10 × 106/ 50 × 106 | 1 | None | None | KL III–IV | 1 | 6 months | Pain reduction and function improvement observed in all cases but statistical significance only observed for the low-dose group. | [74] |
| Autologous BMMSCs | 10 × 106/ 100 × 106 | Unavailable | Hyaluronic acid | HA alone | KL II–IV | 1 | 12 months/4 years | 12 months: better X-ray and MRI findings only in HA + high-dose group; no effects in the control group./ 4 years: better clinical improvement in high- and low-dose groups. The low-dose group induced higher level of pain reduction. | [75] / [76] |
| Autologous ADMSC | 10 × 106/ 20 × 106/ 50 × 106 | 4 | None | None | KL II–IV | 3 (0–6–48 weeks) | 96 weeks (≈22.4 months) | Increased cartilage volume by MRI and significant difference detected in the middle-dose group. The middle-dose group also had the highest functional improvement and SF-36 scores at 96 weeks. | [77] |
| Autologous SVF cells (adipose) | 30 × 106 15 × 106 | 0 | Minimal weight-bearing for 2 days. Full range of motion (non-weight-bearing) was encouraged. Only light activity and previously painful activities should be avoided for the first 3 weeks after injection. | Placebo (zero SVF cells) | KL II–III | 1 | 12 months | Better WOMAC score changes in the high- and low-dose MSCs groups than those in the control (89.5%; 68.2%; 0%). However, no changes in cartilage thickness were detected by MRI. | [84] |
7. Do We Need a Post-Injection Protocol?
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oliveria, S.A.; Felson, D.T.; Reed, J.I.; Cirillo, P.A.; Walker, A.M. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. 1995, 38, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Doyle, E.C.; Wragg, N.M.; Wilson, S.L. Intraarticular injection of bone marrow-derived mesenchymal stem cells enhances regeneration in knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 3827–3842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silverwood, V.; Blagojevic-Bucknall, M.; Jinks, C.; Jordan, J.; Protheroe, J.; Jordan, K. Current evidence on risk factors for knee osteoarthritis in older adults: A systematic review and meta-analysis. Osteoarthr. Cartil. 2015, 23, 507–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef] [Green Version]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, N.; Yan, Z.-P.; Chen, X.-Y.; Ni, G.-X. Infrapatellar Fat Pad and Knee Osteoarthritis. Aging Dis. 2020, 11, 1317–1328. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, J.; Xu, H.; Lin, Z.; Chang, H.; Liu, W.; Kong, L. Mesenchymal stem cells in knee osteoarthritis treatment: A systematic review and meta-analysis. J. Orthop. Transl. 2020, 24, 121–130. [Google Scholar] [CrossRef]
- Mamidi, M.; Das, A.; Zakaria, Z.; Bhonde, R. Mesenchymal stromal cells for cartilage repair in osteoarthritis. Osteoarthr. Cartil. 2016, 24, 1307–1316. [Google Scholar] [CrossRef] [Green Version]
- Koh, Y.-G.; Choi, Y.-J. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee 2012, 19, 902–907. [Google Scholar] [CrossRef]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transpl. 2016, 25, 829–848. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Sekiya, I.; Yagishita, K.; Muneta, T. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheum. 2005, 52, 2521–2529. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Mennan, C.; McCarthy, H.S.; Roberts, S.; Richardson, J.B.; Wright, K.T. Chondrogenic Potency Analyses of Donor-Matched Chondrocytes and Mesenchymal Stem Cells Derived from Bone Marrow, Infrapatellar Fat Pad, and Subcutaneous Fat. Stem. Cells Int. 2016, 2016, 6969726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantripragada, V.P.; Piuzzi, N.S.; Bova, W.A.; Boehm, C.; Obuchowski, N.A.; Lefebvre, V.; Midura, R.J.; Muschler, G.F. Donor-matched comparison of chondrogenic progenitors resident in human infrapatellar fat pad, synovium, and periosteum—implications for cartilage repair. Connect. Tissue Res. 2019, 60, 597–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caplan, A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell. Physiol. 2007, 213, 341–347. [Google Scholar] [CrossRef]
- Brody, L.T. Knee osteoarthritis: Clinical connections to articular cartilage structure and function. Phys. Ther. Sport 2015, 16, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Armiento, A.R.; Alini, M.; Stoddart, M.J. Articular fibrocartilage—Why does hyaline cartilage fail to repair. Adv. Drug Deliv. Rev. 2019, 146, 289–305. [Google Scholar] [CrossRef]
- Aigner, T.; Söder, S.; Gebhard, P.M.; McAlinden, A.; Haag, J. Mechanisms of disease: Role of chondrocytes in the pathogenesis of osteoarthritis--structure, chaos and senescence. Nat. Clin. Pract. Rheumatol. 2007, 3, 391–399. [Google Scholar] [CrossRef]
- Li, G.; Park, S.E.; DeFrate, L.E.; Schutzer, M.E.; Ji, L.; Gill, T.J.; Rubash, H.E. The cartilage thickness distribution in the tibiofemoral joint and its correlation with cartilage-to-cartilage contact. Clin. Biomech. 2005, 20, 736–744. [Google Scholar] [CrossRef]
- Si, L.; Xuan, K.; Zhong, J.; Huo, J.; Xing, Y.; Geng, J.; Hu, Y.; Zhang, H.; Wang, Q.; Yao, W. Knee Cartilage Thickness Differs Alongside Ages: A 3-T Magnetic Resonance Research Upon 2,481 Subjects via Deep Learning. Front. Med. 2020, 7, 600049. [Google Scholar] [CrossRef]
- Sidharthan, S.; Yau, A.; Almeida, B.A.; Shea, K.G.; Greditzer, H.G., 4th; Jones, K.J.; Fabricant, P.D. Patterns of Articular Cartilage Thickness in Pediatric and Adolescent Knees: A Magnetic Resonance Imaging-Based Study. Arthrosc. Sport. Med. Rehabil. 2021, 3, e381–e390. [Google Scholar] [CrossRef]
- Kobayashi, S.; Yonekubo, S.; Kurogouchi, Y. Cryoscanning electron microscopic study of the surface amorphous layer of articular cartilage. J. Anat. 1995, 187 Pt 2, 429–444. [Google Scholar] [PubMed]
- Jay, G.D.; Waller, K.A. The biology of lubricin: Near frictionless joint motion. Matrix. Biol. 2014, 39, 17–24. [Google Scholar] [CrossRef]
- Watkins, A.R.; Reesink, H.L. Lubricin in experimental and naturally occurring osteoarthritis: A systematic review. Osteoarthr. Cartil. 2020, 28, 1303–1315. [Google Scholar] [CrossRef] [PubMed]
- Mills, A. The coefficient of friction, particularly of ice. Phys. Educ. 2008, 43, 392. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, Z.; Yang, H.; Zhong, H.; Peng, W.; Xie, R. Recent Advances in Understanding the Role of Cartilage Lubrication in Osteoarthritis. Molecules 2021, 26, 6122. [Google Scholar] [CrossRef]
- Chan, S.M.; Neu, C.P.; Duraine, G.; Komvopoulos, K.; Reddi, A.H. Atomic force microscope investigation of the boundary-lubricant layer in articular cartilage. Osteoarthr. Cartil. 2010, 18, 956–963. [Google Scholar] [CrossRef] [Green Version]
- Bobic, V. What is Lamina Splendens and What Does it Do. In Proceedings of the ICRS 2015, Chicago, IL, USA, 8–11 May 2015. [Google Scholar]
- Janicka, K.; Beldowski, P.; Majewski, T.; Urbaniak, W.; Petelska, A.D. The Amphoteric and Hydrophilic Properties of Cartilage Surface in Mammalian Joints: Interfacial Tension and Molecular Dynamics Simulation Studies. Molecules 2019, 24, 2248. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.P.; Kirk, T.B.; Zheng, M.H. Study of the collagen structure in the superficial zone and physiological state of articular cartilage using a 3D confocal imaging technique. J. Orthop. Surg. Res. 2008, 3, 29. [Google Scholar] [CrossRef] [Green Version]
- Klausner, R.D.; Kempf, C.; Weinstein, J.N.; Blumenthal, R.; Van Renswoude, J. The folding of ovalbumin. Renaturation in vitro versus biosynthesis in vitro. Biochem. J. 1983, 212, 801–810. [Google Scholar] [CrossRef] [Green Version]
- Mow, V.C.; Holmes, M.H.; Lai, W.M. Fluid transport and mechanical properties of articular cartilage: A review. J. Biomech. 1984, 17, 377–394. [Google Scholar] [CrossRef]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix. Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef] [PubMed]
- Culav, E.M.; Clark, C.H.; Merrilees, M.J. Connective tissues: Matrix composition and its relevance to physical therapy. Phys. Ther. 1999, 79, 308–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bishop, P.N. Molecular Composition of the Vitreous and Aging Changes. In Encyclopedia of the Eye; Academic Press: Waltham, MA, USA, 2010; pp. 37–43. [Google Scholar] [CrossRef]
- Holmes, D.F.; Lu, Y.; Starborg, T.; Kadler, K.E. Collagen Fibril Assembly and Function. Curr. Top. Dev. Biol. 2018, 130, 107–142. [Google Scholar]
- Han, E.H.; Chen, S.S.; Klisch, S.M.; Sah, R.L. Contribution of proteoglycan osmotic swelling pressure to the compressive properties of articular cartilage. Biophys. J. 2011, 101, 916–924. [Google Scholar] [CrossRef] [Green Version]
- Maroudas, A.I. Balance between swelling pressure and collagen tension in normal and degenerate cartilage. Nature 1976, 260, 808–809. [Google Scholar] [CrossRef] [PubMed]
- LaPrade, C.M.; Ellman, M.B.; Rasmussen, M.T.; James, E.W.; Wijdicks, C.A.; Engebretsen, L.; LaPrade, R.F. Anatomy of the anterior root attachments of the medial and lateral menisci: A quantitative analysis. Am. J. Sports Med. 2014, 42, 2386–2392. [Google Scholar] [CrossRef] [PubMed]
- Chahla, J.; Cinque, M.E.; Godin, J.A.; Sanchez, G.; Lebus, G.F.; Whalen, J.M.; Price, M.D.; Kennedy, N.I.; Moatshe, G.; LaPrade, R.F.; et al. Meniscectomy and Resultant Articular Cartilage Lesions of the Knee Among Prospective National Football League Players: An Imaging and Performance Analysis. Am. J. Sports Med. 2018, 46, 200–207. [Google Scholar] [CrossRef]
- Englund, M.; Roos, E.M.; Lohmander, L.S. Impact of type of meniscal tear on radiographic and symptomatic knee osteoarthritis: A sixteen-year followup of meniscectomy with matched controls. Arthritis Rheum. 2003, 48, 2178–2187. [Google Scholar] [CrossRef]
- Vangsness, C.T., Jr.; Farr, J., 2nd; Boyd, J.; Dellaero, D.T.; Mills, C.R.; LeRoux-Williams, M. Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: A randomized, double-blind, controlled study. J. Bone Jt. Surg. 2014, 96, 90–98. [Google Scholar] [CrossRef] [Green Version]
- Centeno, C.J.; Al-Sayegh, H.; Bashir, J.; Goodyear, S.; Freeman, M.D. A dose response analysis of a specific bone marrow concentrate treatment protocol for knee osteoarthritis. BMC Musculoskelet. Disord. 2015, 16, 258. [Google Scholar] [CrossRef]
- Mameri, E.S.; Dasari, S.P.; Fortier, L.M.; Verdejo, F.G.; Gursoy, S.; Yanke, A.B.; Chahla, J. Review of Meniscus Anatomy and Biomechanics. Curr. Rev. Musculoskelet. Med. 2022, 15, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Gee, S.M.; Posner, M. Meniscus Anatomy and Basic Science. Sports Med. Arthrosc. Rev. 2021, 29, e18–e23. [Google Scholar] [CrossRef] [PubMed]
- Markes, A.R.; Hodax, J.D.; Ma, C.B. Meniscus Form and Function. Clin. Sports Med. 2020, 39, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of human knee menisci: Structure, composition, and function. Sports Health 2012, 4, 340–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapiro, S.A.; Kazmerchak, S.E.; Heckman, M.G.; Zubair, A.C.; O’Connor, M.I. A Prospective, Single-Blind, Placebo-Controlled Trial of Bone Marrow Aspirate Concentrate for Knee Osteoarthritis. Am. J. Sports Med. 2017, 45, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Kim, H.J.; Kim, K.I.; Kim, G.B.; Jin, W. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl. Med. 2019, 8, 504–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soltani, S.K.; Forogh, B.; Ahmadbeigi, N.; Kharazi, H.H.; Fallahzadeh, K.; Kashani, L.; Karami, M.; Kheyrollah, Y.; Vasei, M. Safety and efficacy of allogenic placental mesenchymal stem cells for treating knee osteoarthritis: A pilot study. Cytotherapy 2019, 21, 54–63. [Google Scholar] [CrossRef]
- Vega, A.; Martín-Ferrero, M.A.; Del Canto, F.; Alberca, M.; García, V.; Munar, A.; Orozco, L.; Soler, R.; Fuertes, J.J.; Huguet, M.; et al. Treatment of Knee Osteoarthritis With Allogeneic Bone Marrow Mesenchymal Stem Cells: A Randomized Controlled Trial. Transplantation 2015, 99, 1681–1690. [Google Scholar] [CrossRef]
- Matas, J.; Orrego, M.; Amenabar, D.; Infante, C.; Tapia-Limonchi, R.; Cadiz, M.I.; Alcayaga-Miranda, F.; González, P.L.; Muse, E.; Khoury, M.; et al. Umbilical Cord-Derived Mesenchymal Stromal Cells (MSCs) for Knee Osteoarthritis: Repeated MSC Dosing Is Superior to a Single MSC Dose and to Hyaluronic Acid in a Controlled Randomized Phase I/II Trial. Stem Cells Transl. Med. 2019, 8, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Dai, C.; Zhang, Z.; Du, H.; Li, S.; Ye, P.; Fu, Q.; Zhang, L.; Wu, X.; Dong, Y.; et al. Treatment of knee osteoarthritis with intra-articular injection of autologous adipose-derived mesenchymal progenitor cells: A prospective, randomized, double-blind, active-controlled, phase IIb clinical trial. Stem Cell Res. Ther. 2019, 10, 143. [Google Scholar] [CrossRef]
- Kim, Y.S.; Suh, D.S.; Tak, D.H.; Chung, P.K.; Kwon, Y.B.; Kim, T.Y.; Koh, Y.G. Comparative matched-pair cohort analysis of the short-term clinical outcomes of mesenchymal stem cells versus hyaluronic acid treatments through intra-articular injections for knee osteoarthritis. J. Exp. Orthop. 2020, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Lamo-Espinosa, J.M.; Blanco, J.F.; Sánchez, M.; Moreno, V.; Granero-Moltó, F.; Sánchez-Guijo, F.; Crespo-Cullel, Í.; Mora, G.; San Vicente, D.D.; Pompei-Fernández, O.; et al. Phase II multicenter randomized controlled clinical trial on the efficacy of intra-articular injection of autologous bone marrow mesenchymal stem cells with platelet rich plasma for the treatment of knee osteoarthritis. J. Transl. Med. 2020, 18, 356. [Google Scholar] [CrossRef] [PubMed]
- Woodell-May, J.; Matuska, A.; Oyster, M.; Welch, Z.; O′Shaughnessey, K.; Hoeppner, J. Autologous protein solution inhibits MMP-13 production by IL-1β and TNFα-stimulated human articular chondrocytes. J. Orthop. Res. 2011, 29, 1320–1326. [Google Scholar] [CrossRef]
- Tang, X.B.; Dong, P.L.; Wang, J.; Zhou, H.Y.; Zhang, H.X.; Wang, S.Z. Effect of autologous platelet-rich plasma on the chondrogenic differentiation of rabbit adipose-derived stem cells in vitro. Exp. Ther. Med. 2015, 10, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Elder, S.; Thomason, J. Effect of platelet-rich plasma on chondrogenic differentiation in three-dimensional culture. Open Orthop. J. 2014, 8, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Bastos, R.; Mathias, M.; Andrade, R.; Bastos, R.; Balduino, A.; Schott, V.; Rodeo, S.; Espregueira-Mendes, J. Intra-articular injections of expanded mesenchymal stem cells with and without addition of platelet-rich plasma are safe and effective for knee osteoarthritis. Knee Surg. Sport. Traumatol. Arthrosc. 2018, 26, 3342–3350. [Google Scholar] [CrossRef]
- Bastos, R.; Mathias, M.; Andrade, R.; Amaral, R.; Schott, V.; Balduino, A.; Bastos, R.; Miguel Oliveira, J.; Reis, R.L.; Rodeo, S.; et al. Intra-articular injection of culture-expanded mesenchymal stem cells with or without addition of platelet-rich plasma is effective in decreasing pain and symptoms in knee osteoarthritis: A controlled, double-blind clinical trial. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 1989–1999. [Google Scholar] [CrossRef] [Green Version]
- Hernigou, P.; Bouthors, C.; Bastard, C.; Flouzat Lachaniette, C.H.; Rouard, H.; Dubory, A. Subchondral bone or intra-articular injection of bone marrow concentrate mesenchymal stem cells in bilateral knee osteoarthritis: What better postpone knee arthroplasty at fifteen years? A randomized study. Int. Orthop. 2021, 45, 391–399. [Google Scholar] [CrossRef]
- Freitag, J.; Bates, D.; Wickham, J.; Shah, K.; Huguenin, L.; Tenen, A.; Paterson, K.; Boyd, R. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: A randomized controlled trial. Regen. Med. 2019, 14, 213–230. [Google Scholar] [CrossRef] [Green Version]
- Marks, P.W.; Witten, C.M.; Califf, R.M. Clarifying Stem-Cell Therapy′s Benefits and Risks. N. Engl. J. Med. 2017, 376, 1007–1009. [Google Scholar] [CrossRef]
- Jones, I.A.; Togashi, R.; Wilson, M.L.; Heckmann, N.; Vangsness, C.T., Jr. Intra-articular treatment options for knee osteoarthritis. Nat. Rev. Rheumatol. 2019, 15, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Akgun, I.; Unlu, M.C.; Erdal, O.A.; Ogut, T.; Erturk, M.; Ovali, E.; Kantarci, F.; Caliskan, G.; Akgun, Y. Matrix-induced autologous mesenchymal stem cell implantation versus matrix-induced autologous chondrocyte implantation in the treatment of chondral defects of the knee: A 2-year randomized study. Arch. Orthop. Trauma Surg. 2015, 135, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.B.; Wang, V.T.; Chan, Y.H.; Hui, J.H. A novel, minimally-invasive technique of cartilage repair in the human knee using arthroscopic microfracture and injections of mesenchymal stem cells and hyaluronic acid—a prospective comparative study on safety and short-term efficacy. Ann. Acad. Med. Singap. 2012, 41, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Spasovski, D.; Spasovski, V.; Baščarević, Z.; Stojiljković, M.; Vreća, M.; Anđelković, M.; Pavlović, S. Intra-articular injection of autologous adipose-derived mesenchymal stem cells in the treatment of knee osteoarthritis. J. Gene Med. 2018, 20, e3002. [Google Scholar] [CrossRef] [PubMed]
- Kuah, D.; Sivell, S.; Longworth, T.; James, K.; Guermazi, A.; Cicuttini, F.; Wang, Y.; Craig, S.; Comin, G.; Robinson, D.; et al. Safety, tolerability and efficacy of intra-articular Progenza in knee osteoarthritis: A randomized double-blind placebo-controlled single ascending dose study. J. Transl. Med. 2018, 16, 49. [Google Scholar] [CrossRef] [Green Version]
- Koh, Y.G.; Kwon, O.R.; Kim, Y.S.; Choi, Y.J. Comparative outcomes of open-wedge high tibial osteotomy with platelet-rich plasma alone or in combination with mesenchymal stem cell treatment: A prospective study. Arthroscopy 2014, 30, 1453–1460. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kwon, O.R.; Choi, Y.J.; Suh, D.S.; Heo, D.B.; Koh, Y.G. Comparative Matched-Pair Analysis of the Injection Versus Implantation of Mesenchymal Stem Cells for Knee Osteoarthritis. Am. J. Sports Med. 2015, 43, 2738–2746. [Google Scholar] [CrossRef]
- Jo, C.H.; Lee, Y.G.; Shin, W.H.; Kim, H.; Chai, J.W.; Jeong, E.C.; Kim, J.E.; Shim, H.; Shin, J.S.; Shin, I.S.; et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A proof-of-concept clinical trial. Stem Cells 2014, 32, 1254–1266. [Google Scholar] [CrossRef]
- Wong, K.L.; Lee, K.B.; Tai, B.C.; Law, P.; Lee, E.H.; Hui, J.H. Injectable cultured bone marrow-derived mesenchymal stem cells in varus knees with cartilage defects undergoing high tibial osteotomy: A prospective, randomized controlled clinical trial with 2 years′ follow-up. Arthroscopy 2013, 29, 2020–2028. [Google Scholar] [CrossRef]
- Gupta, P.K.; Chullikana, A.; Rengasamy, M.; Shetty, N.; Pandey, V.; Agarwal, V.; Wagh, S.Y.; Vellotare, P.K.; Damodaran, D.; Viswanathan, P.; et al. Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (Stempeucel®): Preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res. Ther. 2016, 18, 301. [Google Scholar] [CrossRef]
- Lu, L.; Dai, C.; Du, H.; Li, S.; Ye, P.; Zhang, L.; Wang, X.; Song, Y.; Togashi, R.; Vangsness, C.T.; et al. Intra-articular injections of allogeneic human adipose-derived mesenchymal progenitor cells in patients with symptomatic bilateral knee osteoarthritis: A Phase I pilot study. Regen. Med. 2020, 15, 1625–1636. [Google Scholar] [CrossRef] [PubMed]
- Pers, Y.M.; Rackwitz, L.; Ferreira, R.; Pullig, O.; Delfour, C.; Barry, F.; Sensebe, L.; Casteilla, L.; Fleury, S.; Bourin, P.; et al. Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. Stem Cells Transl. Med. 2016, 5, 847–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamo-Espinosa, J.M.; Mora, G.; Blanco, J.F.; Granero-Moltó, F.; Nuñez-Córdoba, J.M.; Sánchez-Echenique, C.; Bondía, J.M.; Aquerreta, J.D.; Andreu, E.J.; Ornilla, E.; et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: Multicenter randomized controlled clinical trial (phase I/II). J. Transl. Med. 2016, 14, 246. [Google Scholar] [CrossRef] [Green Version]
- Lamo-Espinosa, J.M.; Mora, G.; Blanco, J.F.; Granero-Moltó, F.; Núñez-Córdoba, J.M.; López-Elío, S.; Andreu, E.; Sánchez-Guijo, F.; Aquerreta, J.D.; Bondía, J.M.; et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: Long-term follow up of a multicenter randomized controlled clinical trial (phase I/II). J. Transl. Med. 2018, 16, 213. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Du, H.; Dai, C.; Zhang, L.; Li, S.; Hunter, D.J.; Lu, L.; Bao, C. Human adipose-derived mesenchymal stem cells for osteoarthritis: A pilot study with long-term follow-up and repeated injections. Regen. Med. 2018, 13, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joswig, A.J.; Mitchell, A.; Cummings, K.J.; Levine, G.J.; Gregory, C.A.; Smith, R., 3rd; Watts, A.E. Repeated intra-articular injection of allogeneic mesenchymal stem cells causes an adverse response compared to autologous cells in the equine model. Stem Cell Res. Ther. 2017, 8, 42. [Google Scholar] [CrossRef] [Green Version]
- van Rhijn-Brouwer, F.; Gremmels, H.; Fledderus, J.O.; Verhaar, M.C. Mesenchymal Stromal Cell Characteristics and Regenerative Potential in Cardiovascular Disease: Implications for Cellular Therapy. Cell Transpl. 2018, 27, 765–785. [Google Scholar] [CrossRef] [Green Version]
- Charif, N.; Li, Y.Y.; Targa, L.; Zhang, L.; Ye, J.S.; Li, Y.P.; Stoltz, J.F.; Han, H.Z.; de Isla, N. Aging of bone marrow mesenchymal stromal/stem cells: Implications on autologous regenerative medicine. Biomed. Mater. Eng. 2017, 28, S57–S63. [Google Scholar] [CrossRef]
- Cui, J.H.; Park, K.; Park, S.R.; Min, B.H. Effects of low-intensity ultrasound on chondrogenic differentiation of mesenchymal stem cells embedded in polyglycolic acid: An in vivo study. Tissue Eng. 2006, 12, 75–82. [Google Scholar] [CrossRef]
- Risbud, M.V.; Albert, T.J.; Guttapalli, A.; Vresilovic, E.J.; Hillibrand, A.S.; Vaccaro, A.R.; Shapiro, I.M. Differentiation of mesenchymal stem cells towards a nucleus pulposus-like phenotype in vitro: Implications for cell-based transplantation therapy. Spine 2004, 29, 2627–2632. [Google Scholar] [CrossRef] [PubMed]
- Garza, J.R.; Campbell, R.E.; Tjoumakaris, F.P.; Freedman, K.B.; Miller, L.S.; Santa Maria, D.; Tucker, B.S. Clinical Efficacy of Intra-articular Mesenchymal Stromal Cells for the Treatment of Knee Osteoarthritis: A Double-Blinded Prospective Randomized Controlled Clinical Trial. Am. J. Sports Med. 2020, 48, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.M.; Pham, P.T.; Bach, T.Q.; Ngo, A.; Nguyen, Q.T.; Phan, T.; Nguyen, G.H.; Le, P.T.; Hoang, V.T.; Forsyth, N.R.; et al. Stem cell-based therapy for human diseases. Signal Transduct. Target. Ther. 2022, 7, 272. [Google Scholar] [PubMed]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef] [Green Version]
- Gibbs, N.; Diamond, R.; Sekyere, E.O.; Thomas, W.D. Management of knee osteoarthritis by combined stromal vascular fraction cell therapy, platelet-rich plasma, and musculoskeletal exercises: A case series. J. Pain Res. 2015, 8, 799–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicuttini, F.M.; Jones, G.; Forbes, A.; Wluka, A.E. Rate of cartilage loss at two years predicts subsequent total knee arthroplasty: A prospective study. Ann. Rheum. Dis. 2004, 63, 1124–1127. [Google Scholar] [CrossRef] [Green Version]
- Graham, B.T.; Moore, A.C.; Burris, D.L.; Price, C. Sliding enhances fluid and solute transport into buried articular cartilage contacts. Osteoarthr. Cartil. 2017, 25, 2100–2107. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Hung, C.T.; Ateshian, G.A. Mechanical response of bovine articular cartilage under dynamic unconfined compression loading at physiological stress levels. Osteoarthr. Cartil. 2004, 12, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Xu, H.; Huang, Y.; Gu, S.; Jiang, J.X. Coupling Effect of Water and Proteoglycans on the In Situ Toughness of Bone. J. Bone Miner. Res. 2016, 31, 1026–1029. [Google Scholar] [CrossRef] [Green Version]
- Samuel, J.; Sinha, D.; Zhao, J.C.; Wang, X. Water residing in small ultrastructural spaces plays a critical role in the mechanical behavior of bone. Bone 2014, 59, 199–206. [Google Scholar] [CrossRef]
- Maroudas, A.; Bullough, P.; Swanson, S.A.; Freeman, M.A. The permeability of articular cartilage. J. Bone Jt. Surg. 1968, 50, 166–177. [Google Scholar] [CrossRef]
- Zhang, L.; Miramini, S.; Smith, D.W.; Gardiner, B.S.; Grodzinsky, A.J. Time evolution of deformation in a human cartilage under cyclic loading. Ann. Biomed. Eng. 2015, 43, 1166–1177. [Google Scholar] [CrossRef]
- Costa, R.A.; de Oliveira, L.M.; Watanabe, S.H.; Jones, A.; Natour, J. Isokinetic assessment of the hip muscles in patients with osteoarthritis of the knee. Clinics 2010, 65, 1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinman, R.S.; Hunt, M.A.; Creaby, M.W.; Wrigley, T.V.; McManus, F.J.; Bennell, K.L. Hip muscle weakness in individuals with medial knee osteoarthritis. Arthritis Care Res. Care Res. Care Res. 2010, 62, 1190. [Google Scholar] [CrossRef] [PubMed]
- Deasy, M.; Leahy, E.; Semciw, A.I. Hip Strength Deficits in People With Symptomatic Knee Osteoarthritis: A Systematic Review With Meta-analysis. J. Orthop. Sport. Phys. Ther. 2016, 46, 629. [Google Scholar] [CrossRef] [Green Version]
- Chang, A.H.; Chmiel, J.S.; Almagor, O.; Hayes, K.W.; Guermazi, A.; Prasad, P.V.; Moisio, K.C.; Zhang, Y.; Szymaszek, J.; Sharma, L. Hip muscle strength and protection against structural worsening and poor function and disability outcomes in knee osteoarthritis. Osteoarthr. Cartil. 2019, 27, 885–894. [Google Scholar] [CrossRef]
- Konow, N.; Roberts, T.J. The series elastic shock absorber: Tendon elasticity modulates energy dissipation by muscle during burst deceleration. Proc. Biol. Sci. 2015, 282, 20142800. [Google Scholar] [CrossRef] [Green Version]
- Hyun, J.S.; Tran, M.C.; Wong, V.W.; Chung, M.T.; Lo, D.D.; Montoro, D.T.; Wan, D.C.; Longaker, M.T. Enhancing stem cell survival in vivo for tissue repair. Biotechnol. Adv. 2013, 31, 736–743. [Google Scholar] [CrossRef]
- Bi, S.; Wang, H.; Kuang, W. Stem cell rejuvenation and the role of autophagy in age retardation by caloric restriction: An update. Mech. Ageing Dev. 2018, 175, 46–54. [Google Scholar] [CrossRef]
- Rutjes, A.W.; Nüesch, E.; Sterchi, R.; Jüni, P. Therapeutic ultrasound for osteoarthritis of the knee or hip. Cochrane Database Syst. Rev. 2010, CD003132. [Google Scholar] [CrossRef]
- Jia, L.; Li, D.; Wei, X.; Chen, J.; Zuo, D.; Chen, W. Efficacy and safety of focused low-intensity pulsed ultrasound versus pulsed shortwave diathermy on knee osteoarthritis: A randomized comparative trial. Sci. Rep. 2022, 12, 12792. [Google Scholar] [CrossRef] [PubMed]
- Atamaz, F.C.; Durmaz, B.; Baydar, M.; Demircioglu, O.Y.; Iyiyapici, A.; Kuran, B.; Oncel, S.; Sendur, O.F. Comparison of the efficacy of transcutaneous electrical nerve stimulation, interferential currents, and shortwave diathermy in knee osteoarthritis: A double-blind, randomized, controlled, multicenter study. Arch. Phys. Med. Rehabil. 2012, 93, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Choi, Y.J.; Suh, D.S.; Heo, D.B.; Kim, Y.I.; Ryu, J.S.; Koh, Y.G. Mesenchymal stem cell implantation in osteoarthritic knees: Is fibrin glue effective as a scaffold. Am. J. Sports Med. 2015, 43, 176–185. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, P.; Bao, R. Intra-Articular Mesenchymal Stem Cell Injection for Knee Osteoarthritis: Mechanisms and Clinical Evidence. Int. J. Mol. Sci. 2023, 24, 59. https://doi.org/10.3390/ijms24010059
Wei P, Bao R. Intra-Articular Mesenchymal Stem Cell Injection for Knee Osteoarthritis: Mechanisms and Clinical Evidence. International Journal of Molecular Sciences. 2023; 24(1):59. https://doi.org/10.3390/ijms24010059
Chicago/Turabian StyleWei, Pengxu, and Ruixue Bao. 2023. "Intra-Articular Mesenchymal Stem Cell Injection for Knee Osteoarthritis: Mechanisms and Clinical Evidence" International Journal of Molecular Sciences 24, no. 1: 59. https://doi.org/10.3390/ijms24010059
APA StyleWei, P., & Bao, R. (2023). Intra-Articular Mesenchymal Stem Cell Injection for Knee Osteoarthritis: Mechanisms and Clinical Evidence. International Journal of Molecular Sciences, 24(1), 59. https://doi.org/10.3390/ijms24010059






