Improvement of Fertilization Capacity and Developmental Ability of Vitrified Bovine Oocytes by JUNO mRNA Microinjection and Cholesterol-Loaded Methyl-β-Cyclodextrin Treatment
Abstract
:1. Introduction
2. Results
2.1. Effect of Vitrification on JUNO Protein Abundance in Bovine Oocytes
2.2. Effect of Vitrification on the Post-Translational Modifications of JUNO Protein Sequence in Bovine Oocytes
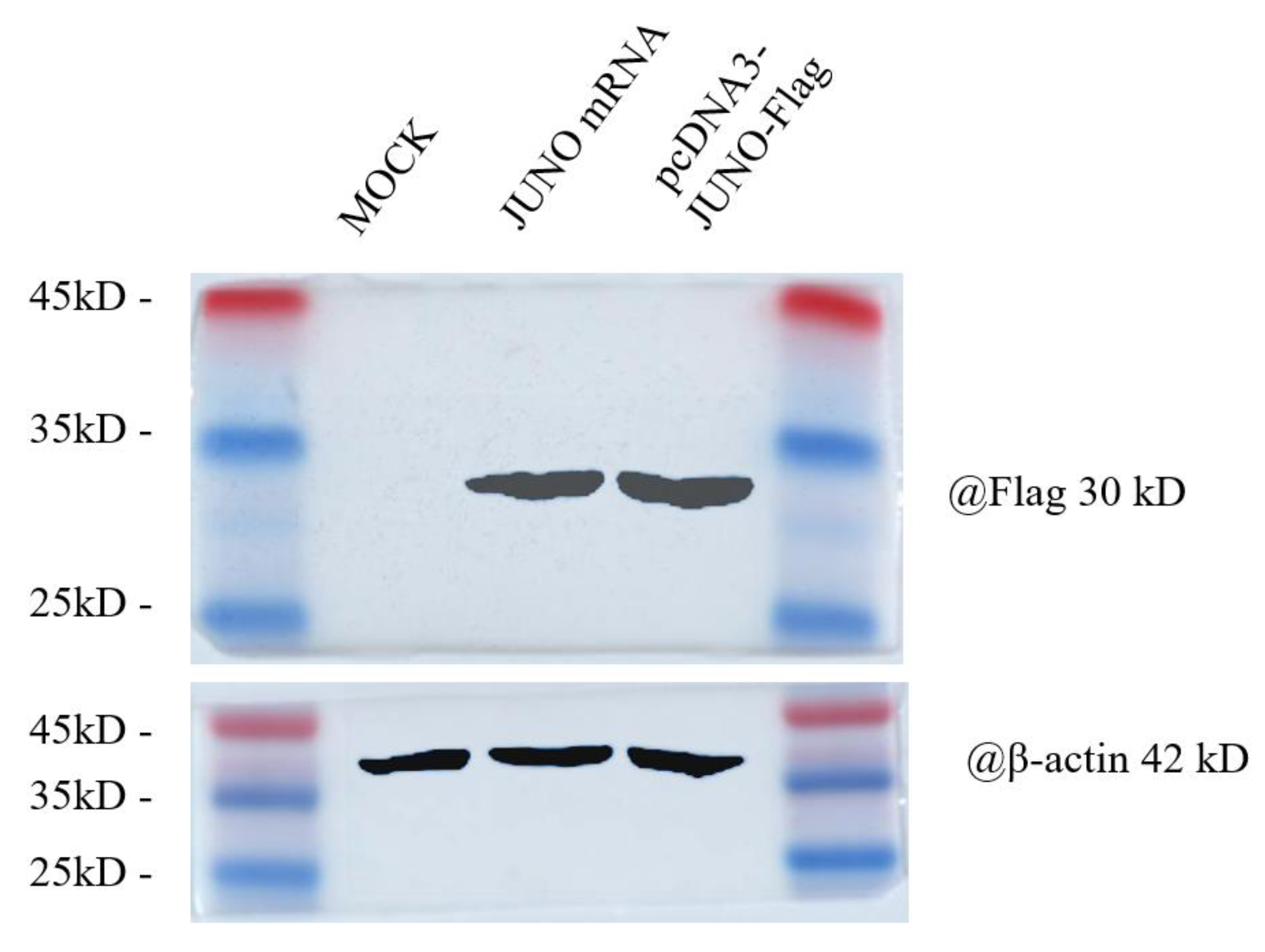
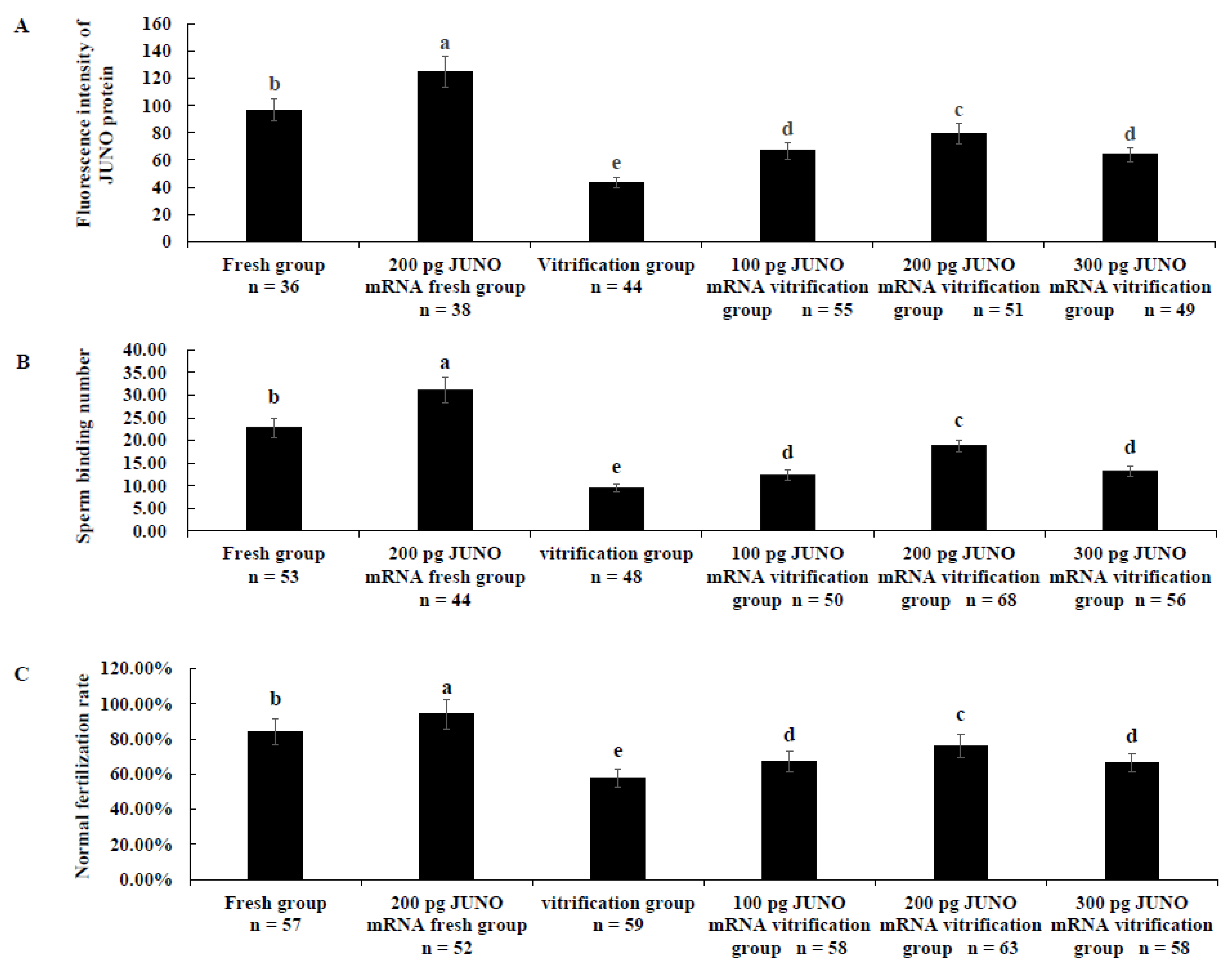
2.3. Effect of JUNO mRNA Microinjection on the Protein Abundance of JUNO in Vitrified Oocytes
2.4. Effect of JUNO mRNA Microinjection on the Development Ability of Vitrified Oocytes
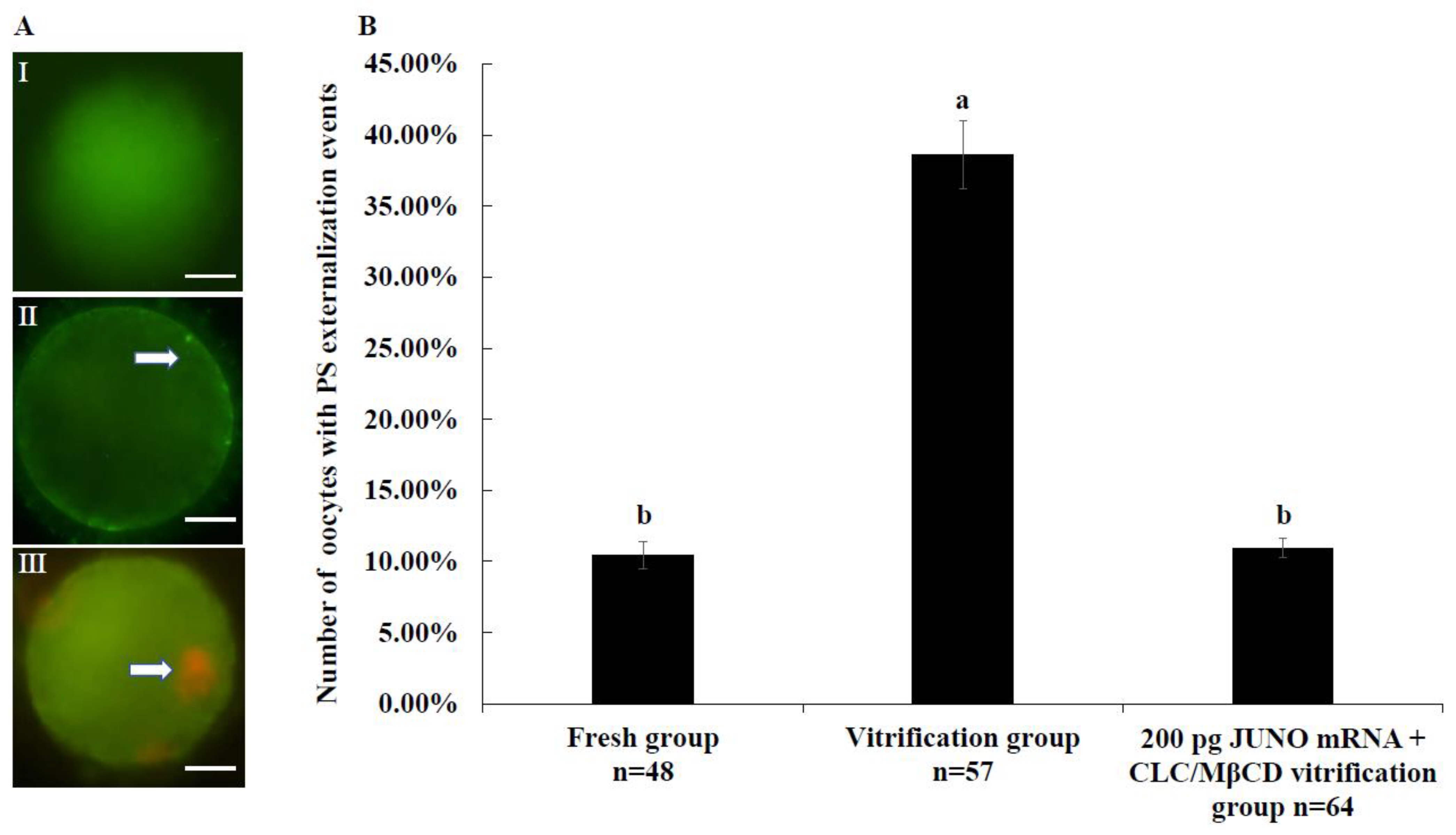
2.5. Effect of the JUNO mRNA + CLC/MβCD Treatment on the Membrane Integrity of Vitrified Oocytes
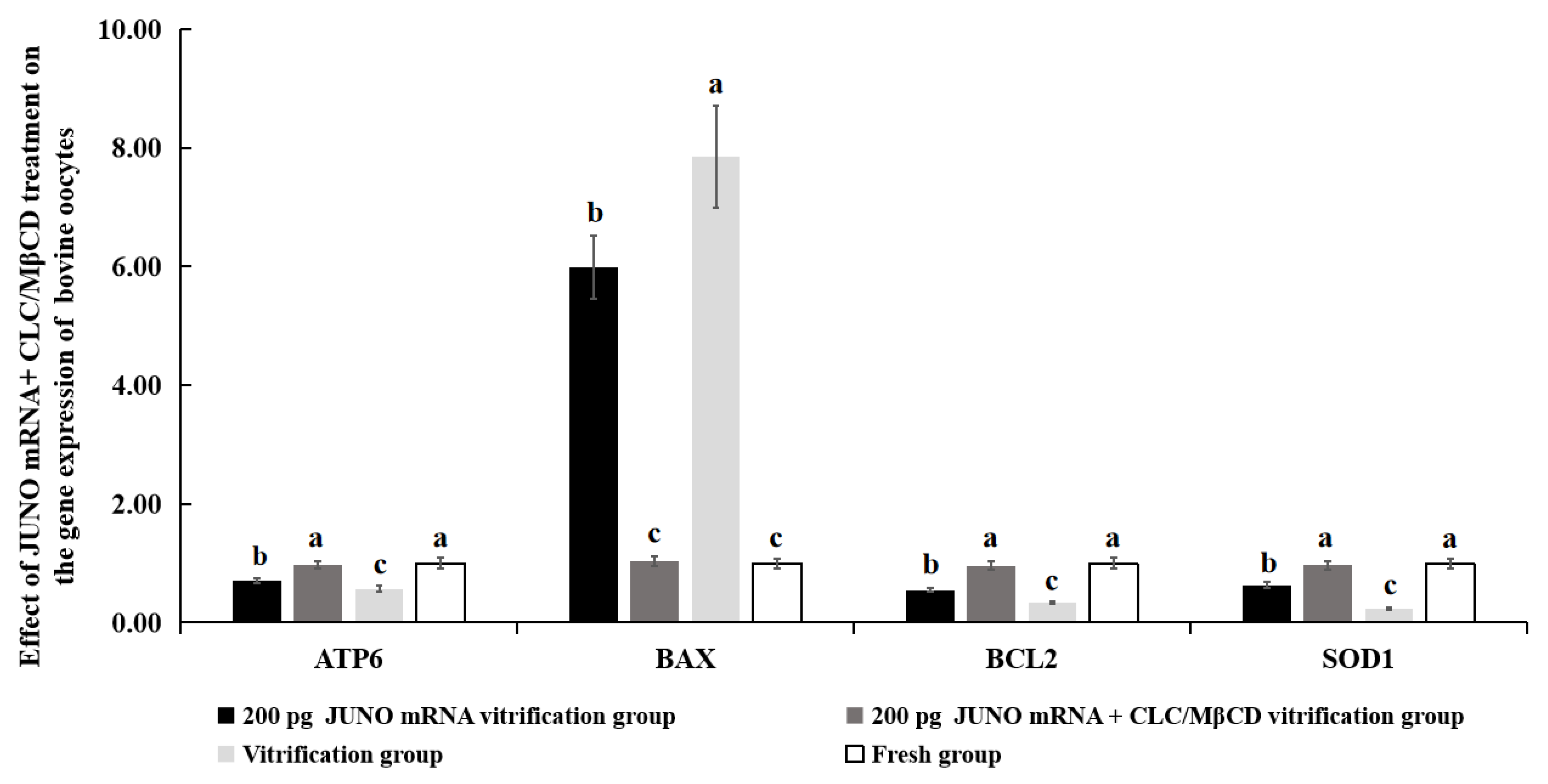
2.6. Effect of the JUNO mRNA + CLC/MβCD Treatment on the Gene Expression of Vitrified Oocytes
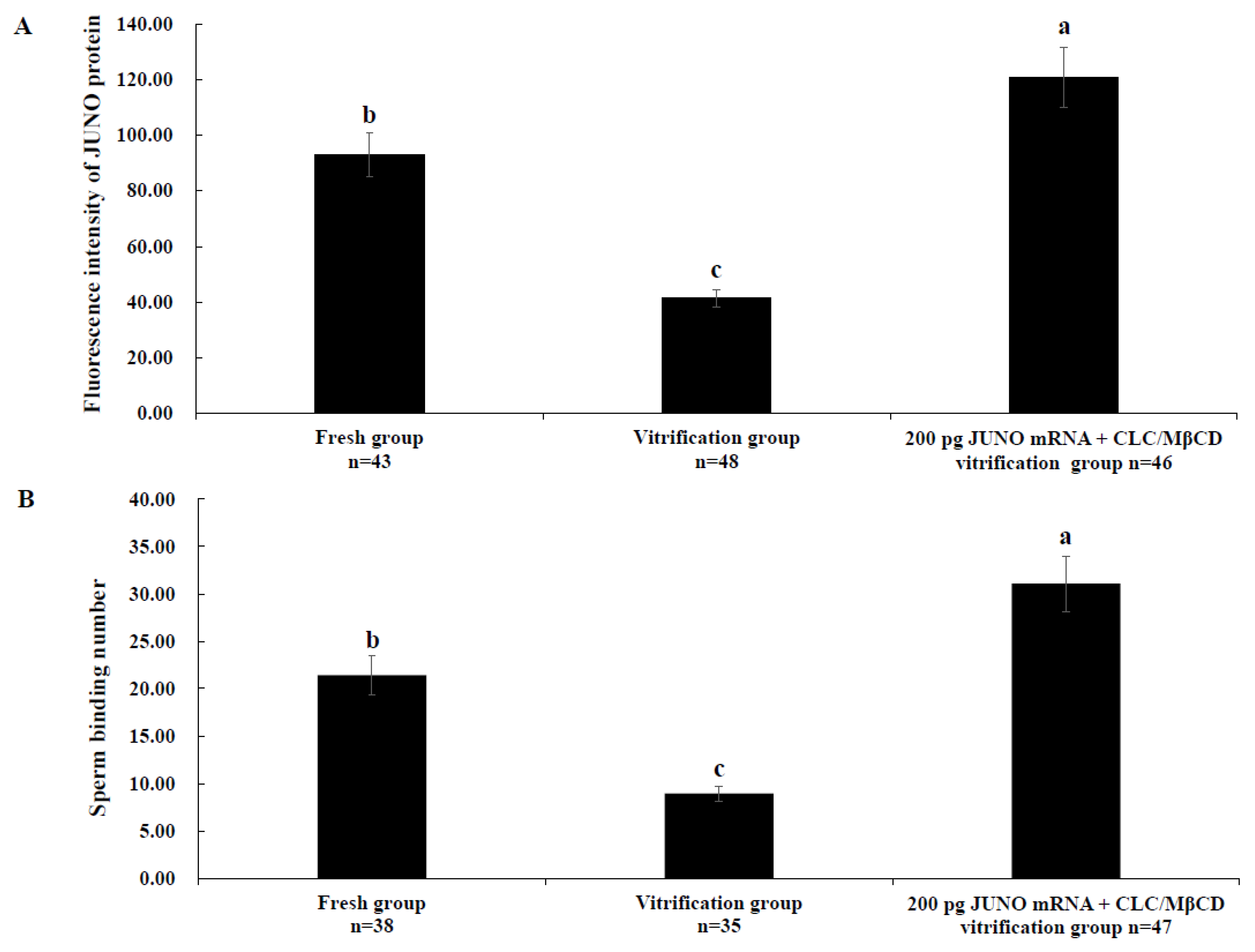
2.7. Effect of the JUNO mRNA + CLC/MβCD Treatment on the JUNO Protein Abundance and Sperm Binding Number of Vitrified Oocytes
2.8. Effect of JUNO mRNA + CLC/MβCD Treatment on the Development Ability of Vitrified Oocytes after IVF
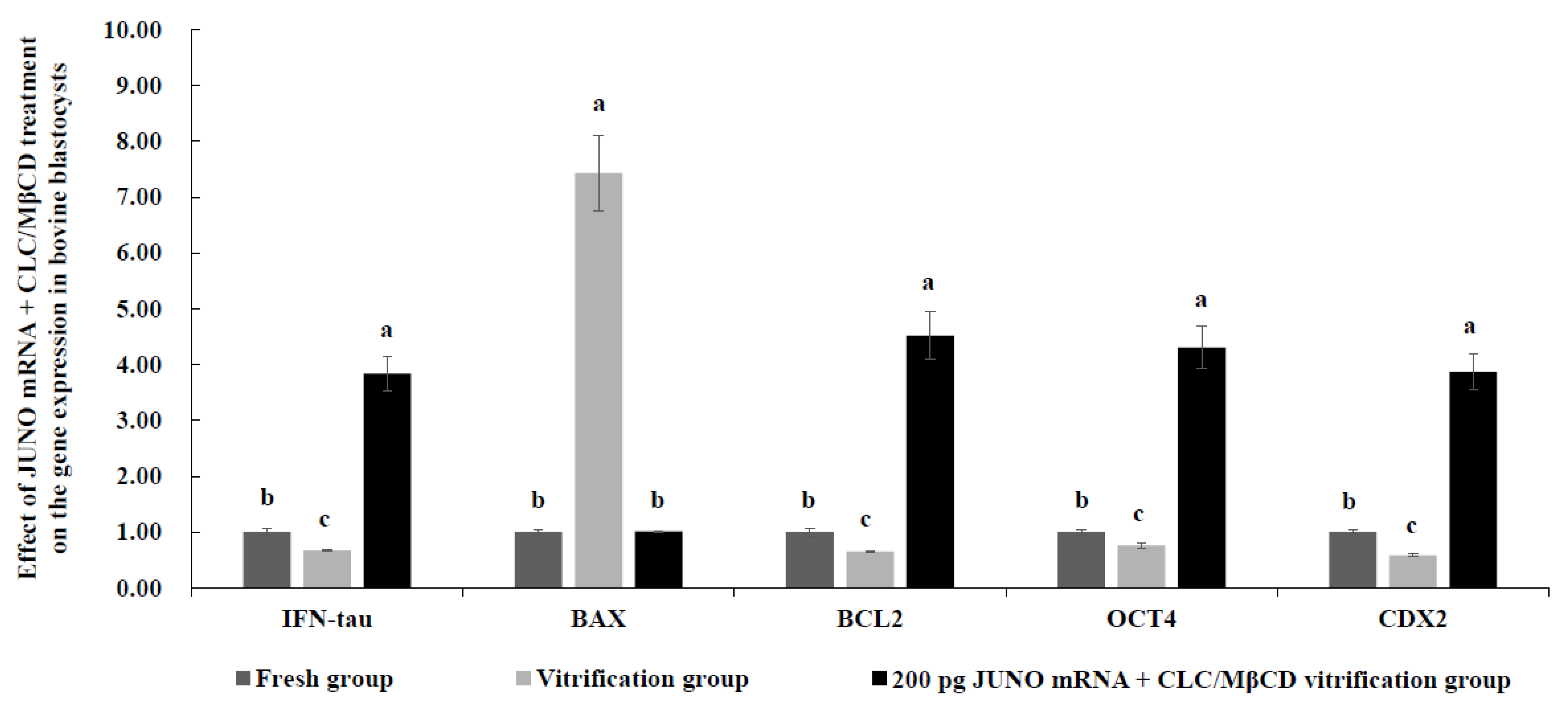
2.9. Effect of JUNO mRNA + CLC/MβCD Treatment on the Gene Expression in Vitrified Blastocysts
3. Discussion
4. Materials and Methods
4.1. In Vitro Maturation of Oocytes (IVM)
4.2. Oocytes Vitrification and Warming
4.3. In Vitro Fertilization Experiments (IVF)
4.4. Assessment of JUNO Protein Abundance in Bovine Oocytes
4.5. JUNO Protein Sequencing and Protein Modification Analysis Based on LC-MS/MS
4.6. Preparation and Validation of JUNO mRNA Used for Microinjection
4.7. The Assessment of Sperm Binding Ability
4.8. The Assessment of Normal Fertilization
4.9. Microinjection of JUNO mRNA
4.10. The Preparation and Addition of CLC/MβCD
4.11. Analysis of Phosphatidylserine (PS) Externalization Events
4.12. Quantitative Real-Time PCR of Candidate Genes
4.13. Total Nuclear Counts of Blastocysts
4.14. Experiment Design
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Argyle, C.E.; Harper, J.C.; Davies, M.C. Oocyte cryopreservation: Where are we now? Hum. Reprod. Update 2016, 22, 440–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rienzi, L.; Gracia, C.; Maggiulli, R.; LaBarbera, A.R.; Kaser, D.J.; Ubaldi, F.M.; Vanderpoel, S.; Racowsky, C. Oocyte, embryo and blastocyst cryopreservation in ART: Systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum. Reprod. Update 2017, 23, 139–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tharasanit, T.; Thuwanut, P. Oocyte cryopreservation in domestic animals and humans: Principles, techniques and updated outcomes. Animals 2021, 11, 2949. [Google Scholar] [CrossRef] [PubMed]
- Angel-Velez, D.; de Coster, T.; Azari-Dolatabad, N.; Fernandez-Montoro, A.; Benedetti, C.; Bogado Pascottini, O.; Woelders, H.; Van Soom, A.; Smits, K. New alternative mixtures of cryoprotectants for equine immature oocyte vitrification. Animals 2021, 11, 3077. [Google Scholar] [CrossRef] [PubMed]
- Hagedorn, M.; Varga, Z.; Walter, R.B.; Tiersch, T.R. Workshop report: Cryopreservation of aquatic biomedical models. Cryobiology 2019, 86, 120–129. [Google Scholar] [CrossRef]
- Somfai, T.; Kikuchi, K. Vitrification of porcine oocytes and zygotes in microdrops on a solid metal surface or liquid nitrogen. Methods Mol. Biol. 2021, 2180, 455–468. [Google Scholar]
- Mara, L.; Casu, S.; Carta, A.; Dattena, M. Cryobanking of farm animal gametes and embryos as a means of conserving livestock genetics. Anim. Reprod. Sci. 2013, 138, 25–38. [Google Scholar] [CrossRef]
- Pitchayapipatkul, J.; Somfai, T.; Matoba, S.; Parnpai, R.; Nagai, T.; Geshi, M.; Vongpralub, T. Microtubule stabilisers docetaxel and paclitaxel reduce spindle damage and maintain the developmental competence of in vitro-mature bovine oocytes during vitrification. Reprod. Fertil. Dev. 2017, 29, 2028–2039. [Google Scholar] [CrossRef]
- Stachecki, J.J.; Cohen, J. An overview of oocyte cryopreservation. Reprod. Biomed. Online 2004, 9, 152–163. [Google Scholar] [CrossRef]
- Abir, R.; Ben-Aharon, I.; Garor, R.; Yaniv, I.; Ash, S.; Stemmer, S.M.; Ben-Haroush, A.; Freud, E.; Kravarusic, D.; Sapir, O.; et al. Cryopreservation of in vitro matured oocytes in addition to ovarian tissue freezing for fertility preservation in paediatric female cancer patients before and after cancer therapy. Hum. Reprod. 2016, 31, 750–762. [Google Scholar] [CrossRef] [Green Version]
- Iussig, B.; Maggiulli, R.; Fabozzi, G.; Bertelle, S.; Vaiarelli, A.; Cimadomo, D.; Ubaldi, F.M.; Rienzi, L. A brief history of oocyte cryopreservation: Arguments and facts. Acta Obs. Gynecol Scand. 2019, 98, 550–558. [Google Scholar] [CrossRef]
- Khalili, M.A.; Shahedi, A.; Ashourzadeh, S.; Nottola, S.A.; Macchiarelli, G.; Palmerini, M.G. Vitrification of human immature oocytes before and after in vitro maturation: A review. J. Assist. Reprod. Genet. 2017, 34, 1413–1426. [Google Scholar] [CrossRef] [Green Version]
- Parnpai, R.; Liang, Y.; Ketudat-Cairns, M.; Somfai, T.; Nagai, T. Vitrification of buffalo oocytes and embryos. Theriogenology 2016, 86, 214–220. [Google Scholar] [CrossRef]
- Eroglu, B.; Szurek, E.A.; Schall, P.; Latham, K.E.; Eroglu, A. Probing lasting cryoinjuries to oocyte-embryo transcriptome. PLoS ONE 2020, 15, e0231108. [Google Scholar] [CrossRef] [Green Version]
- Amidi, F.; Khodabandeh, Z.; Nori Mogahi, M.H. Comparison of the effects of vitrification on gene expression of mature mouse oocytes using cryotop and open pulled straw. Int. J. Fertil. Steril. 2018, 12, 61–67. [Google Scholar]
- Lowther, K.M.; Weitzman, V.N.; Maier, D.; Mehlmann, L.M. Maturation, fertilization, and the structure and function of the endoplasmic reticulum in cryopreserved mouse oocytes. Biol. Reprod. 2009, 81, 147–154. [Google Scholar] [CrossRef]
- Carroll, J.; Depypere, H.; Matthews, C.D. Freeze-thaw-induced changes of the zona pellucida explains decreased rates of fertilization in frozen-thawed mouse oocytes. J. Reprod. Fertil. 1990, 90, 547–553. [Google Scholar] [CrossRef] [Green Version]
- Wood, M.J.; Whittingham, D.G.; Lee, S.H. Fertilization failure of frozen mouse oocytes is not due to premature cortical granule release. Biol. Reprod. 1992, 46, 1187–1195. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos-Neto, P.C.; Vilariño, M.; Cuadro, F.; Barrera, N.; Crispo, M.; Menchaca, A. Cumulus cells during in vitro fertilization and oocyte vitrification in sheep: Remove, maintain or add? Cryobiology 2020, 92, 161–167. [Google Scholar] [CrossRef]
- Gutnisky, C.; Morado, S.; Gadze, T.; Donato, A.; Alvarez, G.; Dalvit, G.; Cetica, P. Morphological, biochemical and functional studies to evaluate bovine oocyte vitrification. Theriogenology 2020, 143, 18–26. [Google Scholar] [CrossRef]
- Le Naour, F.; Rubinstein, E.; Jasmin, C.; Prenant, M.; Boucheix, C. Severely reduced female fertility in CD9-deficient mice. Science 2000, 287, 319–321. [Google Scholar] [CrossRef]
- Bianchi, E.; Doe, B.; Goulding, D.; Wright, G.J. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature 2014, 508, 483–487. [Google Scholar] [CrossRef]
- Inoue, N.; Ikawa, M.; Isotani, A.; Okabe, M. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature 2005, 434, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Miyado, K.; Yamada, G.; Yamada, S.; Hasuwa, H.; Nakamura, Y.; Ryu, F.; Suzuki, K.; Kosai, K.; Inoue, K.; Ogura, A.; et al. Requirement of CD9 on the egg plasma membrane for fertilization. Science 2000, 287, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Hou, Y.; Ma, W.; Yuan, J.X.; Zhang, D.; Sun, Q.Y.; Wang, W.H. Localization of CD9 in pig oocytes and its effects on sperm-egg interaction. Reproduction 2004, 127, 151–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, G.B.; Liu, G.S.; Meng, Q.G.; Liu, Y.; Hou, Y.P.; Wang, X.X.; Li, N.; Zhu, S.-E. Tetraspanin CD9 in bovine oocytes and its role in fertilization. J. Reprod. Dev. 2009, 55, 305–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, N.; Hamada, D.; Kamikubo, H.; Hirata, K.; Kataoka, M.; Yamamoto, M.; Ikawa, M.; Okabe, M.; Hagihara, Y. Molecular dissection of IZUMO1, a sperm protein essential for sperm-egg fusion. Development 2013, 140, 3221–3229. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Lu, Y.; Zhang, M.; Miao, Y.; Zhou, C.; Cui, Z.; Xiong, B. Melatonin improves the fertilization ability of post-ovulatory aged mouse oocytes by stabilizing ovastacin and Juno to promote sperm binding and fusion. Hum. Reprod. 2017, 32, 598–606. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Miao, Y.; Chen, Q.; Cai, M.; Dong, W.; Dai, X.; Lu, Y.; Zhou, C.; Cui, Z.; Xiong, B. BaP exposure causes oocyte meiotic arrest and fertilization failure to weaken female fertility. FASEB J. 2018, 32, 342–352. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Tan, R.; Wang, L.; Shi, X.; Li, Y.; Zhong, X.; He, X.; Xiong, B. Shoutai pills improve the quality of oocytes exposed to the chemotherapeutic drug Hydroxyurea. Aging 2020, 12, 8473–8483. [Google Scholar] [CrossRef]
- Jean, C.; Haghighirad, F.; Zhu, Y.; Chalbi, M.; Ziyyat, A.; Rubinstein, E.; Gourier, C.; Yip, P.; Wolf, J.P.; Lee, J.E.; et al. JUNO, the receptor of sperm IZUMO1, is expressed by the human oocyte and is essential for human fertilisation. Hum. Reprod. 2019, 34, 118–126. [Google Scholar] [CrossRef]
- Hu, W.; Dong, X.; Tian, Z.; Zhang, Z.; Tang, J.; Liang, B.; Liu, Q.; Chu, M. Expression, structure and function analysis of the sperm-oocyte fusion genes Juno and Izumo1 in sheep (Ovis aries). J. Anim. Sci. Biotechnol. 2021, 12, 37. [Google Scholar] [CrossRef]
- Hamze, J.G.; Sánchez, J.M.; O’Callaghan, E.; McDonald, M.; Bermejo-Álvarez, P.; Romar, R.; Lonergan, P.; Jiménez-Movilla, M. JUNO protein coated beads: A potential tool to predict bovine sperm fertilizing ability. Theriogenology 2020, 155, 168–175. [Google Scholar] [CrossRef]
- Hao, T.; Zhang, P.; Hao, H.; Du, W.; Pang, Y.; Zhao, S.; Zou, H.; Zhu, H.; Yu, W.; Li, S.; et al. The combination treatment of cholesterol-loaded methyl-β-cyclodextrin and methyl-β-cyclodextrin significantly improves the fertilization capacity of vitrified bovine oocytes by protecting fertilization protein JUNO. Reprod. Domest. Anim. 2021, 56, 519–530. [Google Scholar] [CrossRef]
- Dai, X.; Zhang, M.; Lu, Y.; Miao, Y.; Zhou, C.; Sun, S.; Xiong, B. Melamine impairs female fertility via suppressing protein level of juno in mouse eggs. PLoS ONE 2015, 10, e0144248. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Zhang, C.; Han, C.; An, Q.; Cheng, Y.; Chen, Y.; Meng, R.; Zhang, Y.; Su, J. Plasticizer Bis(2-ethylhexyl) phthalate causes meiosis defects and decreases fertilization ability of mouse oocytes in vivo. J. Agric. Food Chem. 2019, 67, 3459–3468. [Google Scholar] [CrossRef]
- Moulavi, F.; Saadeldin, I.M.; Swelum, A.A.; Tasdighi, F.; Hosseini-Fahraji, H.; Hosseini, S.M. Oocyte vitrification induces loss of DNA methylation and histone acetylation in the resulting embryos derived using ICSI in dromedary camel. Zygote 2021, 29, 383–392. [Google Scholar] [CrossRef]
- Ma, Y.; Long, C.; Liu, G.; Bai, H.; Ma, L.; Bai, T.; Zuo, Y.; Li, S. WGBS combined with RNA-seq analysis revealed that Dnmt1 affects the methylation modification and gene expression changes during mouse oocyte vitrification. Theriogenology 2022, 177, 11–21. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, Y. TET-mediated active DNA demethylation: Mechanism, function and beyond. Nat. Rev. Genet. 2017, 18, 517–534. [Google Scholar] [CrossRef]
- Uysal, F.; Cinar, O.; Can, A. Knockdown of Dnmt1 and Dnmt3a gene expression disrupts preimplantation embryo development through global DNA methylation. J. Assist. Reprod. Genet. 2021, 38, 3135–3144. [Google Scholar] [CrossRef]
- Maunakea, A.K.; Nagarajan, R.P.; Bilenky, M.; Ballinger, T.J.; D’Souza, C.; Fouse, S.D.; Johnson, B.E.; Hong, C.; Nielsen, C.; Zhao, Y.; et al. Conserved role of intragenic DNA methylation in regulating alternative promoters. Nature 2010, 466, 253–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, S.; Hong, K.; Liu, R.; Shen, L.; Inoue, A.; Diep, D.; Zhang, K.; Zhang, Y. Tet1 controls meiosis by regulating meiotic gene expression. Nature 2012, 492, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Deribe, Y.L.; Pawson, T. Dikic IPost-translational modifications in signal integration. Nat. Struct. Mol. Biol. 2010, 17, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Mann, M.; Jensen, O.N. Proteomic analysis of post-translational modifications. Nat. Biotechnol. 2003, 21, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Woodsmith, J.; Kamburov, A.; Stelzl, U. Dual coordination of post translational modifications in human protein networks. PLoS Comput. Biol. 2013, 9, e1002933. [Google Scholar] [CrossRef]
- Oborná, I.; Fingerová, H. Influence of hCG glycosylation on its functions in female reproduction. Ceska Gynekol. 2017, 82, 42–46. [Google Scholar]
- Bousfield, G.R.; Harvey, D.J. Follicle-stimulating hormone glycobiology. Endocrinology 2019, 160, 1515–1535. [Google Scholar] [CrossRef] [Green Version]
- Saito, S.; Yano, K.; Sharma, S.; McMahon, H.E.; Shimasaki, S. Characterization of the post-translational modification of recombinant human BMP-15 mature protein. Protein Sci. 2008, 17, 362–370. [Google Scholar] [CrossRef] [Green Version]
- Bodensteiner, K.J.; Clay, C.M.; Moeller, C.L.; Sawyer, H.R. Molecular cloning of the ovine growth/differentiation factor-9 gene and expression of growth/differentiation factor-9 in ovine and bovine ovaries. Biol. Reprod. 1999, 60, 381–386. [Google Scholar] [CrossRef]
- Aydin, H.; Sultana, A.; Li, S.; Thavalingam, A.; Lee, J.E. Molecular architecture of the human sperm IZUMO1 and egg JUNO fertilization complex. Nature 2016, 534, 562–565. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.S.; Lin, W.Z.; Wang, T.E.; Lee, W.Y.; Li, S.H.; Lin, F.J.; Nixon, B.; Sipilä, P.; Tsai, P.-S. Polarized epithelium-sperm co-culture system reveals stimulatory factors for the secretion of mouse epididymal quiescin sulfhydryl oxidase 1. J. Reprod. Dev. 2022, 68, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Emelyanov, A.V.; Fyodorov, D.V. Thioredoxin-dependent disulfide bond reduction is required for protamine eviction from sperm chromatin. Genes Dev. 2016, 30, 2651–2656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Huan, Y.; Xie, B.; Wang, J.; Zhao, Y.; Jiao, M.; Huang, T.; Kong, Q.; Liu, Z. Identification and characterization of an oocyte factor required for sperm decondensation in pig. Reproduction 2014, 148, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Julianelli, V.; Farrando, B.; Alvarez Sedó, C.; Calvo, L.; Romanato, M.; Calvo, J.C. Heparin enhances protamine disulfide bond reduction during in vitro decondensation of human spermatozoa. Hum. Reprod. 2012, 27, 1930–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukunoki, A.; Takeo, T.; Nakagata, N. N-acetyl cysteine restores the fertility of vitrified-warmed mouse oocytes derived through ultrasuperovulation. PLoS ONE 2019, 14, e0224087. [Google Scholar] [CrossRef]
- Lindsay, L.L.; Hedrick, J.L. Proteolysis of Xenopus laevis egg envelope ZPA triggers envelope hardening. Biochem. Biophys. Res. Commun. 2004, 324, 648–654. [Google Scholar] [CrossRef]
- Ardito, F.; Giuliani, M.; Perrone, D.; Troiano, G.; Lo Muzio, L. The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy (review). Int. J. Mol. Med. 2017, 40, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Staicu, F.D.; Martínez-Soto, J.C.; Canovas, S.; Matás, C. Nitric oxide-targeted protein phosphorylation during human sperm capacitation. Sci. Rep. 2021, 11, 20979. [Google Scholar] [CrossRef]
- Sudhakaran, S.; Uppangala, S.; Salian, S.R.; Honguntikar, S.D.; Nair, R.; Kalthur, G.; Adiga, S.K. Oocytes recovered after ovarian tissue slow freezing have impaired H2AX phosphorylation and functional competence. Reprod. Fertil. Dev. 2015, 27, 1242–1248. [Google Scholar] [CrossRef]
- Qin, J.; Guo, S.; Yang, J.; Qazi, I.H.; Pan, B.; Lv, T.; Zang, S.; Fang, Y.; Zhou, G. Melatonin promotes in vitro development of vitrified-warmed mouse GV oocytes, potentially by modulating phosphorylation of Drp1. Front. Vet. Sci. 2021, 8, 752001. [Google Scholar] [CrossRef]
- Gou, L.T.; Lim, D.H.; Ma, W.; Aubol, B.E.; Hao, Y.; Wang, X.; Zhao, J.; Liang, Z.; Shao, C.; Zhang, X.; et al. Initiation of parental genome reprogramming in fertilized oocyte by splicing kinase SRPK1-catalyzed protamine phosphorylation. Cell 2020, 180, 1212–1227.e1214. [Google Scholar] [CrossRef]
- Zapata-Carmona, H.; Soriano-Úbeda, C.; París-Oller, E.; Matás, C. Periovulatory oviductal fluid decreases sperm protein kinase a activity, tyrosine phosphorylation, and in vitro fertilization in pig. Andrology 2020, 8, 756–768. [Google Scholar] [CrossRef]
- Urner, F.; Sakkas, D. Protein phosphorylation in mammalian spermatozoa. Reproduction 2003, 125, 17–26. [Google Scholar] [CrossRef]
- Li, H.; You, L.; Tian, Y.; Guo, J.; Fang, X.; Zhou, C.; Shi, L.; Su, Y. DPAGT1-mediated protein N-glycosylation is indispensable for oocyte and follicle development in mice. Adv. Sci. 2020, 7, 2000531. [Google Scholar] [CrossRef]
- Van den Steen, P.; Rudd, P.M.; Dwek, R.A.; Opdenakker, G. Concepts and principles of O-linked glycosylation. Crit. Rev. Biochem. Mol. Biol. 1998, 33, 151–208. [Google Scholar] [CrossRef]
- Yurewicz, E.C.; Pack, B.A.; Sacco, A.G. Isolation, composition, and biological activity of sugar chains of porcine oocyte zona pellucida 55K glycoproteins. Mol. Reprod. Dev. 1991, 30, 126–134. [Google Scholar] [CrossRef]
- Yonezawa, N.; Kanai, S.; Nakano, M. Structural significance of N-glycans of the zona pellucida on species-selective recognition of spermatozoa between pig and cattle. Soc. Reprod. Fertil Suppl. 2007, 63, 217–228. [Google Scholar]
- Suzuki, B.; Sugano, Y.; Ito, J.; Saito, H.; Niimura, S.; Yamashiro, H. Location and expression of JUNO in mice oocytes during maturation. JBRA Assist. Reprod. 2017, 21, 321–326. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.M.; Wang, N.; Hao, H.S.; Li, C.Y.; Zhao, Y.H.; Yan, C.L.; Wang, H.-Y.; Du, W.-H.; Wang, D.; Liu, Y.; et al. Melatonin improves the fertilization capacity and developmental ability of bovine oocytes by regulating cytoplasmic maturation events. J. Pineal Res. 2018, 64, e12445. [Google Scholar] [CrossRef]
- Yun, Y.; An, P.; Ning, J.; Zhao, G.M.; Yang, W.L.; Lei, A.M. H1foo is essential for in vitro meiotic maturation of bovine oocytes. Zygote 2015, 23, 416–425. [Google Scholar] [CrossRef]
- Sive, H.L.; Grainger, R.M.; Harland, R.M. Microinjection of Xenopus oocytes. Cold Spring Harb. Protoc. 2010, 2010, pdb.prot5536. [Google Scholar] [CrossRef] [PubMed]
- Inoue, N. Novel insights into the molecular mechanism of sperm-egg fusion via IZUMO1. J. Plant Res. 2017, 130, 475–478. [Google Scholar] [CrossRef]
- Sui, L.; Yan, K.; Zhang, H.; Nie, J.; Yang, X.; Xu, C.L.; Liang, X. Mogroside V alleviates oocyte meiotic defects and quality deterioration in benzo(a)pyrene-exposed mice. Front. Pharmacol. 2021, 12, 722779. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.B.; Li, N. Bovine oocytes cryoinjury and how to improve their development following cryopreservation. Anim. Biotechnol. 2013, 24, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.I.; Squires, E.L.; Graham, J.K. Adding cholesterol to the stallion sperm plasma membrane improves cryosurvival. Cryobiology 2005, 51, 241–249. [Google Scholar] [CrossRef]
- Zidovetzki, R.; Levitan, I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: Evidence, misconceptions and control strategies. Biochim. Biophys. Acta 2007, 1768, 1311–1324. [Google Scholar] [CrossRef] [Green Version]
- Horvath, G.; Seidel, G.E., Jr. Vitrification of bovine oocytes after treatment with cholesterol-loaded methyl-beta-cyclodextrin. Theriogenology 2006, 66, 1026–1033. [Google Scholar] [CrossRef]
- Buschiazzo, J.; Ríos, G.L.; Canizo, J.R.; Antollini, S.S.; Alberio, R.H. Free cholesterol and cholesterol esters in bovine oocytes: Implications in survival and membrane raft organization after cryopreservation. PLoS ONE 2017, 12, e0180451. [Google Scholar] [CrossRef] [Green Version]
- Casao, A.; Mendoza, N.; Pérez-Pé, R.; Grasa, P.; Abecia, J.A.; Forcada, F.; Cebrián-Pérez, J.A.; Muino-Blanco, T. Melatonin prevents capacitation and apoptotic-like changes of ram spermatozoa and increases fertility rate. J. Pineal Res. 2010, 48, 39–46. [Google Scholar] [CrossRef]
- Yadav, H.P.; Kumar, A.; Shah, N.; Chauhan, D.S.; Saxena, A.; Yadav, S.; Swain, D.K. Effect of cholesterol loaded cyclodextrin supplementation on tyrosine phosphorylation and apoptosis like changes in frozen thawed Hariana bull spermatozoa. Theriogenology 2017, 96, 164–171. [Google Scholar] [CrossRef]
- Zhao, X.M.; Min, J.T.; Du, W.H.; Hao, H.S.; Liu, Y.; Qin, T.; Wang, D.; Zhu, H. Melatonin enhances the in vitro maturation and developmental potential of bovine oocytes denuded of the cumulus oophorus. Zygote 2015, 23, 525–536. [Google Scholar] [CrossRef]
- Canosa, S.; Bergandi, L.; Macrì, C.; Charrier, L.; Paschero, C.; Carosso, A.; Di Segni, N.; Silvagno, F.; Gennarelli, G.; Benedetto, C.; et al. Morphokinetic analysis of cleavage stage embryos and assessment of specific gene expression in cumulus cells independently predict human embryo development to expanded blastocyst: A preliminary study. J. Assist. Reprod. Genet. 2020, 37, 1409–1420. [Google Scholar] [CrossRef]
- Rival, C.M.; Xu, W.; Shankman, L.S.; Morioka, S.; Arandjelovic, S.; Lee, C.S.; Wheeler, K.M.; Smith, R.P.; Haney, L.B.; Isakson, B.E.; et al. Phosphatidylserine on viable sperm and phagocytic machinery in oocytes regulate mammalian fertilization. Nat. Commun. 2019, 10, 4456. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.J.; Sun, A.G.; Zhao, S.G.; Liu, H.; Ma, S.Y.; Li, M.; Huai, Y.-X.; Zhao, H.; Liu, H.-B. Resveratrol improves in vitro maturation of oocytes in aged mice and humans. Fertil. Steril. 2018, 109, 900–907. [Google Scholar] [CrossRef]
- Khatun, H.; Wada, Y.; Konno, T.; Tatemoto, H.; Yamanaka, K.I. Endoplasmic reticulum stress attenuation promotes bovine oocyte maturation in vitro. Reproduction 2020, 159, 361–370. [Google Scholar] [CrossRef]
- Carro, M.L.M.; Peñalva, D.A.; Antollini, S.S.; Hozbor, F.A.; Buschiazzo, J. Cholesterol and desmosterol incorporation into ram sperm membrane before cryopreservation: Effects on membrane biophysical properties and sperm quality. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183357. [Google Scholar] [CrossRef]
- Buschiazzo, J.; Ialy-Radio, C.; Auer, J.; Wolf, J.P.; Serres, C.; Lefèvre, B.; Ziyyat, A. Cholesterol depletion disorganizes oocyte membrane rafts altering mouse fertilization. PLoS ONE 2013, 8, e62919. [Google Scholar] [CrossRef]
- Chen, X.; Dong, H.; Cheng, M.; Wang, Q.; Jin, Y. Addition of cholesterol loaded cyclodextrin prior to GV-phase vitrification improves the quality of mature porcine oocytes in vitro. Cryobiology 2019, 90, 54–62. [Google Scholar] [CrossRef]
- Sprícigo, J.F.; Morais, K.S.; Yang, B.S.; Dode, M.A. Effect of the exposure to methyl-β-cyclodextrin prior to chilling or vitrification on the viability of bovine immature oocytes. Cryobiology 2012, 65, 319–325. [Google Scholar] [CrossRef] [Green Version]
- Roberts, R.M. Interferon-tau, a type 1 interferon involved in maternal recognition of pregnancy. Cytokine Growth Factor Rev. 2007, 18, 403–408. [Google Scholar] [CrossRef] [Green Version]
- Simmet, K.; Zakhartchenko, V.; Philippou-Massier, J.; Blum, H.; Klymiuk, N.; Wolf, E. OCT4/POU5F1 is required for NANOG expression in bovine blastocysts. Proc. Natl. Acad. Sci. USA 2018, 115, 2770–2775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.; Choi, K.H.; Oh, J.N.; Kim, S.H.; Lee, D.K.; Choe, G.C.; Jeong, J.; Lee, C. SOX2 plays a crucial role in cell proliferation and lineage segregation during porcine pre-implantation embryo development. Cell Prolif. 2021, 54, e13097. [Google Scholar] [CrossRef] [PubMed]
- Shyam, S.; Goel, P.; Kumar, D.; Malpotra, S.; Singh, M.K.; Lathwal, S.S.; Chand, S.; Palta, P. Effect of Dickkopf-1 and colony stimulating factor-2 on the developmental competence, quality, gene expression and live birth rate of buffalo (Bubalus bubalis) embryos produced by hand-made cloning. Theriogenology 2020, 157, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.M.; Ren, J.J.; Du, W.H.; Hao, H.S.; Wang, D.; Qin, T.; Liu, Y.; Zhu, H.-B. Effect of vitrification on promoter CpG island methylation patterns and expression levels of DNA methyltransferase 1o, histone acetyltransferase 1, and deacetylase 1 in metaphase II mouse oocytes. Fertil. Steril. 2013, 100, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Brackett, B.G.; Oliphant, G. Capacitation of rabbit spermatozoa in vitro. Biol. Reprod. 1975, 12, 260–274. [Google Scholar] [CrossRef] [Green Version]
- Waniwan, J.T.; Chen, Y.J.; Capangpangan, R.; Weng, S.H.; Chen, Y.J. Glycoproteomic alterations in drug-resistant nonsmall cell lung cancer cells revealed by lectin magnetic nanoprobe-based mass spectrometry. J. Proteome Res. 2018, 17, 3761–3773. [Google Scholar] [CrossRef]
- Conover, J.C.; Gwatkin, R.B. Pre-loading of mouse oocytes with DNA-specific fluorochrome (Hoechst 33342) permits rapid detection of sperm-oocyte fusion. J. Reprod. Fertil. 1988, 82, 681–690. [Google Scholar] [CrossRef]
- Purdy, P.H.; Graham, J.K. Effect of adding cholesterol to bull sperm membranes on sperm capacitation, the acrosome reaction, and fertility. Biol. Reprod. 2004, 71, 522–527. [Google Scholar] [CrossRef] [Green Version]
- Anguita, B.; Vandaele, L.; Mateusen, B.; Maes, D.; van Soom, A. Developmental competence of bovine oocytes is not related to apoptosis incidence in oocytes, cumulus cells and blastocysts. Theriogenology 2007, 67, 537–549. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Zhao, X.M.; Hao, H.S.; Du, W.H.; Zhao, S.J.; Wang, H.Y.; Wang, N.; Wang, D.; Liu, Y.; Qin, T.; Zhu, H.-B. Melatonin inhibits apoptosis and improves the developmental potential of vitrified bovine oocytes. J. Pineal Res. 2016, 60, 132–141. [Google Scholar] [CrossRef]
- Su, J.; Wang, Y.; Li, Y.; Li, R.; Li, Q.; Wu, Y.; Quan, F.; Liu, J.; Guo, Z.; Zhang, Y. Oxamflatin significantly improves nuclear reprogramming, blastocyst quality, and in vitro development of bovine SCNT embryos. PLoS ONE 2011, 6, e23805. [Google Scholar] [CrossRef]

 : The phosphorylation site was detected in only the vitrification group.
: The phosphorylation site was detected in only the vitrification group.  : The glycosylation site was detected in both the fresh group and the vitrification group.
: The glycosylation site was detected in both the fresh group and the vitrification group.  : The glycosylation sites were detected in only the vitrification group.
: The glycosylation sites were detected in only the vitrification group.
 : The phosphorylation site was detected in only the vitrification group.
: The phosphorylation site was detected in only the vitrification group.  : The glycosylation site was detected in both the fresh group and the vitrification group.
: The glycosylation site was detected in both the fresh group and the vitrification group.  : The glycosylation sites were detected in only the vitrification group.
: The glycosylation sites were detected in only the vitrification group.
| Groups | Site | Peptide | Protein Type |
|---|---|---|---|
| Fresh group | 18–179 | AVPPTCTGHELL (6)-PTPADLCERIW (7) | Intra |
| 18–165 | AVPPTCTGHELL (6)-EPCRPF (3) | Intra | |
| 27–125 | APLCLEDCE (4)-LLNVCMNAK (5) | Intra | |
| 79–47 | SLVHCGL (5)-EECIPW (3) | Intra | |
| 79–79 | SLVHCGL (5)-SLVHCGL (5) | Inter | |
| 79–99 | SLVHCGLMM (5)-YQCSPNL (3) | Intra | |
| 95–165 | LQAICFY (5)-EPCRPF (3) | Intra | |
| 99–165 | EPCRPFPHHF (3)-QCSPNL (2) | Intra | |
| Vitrification group | 79–79 | NFSLVHCGLM (7)-NFSLVHCGLM (7) | Inter |
| 79–179 | PTPADLCERIW (7)-SLVHCGLMM (5) | Intra | |
| 79–229 | ARCPGLLAFPL (3)-NFSLVHCGL (7) | Intra | |
| 95–95 | LQAICFY (5)-LQAICFY (5) | Inter | |
| 125–142 | VLDAPLCLEDCE (7)-TSHTCKC (5) | Intra |
| Groups | No. of Oocytes | No. of Cleavage Embryos (%) | No. of Blastocysts (%) |
|---|---|---|---|
| Fresh group | 126 | 108 (85.71 ± 7.83%) b | 49 (45.37 ± 4.09%) b |
| 200 pg JUNO mRNA fresh group | 119 | 115 (96.64 ± 8.17%) a | 65 (56.52 ± 4.87%) a |
| Vitrification group | 132 | 72 (54.55 ± 4.39%) d | 8 (11.11 ± 1.02%) d |
| 200 pg JUNO mRNA vitrification group | 145 | 102 (70.34 ± 5.92%) c | 27 (26.47 ± 2.45%) c |
| Groups | No. of Oocytes | No. of Cleavage Embryos (%) | No. of Blastocysts (%) | Cell Number Per Blastocyst |
|---|---|---|---|---|
| Fresh group | 224 | 193 (86.16 ± 7.34%) a | 83 (43.01 ± 3.08%) a | 102.32 ± 9.02 (n = 30) a |
| Vitrification group | 675 | 385 (57.04 ± 4.92%) b | 50 (12.99 ± 1.12%) b | 80.87 ± 7.38 (n = 30) b |
| 200 pg JUNO mRNA + CLC/MβCD vitrification group | 284 | 228 (80.28 ± 4.92%) a | 91 (39.91 ± 3.27%) a | 99.03 ± 8.38 (n = 30) a |
| Genes | Primer Sequences (5′-3′) |
|---|---|
| JUNO | T7 pro F: TAATACGACTCACTATAGGG BGH-rev: TAGAAGGCACAGTCGAGG |
| Genes | Primer Sequences (5′-3′) | References |
|---|---|---|
| JUNO | F: GGTTCACTGCGGACTAATGATG R: GGGAGCACTGGTAGAAGCAGAT | Hao [34] |
| ATP6 | F: GAACACCCACTCCACTAATCCCAAT R: GTGCAAGTGTAGCTCCTCCGATT | Zhao [81] |
| BAX | F: TTTCTGACGGCAACTTCAACTG R: GGTGCACAGGGCCTTGAG | Zhao [101] |
| BCL2 | F: TCAATTGTCGTGGCATCAAAA R: CCCCCGACACCTGTTAGCTT | Zhao [101] |
| SOD1 | F: CATCCACTTCGAGGCAAAGG R: GGTCTCCAACATGCCTCTCT | Zhao [69] |
| IFN-tau | F: GCTCCAGAAGGATCAGGCTATC R: TGTTCCAAGCAGCAGACGAGT | Zhao [81] |
| OCT4 | F: CCACCCTGCAGCAAATTAGC R: CCACACTCGGACCACGTCTT | Su [102] |
| CDX2 | F: GCAAAGGAAAGGAAAATCAACAA R: GGGCTCTGGGACGCTTCT | Su [102] |
| β-ACTIN | F: TCCTGGGCATGGAATCCTG R: GGCGCGATGATCTTGATCTTC | Zhao [101] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Hao, T.; Komba, E.; Yang, B.; Hao, H.; Du, W.; Zhu, H.; Zhang, H.; Zhao, X. Improvement of Fertilization Capacity and Developmental Ability of Vitrified Bovine Oocytes by JUNO mRNA Microinjection and Cholesterol-Loaded Methyl-β-Cyclodextrin Treatment. Int. J. Mol. Sci. 2023, 24, 590. https://doi.org/10.3390/ijms24010590
Xu X, Hao T, Komba E, Yang B, Hao H, Du W, Zhu H, Zhang H, Zhao X. Improvement of Fertilization Capacity and Developmental Ability of Vitrified Bovine Oocytes by JUNO mRNA Microinjection and Cholesterol-Loaded Methyl-β-Cyclodextrin Treatment. International Journal of Molecular Sciences. 2023; 24(1):590. https://doi.org/10.3390/ijms24010590
Chicago/Turabian StyleXu, Xi, Tong Hao, Emma Komba, Baigao Yang, Haisheng Hao, Weihua Du, Huabin Zhu, Hang Zhang, and Xueming Zhao. 2023. "Improvement of Fertilization Capacity and Developmental Ability of Vitrified Bovine Oocytes by JUNO mRNA Microinjection and Cholesterol-Loaded Methyl-β-Cyclodextrin Treatment" International Journal of Molecular Sciences 24, no. 1: 590. https://doi.org/10.3390/ijms24010590
APA StyleXu, X., Hao, T., Komba, E., Yang, B., Hao, H., Du, W., Zhu, H., Zhang, H., & Zhao, X. (2023). Improvement of Fertilization Capacity and Developmental Ability of Vitrified Bovine Oocytes by JUNO mRNA Microinjection and Cholesterol-Loaded Methyl-β-Cyclodextrin Treatment. International Journal of Molecular Sciences, 24(1), 590. https://doi.org/10.3390/ijms24010590






