Synthesis, Photochemistry, Computational Study and Potential Application of New Styryl-Thiophene and Naphtho-Thiophene Benzylamines
Abstract
1. Introduction
2. Results and Discussion
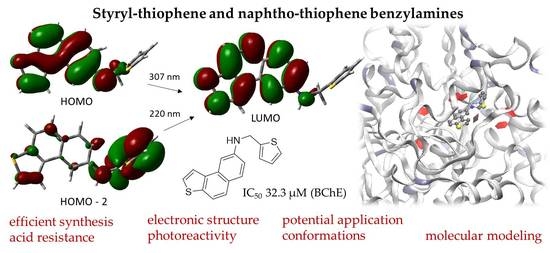
2.1. Synthesis, Photophysical Properties, and Photochemistry of Styryl-Thiophene Benzylamines 2–8 toward Photocyclization Products 9–15
2.2. Computational Study of the Electronic Structure of Styryl-Thiophene and Naphtho-Thiophene Benzylamines 2–15
2.3. Biological Potential of Styryl-Thiophene and Naphtho-Thiophene Benzylamines 2–15
3. Materials and Methods
3.1. General Remarks
3.2. General Procedure for the Synthesis of Starting 2-(4-Chlorostyryl)Thiophene (1)

3.3. General Procedure for the Synthesis of New 4-(2-(Thiophen-2-yl)Vinyl)Anilines (2–8) by Buchwald-Hartwig Amination







3.4. General Procedure for the Synthesis of the Electrocyclization Photoproducts 9–15


3.5. Computational Details
3.6. Cholinesterase Inhibition Activity Measurements
3.7. In Vitro Biological Activity Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saltiel, J.; Tarkalanov, N.; Sears, D.F., Jr. Conformer-Specific Adiabatic cis–trans photoisomerization of cis-l-(2-Naphthyl)-2-phenylethene. A striking application of the NEER Principle. J. Am. Chem. Soc. 1995, 117, 5586–5587. [Google Scholar] [CrossRef]
- Bartocci, G.; Galiazzo, G.; Marri, E.; Mazzucato, U.; Spalletti, A. Role of adiabatic pathways in the photoisomerization of aromatic olefins. Inorg. Chim. Acta 2007, 360, 961–969. [Google Scholar] [CrossRef]
- Majima, T.; Tojo, S.; Ishida, A.; Takamuku, S. Cis-trans isomerization and oxidation of radical cations of stilbene derivatives. J. Org. Chem. 1996, 61, 7793–7800. [Google Scholar] [CrossRef] [PubMed]
- Imamoto, Y.; Kuroda, T.; Kataoka, M.; Shevyakov, S.; Krishnamoorthy, G.; Liu, R.S.H. Photoisomerization by hula twist: 2,2′-dimethylstilbene and a ring-fused analogue. Angew. Chem. 2003, 115, 3758–3761. [Google Scholar] [CrossRef]
- Meier, H. The photochemistry of stilbenoid compounds and their role in materials technology. Angew. Chem. Int. Ed. 1992, 31, 1399–1420. [Google Scholar] [CrossRef]
- Vachon, J.; Carroll, G.T.; Pollard, M.M.; Mes, E.M.; Brouwer, A.M.; Feringa, B.L. An ultrafast surface-bound photo-active molecular motor. Photochem. Photobiol. Sci. 2014, 13, 241–246. [Google Scholar] [CrossRef]
- Van Delden, R.L.; Ter Wiel, M.K.J.; Pollard, M.M.; Vicario, J.; Koumura, N.; Feringa, B.L. Unidirectional molecular motor on a gold surface. Nature 2005, 437, 1337–1340. [Google Scholar] [CrossRef]
- Segura, J.L.; Martin, N. Functionalized oligoarylenes as building blocks for new organic materials. J. Mater. Chem. 2000, 10, 2403–2435. [Google Scholar] [CrossRef]
- Horspool, W.M.; Lenci, F. CRC Handbook of Organic Photochemistry and Photobiology, 2nd ed.; CRC: Boca Raton, FL, USA, 2004. [Google Scholar]
- Griesbeck, A.; Oelgemöller, M.; Ghetti, F. CRC Handbook of Organic Photochemistry and Photobiology, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Qu, J.; Cao, C.T.; Cao, C. Determining the excited-state substituent constants of furyl and thienyl groups. J. Phys. Org. Chem. 2018, 31, 3799. [Google Scholar] [CrossRef]
- Mencaroni, L.; Cesaretti, A.; Carlotti, B.; Alebardi, M.; Elisei, F.; Ratković, A.; Škorić, I.; Spalletti, A. Tuning the Photophysics of Two-Arm Bis[(dimethylamino)styryl]benzene Derivatives by Heterocyclic Substitution. Molecules 2022, 27, 8725. [Google Scholar] [CrossRef]
- Mencaroni, L.; Cesaretti, A.; Elisei, F.; Škorić, I.; Mlakić, M.; Spalletti, A. Acid–base strength and acido(fluoro)chromism of three push–pull derivatives of 2,6-distyrylpyridine. Photochem. Photobiol. Sci. 2022, 21, 935–947. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, A.; Yariv, A. Quantum well lasers-gain, spectra, dynamics. IEEE J. Quantum Electron. 1986, 22, 1887–1899. [Google Scholar] [CrossRef]
- Wang, P.; Pu, H.; Jin, M. Single-chain nanoparticles with well-defined structure via intramolecular crosslinking of linear polymers with pendant benzoxazine groups. J. Polym. Sci A: Polym. Chem. 2011, 49, 5133–5141. [Google Scholar] [CrossRef]
- Lumpi, D.; Glöcklhofer, F.; Holzer, B.; Stöger, B.; Hametner, C.; Reider, G.A.; Fröhlich, J. Systematic investigations on 1,2,3-triazole-based compounds capable of second harmonic generation. Cryst. Growth Des. 2014, 14, 1018–1031. [Google Scholar] [CrossRef]
- Šagud, I.; Šindler-Kulyk, M.; Škorić, I.; Kelava, V.; Marinić, Ž. Synthesis of naphthoxazoles by photocyclization of 4-/5-(phenylethenyl)oxazoles. Eur. J. Org. Chem. 2018, 25, 3326–3335. [Google Scholar] [CrossRef]
- Škorić, I.; Flegar, I.; Marinić, Ž.; Šindler-Kulyk, M. Synthesis of the novel conjugated ω, ω’-diaryl/heteroaryl hexatriene system with the central double bond in a heteroaromatic ring: Photochemical transformation of 2,3-divinylfuran derivatives. Tetrahedron 2006, 62, 7396–7407. [Google Scholar] [CrossRef]
- Ciorba, S.; Carlotti, B.; Škorić, I.; Šindler-Kulyk, M.; Spalletti, A. Spectral properties and photobehaviour of 2,5-distyrylfuran derivatives. J. Photochem. Photobiol. A Chem. 2011, 219, 1–9. [Google Scholar] [CrossRef]
- Carlotti, B.; Cesaretti, A.; Cacioppa, G.; Elisei, F.; Odak, I.; Škorić, I.; Spalletti, A. Fluorosolvatochromism and hyperpolarizability of one-arm and two-arms nitrocompounds bearing heterocyclic rings. J. Photochem. Photobiol. A Chem. 2019, 368, 190–199. [Google Scholar] [CrossRef]
- Mencaroni, L.; Carlotti, B.; Cesaretti, A.; Elisei, F.; Grgičević, A.; Škorić, I.; Spalletti, A. Competition between fluorescence and triplet production ruled by nitro groups in one-arm and two-arm styrylbenzene heteroanalogues. Photochem. Photobiol. Sci. 2020, 19, 1665–1676. [Google Scholar] [CrossRef]
- Wan, M.; Luo, Y.; Tong, Z.; Geng, Q.; Hua, L. Novel amino acid-stilbene quaternary ammonium salt fluorescent whitening agents: Synthesis, optical properties, acid resistance and antibacterial activity. Coloration Technol. 2022, 138, 201–209. [Google Scholar] [CrossRef]
- Um, S.I.; Lee, J.K.; Kang, Y.; Baek, D.J. The synthesis and properties of triazine–stilbene fluorescent brighteners containing the phenolic antioxidant [II]. Dyes Pigm. 2006, 70, 84–90. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, X.; Fei, Q.; Yu, Y.; Tian, S.; Wang, K.; Jiang, J.; Song, D.; Yu, A.; Zhang, Z. On-site determination of the migration amount of fluorescent whitening agents from paper to finger by fluorescence spectrophotometry. Anal. Methods. 2017, 9, 465–472. [Google Scholar] [CrossRef]
- Matić, J.; Tumir, L.M.; Radić Stojković, M.; Piantanida, I. Advances in peptide-based DNA/RNA-intercalators. Curr. Protein Peptide Sci. 2016, 17, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.Y.; Mellerup, S.K.; Yuan, K.; Hu, G.F.; Sauriol, F.; Peng, T.; Wang, N.; Chen, P.; Wang, S. Stabilising fleeting intermediates of stilbene photocyclization with amino-borane functionalisation: The rare isolation of presistent dihydrophenanthrenes and their [1,5] H-shift isomers. Chem. Sci. 2018, 9, 3844–3855. [Google Scholar] [CrossRef]
- Šagud, I.; Maček Hrvat, N.; Grgičević, A.; Čadež, T.; Hodak, J.; Dragojević, M.; Lasić, K.; Kovarik, Z.; Škorić, I. Design, synthesis and cholinesterase inhibitory properties of new oxazole benzylamine derivatives. J. Enzyme Inhib. Med. Chem. 2020, 1, 460–467. [Google Scholar] [CrossRef]
- Mlakić, M.; Odak, I.; Faraho, I.; Talić, S.; Bosnar, M.; Lasić, K.; Barić, D.; Škorić, I. New naphtho/thienobenzo-triazoles with interconnected anti-inflammatory and cholinesterase inhibitory activity. Eur. J. Med. Chem. 2022, 241, 114616. [Google Scholar] [CrossRef]
- Mlakić, M.; Faraho, I.; Odak, I.; Talić, S.; Vukovinski, A.; Raspudić, A.; Bosnar, M.; Zadravec, R.; Ratković, A.; Lasić, K.; et al. Synthesis, photochemistry and computational study of novel 1,2,3-triazole heterostilbenes: Expressed biological activity of their electrocyclization photoproducts. Bioorg. Chem. 2022, 121, 105701. [Google Scholar] [CrossRef]
- Deligeorgiev, T.; Vasilev, A.; Kaloyanova, S.; Vaquero, J.J. Styryl dyes—Synthesis and applications during the last 15 years, Society of Dyers and Colourists. Color. Technol. 2010, 126, 55–80. [Google Scholar] [CrossRef]
- Xie, X.; Choi, B.; Largy, E.; Guillot, R.; Granzhan, A.; Teulade-Fichou, M.P. Asymmetric distyrylpyridinium dyes as red-emitting fluorescent probes for quadruplex DNA. Chem. Eur. J. 2013, 19, 1214–1226. [Google Scholar] [CrossRef]
- Botti, V.; Cesaretti, A.; Ban, Ž.; Crnolatac, I.; Consiglio, G.; Elisei, F.; Piantanida, I. Fine structural tuning of styryl-based dyes for fluorescence and CD-based sensing of various ds-DNA/RNA sequences, The Royal Society of Chemistry. Org. Biomol. Chem. 2019, 17, 8243–8258. [Google Scholar] [CrossRef]
- Mazzoli, A.; Carlotti, B.; Consiglio, G.; Fortuna, G.C.; Miolo, G.; Spalletti, A. Photobehaviour of methyl-pyridinium and quinolinium iodides derivatives, free and complexed with DNA. A case of bisintercalation. Photochem. Photobiol. Sci. 2014, 13, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Mlakić, M.; Čadež, T.; Barić, D.; Puček, I.; Ratković, A.; Marinić, Ž.; Lasić, K.; Kovarik, Z.; Škorić, I. New uncharged 2-thienostilbene oximes as reactivators of organophosphate-inhibited cholinesterases. Pharmaceuticals 2021, 14, 1147. [Google Scholar] [CrossRef] [PubMed]
- Ratković, A.; Kelava, V.; Marinić, Ž.; Škorić, I. Buchwald-Hartwig amination of the chloro substituted benzobicyclo [3.2.1]octadiene skeleton using primary benzylic amines. J. Mol. Struct. 2019, 1179, 597–607. [Google Scholar] [CrossRef]
- Runge, E.; Gross, E.K.U. Density-functional theory for time-dependent systems. Phys. Rev. Lett. 1984, 52, 997–1000. [Google Scholar] [CrossRef]
- Plantone, D.; Koudriavtseva, T. Current and future use of chloroquine and hydroxychloroquine in infectious, immune, neoplastic, and neurological diseases: A minireview. Clin. Drug Investig. 2018, 38, 653–671. [Google Scholar] [CrossRef] [PubMed]
- Hamulakova, S.; Janovec, L.; Soukup, O.; Jun, D.; Janockova, J.; Hrabinova, M.; Sepsova, V.; Kuca, K. Tacrine-coumarin and tacrine-7-chloroquinoline hybrids with thiourea linkers: Cholinesterase inhibition properties, kinetic study, molecular docking and permeability assay for blood-brain barrier. Curr. Alzheimer Res. 2018, 15, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Mlakić, M.; Fodor, L.; Odak, I.; Horváth, O.; Lovrić, M.J.; Barić, D.; Milašinović, V.; Molčanov, K.; Marinić, Ž.; Lasić, Z.; et al. Resveratrol-maltol and resveratrol-thiophene hybrids as cholinesterase inhibitors and antioxidants: Synthesis, bio-metal chelating capability and crystal structure. Molecules 2022, 27, 6379. [Google Scholar] [CrossRef]
- Gokarn, K.; Pal, R.B. Activity of siderophores against drug-resistant Gram-positive and Gram-negative bacteria. Infect. Drug Resist. 2018, 11, 61–75. [Google Scholar] [CrossRef]
- Thompson, M.G.; Corey, B.W.; Si, Y.; Craft, D.W.; Zurawski, D.V. Antibacterial activities of iron chelators against common nosocomial pathogens. Antimicrob. Agents Chemother. 2012, 56, 5419–5421. [Google Scholar] [CrossRef]
- Vinuesa, V.; McConnell, M.J. Recent Advances in Iron Chelation and Gallium-Based Therapies for Antibiotic Resistant Bacterial Infections. Int. J. Mol. Sci. 2021, 22, 2876. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- Barone, V.; Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Raves, M.L.; Giles, K.; Schrag, J.D.; Schmid, M.F.; Phillips, G.N., Jr.; Wah, C.; Howard, A.J.; Silman, I.; Sussman, J.L. Quaternary structure of tetrameric acetylcholinesterase. In Structure and Function of Cholinesterases and Related Proteins; Doctor, B.P., Quinn, D.M., Rotundo, R.L., Taylor, P., Eds.; Springer: Boston, MA, USA, 1998; pp. 351–356. [Google Scholar]
- Nicolet, Y.; Lockridge, O.; Masson, P.; Fontecilla-Camps, J.C.; Nachon, F. Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J. Biol. Chem. 2013, 278, 41141–41147. [Google Scholar] [CrossRef] [PubMed]
- Repasky, M.P.; Chandrasekhar, J.; Jorgensen, W.L. PDDG/PM3 and PDDG/MNDO: Improved semiempirical methods. J. Comp. Chem. 2002, 23, 1601–1622. [Google Scholar] [CrossRef]
- Siegbahn, P.E.M.; Himo, F. The quantum chemical cluster approach for modeling enzyme reactions. Comput. Mol. Sci. 2011, 1, 323–336. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtnex, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]













| Compound | λexp/nm | λcalc/nm | Assignment |
|---|---|---|---|
| cis-2 | 322 | 323 | H → L |
| cis-3 | 327 | 323 | H → L |
| cis-4 | 323 | 323 | H → L |
| cis-5 | 325 | 323 | H → L |
| cis-6 | 327 | 325 | H → L |
| cis-7 | - 1 | 324 | H → L |
| cis-8 | 325 | 322 | H → L |
| Compound | λexp/nm | λcalc/nm | Assignment |
|---|---|---|---|
| trans-2 | 355 | 340 | H → L |
| trans-3 | 355 | 340 | H → L |
| trans-4 | 357 | 340 | H → L |
| trans-5 | 355 | 340 | H → L |
| trans-6 | 358 | 342 | H → L |
| trans-7 | - 1 | 341 | H → L |
| trans-8 | 355 | 339 | H → L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mlakić, M.; Odak, I.; Faraho, I.; Bosnar, M.; Banjanac, M.; Lasić, Z.; Marinić, Ž.; Barić, D.; Škorić, I. Synthesis, Photochemistry, Computational Study and Potential Application of New Styryl-Thiophene and Naphtho-Thiophene Benzylamines. Int. J. Mol. Sci. 2023, 24, 610. https://doi.org/10.3390/ijms24010610
Mlakić M, Odak I, Faraho I, Bosnar M, Banjanac M, Lasić Z, Marinić Ž, Barić D, Škorić I. Synthesis, Photochemistry, Computational Study and Potential Application of New Styryl-Thiophene and Naphtho-Thiophene Benzylamines. International Journal of Molecular Sciences. 2023; 24(1):610. https://doi.org/10.3390/ijms24010610
Chicago/Turabian StyleMlakić, Milena, Ilijana Odak, Ivan Faraho, Martina Bosnar, Mihailo Banjanac, Zlata Lasić, Željko Marinić, Danijela Barić, and Irena Škorić. 2023. "Synthesis, Photochemistry, Computational Study and Potential Application of New Styryl-Thiophene and Naphtho-Thiophene Benzylamines" International Journal of Molecular Sciences 24, no. 1: 610. https://doi.org/10.3390/ijms24010610
APA StyleMlakić, M., Odak, I., Faraho, I., Bosnar, M., Banjanac, M., Lasić, Z., Marinić, Ž., Barić, D., & Škorić, I. (2023). Synthesis, Photochemistry, Computational Study and Potential Application of New Styryl-Thiophene and Naphtho-Thiophene Benzylamines. International Journal of Molecular Sciences, 24(1), 610. https://doi.org/10.3390/ijms24010610