Autophagy and Apoptosis: Current Challenges of Treatment and Drug Resistance in Multiple Myeloma
Abstract
1. Introduction
2. Dual Role of Autophagy in Cancer
3. Autoghagy in MM
4. Role of Apoptosis in Cancer
5. Apoptotic Pathways in MM
6. Apoptosis and Autophagy Crosslink in MM
7. Co-Targeting of Apoptosis and Autophagy in MM
8. Conclusions and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 3-BrPA | 3-bromopyruvate |
| 3-MA | 3-Methyladenine |
| ADCC | Antibody-dependent cellular cytotoxicity |
| AMBRA1 | Autophagy and Beclin 1 Regulator 1 |
| AML | Acute myeloid leukemia |
| AMPK | AMP-activated protein kinase |
| AP-1 | Activator protein 1 |
| APRIL | A proliferation inducing ligand |
| ATG | Autophagy-related protein |
| ATM | Ataxia-telangiectasia mutated |
| BAFF | B cell activating factor |
| Bcl-2 | B-cell lymphoma 2 |
| BCLAF-1 | Bcl-2 associated transcription factor |
| Bcl-xL | B-cell lymphoma-extra large |
| BM | Bone marrow |
| BMM | Bone marrow microenvironment |
| BMSCs | BM stromal cells |
| BTK | Bruton tyrosine kinase |
| CACR4 | C-X-C chemokine receptor type 4 |
| CDC | Complement-dependent cytotoxicity |
| cIAP-2 | The cellular inhibitor of apoptosis 2 |
| CLL | Chronic lymphocytic leukemia |
| CLRs | C-type lectin receptors |
| CMA | Chaperone-mediated autophagy |
| CML | Chronic myeloid leukemia |
| CQ | Chloroquine |
| CRBN | Cereblon |
| CSC | Cancer stem cells |
| DAMPs | Damage-associated molecular patterns |
| DAPK | Damage-associated molecular pattern |
| DDR | DNA damage response |
| Deptor | DEP Domain Containing MTOR Interacting Protein |
| DHA | Dihydroartemisinin |
| DISC | Death-inducing signaling complex |
| EGFR | Epidermal growth factor receptor |
| ER | Endoplasmic reticulum |
| ERK | Extracellular signal-regulated kinases |
| FADD | Fas associated via death domain |
| FAK | Focal adhesion kinase |
| FLIP | FLICE-like inhibitory protein |
| FZD | Frizzled |
| HCQ | Hydroxychloroquine |
| HIF-1 | Hypoxia-inducible factor-1 |
| HIF-1 | Hypoxia-inducible factor |
| HK2 | Hypoxia inducible glycolytic enzyme hexokinase-2 |
| HMGB1 | High mobility group box 1 |
| IGF-1 | Insulin like growth factor-1 |
| IL-1 | Interleukin-1 |
| IP3 | Inositol trisphosphate |
| IRF4 | Interferon Regulatory Factor 4 |
| JAK | Janus tyrosine kinase |
| JNK | c-Jun N-terminal kinases |
| LAMP2A | Lysosomal-associated membrane protein 2A |
| LC3 | Microtubule-associated protein-1 light chain kinase 3 |
| LKB1 | Liver kinase B1 |
| MAPK | Mitogen-associated protein kinase |
| MARCKS | Myristoylated alanine-rich C-kinase substrate |
| Mcl-1 | Myeloid cell leukemia-1 |
| MDS | Myelodysplastic syndromes |
| MGUS | Monoclonal gammopathy of undetermined significance |
| MHC class II | Major histocompatibility complex class II |
| MM | Multiple myeloma |
| MOMP | Mitochondrial outer membrane permeabilization |
| MRN | Mre11-Rad50-Nbs1 complex |
| mTOR | Mammalian target of rapamycin |
| NEK2 | Never in mitosis-related kinase 2 |
| NEMO | NF-κB essential modulator |
| NF-κβ | Nuclear factor-κB |
| NK | Natural Killer |
| PCs | Plasma cells |
| PI | Proteasome inhibitors |
| PI(3)P, PtdIns3P | Phosphatidylinositol 3-phosphate |
| PI3K class III | Phosphatidylinositol-3 kinase |
| PI3K/Akt/mTOR | Phosphatidylinositol-3-kinase (PI3K)/Akt/ mammalian target of rapamycin (mTOR) |
| PTEN | Phosphatase and tensin analog |
| R/R | Relapse/Refractory |
| RB1CC1, FIP200 | RB1-inducible coiled-coil 1 |
| Redd1 | Regulated in development and DNA damage responses 1 |
| RLRs | Retinoic acid-inducible gene (RIG)-I-like receptors |
| ROS | Reactive oxygen species |
| SERCA | Sarco/Endoplasmic reticulum Ca²⁺ ATPase |
| SIRT1 | Sirtuin 1 |
| SLAM7 | SLAM Family Member 7 |
| SMM | Smoldering multiple myeloma |
| STAT3 | Signal transducer and activator of transcription 3 |
| TAK1 | Transforming growth factor-β (TGF-β)-activated kinase 1 |
| TFP | Trifluoperazine |
| TLR | Toll-like receptor |
| TNF | Tumor necrosis factor |
| TNF-α | Tumor necrosis factor α |
| TORC1 | TOR-Autophagy Spatial Coupling Compartment |
| TRAIL | TNF-related apoptosis-inducing ligand |
| TSC2 | TNF receptor-associated factor interacting protein 2 |
| TSPs | Tumor suppressor proteins |
| ULK1/2 | UNC-51-like autophagy-activating kinase |
| UPR | Unfolded protein response |
| UPS | Ubiquitin-proteasome system |
| UVRAG | UV radiation resistance-associated gene |
| VEGF | Vascular endothelial growth factors |
| VLA4 | Very late antigen-4 |
| VTX | Venetoclax |
| XBP1 | X-box binding protein 1 |
| XIAP | X-linked inhibitor of apoptosis protein |
References
- Palumbo, A.; Anderson, K. Venous thromboembolism in the patient with cancer: Focus on burden of disease and benefits of thromboprophylaxis. N. Engl. J. Med. 2011, 364, 1046–1060. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Al-Odat, O.; von Suskil, M.; Chitren, R.; Elbezanti, W.; Srivastava, S.; Budak-Alpddogan, T.; Jonnalagadda, S.; Aggarwal, B.; Pandey, M. Mcl-1 Inhibition: Managing Malignancy in Multiple Myeloma. Front. Pharmacol. 2021, 12, 699629. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.; Amin, S.; Zangari, M.; Talamo, G. Drug resistance in multiple myeloma: How to cross the border. Ann. Hematol. Oncol. 2015, 2, 1025. [Google Scholar]
- Esma, F.; Salvini, M.; Troia, R.; Boccadoro, M.; Larocca, A.; Pautasso, C. Melphalan hydrochloride for the treatment of multiple myeloma. Expert Opin. Pharmacother. 2017, 18, 1127–1136. [Google Scholar] [CrossRef]
- Cho, H.J.; Mei, A.; Fukui, J.; Tung, K.; Leshchenko, V.V.; Lagana, A.; Patel, M.; Kim-Schulze, S.; Perumal, D.; Chari, A. MAGE-a mediate resistance to chemotherapy in multiple myeloma through regulation of Bcl-2 proteins. Blood 2016, 128, 3277. [Google Scholar] [CrossRef]
- Tu, Y.; Renner, S.; Xu, F.-H.; Fleishman, A.; Taylor, J.; Weisz, J.; Vescio, R.; Rettig, M.; Berenson, J.; Krajewski, S. BCL-X expression in multiple myeloma: Possible indicator of chemoresistance. Cancer Res. 1998, 58, 256–262. [Google Scholar]
- Saltarella, I.; Lamanuzzi, A.; Reale, A.; Vacca, A.; Ria, R. Identify multiple myeloma stem cells: Utopia? World J. Stem Cells 2015, 7, 84. [Google Scholar] [CrossRef]
- Franqui-Machin, R.; Wendlandt, E.B.; Janz, S.; Zhan, F.; Tricot, G. Cancer stem cells are the cause of drug resistance in multiple myeloma: Fact or fiction? Oncotarget 2015, 6, 40496. [Google Scholar] [CrossRef]
- Damiano, J.S.; Cress, A.E.; Hazlehurst, L.A.; Shtil, A.A.; Dalton, W.S. Cell adhesion mediated drug resistance (CAM-DR): Role of integrins and resistance to apoptosis in human myeloma cell lines. Blood J. Am. Soc. Hematol. 1999, 93, 1658–1667. [Google Scholar]
- Masaki, R. Mechanism of action of bortezomib in multiple myeloma therapy. Inte. J. Myeloma 2016, 6, 1–6. [Google Scholar]
- Mohan, M.; Matin, A.; Davies, F.E. Update on the optimal use of bortezomib in the treatment of multiple myeloma. Cancer Manag. Res. 2017, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Moreau, P.; Palumbo, A.; Joshua, D.; Pour, L.; Hájek, R.; Facon, T.; Ludwig, H.; Oriol, A.; Goldschmidt, H. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): A randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016, 17, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Franken, B.; van de Donk, N.; Cloos, J.; Zweegman, S.; Lokhorst, H. A clinical update on the role of carfilzomib in the treatment of relapsed or refractory multiple myeloma. Ther. Adv. Hematol. 2016, 7, 330–344. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Frezza, M.; Schmitt, S.; Kanwar, J.; Dou, Q.P. Bortezomib as the first proteasome inhibitor anticancer drug: Current status and future perspectives. Curr. Cancer Drug Targets 2011, 11, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.G.; Barlogie, B.; Berenson, J.; Singhal, S.; Jagannath, S.; Irwin, D.; Rajkumar, S.V.; Srkalovic, G.; Alsina, M.; Alexanian, R. A phase 2 study of bortezomib in relapsed, refractory myeloma. N. Engl. J. Med. 2003, 348, 2609–2617. [Google Scholar] [CrossRef]
- Kuhn, D.J.; Chen, Q.; Voorhees, P.M.; Strader, J.S.; Shenk, K.D.; Sun, C.M.; Demo, S.D.; Bennett, M.K.; Van Leeuwen, F.W.; Chanan-Khan, A.A. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood J. Am. Soc. Hematol. 2007, 110, 3281–3290. [Google Scholar] [CrossRef]
- Kubiczkova, L.; Pour, L.; Sedlarikova, L.; Hajek, R.; Sevcikova, S. Proteasome inhibitors–molecular basis and current perspectives in multiple myeloma. J. Cell. Mol. Med. 2014, 18, 947–961. [Google Scholar] [CrossRef]
- Wuilleme-Toumi, S.; Robillard, N.; Gomez, P.; Moreau, P.; Le Gouill, S.; Avet-Loiseau, H.; Harousseau, J.; Amiot, M.; Bataille, R. Mcl-1 is overexpressed in multiple myeloma and associated with relapse and shorter survival. Leukemia 2005, 19, 1248–1252. [Google Scholar] [CrossRef]
- Nikesitch, N.; Ling, S.C. Molecular mechanisms in multiple myeloma drug resistance. J. Clin. Pathol. 2016, 69, 97–101. [Google Scholar] [CrossRef]
- Gambella, M.; Rocci, A.; Passera, R.; Gay, F.; Omedè, P.; Crippa, C.; Corradini, P.; Romano, A.; Rossi, D.; Ladetto, M. High XBP1 expression is a marker of better outcome in multiple myeloma patients treated with bortezomib. Haematologica 2014, 99, e14. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, M.; Jain, S.; Stühmer, T.; Andrulis, M.; Ungethüm, U.; Kuban, R.J.; Lorentz, H.; Bommert, K.; Topp, M.; Krämer, D. STAT3 and MAPK signaling maintain overexpression of heat shock proteins 90α and β in multiple myeloma cells, which critically contribute to tumor-cell survival. Blood 2007, 109, 720–728. [Google Scholar] [CrossRef]
- Hao, M.; Zhang, L.; An, G.; Sui, W.; Yu, Z.; Zou, D.; Xu, Y.; Chang, H.; Qiu, L. Suppressing miRNA-15a/-16 expression by interleukin-6 enhances drug-resistance in myeloma cells. J. Hematol. Oncol. 2011, 4, 37. [Google Scholar] [CrossRef] [PubMed]
- Roccaro, A.M.; Sacco, A.; Maiso, P.; Azab, A.K.; Tai, Y.-T.; Reagan, M.; Azab, F.; Flores, L.M.; Campigotto, F.; Weller, E. BM mesenchymal stromal cell–derived exosomes facilitate multiple myeloma progression. J. Clin. Investig. 2013, 123, 1542–1555. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xu, F.; Lu, T.; Duan, Z.; Zhang, Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat. Rev. 2012, 38, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, Y.; Saha, M.; Chen, J.; Evans, K.; Qiu, L.; Reece, D.; Chen, G.A.; Chang, H. Targeting phospho-MARCKS overcomes drug-resistance and induces antitumor activity in preclinical models of multiple myeloma. Leukemia 2015, 29, 715–726. [Google Scholar] [CrossRef]
- Stessman, H.; Mansoor, A.; Zhan, F.; Janz, S.; Linden, M.; Baughn, L.; Van Ness, B. Reduced CXCR4 expression is associated with extramedullary disease in a mouse model of myeloma and predicts poor survival in multiple myeloma patients treated with bortezomib. Leukemia 2013, 27, 2075–2077. [Google Scholar] [CrossRef]
- Stessman, H.A.; Baughn, L.B.; Sarver, A.; Xia, T.; Deshpande, R.; Mansoor, A.; Walsh, S.A.; Sunderland, J.J.; Dolloff, N.G.; Linden, M.A. Profiling bortezomib resistance identifies secondary therapies in a mouse myeloma model. Mol. Cancer Ther. 2013, 12, 1140–1150. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Kortuem, K.M.; Stewart, A.K. Molecular mechanism of action of immune-modulatory drugs thalidomide, lenalidomide and pomalidomide in multiple myeloma. Leuk. Lymphoma 2013, 54, 683–687. [Google Scholar] [CrossRef]
- Hu, S.; Yuan, L.; Yan, H.; Li, Z. Design, synthesis and biological evaluation of Lenalidomide derivatives as tumor angiogenesis inhibitor. Bioorg. Med. Chem. Lett. 2017, 27, 4075–4081. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Shi, C.-X.; Bruins, L.A.; Wang, X.; Riggs, D.L.; Porter, B.; Ahmann, J.M.; de Campos, C.B.; Braggio, E.; Bergsagel, P.L. Identification of lenalidomide resistance pathways in myeloma and targeted resensitization using cereblon replacement, inhibition of STAT3 or targeting of IRF4. Blood Cancer J. 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Afifi, S.; Michael, A.; Azimi, M.; Rodriguez, M.; Lendvai, N.; Landgren, O. Role of histone deacetylase inhibitors in relapsed refractory multiple myeloma: A focus on vorinostat and panobinostat. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2015, 35, 1173–1188. [Google Scholar] [CrossRef] [PubMed]
- Yee, A.J.; Raje, N.S. Panobinostat and multiple myeloma in 2018. Oncologist 2018, 23, 516–517. [Google Scholar] [CrossRef] [PubMed]
- Laubach, J.P.; Moreau, P.; San-Miguel, J.F.; Richardson, P.G. Panobinostat for the treatment of multiple myeloma. Clin. Cancer Res. 2015, 21, 4767–4773. [Google Scholar] [CrossRef]
- Mithraprabhu, S.; Khong, T.; Spencer, A. Overcoming inherent resistance to histone deacetylase inhibitors in multiple myeloma cells by targeting pathways integral to the actin cytoskeleton. Cell Death Dis. 2014, 5, e1134. [Google Scholar] [CrossRef]
- Varga, C.; Maglio, M.; Ghobrial, I.M.; Richardson, P.G. Current use of monoclonal antibodies in the treatment of multiple myeloma. Br. J. Haematol. 2018, 181, 447–459. [Google Scholar] [CrossRef]
- De Weers, M.; Tai, Y.-T.; Van Der Veer, M.S.; Bakker, J.M.; Vink, T.; Jacobs, D.C.; Oomen, L.A.; Peipp, M.; Valerius, T.; Slootstra, J.W. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. J. Immunol. 2011, 186, 1840–1848. [Google Scholar] [CrossRef]
- Krejcik, J.; Casneuf, T.; Nijhof, I.S.; Verbist, B.; Bald, J.; Plesner, T.; Syed, K.; Liu, K.; van de Donk, N.W.; Weiss, B.M. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood J. Am. Soc. Hematol. 2016, 128, 384–394. [Google Scholar] [CrossRef]
- Overdijk, M.B.; Jansen, J.M.; Nederend, M.; van Bueren, J.J.L.; Groen, R.W.; Parren, P.W.; Leusen, J.H.; Boross, P. The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via Fcγ receptor–mediated cross-linking. J. Immunol. 2016, 197, 807–813. [Google Scholar] [CrossRef]
- Collins, S.M.; Bakan, C.E.; Swartzel, G.D.; Hofmeister, C.C.; Efebera, Y.A.; Kwon, H.; Starling, G.C.; Ciarlariello, D.; Bhaskar, S.; Briercheck, E.L. Elotuzumab directly enhances NK cell cytotoxicity against myeloma via CS1 ligation: Evidence for augmented NK cell function complementing ADCC. Cancer Immunol. Immunother. 2013, 62, 1841–1849. [Google Scholar] [CrossRef]
- Overdijk, M.B.; Verploegen, S.; Bögels, M.; van Egmond, M.; van Bueren, J.J.L.; Mutis, T.; Groen, R.W.; Breij, E.; Martens, A.C.; Bleeker, W.K. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab in lymphoma and multiple myeloma. In MAbs; Taylor & Francis: Abingdon, UK, 2015; pp. 311–320. [Google Scholar]
- van de Donk, N.W.; Moreau, P.; Plesner, T.; Palumbo, A.; Gay, F.; Laubach, J.P.; Malavasi, F.; Avet-Loiseau, H.; Mateos, M.-V.; Sonneveld, P. Clinical efficacy and management of monoclonal antibodies targeting CD38 and SLAMF7 in multiple myeloma. Blood J. Am. Soc. Hematol. 2016, 127, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Siegel, D.; Gutierrez, M.; Jacoby, M.; Hofmeister, C.C.; Gabrail, N.; Baz, R.; Mau-Sorensen, M.; Berdeja, J.G.; Savona, M. Safety and efficacy of selinexor in relapsed or refractory multiple myeloma and Waldenstrom macroglobulinemia. Blood J. Am. Soc. Hematol. 2018, 131, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Chari, A.; Vogl, D.T.; Gavriatopoulou, M.; Nooka, A.K.; Yee, A.J.; Huff, C.A.; Moreau, P.; Dingli, D.; Cole, C.; Lonial, S. Oral selinexor–dexamethasone for triple-class refractory multiple myeloma. N. Engl. J. Med. 2019, 381, 727–738. [Google Scholar] [CrossRef]
- Vogl, D.T.; Dingli, D.; Cornell, R.F.; Huff, C.A.; Jagannath, S.; Bhutani, D.; Zonder, J.; Baz, R.; Nooka, A.; Richter, J. Selective inhibition of nuclear export with oral selinexor for treatment of relapsed or refractory multiple myeloma. J. Clin. Oncol. 2018, 36, 859. [Google Scholar] [CrossRef] [PubMed]
- Greenstein, S.; Ghias, K.; Krett, N.L.; Rosen, S.T. Mechanisms of glucocorticoid-mediated apoptosis in hematological malignancies. Clin. Cancer Res. 2002, 8, 1681–1694. [Google Scholar]
- Rees-Unwin, K.S.; Craven, R.A.; Davenport, E.; Hanrahan, S.; Totty, N.F.; Dring, A.M.; Banks, R.E.; Morgan, G.J.; Davies, F.E. Proteomic evaluation of pathways associated with dexamethasone-mediated apoptosis and resistance in multiple myeloma. Br. J. Haematol. 2007, 139, 559–567. [Google Scholar] [CrossRef]
- Yang, Z.; Klionsky, D.J. Mammalian autophagy: Core molecular machinery and signaling regulation. Curr. Opin. Cell Biol. 2010, 22, 124–131. [Google Scholar] [CrossRef]
- Feng, Y.; He, D.; Yao, Z.; Klionsky, D.J. The machinery of macroautophagy. Cell Res. 2014, 24, 24–41. [Google Scholar] [CrossRef]
- Klionsky, D.J. The molecular machinery of autophagy: Unanswered questions. J. Cell Sci. 2005, 118, 7–18. [Google Scholar] [CrossRef]
- Parzych, K.R.; Klionsky, D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef]
- Schuck, S. Microautophagy–distinct molecular mechanisms handle cargoes of many sizes. J. Cell Sci. 2020, 133, jcs246322. [Google Scholar] [CrossRef] [PubMed]
- Dice, J.F. Peptide sequences that target cytosolic proteins for lysosomal proteolysis. Trends Biochem. Sci. 1990, 15, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Orenstein, S.J.; Cuervo, A.M. Chaperone-mediated autophagy: Molecular mechanisms and physiological relevance. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2010; pp. 719–726. [Google Scholar]
- Klionsky, D.J.; Baehrecke, E.H.; Brumell, J.H.; Chu, C.T.; Codogno, P.; Cuervo, A.M.; Debnath, J.; Deretic, V.; Elazar, Z.; Eskelinen, E.-L. A comprehensive glossary of autophagy-related molecules and processes. Autophagy 2011, 7, 1273–1294. [Google Scholar] [CrossRef]
- Mizushima, N. The pleiotropic role of autophagy: From protein metabolism to bactericide. Cell Death Differ. 2005, 12, 1535–1541. [Google Scholar] [CrossRef]
- Mizushima, N. Autophagy: Process and function. Genes Dev. 2007, 21, 2861–2873. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.H.; Young, L.N. Mechanisms of autophagy initiation. Annu. Rev. Biochem. 2017, 86, 225. [Google Scholar] [CrossRef]
- Cuervo, A.M. Autophagy: In sickness and in health. Trends Cell Biol. 2004, 14, 70–77. [Google Scholar] [CrossRef]
- Hippert, M.M.; O’Toole, P.S.; Thorburn, A. Autophagy in cancer: Good, bad, or both? Cancer Res. 2006, 66, 9349–9351. [Google Scholar] [CrossRef]
- Condello, M.; Pellegrini, E.; Caraglia, M.; Meschini, S. Targeting autophagy to overcome human diseases. Int. J. Mol. Sci. 2019, 20, 725. [Google Scholar] [CrossRef]
- Shintani, T.; Klionsky, D.J. Autophagy in health and disease: A double-edged sword. Science 2004, 306, 990–995. [Google Scholar] [CrossRef]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Usman, R.M.; Razzaq, F.; Akbar, A.; Farooqui, A.A.; Iftikhar, A.; Latif, A.; Hassan, H.; Zhao, J.; Carew, J.S.; Nawrocki, S.T. Role and mechanism of autophagy-regulating factors in tumorigenesis and drug resistance. Asia-Pac. J. Clin. Oncol. 2021, 17, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Ren, X.; Hait, W.N.; Yang, J.M. Therapeutic targeting of autophagy in disease: Biology and pharmacology. Pharmacol. Rev. 2013, 65, 1162–1197. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, M.T.; Ryan, K.M. The multiple roles of autophagy in cancer. Carcinogenesis 2011, 32, 955–963. [Google Scholar] [CrossRef]
- Liang, X.H.; Jackson, S.; Seaman, M.; Brown, K.; Kempkes, B.; Hibshoosh, H.; Levine, B. Induction of autophagy and inhibition of tumorigenesis by Beclin1. Nature 1999, 402, 672–676. [Google Scholar] [CrossRef]
- Qu, X.; Yu, J.; Bhagat, G.; Furuya, N.; Hibshoosh, H.; Troxel, A.; Rosen, J.; Eskelinen, E.-L.; Mizushima, N.; Ohsumi, Y. Promotion of tumorigenesis by heterozygous disruption of the Beclin1 autophagy gene. J. Clin. Investig. 2003, 112, 1809–1820. [Google Scholar] [CrossRef]
- Liang, C.; Feng, P.; Ku, B.; Dotan, I.; Canaani, D.; Oh, B.-H.; Jung, J.U. Autophagic and tumour suppressor activity of a novel Beclin1-binding protein UVRAG. Nat. Cell Biol. 2006, 8, 688–698. [Google Scholar] [CrossRef]
- Degenhardt, K.; Mathew, R.; Beaudoin, B.; Bray, K.; Anderson, D.; Chen, G.; Mukherjee, C.; Shi, Y.; Gélinas, C.; Fan, Y. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell 2006, 10, 51–64. [Google Scholar] [CrossRef]
- Wei, H.; Wei, S.; Gan, B.; Peng, X.; Zou, W.; Guan, J.-L. Suppression of autophagy by FIP200 deletion inhibits mammary tumorigenesis. Genes Dev. 2011, 25, 1510–1527. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, L.; Fu, X.; Wang, W.; Ma, J.; Tian, T.; Nan, K.; Liang, X. Cytoplasmic liver kinase B1 promotes the growth of human lung adenocarcinoma by enhancing autophagy. Cancer Sci. 2018, 109, 3055–3067. [Google Scholar] [CrossRef]
- Aquila, S.; Santoro, M.; Caputo, A.; Panno, M.L.; Pezzi, V.; De Amicis, F. The tumor suppressor PTEN as molecular switch node regulating cell metabolism and autophagy: Implications in immune system and tumor microenvironment. Cells 2020, 9, 1725. [Google Scholar] [CrossRef] [PubMed]
- Daskalaki, I.; Gkikas, I.; Tavernarakis, N. Hypoxia and selective autophagy in cancer development and therapy. Front. Cell Dev. Biol. 2018, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.L. Chemical genetics resulting from a passion for synthetic organic chemistry. Bioorg. Med. Chem. 1998, 6, 1127–1152. [Google Scholar] [CrossRef] [PubMed]
- Deretic, V.; Saitoh, T.; Akira, S. Autophagy in infection, inflammation and immunity. Nat. Rev. Immunol. 2013, 13, 722–737. [Google Scholar] [CrossRef]
- Montecino-Rodriguez, E.; Dorshkind, K. New perspectives in B-1 B cell development and function. Trends Immunol. 2006, 27, 428–433. [Google Scholar] [CrossRef]
- Raza, I.G.; Clarke, A.J. B cell metabolism and autophagy in autoimmunity. Front. Immunol. 2021, 2157. [Google Scholar] [CrossRef]
- Pengo, N.; Scolari, M.; Oliva, L.; Milan, E.; Mainoldi, F.; Raimondi, A.; Fagioli, C.; Merlini, A.; Mariani, E.; Pasqualetto, E. Plasma cells require autophagy for sustainable immunoglobulin production. Nat. Immunol. 2013, 14, 298–305. [Google Scholar] [CrossRef]
- Halliley, J.L.; Tipton, C.M.; Liesveld, J.; Rosenberg, A.F.; Darce, J.; Gregoretti, I.V.; Popova, L.; Kaminiski, D.; Fucile, C.F.; Albizua, I. Long-lived plasma cells are contained within the CD19− CD38hiCD138+ subset in human bone marrow. Immunity 2015, 43, 132–145. [Google Scholar] [CrossRef]
- Benbrook, D.; Long, A. Integration of autography, proteasomal degradation, unfolded protein responce and apoptosis. Exp. Oncol. 2012, 3, 286–297. [Google Scholar]
- Hoang, B.; Benavides, A.; Shi, Y.; Frost, P.; Lichtenstein, A. Effect of autophagy on multiple myeloma cell viabilityAutophagy and Myeloma. Mol. Cancer Ther. 2009, 8, 1974–1984. [Google Scholar] [CrossRef]
- Ho, M.; Patel, A.; Hanley, C.; Murphy, A.; McSweeney, T.; Zhang, L.; McCann, A.; O’Gorman, P.; Bianchi, G. Exploiting autophagy in multiple myeloma. J. Cancer Metastasis Treat. 2019, 5, 70. [Google Scholar] [CrossRef][Green Version]
- Bernales, S.; McDonald, K.L.; Walter, P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol. 2006, 4, e423. [Google Scholar] [CrossRef] [PubMed]
- Milan, E.; Perini, T.; Resnati, M.; Orfanelli, U.; Oliva, L.; Raimondi, A.; Cascio, P.; Bachi, A.; Marcatti, M.; Ciceri, F. A plastic SQSTM1/p62-dependent autophagic reserve maintains proteostasis and determines proteasome inhibitor susceptibility in multiple myeloma cells. Autophagy 2015, 11, 1161–1178. [Google Scholar] [CrossRef]
- Jung, G.; Roh, J.; Lee, H.; Gil, M.; Yoon, D.H.; Suh, C.; Jang, S.; Park, C.-J.; Huh, J.; Park, C.-S. Autophagic markers BECLIN1 and LC3 are associated with prognosis of multiple myeloma. Acta Haematol. 2015, 134, 17–24. [Google Scholar] [CrossRef]
- Tucci, M.; Stucci, S.; Savonarola, A.; Resta, L.; Cives, M.; Rossi, R.; Silvestris, F. An imbalance between Beclin-1 and p62 expression promotes the proliferation of myeloma cells through autophagy regulation. Exp. Hematol. 2014, 42, 897–908. [Google Scholar] [CrossRef]
- Gourzones-Dmitriev, C.; Kassambara, A.; Sahota, S.; Rème, T.; Moreaux, J.; Bourquard, P.; Hose, D.; Pasero, P.; Constantinou, A.; Klein, B. DNA repair pathways in human multiple myeloma: Role in oncogenesis and potential targets for treatment. Cell Cycle 2013, 12, 2760–2773. [Google Scholar] [CrossRef]
- Eliopoulos, A.G.; Havaki, S.; Gorgoulis, V.G. DNA damage response and autophagy: A meaningful partnership. Front. Genet. 2016, 7, 204. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.G.; Macleod, K.F. Autophagy, cancer stem cells and drug resistance. J. Pathol. 2019, 247, 708–718. [Google Scholar] [CrossRef]
- Malek, M.A.A.; Jagannathan, S.; Malek, E.; Sayed, D.M.; Elgammal, S.A.; Abd El-Azeem, H.G.; Thabet, N.M.; Driscoll, J.J. Molecular chaperone GRP78 enhances aggresome delivery to autophagosomes to promote drug resistance in multiple myeloma. Oncotarget 2015, 6, 3098. [Google Scholar] [CrossRef]
- Hamouda, M.-A.; Belhacene, N.; Puissant, A.; Colosetti, P.; Robert, G.; Jacquel, A.; Mari, B.; Auberger, P.; Luciano, F. The small heat shock protein B8 (HSPB8) confers resistance to bortezomib by promoting autophagic removal of misfolded proteins in multiple myeloma cells. Oncotarget 2014, 5, 6252. [Google Scholar] [CrossRef]
- Shanmugam, M.; McBrayer, S.K.; Qian, J.; Raikoff, K.; Avram, M.J.; Singhal, S.; Gandhi, V.; Schumacker, P.T.; Krett, N.L.; Rosen, S.T. Targeting glucose consumption and autophagy in myeloma with the novel nucleoside analogue 8-aminoadenosine. J. Biol. Chem. 2009, 284, 26816–26830. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Miyazawa, K.; Moriya, S.; Ohtomo, T.; Che, X.-F.; Naito, M.; Itoh, M.; Tomoda, A. Combined treatment with bortezomib plus bafilomycin A1 enhances the cytocidal effect and induces endoplasmic reticulum stress in U266 myeloma cells: Crosstalk among proteasome, autophagy-lysosome and ER stress. Int. J. Oncol. 2011, 38, 643–654. [Google Scholar] [PubMed]
- Wang, G.; Zhou, P.; Chen, X.; Zhao, L.; Tan, J.; Yang, Y.; Fang, Y.; Zhou, J. The novel autophagy inhibitor elaiophylin exerts antitumor activity against multiple myeloma with mutant TP53 in part through endoplasmic reticulum stress-induced apoptosis. Cancer Biol. Ther. 2017, 18, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, W.; Yan, Z.; Zhao, W.; Mi, J.; Li, J.; Yan, H. Metformin induces autophagy and G0/G1 phase cell cycle arrest in myeloma by targeting the AMPK/mTORC1 and mTORC2 pathways. J. Exp. Clin. Cancer Res. 2018, 37, 63. [Google Scholar] [CrossRef] [PubMed]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Riedl, S.J.; Salvesen, G.S. The apoptosome: Signalling platform of cell death. Nat. Rev. Mol. Cell Biol. 2007, 8, 405–413. [Google Scholar] [CrossRef]
- Fulda, S.; Debatin, K.M. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene 2006, 25, 4798–4811. [Google Scholar] [CrossRef]
- Chan, S.-L.; Yu, V.C. Proteins of the BCL-2 family in apoptosis signalling: From mechanistic insights to therapeutic opportunities. Clin. Exp. Pharmacol. Physiol. 2004, 31, 119–128. [Google Scholar] [CrossRef]
- Kaufmann, T.; Strasser, A.; Jost, P.J. Fas death receptor signalling: Roles of Bid and XIAP. Cell Death Differ. 2012, 19, 42–50. [Google Scholar] [CrossRef]
- Zhou, L. Caspase-8: Friend or Foe in Bortezomib/Lenalidomide-Based Therapy for Myeloma. Front. Oncol. 2022, 12, 861709. [Google Scholar] [CrossRef]
- Tummers, B.; Green, D.R. Caspase-8: Regulating life and death. Immunol. Rev. 2017, 277, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Shukla, N.; Singh, S.S.; Kushwaha, S.; Shrivastava, R. Mechanism of interaction between autophagy and apoptosis in cancer. Apoptosis 2021, 26, 512–533. [Google Scholar] [CrossRef] [PubMed]
- Costello, R.T.; Mallet, F.; Gaugler, B.; Sainty, D.; Arnoulet, C.; Gastaut, J.-A.; Olive, D. Human acute myeloid leukemia CD34+/CD38− progenitor cells have decreased sensitivity to chemotherapy and Fas-induced apoptosis, reduced immunogenicity, and impaired dendritic cell transformation capacities. Cancer Res. 2000, 60, 4403–4411. [Google Scholar]
- Ozaki, T.; Nakagawara, A. Role of p53 in Cell Death and Human Cancers. Cancers 2011, 3, 994–1013. [Google Scholar] [CrossRef] [PubMed]
- Pflaum, J.; Schlosser, S.; Müller, M. p53 family and cellular stress responses in cancer. Front. Oncol. 2014, 4, 285. [Google Scholar] [CrossRef]
- Oh, Y.-T.; Sun, S.-Y. Regulation of cancer metastasis by trail/death receptor signaling. Biomolecules 2021, 11, 499. [Google Scholar] [CrossRef]
- Miles, M.A.; Hawkins, C.J. Executioner caspases and CAD are essential for mutagenesis induced by TRAIL or vincristine. Cell Death Dis. 2017, 8, e3062. [Google Scholar] [CrossRef]
- Slomp, A.; Moesbergen, L.M.; Gong, J.-N.; Cuenca, M.; von dem Borne, P.A.; Sonneveld, P.; Huang, D.C.; Minnema, M.C.; Peperzak, V. Multiple myeloma with 1q21 amplification is highly sensitive to MCL-1 targeting. Blood Adv. 2019, 3, 4202–4214. [Google Scholar] [CrossRef]
- Podar, K.; Gouill, S.L.; Zhang, J.; Opferman, J.T.; Zorn, E.; Tai, Y.T.; Hideshima, T.; Amiot, M.; Chauhan, D.; Harousseau, J.L.; et al. A pivotal role for Mcl-1 in Bortezomib-induced apoptosis. Oncogene 2008, 27, 721–731. [Google Scholar] [CrossRef][Green Version]
- Dankbar, B.; Padró, T.; Leo, R.; Feldmann, B.; Kropff, M.; Mesters, R.M.; Serve, H.; Berdel, W.E.; Kienast, J. Vascular endothelial growth factor and interleukin-6 in paracrine tumor-stromal cell interactions in multiple myeloma. Blood J. Am. Soc. Hematol. 2000, 95, 2630–2636. [Google Scholar]
- Mitsiades, C.S.; Mitsiades, N.; Poulaki, V.; Schlossman, R.; Akiyama, M.; Chauhan, D.; Hideshima, T.; Treon, S.P.; Munshi, N.C.; Richardson, P.G.; et al. Activation of NF-κB and upregulation of intracellular anti-apoptotic proteins via the IGF-1/Akt signaling in human multiple myeloma cells: Therapeutic implications. Oncogene 2002, 21, 5673–5683. [Google Scholar] [CrossRef]
- De Bruyne, E.; Bos, T.J.; Schuit, F.; Van Valckenborgh, E.; Menu, E.; Thorrez, L.; Atadja, P.; Jernberg-Wiklund, H.; Vanderkerken, K. IGF-1 suppresses Bim expression in multiple myeloma via epigenetic and posttranslational mechanisms. Blood J. Am. Soc. Hematol. 2010, 115, 2430–2440. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, D.J.; Berkova, Z.; Jones, R.J.; Woessner, R.; Bjorklund, C.C.; Ma, W.; Davis, R.E.; Lin, P.; Wang, H.; Madden, T.L.; et al. Targeting the insulin-like growth factor-1 receptor to overcome bortezomib resistance in preclinical models of multiple myeloma. Blood 2012, 120, 3260–3270. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, P.; Zhao, W.; Zhu, L.; Sui, J.; Dai, Y.; Lai, Y. MiR-197-3p reduces bortezomib resistance in multiple myeloma by inhibiting IL-6 expression in a MEAF6-dependent manner. Leuk. Res. 2022, 114, 106785. [Google Scholar] [CrossRef] [PubMed]
- Eccles, D.; Russell, S.; Haites, N.; Atkinson, R.; Bell, D.W.; Gruber, L.; Hickey, I.; Kelly, K.; Kitchener, H.; Leonard, R. Early loss of heterozygosity on 17q in ovarian cancer. The Abe Ovarian Cancer Genetics Group. Oncogene 1992, 7, 2069–2072. [Google Scholar]
- Futreal, P.; Söderkvist, P.; Marks, J.; Iglehart, J.; Cochran, C.; Barrett, J.; Wiseman, R. Detection of frequent allelic loss on proximal chromosome 17q in sporadic breast carcinoma using microsatellite length polymorphisms. Cancer Res. 1992, 52, 2624–2627. [Google Scholar]
- Gao, X.; Zacharek, A.; Salkowski, A.; Grignon, D.J.; Sakr, W.; Porter, A.T.; Honn, K.V. Loss of heterozygosity of the BRCA1 and other loci on chromosome 17q in human prostate cancer. Cancer Res. 1995, 55, 1002–1005. [Google Scholar]
- Saito, H.; Inazawa, J.; Saito, S.; Kasumi, F.; Koi, S.; Sagae, S.; Kudo, R.; Saito, J.; Noda, K.; Nakamura, Y. Detailed deletion mapping of chromosome 17q in ovarian and breast cancers: 2-cM region on 17q21. 3 often and commonly deleted in tumors. Cancer Res. 1993, 53, 3382–3385. [Google Scholar]
- Strappazzon, F.; Vietri-Rudan, M.; Campello, S.; Nazio, F.; Florenzano, F.; Fimia, G.M.; Piacentini, M.; Levine, B.; Cecconi, F. Mitochondrial BCL-2 inhibits AMBRA1-induced autophagy. EMBO J. 2011, 30, 1195–1208. [Google Scholar] [CrossRef]
- Sun, W.L. Ambra1 in autophagy and apoptosis: Implications for cell survival and chemotherapy resistance (Review). Oncol. Lett. 2016, 12, 367–374. [Google Scholar] [CrossRef]
- Takahashi, Y.; Coppola, D.; Matsushita, N.; Cualing, H.D.; Sun, M.; Sato, Y.; Liang, C.; Jung, J.U.; Cheng, J.Q.; Mul, J.J.; et al. Bif-1 interacts with Beclin1 through UVRAG and regulates autophagy and tumorigenesis. Nat. Cell Biol. 2007, 9, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Liang, L.; Xiao, X.; Peng, Y.; Luo, Y.; Zhou, W.; Zhang, J.; Qiu, L.; Zhang, S.; Liu, F.; et al. Lycorine Downregulates HMGB1 to Inhibit Autophagy and Enhances Bortezomib Activity in Multiple Myeloma. Theranostics 2016, 6, 2209–2224. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Livesey, K.M.; Cheh, C.W.; Farkas, A.; Loughran, P.; Hoppe, G.; Bianchi, M.E.; Tracey, K.J.; Zeh, H.J., 3rd; et al. Endogenous HMGB1 regulates autophagy. J. Cell Biol. 2010, 190, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Cao, L.; Peng, Y.; Tan, Y.; Xie, M.; Kang, R.; Livesey, K.M.; Tang, D. A critical role for UVRAG in apoptosis. Autophagy 2011, 7, 1242–1244. [Google Scholar] [CrossRef]
- Sui, X.; Kong, N.; Ye, L.; Han, W.; Zhou, J.; Zhang, Q.; He, C.; Pan, H. p38 and JNK MAPK pathways control the balance of apoptosis and autophagy in response to chemotherapeutic agents. Cancer Lett. 2014, 344, 174–179. [Google Scholar] [CrossRef]
- You, L.; Wang, Z.; Li, H.; Shou, J.; Jing, Z.; Xie, J.; Sui, X.; Pan, H.; Han, W. The role of STAT3 in autophagy. Autophagy 2015, 11, 729–739. [Google Scholar] [CrossRef]
- Winter, J.N.; Jefferson, L.S.; Kimball, S.R. ERK and Akt signaling pathways function through parallel mechanisms to promote mTORC1 signaling. Am. J. Physiol.-Cell Physiol. 2011, 300, C1172–C1180. [Google Scholar] [CrossRef] [PubMed]
- Hoang, B.; Benavides, A.; Shi, Y.; Yang, Y.; Frost, P.; Gera, J.; Lichtenstein, A. The PP242 mammalian target of rapamycin (mTOR) inhibitor activates extracellular signal-regulated kinase (ERK) in multiple myeloma cells via a target of rapamycin complex 1 (TORC1)/eukaryotic translation initiation factor 4E (eIF-4E)/RAF pathway and activation is a mechanism of resistance. J. Biol. Chem. 2012, 287, 21796–21805. [Google Scholar]
- Ramakrishnan, V.; Kumar, S. PI3K/AKT/mTOR pathway in multiple myeloma: From basic biology to clinical promise. Leuk. Lymphoma 2018, 59, 2524–2534. [Google Scholar] [CrossRef]
- Mills, J.R.; Hippo, Y.; Robert, F.; Chen, S.M.; Malina, A.; Lin, C.-J.; Trojahn, U.; Wendel, H.-G.; Charest, A.; Bronson, R.T. mTORC1 promotes survival through translational control of Mcl-1. Proc. Natl. Acad. Sci. USA 2008, 105, 10853–10858. [Google Scholar] [CrossRef] [PubMed]
- Pradelli, L.; Beneteau, M.; Chauvin, C.; Jacquin, M.; Marchetti, S.; Munoz-Pinedo, C.; Auberger, P.; Pende, M.; Ricci, J. Glycolysis inhibition sensitizes tumor cells to death receptors-induced apoptosis by AMP kinase activation leading to Mcl-1 block in translation. Oncogene 2010, 29, 1641–1652. [Google Scholar] [CrossRef] [PubMed]
- Betin, V.M.; Lane, J.D. Atg4D at the interface between autophagy and apoptosis. Autophagy 2009, 5, 1057–1059. [Google Scholar] [CrossRef] [PubMed]
- Radoshevich, L.; Murrow, L.; Chen, N.; Fernandez, E.; Roy, S.; Fung, C.; Debnath, J. ATG12 conjugation to ATG3 regulates mitochondrial homeostasis and cell death. Cell 2010, 142, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S.; Perozzo, R.; Schmid, I.; Ziemiecki, A.; Schaffner, T.; Scapozza, L.; Brunner, T.; Simon, H.-U. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat. Cell Biol. 2006, 8, 1124–1132. [Google Scholar] [CrossRef]
- Liu, H.; He, Z.; von Rütte, T.; Yousefi, S.; Hunger, R.E.; Simon, H.-U. Down-regulation of autophagy-related protein 5 (ATG5) contributes to the pathogenesis of early-stage cutaneous melanoma. Sci. Transl. Med. 2013, 5, ra123–ra202. [Google Scholar] [CrossRef]
- Milan, E.; Fabbri, M.; Cenci, S. Autophagy in Plasma Cell Ontogeny and Malignancy. J. Clin. Immunol. 2016, 36 (Suppl. S1), 18–24. [Google Scholar] [CrossRef]
- Lamy, L.; Ngo, V.N.; Emre, N.C.; Shaffer, A.L., 3rd; Yang, Y.; Tian, E.; Nair, V.; Kruhlak, M.J.; Zingone, A.; Landgren, O.; et al. Control of autophagic cell death by caspase-10 in multiple myeloma. Cancer Cell 2013, 23, 435–449. [Google Scholar] [CrossRef]
- Zeng, R.; Chen, Y.; Zhao, S.; Cui, G.-H. Autophagy counteracts apoptosis in human multiple myeloma cells exposed to oridonin in vitro via regulating intracellular ROS and SIRT1. Acta Pharmacol. Sin. 2012, 33, 91–100. [Google Scholar] [CrossRef]
- Li, A.; Chen, X.; Jing, Z.; Chen, J. Trifluoperazine induces cellular apoptosis by inhibiting autophagy and targeting NUPR1 in multiple myeloma. FEBS Open Bio. 2020, 10, 2097–2106. [Google Scholar] [CrossRef]
- Xia, J.; He, Y.; Meng, B.; Chen, S.; Zhang, J.; Wu, X.; Zhu, Y.; Shen, Y.; Feng, X.; Guan, Y.; et al. NEK2 induces autophagy-mediated bortezomib resistance by stabilizing Beclin-1 in multiple myeloma. Mol. Oncol. 2020, 14, 763–778. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Abe, F.; Matsuda, Y.; Kitadate, A.; Takahashi, N.; Tagawa, H. Hypoxia-inducible hexokinase-2 enhances anti-apoptotic function via activating autophagy in multiple myeloma. Cancer Sci. 2020, 111, 4088–4101. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Z.; Chen, J.M.; Zeng, Z.Y.; Qiu, D.B. Effects of Autophagy Regulating Drugs on Proliferation, Apoptosis and Autophagy of Multiple Myeloma Cells. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2018, 26, 817–823. [Google Scholar] [CrossRef]
- Ma, Y.; Jin, Z.; Yu, K.; Liu, Q. NVP-BEZ235-induced autophagy as a potential therapeutic approach for multiple myeloma. Am. J. Transl. Res. 2019, 11, 87–105. [Google Scholar] [PubMed]
- Wu, X.; Liu, Y.; Zhang, E.; Chen, J.; Huang, X.; Yan, H.; Cao, W.; Qu, J.; Gu, H.; Xu, R.; et al. Dihydroartemisinin Modulates Apoptosis and Autophagy in Multiple Myeloma through the P38/MAPK and Wnt/β-Catenin Signaling Pathways. Oxidative Med. Cell. Longev. 2020, 2020, 6096391. [Google Scholar] [CrossRef]
- Liao, A.; Hu, R.; Zhao, Q.; Li, J.; Li, Y.; Yao, K.; Zhang, R.; Wang, H.; Yang, W.; Liu, Z. Autophagy induced by FTY720 promotes apoptosis in U266 cells. Eur. J. Pharm. Sci. 2012, 45, 600–605. [Google Scholar] [CrossRef]
- Wu, H.; Liu, C.; Yang, Q.; Xin, C.; Du, J.; Sun, F.; Zhou, L. MIR145-3p promotes autophagy and enhances bortezomib sensitivity in multiple myeloma by targeting HDAC4. Autophagy 2020, 16, 683–697. [Google Scholar] [CrossRef]
- Yang, Y.; Li, F.; Saha, M.N.; Abdi, J.; Qiu, L.; Chang, H. miR-137 and miR-197 Induce Apoptosis and Suppress Tumorigenicity by Targeting MCL-1 in Multiple Myeloma. Clin. Cancer Res. 2015, 21, 2399–2411. [Google Scholar] [CrossRef]
- Ma, R.; Yu, D.; Peng, Y.; Yi, H.; Wang, Y.; Cheng, T.; Shi, B.; Yang, G.; Lai, W.; Wu, X.; et al. Resveratrol induces AMPK and mTOR signaling inhibition-mediated autophagy and apoptosis in multiple myeloma cells. Acta Biochim. Biophys. Sin. 2021, 53, 775–783. [Google Scholar] [CrossRef]
- Kharaziha, P.; De Raeve, H.; Fristedt, C.; Li, Q.; Gruber, A.; Johnsson, P.; Kokaraki, G.; Panzar, M.; Laane, E.; Österborg, A.; et al. Sorafenib Has Potent Antitumor Activity against Multiple Myeloma In Vitro, Ex Vivo, and In Vivo in the 5T33MM Mouse Model. Cancer Res. 2012, 72, 5348–5362. [Google Scholar] [CrossRef]
- Al-Odat, O.S. Selective Small Molecule Targeting of MCL-1 in Multiple Myeloma. Ph.D. Thesis, Rowan University, Glassboro, NJ, USA, 2021. [Google Scholar]
- Al-Odat, O.S.; Tripathi, R.S.; Srivastava, S.K.; Gowda, K.; Amin, S.G.; Budak-Alpdogan, T.; Jonnalagadda, S.C.; Pandey, M.K. A novel Mcl-1 inhibitor induces cells death in a caspase-dependent manner and increases the efficacies of Venetoclax and ABT-737 in multiple myeloma cells. Cancer Res. 2022, 82, 3952. [Google Scholar] [CrossRef]
- Sulkshane, P.; Teni, T. BH3 mimetic Obatoclax (GX15-070) mediates mitochondrial stress predominantly via MCL-1 inhibition and induces autophagy-dependent necroptosis in human oral cancer cells. Oncotarget 2017, 8, 60060–60079. [Google Scholar] [CrossRef] [PubMed]
- Satta, T.; Grant, S. Enhancing venetoclax activity in hematological malignancies. Expert Opin. Investig. Drugs 2020, 29, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.Y.; Chim, C.S. Venetoclax, bortezomib and S63845, an MCL1 inhibitor, in multiple myeloma. J. Pharm. Pharmacol. 2020, 72, 728–737. [Google Scholar] [CrossRef]
- Algarín, E.M.; Díaz-Tejedor, A.; Mogollón, P.; Hernández-García, S.; Corchete, L.A.; San-Segundo, L.; Martín-Sánchez, M.; González-Méndez, L.; Schoumacher, M.; Banquet, S.; et al. Preclinical evaluation of the simultaneous inhibition of MCL-1 and BCL-2 with the combination of S63845 and venetoclax in multiple myeloma. Haematologica 2020, 105, e116–e120. [Google Scholar] [CrossRef]
- Spaan, I.; Timmerman, L.M.; Kimman, T.; Slomp, A.; Cuenca, M.; van Nieuwenhuijzen, N.; Moesbergen, L.M.; Minnema, M.C.; Raymakers, R.A.; Peperzak, V. Direct P70S6K1 inhibition to replace dexamethasone in synergistic combination with MCL-1 inhibition in multiple myeloma. Blood Adv. 2021, 5, 2593–2607. [Google Scholar] [CrossRef]
- Ookura, M.; Fujii, T.; Yagi, H.; Ogawa, T.; Kishi, S.; Hosono, N.; Shigemi, H.; Yamauchi, T.; Ueda, T.; Yoshida, A. YM155 exerts potent cytotoxic activity against quiescent (G(0)/G(1)) multiple myeloma and bortezomib resistant cells via inhibition of survivin and Mcl-1. Oncotarget 2017, 8, 111535–111550. [Google Scholar] [CrossRef]
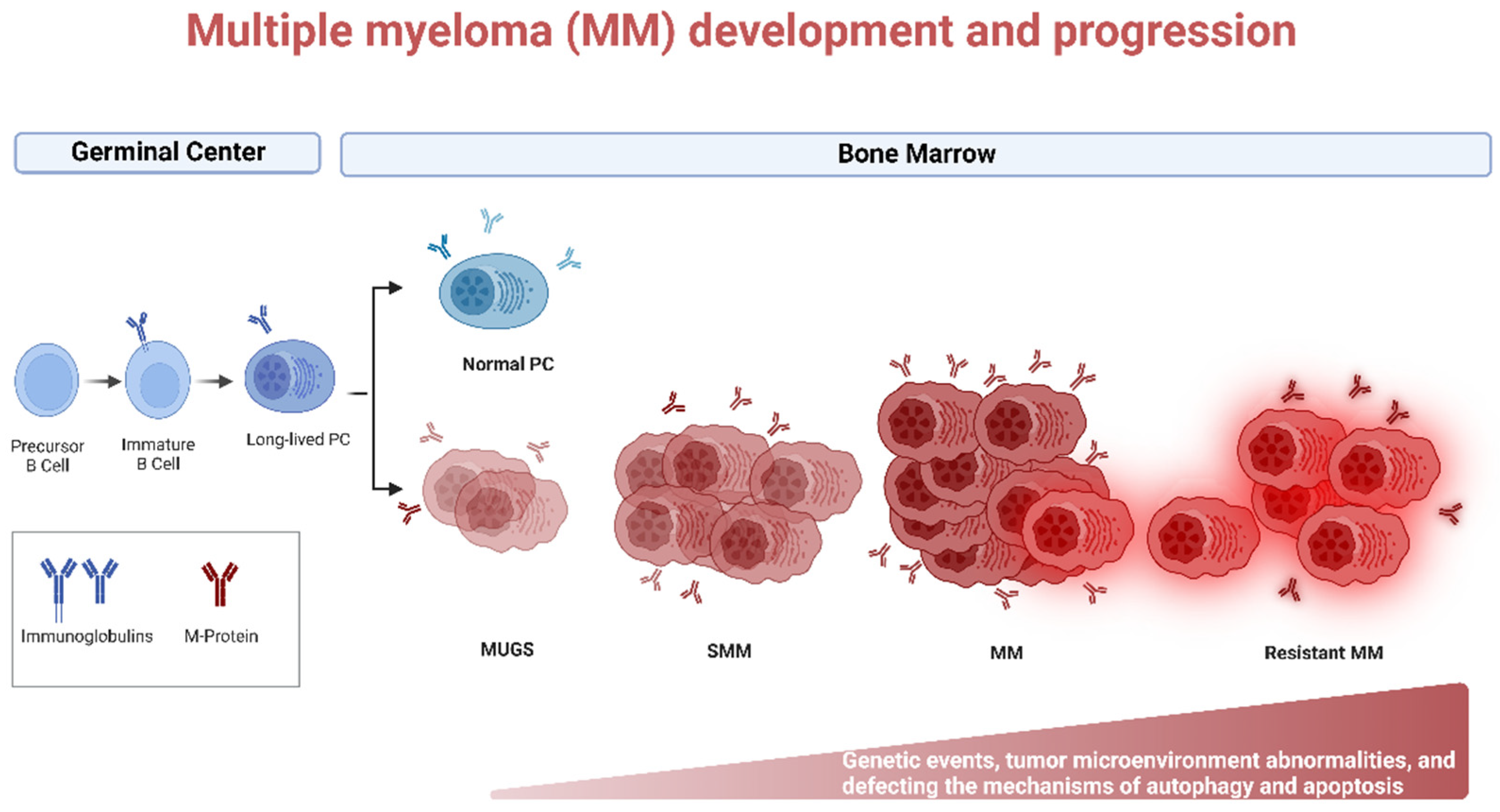

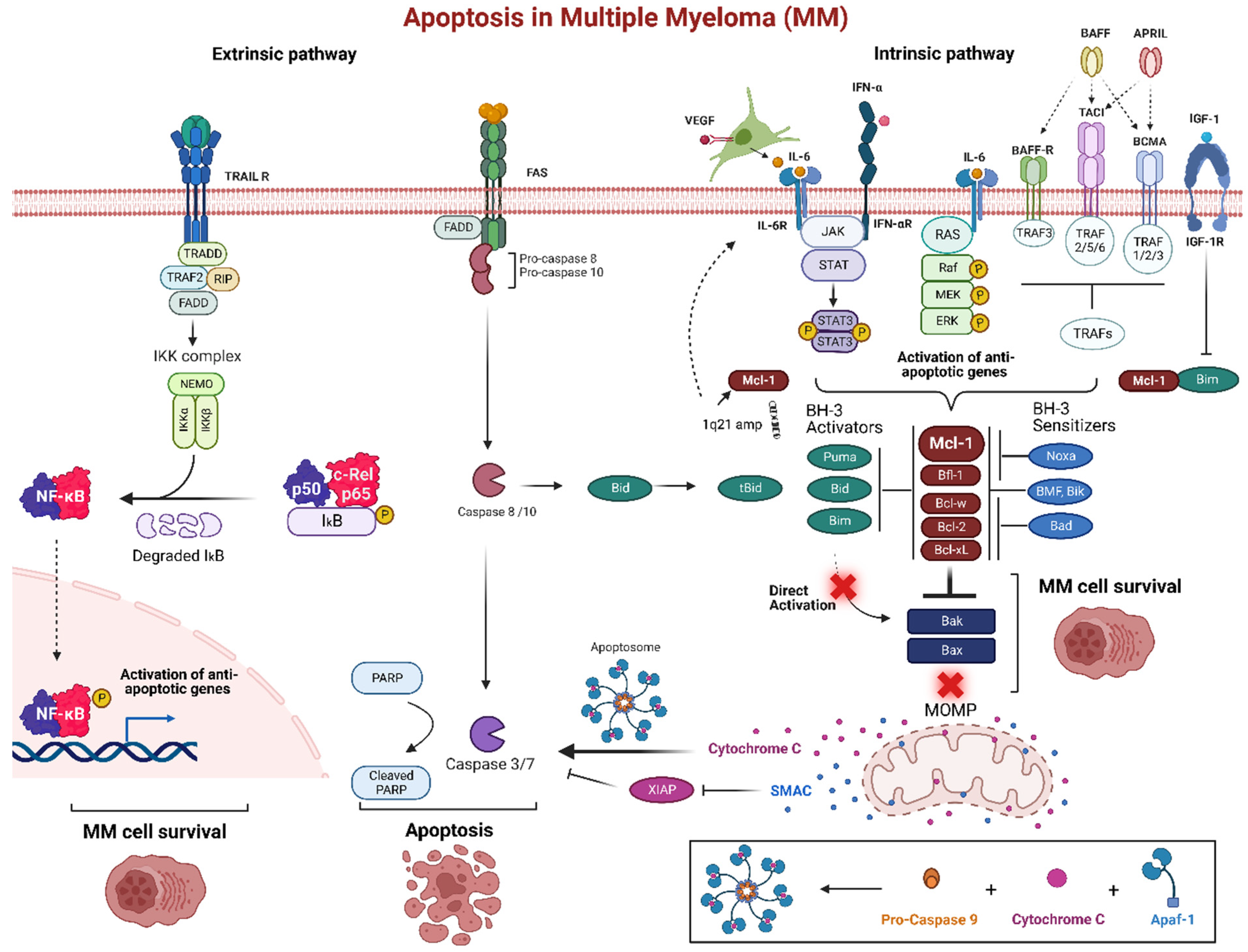

| Class | Drugs | Mechanism of Action | Type of Resistance | Mechanism of Resistance |
|---|---|---|---|---|
| Alkalyting agents Anthracyclines | Melphalan Cyclophosphamide Doxorubicin | Impairment of DNA synthesis and cell replication, immunostimulatory activity by inhibiting interleukin-6 (IL6), interaction with dendritic cells, and immunogenic effects in the tumor microenvironment [4,5]. Topoisomerase II inhibition (Doxorubicin). | Alters autophagy and apoptosis signaling pathways Cancer stem cells and bone marrow microenvironment | Upregulation of anti-apoptotic proteins (Mcl-1, Bcl-2, Bcl-xL) [6,7]. Stem cell-like phenotype with triggering of ALDH1A1 enzymatic activity and upregulation of BTK [8,9]. Increase of cell adhesion molecules (VLA4) [10]. |
| Proteasome inhibitors | Bortezomib Carfilzomib Ixazomib | Inhibition of proteasome activity; inhibition of NF-κβ activity; induction of apoptosis by activation of caspase-8 and -9; upregulation of pro-apoptotic protein Noxa; downregulation of adhesion molecules on myeloma cells [4,11,12,13,14,15,16,17,18]. | Alters autophagy and apoptosis signaling pathways Cancer stem cells and bone marrow microenvironment | Upregulation of the proteasomal system; Upregulation of anti-apoptotic proteins (Mcl-1, Bcl-2, Bcl-xL); activation of autophagy pathway; induction of NF-κβ; unfolded protein response (UPR) transcription factor XBP1 suppression; overexpression of heat shock proteins [3,19,20,21,22]. Stem cell-like phenotype with triggering of ALDH1A1 enzymatic activity and upregulation of BTK [8,9]. Secreting a group of extracellular signaling cues including IL-6, growth factors such as vascular endothelial growth factor (VEGF), and Insulin-like growth factor 1 (IGF-1); trigger and modulate multiple keys of the transcriptional pathway including Ras/MAPK, JAK/STAT3, and PI3/Akt; Increase of pro-inflammatory TNF-α; Increase of different cell adhesion molecules; overexpression of CXCR4; overexpression of MARCKS [22,23,24,25,26,27,28]. |
| Immunomodulatory agents | Thalidomide Lenalidomide Pomalidomide | Induction of apoptosis by activation of cspase-8 and -9; interaction with BMM and downregulation of adhesion molecules; affecting cereblon (CRBN) and downstream targets; regulation of T and natural killer (NK) cells activity; anti-angiogenic activity [29,30]. | Cancer stem cells and bone marrow microenvironment | Stem cell-like phenotype with triggering of ALDH1A1 enzymatic activity and upregulation of BTK [8,9]. Downregulation of CRBN expression and deregulation of IRF4 expression; increased IL-6 expression and constitutive STAT3 activation [31]. |
| Histone deacetylase inhibitors | Panobinostat Vorinostat | Increasing chromatin structure opening, end with activation of tumor suppressor genes [32,33,34]. | Bone marrow microenvironment and disruption intracellular signaling pathways | Regulation of actin cytoskeleton and protein processing in endoplasmic reticulum (triggering of MEK/ERK, PI3K, and FAK pathways) [35]. |
| Monoclonal antibodies | Daratumumab Elotuzumab Isatuximab | Antibody-dependent cellular cytotoxicity (ADCC); complement-dependent cytotoxicity (CDC); modulation of target antigen enzymatic activity; macrophage-mediated pagocytosis; apoptosis via Fcγ receptor-mediated crosslinking; stimulation of immune cells activity, particularly T and NK cells [36,37,38,39,40,41]. | Bone marrow microenvironment | Competition by the soluble extracellular forms of CD38 and SLAM7 [42]. |
| Selective Exportin 1 (XPO1) inhibitor | Selinexor | Induces apoptosis through nuclear retention and functional activation of tumor suppressor proteins (TSPs), inhibits NF-κβ, and the translation of oncoprotein mRNAs [43,44,45]. | - | - |
| Corticosteroids | Dexamethasone Prednisolone Methylprednisolone | Induction of apoptosis [46]. | Bone marrow microenvironment | Increased secretion of pro-survival cytokines bone marrow microenvironment [4,47]. |
| Drug | Mechanism of Action |
|---|---|
| Autophagy inhibitors | |
| 3-Methyladenine (3-MA) Wortmannin LY294002 | Class III PI3K inhibitors |
| Chloroquine (CQ) Hydroxychloroquine (HCQ) Lys05 (CQ derivative) | Lysosomal alkalizer |
| Bafilomycin A1 Concanamycin A | Vacuolar H⁺-ATPases inhibitors |
| Elaiophylin 4-Acetylantroquinonol B | Inhibition of autophagy flux |
| Thymoquinone | Permeabilization of the lysosome membrane |
| Pepstatin A E64d Leupeptin | Lysosomal proteolysis (hydrolases and proteases) inhibitor |
| Thapsigargin | Sarco/Endoplasmic reticulum Ca²⁺ ATPase (SERCA) inhibitor |
| Paclitaxel | VPS34 kinase inhibitor and blocking autophosome-lysosome fusion |
| PT21 | VPS34 Kinase inhibitor |
| Rapamycin Everolimus Deforolimus Temsirolimus (CCI-779) | mTORC1 inhibitors |
| Autophagy inducers | |
| AZD8055 Torin1 PP242 | mTORC1 and mTORC2 inhibitors |
| GDC-0980 | Class I PI3K and mTORC1/2 inhibitor |
| CH5132799 GDC-0941 | Class I PI3K inhibitor |
| Metformin Spermidine | AMPK activators |
| Ibrutinib | BTK inhibitor |
| Vorinostat (SAHA) | Histone deacetylase inhibitor |
| Perifosine | AKT inhibitors |
| Tat-beclin 1 peptide | Autophagy inducing peptide |
| Resveratrol | Sirtuin 1 and S6 kinase inhibitor |
| Drug | Study Design | Clinical Trial Status |
|---|---|---|
| Autophagy inhibitors | ||
| Chloroquine (CQ) | In combination with bortezomib and cyclophosphamide in R/R MM patients | Phase II (NCT01438177) |
| Hydroxychloroquine (HCQ) | In combination with bortezomib and in R/R MM patients | Phase I/II (NCT00568880) |
| 3-Methyladenine (3-MA) | Human MM cell lines (U266, MM.1S, RPMI8226, and ARH 77) | Preclinical [93] |
| Bafilomycin A1 | In combination with bortezomib in U266 MM cell line | Preclinical [94] |
| Elaiophylin | Human MM cell lines (U266, RPMI8226, KMS11, and H929) | Preclinical [95] |
| Autophagy inducers | ||
| Metformin | Human MM cell lines (RPMI8226 and U266) and in vivo NOD-SCID murine xenograft MM model | Preclinical [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Odat, O.S.; Guirguis, D.A.; Schmalbach, N.K.; Yao, G.; Budak-Alpdogan, T.; Jonnalagadda, S.C.; Pandey, M.K. Autophagy and Apoptosis: Current Challenges of Treatment and Drug Resistance in Multiple Myeloma. Int. J. Mol. Sci. 2023, 24, 644. https://doi.org/10.3390/ijms24010644
Al-Odat OS, Guirguis DA, Schmalbach NK, Yao G, Budak-Alpdogan T, Jonnalagadda SC, Pandey MK. Autophagy and Apoptosis: Current Challenges of Treatment and Drug Resistance in Multiple Myeloma. International Journal of Molecular Sciences. 2023; 24(1):644. https://doi.org/10.3390/ijms24010644
Chicago/Turabian StyleAl-Odat, Omar S., Daniel A. Guirguis, Nicole K. Schmalbach, Gabriella Yao, Tulin Budak-Alpdogan, Subash C. Jonnalagadda, and Manoj K. Pandey. 2023. "Autophagy and Apoptosis: Current Challenges of Treatment and Drug Resistance in Multiple Myeloma" International Journal of Molecular Sciences 24, no. 1: 644. https://doi.org/10.3390/ijms24010644
APA StyleAl-Odat, O. S., Guirguis, D. A., Schmalbach, N. K., Yao, G., Budak-Alpdogan, T., Jonnalagadda, S. C., & Pandey, M. K. (2023). Autophagy and Apoptosis: Current Challenges of Treatment and Drug Resistance in Multiple Myeloma. International Journal of Molecular Sciences, 24(1), 644. https://doi.org/10.3390/ijms24010644







