Endoglin Is an Important Mediator in the Final Common Pathway of Chronic Kidney Disease to End-Stage Renal Disease
Abstract
1. Introduction
2. Results
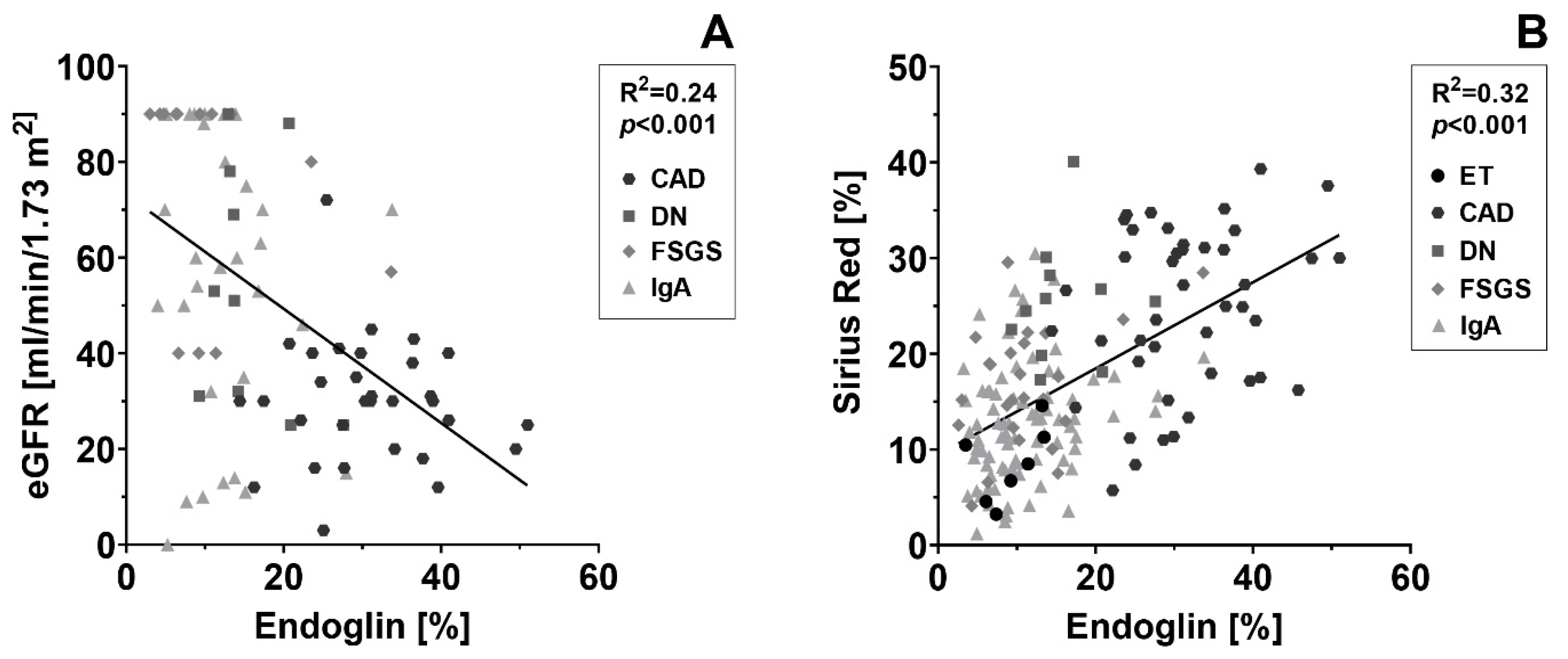
2.1. Endoglin Expression Is Increased in Various Chronic Kidney Diseases and Correlates with Renal Function and the Amount of Interstitial Fibrosis
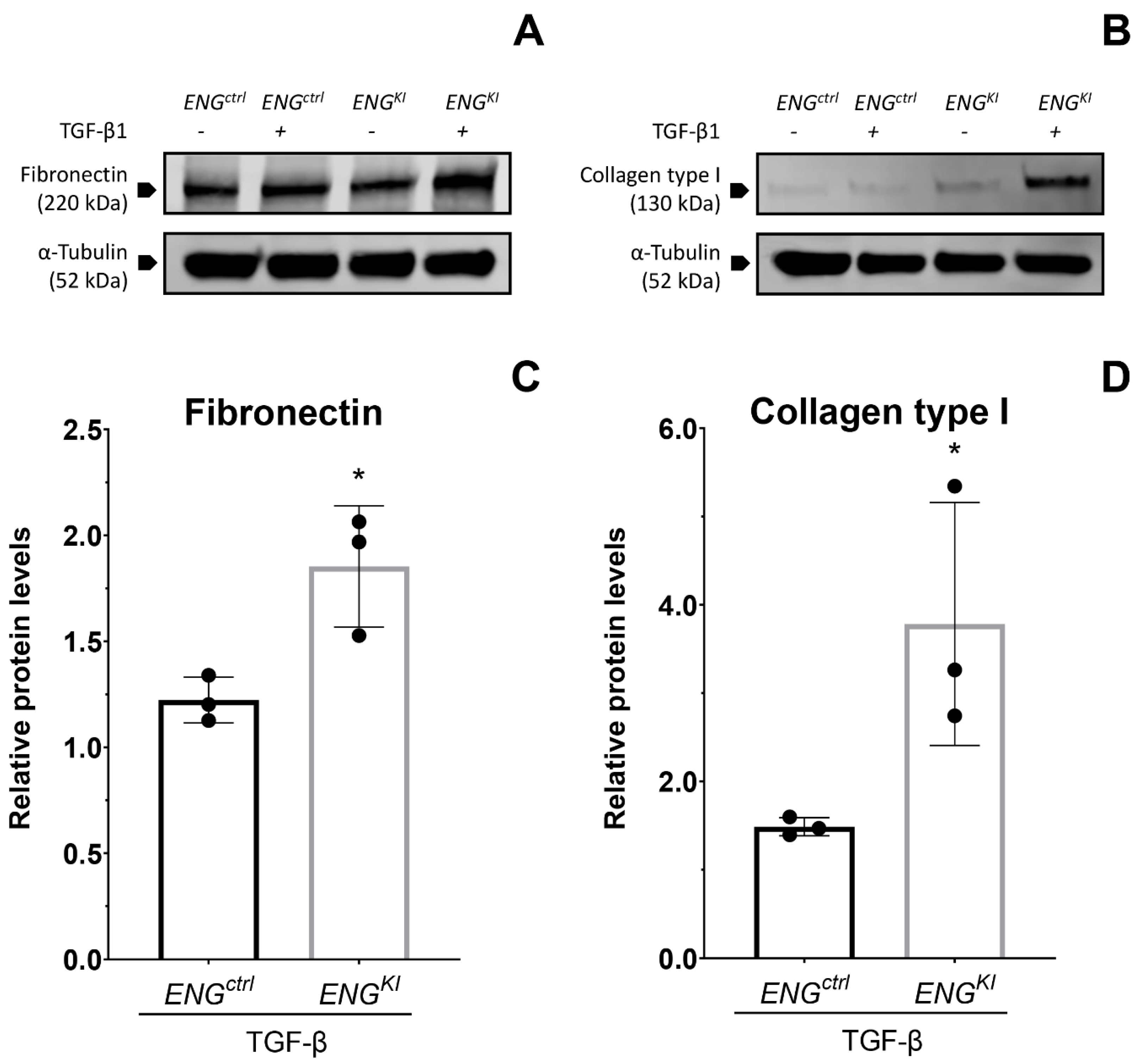
2.2. Endoglin Overexpression Increases the TGF-β-Induced Profibrotic Response in Human Kidney Fibroblast
3. Discussion
4. Materials and Methods
4.1. Patient Cohorts
4.2. Histology, Immunohistochemistry and Analysis of Sections
4.3. Cell Lines and Cell Culture
4.4. Lentiviral Transduction of TK173 Fibroblasts
4.5. Analysis of Transduction Efficiency
4.6. Analysis of TGF-β-Induced Changes in Gene and Protein Expression
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CKD | Chronic kidney disease |
| ESRD | End stage renal disease |
| DN | Diabetic nephropathy |
| FSGS | Focal segmental glomerular sclerosis |
| eGFR | Estimated glomerular filtration rate |
| ECM | Extra cellular matrix |
| COL1A1 | Collagen type I (gene ID) |
| FN1 | Fibronectin (gene ID) |
| αSMA | Alpha smooth muscle actin |
| ACTA2 | αSMA (gene ID) |
| TGF-β | Transforming growth factor-beta |
| TβR | TGF-β receptor |
| CCN2 | Connective tissue growth factor (gene ID) |
| SERPINE1 | Plasminogen activator inhibitor-1 (gene ID) |
| ENG | Endoglin (gene ID) |
| UUO | Unilateral ureter obstruction |
| CAD | Chronic allograft dysfunction |
| ET | Excluded for transplantation |
| mRNA | Messenger Ribonucleic acid |
| GFP | Green Fluorescent Protein (gene ID) |
| qPCR | Quantitative real-time PCR |
| LUMC | Leiden University Medical Centre |
| Tris | Tromethamine |
| EDTA | Ethylenediamine tetraacetic acid buffer |
| HPRT1 | Hypoxanthine phosphoribosyltransferase 1 (gene ID) |
| Ct | Cycle threshold |
| PBS | Phosphate-buffered saline |
| TBS | Tris-buffered saline |
| SDS | Sodium dodecyl sulfate |
| BSA | Bovine serum albumin |
References
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, F.A.; Al-Khader, A.A. Epidemiology and causes of end stage renal disease (ESRD). Saudi J. Kidney Dis. Transpl. 2005, 163, 277–281. [Google Scholar]
- Taherkhani, A.; Farrokhi Yekta, R.; Mohseni, M.; Saidijam, M.; Arefi Oskouie, A. Chronic kidney disease: A review of proteomic and metabolomic approaches to membranous glomerulonephritis, focal segmental glomerulosclerosis, and IgA nephropathy biomarkers. Proteome Sci. 2019, 17, 7. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Coresh, J. Chronic kidney disease. Lancet 2012, 379, 165–180. [Google Scholar] [CrossRef]
- Lopez-Hernandez, F.J.; Lopez-Novoa, J.M. Role of TGF-beta in chronic kidney disease: An integration of tubular, glomerular and vascular effects. Cell Tissue Res. 2012, 3471, 141–154. [Google Scholar] [CrossRef]
- Bulow, R.D.; Boor, P. Extracellular Matrix in Kidney Fibrosis: More Than Just a Scaffold. J. Histochem. Cytochem. 2019, 679, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Kuppe, C.; Ibrahim, M.M.; Kranz, J.; Zhang, X.; Ziegler, S.; Perales-Patón, J.; Jansen, J.; Reimer, K.C.; Smith, J.R.; Dobie, R.; et al. Decoding myofibroblast origins in human kidney fibrosis. Nature 2021, 589, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Meran, S.; Steadman, R. Fibroblasts and myofibroblasts in renal fibrosis. Int. J. Exp. Pathol. 2011, 923, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-beta: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 126, 325–338. [Google Scholar] [CrossRef]
- Ina, K.; Kitamura, H.; Tatsukawa, S.; Takayama, T.; Fujikura, Y.; Shimada, T. Transformation of interstitial fibroblasts and tubulointerstitial fibrosis in diabetic nephropathy. Med. Electron. Microsc. 2002, 352, 87–95. [Google Scholar] [CrossRef]
- Roberts, A.B.; Kim, S.J.; Noma, T.; Glick, A.B.; Lafyatis, R.; Lechleider, R.; Jakowlew, S.B.; Geiser, A.; O’Reilly, M.A.; Danielpour, D.; et al. Multiple forms of TGF-beta: Distinct promoters and differential expression. Ciba Found. Symp. 1991, 157, 7–15; discussion 15–28. [Google Scholar]
- Sureshbabu, A.; Muhsin, S.A.; Choi, M.E. TGF-beta signaling in the kidney: Profibrotic and protective effects. Am. J. Physiol. Renal. Physiol. 2016, 3107, F596–F606. [Google Scholar] [CrossRef]
- Lan, H.Y.; Chung, A.C. TGF-beta/Smad signaling in kidney disease. Semin. Nephrol. 2012, 323, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Gougos, A.; Letarte, M. Primary structure of endoglin, an RGD-containing glycoprotein of human endothelial cells. J. Biol. Chem. 1990, 26515, 8361–8364. [Google Scholar] [CrossRef]
- Barbara, N.P.; Wrana, J.L.; Letarte, M. Endoglin is an accessory protein that interacts with the signaling receptor complex of multiple members of the transforming growth factor-beta superfamily. J. Biol. Chem. 1999, 2742, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Cheifetz, S.; Bellón, T.; Calés, C.; Vera, S.; Bernabeu, C.; Massague, J.; Letarte, M. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J. Biol. Chem. 1992, 267, 19027–19030. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Y.; Sorensen, L.K.; Brooke, B.S.; Urness, L.D.; Davis, E.C.; Taylor, D.G.; Boak, B.B.; Wendel, D.P. Defective Angiogenesis in Mice Lacking Endoglin. Science 1999, 284, 1534–1537. [Google Scholar] [CrossRef]
- McAllister, K.A.; Baldwin, M.A.; Thukkani, A.K.; Gallione, C.J.; Berg, J.N.; Porteous, M.E.; Guttmacher, A.E.; Marchuk, D.A. Six novel mutations in the endoglin gene in hereditary hemorrhagic telangiectasia type 1 suggest a dominant-negative effect of receptor function. Hum. Mol. Genet. 1995, 4, 1983–1985. [Google Scholar] [CrossRef]
- Nassiri, F.; Cusimano, M.D.; Scheithauer, B.W.; Rotondo, F.; Fazio, A.; Yousef, G.M.; Syro, L.V.; Kovacs, K.; Lloyd, R.V. Endoglin (CD105): A review of its role in angiogenesis and tumor diagnosis, progression and therapy. Anticancer Res. 2011, 31, 2283–2290. [Google Scholar]
- Shovlin, C.L.; Hughes, J.M.; Scott, J.; Seidman, C.E.; Seidman, J.G. Characterization of endoglin and identification of novel mutations in hereditary hemorrhagic telangiectasia. Am. J. Hum. Genet. 1997, 611, 68–79. [Google Scholar] [CrossRef]
- Schoonderwoerd, M.J.A.; Goumans, M.T.H.; Hawinkels, L. Endoglin: Beyond the Endothelium. Biomolecules 2020, 10, 289. [Google Scholar] [CrossRef]
- Alsamman, M.; Sterzer, V.; Meurer, S.K.; Sahin, H.; Schaeper, U.; Kuscuoglu, D.; Strnad, P.; Weiskirchen, R.; Trautwein, C.; Scholten, D. Endoglin in human liver disease and murine models of liver fibrosis-A protective factor against liver fibrosis. Liver Int. 2018, 38, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Kapur, N.K.; Wilson, S.; Yunis, A.A.; Qiao, X.; Mackey, E.; Paruchuri, V.; Baker, C.; Aronovitz, M.J.; Karumanchi, S.A.; Letarte, M.; et al. Reduced endoglin activity limits cardiac fibrosis and improves survival in heart failure. Circulation 2012, 125, 2728–2738. [Google Scholar] [CrossRef] [PubMed]
- Leask, A.; Abraham, D.J.; Finlay, D.R.; Holmes, A.; Pennington, D.; Shi-Wen, X.; Chen, Y.; Venstrom, K.; Dou, X.; Ponticos, M.; et al. Dysregulation of transforming growth factor beta signaling in scleroderma: Overexpression of endoglin in cutaneous scleroderma fibroblasts. Arthritis Rheum. 2002, 46, 1857–1865. [Google Scholar] [CrossRef]
- Docherty, N.; López-Novoa, J.M.; Arevalo, M.; Düwel, A.; Rodriguez-Peña, A.; Pérez-Barriocanal, F.; Bernabeu, C.; Eleno, N. Endoglin regulates renal ischaemia–reperfusion injury. Nephrol. Dial. Transplant. 2006, 21, 2106–2119. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Peña, A.; Eleno, N.; Düwell, A.; Arévalo, M.; Pérez-Barriocanal, F.; Flores, O.; Docherty, N.; Bernabeu, C.; Letarte, M.; Lopez-Novoa, J.M. Endoglin Upregulation During Experimental Renal Interstitial Fibrosis in Mice. Hypertension 2002, 40, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Prieto, M.; Rodriguez-Pena, A.B.; Düwel, A.; Rivas, J.V.; Docherty, N.; Pérez-Barriocanal, F.; Arévalo, M.; Vary, C.P.H.; Bernabeu, C.; López-Novoa, J.M.; et al. Temporal changes in renal endoglin and TGF-beta1 expression following ureteral obstruction in rats. J. Physiol. Biochem. 2005, 61, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Oujo, B.; Muñoz-Félix, J.M.; Arevalo, M.; Nuñez-Gomez, E.; Perez-Roque, L.; Pericacho, M.; Gonzalez-Nunez, M.; Langa, C.; Martinez-Salgado, C.; Pérez-Barriocanal, F.; et al. L-Endoglin Overexpression Increases Renal Fibrosis after Unilateral Ureteral Obstruction. PLoS ONE 2014, 9, e110365. [Google Scholar] [CrossRef]
- Scharpfenecker, M.; Floot, B.; Russell, N.S.; Coppes, R.P.; Stewart, F.A. Endoglin haploinsufficiency attenuates radiation-induced deterioration of kidney function in mice. Radiother. Oncol. 2013, 108, 464–468. [Google Scholar] [CrossRef]
- Roy-Chaudhury, P.; Simpson, J.G.; Power, D.A. Endoglin, a transforming growth factor-beta-binding protein, is upregulated in chronic progressive renal disease. Exp. Nephrol. 1997, 5, 55–60. [Google Scholar]
- Gerrits, T.; Zandbergen, M.; Wolterbeek, R.; Bruijn, J.A.; Baelde, H.J.; Scharpfenecker, M. Endoglin Promotes Myofibroblast Differentiation and Extracellular Matrix Production in Diabetic Nephropathy. Int. J. Mol. Sci. 2020, 21, 7713. [Google Scholar] [CrossRef] [PubMed]
- Hinz, B.; Phan, S.H.; Thannickal, V.J.; Galli, A.; Bochaton-Piallat, M.L.; Gabbiani, G. The myofibroblast: One function, multiple origins. Am. J. Pathol. 2007, 1706, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Abreu, J.G.; Ketpura, N.I.; Reversade, B.; De Robertis, E.M. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat. Cell Biol. 2002, 48, 599–604. [Google Scholar] [CrossRef]
- Samarakoon, R.; Overstreet, J.M.; Higgins, S.P.; Higgins, P.J. TGF-beta1 --> SMAD/p53/USF2 --> PAI-1 transcriptional axis in ureteral obstruction-induced renal fibrosis. Cell Tissue Res. 2012, 3471, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Conley, B.A.; Smith, J.D.; Guerrero-Esteo, M.; Bernabeu, C.; Vary, C.P. Endoglin, a TGF-beta receptor-associated protein, is expressed by smooth muscle cells in human atherosclerotic plaques. Atherosclerosis 2000, 153, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.P.; Watson, R.W.; Mulsow, J.J.; Docherty, N.G.; Coffey, J.C.; O’Connell, P.R. Endoglin negatively regulates transforming growth factor beta1-induced profibrotic responses in intestinal fibroblasts. Br. J. Surg. 2010, 976, 892–901. [Google Scholar] [CrossRef]
- Scharpfenecker, M.; Floot, B.; Russell, N.S.; Stewart, F.A. The TGF-beta co-receptor endoglin regulates macrophage infiltration and cytokine production in the irradiated mouse kidney. Radiother. Oncol. 2012, 1053, 313–320. [Google Scholar] [CrossRef]
- Scharpfenecker, M.; Floot, B.; Russell, N.S.; Ten Dijke, P.; Stewart, F.A. Endoglin haploinsufficiency reduces radiation-induced fibrosis and telangiectasia formation in mouse kidneys. Radiother. Oncol. 2009, 923, 484–491. [Google Scholar] [CrossRef]
- Yamamoto, T.; Watanabe, T.; Ikegaya, N.; Fujigaki, Y.; Matsui, K.; Masaoka, H.; Nagase, M.; Hishida, A. Expression of types I, II, and III TGF-beta receptors in human glomerulonephritis. J. Am. Soc. Nephrol. 1998, 9, 2253–2261. [Google Scholar] [CrossRef]
- Shihab, F.S.; Yamamoto, T.; Nast, C.C.; Cohen, A.H.; Noble, N.A.; Gold, L.I.; Border, W.A. Transforming growth factor-beta and matrix protein expression in acute and chronic rejection of human renal allografts. J. Am. Soc. Nephrol. 1995, 6, 286–294. [Google Scholar] [CrossRef]
- Djudjaj, S.; Boor, P. Cellular and molecular mechanisms of kidney fibrosis. Mol. Asp. Med. 2019, 65, 16–36. [Google Scholar] [CrossRef] [PubMed]
- Nangaku, M. Chronic hypoxia and tubulointerstitial injury: A final common pathway to end-stage renal failure. J. Am. Soc. Nephrol. 2006, 17, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Bechtel, W.; McGoohan, S.; Zeisberg, E.M.; Müller, G.A.; Kalbacher, H.; Salant, D.J.; Müller, C.A.; Kalluri, R.; Zeisberg, M. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat. Med. 2010, 16, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Herrera, J.; Henke, C.A.; Bitterman, P.B. Extracellular matrix as a driver of progressive fibrosis. J. Clin. Investig. 2018, 128, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Seon, B.K.; Haba, A.; Matsuno, F.; Takahashi, N.; Tsujie, M.; She, X.; Harada, N.; Uneda, S.; Tsujie, T.; Toi, H.; et al. Endoglin-targeted cancer therapy. Curr. Drug Deliv. 2011, 8, 135–143. [Google Scholar] [CrossRef]
- Rosen, L.S.; Hurwitz, H.I.; Wong, M.K.; Goldman, J.; Mendelson, D.S.; Figg, W.D.; Spencer, S.; Adams, B.J.; Alvarez, D.; Seon, B.K.; et al. A Phase I First-in-Human Study of TRC105 (Anti-Endoglin Antibody) in Patients with Advanced Cancer. Clin. Cancer Res. 2012, 18, 4820–4829. [Google Scholar] [CrossRef]
- Liu, Y.; Paauwe, M.; Nixon, A.B.; Hawinkels, L.J. Endoglin Targeting: Lessons Learned and Questions That Remain. Int. J. Mol. Sci. 2020, 22, 147. [Google Scholar] [CrossRef]
- Paauwe, M.; Schoonderwoerd, M.J.; Helderman, R.F.; Harryvan, T.J.; Groenewoud, A.; van Pelt, G.W.; Bor, R.; Hemmer, D.M.; Versteeg, H.H.; Snaar-Jagalska, B.E.; et al. Endoglin Expression on Cancer-Associated Fibroblasts Regulates Invasion and Stimulates Colorectal Cancer Metastasis. Clin. Cancer Res. 2018, 24, 6331–6344. [Google Scholar] [CrossRef]
- Huang, Q.; Xiao, R.; Lu, J.; Zhang, Y.; Xu, L.; Gao, J.; Sun, J.; Wang, H. Endoglin aggravates peritoneal fibrosis by regulating the activation of TGF-beta/ALK/Smads signaling. Front. Pharmacol. 2022, 13, 973182. [Google Scholar] [CrossRef]
- Muller, G.A.; Frank, J.; Rodemann, H.P.; Engler-Blum, G. Human renal fibroblast cell lines (tFKIF and tNKF) are new tools to investigate pathophysiologic mechanisms of renal interstitial fibrosis. Exp. Nephrol. 1995, 3, 127–133. [Google Scholar]
- Lou, E.; Fujisawa, S.; Morozov, A.; Barlas, A.; Romin, Y.; Dogan, Y.; Gholami, S.; Moreira, A.L.; Manova-Todorova, K.; Moore, M.A.S. Tunneling Nanotubes Provide a Unique Conduit for Intercellular Transfer of Cellular Contents in Human Malignant Pleural Mesothelioma. PLoS ONE 2012, 7, e33093. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, S.S.; Sauer, M.G. Molecular cloning using polymerase chain reaction, an educational guide for cellular engineering. J. Biol. Eng. 2015, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.A.; Dykxhoorn, D.M.; Palliser, D.; Mizuno, H.; Yu, E.Y.; An, D.S.; Sabatini, D.M.; Chen, I.S.; Hahn, W.C.; Sharp, P.A.; et al. Lentivirus-delivered stable gene silencing by RNAi in primary cells. RNA 2003, 9, 493–501. [Google Scholar] [CrossRef]
- Dull, T.; Zufferey, R.; Kelly, M.; Mandel, R.J.; Nguyen, M.; Trono, D.; Naldini, L. A third-generation lentivirus vector with a conditional packaging system. J. Virol. 1998, 72, 8463–8471. [Google Scholar] [CrossRef] [PubMed]
- Sokal, R.R.; Rohlf, J.F. Biometry: The Principles and Practice of Statistics in Biological Research, 2nd ed.; W. H. Freeman and Company: San Francisco, CA, USA, 1981; 859p. [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 7th ed.; The Iowa State University Press: Ames, IA, USA, 1980. [Google Scholar]



| Characteristics | (n = 170) | CKD (n = 163) | ET (n = 7) | |
|---|---|---|---|---|
| Male/Female sex | n/n (%/%) | 96/67 (58.8/41.2) | 3/4 (42.9/57.1) | |
| CAD (n = 43) | 22/21 (51.2/48.8) | |||
| DN (n = 11) | 9/2 (81.8/18.2) | |||
| FSGS (n = 25) | 10/15 (40.0/60.0) | |||
| IgA (n = 84) | 59/25 (70.2/29.8) | |||
| Age (Years) | Mean ± SD | 42.8 ± 20.3 | 54.8 ± 23.6 | |
| CAD | 50.1 ± 13.1 | |||
| DN | 57.7 ± 12.1 | |||
| FSGS | 33.7 ± 22.1 | |||
| IgA | 39.9 ± 21.6 | |||
| eGFR (mL/min/1.73 m2) | Mean ± SD | 50.4 ± 28.4 | N.A. | |
| CAD | 30.0 ± 12.9 | |||
| DN | 54.2 ± 25.7 | |||
| FSGS | 75.2 ± 22.0 | |||
| IgA | 58.4 ± 30.4 | |||
| Years after kidney transplantation | CAD | Mean ± SD | 6.1 ± 4.9 | |
| Type 1 Diabetes | DN | n (%) | 3 (27.3) | |
| Primer Set | Forward | Reverse |
|---|---|---|
| ACTA2 | 5′-TTCAATGTCCCAGCCATGTA-3′ | 5′-GAAGGAATAGCCACGCTCAG-3′ |
| COL1A1 | 5′-GTGCTAAAGGTGCCAATGGT-3′ | 5′-CTCCTCGCTTTCCTTCCTCT-3′ |
| CCN2 | 5′-CCTGGTCCAGACCACAGAGT-3′ | 5′-TGGAGATTTTGGGAGTACGG-3′ |
| ENG | 5′-CACTAGCCAGGTCTCGAAGG-3′ | 5′-CTGAGGACCAGAAGCACCTC-3′ |
| FN1 | 5′-ACCAACCTACGGATGACTCG-3′ | 5′-GCTCATCATCTGGCCATTTT-3′ |
| GFP | 5′-CCCGACACCCACTACCTGAG-3′ | 5′-GTCCATGCCGAGAGTGATCC-3′ |
| HPRT1 | 5′-AGATGGTCAAGGTCGCAAGC-3′ | 5′-TCAAGGGCATATCCTACAACAAAC-3′ |
| SERPINE1 | 5′-ACTGGAAAGGCAACATGACC-3′ | 5′-TGACAGCTGTGGATGAGGAG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerrits, T.; Brouwer, I.J.; Dijkstra, K.L.; Wolterbeek, R.; Bruijn, J.A.; Scharpfenecker, M.; Baelde, H.J. Endoglin Is an Important Mediator in the Final Common Pathway of Chronic Kidney Disease to End-Stage Renal Disease. Int. J. Mol. Sci. 2023, 24, 646. https://doi.org/10.3390/ijms24010646
Gerrits T, Brouwer IJ, Dijkstra KL, Wolterbeek R, Bruijn JA, Scharpfenecker M, Baelde HJ. Endoglin Is an Important Mediator in the Final Common Pathway of Chronic Kidney Disease to End-Stage Renal Disease. International Journal of Molecular Sciences. 2023; 24(1):646. https://doi.org/10.3390/ijms24010646
Chicago/Turabian StyleGerrits, Tessa, Isabella J. Brouwer, Kyra L. Dijkstra, Ron Wolterbeek, Jan A. Bruijn, Marion Scharpfenecker, and Hans J. Baelde. 2023. "Endoglin Is an Important Mediator in the Final Common Pathway of Chronic Kidney Disease to End-Stage Renal Disease" International Journal of Molecular Sciences 24, no. 1: 646. https://doi.org/10.3390/ijms24010646
APA StyleGerrits, T., Brouwer, I. J., Dijkstra, K. L., Wolterbeek, R., Bruijn, J. A., Scharpfenecker, M., & Baelde, H. J. (2023). Endoglin Is an Important Mediator in the Final Common Pathway of Chronic Kidney Disease to End-Stage Renal Disease. International Journal of Molecular Sciences, 24(1), 646. https://doi.org/10.3390/ijms24010646






