Identification and Pharmacological Characterization of Two Serotonin Type 7 Receptor Isoforms from Mythimna separata
Abstract
1. Introduction
2. Results
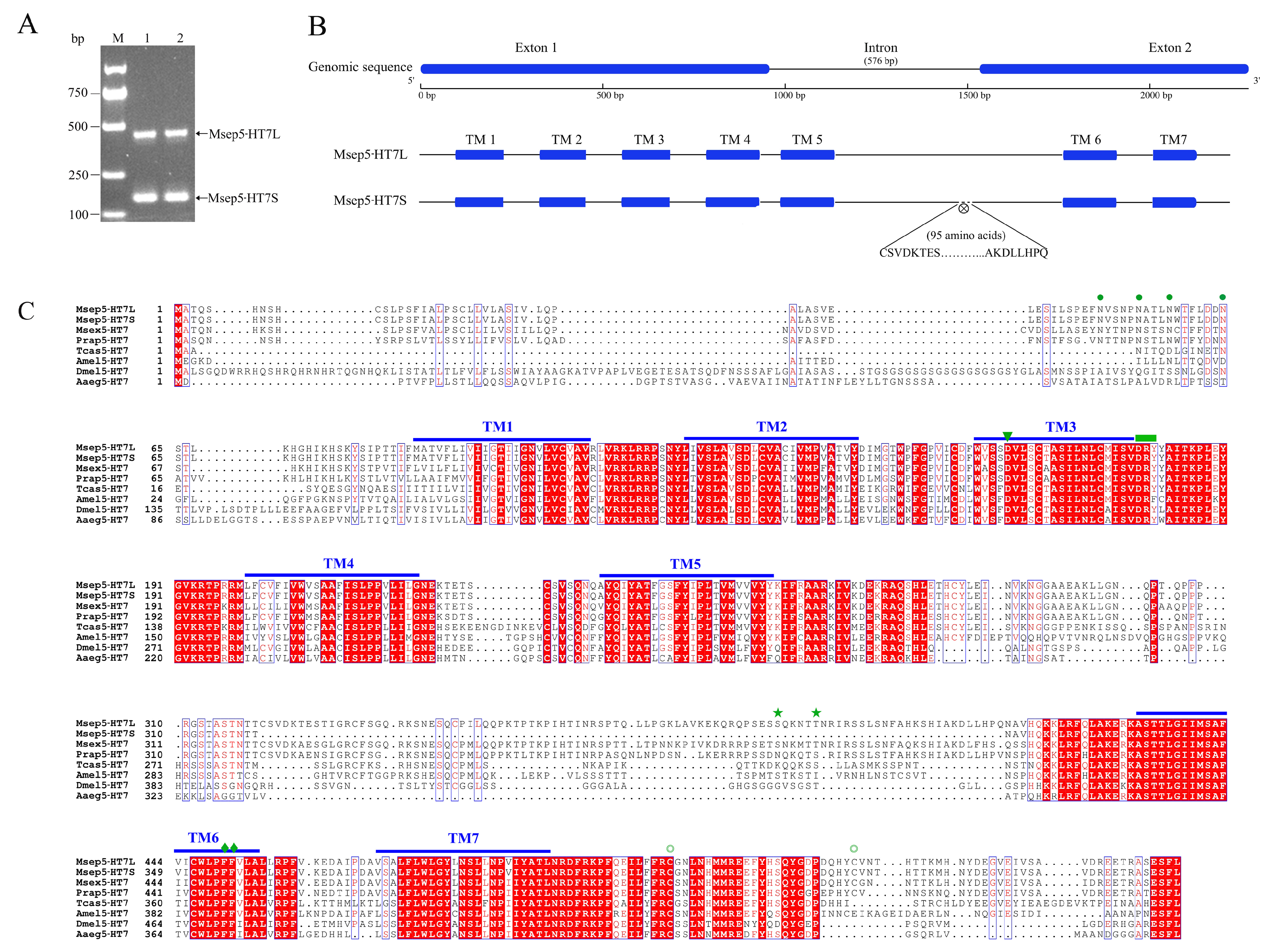
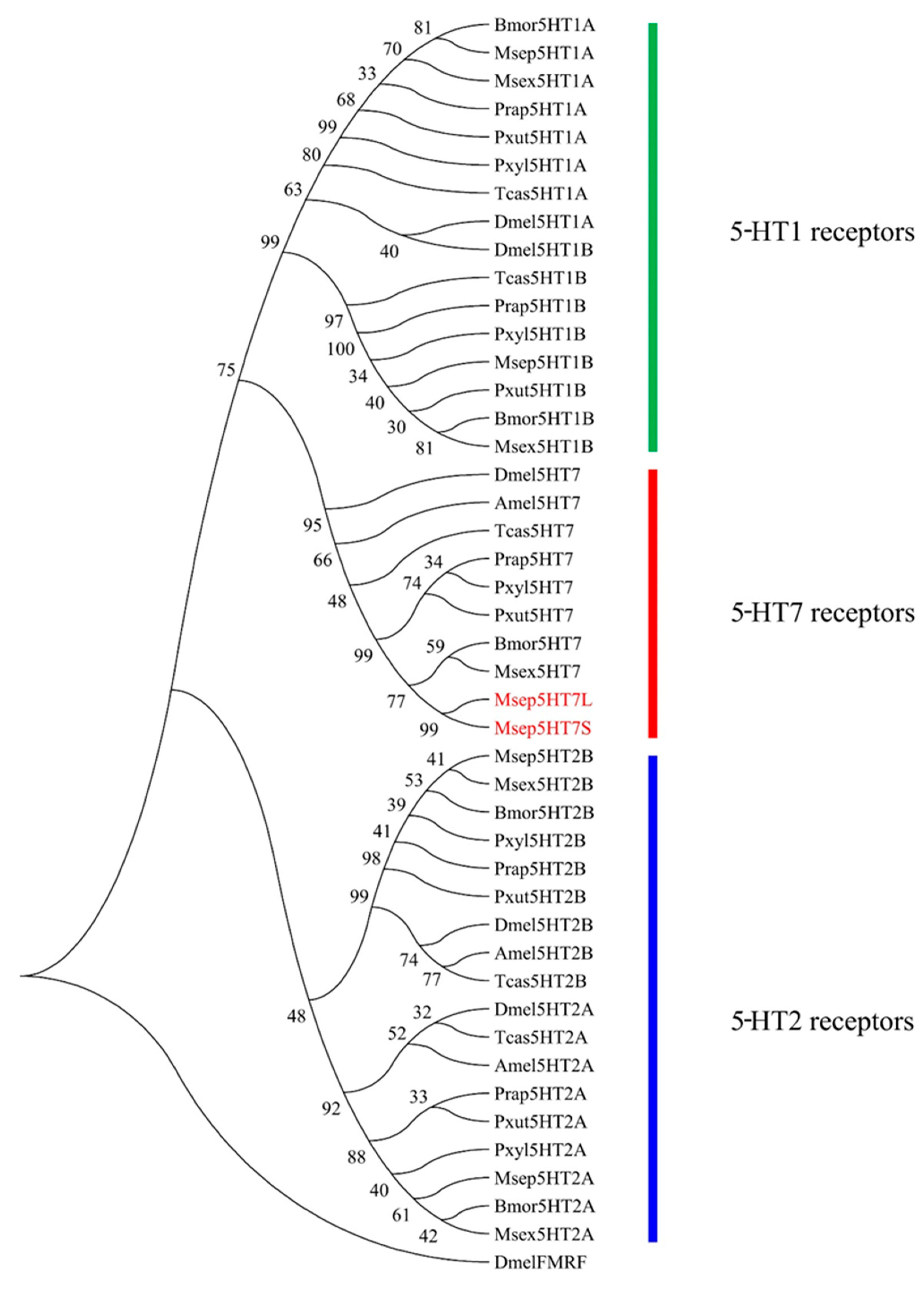
2.1. Molecular Features and Phylogenetic Analysis
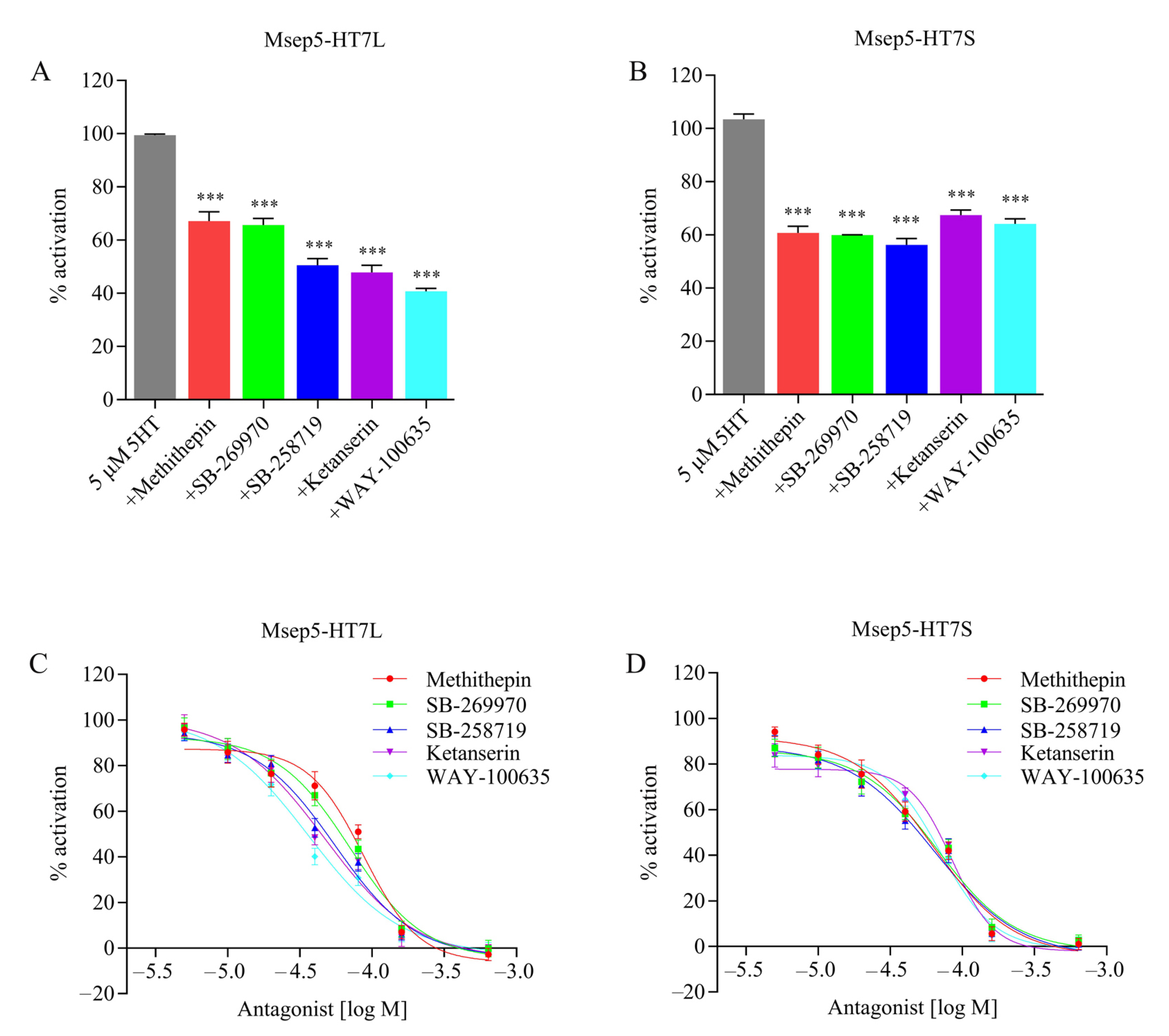
2.2. Pharmacological Characterization of Two Msep5-HT7 Receptor Isoforms
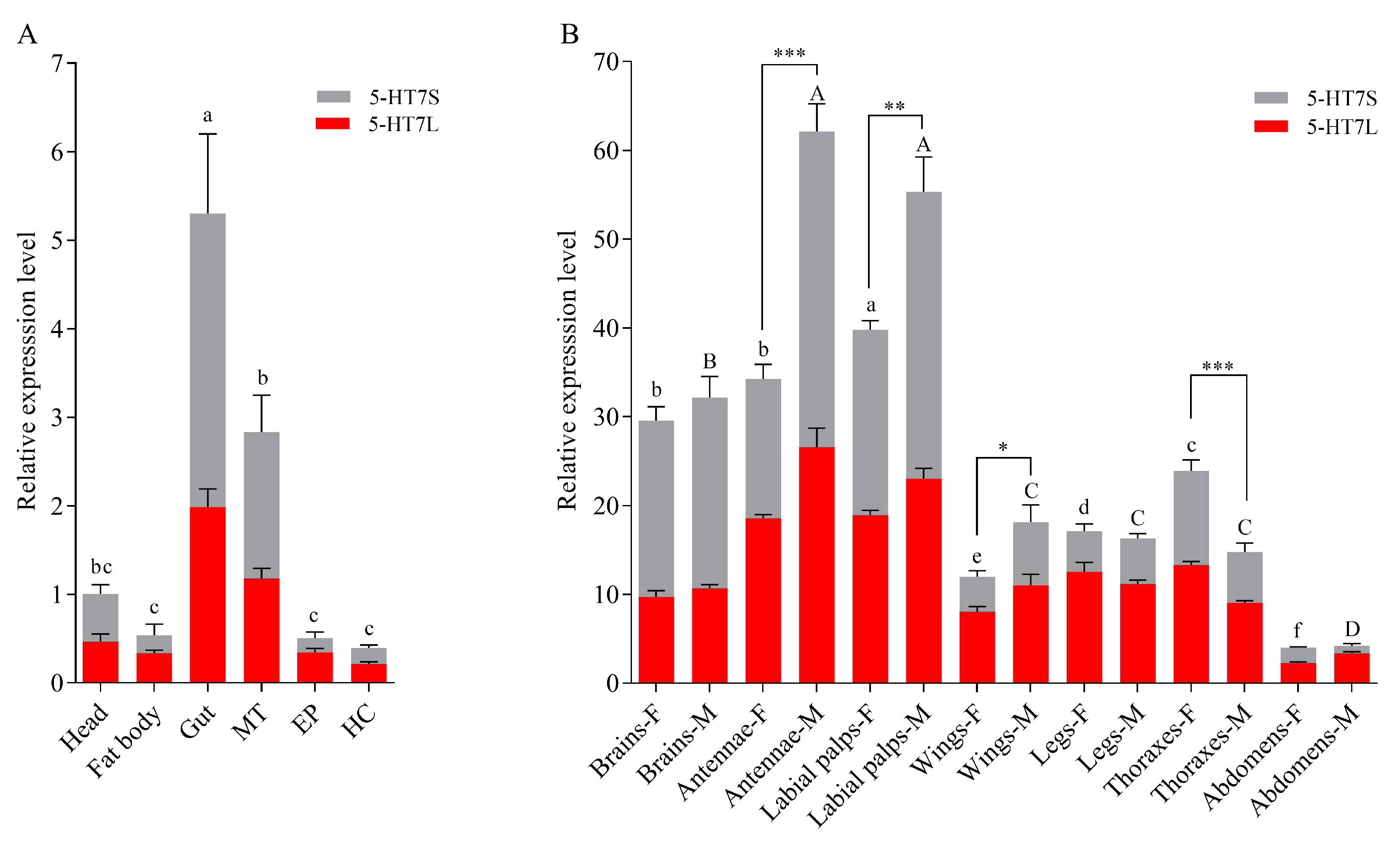
2.3. Expression Profile Analysis of Msep5-HT7 Genes
3. Discussion
4. Materials and Methods
4.1. Insects Rearing, Tissue Collection and Reagents
4.2. Cloning of M. separata 5-HT7 Receptor Gene
4.3. Multiple Sequence and Phylogenetic Analysis
4.4. Construction of Expression Plasmids
4.5. Cell Culture, Transfection, and Creation of Stable Cell Lines
4.6. cAMP Assays
4.7. Expression Profiles Analysis by qPCR
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT | serotonin |
| OA | octopamine |
| TA | tyramine |
| DA | dopamine |
| 8-OH-DPAT | 8-Hydroxy-DPAT |
| 5-MT | 5-methoxytryptamine |
| αm-5-HT | α-methylserotonin |
| TM | transmembrane |
| GPCRs | G protein-coupled receptors |
| HEK 293 | Human Embryonic Kidney 293 |
| cAMP | cyclic adenosine monophosphate |
| Ac | adenylyl cyclase |
| IBMX | 3-isobutyl-1-methylxanthine |
| PBS | phosphate buffered solution |
| DPBS | Dulbecco’s phosphate buffered solution |
| FBS | fetal bovine serum |
| D-MEM | Dulbecco’s Modified Eagle Medium |
| RT-PCR | reverse transcription-polymerase chain reaction |
| qPCR | quantitative real-time polymerase chain reaction |
| NCBI | National Center for Biotechnology Information |
References
- Nichols, D.E.; Nichols, C.D. Serotonin receptors. Chem. Rev. 2008, 108, 1614–1641. [Google Scholar] [CrossRef] [PubMed]
- Gellynck, E.; Heyninck, K.; Andressen, K.W.; Haegeman, G.; Levy, F.O.; Vanhoenacker, P.; Craenenbroeck, K.V. The serotonin 5-HT7 receptors: Two decades of research. Exp. Brain Res. 2013, 230, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Vleugels, R.; Velinden, H.; Broeck, J.V. Serotonin, serotonin receptors and their actions in insects. Neurotransimitter 2015, 2, e314. [Google Scholar]
- Qi, Y.X.; Jin, M.; Ni, X.Y.; Ye, G.Y.; Lee, Y.; Huang, J. Characterization of three serotonin receptors from the small white butterfly, Pieris rapae. Insect Biochem. Mol. 2017, 87, 107–116. [Google Scholar] [CrossRef]
- Saifullah, A.S.M.; Tomioka, K. Serotonin sets the day state in the neurons that control coupling between the optic lobe circadian pacemakers in the cricket Gryllus Bimaculatus. J. Exp. Biol. 2002, 205, 1305–1314. [Google Scholar] [CrossRef]
- Yuan, Q.; Lin, F.; Zheng, X.; Sehgal, A. Serotonin modulates circadian entrainment in Drosophila. Neuron 2005, 47, 115–127. [Google Scholar] [CrossRef]
- Yuan, Q.; Joiner, W.J.; Sehgal, A. A sleep-promoting role for the Drosophila serotonin receptor 1A. Curr. Biol. 2006, 16, 1051–1062. [Google Scholar] [CrossRef]
- Dierick, H.A.; Greenspan, R.J. Serotonin and neuropeptide F have opposite modulatory effects on fly aggression. Nat. Genet. 2007, 39, 678–682. [Google Scholar] [CrossRef]
- Sitaraman, D.; Zars, M.; Laferriere, H.; Chen, Y.C.; Sable-Smith, A.; Kitamoto, T.; Rottinghaus, G.E.; Zars, T. Serotonin is necessary for place memory in Drosophila. Proc. Natl. Acad. Sci. USA 2008, 105, 5579–5584. [Google Scholar] [CrossRef]
- Sitaraman, D.; Laferriere, H.; Birman, S.; Zars, T. Serotonin is critical for rewarded olfactory short-term memory in Drosophila. J. Neurogenet. 2012, 26, 238–244. [Google Scholar] [CrossRef]
- Wright, G.A. The role of dopamine and serotonin in conditioned food aversion learning in the honeybee. Commun. Integr. Biol. 2011, 4, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Gasque, G.; Conway, S.; Huang, J.; Rao, Y.; Vosshall, L.B. Small molecule drug screening in Drosophila identifies the 5HT2A receptor as a feeding modulation target. Sci. Rep. 2013, 3, 2120. [Google Scholar] [CrossRef] [PubMed]
- French, A.S.; Simcock, K.L.; Rolke, D.; Gartside, S.E.; Blenau, W.; Wright, G.A. The role of serotonin in feeding and gut contractions in the honeybee. J. Insect Physiol. 2014, 61, 8–15. [Google Scholar] [CrossRef]
- Kloppenburg, P.; Mercer, A.R. Serotonin modulation of moth central olfactory neurons. Annu. Rev. Entomol. 2008, 53, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gaudry, Q. Functional integration of a serotonergic neuron in the Drosophila antennal lobe. eLife 2016, 5, e16836. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, A.; Cheng, Q.; Bajaj, J.; Lee, D. Serotonin receptor 5-HT7 in Drosophila mushroom body neurons mediates larval appetitive olfactory learning. Sci. Rep. 2020, 10, 21267. [Google Scholar] [CrossRef] [PubMed]
- Vleugels, R.; Lenaerts, C.; Baumann, A.; Vanden Broeck, J.; Verlinden, H. Pharmacological characterization of a 5-HT1-type serotonin receptor in the red flour beetle, Tribolium castaneum. PLoS ONE 2013, 8, e65052. [Google Scholar] [CrossRef]
- Tierney, A.J. Invertebrate serotonin receptors: A molecular perspective on classification and pharmacology. J. Exp. Biol. 2018, 221, 184838. [Google Scholar] [CrossRef]
- Thamm, M.; Rolke, D.; Jordan, N.; Balfanz, S.; Schiffer, C.; Baumann, A.; Blenau, W. Function and distribution of 5-HT2 receptors in the honeybee (Apis mellifera). PLoS ONE 2013, 8, e82407. [Google Scholar] [CrossRef]
- Qi, Y.X.; Xia, R.Y.; Wu, Y.S.; Stanley, D.; Huang, J.; Ye, G.Y. Larvae of the small white butterfly, Pieris rapae, express a novel serotonin receptor. J. Neurochem. 2014, 131, 767–777. [Google Scholar] [CrossRef]
- Tierney, A.J. Structure and function of invertebrate 5-HT receptors: A review. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 128, 791–804. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.Y.; Zhong, L.; Feng, F.; Huang, Q.C.; Shao, X.S.; Song, G.H. Design and synthesis of novel insecticides based on the serotonergic ligand 1-[(4-aminophenyl) ethyl]-4-[3-(trifluoromethyl) phenyl] piperazine (PAPP). J. Agric. Food Chem. 2010, 58, 2624–2629. [Google Scholar] [CrossRef] [PubMed]
- Matthys, A.; Haegeman, G.; Van Craenenbroeck, K.; Vanhoenacker, P. Role of the 5-HT7 receptor in the central nervous system: From current status to future perspectives. Mol. Neurobiol. 2011, 43, 228–253. [Google Scholar] [CrossRef] [PubMed]
- Gellynck, E.; Laenen, K.; Andressen, K.W.; Lintermans, B.; Martelaere, K.D.; Matthys, A.; Levy, F.O.; Haegeman, G.; Vanhoenacker, P.; Craenenbroeck, K.V. Cloning, genomic organization and functionality of 5-HT7 receptor splice variants from mouse brain. Gene 2008, 426, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Leopoldo, M.; Lacivita, E.; Berardi, F.; Perrone, R.; Hedlund, P.B. Serotonin 5-HT7 receptor agents: Structure-activity relationships and potential therapeutic applications in central nervous system disorders. Pharmacol. Therapeut. 2011, 129, 120–148. [Google Scholar] [CrossRef]
- Becnel, J.; Johnson, O.; Luo, J.N.; Nassel, D.R.; Nichols, C.D. The serotonin 5-HT7Dro receptor is expressed in the brain of Drosophila, and is essential for normal courtship and mating. PLoS ONE 2011, 6, e20800. [Google Scholar] [CrossRef]
- Schlenstedt, J.; Balfanz, S.; Baumann, A.; Blenau, W. Am5-HT7: Molecular and pharmacological characterization of the first serotonin receptor of the honeybee (Apis mellifera). J. Neurochem. 2006, 98, 1985–1998. [Google Scholar] [CrossRef]
- Röser, C.; Jordan, N.; Balfanz, S.; Baumann, A.; Walz, B.; Baumann, O.; Blenau, W. Molecular and pharmacological characterization of serotonin 5-HT2α and 5-HT7 receptors in the salivary glands of the blowfly Calliphora vicina. PLoS ONE 2012, 7, e49459. [Google Scholar] [CrossRef]
- Wu, S.X. Pesticide market and target control in China. Pesticides 2000, 39, 7–10. [Google Scholar]
- Heidmann, D.E.; Metcalf, M.A.; Kohen, R.; Hamblin, M.W. Four 5-hydroxytryptamine7 (5-HT7) receptor isoforms in human and rat produced by alternative splicing: Species differences due to altered intron-exon organization. J. Neurochem. 1997, 68, 1372–1381. [Google Scholar] [CrossRef]
- Heidmann, D.E.; Szot, P.; Kohen, R.; Hamblin, M.W. Function and distribution of three rat 5-hydroxytryptamine7 (5-HT7) receptor isoforms produced by alternative splicing. Neuropharmacology 1998, 37, 1621–1632. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Irving, H.R.; Coupar, I.M. Expression patterns of 5-HT7 receptor isoforms in the rat digestive tract. Life Sci. 2001, 69, 2467–2475. [Google Scholar] [CrossRef] [PubMed]
- Witz, P.; Amlaiky, N.; Plassat, J.L.; Maroteaux, L.; Borrelli, E.; Hen, R. Cloning and characterization of a Drosophila serotonin receptor that activates adenylate cyclase. Proc. Natl. Acad. Sci. USA 1990, 87, 8940–8944. [Google Scholar] [CrossRef] [PubMed]
- Pietrantonio, P.V.; Jagge, C.; McDowell, C. Cloning and expression analysis of a 5HT7-like serotonin receptor cDNA from mosquito Aedes aegypti female excretory and respiratory systems. Insect Mol. Biol. 2001, 10, 357–369. [Google Scholar] [CrossRef]
- Dacks, A.M.; Reale, V.; Pi, Y.L.; Zhang, W.J.; Dacks, J.B.; Nighorn, A.J.; Evans, P.D. A characterization of the Manduca sexta serotonin receptors in the context of olfactory neuromodulation. PLoS ONE 2013, 8, e69422. [Google Scholar] [CrossRef]
- Vleugels, R.; Lenaerts, C.; Vanden, B.J.; Verlinden, H. Signalling properties and pharmacology of a 5-HT7 -type serotonin receptor from Tribolium castaneum. Insect Mol. Biol. 2014, 23, 230–243. [Google Scholar] [CrossRef]
- Liu, T.; Zhan, X.; Yu, Y.; Wang, S.; Lu, C.; Lin, G.; Zhu, X.; He, W.; You, M.; You, S. Molecular and pharmacological characterization of biogenic amine receptors from the diamondback moth, Plutella xylostella. Pest Manag. Sci. 2021, 77, 4462–4475. [Google Scholar] [CrossRef]
- Blenau, W.; Baumann, A. Molecular and pharmacological properties of insect biogenic amine receptors: Lessons from Drosophila melanogaster and Apis mellifera. Arch. Insect Biochem. 2001, 48, 13–38. [Google Scholar] [CrossRef]
- Thamm, M.; Balfanz, S.; Scheiner, R.; Baumann, A.; Blenau, W. Characterization of the 5-HT1A receptor of the honeybee (Apis mellifera) and involvement of serotonin in phototactic behavior. Cell. Mol. Life Sci. 2010, 67, 2467–2479. [Google Scholar] [CrossRef]
- Ferguson, S. Evolving concepts in G protein-coupled receptor endocytosis: The role in receptor desensitization and signaling. Pharmacol. Rev. 2001, 53, 1–24. [Google Scholar]
- Fritze, O.; Filipek, S.; Kuksa, V.; Palczewski, K.; Hofmann, K.P.; Ernst, O.P. Role of the conserved NPxxY(x)5,6F motif in the rhodopsin ground state and during activation. Proc. Natl. Acad. Sci. USA 2003, 100, 2290–2295. [Google Scholar] [CrossRef] [PubMed]
- Rovati, G.E.; Capra, V.; Neubig, R.R. The highly conserved DRY motif of class A G protein-coupled receptors: Beyond the ground state. Mol. Pharmacol. 2007, 71, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Pietrantonio, P.V. In vitro expression and pharmacology of the 5-HT7-like receptor present in the mosquito Aedes aegypti tracheolar cells and hindgut-associated nerves. Insect Mol. Biol. 2003, 12, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, C.C.; Van Heerikhuizen, H. Functional characteristics of heterologously expressed 5-HT receptors. Eur. J. Pharmacol. 1997, 334, 1–23. [Google Scholar] [CrossRef]
- Colas, J.F.; Launay, J.M.; Kellermann, O.; Rosay, P.; Maroteaux, L. Drosophila 5-HT2 serotonin receptor: Coexpression with fushi-tarazu during segmentation. Proc. Natl. Acad. Sci. USA 1995, 92, 5441–5445. [Google Scholar] [CrossRef]
- Hobson, R.J.; Geng, J.; Gray, A.D.; Komuniecki, R.W. SER-7b, a constitutively active Galphas coupled 5-HT7-like receptor expressed in the Caenorhabditis elegans M4 pharyngeal motorneuron. J. Neurochem. 2003, 87, 22–29. [Google Scholar] [CrossRef]
- Troppmann, B.; Balfanz, S.; Baumann, A.; Blenau, W. Inverse agonist and neutral antagonist actions of synthetic compounds at an insect 5-HT1 receptor. Brit. J. Pharmacol. 2010, 159, 1450–1462. [Google Scholar] [CrossRef]
- Lovell, P.J.; Bromidge, S.M.; Dabbs, S.; Duckworth, D.M.; Forbes, I.T.; Jennings, A.J.; King, F.D.; Middlemiss, D.N.; Rahman, S.K.; Saunders, D.V.; et al. A novel, potent, and selective 5-HT7 antagonist: (R)-3- (2- (2- (4-methylpiperidin-1-yl) ethyl) pyrrolidine-1-sulfonyl) phenol (SB-269970). J. Med. Chem. 2000, 43, 342–345. [Google Scholar] [CrossRef]
- Thomas, D.R.; Gittins, S.A.; Collin, L.L.; Middlemiss, D.N.; Riley, G.; Hagan, J.; Gloger, I.; Ellis, C.E.; Forbes, I.T.; Brown, A.M. Functional characterisation of the human cloned 5-HT7 receptor (long form); antagonist profile of SB-258719. Br. J. Pharmacol. 1998, 124, 1300–1306. [Google Scholar] [CrossRef]
- Thomas, D.R.; Atkinson, P.J.; Ho, M.; Bromidge, S.M.; Lovell, P.J.; Villani, A.J.; Hagan, J.J.; Middlemiss, D.N.; Price, G.W. [3H]-SB-269970-A selective antagonist radioligand for 5-HT7 receptors. Br. J. Pharmacol. 2000, 130, 409–417. [Google Scholar] [CrossRef]
- Watanabe, T.; Sadamoto, H.; Aonuma, H. Identification and expression analysis of the genes involved in serotonin biosynthesis and transduction in the field cricket Gryllus bimaculatus. Insect Mol. Biol. 2011, 20, 619–635. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Xi, G.S.; Wang, G.R. Molecular cloning and expression analysis of 5-hydroxytryptamine receptor 7 in ant Polyrhachis vicina Roger (Hymenoptera: Formicidae). J. Insect Sci. 2019, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Dacks, A.M.; Christensen, T.A.; Hildebrand, J.G. Modulation of olfactory information processing in the antennal lobe of Manduca sexta by serotonin. J. Neurophysiol. 2008, 99, 2077–2085. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Davies, J. The Oriental Armyworm, Mythimna separata (Wlk.). Distribution, Biology and Control: A Literature Review; Miscellaneous Reports; Centre for Overseas Pest Research: London, UK, 1983; Volume 59, pp. 1–24. [Google Scholar]
- Jiang, X.; Luo, L.; Zhang, L.; Sappington, T.W.; Hu, Y. Regulation of migration in Mythimna separata (Walker) in China: A review integrating environmental, physiological, hormonal, genetic, and molecular factors. Environ. Entomol. 2011, 40, 516–533. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, L.; Cheng, Y.; Luo, L. Current status and trends in research on the oriental armyworm, Mythimna separata (Walker) in China. Chin. J. Appl. Entomol. 2014, 51, 881–889. [Google Scholar]
- Liu, Y.; Qi, M.; Chi, Y.; Wuriyanghan, H. De novo assembly of the transcriptome for oriental armyworm Mythimna separata (Lepidoptera: Noctuidae) and analysis on insecticide resistance-related genes. J. Insect Sci. 2016, 16, 92. [Google Scholar] [CrossRef][Green Version]
- Lu, Z.Y.; Ran, H.F.; Liu, W.X.; Qu, Z.G.; Li, J.C. Mass rearing methods of Mythimna separata (Walker) and its parasitoid, Microplitis tuberculifer (Wesmael). J. Environ. Entomol. 2013, 35, 683–687. [Google Scholar]
- Chen, W.B.; Du, L.X.; Gao, X.Y.; Sun, L.L.; Chen, L.L.; Xie, G.Y.; An, S.H.; Zhao, X.C. Identification of odorant-binding and chemosensory protein genes in Mythimna separata adult brains using transcriptome analyses. Front. Physiol. 2022, 13, 839559. [Google Scholar] [CrossRef]
- Gouet, P.; Courcelle, E.; Stuart, D.I.; Métoz, F. ESPript: Analysis of multiple sequence alignments in PostScript. Bioinformatics 1999, 15, 305–308. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Kozak, M. At least six nucleotides preceding the AUG initiator codon enhance translation in mammalian cells. J. Mol. Biol. 1987, 196, 947–950. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Hamasaki, T.; Ozoe, F.; Ohta, H.; Enomoto, K.; Kataoka, H.; Sawa, Y.; Hirota, A.; Ozoe, Y. Identification of critical structural determinants responsible for octopamine binding to the α-adrenergic-like Bombyx mori octopamine receptor. Biochemistry 2007, 46, 5896–5903. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.F.; Xu, G.; Qi, Y.X.; Xia, R.Y.; Huang, J.; Ye, G.Y. Two splicing variants of a novel family of octopamine receptors with different signaling properties. J. Neurochem. 2014, 129, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

| Agonist | 5-HT7L | 5-HT7S | ||
|---|---|---|---|---|
| EC50 (μM) | logEC50 (Mean ± SEM) | EC50 (μM) | logEC50 (Mean ± SEM) | |
| 5-HT | 0.092 | −7.03 ± 0.10 | 0.035 | −7.46 ± 0.19 |
| 5-MT | 20.91 | −4.68 ± 0.07 | 19.42 | −4.71 ± 0.22 |
| αm-5-HT | 13.89 | −4.86 ± 0.04 | 5.90 | −5.23 ± 0.29 |
| 8-OH-DPAT | 10.06 | −5.00 ± 0.11 | 24.82 | −4.61 ± 0.30 |
| Antagonist | 5-HT7L | 5-HT7S | ||
|---|---|---|---|---|
| IC50 (μM) | logIC50 (Mean ± SEM) | IC50 (μM) | logIC50 (Mean ± SEM) | |
| Methithepin | 87.21 | −4.06 ± 0.03 | 62.86 | −4.20 ± 0.03 |
| Ketanserin | 46.91 | −4.33 ± 0.04 | 84.94 | −4.07 ± 0.02 |
| WAY-100635 | 36.15 | −4.44 ± 0.04 | 70.33 | −4.15 ± 0.02 |
| SB-258719 | 55.26 | −4.26 ± 0.03 | 64.04 | −4.19 ± 0.04 |
| SB-269970 | 70.36 | −4.15 ± 0.03 | 67.78 | −4.17 ± 0.03 |
| Tissue | Sex | Expression Ratio (Mean ± SEM%) a | pb |
|---|---|---|---|
| Brain | F | 66.39 ± 0.60 | 0.71 |
| M | 67.19 ± 1.95 | ||
| Antennae | F | 44.87 ± 0.94 | 0.02 |
| M | 56.33 ± 2.64 | ||
| Labial palp | F | 52.42 ± 1.01 | 0.04 |
| M | 57.95 ± 1.61 | ||
| Thorax | F | 42.86 ± 4.95 | 0.35 |
| M | 36.81 ± 2.74 | ||
| Wing | F | 34.96 ± 3.88 | 0.47 |
| M | 38.48 ± 2.13 | ||
| Leg | F | 33.48 ± 4.49 | 0.74 |
| M | 31.06 ± 5.16 | ||
| Abdomen | F | 43.46 ± 1.46 | 0.01 |
| M | 22.87 ± 3.82 |
| Primer Names | Primer Sequence (5′-3′) |
|---|---|
| For complete cDNA and gDNA cloning | |
| 5-HT7-comp-F | ATGGCGACTCAAAGTCATAACTCCCACTGTTCAC |
| 5-HT7-comp-R | TAGAAAACTTTCCGAAGCCCGCGTCTCCTCTC |
| For RT-PCR and real-time PCR | |
| 5-HT7-RTF | CCTCCTCCGAGAGGATCTACTGC |
| 5-HT7-RTR | TTGGCTAGTTGGAACCGCAGC |
| 5-HT7-qPCR-F | GGACCTTCTTAGACGACAACTCCAC |
| 5-HT7-qPCR-R | TCGCTTACTGCCAGCGACACTATC |
| 5-HT7L-qPCR-F | CGAACGAGTCCCAGTGTCCTATCT |
| 5-HT7L-qPCR-R | GATCGGATTCTGTTCGTCGTATTCT |
| β-actin-qPCR-F | AACTTCCCGACGGTCAAGTCAT |
| β-actin-qPCR-R | TGTTGGCGTACAAGTCCTTACG |
| GAPDH-qPCR-F | ATGTTCGTGTGCGGAGTCAAC |
| GAPDH-qPCR-R | TCTTCTGGGTAGCGGTGGTAG |
| For eukaryotic expression | |
| Msep5-HT7-KpnⅠ-F | GGGGTACCGCCACCATGGCGACTCAAAGTCATAACTCC |
| Msep5-HT7-XhoⅠ-R | CCGCTCGAGTCATAGAAAACTTTCCGAAGCCCGCGTCTCCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Gao, X.; Wang, H.; Xie, G.; An, S.; Du, Y.; Zhao, X. Identification and Pharmacological Characterization of Two Serotonin Type 7 Receptor Isoforms from Mythimna separata. Int. J. Mol. Sci. 2023, 24, 655. https://doi.org/10.3390/ijms24010655
Chen W, Gao X, Wang H, Xie G, An S, Du Y, Zhao X. Identification and Pharmacological Characterization of Two Serotonin Type 7 Receptor Isoforms from Mythimna separata. International Journal of Molecular Sciences. 2023; 24(1):655. https://doi.org/10.3390/ijms24010655
Chicago/Turabian StyleChen, Wenbo, Xiaoyan Gao, Huixin Wang, Guiying Xie, Shiheng An, Yongkun Du, and Xincheng Zhao. 2023. "Identification and Pharmacological Characterization of Two Serotonin Type 7 Receptor Isoforms from Mythimna separata" International Journal of Molecular Sciences 24, no. 1: 655. https://doi.org/10.3390/ijms24010655
APA StyleChen, W., Gao, X., Wang, H., Xie, G., An, S., Du, Y., & Zhao, X. (2023). Identification and Pharmacological Characterization of Two Serotonin Type 7 Receptor Isoforms from Mythimna separata. International Journal of Molecular Sciences, 24(1), 655. https://doi.org/10.3390/ijms24010655







