Volumetric Absorptive Microsampling in Therapeutic Drug Monitoring of Immunosuppressive Drugs—From Sampling and Analytical Issues to Clinical Application
Abstract
:1. Therapeutic Drug Monitoring of Immunosuppressive Agents
2. Volumetric Absorptive Microsampling (VAMS) in the Current Literature (2014–2022)
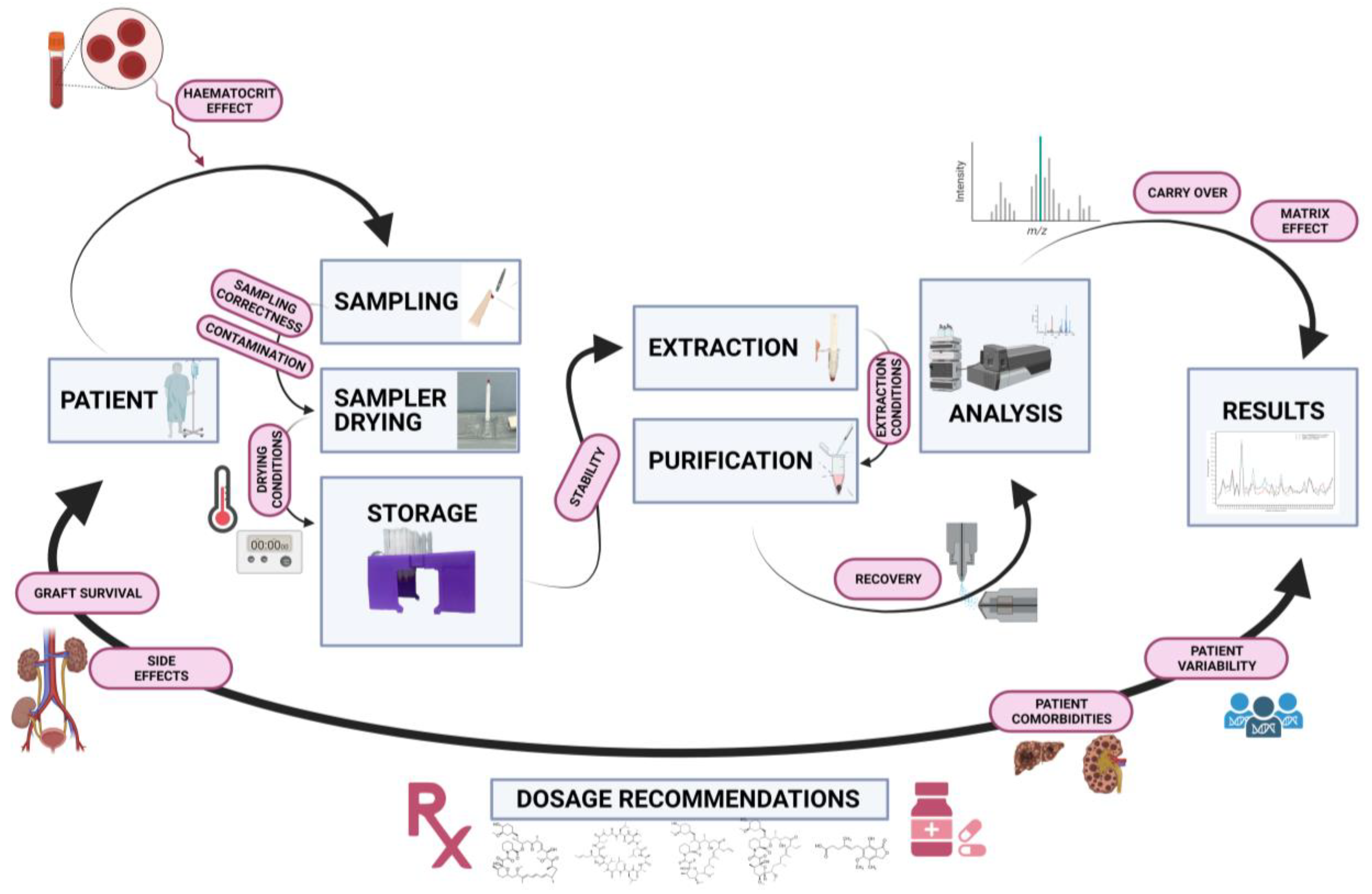
2.1. Pre-Sampling
2.2. Sampling
| Drug Name | Matrix | Sample Volume | Storage | Drying Method | Sampling Correctness | Microsampling Device | Reference |
|---|---|---|---|---|---|---|---|
| CSA | fingerprick CB | 20 μL | n.d | n.d | n.t. | Mitra™ | [22] |
| fingerprick CB | 20 μL | zip-lock bags with desiccant (−20 °C until analysis) | RT, 2 h | n.t. | Mitra™ | [23] | |
| fingerprick CB | 20 μL | RT, ambient conditions | RT, 24 h | n.t. | Mitra™ | [26] | |
| fingerprick CB | 20 μL | zip-lock bags with desiccant (−20 °C until analysis) | RT, 2 h | n.t. | Mitra™ | [27] | |
| TAC | fingerprick CB | 10 μL | RT, ambient conditions | RT, 3 h | satisfactory | Mitra™ | [19] |
| fingerprick CB | 10 μL | RT, ambient conditions | RT, 2 h | n.t. | Mitra™ | [20] | |
| tail prick CB | 10 μL | freezing in tube | RT, 2 h | n.t. | Mitra™ | [21] | |
| fingerprick CB | 20 μL | n.d | n.d | n.t. | Mitra™ | [22] | |
| fingerprick CB | 20 μL | zip-lock bags with desiccant (−20 °C until analysis) | RT, 2 h | MS: 95.20% SS: 70.06% | Mitra™ | [23] | |
| fingerprick CB | 20 μL | RT, ambient conditions | RT, 24 h | n.t. | Mitra™ | [26] | |
| fingerprick CB | 20 μL | zip-lock bags with desiccant (−20 °C until analysis) | RT, 2 h | n.t. | Mitra™ | [27] | |
| fingerprick CB | 20 μL | zip-lock bags with desiccant (−20 °C until analysis) | RT, 24 h | 67.7% | Mitra™ | [28] | |
| fingerprick CB | 10μL | RT, ambient conditions | RT, 2 h | n.t. | Mitra™ | [29] | |
| fingerprick CB | 20μL | zip-lock bags with desiccant | RT, 2 h | n.t. | Mitra™ | [30] | |
| fingerprick CB | 10 μL | 4 °C, darkness | RT, 1 h | n.t. | Mitra™ | [18] | |
| fingerprick CB | 20 μL | RT, ambient conditions | RT, 24 h | n.t. | Mitra™ | [31] | |
| fingerprick CB | 10 μL | At least 24 h in a specimen bag | RT, 24 h | n.t. | HemaXis™ | [32] | |
| MPA | fingerprick CB | 20 μL | n.d | n.d | n.t. | Mitra™ | [22] |
| fingerprick CB | 20 μL | zip-lock bags with desiccant (−20 °C until analysis) | RT, 2 h | MS: 95.20% SS: 70.06% | Mitra™ | [23] | |
| fingerprick CB | 20 μL | zip-lock bags with desiccant | RT, 2 h | n.t. | Mitra™ | [30] | |
| fingerprick CB | 10 μL | RT, ambient conditions | RT, 24 h | n.t. | HemaXis™ | [32] | |
| EVE | fingerprick CB | 20 μL | n.d | n.d | n.t. | Mitra™ | [22] |
| fingerprick CB | 20 μL | zip-lock bags with desiccant (−20 °C until analysis) | RT, 2 h | n.t. | Mitra™ | [23] | |
| fingerprick CB | 20 μL | RT, ambient conditions | RT, 24 h | n.t. | Mitra™ | [26] | |
| SIR | fingerprick CB | 20 μL | n.d | n.d | n.t. | Mitra™ | [22] |
| fingerprick CB | 20 μL | zip-lock bags with desiccant (−20 °C until analysis) | RT, 2 h | n.t. | Mitra™ | [23] | |
| fingerprick CB | 20 μL | RT, ambient conditions | RT, 24 h | n.t. | Mitra™ | [26] | |
| fingerprick CB | 10 μL | n.d. | n.d. | n.t. | Mitra™ | [33] | |
| fingerprick CB | 10 or 20 μL | 20 ± 5 °C, <40% humidity, zip-lock bags with desiccant | RT, n.d. | MS: 39.1% (reduced to 13.6%) | Mitra™ | [34] |
2.3. Sample Preparation (Extraction and Purification)
| Drug Name | Microsampling Method | Extraction Solvent | Extraction Conditions | Solvent for Sample Purification | Purification Conditions | Additional Steps | Reference |
|---|---|---|---|---|---|---|---|
| CSA | Mitra™ | methanol: water (with IS); (40:60, v/v%) | sonication (30 min) | methanol | vortexing (15 min, low speed, 1 min maximal speed), sonication (15 min), vortexing (the same conditions as above), centrifugation (5 min, 10,000 g), and storage at −20 °C (10 min), centrifugation (the same conditions above) | n.d. | [22] |
| Mitra™ | methanol (with IS); (62.5:37.5, v/v) | sonication (15 min) | methanol | sonication (15 min), centrifugation (5 min, 14,500 g) | evaporation to dry, reconstitution with the mobile phase | [23] | |
| Mitra™ | methanol: water (with IS); (80:20, v/v) | sonication (15 min), vortexing (60 min), centrifugation (10 min, 18,403.2 g) | n.d. | vortexing (15 min), centrifugation (10 min, 18,403.2 g) | evaporation to dry, reconstitution with the mobile phase | [26] | |
| Mitra™ | IS solution | sonication (30 min) | methanol | vortexing (15 min), sonication (15 min), centrifugation (5 min, 13,000 g), storage at −20 °C (10 min), centrifugation (the same conditions above) | n.d. | [27] | |
| TAC | Mitra™ | water | shaking (15 min) | methanol: zinc sulphate (2:1, v/v) | shaking (6 min) centrifugation (2000 g, 10 min, 4 °C) | n.d. | [19] |
| Mitra™ | methanol: water (1:1, v/v) | sonication (15 min) | methanol: acetonitrile (1:1, v/v) | centrifugation (13,000 g, 5–15 min, 4 °C), | n.d. | [20] | |
| Mitra™ | methanol: water (1:1, v/v) | sonication (15 min) | methanol: acetonitrile (1:1, v/v) | centrifugation (13,000 g, 15 min, 4 °C), | n.d. | [21] | |
| Mitra™ | methanol: water (with IS); (40:60, v/v%) | sonication (30 min) | methanol | vortexing (15 min, low speed, 1 min maximal seed), sonication (15 min), vortexing (the same conditions as above), centrifugation (10,000 g), and storage at −20 °C (10 min), centrifugation (the same conditions above) | n.d. | [22] | |
| Mitra™ | methanol (with IS); (62.5:37.5, v/v) | sonication (15 min) | methanol | sonication (15 min), centrifugation (5 min, 14,500 g) | evaporation to dry, reconstitution with the mobile phase | [23] | |
| Mitra™ | methanol: water (with IS); (80:20, v/v) | sonication (15 min), vortexing (60 min), centrifugation (10 min, 18,403.2 g) | n.d. | vortexing (15 min), centrifugation (10 min, 18,403.2 g) | evaporation to dry, reconstitution with the mobile phase | [26] | |
| Mitra™ | the internal standard solution | sonication (30 min) | methanol | vortexing (15 min), sonication (15 min), centrifugation (5 min, 13,000 g), storage at −20 °C (10 min), centrifugation (the same conditions above) | n.d. | [27] | |
| Mitra™ | methanol: water (with IS), (80:20, v/v) | sonication (30 min) | methanol and zinc sulphate solution | vortexing (15 min), sonication (15 min), vortexing (15 min), centrifugation (10,000 g, 5 min), and storage at −20 °C (10 min), centrifugation (the same conditions above) | n.d. | [28] | |
| Mitra™ | water with IS (50:50, v/v) | shaking (15 min), sonication (10 min), | acetonitrile and zinc sulphate mixture (1:1, v/v) | centrifugation (16,260 g, 5 min, 8 °C) | salting out with ammonium sulphate | [29] | |
| Mitra™ | 50% methanol solution | sonication (10 min), vortexing (20 min) | acetonitrile and zinc sulphate mixture (1:1, v/v) with IS | shaking (10 min) centrifugation (2900 rpm, 5 min) | n.d. | [30] | |
| Mitra™ | water | shaking (60 min) | acetonitrile and zinc sulphate mixture (1:1, v/v) | shaking (10 min) centrifugation (3500 rpm, 10 min, 4 °C) | n.d. | [18] | |
| Mitra™ | acetonitrile: water (40:60, v/v%) | vortexing (10 min), sonication (15 min), vortexing (10 min) | acetonitrile with IS | vortexing (5 min), centrifugation (11,337 g, 5 min) | n.d. | [31] | |
| HemaXis™ | IS solution in methanol | vortexing (15 min) | zinc sulphate solution | centrifugation (16,000 g, 5 min) | n.d. | [32] | |
| MPA | Mitra™ | methanol: water (with IS); (40:60, v/v%) | sonication (30 min) | methanol | vortexing (15 min, low speed, 1 min maximal seed), sonication (15 min), vortexing (the same conditions as above), centrifugation (10,000 g), and storage at −20 °C (10 min), centrifugation (the same conditions above) | n.d. | [22] |
| Mitra™ | methanol (with IS); (62.5:37.5, v/v) | sonication (15 min) | methanol | sonication (15 min), centrifugation (5 min, 14,500 g) | evaporation to dry, reconstitution with the mobile phase | [23] | |
| Mitra™ | 50% methanol solution | sonication (10 min), vortexing (20 min) | acetonitrile and zinc sulphate mixture (1:1, v/v) with IS | shaking (10 min) centrifugation (2900 rpm, 5 min) | n.d. | [30] | |
| HemaXis™ | IS solution in methanol | vortexing (15 min) | zinc sulphate solution | centrifugation (16,000 g, 5 min) | n.d. | [32] | |
| EVE | Mitra™ | methanol: water (with IS); (40:60, v/v%) | sonication (30 min) | methanol | vortexing (15 min, low speed, 1 min maximal seed), sonication (15 min), vortexing (the same conditions as above), centrifugation (10,000 g), and storage at −20 °C (10 min), centrifugation (the same conditions above) | n.d. | [22] |
| Mitra™ | methanol (with IS); (62.5:37.5, v/v) | sonication (15 min) | methanol | sonication (15 min), centrifugation (5 min, 14,500 g) | evaporation to dry, reconstitution with the mobile phase | [23] | |
| Mitra™ | methanol: water (with IS); (80:20, v/v) | sonication (15 min), vortexing (60 min), centrifugation (10 min, 18,403.2 g) | n.d. | vortexing (15 min), centrifugation (10 min, 18,403.2 g) | evaporation to dry, reconstitution with the mobile phase | [26] | |
| SIR | Mitra™ | methanol: water (with IS); (40:60, v/v%) | sonication (30 min) | methanol | vortexing (15 min, low speed, 1 min maximal seed), sonication (15 min), vortexing (the same conditions as above), centrifugation (10,000 g), and storage at −20 °C (10 min), centrifugation (the same conditions above) | n.d. | [22] |
| Mitra™ | methanol (with IS); (62.5:37.5, v/v) | sonication (15 min) | methanol | sonication (15 min), centrifugation (5 min, 14,500 g) | evaporation to dry, reconstitution with the mobile phase | [23] | |
| Mitra™ | methanol: water (with IS); (80:20, v/v) | sonication (15 min), vortexing (60 min), centrifugation (10 min, 18,403.2 g) | n.d. | vortexing (15 min), centrifugation (10 min, 18,403.2 g) | evaporation to dry, reconstitution with the mobile phase | [26] | |
| Mitra™ | methanol with IS | sonication (15 min) | n.d. | vortexing, centrifugation (10 min, 15,000 g) | evaporation to dry, reconstitution with the mobile phase | [33] | |
| Mitra™ | water with IS (20:1, v/v) | sonication (20 min) | LLE with tert-butyl-methyl-ether | freezing (−60 °C) | evaporation to dry, reconstitution with the mobile phase | [34] |
2.4. Analytical Assay Characteristics
2.5. Clinical Outcome
3. Around VAMS—Strengths, Weaknesses, and Relevant Aspects
3.1. Hematocrit Effect
3.2. Automatization of the VAMS Methods
3.3. Sampling in the Home–Point-of-Care (POC) as a Method for Adherence Improvement
3.4. Proficiency Testing as a Method for Global Standardization
3.5. Future Perspective on VAMS for Immunosuppressants TDM
4. Other Volumetric Microsampling Methods
4.1. Capillary Microsampling (CMS)
4.2. Dried Blood Spots (DBS)
4.3. CapitainerTM
4.4. HemaxisTM
4.5. HemaPenTM
5. Application of Microsampling for Other Matrices Collecting
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Seger, C.; Shipkova, M.; Christians, U.; Billaud, E.M.; Wang, P.; Holt, D.W.; Brunet, M.; Kunicki, P.K.; Pawiński, T.; Langman, L.J.; et al. Assuring the Proper Analytical Performance of Measurement Procedures for Immunosuppressive Drug Concentrations in Clinical Practice: Recommendations of the International Association of Therapeutic Drug Monitoring and Clinical Toxicology Immunosuppressive Drug Scientific Committee. Ther. Drug Monit. 2016, 38, 170–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergan, S.; Brunet, M.; Hesselink, D.A.; Johnson-Davis, K.L.; Kunicki, P.K.; Lemaitre, F.; Marquet, P.; Molinaro, M.; Noceti, O.; Pattanaik, S.; et al. Personalized Therapy for Mycophenolate: Consensus Report by the International Association of Therapeutic Drug Monitoring and Clinical Toxicology. Ther. Drug Monit. 2021, 43, 150–200. [Google Scholar] [CrossRef] [PubMed]
- Milone, M.C. Overview of the pharmacology and toxicology of immunosuppressant agents that require therapeutic drug monitoring. In Personalized Immunosuppression in Transplantation; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–27. [Google Scholar]
- Dasgupta, A. Limitations of immunoassays used for therapeutic drug monitoring of immunosuppressants. In Personalized Immunosuppression in Transplantation; Elsevier: Amsterdam, The Netherlands, 2016; pp. 29–56. [Google Scholar]
- Dasgupta, A. Monitoring free mycophenolic acid concentration: Is there any clinical advantage? In Personalized Immunosuppression in Transplantation; Elsevier: Amsterdam, The Netherlands, 2016; pp. 83–107. [Google Scholar]
- Brunet, M.; Van Gelder, T.; Åsberg, A.; Haufroid, V.; Hesselink, D.A.; Langman, L.; Lemaitre, F.; Marquet, P.; Seger, C.; Shipkova, M.; et al. Therapeutic Drug Monitoring of Tacrolimus-Personalized Therapy: Second Consensus Report. Ther. Drug Monit. 2019, 41, 261–307. [Google Scholar] [CrossRef] [PubMed]
- Kahan, B.D.; Keown, P.; Levy, G.A.; Johnston, A. Therapeutic drug monitoring of immunosuppressant drugs in clinical practice. Clin. Ther. 2002, 24, 330–350. [Google Scholar] [CrossRef] [PubMed]
- Shipkova, M.; Hesselink, D.A.; Holt, D.W.; Billaud, E.M.; van Gelder, T.; Kunicki, P.K.; Brunet, M.; Budde, K.; Barten, M.J.; De Simone, P.; et al. Therapeutic Drug Monitoring of Everolimus. Ther. Drug Monit. 2016, 38, 143–169. [Google Scholar] [CrossRef] [Green Version]
- Delahaye, L.; Veenhof, H.; Koch, B.C.P.; Alffenaar, J.C.; Linden, R.; Stove, C. Alternative Sampling Devices to Collect Dried Blood Microsamples: State-of-the-Art. Ther. Drug Monit. 2021, 43, 310–321. [Google Scholar] [CrossRef]
- Capiau, S.; Veenhof, H.; Koster, R.A.; Bergqvist, Y.; Boettcher, M.; Halmingh, O.; Keevil, B.G.; Koch, B.C.; Linden, R.; Pistos, C.; et al. Official International Association for Therapeutic Drug Monitoring and Clinical Toxicology Guideline: Development and Validation of Dried Blood Spot–Based Methods for Therapeutic Drug Monitoring. Ther. Drug Monit. 2019, 41, 409–430. [Google Scholar] [CrossRef]
- Lei, B.U.W.; Prow, T.W. A review of microsampling techniques and their social impact. Biomed. Microdevices 2019, 21, 81. [Google Scholar] [CrossRef] [Green Version]
- Spooner, N.; Anderson, K.D.; Siple, J.; Wickremsinhe, E.R.; Xu, Y.; Lee, M. Microsampling: Considerations for its use in pharmaceutical drug discovery and development. Bioanalysis 2019, 11, 1015–1038. [Google Scholar] [CrossRef]
- Protti, M.; Mandrioli, R.; Mercolini, L. Tutorial: Volumetric absorptive microsampling (VAMS). Anal. Chim. Acta 2018, 1046, 32–47. [Google Scholar] [CrossRef]
- Denniff, P.; Spooner, N. Volumetric absorptive microsampling: A dried sample collection technique for quantitative bioanalysis. Anal. Chem. 2014, 86, 8489–8495. [Google Scholar] [CrossRef] [PubMed]
- How to Collect a Microsample. Available online: https://www.neoteryx.com/volumetrically-accurate-micro-sampling-vams-collection-devices?hsCtaTracking=369dbff8-4756-43aa-b4b8-80149a237d14%7Cb6bba9aa-da63-423c-b404-e6f2935e3852 (accessed on 25 November 2022).
- Londhe, V.; Rajadhyaksha, M. Opportunities and obstacles for microsampling techniques in bioanalysis: Special focus on DBS and VAMS. J. Pharm. Biomed. Anal. 2020, 182, 113102. [Google Scholar] [CrossRef]
- Abu-Rabie, P.; Neupane, B.; Spooner, N.; Rudge, J.; Denniff, P.; Mulla, H.; Pandya, H. Validation of methods for determining pediatric midazolam using wet whole blood and volumetric absorptive microsampling. Bioanalysis 2019, 11, 1737–1754. [Google Scholar] [CrossRef]
- Kocur, A.; Marszałek, D.; Rubik, J.; Czajkowska, A.; Pawiński, T. Volumetric Absorptive Microsampling as a New Approach for Therapeutic Drug Monitoring of Tacrolimus in Pediatric Renal Transplant Recipients: LC-MS/MS Method Development, Validation, and Clinical Application. Pharmaceutics, 2022; submitted/under review. [Google Scholar]
- Vethe, N.T.; Gustavsen, M.T.; Midtvedt, K.; Lauritsen, M.E.; Andersen, A.M.; Åsberg, A.; Bergan, S. Tacrolimus Can Be Reliably Measured with Volumetric Absorptive Capillary Microsampling Throughout the Dose Interval in Renal Transplant Recipients. Ther. Drug Monit. 2019, 41, 607–614. [Google Scholar] [CrossRef]
- Kita, K.; Noritake, K.; Mano, Y. Application of a Volumetric Absorptive Microsampling Device to a Pharmacokinetic Study of Tacrolimus in Rats: Comparison with Wet Blood and Plasma. Eur. J. Drug Metab. Pharmacokinet. 2018, 44, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Kita, K.; Mano, Y. Application of volumetric absorptive microsampling device for quantification of tacrolimus in human blood as a model drug of high blood cell partition. J. Pharm. Biomed. Anal. 2017, 143, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Koster, R.A.; Niemeijer, P.; Veenhof, H.; Hateren, K.V.; Alffenaar, J.C.; Touw, D.J. A volumetric absorptive microsampling LC-MS/MS method for five immunosuppressants and their hematocrit effects. Bioanalysis 2019, 11, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Paniagua-González, L.; Díaz-Louzao, C.; Lendoiro, E.; Otero-Antón, E.; Cadarso-Suárez, C.; López-Rivadulla, M.; Cruz, A.; de-Castro-Ríos, A. Volumetric Absorptive Microsampling (VAMS) for assaying immunosuppressants from venous whole blood by LC-MS/MS using a novel atmospheric pressure ionization probe (UniSpray™). J. Pharm. Biomed. Anal. 2020, 189, 113422. [Google Scholar] [CrossRef]
- Ye, Z.; Gao, H. Evaluation of sample extraction methods for minimizing hematocrit effect on whole blood analysis with volumetric absorptive microsampling. Bioanalysis 2017, 9, 349–357. [Google Scholar] [CrossRef]
- Capiau, S.; Stove, C. Hematocrit prediction in volumetric absorptive microsamples. J. Pharm. Biomed. Anal. 2020, 190, 113491. [Google Scholar] [CrossRef]
- Gruzdys, V.; Merrigan, S.D.; Johnson-Davis, K.L. Feasibility of Immunosuppressant Drug Monitoring by a Microsampling Device. J. Appl. Lab. Med. 2019, 4, 241–246. [Google Scholar] [CrossRef]
- Zwart, T.C.; Metscher, E.; van der Boog, P.J.M.; Swen, J.J.; de Fijter, J.W.; Guchelaar, H.; de Vries, A.P.J.; Moes, D.J.A.R. Volumetric microsampling for simultaneous remote immunosuppressant and kidney function monitoring in outpatient kidney transplant recipients. Br. J. Clin. Pharmacol. 2022, 88, 4854–4869. [Google Scholar] [CrossRef] [PubMed]
- Veenhof, H.; Koster, R.A.; Junier, L.A.; Berger, S.P.; Bakker, S.J.; Touw, D.J. Volumetric absorptive microsampling and dried blood spot microsampling vs. conventional venous sampling for tacrolimus trough concentration monitoring. Clin. Chem. Lab. Med. 2020, 58, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Tron, C.; Ferrand-Sorre, M.-J.; Querzerho-Raguideau, J.; Chemouny, J.M.; Houssel-Debry, P.; Verdier, M.-C.; Bellissant, E.; Lemaitre, F. Volumetric absorptive microsampling for the quantification of tacrolimus in capillary blood by high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2021, 1165, 122521. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, C.; Parker, S.; Jacks, M.; John, G.T.; McWhinney, B.; Ungerer, J.; Mallett, A.; Healy, H.; Roberts, J.; Staatz, C. Finger-prick microsampling methods can replace venepuncture for simultaneous therapeutic drug monitoring of tacrolimus, mycophenolic acid, and prednisolone concentrations in adult kidney transplant patients. Ther. Drug Monit. 2022. [Google Scholar] [CrossRef]
- Mathew, B.S.; Mathew, S.K.; Aruldhas, B.W.; Prabha, R.; Gangadharan, N.; David, V.G.; Varughese, S.; John, G.T. Analytical and clinical validation of dried blood spot and volumetric absorptive microsampling for measurement of tacrolimus and creatinine after renal transplantation. Clin. Biochem. 2022, 105–106, 25–34. [Google Scholar] [CrossRef]
- Zwart, T.C.; Gokoel, S.R.; van der Boog, P.J.; de Fijter, J.W.; Kweekel, D.M.; Swen, J.J.; Guchelaar, H.-J.; Moes, D.J.A. Therapeutic drug monitoring of tacrolimus and mycophenolic acid in outpatient renal transplant recipients using a volumetric dried blood spot sampling device. Br. J. Clin. Pharmacol. 2018, 84, 2889–2902. [Google Scholar] [CrossRef] [Green Version]
- Verheijen, R.; Thijssen, B.; Atrafi, F.; Schellens, J.; Rosing, H.; de Vries, N.; Beijnen, J.; Mathijssen, R.; Steeghs, N.; Huitema, A. Validation and clinical application of an LC-MS/MS method for the quantification of everolimus using volumetric absorptive microsampling. J. Chromatogr. B 2018, 1104, 234–239. [Google Scholar] [CrossRef]
- Yoo, S.; Kim, G.; Kim, S.; Ha, J.; Cho, B.S.; Joo, D.J.; Lee, J.I. Volumetric Absorptive Microsampling for the Therapeutic Drug Monitoring of Everolimus in Patients who have Undergone Liver Transplant. Ther. Drug Monit. 2022; ahead of print. [Google Scholar] [CrossRef]
- Veenhof, H.; Koster, R.A.; Junier, L.A.; Zweipfenning, P.; Touw, D.J. Results from a proficiency testing pilot for immunosuppressant microsampling assays. Ther. Drug Monit. 2022; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- van den Broek, I.; Qin, F.; Stuart, K.; Kowalski, M.P.; Millis, K.; Percy, A.; Holewinski, J.R.; Venkatraman, V.; yan Eyk, J.E. Application of volumetric absorptive microsampling for robust, high-throughput mass spectrometric quantification of circulating protein biomarkers. Clin. Mass Spectrom. 2017, 4, 25–33. [Google Scholar] [CrossRef]
- De Geest, S.; Zullig, L.L.; Dunbar-Jacob, J.; Hughes, D.; Wilson, I.B.; Vrijens, B. Improving Medication Adherence Research Reporting: European Society for Patient Adherence, Compliance and Persistence Medication Adherence Reporting Guideline. J. Cardiovasc. Nurs. 2019, 34, 199–200. [Google Scholar] [CrossRef]
- Blowey, D.L.; Hébert, D.; Arbus, G.S.; Pool, R.; Korus, M.; Koren, G. Compliance with cyclosporine in adolescent renal transplant recipients. Pediatr. Nephrol. 1997, 11, 547–551. [Google Scholar] [CrossRef]
- Rianthavorn, P. Noncompliance with immunosuppressive medications in pediatric and adolescent patients receiving solid-organ transplants. Transplantation 2004, 77, 778–782. [Google Scholar] [CrossRef]
- Christians, U.; Vinks, A.A.; Langman, L.J.; Clarke, W.; Wallemacq, P.; van Gelder, T.; Renjen, V.; Marquet, P.; Meyer, E.J. Impact of Laboratory Practices on Interlaboratory Variability in Therapeutic Drug Monitoring of Immunosuppressive Drugs. Ther. Drug Monit. 2015, 37, 718–724. [Google Scholar] [CrossRef]
- Microsampling—2 Years. Available online: https://www.bioanalysis-zone.com/microsampling-2-years/ (accessed on 25 November 2022).
- National Library of Medicine. Available online: https://pubmed.ncbi.nlm.nih.gov/?term=volumetric+absorptive+microsampling&sort=jour&size=200 (accessed on 25 November 2022).
- Rajadhyaksha, M.; Londhe, V. Microsampling: A role to play in COVID-19 diagnosis, surveillance, treatment and clinical trials. Drug Test. Anal. 2021, 13, 1238–1248. [Google Scholar] [CrossRef]
- Gustavsen, M.T.; Midtvedt, K.; Vethe, N.T.; Robertsen, I.; Bergan, S.; Åsberg, A. Tacrolimus Area Under the Concentration Versus Time Curve Monitoring, Using Home-Based Volumetric Absorptive Capillary Microsampling. Ther. Drug Monit. 2020, 42, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Tron, C.; Lemaitre, F. Perspective on the Use of Limited Sampling Strategies to Assess Drug Exposure in the Era of Microsampling. Ther. Drug Monit. 2021, 43, 812–813. [Google Scholar] [CrossRef]
- Kindem, I.A.; Bjerre, A.; Åsberg, M.A.; Midtvedt, K.; Bergan, M.S.; Vethe, M.N.T. Tacrolimus measured in capillary volumetric microsamples in pediatric patients-a cross-validation study. Ther. Drug Monit. 2021, 43, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Yang, H.; Choi, J.R.; Gong, Y.; You, M.; Wen, T.; Li, A.; Li, X.; Xu, B.; Zhang, S.; et al. Capillary blood for point-of-care testing. Crit. Rev. Clin. Lab. Sci. 2017, 54, 294–308. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, B.; Chadwick, K.D.; Su, T.; Mangipudy, R.; Pillutla, R.C.; Qin, C.J. A practical workflow for capillary microsampling in nonclinical studies. Bioanalysis 2019, 11, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Velghe, S.; Stove, C.P. Evaluation of the Capitainer-B Microfluidic Device as a New Hematocrit-Independent Alternative for Dried Blood Spot Collection. Anal. Chem. 2018, 90, 12893–12899. [Google Scholar] [CrossRef] [PubMed]
- Capitainer Microsampling Device Manual. Available online: https://capitainer.se/ (accessed on 25 November 2022).
- Hemaxis Microsampling Device Manual. Available online: https://hemaxis.com/ (accessed on 25 November 2022).
- Trajan Microsampling Device Manual. Available online: https://www.trajanscimed.com/pages/hemapen (accessed on 25 November 2022).


| Drug Name | Analytical Method | Injection Volume | Selected Chromatographic Conditions | Internal Standard | Selected Apparatus Conditions | Calibration Range (Linearity) | Reference |
|---|---|---|---|---|---|---|---|
| CSA | (+)ESI-LC-MS/MS | 20 μL | C18 chromatographic column, mobile phase: ammonium formate buffer pH 3.5 and methanol, flow rate: 1 mL/min, gradient flow | D12-CSA | RF lens = 93 V CE = 15 eV positive mode (ammonium adduct monitoring) | 10–500 ng/mL | [22] |
| (+)ESI-LC-MS/MS and US-LC-MS/MS | 20 μL | C18 chromatographic column, mobile phase: ammonium formate and formic acid in water and acetonitrile flow rate: 0.5 mL/min, gradient flow | D4-CSA | CV = 20 V CE = 19 eV positive mode (ammonium adduct monitoring) | 20–2000 ng/mL | [23] | |
| (+)ESI-LC-MS/MS | 35 μL | C18 chromatographic column, mobile phase: n.d., flow rate: n.d. gradient flow | D12-CSA | RF lens = n.d. CE = n.d. positive mode (ammonium adduct monitoring) | 22.7–937.0 ng/mL | [26] | |
| (+)ESI-LC-MS/MS | 40 μL | C18 chromatographic column, mobile phase: formic acid, ammonium in water and methanol, flow rate: 0.45 mL/min, gradient flow | D12-CSA | RF lens = n.d. CE = n.d. positive mode (ammonium adduct monitoring) | 0–1904 μg/L | [27] | |
| TAC | (+)ESI-LC-MS/MS | 20 μL | C8 chromatographic column, mobile phase: water with formic acid and ammonium acetate and methanol with formic acid ammonium acetate., flow rate: 0.60mL/min, gradient flow | 13C,D2-TAC | RF lens = 82 V CE = 23 eV positive mode (ammonium adduct monitoring) | 1.3–60 μg/L | [19] |
| (+)ESI-LC-MS/MS | 3 μL | C18 chromatographic column, mobile phase: water, and methanol (with acetic acid), flow rate: 0.25 mL/min, gradient flow | ASC | RF lens = 60 V CE = 40 eV positive mode | 1–250 ng/mL | [20] | |
| (+)ESI-LC-MS/MS | 3 μL | C18 chromatographic column, mobile phase: water and methanol (with ammonium acetate and acetic acid) flow rate: 0.35 mL/min, gradient flow | ASC | RF lens = 40 V CE = 27 eV positive mode (ammonium adduct monitoring) | 0.2–250 ng/mL | [21] | |
| (+)ESI-LC-MS/MS | 20 μL | C18 chromatographic column, mobile phase: ammonium formate buffer pH 3.5 and methanol, flow rate: 1 mL/min, gradient flow | 13C,D2-TAC | RF lens = 82 V CE = 20 eV positive mode (ammonium adduct monitoring) | 1–50 μg/L | [22] | |
| (+)ESI-LC-MS/MS and US-LC-MS/MS | 20 μL | C18 chromatographic column, mobile phase: ammonium formate and formic acid in water and acetonitrile flow rate: 0.5 mL/min, gradient flow | 13C,D2-TAC | CV = 22 V CE = 20 eV positive mode (ammonium adduct monitoring) | 0.5–50 ng/mL | [23] | |
| (+)ESI-LC-MS/MS | 35 μL | C18 chromatographic column, mobile phase: n.d., flow rate: n.d. gradient flow | 13C,D2-TAC | RF lens = n.d. CE = n.d. positive mode (ammonium adduct monitoring) | 2.20–41.30 ng/mL | [26] | |
| (+)ESI-LC-MS/MS | 40 μL | C18 chromatographic column, mobile phase: formic acid, ammonium in water and methanol, flow rate: 0.45 mL/min, gradient flow | 13C,D2-TAC | RF lens = n.d. CE = n.d. positive mode (ammonium adduct monitoring) | 2.18–42.4 μg/L | [27] | |
| (+)ESI-LC-MS/MS | 20 μL | C18 chromatographic column, mobile phase: ammonium formate buffer pH 3.5 and methanol, flow rate: 1 mL/min, gradient flow | 13C,D2-TAC | RF lens = 82 V CE = 20 eV positive mode (ammonium adduct monitoring) | 1–50 μg/L | [28] | |
| (+)ESI-LC-MS/MS | 10 μL | C18 chromatographic column, mobile phase: 95% acetonitrile and 5% 10 mM ammonium acetate in water, flow rate: 0.1–0.6 mL/min, isocratic flow | ASC | RF lens = 135 V CE = 20 eV positive mode (ammonium adduct monitoring) | 2.25–42.9 ng/mL | [29] | |
| (+)ESI-LC-MS/MS | n.d. | C18 chromatographic column, mobile phase: ammonium acetate with formic acid in water and methanol, flow rate: 0.4 mL/min, gradient flow | 13C,D2-TAC | RF lens = n.d. CE = n.d. positive mode (ammonium adduct monitoring) | 0–40 μg/L | [30] | |
| (+)ESI-LC-MS/MS | 10 μL | C18 chromatographic column, mobile phase: water with ammonium fluoride and formic acid, and methanol: acetonitrile with ammonium fluoride and formic acid, flow rate: 0.75 mL/min, gradient flow | ASC | CV = n.d. CE = 22 eV positive mode (ammonium adduct monitoring) | 0–60 μg/L | [18] | |
| (+)ESI-LC-MS/MS | 20 μL | C18 chromatographic column, mobile phase: ammonium acetate buffer with 0.1% formic acid and ammonium acetate in methanol with 0.1% formic acid, flow rate: 0.4 mL/min, gradient flow | ASC | CV = 27 V CE = 20 eV positive mode (ammonium adduct monitoring) | 1.45–29.28 μg/L | [31] | |
| (+)ESI-LC-MS/MS | 50 μL | C18 chromatographic column, mobile phase: formic acid, ammonium in water and methanol, flow rate: 0.45 mL/min, gradient flow | ASC | RF lens = 35 V CE = n.d. positive mode (ammonium adduct monitoring) | 2.23–84 μg/L | [32] | |
| MPA | (+)ESI-LC-MS/MS | 20 μL | C18 chromatographic column, mobile phase: 20 ammonium formate buffer pH 3.5 and methanol, flow rate: 1 mL/min, gradient flow | 13C,D3-MPA | RF lens = 58 V CE = 22 eV positive mode (ammonium adduct monitoring) | 100–1500 ng/mL | [22] |
| (+)ESI-LC-MS/MS and US-LC-MS/MS | 20 μL | C18 chromatographic column, mobile phase: ammonium formate and formic acid in water and acetonitrile flow rate: 0.5 mL/min, gradient flow | 13C,D3-MPA | CV = 30 V CE = 15 eV positive mode (ammonium adduct monitoring) | 75–7500 ng/mL | [23] | |
| (+)ESI-LC-MS/MS | 10 μL | C18 chromatographic column, mobile phase: formic acid, ammonium in water and methanol, flow rate: 0.45 mL/min, gradient flow | 13C,D3-MPA | RF lens = n.d. CE = n.d. positive mode (ammonium adduct monitoring) | 0.5–20 mg/L | [27] | |
| (+)ESI-LC-MS/MS | n.d. | C18 chromatographic column, mobile phase: ammonium acetate with formic acid in water and methanol, flow rate: 0.4 mL/min, gradient flow | 13C,D3-MPA | RF lens = n.d. CE = n.d. positive mode (ammonium adduct monitoring) | 0–20 mg/L | [30] | |
| (+)ESI-LC-MS/MS | 50 μL | C18 chromatographic column, mobile phase: formic acid, ammonium in water and methanol, flow rate: 0.45 mL/min, gradient flow | 13C,D3-MPA | RF lens = 35 V CE = n.d. positive mode (ammonium adduct monitoring) | 0–20 mg/L | [32] | |
| EVE | (+)ESI-LC-MS/MS | 20 μL | C18 chromatographic column, mobile phase: ammonium formate buffer pH 3.5 and methanol, flow rate: 1 mL/min, gradient flow | 13C2,D4-EVE | RF lens = 88 V CE = 16 eV positive mode (ammonium adduct monitoring) | 1–50 μg/L | [22] |
| (+)ESI-LC-MS/MS and US-LC-MS/MS | 20 μL | C18 chromatographic column, mobile phase: ammonium formate and formic acid in water and acetonitrile flow rate: 0.5 mL/min, gradient flow | 13C2,D4-EVE | CV = 20 V CE = 16 eV positive mode (ammonium adduct monitoring) | 0.5–50 ng/mL | [23] | |
| (+)ESI-LC-MS/MS | 35 μL | C18 chromatographic column, mobile phase: n.d., flow rate: n.d. gradient flow | 13C2,D4-EVE | RF lens = n.d. CE = n.d. positive mode (ammonium adduct monitoring) | 2.3–44.2 ng/mL | [26] | |
| (+)ESI-LC-MS/MS | 40 μL | C18 chromatographic column, mobile phase: formic acid, ammonium in water and methanol, flow rate: 0.45 mL/min, gradient flow | 13C2,D4-EVE | RF lens = n.d. CE = n.d. positive mode (ammonium adduct monitoring) | 0–41.6 μg/L | [27] | |
| (+)ESI-LC-MS/MS | 5 μL | C18 chromatographic column, mobile phase: 20 mM ammonium formate in water and methanol flow rate: 0.4 mL/min, gradient flow | 13C2,D4-EVE | RF lens = n.d. CE = 30 eV positive mode (ammonium adduct monitoring) | 2.5–100 μg/L | [33] | |
| SIR | (+)ESI-LC-MS/MS | 20 μL | C18 chromatographic column, mobile phase: ammonium formate buffer pH 3.5 and methanol, flow rate: 1 mL/min, gradient flow | temsirolimus | RF lens = 83 V CE = 15 eV positive mode (ammonium adduct monitoring) | 1–50 μg/L | [22] |
| (+)ESI-LC-MS/MS and US-LC-MS/MS | 20 μL | C18 chromatographic column, mobile phase: ammonium formate and formic acid in water and acetonitrile flow rate: 0.5 mL/min, gradient flow | 13C2,D4-EVE | CV = 22 V CE = 16 eV positive mode (ammonium adduct monitoring) | 0.5–50 ng/mL | [23] | |
| (+)ESI-LC-MS/MS | 35 μL | C18 chromatographic column, mobile phase: n.d., flow rate: n.d. gradient flow | 13C,D3-SIR | RF lens = n.d. CE = n.d. positive mode (ammonium adduct monitoring) | 2.20–47.20 ng/mL | [26] | |
| (+)ESI-LC-MS/MS | 40 μL | C18 chromatographic column, mobile phase: formic acid, ammonium in water and methanol, flow rate: 0.45 mL/min, gradient flow | 13C,D3-SIR | RF lens = n.d. CE = n.d. positive mode (ammonium adduct monitoring) | 0–47 μg/L | [27] | |
| (+)ESI-LC-MS/MS | 10 μL | C18 chromatographic column, mobile phase: 0.1% formic acid and methanol, flow rate: 0.6 mL/min, gradient flow | 13C,D3-SIR | RF lens = 100 V CE = 58 eV positive mode (ammonium adduct monitoring) | 1–250 ng/mL | [34] |
| Difficulty | Workable Solutions |
|---|---|
| hematocrit effect | Testing hematocrit effect in case of validation and every method modification and introducing correction formula based on, e.g., potassium level and/or Modification attempts in extraction parameters and/or Monitoring of self-sampling correctness with a simple questionnaire and/or Monitoring of hematocrit levels according to drug concentrations regularly |
| mistakes in self-sampling | Regular revision of sampling training for patients and/or Good availability of explicit sampling instruction for patients and/or Additional resources, i.e., as tutorial videos for patients and/or Monitoring of self-sampling correctness with a simple questionnaire and/or Responsible guardians/parents care during sample collection (in the case of pediatric patients) |
| limited sample stability | Limited whole blood dilution through low volumes of calibrators and other solutions addition (particularly <5% of sample volume) and/or Clear guidelines about sample storage and preparing to send for patients (according to desiccant and drying) and/or Modification of method protocol—controlling every step according to influence for sample stability (During validation, according to EMA/FDA guidelines) |
| IS incorporation step | Impregnation of the sampler with IS before sample collecting or Spiking the samples before or after the extraction process or/and The two-stage approach according to liquid–liquid extraction or/and Changing of internal standard (another structural analog or isotope-stable internal standard) |
| drying conditions (time, temperature, and humidity) | Testing selected parameters during method development (in-vitro conditions) and/or Drying and sample storage in controlled conditions and/or Clear guidelines about sample storage and preparing to send for patients (according to desiccant and drying) |
| sample reanalysis necessity | Collecting a few samples at the same time (Simultaneously, replicate samples) and/or Collection of the higher volume of whole blood prior to microsampling |
| sampler contamination | In-vitro validation according to potential chemical contamination (creams, petroleum, etc.) and/or Appropriate disinfection of hands before fingerprick by patients and/or Using another microsampling method with limited contamination risk (i.e., HemaPen™) and/or Responsible guardians/parents care during sample collection (in the case of pediatric patients) |
| analytical method sensitivity | Optimization analytes recovery and/or Optimization sample purification and extraction protocol and/or Changing analytical method/apparatus/chromatographic column/detector conditions, etc. and/or The balance between sample injection volume and chromatographic parameters and/or Testing of method automatization |
| Feature | Venipuncture | CMS | DBS | VAMS Mitra™ | VAMS HemaPen™ | qDBS Capitainer™ | VAMS/qDBS hemaXis™ |
|---|---|---|---|---|---|---|---|
| Type of matrix | whole blood | capillary blood | capillary blood | capillary blood | capillary blood | capillary blood | capillary blood |
| Sampling | invasive (venipuncture) | noninvasive (fingerprick) | noninvasive (fingerprick) | noninvasive (fingerprick) | noninvasive (fingerprick) | noninvasive (fingerprick) | noninvasive (fingerprick) |
| Sample self-collection | impossible | impossible | possible (after training) | possible (after training) | possible (after training) | possible (after training) | possible (after training) |
| Sample volume | inaccurate (non-volumetric) | inaccurate (non-volumetric) or quantitative | inaccurate (non-volumetric) | quantitative (10, 20 or 30 μL) with RSD <4% | quantitative (2.74 μL) | quantitative (10 μL) | quantitative (10 μL) |
| Risk of sample contamination | possible (except vacuum and closed devices) | extremely high | high | high | extremely low | high | high |
| Visual control of blood loading | possible | possible | confined | possible | possible | possible (with control of sample volume) | possible (with control of sample volume) |
| Sample storage in RT | undesirable | undesirable | possible | possible, but with desiccant and in the dark | possible | possible, but with desiccant and in the dark | possible |
| Sample transportation | cold chain is required | cold chain is required | except for special conditions | except for special conditions | except for special conditions | except for special conditions | except for special conditions |
| Shipping by post | impossible | impossible | possible | possible | possible | possible | possible |
| Visualization |  |  |  |  |  |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocur, A.; Pawiński, T. Volumetric Absorptive Microsampling in Therapeutic Drug Monitoring of Immunosuppressive Drugs—From Sampling and Analytical Issues to Clinical Application. Int. J. Mol. Sci. 2023, 24, 681. https://doi.org/10.3390/ijms24010681
Kocur A, Pawiński T. Volumetric Absorptive Microsampling in Therapeutic Drug Monitoring of Immunosuppressive Drugs—From Sampling and Analytical Issues to Clinical Application. International Journal of Molecular Sciences. 2023; 24(1):681. https://doi.org/10.3390/ijms24010681
Chicago/Turabian StyleKocur, Arkadiusz, and Tomasz Pawiński. 2023. "Volumetric Absorptive Microsampling in Therapeutic Drug Monitoring of Immunosuppressive Drugs—From Sampling and Analytical Issues to Clinical Application" International Journal of Molecular Sciences 24, no. 1: 681. https://doi.org/10.3390/ijms24010681
APA StyleKocur, A., & Pawiński, T. (2023). Volumetric Absorptive Microsampling in Therapeutic Drug Monitoring of Immunosuppressive Drugs—From Sampling and Analytical Issues to Clinical Application. International Journal of Molecular Sciences, 24(1), 681. https://doi.org/10.3390/ijms24010681







