3D Hierarchical Porous and N-Doped Carbonized Microspheres Derived from Chitin for Remarkable Adsorption of Congo Red in Aqueous Solution
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fabrication of N-Doped CM-Chitin
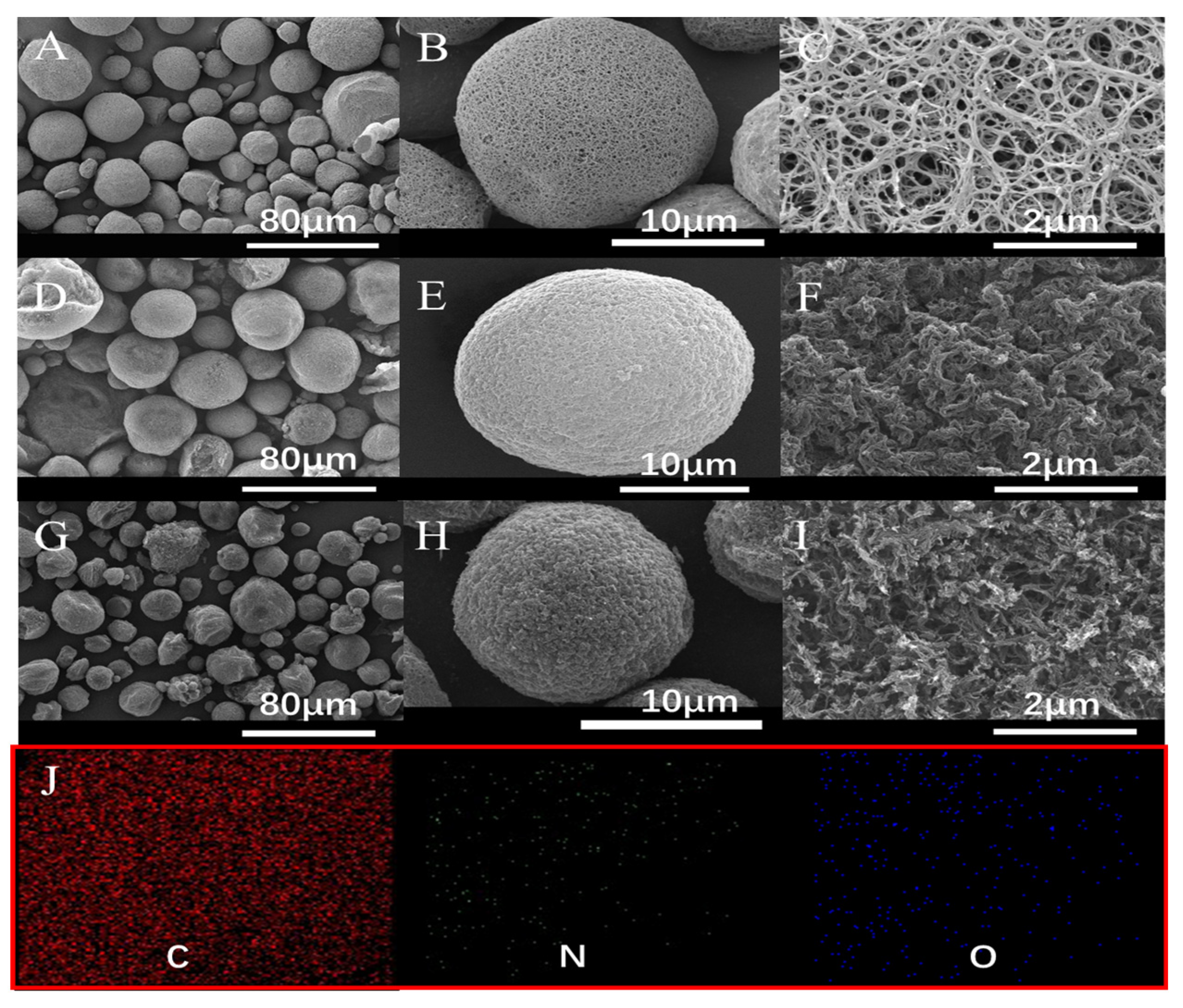
2.2. Characterizations
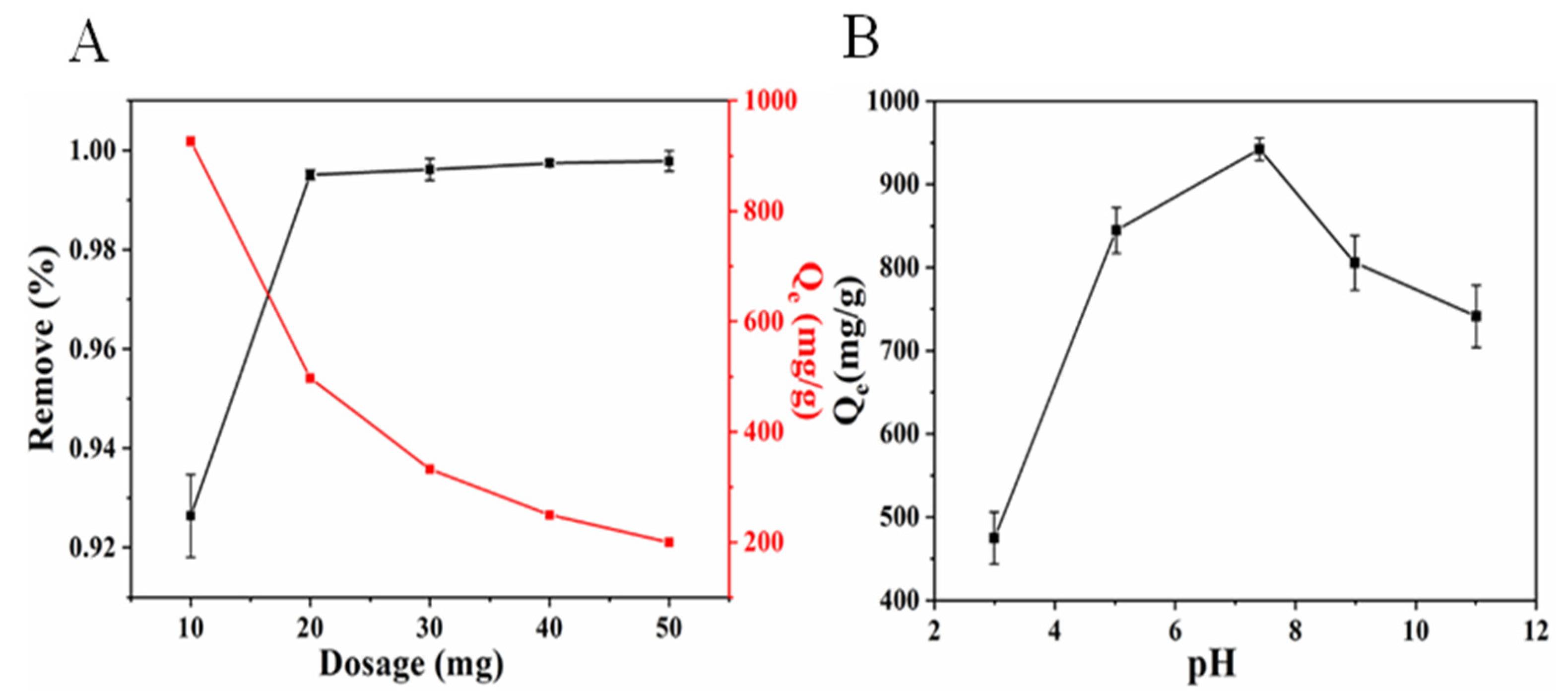
2.3. Optimization of Adsorption Conditions
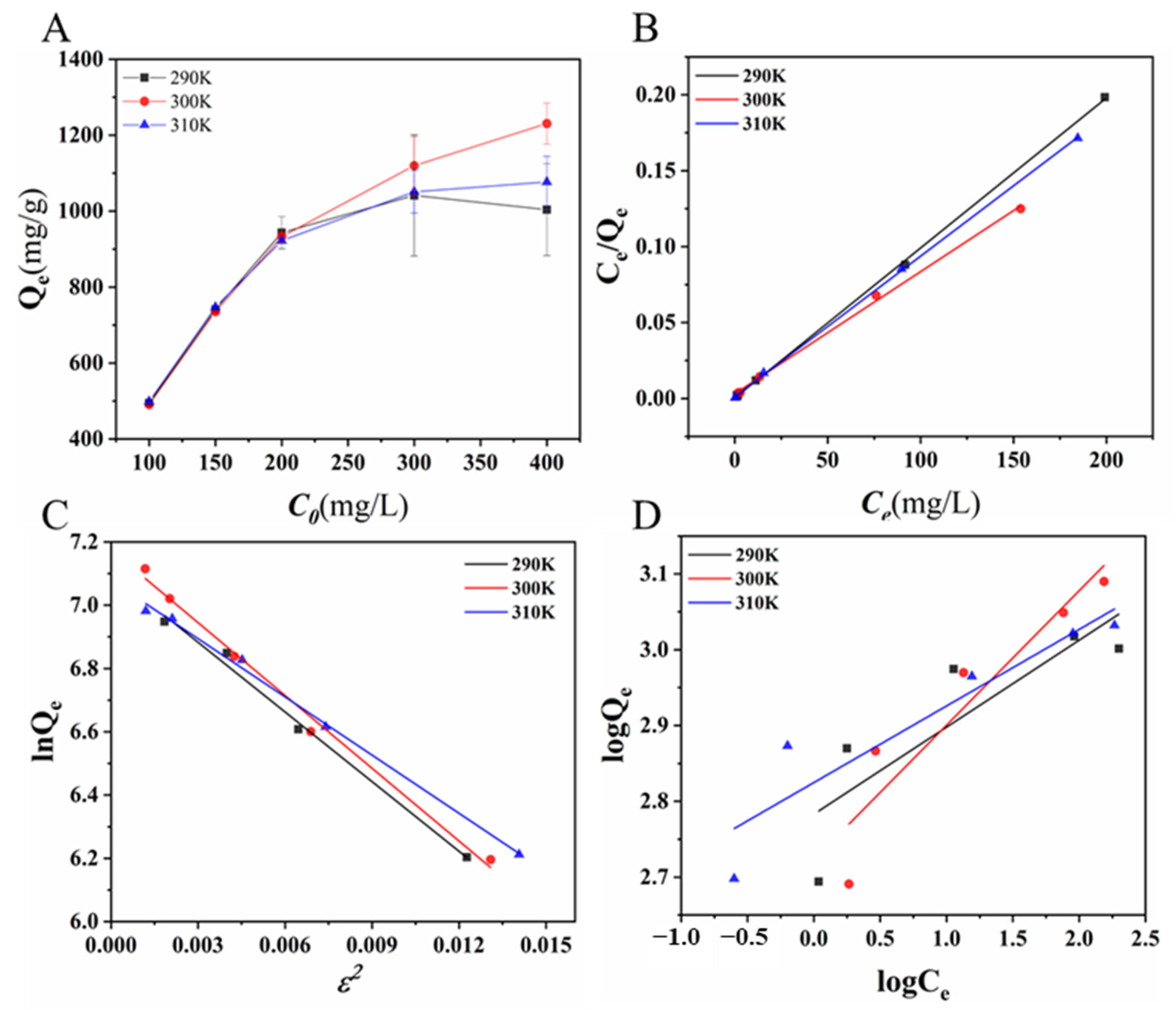
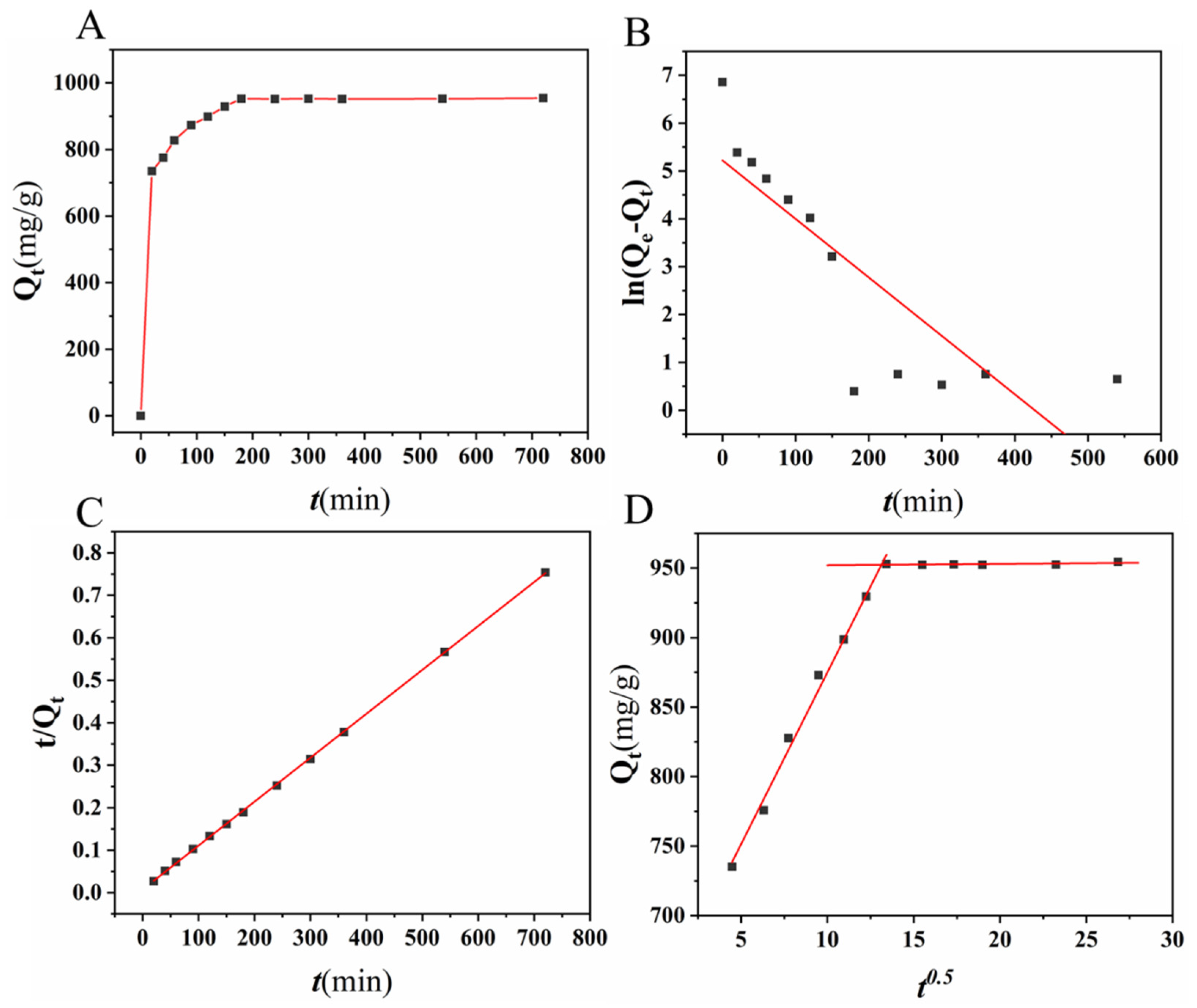
2.4. Adsorption Isotherms, Kinetics, and Thermodynamics
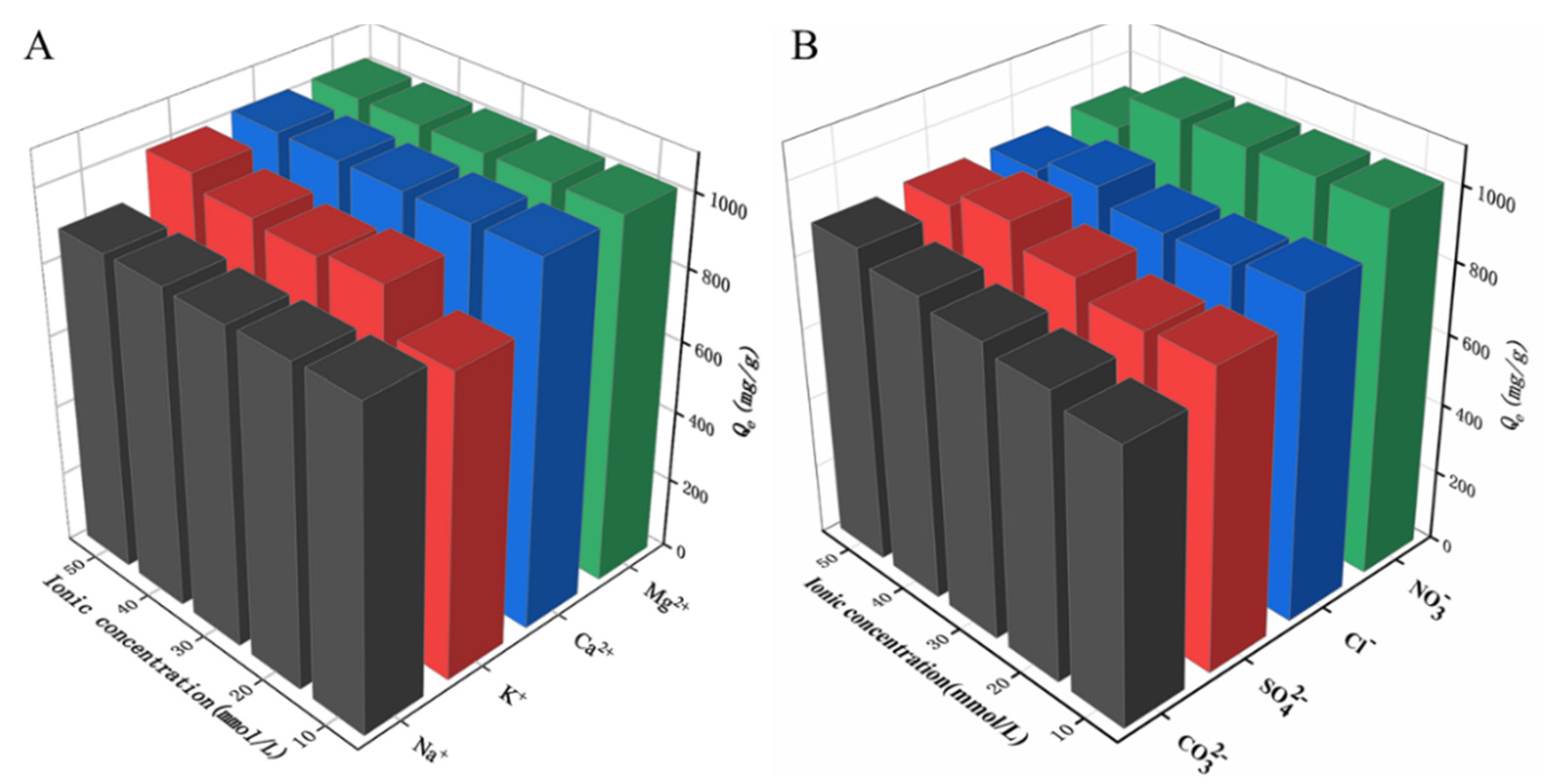
2.5. Effect of Salt Ions
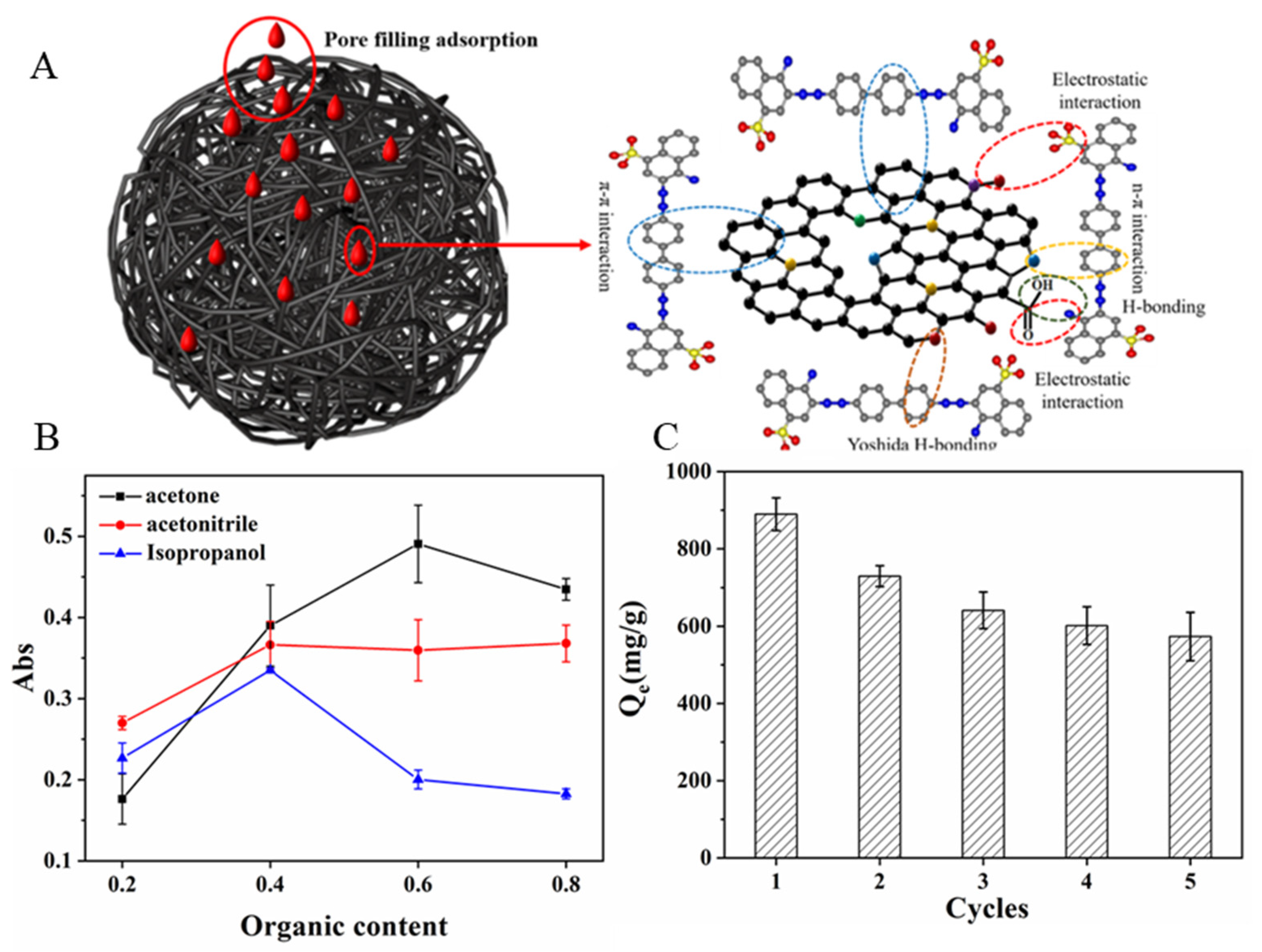
2.6. Possible Adsorption Mechanism
2.7. Regeneration and Reuse
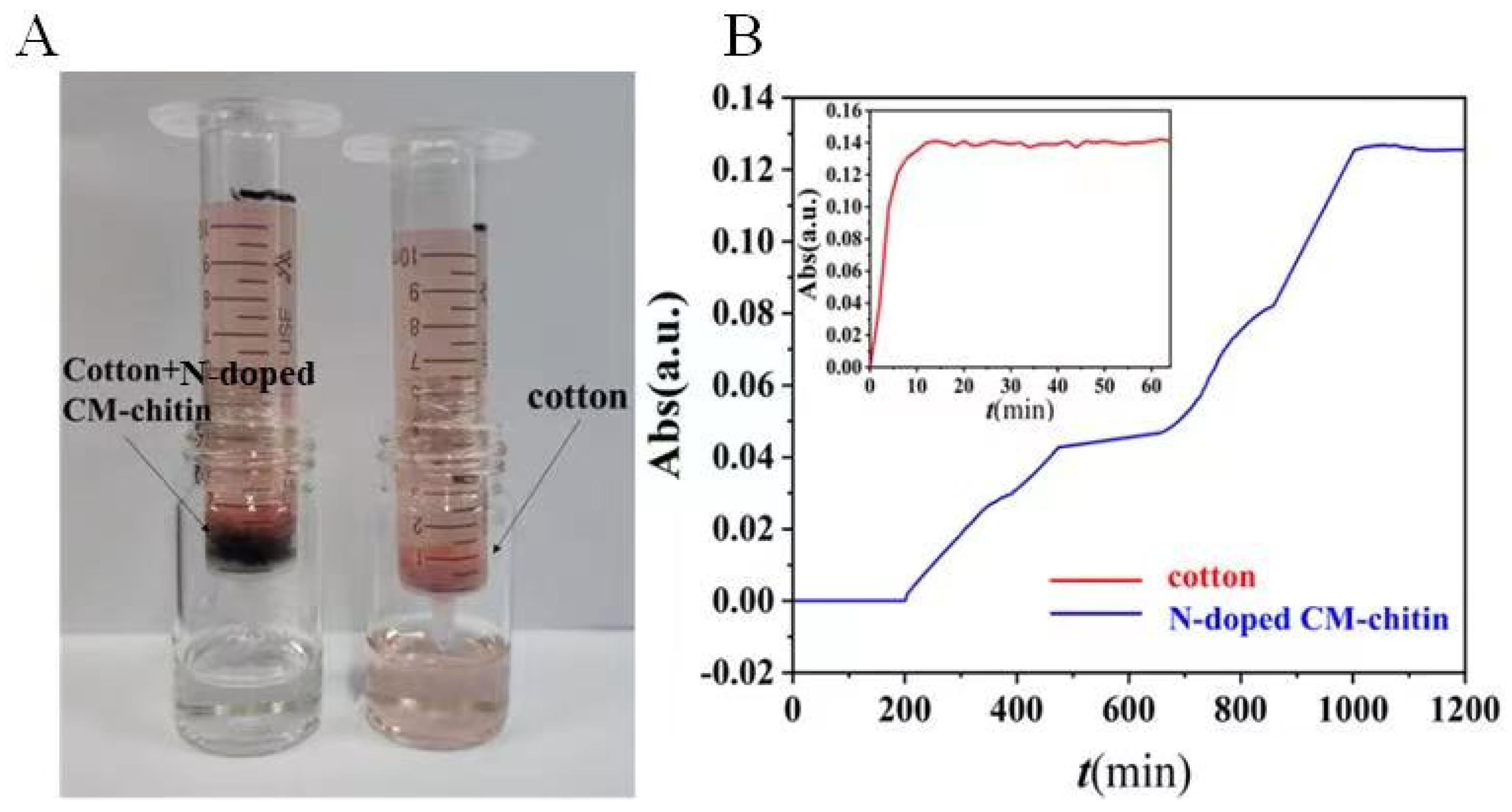
2.8. Dynamic Removal of N-Doped CM-Chitin toward CR
2.9. Comparison of Adsorption Capacity with Different Sorbents
3. Materials and Methods
3.1. Materials
3.2. Fabrication of N-Doped CM-Chitin
3.3. Characterizations
3.4. Adsorption Experiments
3.5. Desorption, Regeneration, and Re-Usability of N-Doped CM-Chitin
3.6. Dynamic Removal of N-Doped CM-Chitin toward CR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azhdari, R.; Mousavi, S.M.; Hashemi, S.A.; Bahrani, S.; Ramakrishna, S. Decorated graphene with aluminum fumarate metal organic framework as a superior non-toxic agent for efficient removal of Congo Red dye from wastewater. J. Environ. Eng. 2019, 7, 103437. [Google Scholar] [CrossRef]
- Gupta, V.K.; Kumar, R.; Nayak, A.; Saleh, T.A.; Barakat, M.A. Adsorption removal of dyes from aqueous solution onto carbon nanotubes: A review. Adv. Colloid Interface Sci. 2013, 193–194, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, K.; AlSalhi, M.S.; Sanganyado, E.; Devanesan, S.; Arulprakash, A.; Rajasekar, A. Sequential electrochemical oxidation and bio-treatment of the azo dye congo red and textile effluent. J. Photoch. Photobio. B 2019, 200, 111655. [Google Scholar] [CrossRef] [PubMed]
- Shaban, M.; Abukhadra, M.R.; Shahien, M.G.; Ibrahim, S.S. Novel bentonite/zeolite-NaP composite efficiently removes methylene blue and Congo red dyes. Environ. Chem. Lett. 2018, 16, 275–280. [Google Scholar] [CrossRef]
- Chowdhyry, A.; Kumari, S.; Khan, A.A.; Hussain, S. Synthesis of mixed phase crystalline CoN12S4 nanomaterial and selective mechanism for adsorption of Congo red from aqueous solution. J. Environ. Chem. Eng. 2021, 9, 106554. [Google Scholar] [CrossRef]
- Sharma, G.; AlGarni, T.S.; Kumar, P.S.; Bhogal, S.; Kumar, A.; Sharma, S.; Stadler, F.J. Utilization of Ag2O–Al2O3–ZrO2 decorated onto rGO as adsorbent for the removal of Congo red from aqueous solution. Environ. Res. 2021, 197, 111179. [Google Scholar] [CrossRef]
- Manzoor, J.; Sharma, M. Impact of textile dyes on human health and environment. In Impact of Textile Dyes on Public Health and the Environment; IGI Global: Hershey, PA, USA, 2020; pp. 162–169. [Google Scholar]
- Sathiyavimal, S.; Vasantharaj, S.; Shanmugavel, M.; Manikandan, E.; Nguyen-Tri, P.; Brindhadevi, K.; Pugazhendhi, A. Facile synthesis and characterization of hydroxyapatite from fish bones: Photocatalytic degradation of industrial dyes (crystal violet and Congo red). Prog. Org. Coat. 2020, 148, 105890. [Google Scholar] [CrossRef]
- Raval, N.P.; Shah, P.U.; Ladha, D.G.; Wadhwani, P.M.; Shah, N.K. Comparative study of chitin and chitosan beads for the adsorption of hazardous anionic azo dye Congo Red from wastewater. Desalin. Water Treat. 2016, 57, 9247–9262. [Google Scholar] [CrossRef]
- Shetti, N.P.; Malode, S.J.; Malladi, R.S.; Nargund, S.L.; Shukla, S.S.; Aminabhavi, T.M. Electrochemical detection and degradation of textile dye Congo red at graphene oxide modified electrode. Microchem. J. 2019, 146, 387–392. [Google Scholar] [CrossRef]
- Buthiyappan, A.; Aziz, A.R.A.; Daud, W.M.A.W. Recent advances and prospects of catalytic advanced oxidation process in treating textile effluents. Rev. Chem. Eng. 2016, 32, 1–47. [Google Scholar] [CrossRef]
- Najafi, M.; Bastami, T.R.; Binesh, N.; Ayati, A.; Emamverdi, S. Sono-sorption versus adsorption for the removal of congo red from aqueous solution using NiFeLDH/Au nanocoposite: Kinetics, thermodynamics, isotherm studies, and optimization of process parameters. J. Ind. Eng. Chem. 2022, 116, 489–503. [Google Scholar] [CrossRef]
- Sharma, A.; Siddiqui, Z.M.; Dhar, S.; Mehta, P.; Pathania, D. Adsorptive removal of congo red dye (CR) from aqueous solution by Cornulaca monacantha stem and biomass-based activated carbon: Isotherm, kinetics and thermodynamics. Sep. Sci. Technol. 2019, 54, 916–929. [Google Scholar] [CrossRef]
- Amran, F.; Zaini, M.A.A. Sodium hydroxide-activated Casuarina empty fruit: Isotherm, kinetics and thermodynamics of methylene blue and congo red adsorption. Environ. Technol. Innov. 2021, 23, 101727. [Google Scholar] [CrossRef]
- Zhao, X.R.; Wang, W.; Zhang, Y.J.; Wu, S.Z.; Li, F.; Liu, J.P. Synthesis and characterization of gadolinium doped cobalt ferrite nanoparticles with enhanced adsorption capability for Congo Red. Chem. Eng. J. 2014, 250, 164–174. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Xie, J.; Oh, J.M. Synthesis of a mesoporous Mg–Al–mixed metal oxide with P123 template for effective removal of Congo red via aggregation-driven adsorption. J. Solid State Chem. 2021, 293, 121758. [Google Scholar]
- Achour, Y.; Bahsis, L.; Ablouh, E.; Yazid, H.; Laanari, M.R.; Haddad, M.E. Insight into adsorption mechanism of Congo red dye onto Bombax Buonopozense bark activated-carbon using central composite design and DFT studies. Surf. Interfaces 2021, 23, 100977. [Google Scholar] [CrossRef]
- Singh, S.; Perween, S.; Ranjan, A. Dramatic enhancement in adsorption of congo red dye in polymer-nanoparticles composite of polyaniline-zinc titanate. J. Environ. Chem. Eng. 2021, 9, 105149. [Google Scholar] [CrossRef]
- Nodehi, R.; Shayesteh, H.; Kelishami, A.R. Enhanced adsorption of congo red using cationic surfactant functionalized zeolite particles. Microchem. J. 2020, 153, 104281. [Google Scholar] [CrossRef]
- Jagusiak, A.; Goclon, J.; Panczyk, T. Adsorption of Evans blue and Congo red on carbon nanotubes and its influence on the fracture parameters of defective and functionalized carbon nanotubes studied suing computational methods. Appl. Surf. Sci. 2021, 539, 148236. [Google Scholar] [CrossRef]
- Li, Z.C.; Hanafy, H.; Zhang, L.; Sellaoui, L.; Netto, M.S.; Oliveira, M.L.S.; Seliem, M.K.; Dotto, G.L.; Bonilla-Petriciolet, A.; Li, Q. Adsorption of congo red and methylene blue dyes on an ashitaba waste and a walnut shell-based activated carbon from aqueous solutions: Experiments, characterization and physical interpretations. Chem. Eng. J. 2020, 388, 124263. [Google Scholar] [CrossRef]
- Ahma, R.; Ansari, K. Comparative study for adsorption of congo red and methylene blue dye on chitosan modified hybrid nanocomposite. Process Biochem. 2021, 108, 90–102. [Google Scholar] [CrossRef]
- Chatterjee, S.; Lee, D.S.; Lee, M.W.; Woo, S.H. Enhanced adsorption of congo red from aqueous solutions by chitosan hydrogel beads impregnated with cetyl trimethyl ammonium bromide. Bioresour. Technol. 2009, 100, 2803–2809. [Google Scholar] [CrossRef] [PubMed]
- Vimonses, V.; Lei, S.; Jin, B.; Chow, C.W.K.; Saint, C. Kinetic study and equilibrium isotherm analysis of Congo Red adsorption by clay materials. Chem. Eng. J. 2009, 148, 354–364. [Google Scholar] [CrossRef]
- Ummartyotin, S.; Pechyen, C. Strategies for development and implementation of bio-based materials as effective renewable resources of energy: A comprehensive review on adsorbent technology. Renew. Sust. Energ. Rev. 2016, 62, 654–664. [Google Scholar] [CrossRef]
- Abdel-Rahman, L.H.; Abu-Dief, A.M.; Sayed, M.A.A.E.; Zikry, M.M. Nano sized moringa oleifera an effective strategy for Pb(II) inos removal from aqueous solution. Chem. Mater. Res. 2016, 8, 8–22. [Google Scholar]
- Gholami, M.; Zare-Hoseinabadi, A.; Mohammadi, M.; Taghizadeh, S.; Behbahani, A.B.; Amani, A.M.; Solghar, R.A. Preparation of ZnXFe3-XO4@ chitosan Nanoparticles as an Adsorbent for Methyl Orange and Phenol. J. Environ. Treat. Tech. 2019, 7, 245–249. [Google Scholar]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived nanomaterials for sustainable water treatment: A review. Carbohydr. Polym. 2021, 251, 116986. [Google Scholar] [CrossRef]
- Hammi, N.; Chen, S.; Dumeignil, F.; Royer, S.; Kadib, A.E. Chitosan as s sustainable precursor for nitrogencontaining carbon nanomaterials: Synthsis and uses. Materals Today Sustain. 2020, 10, 100053. [Google Scholar] [CrossRef]
- Kaya, M.; Baran, T.; Mentes, A.; Asaroglu, M.; Sezen, G.; Tozak, K.O. Extraction and characterization of α-chitin and chitosan from six different aquatic invertebrates. Food Biophys. 2014, 9, 145–157. [Google Scholar] [CrossRef]
- Zamora, A.Z.; Mena, G.J.; Gonzalez, E.G. Removal of congo red from the aqueous phase by chitin and chitosan from waste shrimp. Desalination and Water Treatment. Desalination Water Treat. 2016, 57, 14674–14685. [Google Scholar] [CrossRef]
- Zheng, S.; Cui, Y.; Zhang, J.W.; Gu, Y.X.; Shi, X.W.; Peng, C.; Wang, D.H. Ntrogen doped microporous carbon nanospheres derived from chitin nanogels as attractive materials for supercapacitors. RSC Adv. 2019, 9, 10976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.; Goepel, M.; Colmenares, J.C.; Glaser, R. Chitosan-based N-doped carbon materials for electrocatalytic and photocatalytica applications. ACS Sustain. Chem. Eng. 2020, 8, 4708–4727. [Google Scholar] [CrossRef] [Green Version]
- Zheng, S.; Zhang, J.W.; Deng, H.B.; Du, Y.M.; Shi, X.W. Chitin derived nitrogen-doped porous carbons with ultrahigh specific surface area and tailored hierarchical porosity for high performance supercapacitors. J. Bioresour. Bioprod. 2021, 6, 142–151. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Hameed, B.H.; Hummadi, E.H. Review on recent progress in chiosan/chitin-carbonaceous material composites for the adsrortpion of water pollutants. Carbohydr. Polym. 2020, 247, 116690. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Duan, B.; Lu, A.; Zhang, L.N. Pd/TiO2@ carbon microspheres derived from chitin for highly efficient photocatalytic degradation of volatile organic compounds. ACS Sustain. Chem. Eng. 2018, 7, 1658–1666. [Google Scholar] [CrossRef]
- Zhou, Y.S.; Cai, T.M.; Liu, S.; Liu, Y.Y.; Chen, H.J.; Li, Z.T.; Peng, H.L. N-doped magnetic three-dimensional carbon microspheres@ TiO2 with a porous architecture for enhanced degradation of tetracycline and methyl orange via adsorption/photocatalysis synergy. Chem. Eng. J. 2021, 411, 128615. [Google Scholar] [CrossRef]
- Duan, B.; Liu, F.; He, M.; Zhang, L.N. Ag–Fe3O4 nanocomposites@chitin microspheres constructed by in situ one-pot synthesis for rapid hydrogenation catalysis. Green Chem. 2014, 16, 2835–2845. [Google Scholar] [CrossRef]
- Duan, B.; Gao, X.; Yao, X.; Fang, Y.; Huang, L.; Zhou, J.; Zhang, L.N. Unique elastic N-doped carbon nanofibrous microspheres with hierarchical porosity derived from renewable chitin for high rate supercapacitors. Nano Energy 2016, 27, 482–491. [Google Scholar] [CrossRef]
- Cui, W.G.; Lai, Q.B.; Zhang, L.; Wang, F.M. Quantitative measurements of sp3 content in DLC films with Raman spectroscopy. Surf. Coat. Tech. 2010, 205, 1995–1999. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B. 2000, 61, 14095. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, Y.S.; Hasegawa, K.; Hasegawa, Y.; Takahashi, N.; Kitahama, Y.; Fukuoka, S.; Itoh, T. Direct conversion of silver complexes to nanoscale hexagonal columns on a copper alloy for plasmonic applications. Phys. Chem. Chem. Phys. 2013, 15, 14611–14615. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Liu, J.Y.; Kelly, S.J.; Huang, X.J.; Liu, J.H. Biomimetic snowflake-shaped magnetic micro-/nanostructures for highly efficient adsorption of heavy metal ions and organic pollutants from aqueous solution. J. Mater. Chem. A 2014, 2, 11759–11767. [Google Scholar] [CrossRef]
- Hu, D.Y.; Wang, P.; Li, J.; Wang, L.J. Functionalization of microcrystalline cellulose with n; n-dimethyldodecylamine for the removal of Congo red dye from an aqueous solution. Bioresources 2014, 9, 5951–5962. [Google Scholar] [CrossRef]
- Kammah, M.E.; Elkhatib, E.; Gouveia, S.; Cameselle, C.; Aboukila, E. Cost-effective ecofriendly nanoparticles for rapid and efficient indigo carmine dye removal from wastewater: Adsorption equilibrium, kinetics and mechanism. Environ. Technol. Innov. 2022, 28, 102595. [Google Scholar] [CrossRef]
- Sun, X.F.; Wang, S.G.; Liu, X.W.; Gong, W.X.; Bao, N.; Ma, Y. The effects of pH and ionic strength on fulvic acid uptake by chitosan hydrogel beads. Colloid. Surface. A 2008, 324, 28–34. [Google Scholar] [CrossRef]
- Dichiara, A.B.; Smith, J.B.; Rogers, R.E. Enhanced adsorption of carbon nanocomposites exhausted with 2,4-dichlorophenoxyacetic acid after regeneration by thermal oxidation and microwave irradiation. Environ. Sci. Nano 2014, 1, 113–116. [Google Scholar] [CrossRef]
- Good man, S.M.; Bura, R.; Dichiara, A.B. Facile impregnation of graphene into porous wood filters for the dynamic removal and recovery of dyes from aqueous soluitons. ACS Appl. Nano Mater. 2018, 1, 5682–5690. [Google Scholar] [CrossRef]
- Dichiara, A.B.; Harlander, S.F.; Rogers, R.E. Fixed bed adsorption of diquat dibromide from aqueous solution using carbon nanotubes. RSC Adv. 2015, 5, 61508–61512. [Google Scholar] [CrossRef]
- Hou, F.; Wang, D.; Ma, X.; Fan, L.; Ding, T.; Ye, X.; Liu, D. Enhanced adsorption of Congo red using chitin suspension after sonoenzymolysis. Ultrason. Sonochem. 2021, 70, 105327. [Google Scholar] [CrossRef]
- Zolgharnein, J.; Asanjarani, N.; Mousavi, S. Optimization and Characterization of Tl(I) Adsorption onto Modified Ulmus carpinifolia Tree Leaves. Clean Soil Air Water 2011, 39, 250–258. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, Y.; Chen, J. Zirconium/polyvinyl alcohol modified flat-sheet polyvinyldene fluoride membrane for decontamination of arsenic: Material design and optimization, study of mechanisms, and application prospects. Chemosphere 2016, 155, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Miraboutalebi, S.; Nikouzad, S.; Peydayesh, M.; Allahgholi, N.; Vafajoo, L.; McKay, G. Methylene blue adsorption via maize silk powder: Kinetic, equilibrium, thermodynamic studies and residual error analysis. Process Saf. Environ. Prot. 2017, 106, 191–202. [Google Scholar] [CrossRef]
- Lim, L.; Priyantha, N.; Tennakoon, D.; Chieng, H.; Dahri, M.; Suklueng, M. Breadnut peel as a highly effective low-cost biosorbent for methylene blue: Equilibrium, thermodynamic and kinetic studies. Arab. J. Chem. 2017, 10, S3216–S3228. [Google Scholar] [CrossRef] [Green Version]
- Lian, L.L.; Guo, L.P.; Guo, C.J. Adsorption of Congo red from aqueous solutions onto Ca-bentonite. J. Hazard. Mater. 2009, 161, 126–131. [Google Scholar] [CrossRef]
- Mohebali, S.; Bastani, D.; Shayesteh, H. Equilibrium, kinetic and thermodynamic studies of a low-cost biosorbent for the removal of Congo red dye: Acid and CTAB-acid modified celery (Apium graveolens). J. Mol. Struct. 2019, 1176, 181–193. [Google Scholar] [CrossRef]
- Lei, C.S.; Pi, M.; Jiang, C.J.; Cheng, B.; Yu, J.G. Synthesis of hierarchical porous zinc oxide (ZnO) microspheres with highly efficient adsorption of Congo red. J. Colloid. Interf. Sci. 2017, 490, 242–251. [Google Scholar] [CrossRef]
- Hu, H.M.; Deng, C.H.; Sun, M.; Zhang, K.H.; Wang, M.; Xu, J.Y.; Le, H.R. Facile template-free synthesis of hierarchically porous NiO hollow architectures with high-efficiency adsorptive removal of Congo red. J. Porous. Mat. 2019, 26, 1743–1753. [Google Scholar] [CrossRef]
- Liu, J.Y.; Yu, H.J.; Wang, L. Superior absorption capacity of tremella like ferrocene based metal-organic framework in removal of organic dye from water. J. Hazard. Mater. 2020, 392, 122274. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Wang, G.; Yang, W.N.; Yang, J.; Liu, Y. Bioinspired zeolitic imidazolate framework (ZIF-8) magnetic micromotors for highly efficient removal of organic pollutants from water. J. Colloid Interface Sci. 2019, 555, 234–244. [Google Scholar] [CrossRef]
- Lei, C.S.; Zhu, X.F.; Zhu, B.C.; Jiang, C.J.; Le, Y.; Yu, J.G. Superb adsorption capacity of hierarchical calcined Ni/Mg/Al layered double hydroxides for Congo red and Cr (VI) ions. J. Hazard. Mater. 2017, 321, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fan, Q.H.; Wu, Y.J.; Wang, X.; Chen, C.L.; Tang, Z.Y.; Wang, X.K. Magnetic polydopamine decorated with Mg–Al LDH nanoflakes as a novel bio-based adsorbent for simultaneous removal of potentially toxic metals and anionic dyes. J. Mater. Chem. A 2016, 4, 1737–1746. [Google Scholar] [CrossRef]
- Yu, Z.C.; Hu, C.S.; Dichiara, A.B.; Jiang, W.H.; Gu, J. Cellulose Nanofibril/carbon nanomaterial hybrid aerogels for adsorption removal of cationic and anionic organic dyes. Nanomaterials 2020, 10, 169. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, T.; Chen, H.; Yao, L.; Peng, H. 3D Hierarchical Porous and N-Doped Carbonized Microspheres Derived from Chitin for Remarkable Adsorption of Congo Red in Aqueous Solution. Int. J. Mol. Sci. 2023, 24, 684. https://doi.org/10.3390/ijms24010684
Cai T, Chen H, Yao L, Peng H. 3D Hierarchical Porous and N-Doped Carbonized Microspheres Derived from Chitin for Remarkable Adsorption of Congo Red in Aqueous Solution. International Journal of Molecular Sciences. 2023; 24(1):684. https://doi.org/10.3390/ijms24010684
Chicago/Turabian StyleCai, Taimei, Huijie Chen, Lihua Yao, and Hailong Peng. 2023. "3D Hierarchical Porous and N-Doped Carbonized Microspheres Derived from Chitin for Remarkable Adsorption of Congo Red in Aqueous Solution" International Journal of Molecular Sciences 24, no. 1: 684. https://doi.org/10.3390/ijms24010684





