Regorafenib and Ruthenium Complex Combination Inhibit Cancer Cell Growth by Targeting PI3K/AKT/ERK Signalling in Colorectal Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Cytotoxicity of Regorafenib-Ruthenium Combination on HCT116, HT29, and REG-HCT116-R Cell Lines
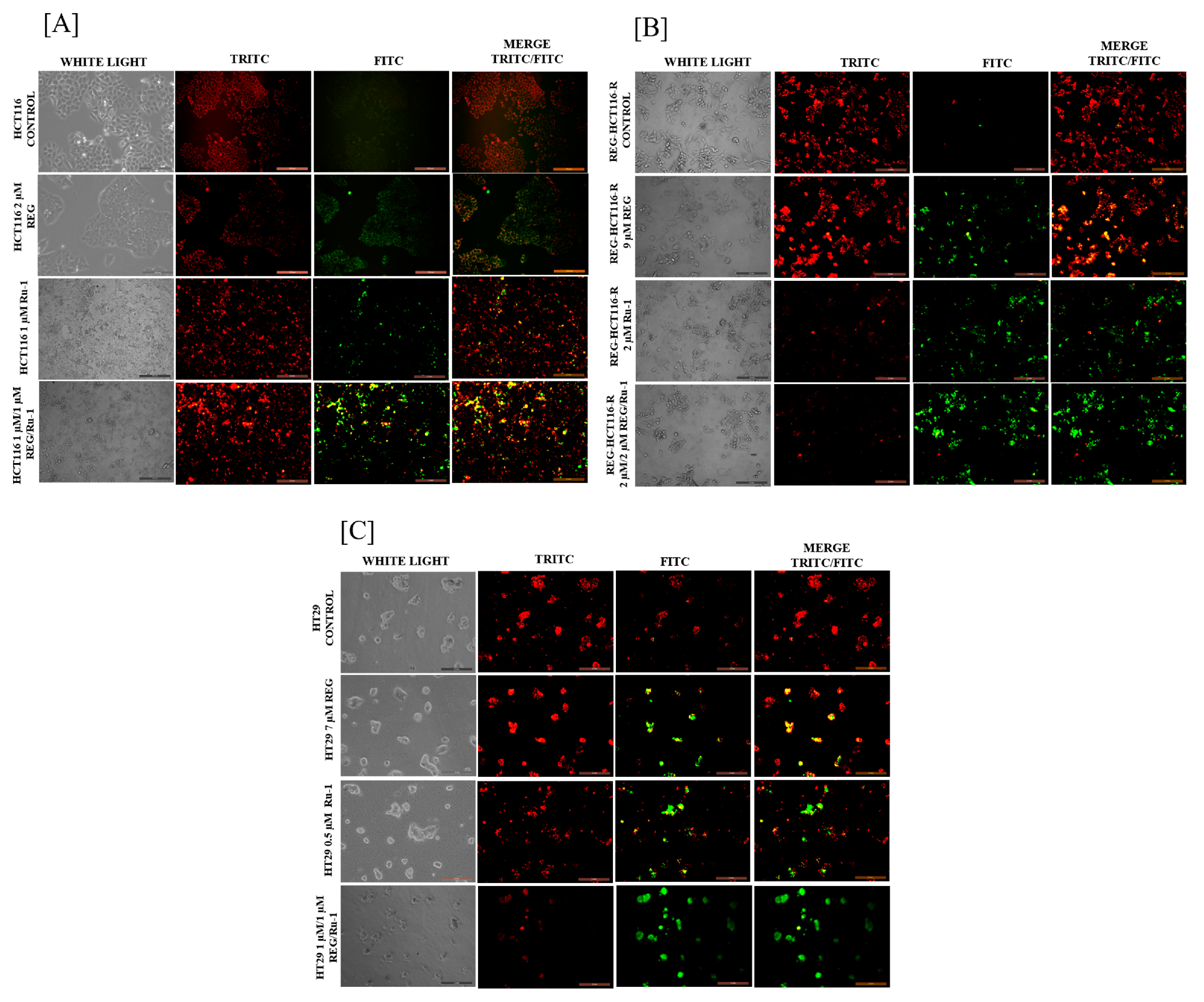
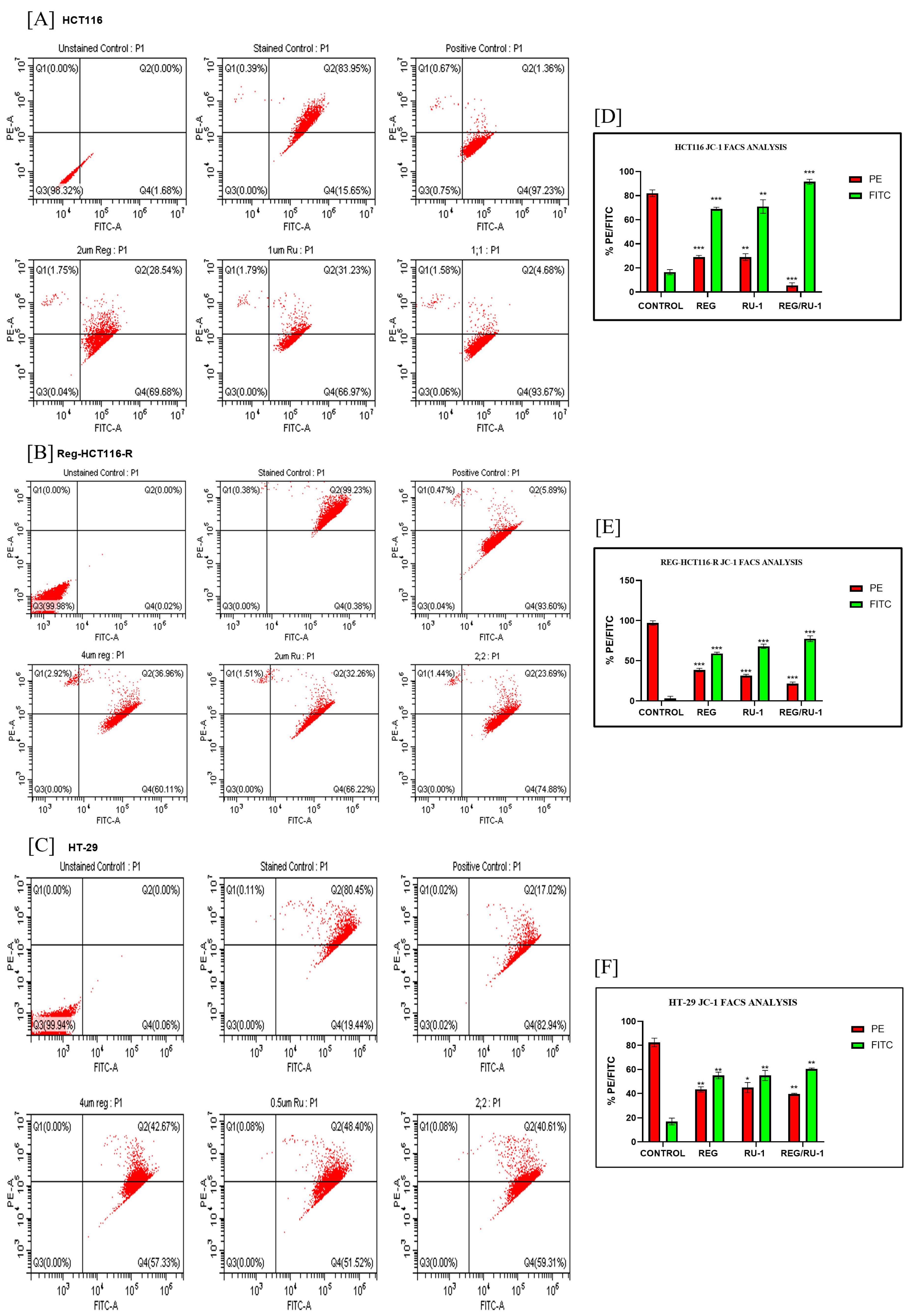
2.2. Regorafenib–Ruthenium Combination-Induced Cell Death
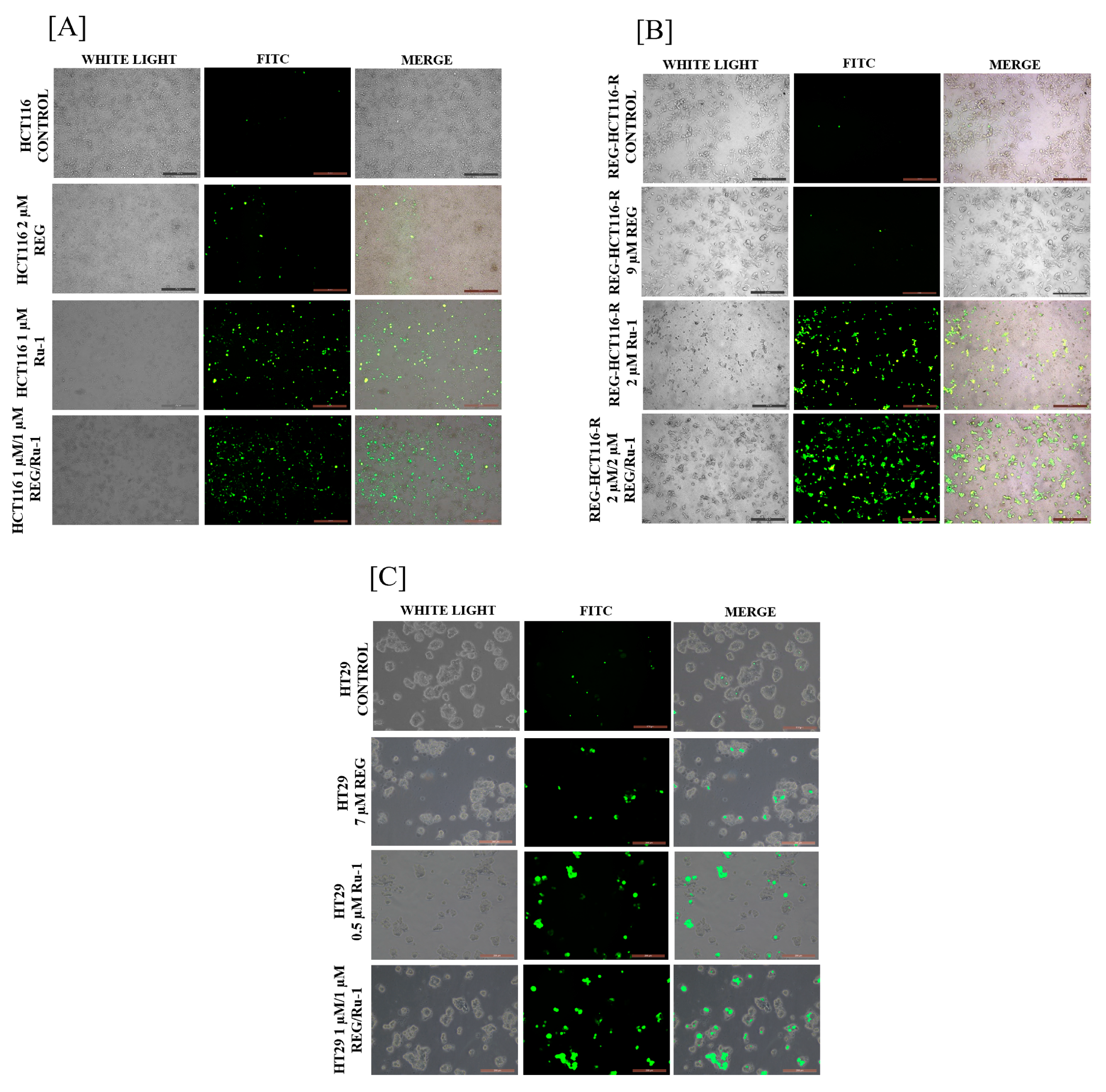
2.3. Reactive Oxygen Species Generation Increases in Reg/Ru-1-Treated Cells
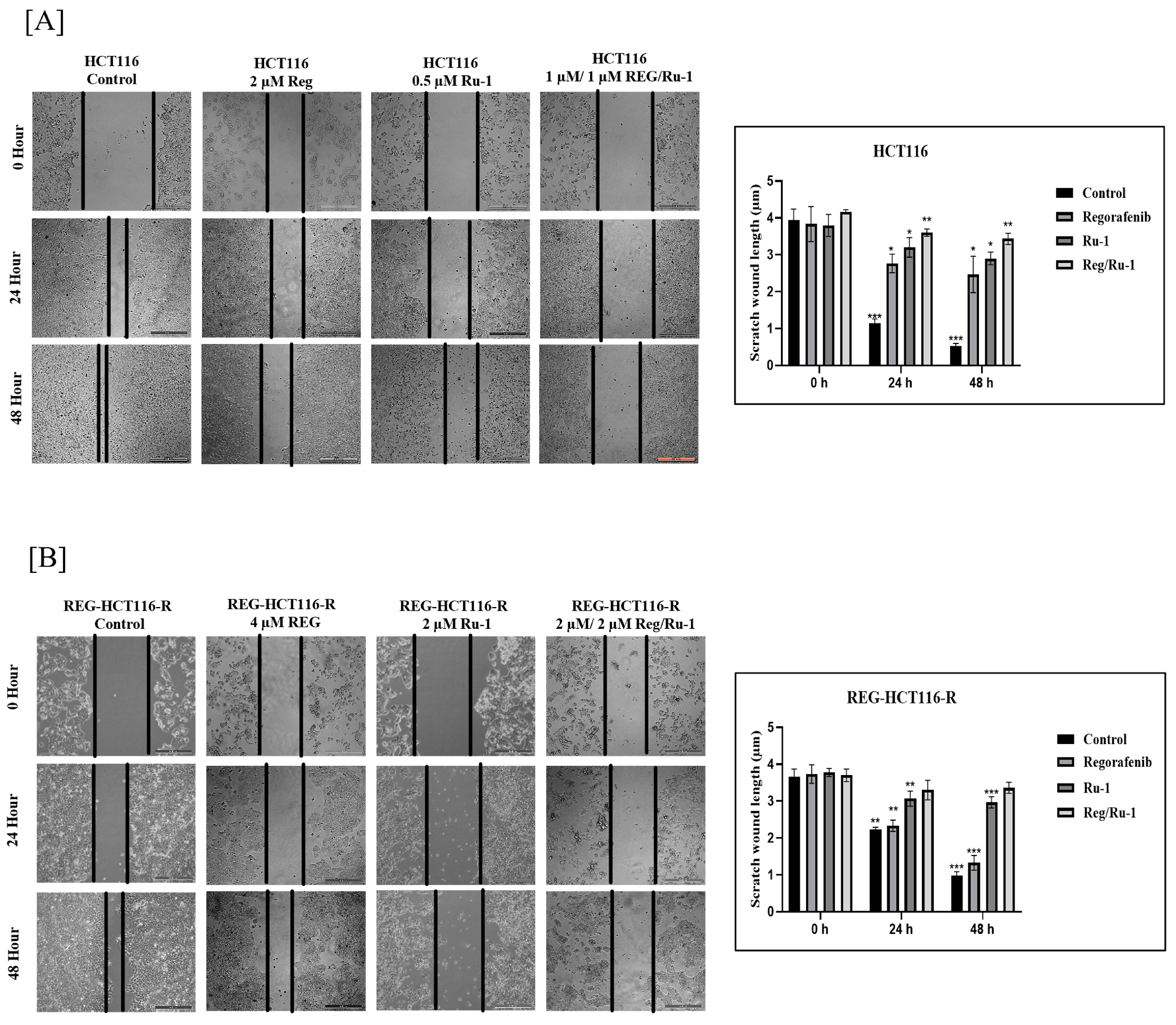
2.4. Drug-Combination-Treated Cells Exhibited Effective Inhibition in Wound-Healing and Cell Migratory Activity
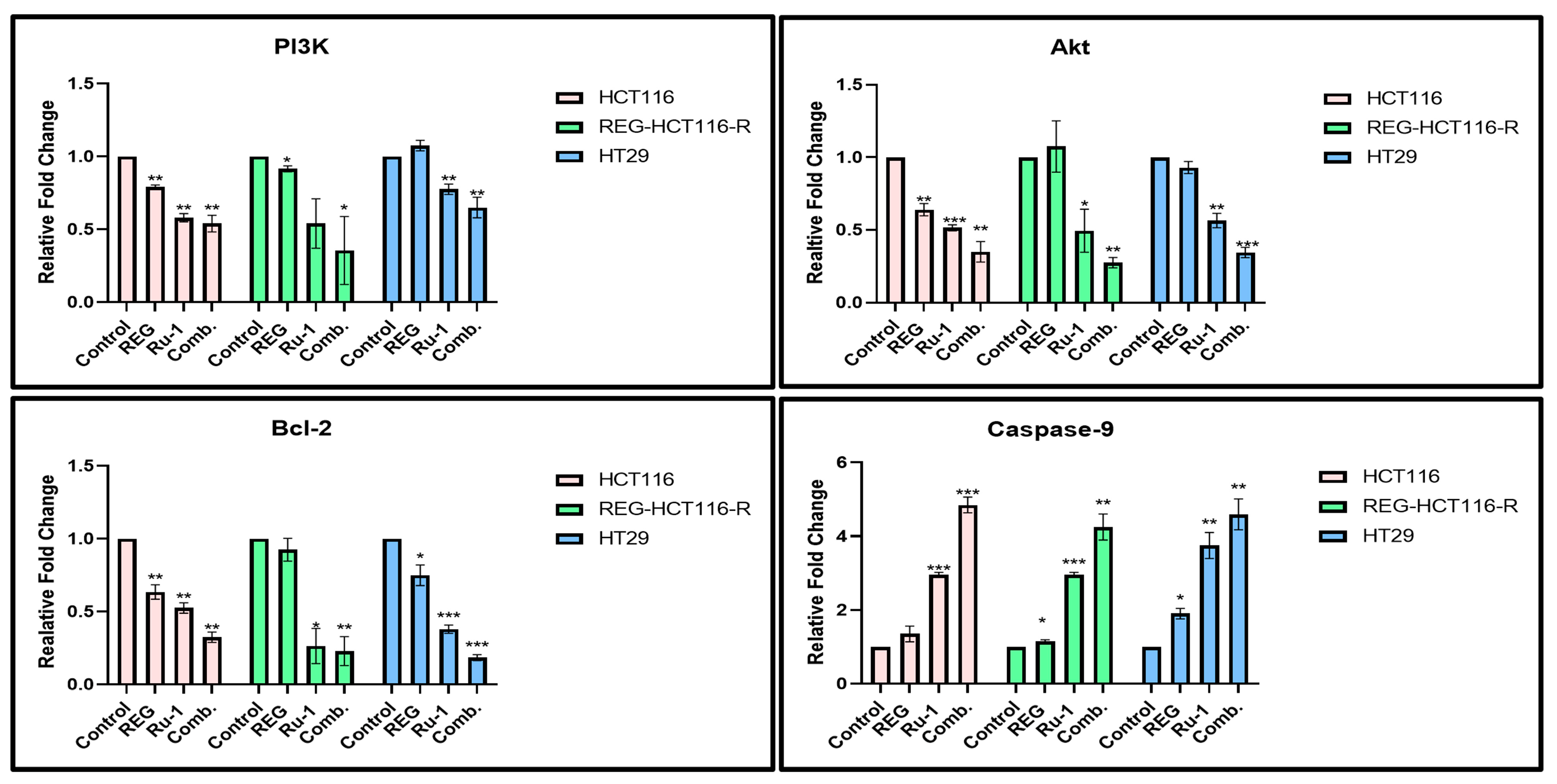
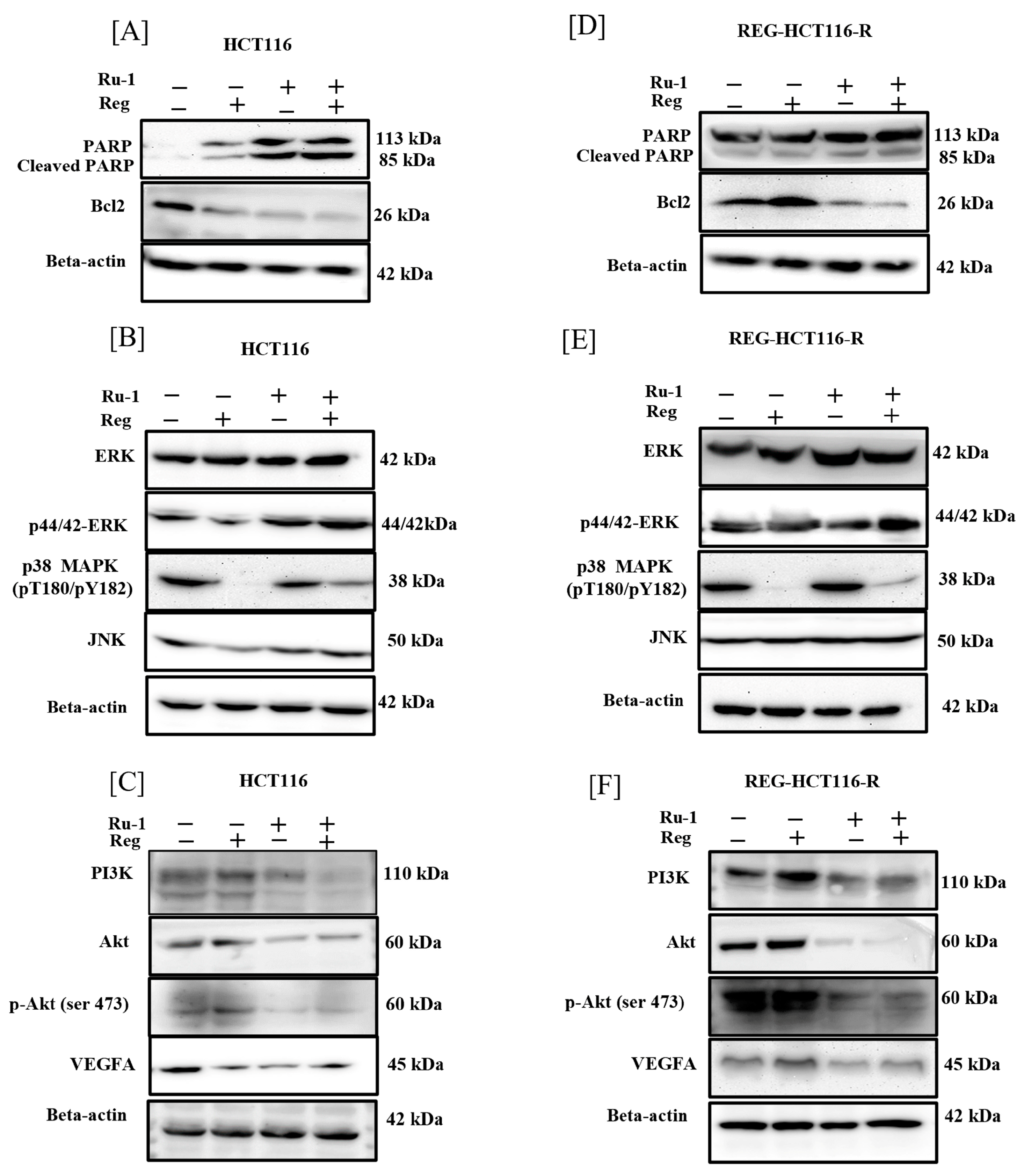
2.5. Change in Apoptosis-Associated Genes and Protein Expression in Reg/Ru-1-Treated Cells
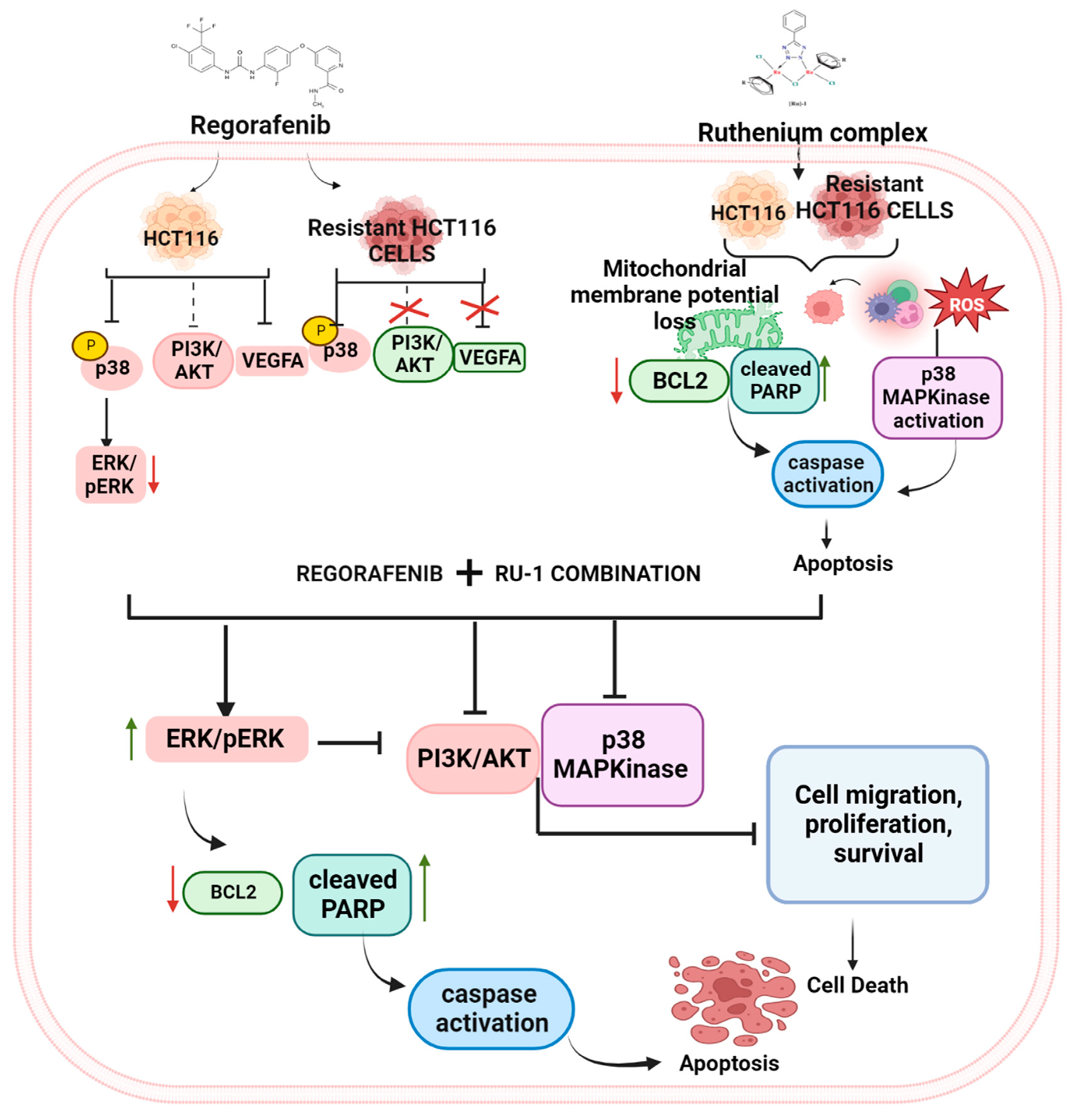
2.6. Reg/Ru-1 Combination Treatment Inhibits the PI3K/Akt Signalling and Activates the ERK-Mediated Cell–Death Pathway
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Media
4.3. Development of Regorafenib-Resistant HCT116 Cell Line
4.4. Cell Viability Assay and Combination Index
4.5. Propidium Iodide Staining (PI)
4.6. Colony Formation Assay
4.7. Mitochondrial Membrane Potential Loss JC-1 Staining
4.8. Reactive Oxygen Species (ROS) Study
4.9. Scratch and Wound Healing Assay
4.10. Real-Time-qPCR
4.11. Immunoblotting
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biller, L.H.; Schrag, D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA 2021, 325, 669–685. [Google Scholar] [CrossRef] [PubMed]
- Dagogo-Jack, I.; Shaw, A.T. Tumour heterogeneity and resistance to cancer therapies. Nat. Rev. Clin. Oncol. 2018, 15, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhou, L.; Xie, N.; Nice, E.C.; Zhang, T.; Cui, Y.; Huang, C. Overcoming cancer therapeutic bottleneck by drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 113. [Google Scholar] [CrossRef] [PubMed]
- Delude, C. Combinations go on trial. Cancer Discov. 2012, 2, 8. [Google Scholar] [CrossRef][Green Version]
- Rosenberg, B.; Van Camp, L.; Krigas, T. Inhibition of Cell Division in Escherichia coli by Electrolysis Products from a Platinum Electrode. Nature 1965, 205, 698–699. [Google Scholar] [CrossRef]
- Wong, E.; Giandornenico, C.M. Current Status of Platinum-Based Antitumor Drugs. Chem. Rev. 1999, 99, 2451–2466. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Guo, Z. Functionalization of Platinum Complexes for Biomedical Applications. Acc. Chem. Res. 2015, 48, 2622–2631. [Google Scholar] [CrossRef]
- Bergamo, A.; Gaiddon, C.; Schellens, J.H.M.; Beijnen, J.H.; Sava, G. Approaching tumour therapy beyond platinum drugs: Status of the art and perspectives of ruthenium drug candidates. J. Inorg. Biochem. 2012, 106, 90–99. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Jin, S.; Muhammad, N.; Guo, Z. Stimuli-Responsive Therapeutic Metallodrugs. Chem. Rev. 2019, 119, 1138–1192. [Google Scholar] [CrossRef]
- Thota, S.; Rodrigues, D.A.; Crans, D.C.; Barreiro, E.J. Ru(II) Compounds: Next-Generation Anticancer Metallotherapeutics? J. Med. Chem. 2018, 61, 5805–5821. [Google Scholar] [CrossRef]
- Zhao, Z.; Gao, P.; You, Y.; Chen, T. Cancer-Targeting Functionalization of Selenium-Containing Ruthenium Conjugate with Tumor Microenvironment-Responsive Property to Enhance Theranostic Effects. Chem. Eur. J. 2018, 24, 3289–3298. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Gupta, P.; Chen, Y.; Wang, E.; Ji, L.; Chao, H.; Chen, Z.-S. The development of anticancer ruthenium(II) complexes: From single molecule compounds to nanomaterials. Chem. Soc. Rev. 2017, 46, 5771–5804. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, Y.; Zheng, W.J.; Liu, J.; Wong, Y.S. Ruthenium polypyridyl complexes that induce mitochondria-mediated apoptosis in cancer cells. Inorg. Chem. 2010, 49, 6366–6368. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Gao, P.; Yu, L.; Ma, B.; You, Y.; Chan, L.; Mei, C.; Chen, T. Ruthenium complexes with phenylterpyridine derivatives target cell membrane and trigger death receptors-mediated apoptosis in cancer cells. Biomaterials 2017, 129, 111–126. [Google Scholar] [CrossRef]
- Meng, X.; Leyva, M.L.; Jenny, M.; Gross, I.; Benosman, S.; Fricker, B.; Harlepp, S.; Hébraud, P.; Boos, A.; Wlosik, P.; et al. A ruthenium-containing organometallic compound reduces tumor growth through induction of the endoplasmic reticulum stress gene CHOP. Cancer Res. 2009, 69, 5458–5466. [Google Scholar] [CrossRef]
- Li, G.; Sasaki, T.; Asahina, S.; Roy, M.C.; Mochizuki, T.; Koizumi, K. Patching of Lipid Rafts by Molecular Self-Assembled Nanofibrils Suppresses Cancer Cell Migration. Chem 2017, 2, 283–298. [Google Scholar] [CrossRef]
- Hu, X.; Lu, Y.; Shi, X.; Yao, T.; Dong, C.; Shi, S. Integrating in situ formation of nanozymes with mesoporous polydopamine for combined chemo, photothermal and hypoxia-overcoming photodynamic therapy. Chem. Commun. 2019, 55, 14785–14788. [Google Scholar] [CrossRef]
- Hu, X.; Lu, Y.; Dong, C.; Zhao, W.; Wu, X.; Zhou, L.; Chen, L.; Yao, T.; Shi, S. A RuII Polypyridyl Alkyne Complex Based Metal–Organic Frameworks for Combined Photodynamic/Photothermal/Chemotherapy. Chem. Eur. J. 2020, 26, 1668–1675. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Y.; Li, G.; Zhang, P.; Jin, C.; Zeng, L.; Ji, L.; Chao, H. Ruthenium(II) polypyridyl complexes as mitochondria-targeted two-photon photodynamic anticancer agents. Biomaterials 2015, 56, 140–153. [Google Scholar] [CrossRef]
- Chakrabortty, S.; Agrawalla, B.K.; Stumper, A.; Vegi, N.M.; Fischer, S.; Reichardt, C.; Kögler, M.; Dietzek, B.; Feuring-Buske, M.; Buske, C.; et al. Mitochondria Targeted Protein-Ruthenium Photosensitizer for Efficient Photodynamic Applications. J. Am. Chem. Soc. 2017, 139, 2512–2519. [Google Scholar] [CrossRef]
- Vyas, K.M.; Sharma, D.; Magani, S.K.J.; Mobin, S.M.; Mukhopadhyay, S. In vitro evaluation of cytotoxicity and antimetastatic properties of novel arene ruthenium(II)-tetrazolato compounds on human cancer cell lines. Appl. Organomet. Chem. 2021, 35, e6187. [Google Scholar] [CrossRef]
- Wei, N.; Chu, E.; Wu, S.Y.; Wipf, P.; Schmitz, J.C. The cytotoxic effects of regorafenib in combination with protein kinase D inhibition in human colorectal cancer cells. Oncotarget 2015, 6, 4745–4756. [Google Scholar] [CrossRef] [PubMed]
- Victorelli, S.; Passos, J.F. Reactive Oxygen Species Detection in Senescent Cells. Methods Mol. Biol. 2019, 1896, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhou, L.; Zhao, T.; Liu, X.; Zhang, P.; Liu, Y.; Zheng, X.; Li, Q. Caspase-9: Structure, mechanisms and clinical application. Oncotarget 2017, 8, 23996. [Google Scholar] [CrossRef]
- Subramonian, D.; Phanhthilath, N.; Rinehardt, H.; Flynn, S.; Huo, Y.; Zhang, J.; Messer, K.; Mo, Q.; Huang, S.; Lesperance, J.; et al. Regorafenib is effective against neuroblastoma in vitro and in vivo and inhibits the RAS/MAPK, PI3K/Akt/mTOR and Fos/Jun pathways. Br. J. Cancer 2020, 123, 568–579. [Google Scholar] [CrossRef]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 26. [Google Scholar] [CrossRef]
- Berg, K.C.G.; Eide, P.W.; Eilertsen, I.A.; Johannessen, B.; Bruun, J.; Danielsen, S.A.; Bjørnslett, M.; Meza-Zepeda, L.A.; Eknæs, M.; Lind, G.E.; et al. Multi-omics of 34 colorectal cancer cell lines—A resource for biomedical studies. Mol. Cancer 2017, 16, 116. [Google Scholar] [CrossRef]
- Weng, M.C.; Li, M.-H.; Chung, J.G.; Liu, Y.-C.; Wu, J.-Y.; Hsu, F.-T.; Wang, H.-E. Apoptosis induction and AKT/NF-κB inactivation are associated with regroafenib-inhibited tumor progression in non-small cell lung cancer in vitro and in vivo. Biomed. Pharmacother. 2019, 116, 109032. [Google Scholar] [CrossRef]
- Kumar, A.; Rajendran, V.; Sethumadhavan, R.; Purohit, R. AKT kinase pathway: A leading target in cancer research. Sci. World J. 2013, 2013, 756134. [Google Scholar] [CrossRef]
- Strumberg, D.; Schultheis, B. Regorafenib for cancer. Expert Opin. Investig. Drugs 2012, 21, 879–889. [Google Scholar] [CrossRef]
- Huynh, H.; Ngo, V.C.; Koong, H.N.; Poon, D.; Choo, S.P.; Thng, C.H.; Chow, P.; Ong, H.S.; Chung, A.; Soo, K.C. Sorafenib and rapamycin induce growth suppression in mouse models of hepatocellular carcinoma. J. Cell Mol. Med. 2009, 13, 2673–2683. [Google Scholar] [CrossRef] [PubMed]
- Refolo, M.G.; Lippolis, C.; Carella, N.; Cavallini, A.; Messa, C.; D’Alessandro, R. Chlorogenic Acid Improves the Regorafenib Effects in Human Hepatocellular Carcinoma Cells. Int. J. Mol. Sci. 2018, 19, 1518. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Li, S. Regorafenib delays the proliferation of hepatocellular carcinoma by inducing autophagy. Pharmazie 2018, 73, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, A.S.; Hagan, S.; Rath, O.; Kolch, W. MAP kinase signalling pathways in cancer. Oncogene 2007, 26, 3279–3290. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.M.; Dumas, J.; Adnane, L.; Lynch, M.; Carter, C.A.; Schütz, G.; Thierauch, K.-H.; Zopf, D. Regorafenib (BAY 73-4506): A new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int. J. Cancer 2011, 129, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Kapitza, S.; Jakupec, M.A.; Uhl, M.; Keppler, B.K.; Marian, B. The heterocyclic ruthenium(III) complex KP1019 (FFC14A) causes DNA damage and oxidative stress in colorectal tumor cells. Cancer Lett. 2005, 226, 115–121. [Google Scholar] [CrossRef]
- Thornton, T.M.; Rincon, M. Non-classical p38 map kinase functions: Cell cycle checkpoints and survival. Int. J. Biol. Sci. 2009, 5, 44–52. [Google Scholar] [CrossRef]
- Lin, K.; Rong, Y.; Chen, D.; Zhao, Z.; Bo, H.; Qiao, A.; Hao, X.; Wang, J. Combination of Ruthenium Complex and Doxorubicin Synergistically Inhibits Cancer Cell Growth by Down-Regulating PI3K/AKT Signaling Pathway. Front. Oncol. 2020, 10, 141. [Google Scholar] [CrossRef]
- Baliza, I.R.S.; Silva, S.L.R.; de Santos, L.S.; Neto, J.H.A.; Dias, R.B.; Sales, C.B.S.; Rocha, C.A.G.; Soares, M.B.P.; Batista, A.A.; Bezerra, D.P. Ruthenium Complexes With Piplartine Cause Apoptosis Through MAPK Signaling by a p53-Dependent Pathway in Human Colon Carcinoma Cells and Inhibit Tumor Development in a Xenograft Model. Front. Oncol. 2019, 9, 582. [Google Scholar] [CrossRef]
- Tan, B.J.; Chiu, G.N.C. Role of oxidative stress, endoplasmic reticulum stress and ERK activation in triptolide-induced apoptosis. Int. J. Oncol. 2013, 42, 1605–1612. [Google Scholar] [CrossRef]
- Yu, C.C.; Huang, S.Y.; Chang, S.F.; Liao, K.F.; Chiu, S.C. The Synergistic Anti-Cancer Effects of NVP-BEZ235 and Regorafenib in Hepatocellular Carcinoma. Molecules 2020, 25, 2454. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Zhao, Z.Z.; Bo, H.B.; Hao, X.J.; Wang, J.Q. Applications of ruthenium complex in tumor diagnosis and therapy. Front. Pharmacol. 2018, 9, 1323. [Google Scholar] [CrossRef] [PubMed]
- Tangchirakhaphan, S.; Innajak, S.; Nilwarangkoon, S.; Tanjapatkul, N.; Mahabusrakum, W.; Watanapokasin, R. Mechanism of apoptosis induction associated with ERK1/2 upregulation via goniothalamin in melanoma cells. Exp. Ther. Med. 2018, 15, 3052. [Google Scholar] [CrossRef] [PubMed]
- McCubrey, J.A.; Steelman, L.S.; Chappell, W.H.; Abrams, S.L.; Wong, E.W.T.; Chang, F.; Lehmann, B.; Terrian, D.M.; Milella, M.; Tafuri, A.; et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta Mol. Cell Res. 2007, 1773, 1263–1284. [Google Scholar] [CrossRef] [PubMed]
- Owuor, E.D.; Kong, A.N.T. Antioxidants and oxidants regulated signal transduction pathways. Biochem. Pharmacol. 2002, 64, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Heffeter, P.; Atil, B.; Kryeziu, K.; Groza, D.; Koellensperger, G.; Körner, W.; Jungwirth, U.; Mohr, T.; Keppler, B.K.; Berger, W. The ruthenium compound KP1339 potentiates the anticancer activity of sorafenib in vitro and in vivo. Eur. J. Cancer. 2013, 49, 3366. [Google Scholar] [CrossRef]
- Van Meerloo, J.; Kaspers, G.J.L.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar] [CrossRef]
- Ashton, J.C. Drug combination studies and their synergy quantification using the Chou-Talalay method—Letter. Cancer Res. 2015, 75, 2400. [Google Scholar] [CrossRef]
- Riccardi, C.; Nicoletti, I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat. Protoc. 2006, 1, 1458–1461. [Google Scholar] [CrossRef]
- Rasool, F.; Sharma, D.; Anand, P.S.; Magani, S.K.J.; Tantravahi, S. Evaluation of the Anticancer Properties of Geranyl Isovalerate, an Active Ingredient of Argyreia nervosa Extract in Colorectal Cancer Cells. Front. Pharmacol. 2021, 12, 2396. [Google Scholar] [CrossRef]
- Sivandzade, F.; Bhalerao, A.; Cucullo, L. Analysis of the Mitochondrial Membrane Potential Using the Cationic JC-1 Dye as a Sensitive Fluorescent Probe. Bio-Protocols 2019, 9, e3128. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yotnda, P. Production and Detection of Reactive Oxygen Species (ROS) in Cancers. J. Vis. Exp. 2011, e3357. [Google Scholar] [CrossRef] [PubMed]
- Pijuan, J.; Barceló, C.; Moreno, D.F.; Maiques, O.; Sisó, P.; Marti, M.R.; Macià, A.; Panosa, A. In vitro cell migration, invasion, and adhesion assays: From cell imaging to data analysis. Front. Cell Dev. Biol. 2019, 7, 107. [Google Scholar] [CrossRef] [PubMed]
- Jozefczuk, J.; Adjaye, J. Quantitative Real-Time PCR-Based Analysis of Gene Expression. Methods Enzymol. 2011, 500, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Sharma, D.; Srivastava, A.K.; Varma, A.; Jayadev, M.S.K.; Joshi, R.K. Evaluation of anticancer activity of ferrocene based benzothiazole and β-ketooxothioacetal. J. Organomet. Chem. 2022, 979, 122500. [Google Scholar] [CrossRef]
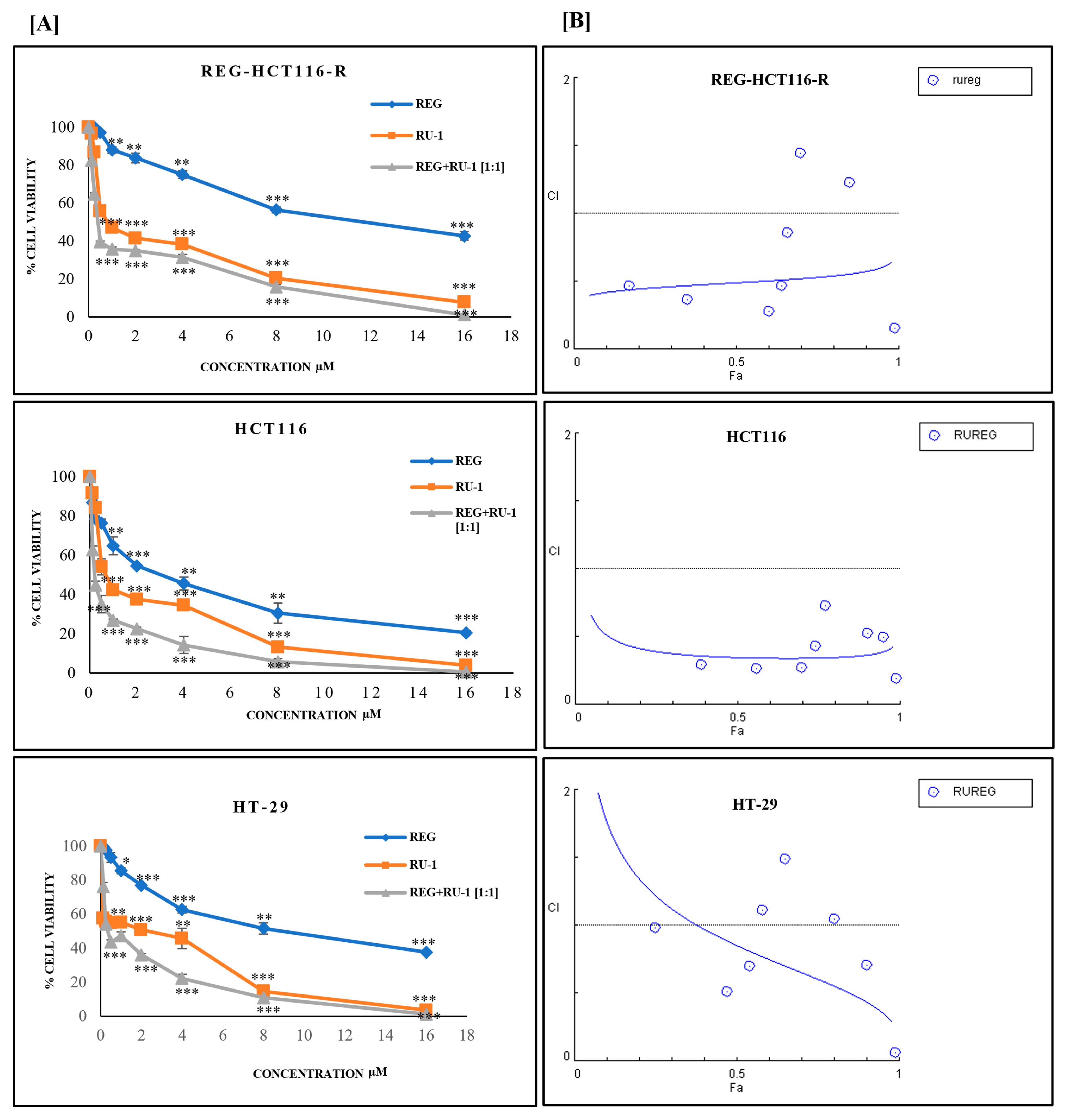
| Cell Line | Ru-1(µM) | REG (µM) | REG/Ru-1 (µM) |
|---|---|---|---|
| HCT116 | 0.9 ± 1.03 | 2.7 ± 0.6 | 0.4 ± 0.9 |
| REG-HCT116-R | 1.4 ± 1.0 | 9.9 ± 1.09 | 1.2 ± 0.96 |
| HT29 | 0.6 ± 0.7 | 7.3 ± 0.98 | 0.9 ± 0.95 |
| RU-1 | REG | HCT116 | REG-HCT116-R | HT29 | |||
|---|---|---|---|---|---|---|---|
| CONC µM | CONC µM | Fa | CI | Fa | CI | Fa | CI |
| 0.125 | 0.125 | 0.39 | 0.2 | 0.17 | 0.4 | 0.25 | 0.9 |
| 0.25 | 0.25 | 0.56 | 0.26 | 0.35 | 0.3 | 0.43 | 0.5 |
| 0.5 | 0.5 | 0.7 | 0.26 | 0.6 | 0.27 | 0.54 | 0.6 |
| 1 | 1 | 0.74 | 0.43 | 0.64 | 0.46 | 0.58 | 1.1 |
| 2 | 2 | 0.77 | 0.7 | 0.66 | 0.86 | 0.65 | 1.4 |
| 4 | 4 | 0.9 | 0.52 | 0.7 | 1.4 | 0.8 | 1.0 |
| 8 | 8 | 0.95 | 0.49 | 0.85 | 1.2 | 0.9 | 0.7 |
| 16 | 16 | 0.99 | 0.05 | 0.99 | 0.1 | 0.99 | 0.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, D.; Rasool, F.; Bharti, M.; Vyas, K.M.; Magani, S.K.J. Regorafenib and Ruthenium Complex Combination Inhibit Cancer Cell Growth by Targeting PI3K/AKT/ERK Signalling in Colorectal Cancer Cells. Int. J. Mol. Sci. 2023, 24, 686. https://doi.org/10.3390/ijms24010686
Sharma D, Rasool F, Bharti M, Vyas KM, Magani SKJ. Regorafenib and Ruthenium Complex Combination Inhibit Cancer Cell Growth by Targeting PI3K/AKT/ERK Signalling in Colorectal Cancer Cells. International Journal of Molecular Sciences. 2023; 24(1):686. https://doi.org/10.3390/ijms24010686
Chicago/Turabian StyleSharma, Deepu, Fayyaz Rasool, Manjri Bharti, Komal M. Vyas, and Sri Krishna Jayadev Magani. 2023. "Regorafenib and Ruthenium Complex Combination Inhibit Cancer Cell Growth by Targeting PI3K/AKT/ERK Signalling in Colorectal Cancer Cells" International Journal of Molecular Sciences 24, no. 1: 686. https://doi.org/10.3390/ijms24010686
APA StyleSharma, D., Rasool, F., Bharti, M., Vyas, K. M., & Magani, S. K. J. (2023). Regorafenib and Ruthenium Complex Combination Inhibit Cancer Cell Growth by Targeting PI3K/AKT/ERK Signalling in Colorectal Cancer Cells. International Journal of Molecular Sciences, 24(1), 686. https://doi.org/10.3390/ijms24010686






