IL-1 Generated by Oral Squamous Cell Carcinoma Stimulates Tumor-Induced and RANKL-Induced Osteoclastogenesis: A Possible Mechanism of Bone Resorption Induced by the Infiltration of Oral Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Results
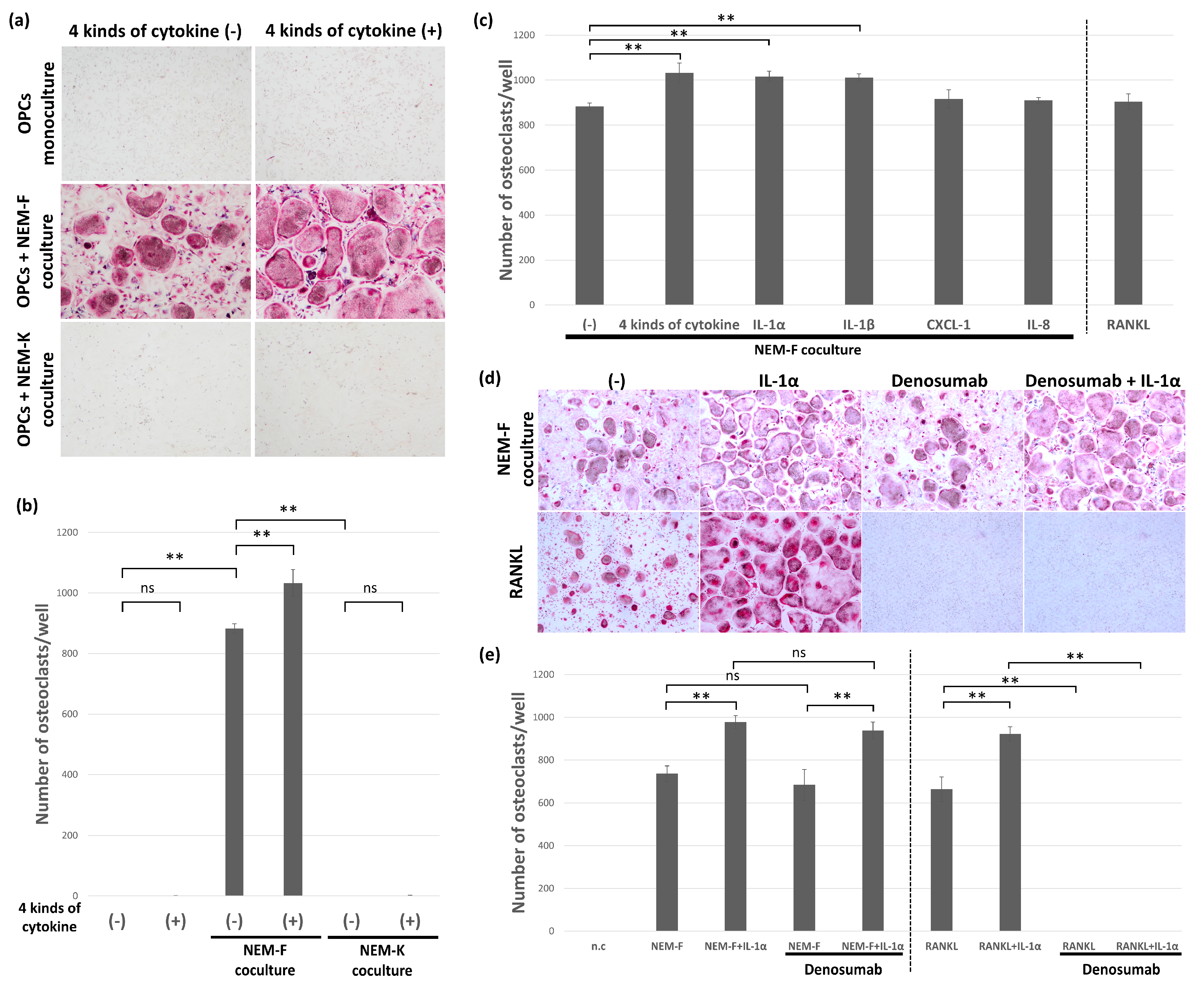
2.1. Induction of Osteoclasts from OPCs by OSCC Cell Lines
2.2. Evaluation of Induction or Stimulation of Osteoclastogenesis with IL-1α, IL-1β, IL-6, IL-8, and CXCL1
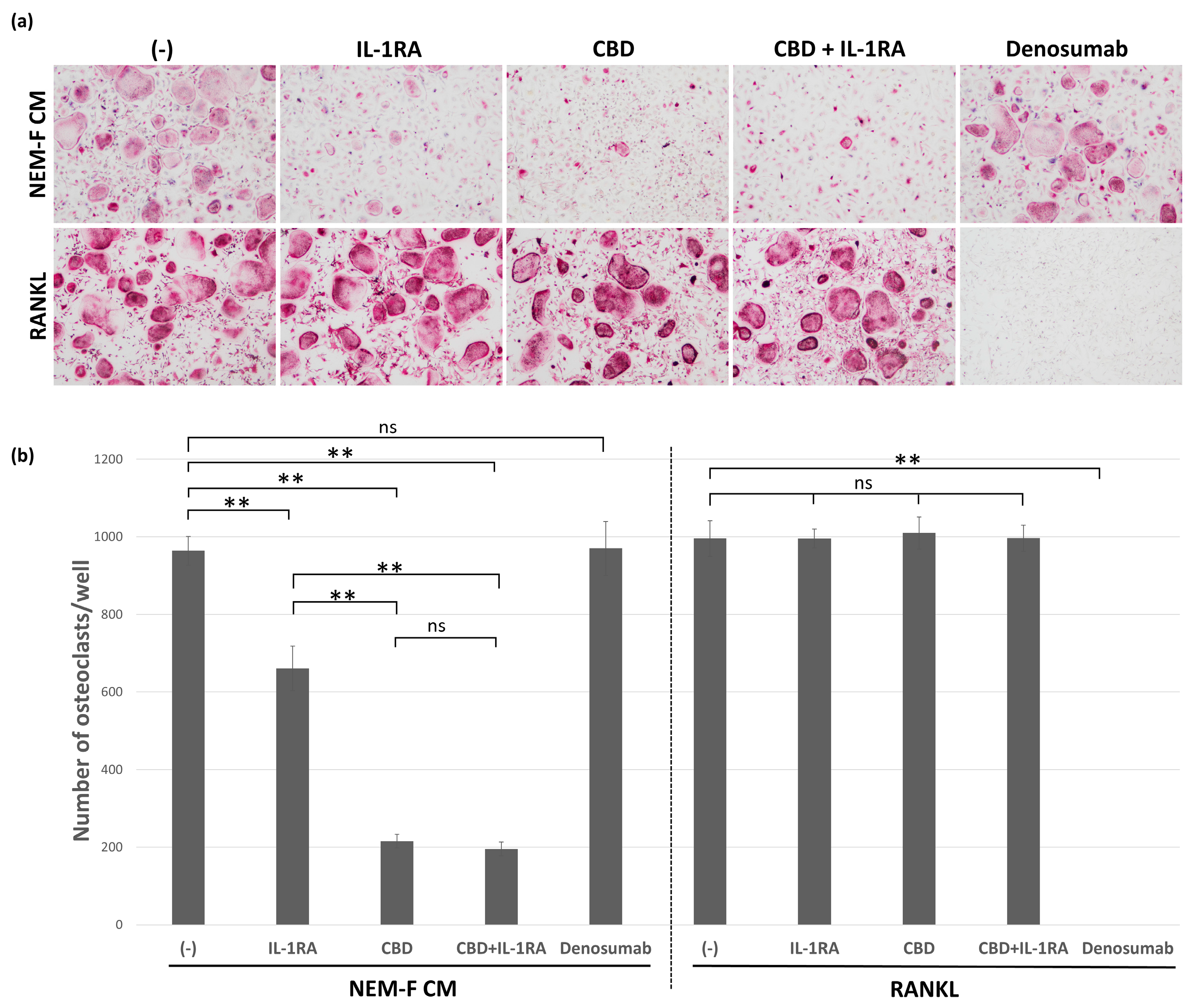
2.3. The Effect of Inhibitors for IL-1α and IL-1β on the Tumor-Induced Osteoclastogenesis
2.4. The Effect of CBD and IL-1RA on Tumor-Induced and RANKL-Induced Osteoclastogenesis
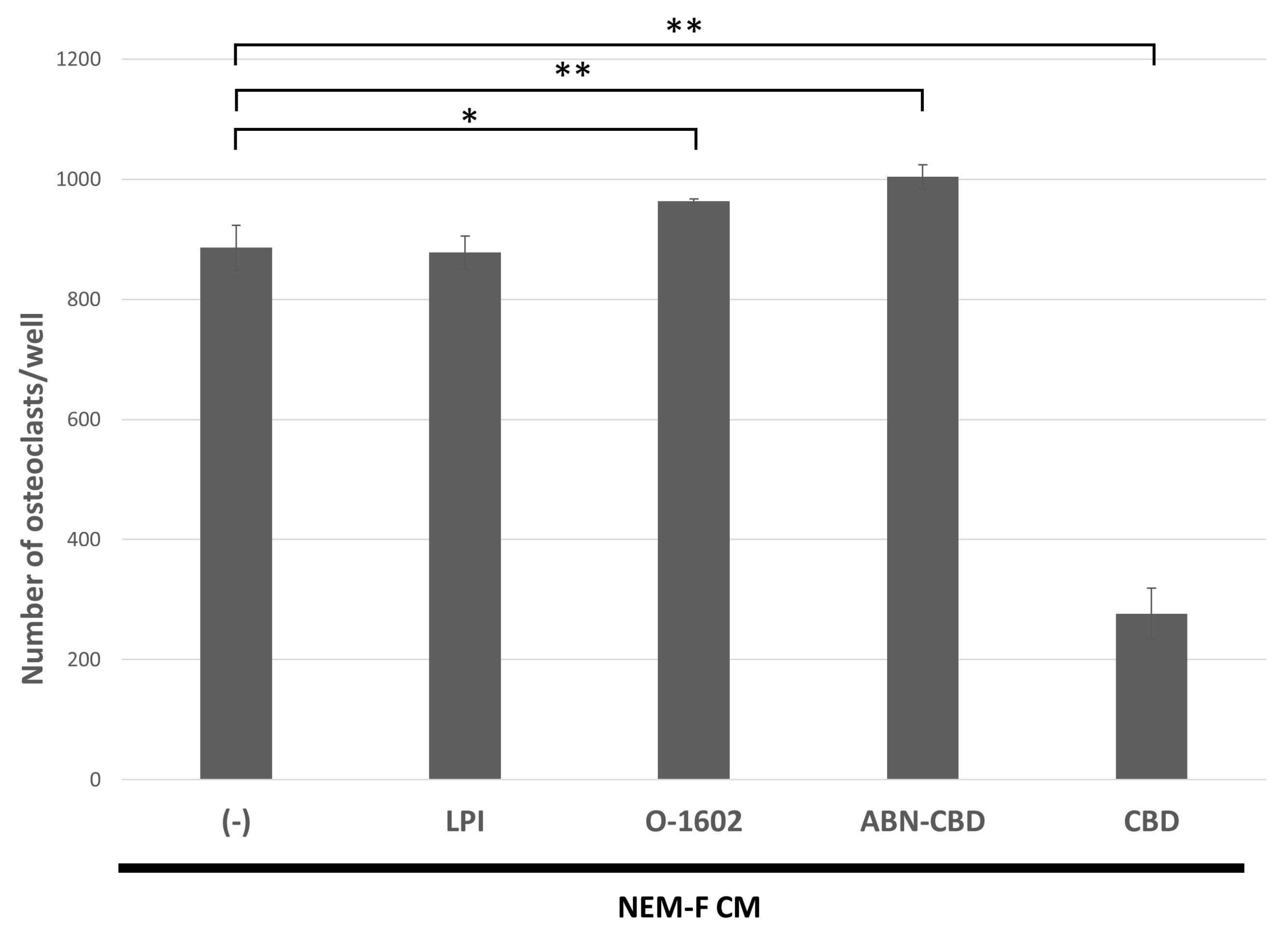
2.5. The Effects of Agonistic Interactors for GPR55 on Tumor-Induced Osteoclastogenesis
3. Discussion
4. Materials and Methods
4.1. Culture of OSCC Cells
4.2. Osteoclastogenesis Assay Using OPCs
4.3. Pit Formation Assay Using OPCs
4.4. Gene Expression Microarray Analysis and Protein Array Analysis
4.5. Quantitative RT-PCR
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ono, T.; Nakashima, T. Recent advances in osteoclast biology. Histochem. Cell Biol. 2018, 149, 325–341. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H. Osteoimmunology: Shared mechanisms and crosstalk between the immune and bone systems. Nat. Rev. Immunol. 2007, 7, 292–304. [Google Scholar] [CrossRef]
- Okamoto, K.; Nakashima, T.; Shinohara, M.; Negishi-Koga, T.; Komatsu, N.; Terashima, A.; Sawa, S.; Nitta, T.; Takayanagi, H. Osteoimmunology: The Conceptual Framework Unifying the Immune and Skeletal Systems. Physiol. Rev. 2017, 97, 1295–1349. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Uehara, S.; Udagawa, N.; Takahashi, N. Regulation of bone metabolism by Wnt signals. J. Biochem. 2016, 159, 387–392. [Google Scholar] [CrossRef] [Green Version]
- von Moos, R.; Costa, L.; Ripamonti, C.I.; Niepel, D.; Santini, D. Improving quality of life in patients with advanced cancer: Targeting metastatic bone pain. Eur. J. Cancer 2017, 71, 80–94. [Google Scholar] [CrossRef] [Green Version]
- Furesi, G.; Rauner, M.; Hofbauer, L.C. Emerging Players in Prostate Cancer-Bone Niche Communication. Trends Cancer 2021, 7, 112–121. [Google Scholar] [CrossRef]
- Satcher, R.L.; Zhang, X.H. Evolving cancer-niche interactions and therapeutic targets during bone metastasis. Nat. Rev. Cancer 2022, 22, 85–101. [Google Scholar] [CrossRef]
- Clézardin, P.; Coleman, R.; Puppo, M.; Ottewell, P.; Bonnelye, E.; Paycha, F.; Confavreux, C.B.; Holen, I. Bone metastasis: Mechanisms, therapies, and biomarkers. Physiol. Rev. 2021, 101, 797–855. [Google Scholar] [CrossRef]
- Vaassen, L.A.A.; Speel, E.M.; Kessler, P. Bone invasion by oral squamous cell carcinoma: Molecular alterations leading to osteoclastogenesis—A review of literature. J. Cranio-Maxillofac. Surg. 2017, 45, 1464–1471. [Google Scholar] [CrossRef]
- Kayamori, K.; Sakamoto, K.; Nakashima, T.; Takayanagi, H.; Morita, K.; Omura, K.; Nguyen, S.T.; Miki, Y.; Iimura, T.; Himeno, A.; et al. Roles of interleukin-6 and parathyroid hormone-related peptide in osteoclast formation associated with oral cancers: Significance of interleukin-6 synthesized by stromal cells in response to cancer cells. Am. J. Pathol. 2010, 176, 968–980. [Google Scholar] [CrossRef]
- Zhang, X.; Junior, C.R.; Liu, M.; Li, F.; D’Silva, N.J.; Kirkwood, K.L. Oral squamous carcinoma cells secrete RANKL directly supporting osteolytic bone loss. Oral Oncol. 2013, 49, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Nagai, M.; Kyakumoto, S.; Sato, N. Cancer cells responsible for humoral hypercalcemia express mRNA encoding a secreted form of ODF/TRANCE that induces osteoclast formation. Biochem. Biophys. Res. Commun. 2000, 269, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Chuang, F.H.; Hsue, S.S.; Wu, C.W.; Chen, Y.K. Immunohistochemical expression of RANKL, RANK, and OPG in human oral squamous cell carcinoma. J. Oral Pathol. Med. 2009, 38, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Shibahara, T.; Nomura, T.; Cui, N.H.; Noma, H. A study of osteoclast-related cytokines in mandibular invasion by squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2005, 34, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Mori, T.; Nomura, T.; Shibahara, T.; Sakamoto, M. Parathyroid-related protein plays a critical role in bone invasion by oral squamous cell carcinoma. Int. J. Oncol. 2010, 36, 1387–1394. [Google Scholar] [PubMed] [Green Version]
- Hwang, Y.S.; Lee, S.K.; Park, K.K.; Chung, W.Y. Secretion of IL-6 and IL-8 from lysophosphatidic acid-stimulated oral squamous cell carcinoma promotes osteoclastogenesis and bone resorption. Oral Oncol. 2012, 48, 40–48. [Google Scholar] [CrossRef]
- Oue, E.; Lee, J.W.; Sakamoto, K.; Iimura, T.; Aoki, K.; Kayamori, K.; Michi, Y.; Yamashiro, M.; Harada, K.; Amagasa, T.; et al. CXCL2 synthesized by oral squamous cell carcinoma is involved in cancer-associated bone destruction. Biochem. Biophys. Res. Commun. 2012, 424, 456–461. [Google Scholar] [CrossRef]
- Takada, H.; Ibaragi, S.; Eguchi, T.; Okui, T.; Obata, K.; Masui, M.; Morisawa, A.; Takabatake, K.; Kawai, H.; Yoshioka, N.; et al. Semaphorin 4D promotes bone invasion in head and neck squamous cell carcinoma. Int. J. Oncol. 2017, 51, 625–632. [Google Scholar] [CrossRef] [Green Version]
- Expression of TNFSF11 in Cancer-Summary-The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000120659-TNFSF11/pathology (accessed on 13 December 2022).
- Expression of TNF in Cancer-Summary-The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000232810-TNF/pathology (accessed on 13 December 2022).
- Amarasekara, D.S.; Yun, H.; Kim, S.; Lee, N.; Kim, H.; Rho, J. Regulation of Osteoclast Differentiation by Cytokine Networks. Immune Netw. 2018, 18, e8. [Google Scholar] [CrossRef]
- Goldring, S.R.; Gravallese, E.M. Pathogenesis of bone erosions in rheumatoid arthritis. Curr. Opin. Rheumatol. 2000, 12, 195–199. [Google Scholar] [CrossRef]
- Kwan Tat, S.; Padrines, M.; Théoleyre, S.; Heymann, D.; Fortun, Y. IL-6, RANKL, TNF-alpha/IL-1: Interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004, 15, 49–60. [Google Scholar] [PubMed]
- Expression of IL1A in Cancer-Summary-The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000115008-IL1A/pathology (accessed on 13 December 2022).
- León, X.; Bothe, C.; García, J.; Parreño, M.; Alcolea, S.; Quer, M.; Vila, L.; Camacho, M. Expression of IL-1α correlates with distant metastasis in patients with head and neck squamous cell carcinoma. Oncotarget 2015, 6, 37398–37409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Jin, H.M.; Kim, K.; Song, I.; Youn, B.U.; Matsuo, K.; Kim, N. The mechanism of osteoclast differentiation induced by IL-1. J. Immunol. 2009, 183, 1862–1870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wada, A.; Tsuchiya, M.; Ozaki-Honda, Y.; Kayamori, K.; Sakamoto, K.; Yamaguchi, A.; Ikeda, T. A new osteoclastogenesis pathway induced by cancer cells targeting osteoclast precursor cells. Biochem. Biophys. Res. Commun. 2019, 509, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, M.; Kayamori, K.; Wada, A.; Komaki, M.; Ohata, Y.; Hamagaki, M.; Sakamoto, K.; Ikeda, T. A Novel, Tumor-Induced Osteoclastogenesis Pathway Insensitive to Denosumab but Interfered by Cannabidiol. Int. J. Mol. Sci. 2019, 20, 6211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizoguchi, T.; Muto, A.; Udagawa, N.; Arai, A.; Yamashita, T.; Hosoya, A.; Ninomiya, T.; Nakamura, H.; Yamamoto, Y.; Kinugawa, S.; et al. Identification of cell cycle-arrested quiescent osteoclast precursors in vivo. J. Cell Biol. 2009, 184, 541–554. [Google Scholar] [CrossRef]
- Azuma, Y.; Kaji, K.; Katogi, R.; Takeshita, S.; Kudo, A. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J. Biol. Chem. 2000, 275, 4858–4864. [Google Scholar] [CrossRef] [Green Version]
- Mikami, S.; Mizuno, R.; Kosaka, T.; Saya, H.; Oya, M.; Okada, Y. Expression of TNF-α and CD44 is implicated in poor prognosis, cancer cell invasion, metastasis and resistance to the sunitinib treatment in clear cell renal cell carcinomas. Int. J. Cancer 2015, 136, 1504–1514. [Google Scholar] [CrossRef]
- Babiuch, K.; Kuśnierz-Cabala, B.; Kęsek, B.; Okoń, K.; Darczuk, D.; Chomyszyn-Gajewska, M. Evaluation of Proinflammatory, NF-kappaB Dependent Cytokines: IL-1α, IL-6, IL-8, and TNF-α in Tissue Specimens and Saliva of Patients with Oral Squamous Cell Carcinoma and Oral Potentially Malignant Disorders. J. Clin. Med. 2020, 9, 867. [Google Scholar] [CrossRef] [Green Version]
- Zhu, G.; Du, Q.; Wang, X.; Tang, N.; She, F.; Chen, Y. TNF-α promotes gallbladder cancer cell growth and invasion through autocrine mechanisms. Int. J. Mol. Med. 2014, 33, 1431–1440. [Google Scholar] [CrossRef]
- Tan, W.; Luo, X.; Li, W.; Zhong, J.; Cao, J.; Zhu, S.; Chen, X.; Zhou, R.; Shang, C.; Chen, Y. TNF-α is a potential therapeutic target to overcome sorafenib resistance in hepatocellular carcinoma. EBioMedicine 2019, 40, 446–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukushima, H.; Jimi, E.; Okamoto, F.; Motokawa, W.; Okabe, K. IL-1-induced receptor activator of NF-kappa B ligand in human periodontal ligament cells involves ERK-dependent PGE2 production. Bone 2005, 36, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Kitaura, H.; Zhou, P.; Ross, F.P.; Teitelbaum, S.L. IL-1 mediates TNF-induced osteoclastogenesis. J. Clin. Investig. 2005, 115, 282–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruscitti, P.; Cipriani, P.; Carubbi, F.; Liakouli, V.; Zazzeroni, F.; Di Benedetto, P.; Berardicurti, O.; Alesse, E.; Giacomelli, R. The role of IL-1β in the bone loss during rheumatic diseases. Mediat. Inflamm. 2015, 2015, 782382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raphael-Mizrahi, B.; Gabet, Y. The Cannabinoids Effect on Bone Formation and Bone Healing. Curr. Osteoporos. Rep. 2020, 18, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Ofek, O.; Karsak, M.; Leclerc, N.; Fogel, M.; Frenkel, B.; Wright, K.; Tam, J.; Attar-Namdar, M.; Kram, V.; Shohami, E.; et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc. Natl. Acad. Sci. USA 2006, 103, 696–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ofek, O.; Attar-Namdar, M.; Kram, V.; Dvir-Ginzberg, M.; Mechoulam, R.; Zimmer, A.; Frenkel, B.; Shohami, E.; Bab, I. CB2 cannabinoid receptor targets mitogenic Gi protein-cyclin D1 axis in osteoblasts. J. Bone Miner. Res. 2011, 26, 308–316. [Google Scholar] [CrossRef] [Green Version]
- Sophocleous, A.; Landao-Bassonga, E.; Van’t Hof, R.J.; Idris, A.I.; Ralston, S.H. The type 2 cannabinoid receptor regulates bone mass and ovariectomy-induced bone loss by affecting osteoblast differentiation and bone formation. Endocrinology 2011, 152, 2141–2149. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.; Yu, B.; Bai, J.; Wang, X.; Guo, X.; Liu, Y.; Lin, J.; Hu, S.; Zhang, W.; Tao, Y.; et al. Cannabinoid Receptor 2 Agonist Prevents Local and Systemic Inflammatory Bone Destruction in Rheumatoid Arthritis. J. Bone Miner. Res. 2019, 34, 739–751. [Google Scholar] [CrossRef]
- de Almeida, D.L.; Devi, L.A. Diversity of molecular targets and signaling pathways for CBD. Pharmacol. Res. Perspect. 2020, 8, e00682. [Google Scholar] [CrossRef]
- Whyte, L.S.; Ryberg, E.; Sims, N.A.; Ridge, S.A.; Mackie, K.; Greasley, P.J.; Ross, R.A.; Rogers, M.J. The putative cannabinoid receptor GPR55 affects osteoclast function in vitro and bone mass in vivo. Proc. Natl. Acad. Sci. USA 2009, 106, 16511–16516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, T.; Kasai, M.; Tatsukawa, E.; Kamitakahara, M.; Shibata, Y.; Yokoi, T.; Nemoto, T.K.; Ioku, K. A bone substitute with high affinity for vitamin D-binding protein--relationship with niche of osteoclasts. J. Cell Mol. Med. 2014, 18, 170–180. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fukawa, Y.; Kayamori, K.; Tsuchiya, M.; Ikeda, T. IL-1 Generated by Oral Squamous Cell Carcinoma Stimulates Tumor-Induced and RANKL-Induced Osteoclastogenesis: A Possible Mechanism of Bone Resorption Induced by the Infiltration of Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2023, 24, 688. https://doi.org/10.3390/ijms24010688
Fukawa Y, Kayamori K, Tsuchiya M, Ikeda T. IL-1 Generated by Oral Squamous Cell Carcinoma Stimulates Tumor-Induced and RANKL-Induced Osteoclastogenesis: A Possible Mechanism of Bone Resorption Induced by the Infiltration of Oral Squamous Cell Carcinoma. International Journal of Molecular Sciences. 2023; 24(1):688. https://doi.org/10.3390/ijms24010688
Chicago/Turabian StyleFukawa, Yuki, Kou Kayamori, Maiko Tsuchiya, and Tohru Ikeda. 2023. "IL-1 Generated by Oral Squamous Cell Carcinoma Stimulates Tumor-Induced and RANKL-Induced Osteoclastogenesis: A Possible Mechanism of Bone Resorption Induced by the Infiltration of Oral Squamous Cell Carcinoma" International Journal of Molecular Sciences 24, no. 1: 688. https://doi.org/10.3390/ijms24010688
APA StyleFukawa, Y., Kayamori, K., Tsuchiya, M., & Ikeda, T. (2023). IL-1 Generated by Oral Squamous Cell Carcinoma Stimulates Tumor-Induced and RANKL-Induced Osteoclastogenesis: A Possible Mechanism of Bone Resorption Induced by the Infiltration of Oral Squamous Cell Carcinoma. International Journal of Molecular Sciences, 24(1), 688. https://doi.org/10.3390/ijms24010688






