Revisiting the Phylogenetic Relationship and Evolution of Gargarini with Mitochondrial Genome (Hemiptera: Membracidae: Centrotinae)
Abstract
:Summary
Abstract
1. Introduction
2. Results
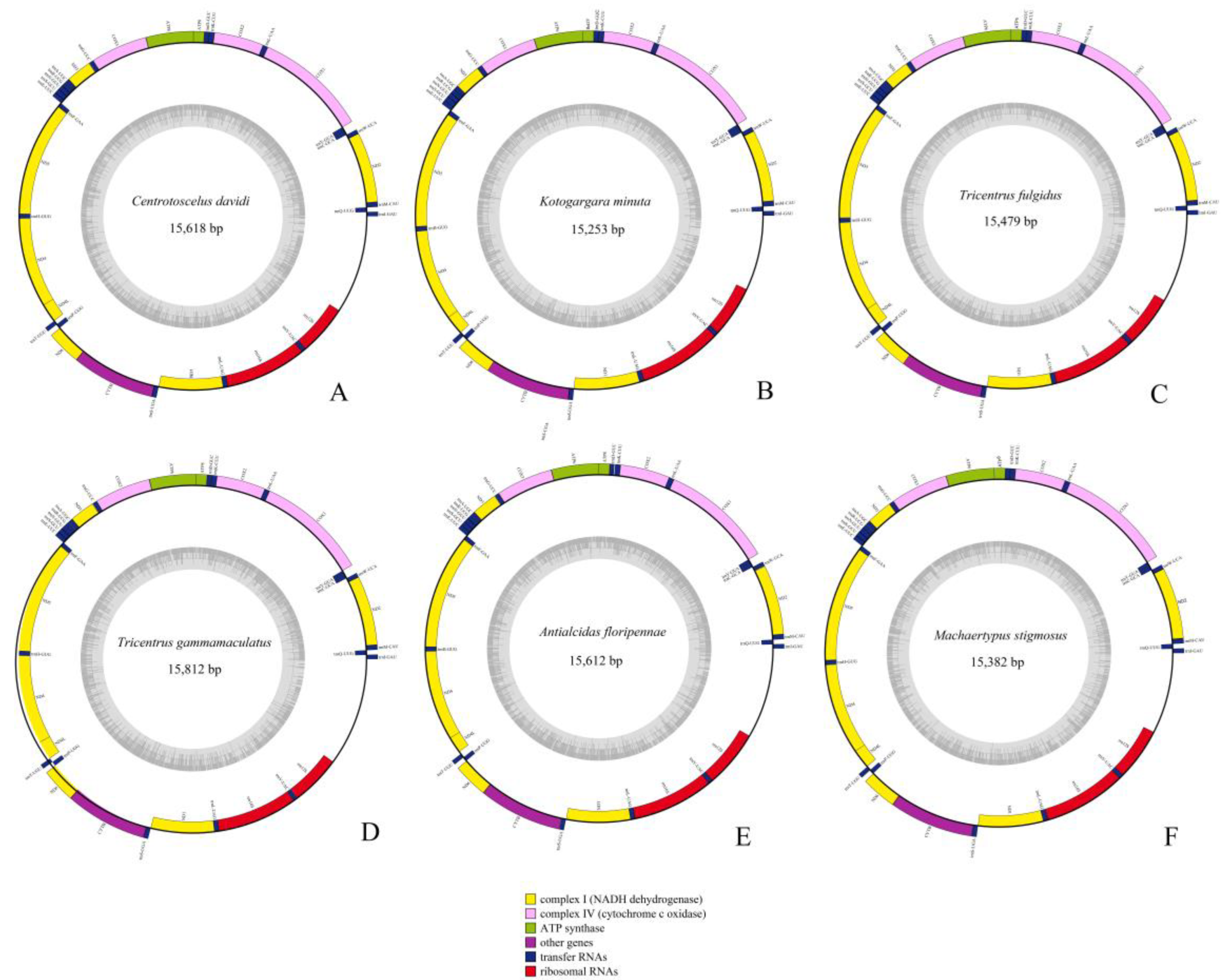
2.1. Genome Organization and Composition
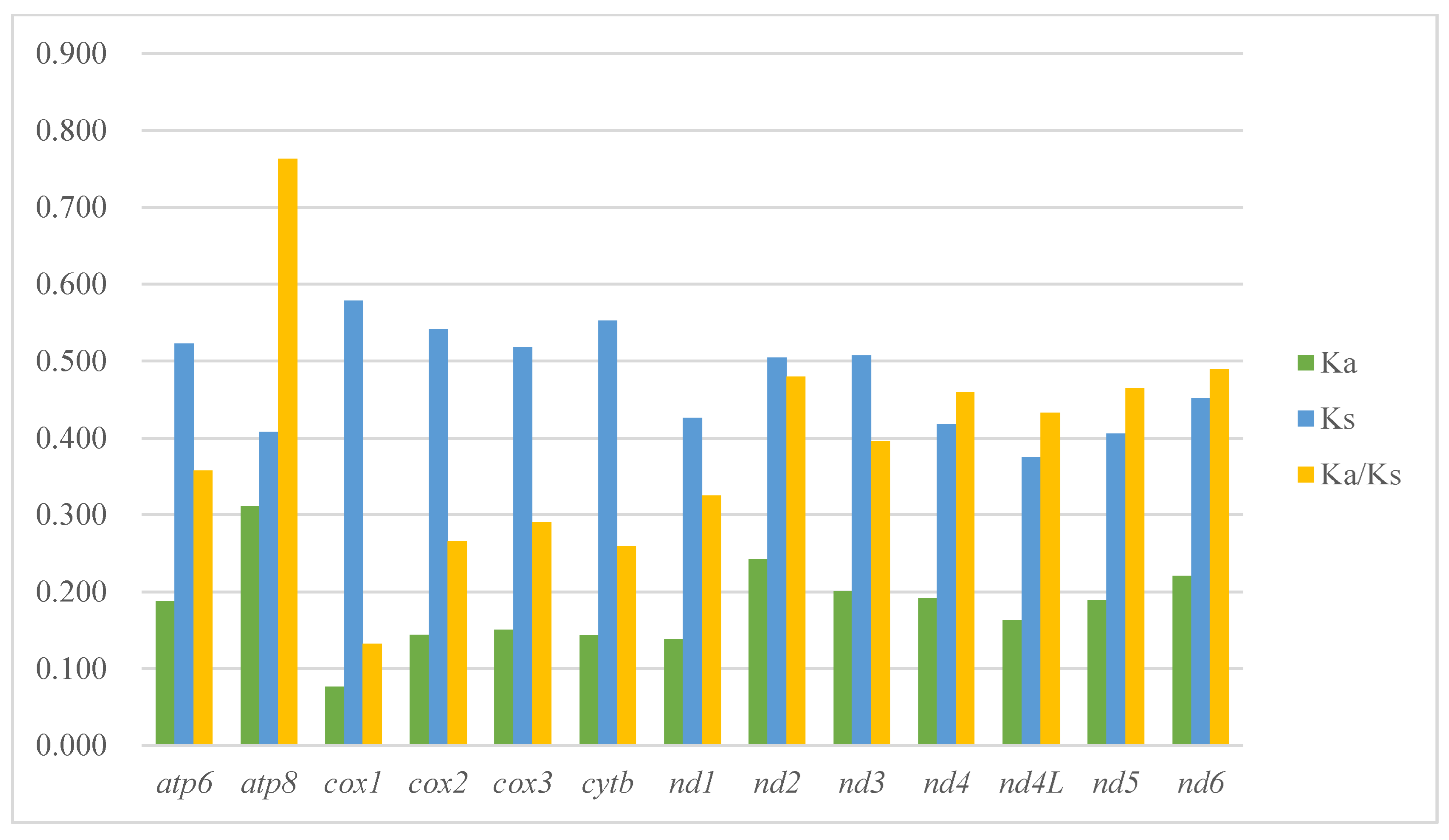
2.2. PCGs and Codon Usage
2.3. Transfer and Ribosomal RNA Genes
2.4. Control Region
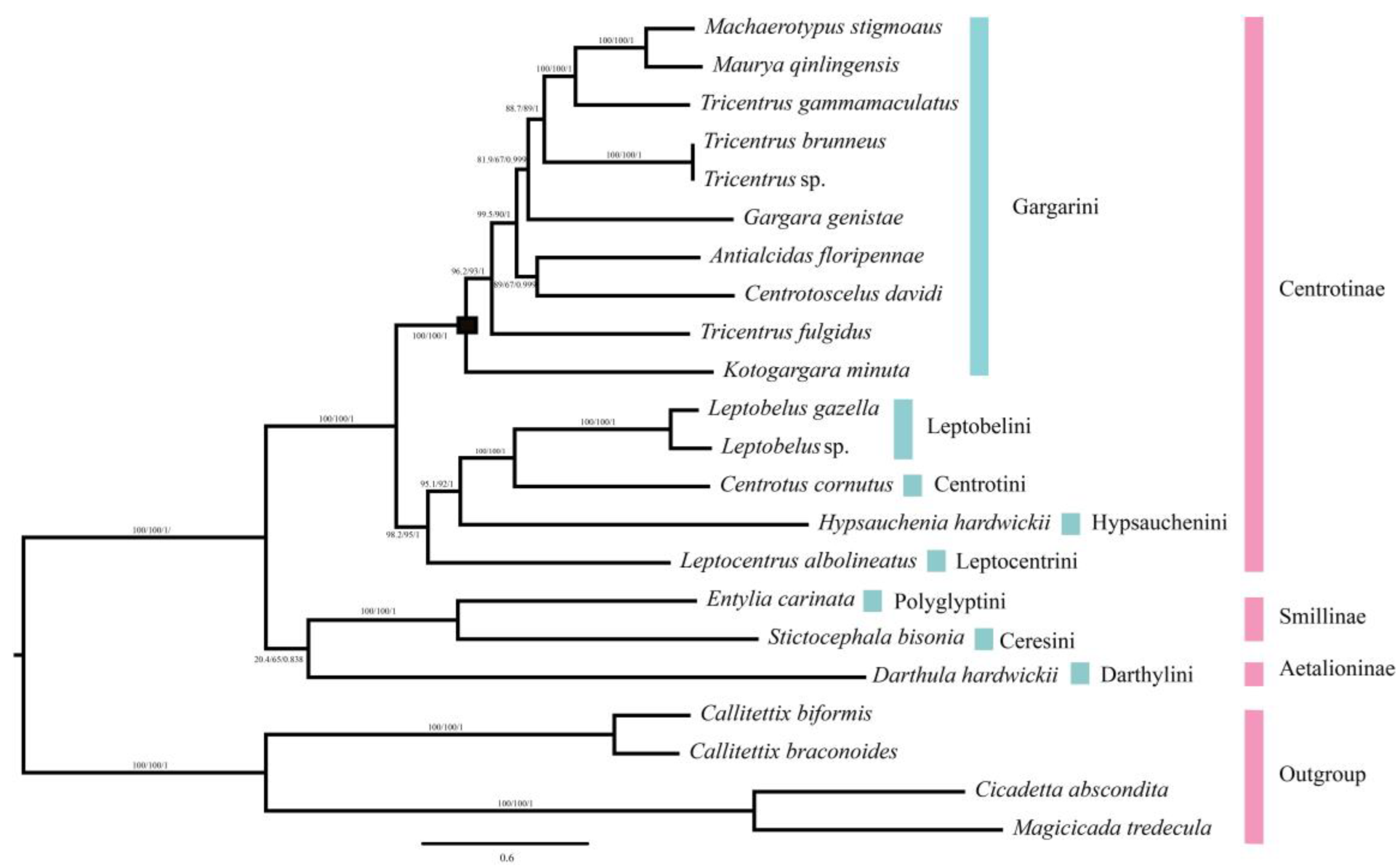
2.5. Phylogenetic Relationship
2.6. Divergence Time Estimates
2.7. Ancestral State Reconstructions
3. Discussion
3.1. Genome Character and Phylogenetic Relationships
3.2. Character Evolution
4. Materials and Methods
4.1. Sample Preparation
4.2. Genomic DNA Extraction, Sequencing
4.3. Data Assembly, Annotation, Analysis
4.4. Phylogenetic Analyses
4.5. Divergence Time Estimates
4.6. Ancestral State Reconstructions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McKamey, S.H. Taxonomic catalogue of the Membracoidea (exclusive of leafhoppers); second supplement to Fascicle 1-Membracidae of the General Catalogue of the Hemiptera. Mem. Am. Entomol. Inst. 1998, 60, 1–377. [Google Scholar]
- Yuan, F.; Chou, I. Fauna Sinica Insecta, Homoptera Membracoidea Aetalionidae Membracidae; Science Press: Beijing, China, 2002; Volume 28, pp. 1–590. [Google Scholar]
- Wallace, M.S.; Deitz, L.L. Phylogeny and Systematics of the Treehopper Subfamily Centrotinae (Hemiptera: Membracidae); Associated Publishers: New York, NY, USA, 2004; Volume 19, pp. 1–377. [Google Scholar]
- Dietrich, C.H.; Allen, J.M.; Alan, R.L.; Emily, M.L.; Takiya, D.M.; Olivia, E.; Walden, K.K.O.; Grady, P.G.S.; Johnson, K.P. Anchored hybrid enrichment-based phylogenomics of leafhoppers and treehoppers (Hemiptera: Cicadomorpha: Membracoidea). Insect Syst. Diver. 2017, 1, 57–72. [Google Scholar] [CrossRef]
- Wallace, M.S.; Deitz, L.L. Australian treehoppers (Hemiptera Membracidae Centrotinae Terentiini) phylogeny and biogeography. Invertebr. Syst. 2006, 20, 163–183. [Google Scholar] [CrossRef]
- Dmitriev, D.A. 3I Interactive Keys and Taxonomic Databases. 2003. Available online: http://dmitriev.speciesfile.org/index.asp/ (accessed on 10 March 2022).
- Ahmad, I.; Yasmeen, N. A new tribe of the subfamily Controtinae Amyot & Serville with comments on its phylogeny. Mitt. Hamburgischen Zool. Mus. Inst. 1974, 71, 175–191. [Google Scholar]
- Deitz, L.L.; Dietrich, C.H. Superfamily Membracoidea (Homoptera: Auchenorrhyncha). 1. Introduction and revised classification with new family-group taxa. Syst Entomol. 1993, 18, 287–296. [Google Scholar] [CrossRef]
- Yu, R.; Feng, L.; Dietrich, C.H.; Yuan, X. Characterization, comparison of four new mitogenomes of Centrotinae (Hemiptera: Membracidae) and phylogenetic implications supports new synonymy. Life 2022, 12, 61. [Google Scholar] [CrossRef]
- Gavin, T.A.; Avise, J.C. Molecular markers, natural history and evolution. J. Wild. Manag. 1995, 58, 798. [Google Scholar] [CrossRef]
- Cameron, S.L. Insect mitochondrial genomics: Implications for evolution and phylogeny. Annu. Rev. Entomol. 2014, 59, 95–117. [Google Scholar] [CrossRef] [Green Version]
- Fenn, J.D.; Song, H.; Cameron, S.L.; Whiting, M.F. A preliminary mitochondrial genome phylogeny of Orthoptera (Insecta) and approaches to maximizing phylogenetic signal found within mitochondrial genome data. Mol. Phylogenet. Evol. 2008, 49, 59–68. [Google Scholar] [CrossRef]
- Cryan, J.R.; Brian, M.W.; Deitz, L.L.; Dietrich, C.H. Phylogeny of the treehoppers (Insecta: Hemiptera: Membracidae): Evidence from two nuclear genes. Mol. Phylogenet. Evol. 2000, 17, 317–334. [Google Scholar] [CrossRef]
- Hu, K.; Yuan, F.; Dietrich, C.H.; Yuan, X.-Q. Structural features and phylogenetic implications of four new mitogenomes of Centrotinae (Hemiptera: Membracidae). Int. J. Bio. Macromol. 2019, 13, 1018–1027. [Google Scholar] [CrossRef] [PubMed]
- Liang, A.-P.; Gao, J.; Zhao, X. Characterization of the complete mitochondrial genome of the treehopper Darthula hardwickii (Hemiptera: Aetalionidae). Mitochondrial DNA A 2016, 27, 3291–3292. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Zhang, H.; Zhao, T. Insights into the phylogeny of Hemiptera from increased mitogenomic taxon sampling. Mol. Phylogenet. Evol. 2019, 137, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liang, A.-P. Complete DNA sequence of the mitochondrial genome of the treehopper Leptobelus gazella (Membracoidea: Hemiptera). Mitochondrial DNA 2016, 27, 3318–3319. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; John, M.L.; Eric, G.C.; Daniel, B.; Song, F.; Jiang, P.; Liu, J.-P.; Zhou, X.-G.; Cai, W.-Z. Mitochondrial phylogenomics of Hemiptera reveals adaptive innovations driving the diversification of true bugs. Proc. Royal Soc. B 2017, 284, 1862. [Google Scholar] [CrossRef] [Green Version]
- Mao, M.; Yang, X.-S.; Gordon, B. The complete mitochondrial genome of Entylia carinata (Hemiptera: Membracidae). Mitochondrial DNA B 2016, 1, 662–663. [Google Scholar] [CrossRef] [Green Version]
- Yu, R.-T.; Feng, L.-N.; Yuan, X.-Q. Complete mitochondrial genome sequence of the global invasive species Stictocephala bisonia (Hemiptera: Membracidae: Smiliinae). Mitochondrial DNA B 2021, 6, 1601–1602. [Google Scholar] [CrossRef]
- Clary, D.O.; Wolstenholme, D.R. The mitochondrial DNA molecule of Drosophila yakuba nucleotide sequence, gene organization, and genetic code. J. Mol. Evol. 1985, 22, 252–271. [Google Scholar] [CrossRef]
- Wolstenholme, D.R. Animal mitochondrial DNA: Structure and evolution. Int. Rev. Cytol. 1992, 141, 173–216. [Google Scholar] [CrossRef]
- Gong, N.; Yang, L.; Chen, X.-S. Structural features and phylogenetic implications of four new mitogenomes of Caliscelidae (Hemiptera: Fulgoromorpha). Int. J. Mol. Sci. 2021, 22, 1348. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, H.-X.; Yu, X.-F.; Yang, M.-F. Comparative analysis of mitochondrial genomes among twelve sibling species of the genus Atkinsoniella Distant, 1908 (Hemiptera: Cicadellidae: Cicadellinae) and phylogenetic analysis. Insects 2022, 13, 254. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-J.; Wang, Z.-Q.; Che, Y.-L. The complete mitogenome of the wood-feeding cockroach Cryptocercus meridianus (Blattodea: Cryptocercidae) and its phylogenetic relationship among cockroach families. Int. J. Mol. Sci. 2017, 18, 2397. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Dietrich, C.H.; Huang, M. Characterization of the complete mitochondrial genomes of two species with preliminary investigation on phylogenetic status of Zyginellini (Hemiptera: Cicadellidae: Typhlocybinae). Insects 2020, 11, 684. [Google Scholar] [CrossRef]
- Lin, S.-H.; Huang, M.; Zhang, Y.-L. Structural features and phylogenetic implications of 11 new mitogenomes of Typhlocybinae (Hemiptera: Cicadellidae). Insects 2021, 12, 678. [Google Scholar] [CrossRef]
- Dietrich, C.H.; Rakitov, R.A.; Holmes, J.L.; Black, W.C. Phylogeny of the major lineages of Membracoidea (Insecta: Hemiptera:Cicadomorpha) based on 28S rDNA sequences. Mol. Phylogenet. Evol. 2001, 18, 293–305. [Google Scholar] [CrossRef]
- Cryan, J.R.; Urban, J.M. Higher-level phylogeny of the insect order Hemiptera: Is Auchenorrhyncha really paraphyletic? Syst. Entomol. 2012, 37, 7–21. [Google Scholar] [CrossRef]
- Johnson, K.P.; Dietrich, C.H.; Friedrich, F.; Beute, R.G.; Wipfler, B.; Peters, R.S.; Allen, J.M.; Petersen, M.; Donath, A.; Walden, K.O.K.; et al. Phylogenomics and the evolution of the insects. Proc. Natl. Acad. Sci. USA 2018, 115, 12775–12780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prud’homme, B.; Minervino, C.; Hocine, M.; Cande, J.D.; Aouane, A.; Dufour, H.D.; Kassner, V.A.; Gompel, N. Body plan innovation in treehoppers through the evolution of an extra wing-like appendage. Nature 2011, 473, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.R.; Wegrzyn, J.L.; Jocjusch, E.L. Co-option of wing-patterning genes underlies the evolution of the treehopper helmet. Nat. Ecol. Evol. 2020, 4, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Skinner, R.K.; Dietrich, C.H.; Walden, K.K.O.; Gordon, E.; Sweet, A.D.; Podsiadlowski, L.; Petersen, M.; Simon, C.; Takiya, D.M.; Johnson, K.P. Phylogenomics of Auchenorrhyncha (Insecta: Hemiptera) using transcriptomes: Examining controversial relationships via degeneracy coding and interrogation of gene conflict. Syst. Entomol. 2020, 45, 85–113. [Google Scholar] [CrossRef]
- Feng, W.-M. Mass extinction at the end of Cretaceous. Fossils 2022, 3, 16–19. [Google Scholar]
- Deitz, L.L. Classification of the higher categories of the New World treehopper (Homoptera: Membracidae). Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, 1975; pp. 1–177. [Google Scholar]
- Dietrich, C.H.; McKamey, S.H.; Deitz, L.L. Morphology-based phylogeny of the treehopper family Membracidae (Hemiptera: Cicadomorpha: Membracoidea). Syst. Entomol. 2001, 26, 213–239. [Google Scholar] [CrossRef]
- Meng, G.; Li, Y.; Yang, C.; Liu, S. MitoZ: A toolkit for animal mitochondrial genome assembly, annotation and visualization. Nucleic Acids Res. 2019, 47, e63. [Google Scholar] [CrossRef] [PubMed]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [Green Version]
- Lowe, T.M.; Chan, P.P. tRNAscan-SE On-line: Integrating search and context for analysis of transfer RNA genes. Nucleic Acids Res. 2016, 44, W54–W57. [Google Scholar] [CrossRef]
- Bernt, M.; Donath, A.; Jühling, F.; Gärtner, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved denovo metazoan mitochondrial genome annotation. Mol. Phylogenet. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef]
- Greiner, S.; Lehwark, P.; Bock, R. Organellar genome DRAW (OGDRAW) version 1.3.1: Expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Res. 2019, 47, W59–W64. [Google Scholar] [CrossRef] [Green Version]
- Benson, G. Tandem repeats finder: A program to analyze DNA sequences. Nucleic Acids Res. 1999, 27, 573–580. [Google Scholar] [CrossRef] [Green Version]
- Perna, N.T.; Kocher, T.D. Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. J. Mol. Evol. 1995, 41, 353–358. [Google Scholar] [CrossRef]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Librado, P.; Rozas, J. DnaSP v5: A software for comprehensive analysis of DNA polymorphism data. Bioinformatics 2009, 25, 1451–1452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Bu, C.-P.; Benjamin, W.; Liang, A.-P. Comparative analysis of the mitochondrial genomes of Callitettixini spittlebugs (Hemiptera: Cercopidae) confirms the overall high evolutionary speed of the AT-Rich region but reveals the presence of short conservative elements at the tribal level. PLoS ONE 2014, 9, e109140. [Google Scholar] [CrossRef] [Green Version]
- Du, Z.-Y.; Hiroki, H.; John, R.-C.; Chris, S.; Yoshimura, J.; Cai, W.-Z.; Teiji, S.; Li, H. Mitochondrial genomics reveals shared phylogeographic patterns and demographic history among three periodical cicada species groups. Mol. Biol. Evol. 2019, 36, 1187–1200. [Google Scholar] [CrossRef]
- Zhang, D.; Gao, F.; Li, W.-X.; Jakovli´c, I.; Zou, H.; Zhang, J.; Wang, G.-T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. bioRxiv 2018, 489088. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, G.; Lohman, D.J.; Meier, R. SequenceMatrix: Concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 2011, 27, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2014, 32, 268–274. [Google Scholar] [CrossRef]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [Green Version]
- Lanfear, R.; Frandsen, P.B.; Wright, A.M.; Senfeld, T.; Calcott, B. PartitionFinder 2: New Methods for Selecting Partitioned Models of Evolution for Molecular and Morphological Phylogenetic Analyses. Mol. Biol. Evol. 2017, 34, 772–773. [Google Scholar] [CrossRef] [Green Version]
- Minh, B.Q.; Nguyen, M.A.T.; Haeseler, A.V. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef] [Green Version]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior summarization in Bayesian phylogenetics using tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mousavi, S.A.; Österman, J.; Wahlberg, N.; Nesme, X.; Lavire, C.; Vial, L.; Paulin, L.; de Lajudie, P.; Lindström, K. Phy-logeny of the Rhizobium-Allorhizobium-Agrobacterium clade supports the delineation of Neorhizobium gen. nov. Systemat. Appl. Microbiol. 2014, 37, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Bouckaert, R.; Heled, J.; Kühnert, D.; Vaughan, T.; Wu, C.H.; Xie, D.; Suchard, M.A.; Rambaut, A.; Drummond, A.J. BEAST 2: A software platform for Bayesian evolutionary analysis. PLoS Comput. Bio. 2014, 10, e1003537. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Harris, A.J.; Blair, C.; He, X.J. RASP (Reconstruct Ancestral State in Phylogenies): A tool for historical biogeography. Mol. Phylogenet. Evol. 2015, 87, 46–49. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.-E.; Yang, L.; Long, J.-K.; Chang, Z.-M.; Chen, X.-S. Revisiting the Phylogenetic Relationship and Evolution of Gargarini with Mitochondrial Genome (Hemiptera: Membracidae: Centrotinae). Int. J. Mol. Sci. 2023, 24, 694. https://doi.org/10.3390/ijms24010694
Li F-E, Yang L, Long J-K, Chang Z-M, Chen X-S. Revisiting the Phylogenetic Relationship and Evolution of Gargarini with Mitochondrial Genome (Hemiptera: Membracidae: Centrotinae). International Journal of Molecular Sciences. 2023; 24(1):694. https://doi.org/10.3390/ijms24010694
Chicago/Turabian StyleLi, Feng-E, Lin Yang, Jian-Kun Long, Zhi-Min Chang, and Xiang-Sheng Chen. 2023. "Revisiting the Phylogenetic Relationship and Evolution of Gargarini with Mitochondrial Genome (Hemiptera: Membracidae: Centrotinae)" International Journal of Molecular Sciences 24, no. 1: 694. https://doi.org/10.3390/ijms24010694





