Silver Nanoparticles Alone or in Combination with Calcium Hydroxide Modulate the Viability, Attachment, Migration, and Osteogenic Differentiation of Human Mesenchymal Stem Cells
Abstract
:1. Introduction
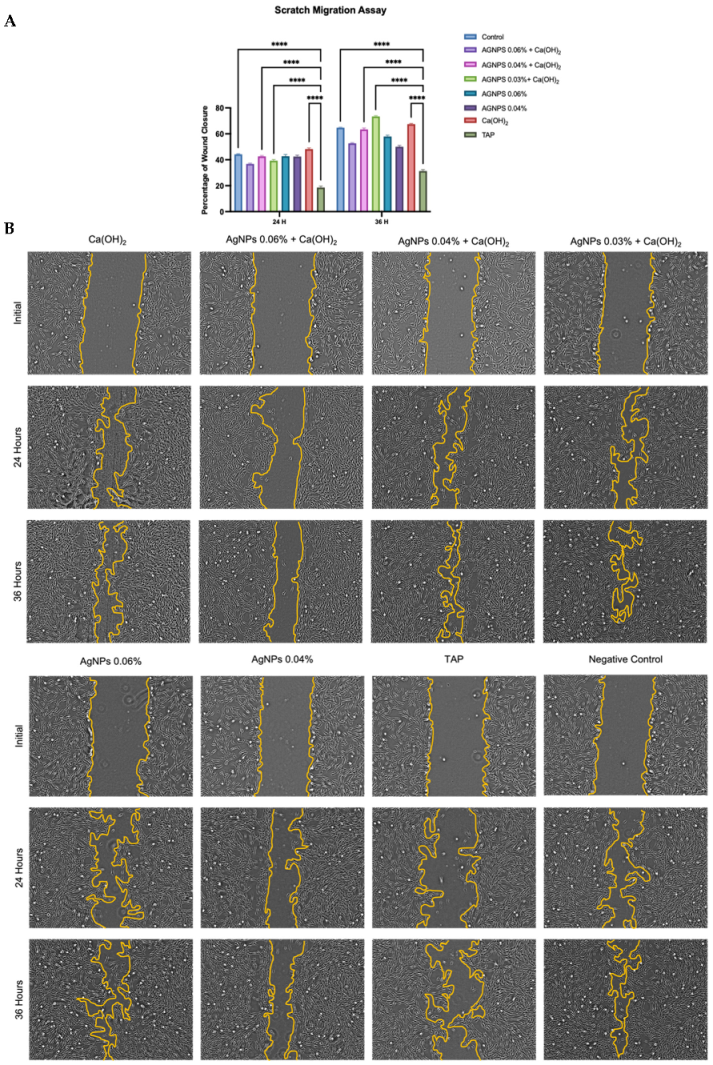
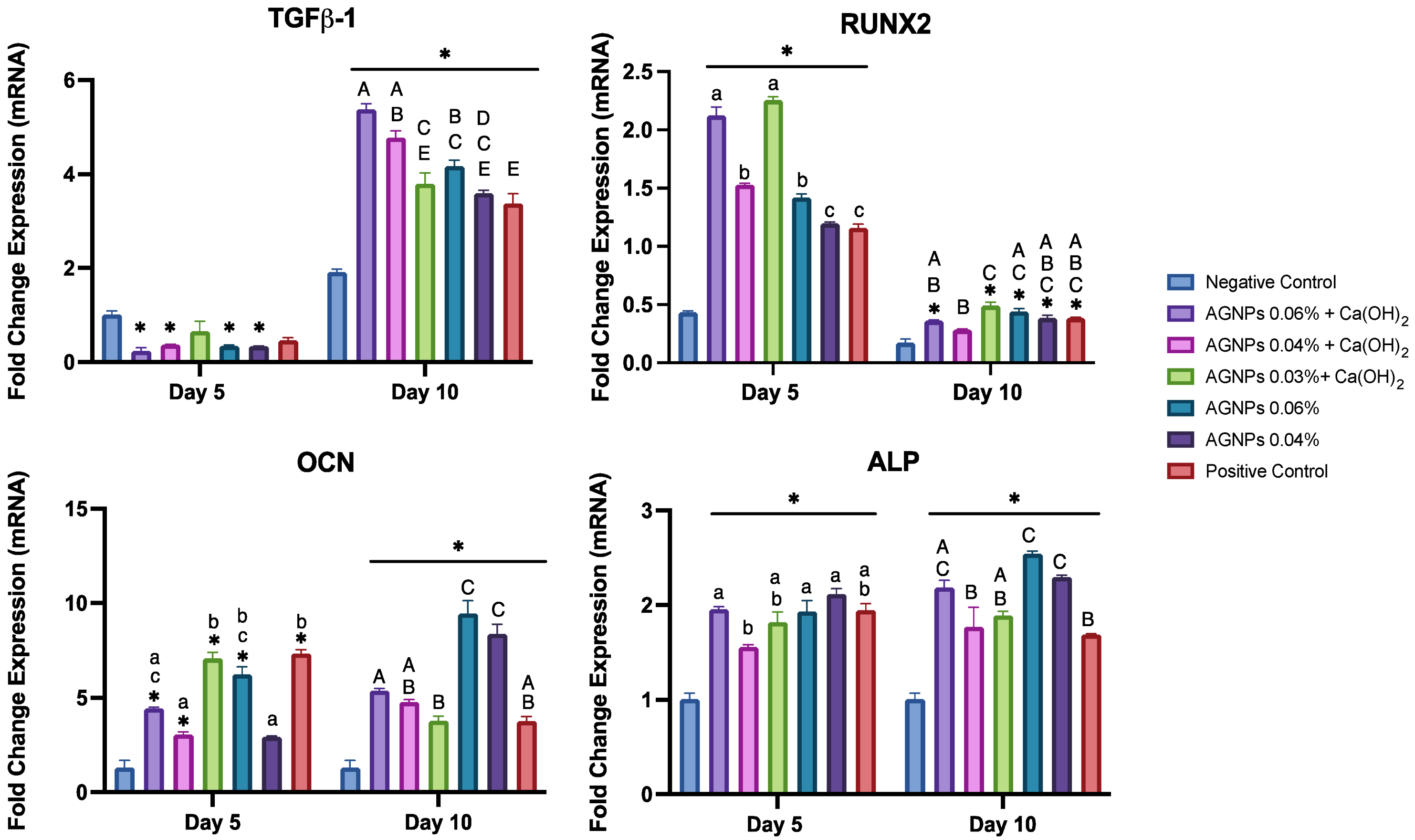
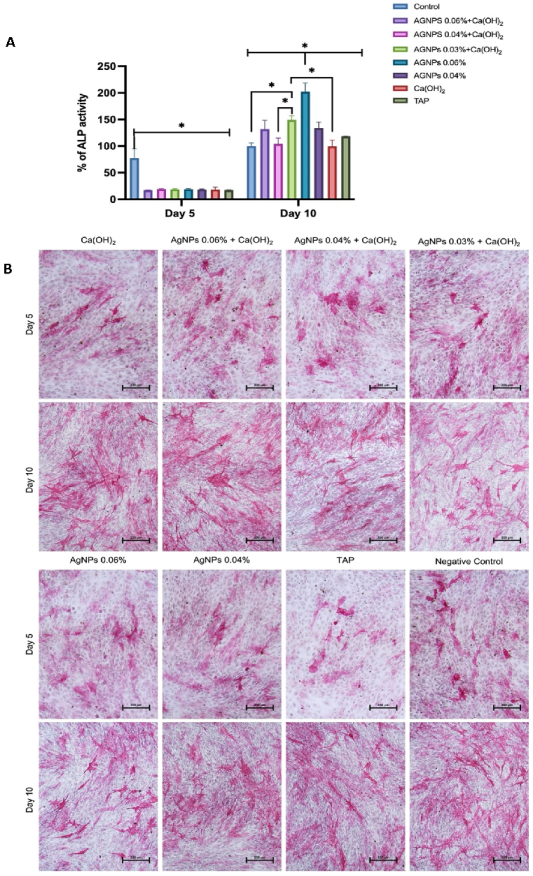
2. Results
2.1. AgNPs Favor Cell Attachment
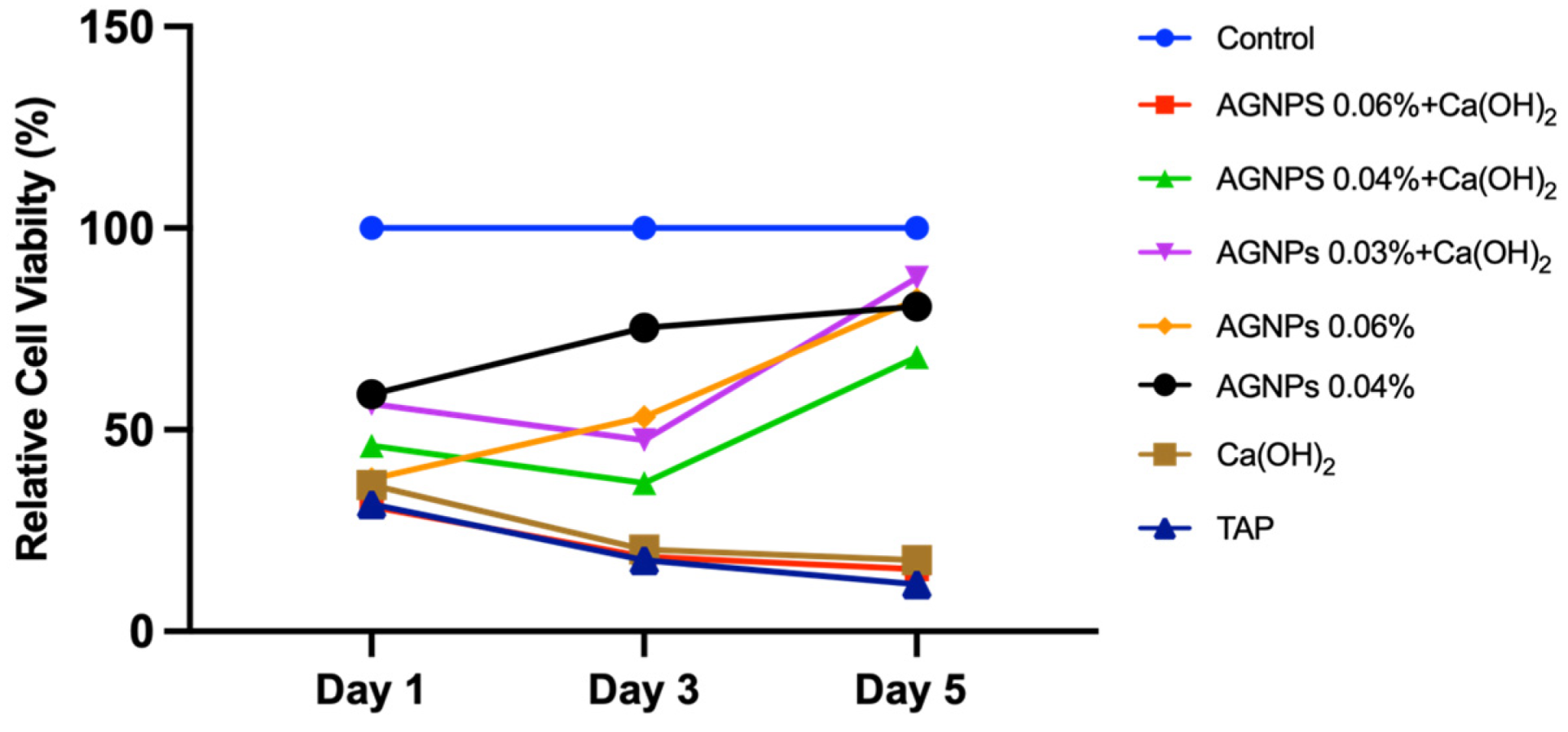
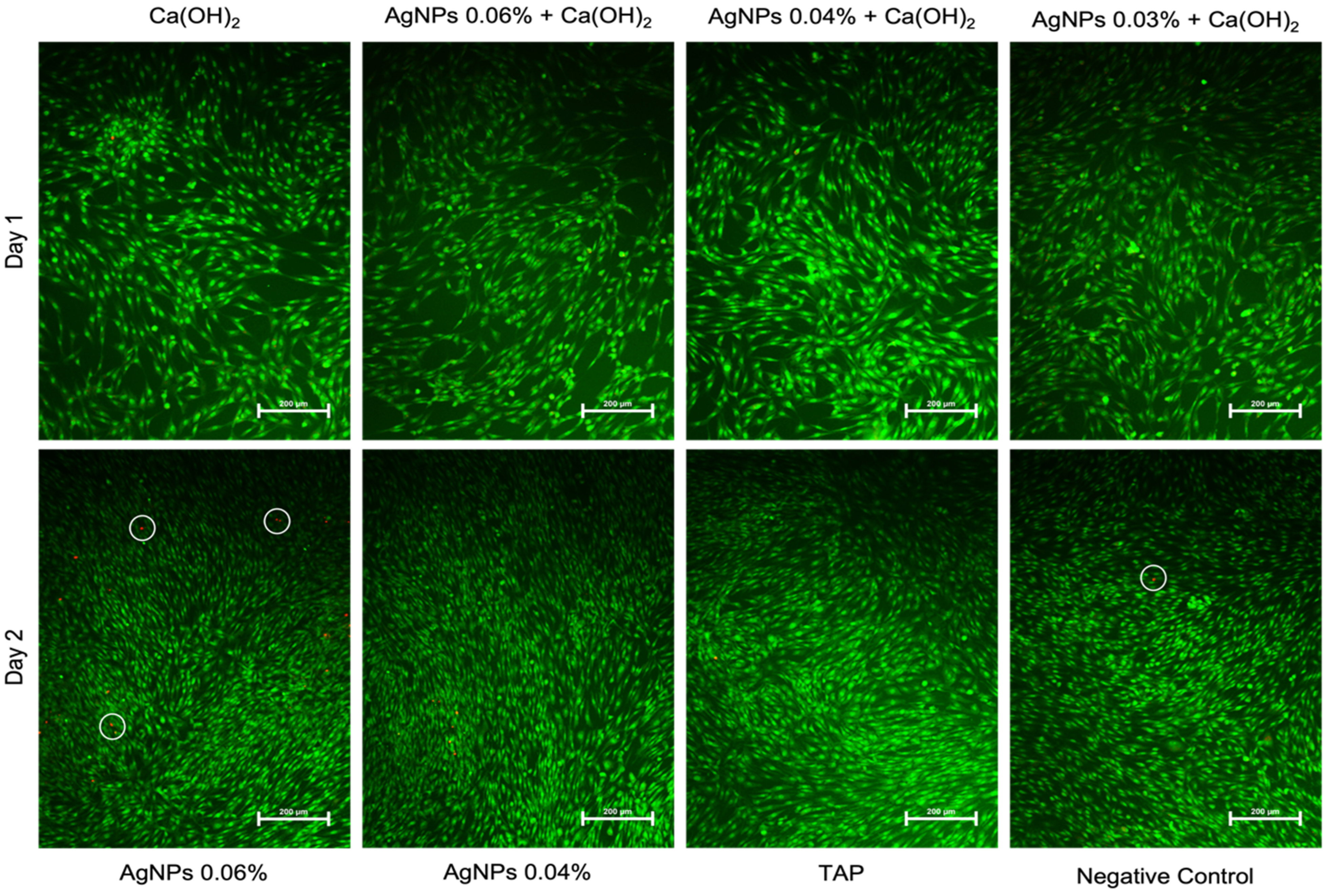
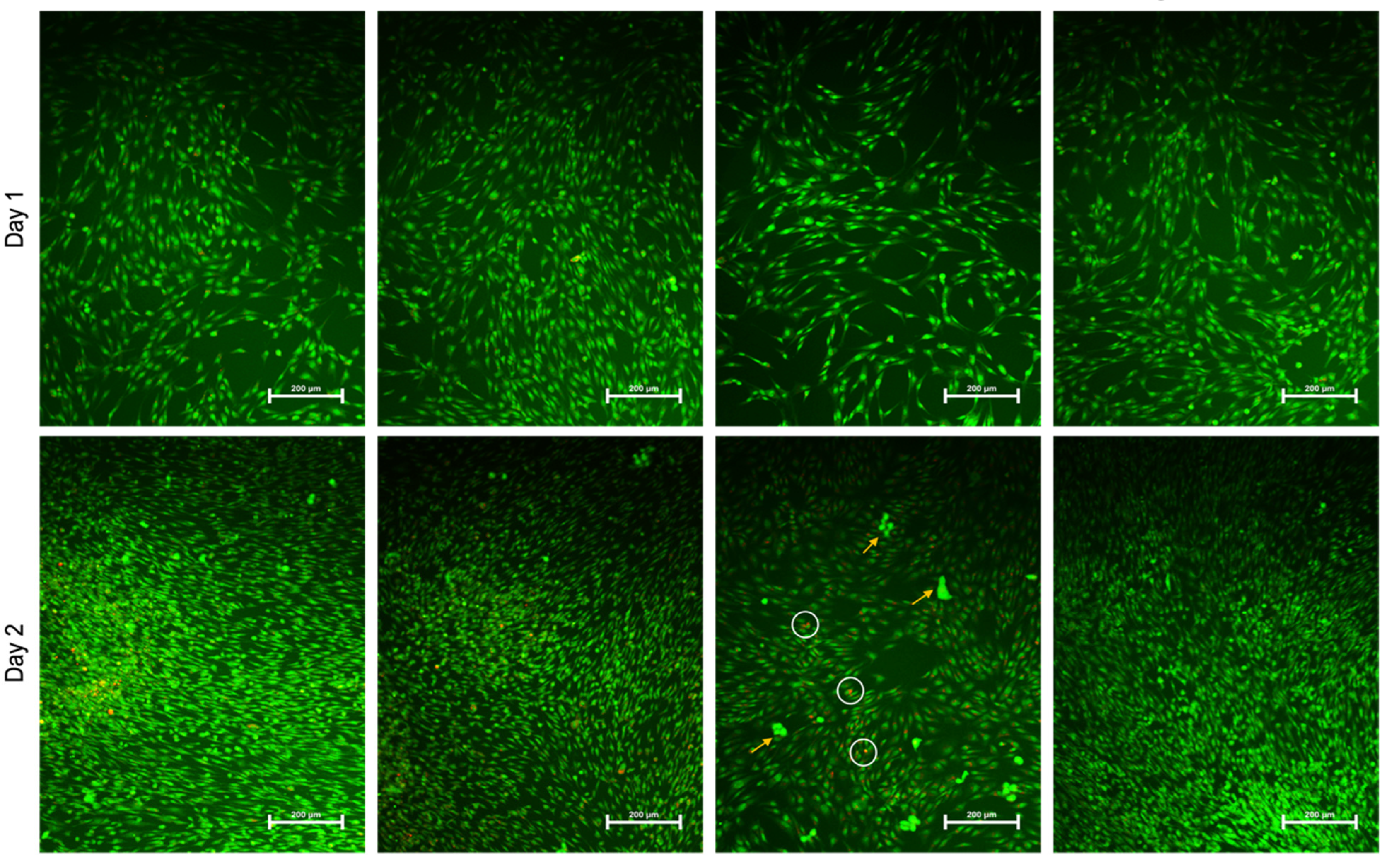
2.2. AgNPs Induce Less Apoptosis
3. Discussion
4. Materials and Methods
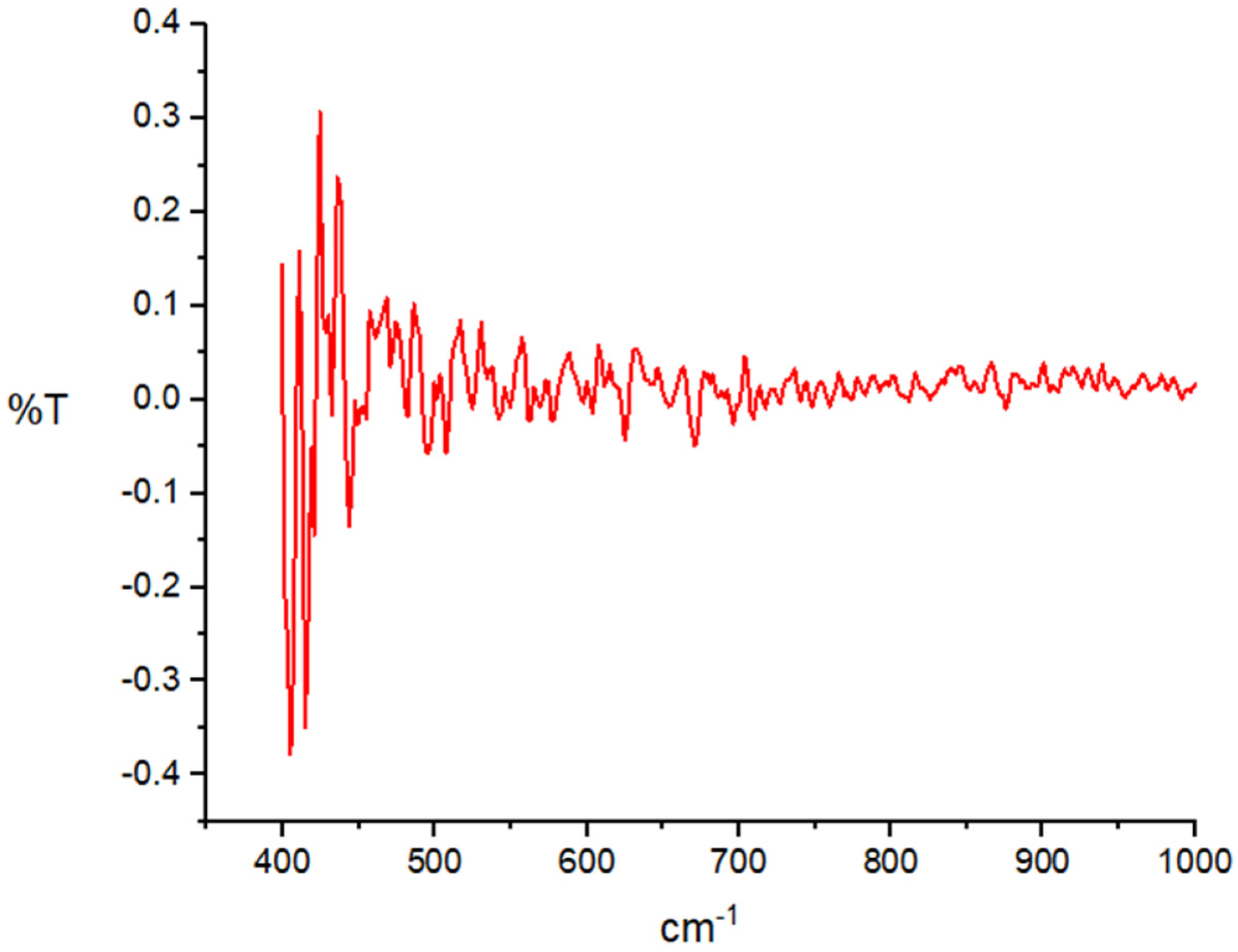
4.1. Characterization of AgNPs
4.2. Medicament Selection and Preparation
- Ca(OH)2 alone (35%);
- AgNPs (0.06%) + Ca(OH)2;
- AgNPs (0.04%) + Ca(OH)2;
- AgNPs (0.03%) + Ca(OH)2;
- AgNPs 0.06%;
- AgNPs 0.04%;
- TAP (1 mg/mL);
- Cells alone as a negative control.
4.3. Cell Culture and Dentin Disk Preparation
4.4. Cell Morphology and Attachment using Scanning Electron Microscopy (SEM)
4.5. Cell Proliferation Assay
4.6. Live–Dead Imaging
4.7. Scratch Migration (Wound healing) Assay
4.8. Osteogenic Differentiation Assays
4.8.1. Quantitative Reverse Transcription Real-Time PCR (qRT-PCR)
4.8.2. Quantification of Alkaline Phosphatase (ALP) Activity
4.8.3. ALP Staining
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frank, A. Therapy for the Divergent Pulpless Tooth by Continued Apical Formation. J. Am. Dent. Assoc. 1966, 72, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Kerekes, K.; Heide, S.; Jacobsen, I. Follow-up Examination of Endodontic Treatment in Traumatized Juvenile Incisors. J. Endod. 1980, 6, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Heithersay, G.S. Stimulation of Root Formation in Incompletely Developed Pulpless Teeth. Oral Surg. Oral Med. Oral Pathol. 1970, 29, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Chivian, N. Clinical Applications of Mineral Trioxide Aggregate. J. Endod. 1999, 25, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Pace, R.; Giuliani, V.; Nieri, M.; Di Nasso, L.; Pagavino, G. Mineral Trioxide Aggregate as Apical Plug in Teeth with Necrotic Pulp and Immature Apices: A 10-Year Case Series. J. Endod. 2014, 40, 1250–1254. [Google Scholar] [CrossRef]
- Bakland, L.K.; Andreasen, J.O. Will Mineral Trioxide Aggregate Replace Calcium Hydroxide in Treating Pulpal and Periodontal Healing Complications Subsequent to Dental Trauma? A Review. Dent. Traumatol. 2012, 28, 25–32. [Google Scholar] [CrossRef]
- Andreasen, J.O.; Farik, B.; Munksgaard, E.C. Long-Term Calcium Hydroxide as a Root Canal Dressing May Increase Risk of Root Fracture. Dent. Traumatol. 2002, 18, 134–137. [Google Scholar] [CrossRef]
- Andreasen, J.O.; Munksgaard, E.C.; Bakland, L.K. Comparison of Fracture Resistance in Root Canals of Immature Sheep Teeth after Filling with Calcium Hydroxide or MTA. Dent. Traumatol. 2006, 22, 154–156. [Google Scholar] [CrossRef]
- Chala, S.; Abouqal, R.; Rida, S. Apexification of Immature Teeth with Calcium Hydroxide or Mineral Trioxide Aggregate: Systematic Review and Meta-Analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2011, 112, e36–e42. [Google Scholar] [CrossRef]
- Bose, R.; Nummikoski, P.; Hargreaves, K. A Retrospective Evaluation of Radiographic Outcomes in Immature Teeth With Necrotic Root Canal Systems Treated With Regenerative Endodontic Procedures. J. Endod. 2009, 35, 1343–1349. [Google Scholar] [CrossRef]
- American Association of Endodontists Glossary of Endodontic Terms, 9th ed.; Gloss. Endod. Terms; American Association of Endodontists: Chicago, IL, USA, 2015.
- Murray, P.E.; Garcia-Godoy, F.; Hargreaves, K.M. Regenerative Endodontics: A Review of Current Status and a Call for Action. J. Endod. 2007, 33, 377–390. [Google Scholar] [CrossRef]
- American Association of Endodontists. AAE Clinical Considerations for a Regenerative Procedure; American Association of Endodontists: Chicago, IL, USA, 2014. [Google Scholar]
- Diogenes, A.; Ruparel, N.B.; Shiloah, Y.; Hargreaves, K.M. Regenerative Endodontics A Way Forward. J. Am. Dent. Assoc. 2016, 147, 372–380. [Google Scholar] [CrossRef]
- Diogenes, A.; Hargreaves, K.M. Microbial Modulation of Stem Cells and Future Directions in Regenerative Endodontics. J. Endod. 2017, 43, S95–S101. [Google Scholar] [CrossRef]
- Almutairi, W.; Yassen, G.H.; Aminoshariae, A.; Williams, K.A.; Mickel, A. Regenerative Endodontics: A Systematic Analysis of the Failed Cases. J. Endod. 2019, 45, 567–577. [Google Scholar] [CrossRef]
- Kakehashi, S.; Stanley, H.R.; Fitzgerald, R.J. The Effects of Surgical Exposures of Dental Pulps in Germ-Free and Conventional Laboratory Rats. Oral Surg. Oral Med. Oral Pathol. 1965, 20, 340–349. [Google Scholar] [CrossRef]
- Moller, A.; Fabricius, L. Influence on Periapical Tissue of Indigenous Oral Bacteria and Cecrotic Pulp in Monkeys. Scand. J. Dent. Res. 1981, 89, 475–484. [Google Scholar]
- AlSaeed, T.; Nosrat, A.; Melo, M.A.; Wang, P.; Romberg, E.; Xu, H.; Fouad, A.F. Antibacterial Efficacy and Discoloration Potential of Endodontic Topical Antibiotics. J. Endod. 2018, 44, 1110–1114. [Google Scholar] [CrossRef]
- Althumairy, R.I.; Teixeira, F.B.; Diogenes, A. Effect of Dentin Conditioning with Intracanal Medicaments on Survival of Stem Cells of Apical Papilla. J. Endod. 2014, 40, 521–525. [Google Scholar] [CrossRef]
- Siqueira, J.F.; Guimarães-Pinto, T.; Rôças, I.N. Effects of Chemomechanical Preparation with 2.5% Sodium Hypochlorite and Intracanal Medication With Calcium Hydroxide on Cultivable Bacteria in Infected Root Canals. J. Endod. 2007, 33, 800–805. [Google Scholar] [CrossRef]
- Ferreira, N.S.; Martinho, F.C.; Cardoso, F.G.R.; Nascimento, G.G.; Carvalho, C.A.T.; Valera, M.C. Microbiological Profile Resistant to Different Intracanal Medications in Primary Endodontic Infections. J. Endod. 2015, 41, 824–830. [Google Scholar] [CrossRef]
- Torabinejad, M.; Nosrat, A.; Verma, P.; Udochukwu, O. Regenerative Endodontic Treatment or Mineral Trioxide Aggregate Apical Plug in Teeth with Necrotic Pulps and Open Apices: A Systematic Review and Meta-Analysis. J. Endod. 2017, 43, 1806–1820. [Google Scholar] [CrossRef] [PubMed]
- Sjögren, U.; Figdor, D.; Spångberg, L.; Sundqvist, G. The Antimicrobial Effect of Calcium Hydroxide as a Short-term Intracanal Dressing. Int. Endod. J. 1991, 24, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Byström, A.; Claesson, R.; Sundqvist, G. The Antibacterial Effect of Camphorated Paramonochlorophenol, Camphorated Phenol and Calcium Hydroxide in the Treatment of Infected Root Canals. Dent. Traumatol. 1985, 1, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Siqueira, J.F.; Rôças, I.N.; Benno, Y. Bacterial Reduction and Persistence after Endodontic Treatment Procedures. Oral Microbiol. Immunol. 2007, 22, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Waltimo, T.M.T.; Sirén, E.K.; Ørstavik, D.; Haapasalo, M.P.P. Susceptibility of Oral Candida Species to Calcium Hydroxide in Vitro. Int. Endod. J. 1999, 32, 94–98. [Google Scholar] [CrossRef]
- Vianna, M.E.; De Almeida Gomes, B.P.F.; Sena, N.T.; Zaia, A.A.; Ferraz, C.C.R.; De Souza Filho, F.J. In Vitro Evaluation of the Susceptibility of Endodontic Pathogens to Calcium Hydroxide Combined with Different Vehicles. Braz. Dent. J. 2005, 16, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Siqueira, J.F.; De Uzeda, M. Disinfection by Calcium Hydroxide Pastes of Dentinal Tubules Infected with Two Obligate and One Facultative Anaerobic Bacteria. J. Endod. 1996, 22, 674–676. [Google Scholar] [CrossRef]
- Rathke, A.; Meisohle, D.; Bokelmann, J.; Haller, B. Antibacterial Activity of Calcium Hydroxide and Chlorhexidine Containing Points against Fusobacterium Nucleatum and Parvimonas Micra. Eur. J. Dent. 2012, 6, 434–439. [Google Scholar] [CrossRef]
- Eldeniz, A.U.; Mustafa, K.; Ørstavik, D.; Dahl, J.E. Cytotoxicity of New Resin-, Calcium Hydrooxide- and Silicone-Based Root Canal Sealers on Fibroblasts Derived from Human Gingiva and L929 Cell Lines. Int. Endod. J. 2007, 40, 329–337. [Google Scholar] [CrossRef]
- Huang, F.M.; Tai, K.W.; Chou, M.Y.; Chang, Y.C. Cytotoxicity of Resin-, Zinc Oxide-Eugenol-, and Calcium Hydroxide-Based Root Canal Sealers on Human Periodontal Ligament Cells and Permanent V79 Cells. Int. Endod. J. 2002, 35, 153–158. [Google Scholar] [CrossRef]
- Kaur, A.; Shah, N.; Logani, A.; Mishra, N. Biotoxicity of Commonly Used Root Canal Sealers: A Meta-Analysis. J. Conserv. Dent. 2015, 18, 83. [Google Scholar] [CrossRef]
- Guven, E.P.; Yalvac, M.E.; Sahin, F.; Yazici, M.M.; Rizvanov, A.A.; Bayirli, G. Effect of Dental Materials Calcium Hydroxide–Containing Cement, Mineral Trioxide Aggregate, and Enamel Matrix Derivative on Proliferation and Differentiation of Human Tooth Germ Stem Cells. J. Endod. 2011, 37, 650–656. [Google Scholar] [CrossRef]
- Himel, V.T.; Brady, J.; Weir, J. Evaluation of Repair of Mechanical Perforations of the Pulp Chamber Floor Using Biodegradable Tricalcium Phosphate or Calcium Hydroxide. J. Endod. 1985, 11, 161–165. [Google Scholar] [CrossRef]
- Song, W.; Ge, S. Application of Antimicrobial Nanoparticles in Dentistry. Molecules 2019, 24, 1033. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, A.; Kishen, A. Antibacterial Nanoparticles in Endodontics: A Review. J. Endod. 2016, 42, 1417–1426. [Google Scholar] [CrossRef]
- Kishen, A. Nanotechnology in Endodontics; Springer International Publishing: Cham, Switzerland, 2015; ISBN 9783319135748. [Google Scholar]
- Bhandi, S.; Mehta, D.; Mashyakhy, M.; Chohan, H.; Testarelli, L.; Thomas, J.; Dhillon, H.; Thirumal Raj, A.; Balaji, T.M.; Varadarajan, S.; et al. Antimicrobial Efficacy of Silver Nanoparticles as Root Canal Irrigant’s: A Systematic Review. J. Clin. Med. 2021, 10, 1152. [Google Scholar] [CrossRef]
- Fong, J.; Wood, F. Nanocrystalline Silver Dressings in Wound Management: A Review. Int. J. Nanomed. 2006, 1, 441. [Google Scholar] [CrossRef] [Green Version]
- Chernousova, S.; Epple, M. Silver as Antibacterial Agent: Ion, Nanoparticle, and Metal. Angew. Chem.-Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef]
- Wu, D.; Fan, W.; Kishen, A.; Gutmann, J.L.; Fan, B. Evaluation of the Antibacterial Efficacy of Silver Nanoparticles against Enterococcus Faecalis Biofilm. J. Endod. 2014, 40, 285–290. [Google Scholar] [CrossRef]
- Rodrigues, C.T.; De Andrade, F.B.; De Vasconcelos, L.R.S.M.; Midena, R.Z. Antibacterial Properties of Silver Nanoparticles as a Root Canal Irrigant against Enterococcus Faecalis Biofilm and Infected Dentinal Tubules. Int. Endod. J. 2018, 51, 901–911. [Google Scholar] [CrossRef]
- Ghahramani, Y.; Yaghoobi, F.; Motamedi, R.; Jamshidzadeh, A.; Abbaszadegan, A. Effect of Endodontic Irrigants and Medicaments Mixed with Silver Nanoparticles against Biofilm Formation of Enterococcus Faecalis. Iran. Endod. J. 2018, 13, 559–564. [Google Scholar] [CrossRef]
- Chan, E.L.K.; Zhang, C.; Cheung, G. Cytotoxicity of a Novel Nano-Silver Particle Endodontic Irrigant. Clin. Cosmet. Investig. Dent. 2015, 7, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Razumova, S.; Brago, A.; Serebrov, D.; Barakat, H.; Kozlova, Y.; Howijieh, A.; Guryeva, Z.; Enina, Y.; Troitskiy, V. The Application of Nano Silver Argitos as a Final Root Canal Irrigation for the Treatment of Pulpitis and Apical Periodontitis. In Vitro Study. Nanomaterials 2022, 12, 248. [Google Scholar] [CrossRef] [PubMed]
- Javidi, M.; Afkhami, F.; Zarei, M.; Ghazvini, K.; Rajabi, O. Efficacy of a Combined Nanoparticulate/Calcium Hydroxide Root Canal Medication on Elimination of Enterococcus faecalis. Aust. Endod. J. 2014, 40, 61–65. [Google Scholar] [CrossRef] [PubMed]
- AlGazlan, A.M.S.; Auda, S.H.; Balto, H.; Alsalleeh, F. Antibiofilm Efficacy of Silver Nanoparticles Alone or Mixed with Calcium Hydroxide as Intracanal Medicaments: An Ex-Vivo Analysis. J. Endod. 2022, 48, 1294–1300. [Google Scholar] [CrossRef]
- Melo, M.A.S.; Cheng, L.; Zhang, K.; Weir, M.D.; Rodrigues, L.K.A.; Xu, H.H.K. Novel Dental Adhesives Containing Nanoparticles of Silver and Amorphous Calcium Phosphate. Dent. Mater. 2013, 29, 199–210. [Google Scholar] [CrossRef] [Green Version]
- Melo, M.A.S.; Cheng, L.; Weir, M.D.; Hsia, R.C.; Rodrigues, L.K.A.; Xu, H.H.K. Novel Dental Adhesive Containing Antibacterial Agents and Calcium Phosphate Nanoparticles. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2013, 101, 620–629. [Google Scholar] [CrossRef] [Green Version]
- Bapat, R.A.; Chaubal, T.V.; Joshi, C.P.; Bapat, P.R.; Choudhury, H.; Pandey, M.; Gorain, B.; Kesharwani, P. An Overview of Application of Silver Nanoparticles for Biomaterials in Dentistry. Mater. Sci. Eng. C 2018, 91, 881–898. [Google Scholar] [CrossRef]
- Espinosa-Cristóbal, L.F.; Martinez-Castanon, G.A.; Téllez-Déctor, E.J.; Niño-Martínez, N.; Zavala-Alonso, N.V.; Loyola-Rodríguez, J.P. Adherence Inhibition of Streptococcus Mutans on Dental Enamel Surface Using Silver Nanoparticles. Mater. Sci. Eng. C 2013, 33, 2197–2202. [Google Scholar] [CrossRef]
- Baras, B.H.; Anne, M.; Melo, S.; Sun, J.; Oates, T.W.; Weir, M.D.; Xie, X.; Bai, Y.; Xu, H.H.K. Novel Endodontic Sealer with Dual Strategies of Dimethylaminohexadecyl Methacrylate and Nanoparticles of Silver to Inhibit Root Canal Biofilms. Dent. Mater. 2019, 35, 1117–1129. [Google Scholar] [CrossRef]
- Baras, B.H.; Sun, J.; Melo, M.A.S.; Tay, F.R.; Oates, T.W.; Zhang, K.; Weir, M.D.; Xu, H.H.K. Novel Root Canal Sealer with Dimethylaminohexadecyl Methacrylate, Nano-Silver and Nano-Calcium Phosphate to Kill Bacteria inside Root Dentin and Increase Dentin Hardness. Dent. Mater. 2019, 35, 1479–1489. [Google Scholar] [CrossRef]
- Gomes-Filho, J.E.; Silva, F.O.; Watanabe, S.; Angelo Cintra, L.T.; Tendoro, K.V.; Dalto, L.G.; Pacanaro, S.V.; Lodi, C.S.; De Melo, F.F.F. Tissue Reaction to Silver Nanoparticles Dispersion as an Alternative Irrigating Solution. J. Endod. 2010, 36, 1698–1702. [Google Scholar] [CrossRef]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver Nanoparticles: The Powerful Nanoweapon against Multidrug-Resistant Bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef]
- Afkhami, F.; Pourhashemi, S.J.; Sadegh, M.; Salehi, Y.; Fard, M.J.K. Antibiofilm Efficacy of Silver Nanoparticles as a Vehicle for Calcium Hydroxide Medicament against Enterococcus Faecalis. J. Dent. 2015, 43, 1573–1579. [Google Scholar] [CrossRef]
- Tülü, G.; Kaya, B.Ü.; Çetin, E.S.; Köle, M. Antibacterial Effect of Silver Nanoparticles Mixed with Calcium Hydroxide or Chlorhexidine on Multispecies Biofilms. Odontology 2021, 109, 802–811. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A Systematic Review on Silver Nanoparticles-Induced Cytotoxicity: Physicochemical Properties and Perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Perde-Schrepler, M.; Florea, A.; Brie, I.; Virag, P.; Fischer-Fodor, E.; Vâlcan, A.; Gurzǎu, E.; Lisencu, C.; Maniu, A. Size-Dependent Cytotoxicity and Genotoxicity of Silver Nanoparticles in Cochlear Cells In Vitro. J. Nanomater. 2019, 2019, 6090259. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ji, Z.; Chang, C.H.; Zhang, H.; Wang, M.; Liao, Y.P.; Lin, S.; Meng, H.; Li, R.; Sun, B.; et al. Use of Coated Silver Nanoparticles to Understand the Relationship of Particle Dissolution and Bioavailability to Cell and Lung Toxicological Potential. Small 2014, 10, 385. [Google Scholar] [CrossRef] [Green Version]
- Kaba, S.I.; Egorova, E.M. In Vitro Studies of the Toxic Effects of Silver Nanoparticles on HeLa and U937 Cells. Nanotechnol. Sci. Appl. 2015, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Piett, C.; Farkas, S.; Qazzaz, M.; Syed, N.I. Silver Nanoparticles (AgNPs) Cause Degeneration of Cytoskeleton and Disrupt Synaptic Machinery of Cultured Cortical Neurons. Mol. Brain 2013, 6, 29. [Google Scholar] [CrossRef] [Green Version]
- Ricucci, D.; Siqueira, J.F. Biofilms and Apical Periodontitis: Study of Prevalence and Association with Clinical and Histopathologic Findings. J. Endod. 2010, 36, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F.; Rôças, I.N.; Ricucci, D. Biofilms in Endodontic Infection. Endod. Top. 2010, 22, 33–49. [Google Scholar] [CrossRef]
- Siqueira, J.F.; Lopes, H.P. Mechanisms of Antimicrobial Activity of Calcium Hydroxide: A Critical Review. Int. Endod. J. 1999, 32, 361–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fayaz, A.M.; Balaji, K.; Girilal, M.; Yadav, R.; Kalaichelvan, P.T.; Venketesan, R. Biogenic Synthesis of Silver Nanoparticles and Their Synergistic Effect with Antibiotics: A Study against Gram-Positive and Gram-Negative Bacteria. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Kora, A.J.; Rastogi, L. Enhancement of Antibacterial Activity of Capped Silver Nanoparticles in Combination with Antibiotics, on Model Gram-Negative and Gram-Positive Bacteria. Bioinorg. Chem. Appl. 2013, 2013, 871097. [Google Scholar] [CrossRef] [Green Version]
- Diogenes, A.; Henry, M.A.; Teixeira, F.B.; Hargreaves, K.M. An Update on Clinical Regenerative Endodontics. Endod. Top. 2013, 28, 2–23. [Google Scholar] [CrossRef]
- Abdallah, B.M.; Haack-Sørensen, M.; Burns, J.S.; Elsnab, B.; Jakob, F.; Hokland, P.; Kassem, M. Maintenance of Differentiation Potential of Human Bone Marrow Mesenchymal Stem Cells Immortalized by Human Telomerase Reverse Transcriptase Gene despite of Extensive Proliferation. Biochem. Biophys. Res. Commun. 2005, 326, 527–538. [Google Scholar] [CrossRef]
- Simonsen, J.L.; Rosada, C.; Serakinci, N.; Justesen, J.; Stenderup, K.; Rattan, S.I.S.; Jensen, T.G.; Kassem, M. Telomerase Expression Extends the Proliferative Life-Span and Maintains the Osteogenic Potential of Human Bone Marrow Stromal Cells. Nat. Biotechnol. 2002, 20, 592–596. [Google Scholar] [CrossRef]
- Shi, S.; Robey, P.G.; Gronthos, S. Comparison of Human Dental Pulp and Bone Marrow Stromal Stem Cells by CDNA Microarray Analysis. Bone 2001, 29, 532–539. [Google Scholar] [CrossRef]
- Huang, G.T.J.; Gronthos, S.; Shi, S. Mesenchymal Stem Cells Derived from Dental Tissues vs. Those from Other Sources: Their Biology and Role in Regenerative Medicine. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef]
- Murakami, M.; Hayashi, Y.; Iohara, K.; Osako, Y.; Hirose, Y.; Nakashima, M. Trophic Effects and Regenerative Potential of Mobilized Mesenchymal Stem Cells from Bone Marrow and Adipose Tissue as Alternative Cell Sources for Pulp/Dentin Regeneration. Cell Transplant. 2015, 24, 1753–1765. [Google Scholar] [CrossRef]
- Al-Nbaheen, M.; Vishnubalaji, R.; Ali, D.; Bouslimi, A.; Al-Jassir, F.; Megges, M.; Prigione, A.; Adjaye, J.; Kassem, M.; Aldahmash, A. Human Stromal (Mesenchymal) Stem Cells from Bone Marrow, Adipose Tissue and Skin Exhibit Differences in Molecular Phenotype and Differentiation Potential. Stem Cell Rev. Rep. 2013, 9, 32–43. [Google Scholar] [CrossRef] [Green Version]
- Afkhami, F.; Akbari, S.; Chiniforush, N. Entrococcus faecalis Elimination in Root Canals Using Silver Nanoparticles, Photodynamic Therapy, Diode Laser, or Laser-Activated Nanoparticles: An In Vitro Study. J. Endod. 2017, 43, 279–282. [Google Scholar] [CrossRef]
- Bo, D.; Kayombo, C.M. Effect of Nanosilver Gel, Chlorhexidine Gluconate, and Camphorated Phenol on Enterococcus faecalis Biofilm. Int. Sch. Res. Not. 2014, 2014, 380278. [Google Scholar] [CrossRef] [Green Version]
- Afkhami, F.; Elahy, S.; Mahmoudi-Nahavandi, A. Spectrophotometric Analysis of Crown Discoloration Following the Use of Silver Nanoparticles Combined with Calcium Hydroxide as Intracanal Medicament. J Clin Exp Dent 2017, 9, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Seung, J.; Weir, M.D.; Melo, M.A.S.; Romberg, E.; Nosrat, A.; Xu, H.H.K.; Tordik, P.A. A Modified Resin Sealer: Physical and Antibacterial Properties. J. Endod. 2018, 44, 1553–1557. [Google Scholar] [CrossRef]
- Chávez-andrade, G.M.; Tanomaru-filho, M.; Rodrigues, E.M.; Gomes-cornélio, A.L.; Faria, G.; Inês, M.; Bernardi, B.; Guerreiro-tanomaru, J.M. Archives of Oral Biology Cytotoxicity, Genotoxicity and Antibacterial Activity of Poly (Vinyl Alcohol)—Coated Silver Nanoparticles and Farnesol as Irrigating Solutions. Arch. Oral Biol. 2017, 84, 89–93. [Google Scholar] [CrossRef] [Green Version]
- Takamiya, A.S.; Monteiro, D.R.; Gorup, L.F.; Gomes-Filho, E.; Camargo, E.R. In Vitro and In Vivo Toxicity Evaluation of Colloidal Silver Nanoparticles Used in Endodontic Treatments. J. Endod. 2016, 42, 953–960. [Google Scholar] [CrossRef] [Green Version]
- Kitikuson, P.; Srisuwan, T. Attachment Ability of Human Apical Papilla Cells to Root Dentin Surfaces Treated with Either 3Mix or Calcium Hydroxide. J. Endod. 2016, 42, 89–94. [Google Scholar] [CrossRef]
- Liu, W.; Wu, Y.; Wang, C.; Li, H.C.; Wang, T.; Liao, C.Y.; Cui, L.; Zhou, Q.F.; Yan, B.; Jiang, G.B. Impact of Silver Nanoparticles on Human Cells: Effect of Particle Size. Nanotoxicology 2010, 4, 319–330. [Google Scholar] [CrossRef]
- Stoehr, L.C.; Gonzalez, E.; Stampfl, A.; Casals, E.; Duschl, A.; Puntes, V.; Oostingh, G.J. Shape Matters: Effects of Silver Nanospheres and Wires on Human Alveolar Epithelial Cells. Part. Fibre Toxicol. 2011, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Wong, K.K.Y.; Ho, C.M.; Lok, C.N.; Yu, W.Y.; Che, C.M.; Chiu, J.F.; Tam, P.K.H. Topical Delivery of Silver Nanoparticles Promotes Wound Healing. ChemMedChem 2007, 2, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, G.; Li, Y.P. TGF-β and BMP Signaling in Osteoblast, Skeletal Development, and Bone Formation, Homeostasis and Disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth Factors and Cytokines in Wound Healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.M.; Jeon, S.H.; Park, J.Y.; Chung, J.H.; Choung, Y.H.; Choung, P.H. Dental Stem Cell Therapy with Calcium Hydroxide in Dental Pulp Capping. Tissue Eng.-Part A 2010, 16, 1823–1833. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Zheng, L.; Jiang, J.; Gui, J.; Zhang, L.; Huang, Y.; Chen, X.; Ji, J.; Fan, Y. Calcium Hydroxide–Induced Proliferation, Migration, Osteogenic Differentiation, and Mineralization via the Mitogen-Activated Protein Kinase Pathway in Human Dental Pulp Stem Cells. J. Endod. 2016, 42, 1355–1361. [Google Scholar] [CrossRef]
- Gong, C.P.; Li, S.C.; Wang, R.Y. Development of Biosynthesized Silver Nanoparticles Based Formulation for Treating Wounds during Nursing Care in Hospitals. J. Photochem. Photobiol. B Biol. 2018, 183, 137–141. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, X.; Lei, W.; Pang, L.; Wan, C.; Shi, Z.; Zhao, L.; Nagy, T.R.; Peng, X.; Hu, J.; et al. TGF-Β1-Induced Migration of Bone Mesenchymal Stem Cells Couples Bone Resorption and Formation. Nat. Med. 2009, 15, 757. [Google Scholar] [CrossRef] [Green Version]
- Laurent, P.; Camps, J.; About, I. Biodentine TM Induces TGF-Β1 Release from Human Pulp Cells and Early Dental Pulp Mineralization. Int. Endod. J. 2012, 45, 439–448. [Google Scholar] [CrossRef]
- Jaunberzins, A.; Gutmann, J.L.; Witherspoon, D.E.; Harper, R.P. TGF-Beta 1 Alone and in Combination with Calcium Hydroxide Is Synergistic to TGF-Beta 1 Production by Osteoblasts In Vitro. Int. Endod. J. 2000, 33, 421–426. [Google Scholar] [CrossRef]
- Nie, X.; Tian, W.; Zhang, Y.; Chen, X.; Dong, R.; Jiang, M.; Chen, F.; Jin, Y. Induction of Transforming Growth Factor-Beta 1 on Dentine Pulp Cells in Different Culture Patterns. Cell Biol. Int. 2006, 30, 295–300. [Google Scholar] [CrossRef]
- Komori, T. Regulation of Proliferation, Differentiation and Functions of Osteoblasts by Runx2. Int. J. Mol. Sci. 2019, 20, 1694. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Sasaki, Y.; Ogata, Y. Calcium Hydroxide Regulates Bone Sialoprotein Gene Transcription in Human Osteoblast-like Saos2 Cells. J. Oral Sci. 2011, 53, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Noda, K.; Yang, Y.; Shen, Z.; Chen, Z.; Ogata, Y. Calcium Hydroxide Regulates Transcription of the Bone Sialoprotein Gene via a Calcium-Sensing Receptor in Osteoblast-like ROS 17/2.8 Cells. Eur. J. Oral Sci. 2018, 126, 13–23. [Google Scholar] [CrossRef]
- Zernik, J.; Twarog, K.; Upholt, W.B. Regulation of Alkaline Phosphatase and Alpha 2(I) Procollagen Synthesis during Early Intramembranous Bone Formation in the Rat Mandible. Differentiation 1990, 44, 207–215. [Google Scholar] [CrossRef]
- Malaval, L.; Modrowski, D.; Gupta, A.K.; Aubin, J.E. Cellular Expression of Bone-Related Proteins during In Vitro Osteogenesis in Rat Bone Marrow Stromal Cell Cultures. J. Cell. Physiol. 1994, 158, 555–572. [Google Scholar] [CrossRef]
- Adeyemi, O.S.; Adewumi, I. Biochemical Evaluation of Silver Nanoparticles in Wistar Rats. Int. Sch. Res. Not. 2014, 2014, 196091. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Zheng, Y.; Feng, Q.; Elkhooly, T.A.; Liu, X.; Yang, X.; Wang, Y.; Xie, Y. Silver Nanoparticles Stimulate Osteogenesis of Human Mesenchymal Stem Cells through Activation of Autophagy. Nanomedicine 2020, 15, 337–353. [Google Scholar] [CrossRef]
- Mahmood, M.; Li, Z.; Casciano, D.; Khodakovskaya, M.V.; Chen, T.; Karmakar, A.; Dervishi, E.; Xu, Y.; Mustafa, T.; Watanabe, F.; et al. Nanostructural Materials Increase Mineralization in Bone Cells and Affect Gene Expression through MiRNA Regulation. J. Cell. Mol. Med. 2011, 15, 2297–2306. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Song, M.Y.; Park, J.D.; Song, K.S.; Ryu, H.R.; Chung, Y.H.; Chang, H.K.; Lee, J.H.; Oh, K.H.; Kelman, B.J.; et al. Subchronic Oral Toxicity of Silver Nanoparticles. Part. Fibre Toxicol. 2010, 7, 20. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Wang, P.; Wu, J. Effect of Exposure of Osteoblast-like Cells to Low-Dose Silver Nanoparticles: Uptake, Retention and Osteogenic Activity. Artif. Cells Nanomed. Biotechnol. 2019, 47, 260–267. [Google Scholar] [CrossRef] [PubMed]
- De Almeida Gomes, B.P.F.; Ferraz, C.C.R.; Vianna, M.E.; Rosalen, P.L.; Zaia, A.A.; Teixeira, F.B.; de Souza-Filho, F.J. In Vitro Antimicrobial Activity of Calcium Hydroxide Pastes and Their Vehicles against Selected Microorganisms. Braz. Dent. J. 2002, 13, 155–161. [Google Scholar] [CrossRef] [PubMed]
- White, J.M.; Goodis, H.E.; Marshall, S.J. Sterilization of Teeth by Gamma Radiation. J. Dent. Res. 1994, 73, 1560–1567. [Google Scholar] [CrossRef] [PubMed]
- Ruparel, N.B.; Teixeira, F.B.; Ferraz, C.C.R.; Diogenes, A. Direct Effect of Intracanal Medicaments on Survival of Stem Cells of the Apical Papilla. J. Endod. 2012, 38, 1372–1375. [Google Scholar] [CrossRef]
- Vishnubalaji, R.; Elango, R.; Al-Toub, M.; Manikandan, M.; Al-Rikabi, A.; Harkness, L.; Ditzel, N.; Atteya, M.; Hamam, R.; Alfayez, M.; et al. Neoplastic Transformation of Human Mesenchymal Stromal Cells Mediated via LIN28B. Sci. Rep. 2019, 9, 8101. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Pijuan, J.; Barceló, C.; Moreno, D.F.; Maiques, O.; Sisó, P.; Marti, R.M.; Macià, A.; Panosa, A. In Vitro Cell Migration, Invasion, and Adhesion Assays: From Cell Imaging to Data Analysis. Front. Cell Dev. Biol. 2019, 7, 107. [Google Scholar] [CrossRef]




| Primer | Forward | Reverse |
|---|---|---|
| TGF-β1 | 5′-GCAGAGCTGTGAAGCCTTGAGA-3′ | 5′-TGCCTTCCTGTTGACTGAGTTG-3′ |
| ALP | 5′-GACGGACCCTCGCCAGTGCT-3′ | 5′-AATCGACGTGGGTGGGAGGGG-3′ |
| OCN | 5′-GGCAGCGAGGTAGTGAAGAG-3′ | 5′-CTCACACACCTCCCTCCTG-3′ |
| RUNX2 | 5′GTA GAT GGA CCT CGG GAA CC3′ | 5′GAG GCG GTC AGA GAA CAA AC3′ |
| GAPDH | 5′-GAAGGTGAAGGTCGGAGT-3′ | 5′-GAAGATGGTGATGGGATTTC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Algazlan, A.S.; Almuraikhi, N.; Muthurangan, M.; Balto, H.; Alsalleeh, F. Silver Nanoparticles Alone or in Combination with Calcium Hydroxide Modulate the Viability, Attachment, Migration, and Osteogenic Differentiation of Human Mesenchymal Stem Cells. Int. J. Mol. Sci. 2023, 24, 702. https://doi.org/10.3390/ijms24010702
Algazlan AS, Almuraikhi N, Muthurangan M, Balto H, Alsalleeh F. Silver Nanoparticles Alone or in Combination with Calcium Hydroxide Modulate the Viability, Attachment, Migration, and Osteogenic Differentiation of Human Mesenchymal Stem Cells. International Journal of Molecular Sciences. 2023; 24(1):702. https://doi.org/10.3390/ijms24010702
Chicago/Turabian StyleAlgazlan, Almaha S., Nihal Almuraikhi, Manikandan Muthurangan, Hanan Balto, and Fahd Alsalleeh. 2023. "Silver Nanoparticles Alone or in Combination with Calcium Hydroxide Modulate the Viability, Attachment, Migration, and Osteogenic Differentiation of Human Mesenchymal Stem Cells" International Journal of Molecular Sciences 24, no. 1: 702. https://doi.org/10.3390/ijms24010702
APA StyleAlgazlan, A. S., Almuraikhi, N., Muthurangan, M., Balto, H., & Alsalleeh, F. (2023). Silver Nanoparticles Alone or in Combination with Calcium Hydroxide Modulate the Viability, Attachment, Migration, and Osteogenic Differentiation of Human Mesenchymal Stem Cells. International Journal of Molecular Sciences, 24(1), 702. https://doi.org/10.3390/ijms24010702








