A Review of Oil–Solid Separation and Oil–Water Separation in Unconventional Heavy Oil Production Process
Abstract
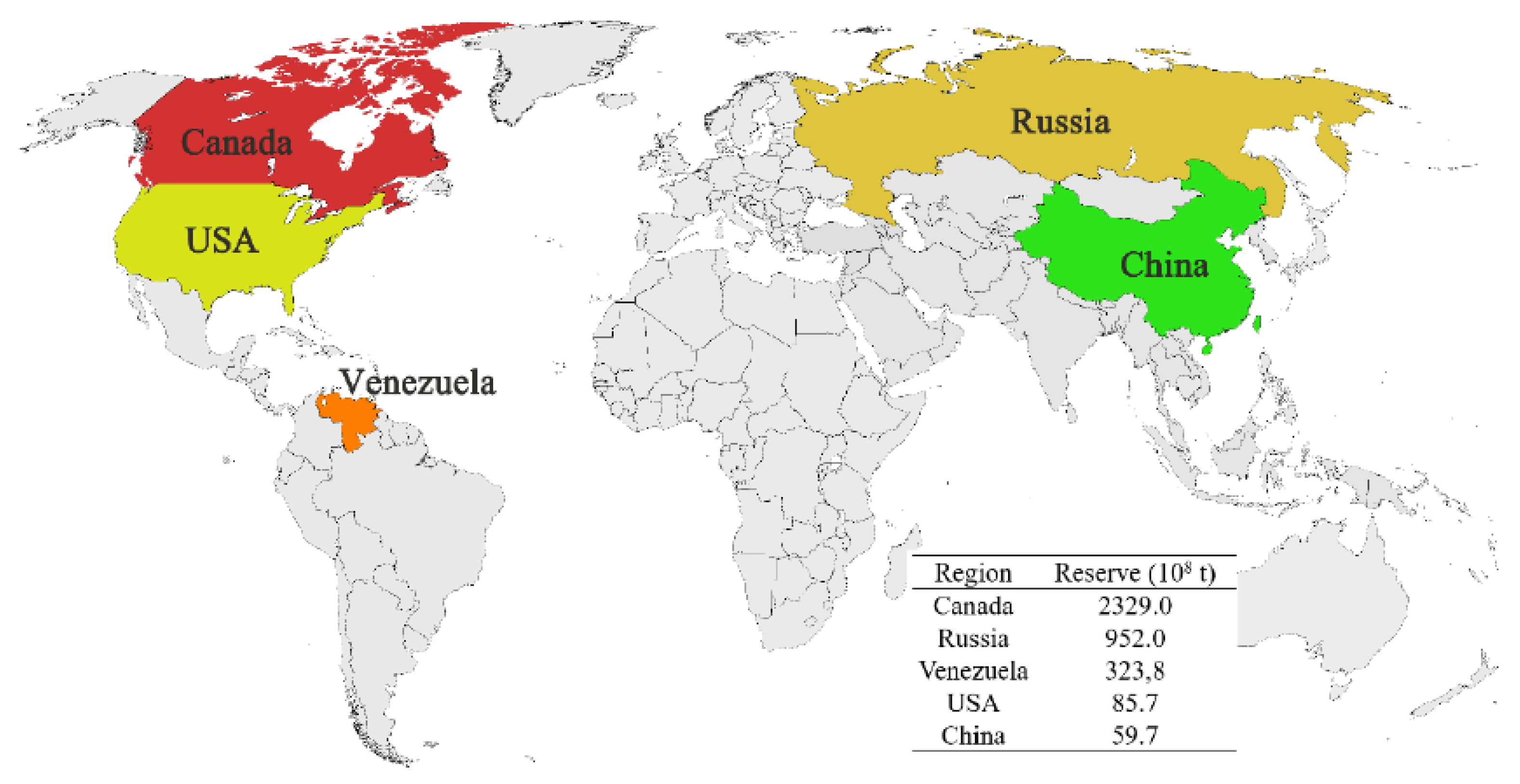
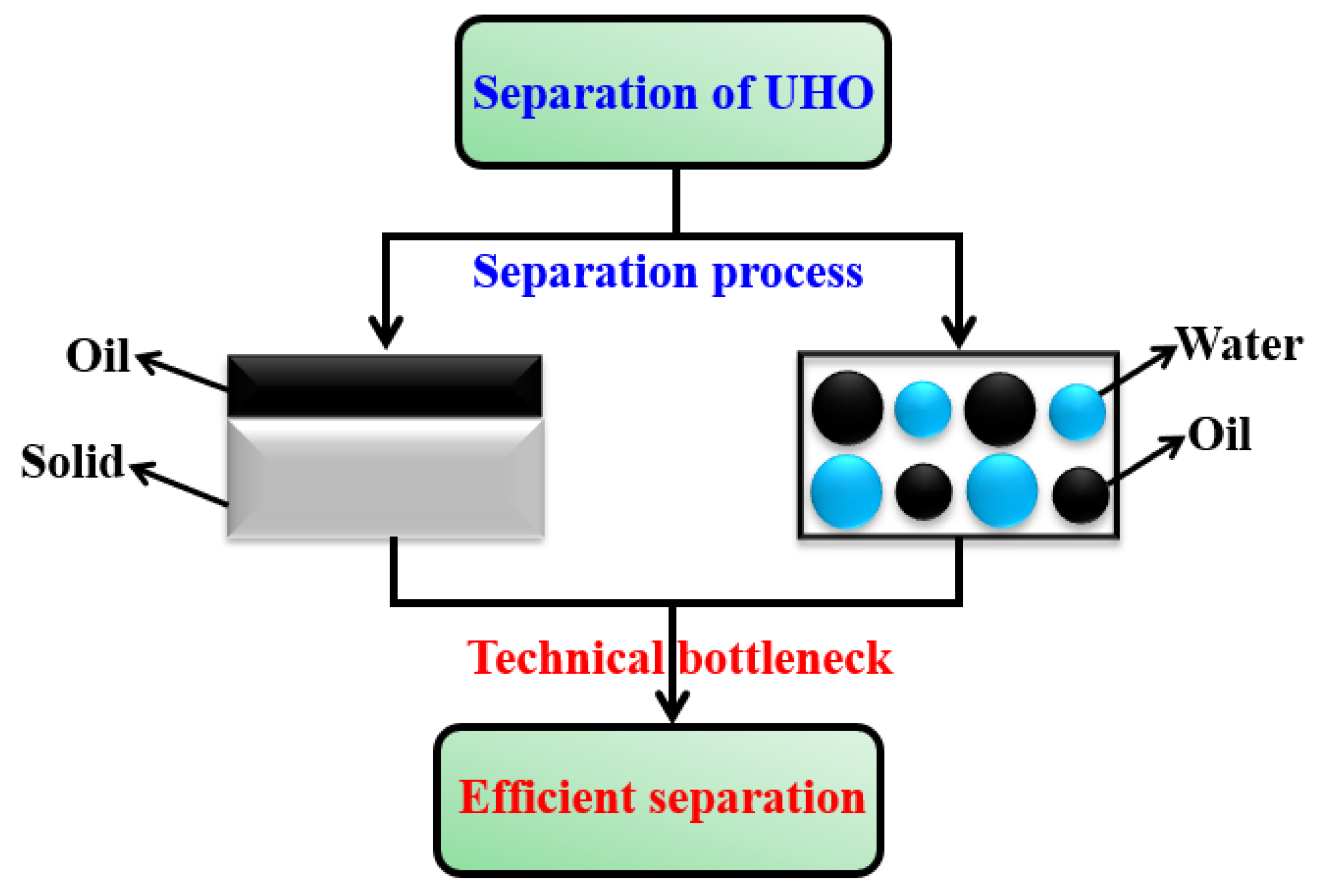
:1. Introduction
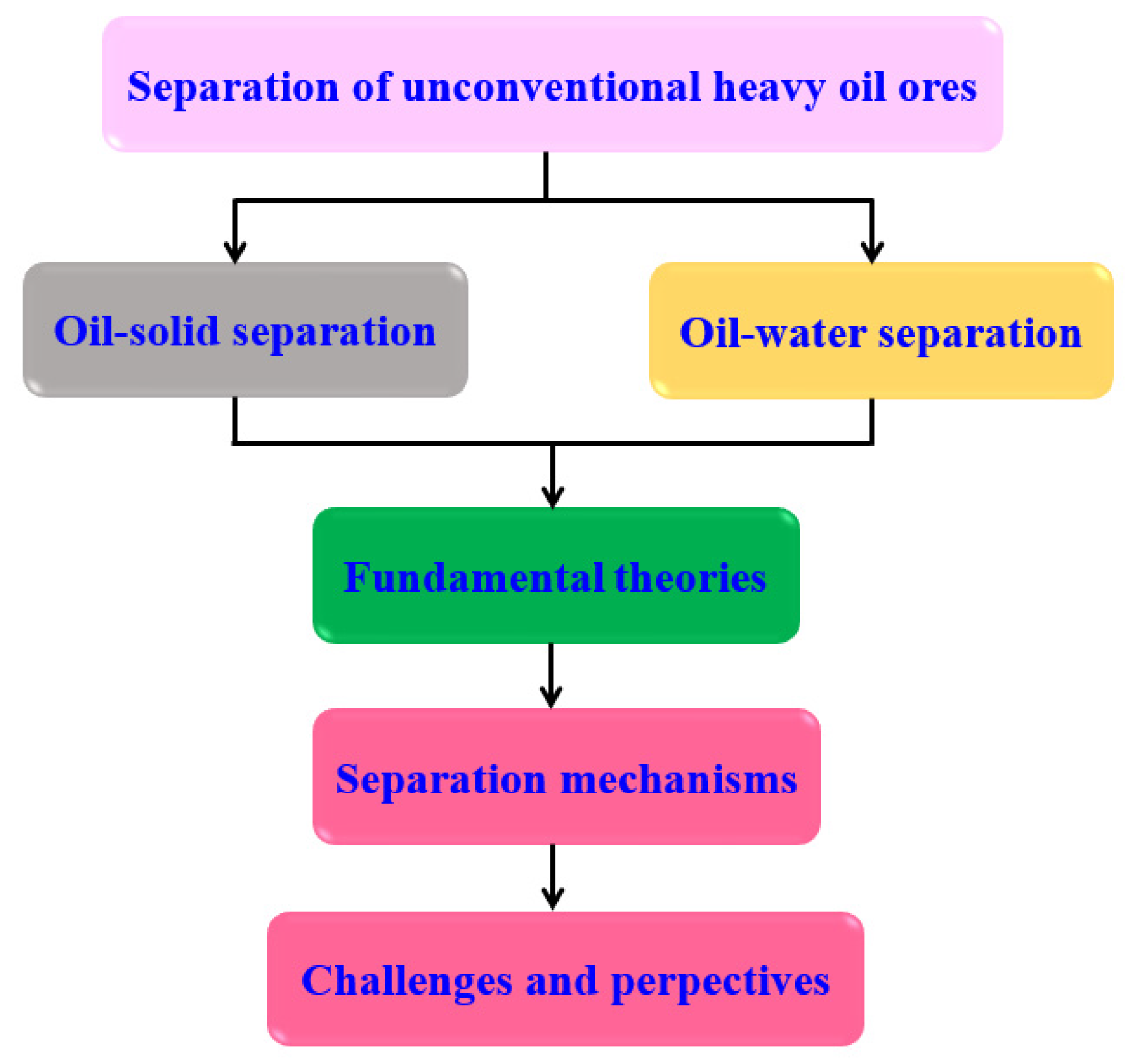
2. Fundamental Theories of OSS and OWS
2.1. Basic Theories of Oil–Solid Separation
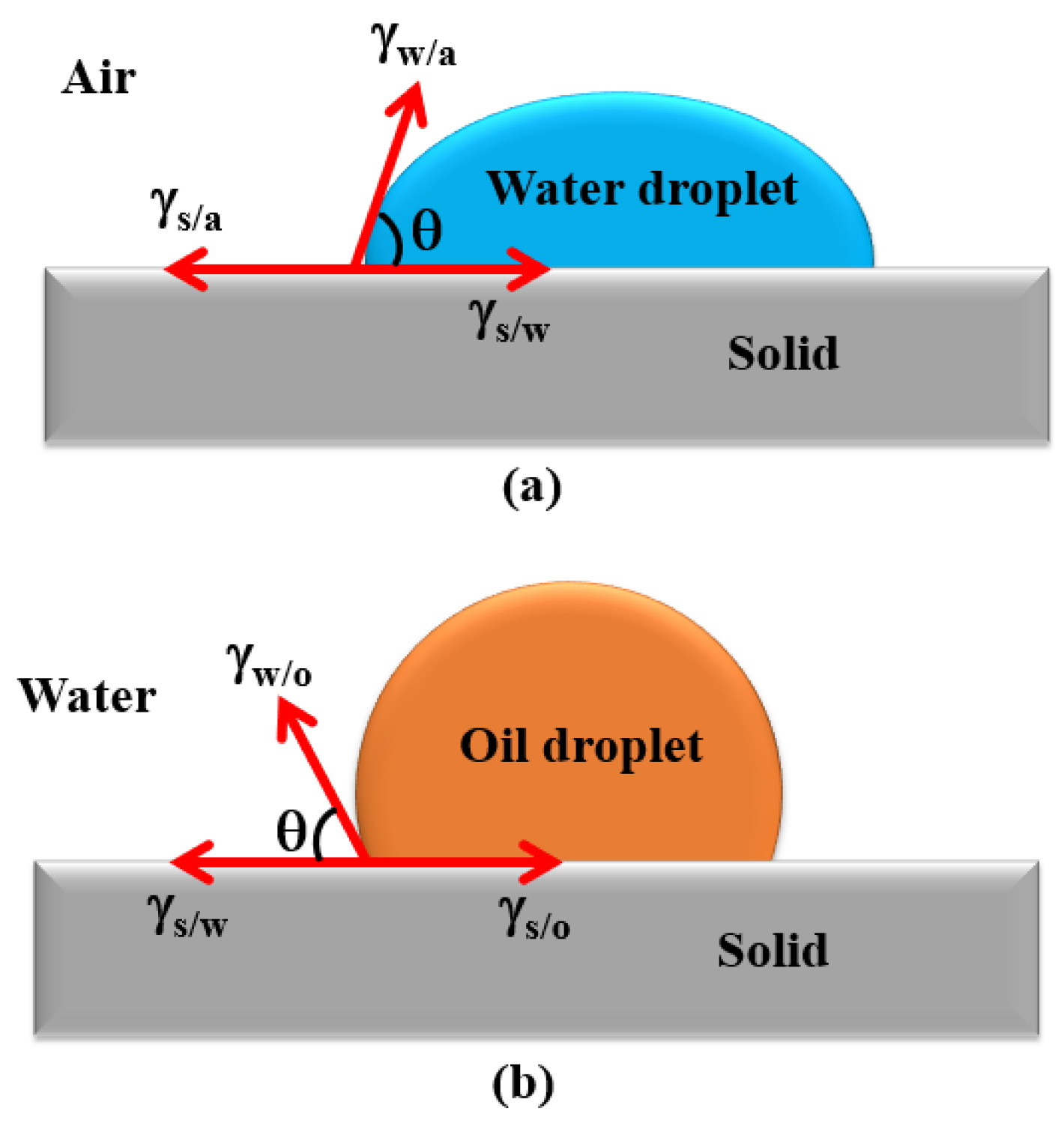
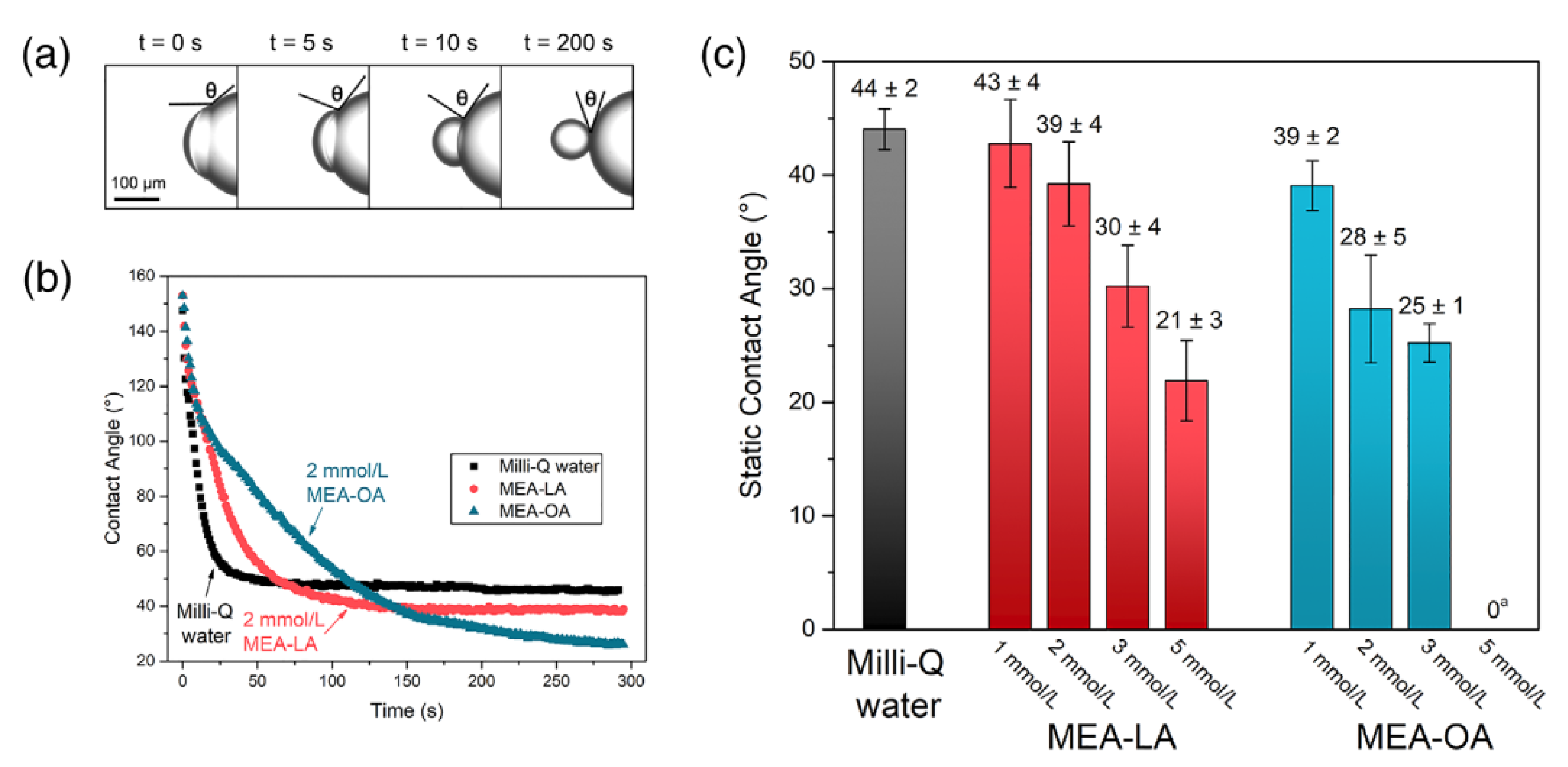
2.1.1. Solid Wettability and Contact Angle
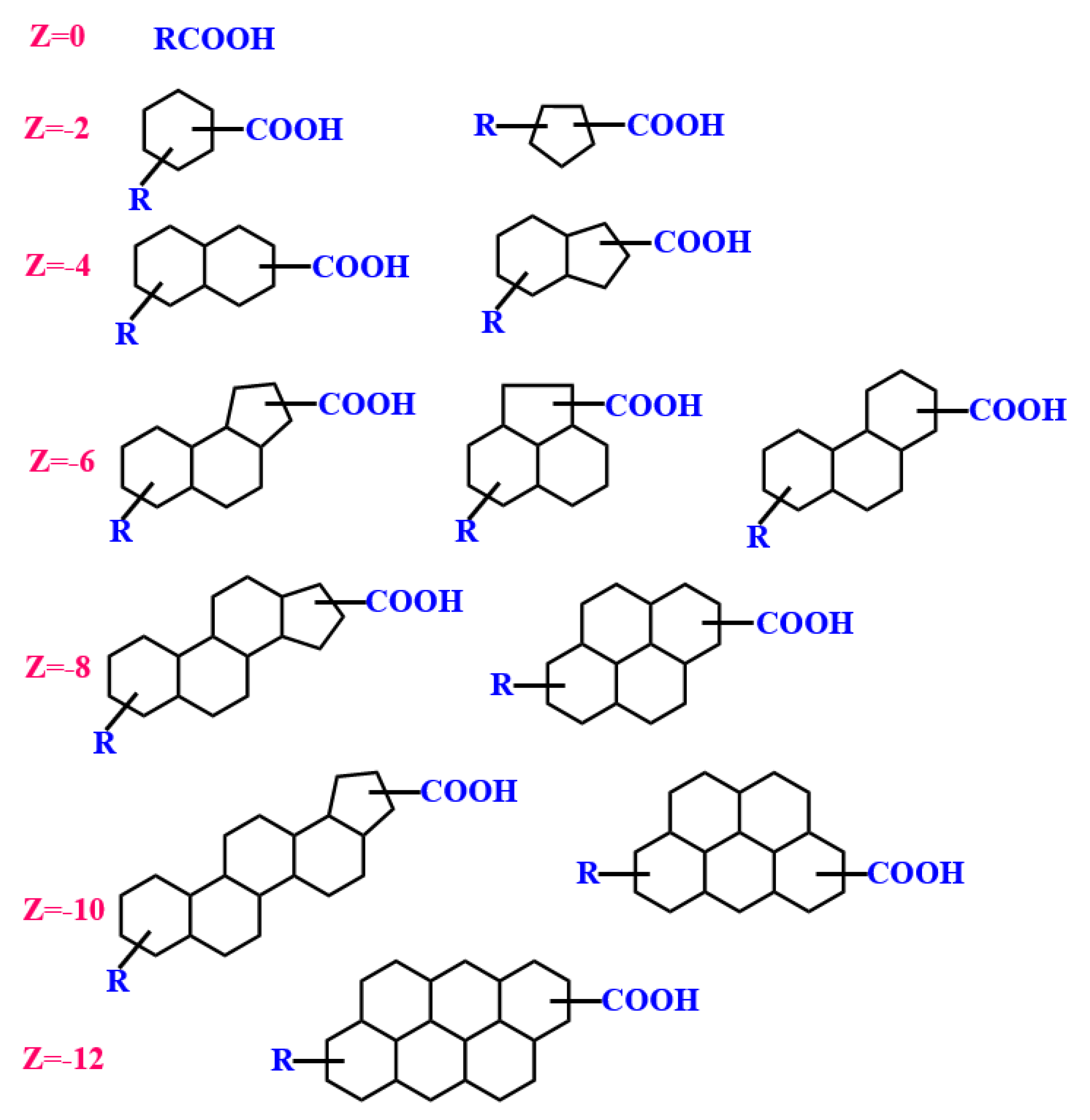
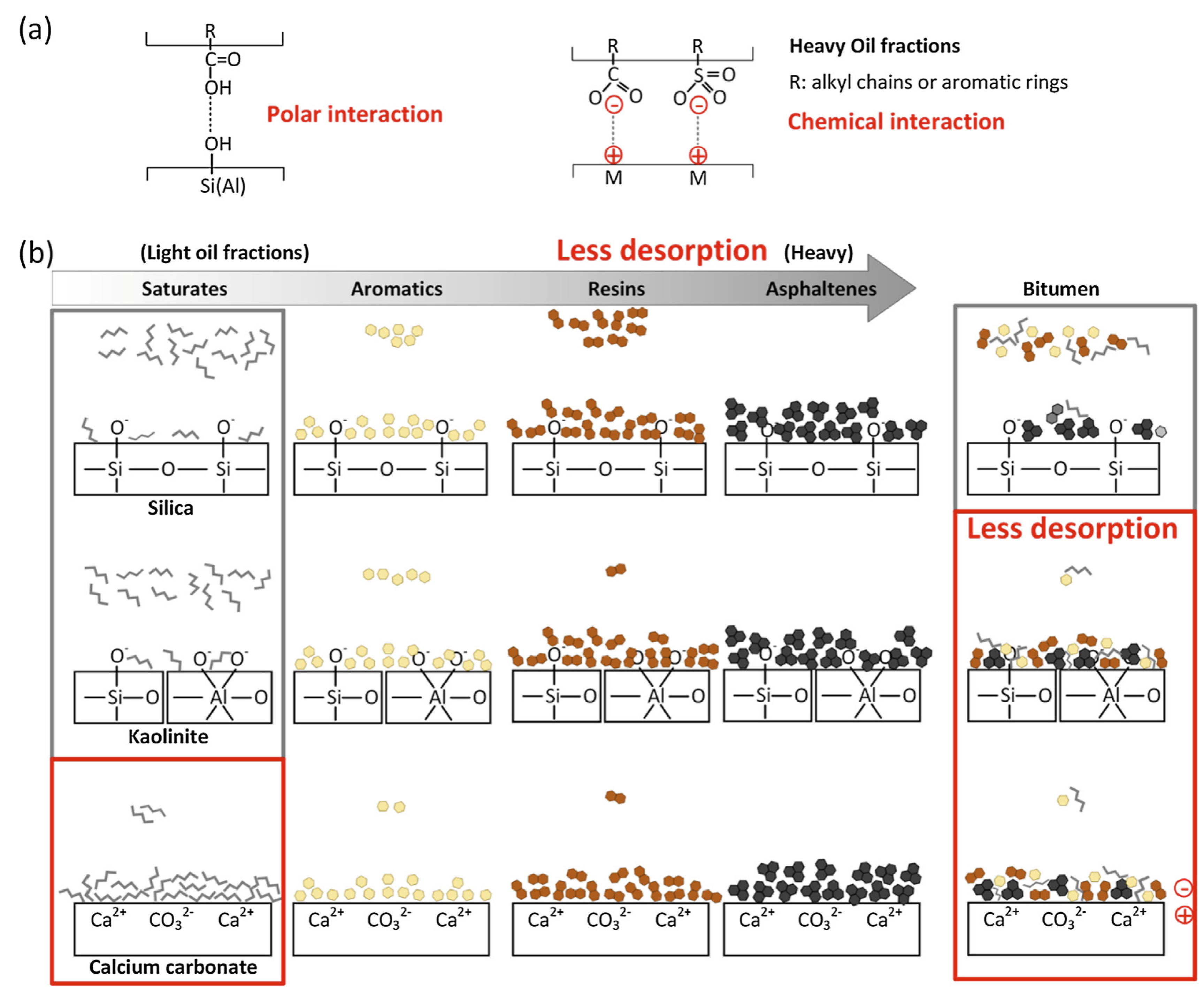
2.1.2. Oil–Solid Interactions
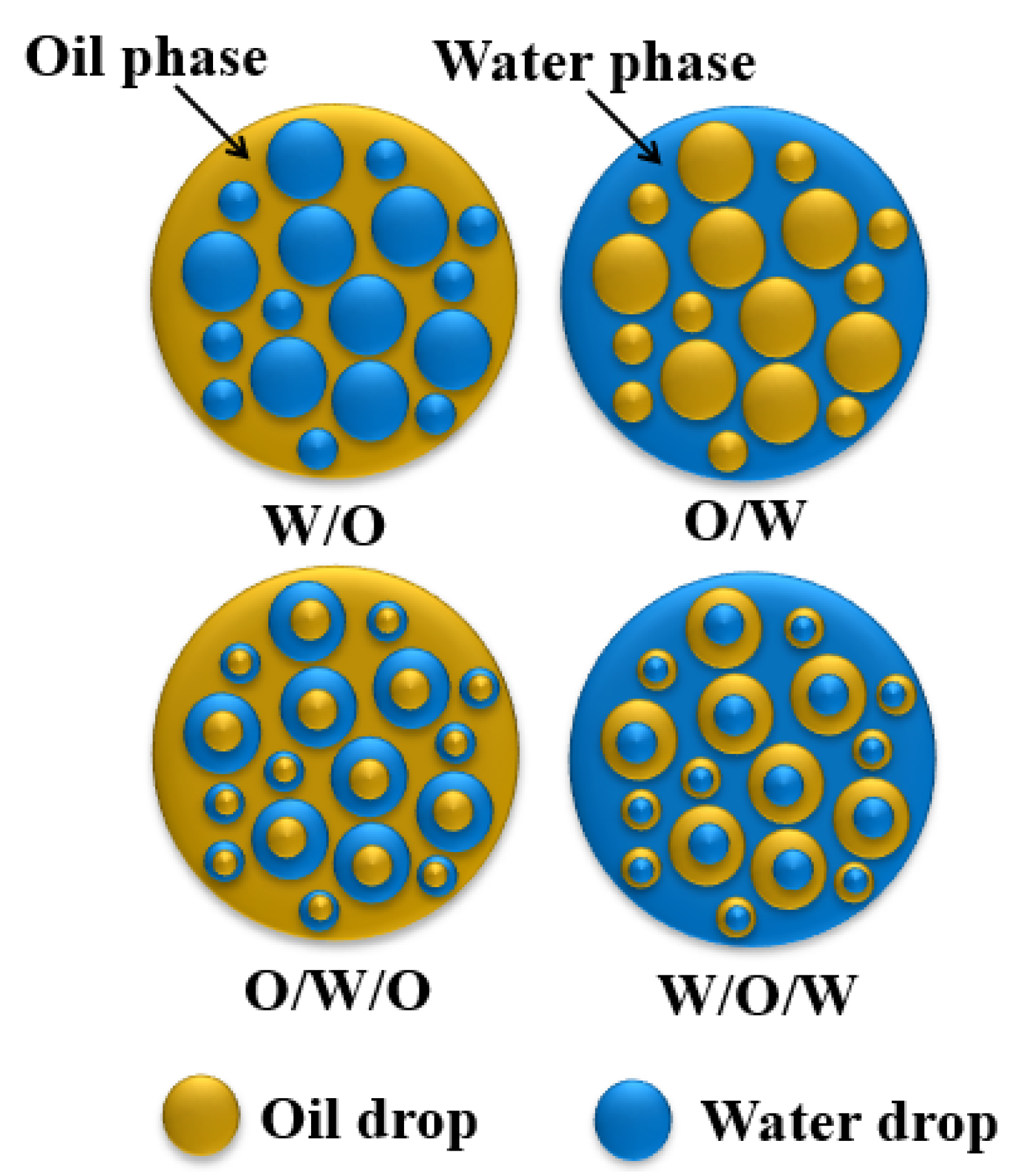
2.2. Basic Theories of Oil–Water Separation
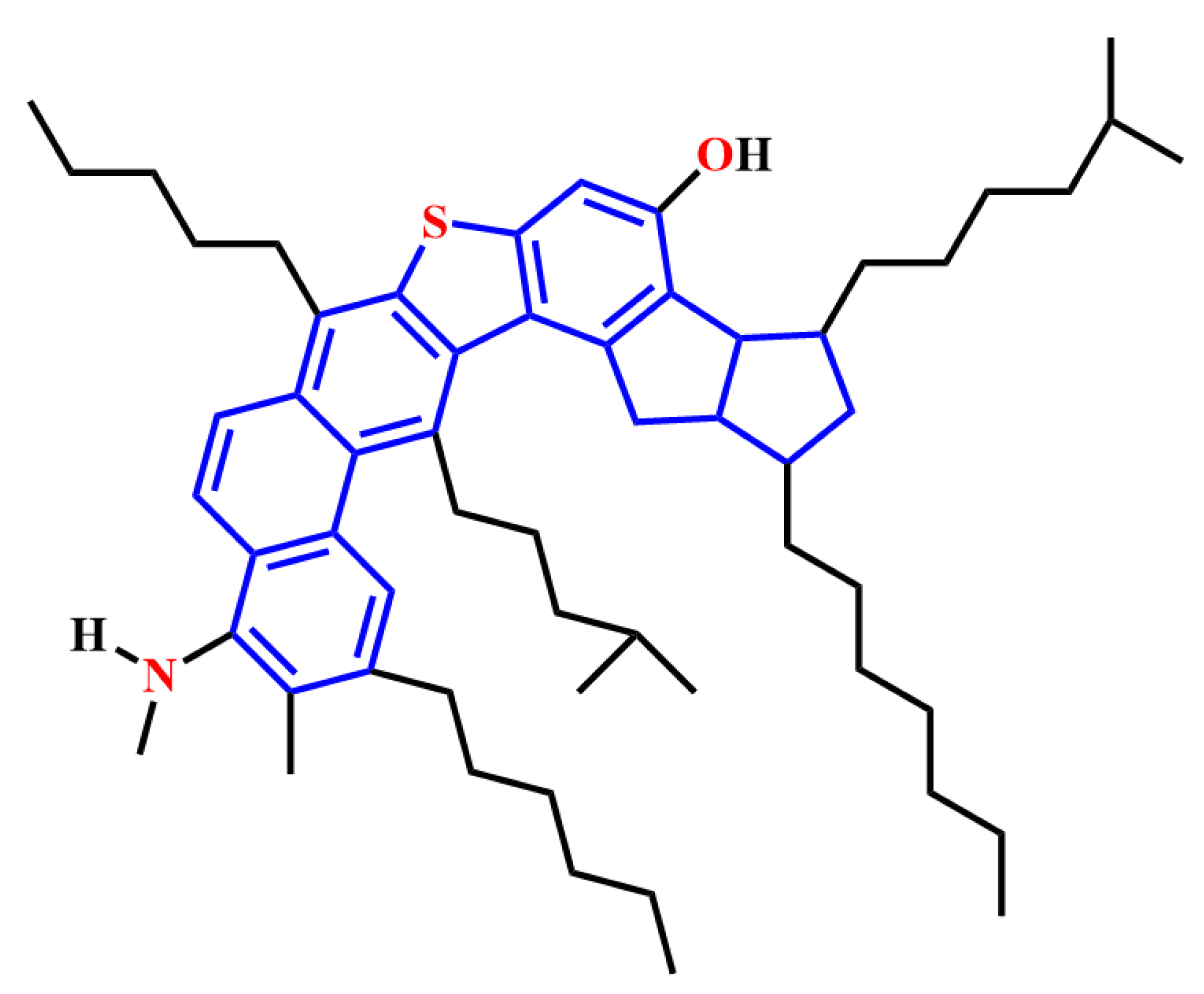
2.2.1. Structural Characteristics of Natural Surfactants
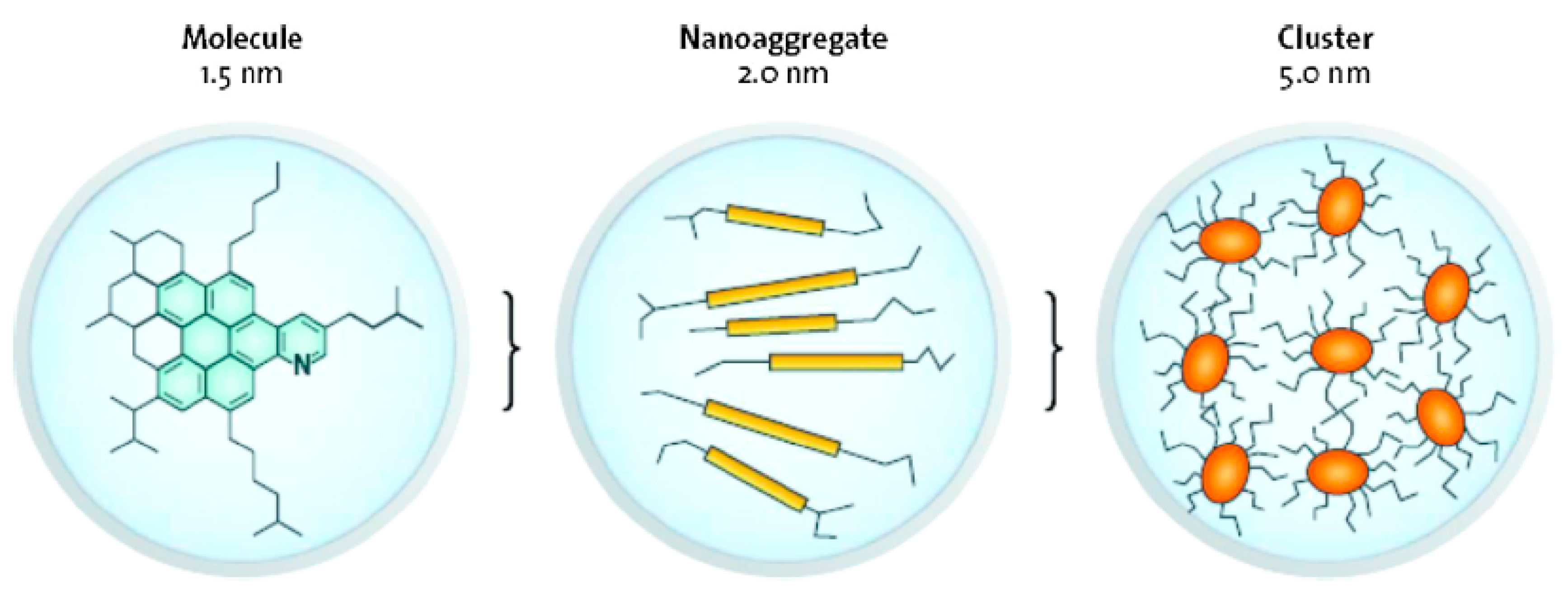
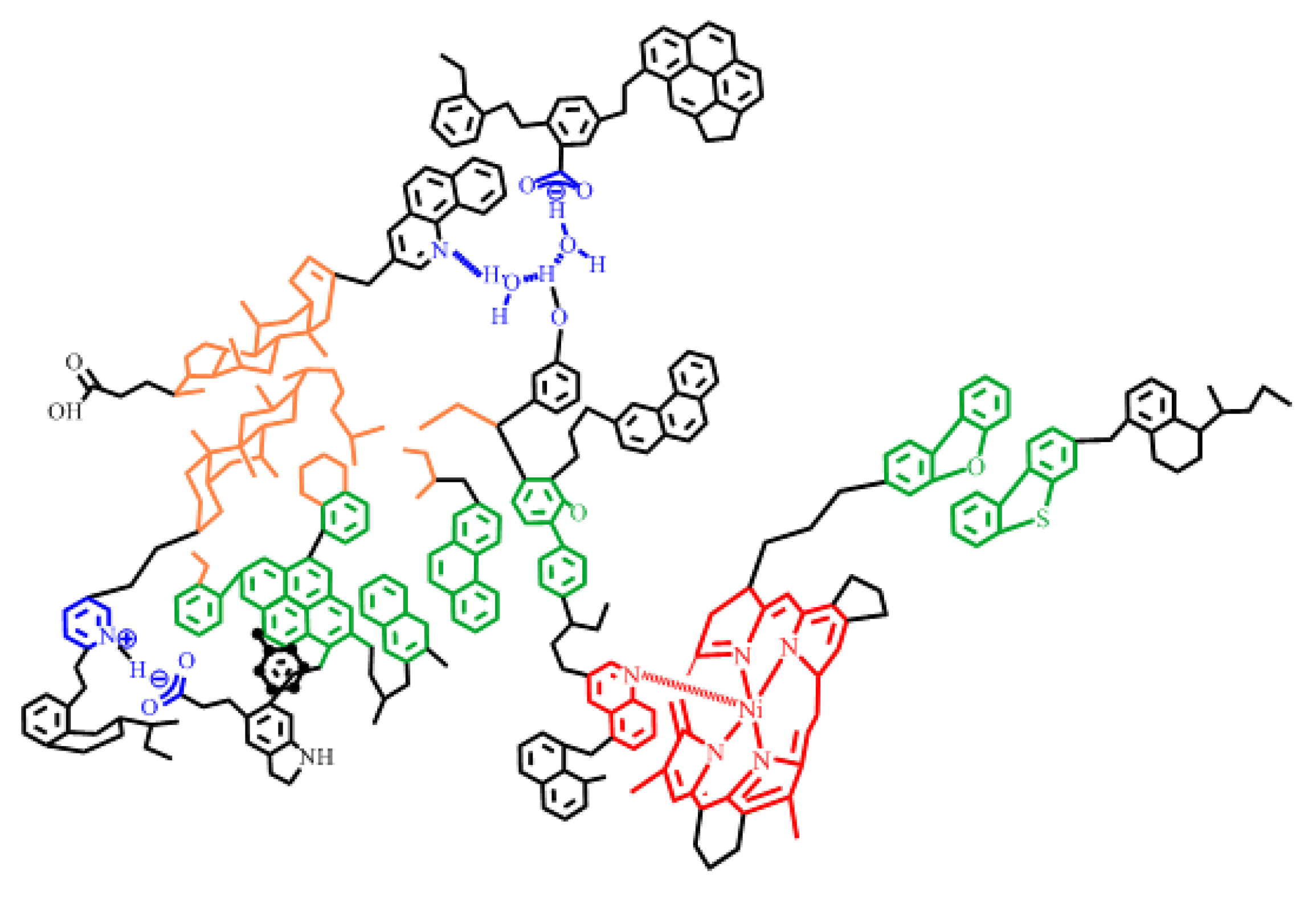
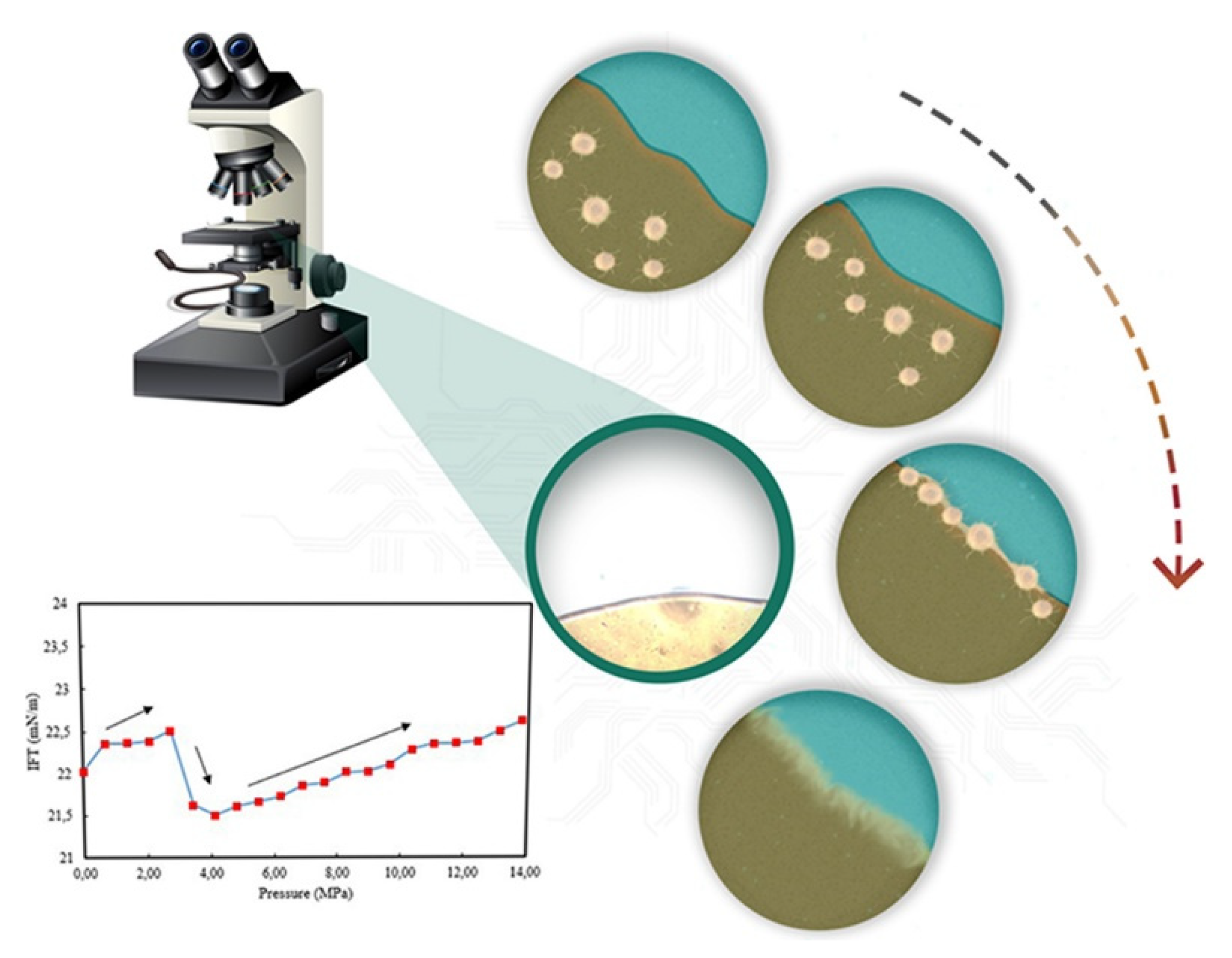
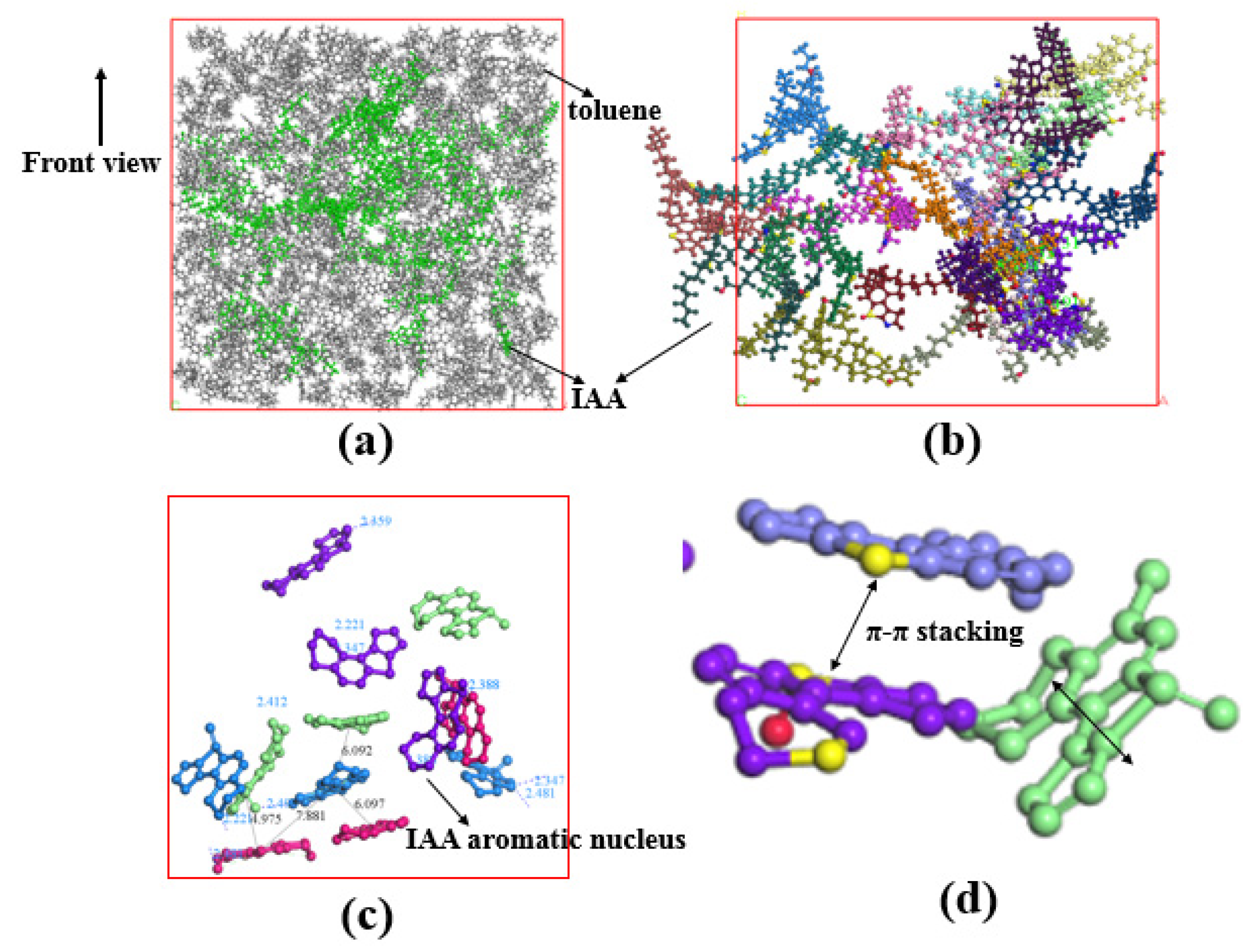
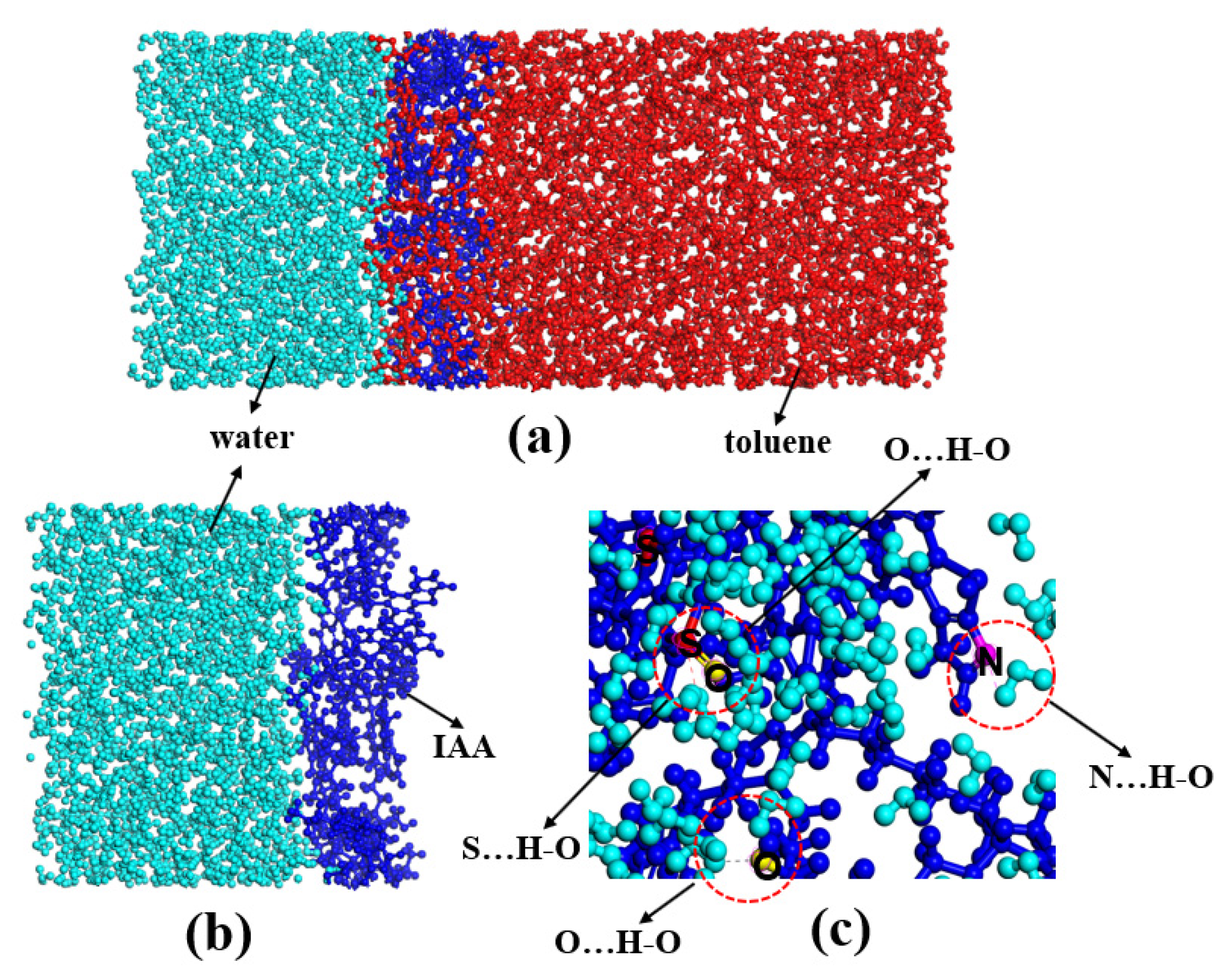
2.2.2. Interface Characteristics of Asphaltene Interfacial Films
3. Separation Mechanism
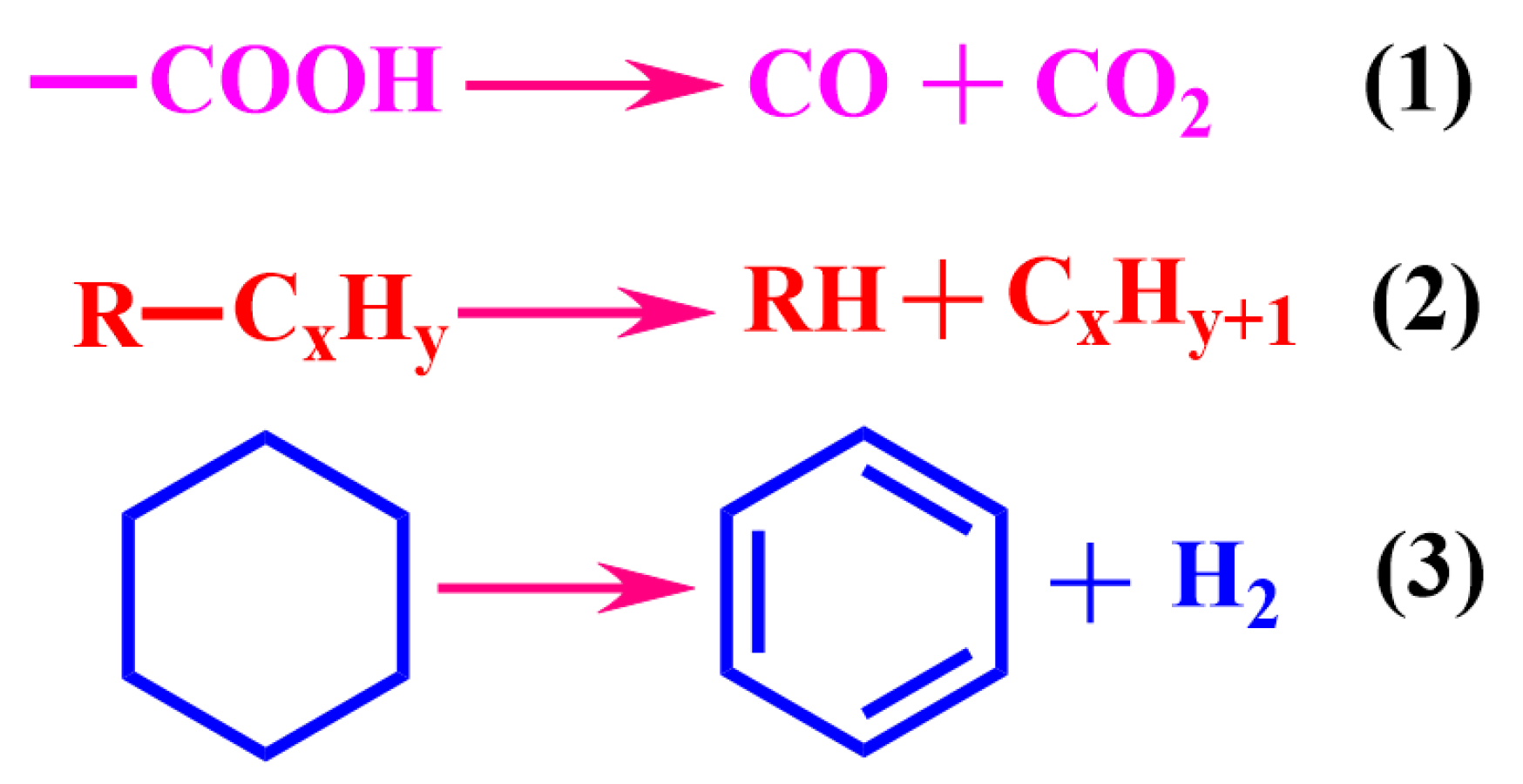
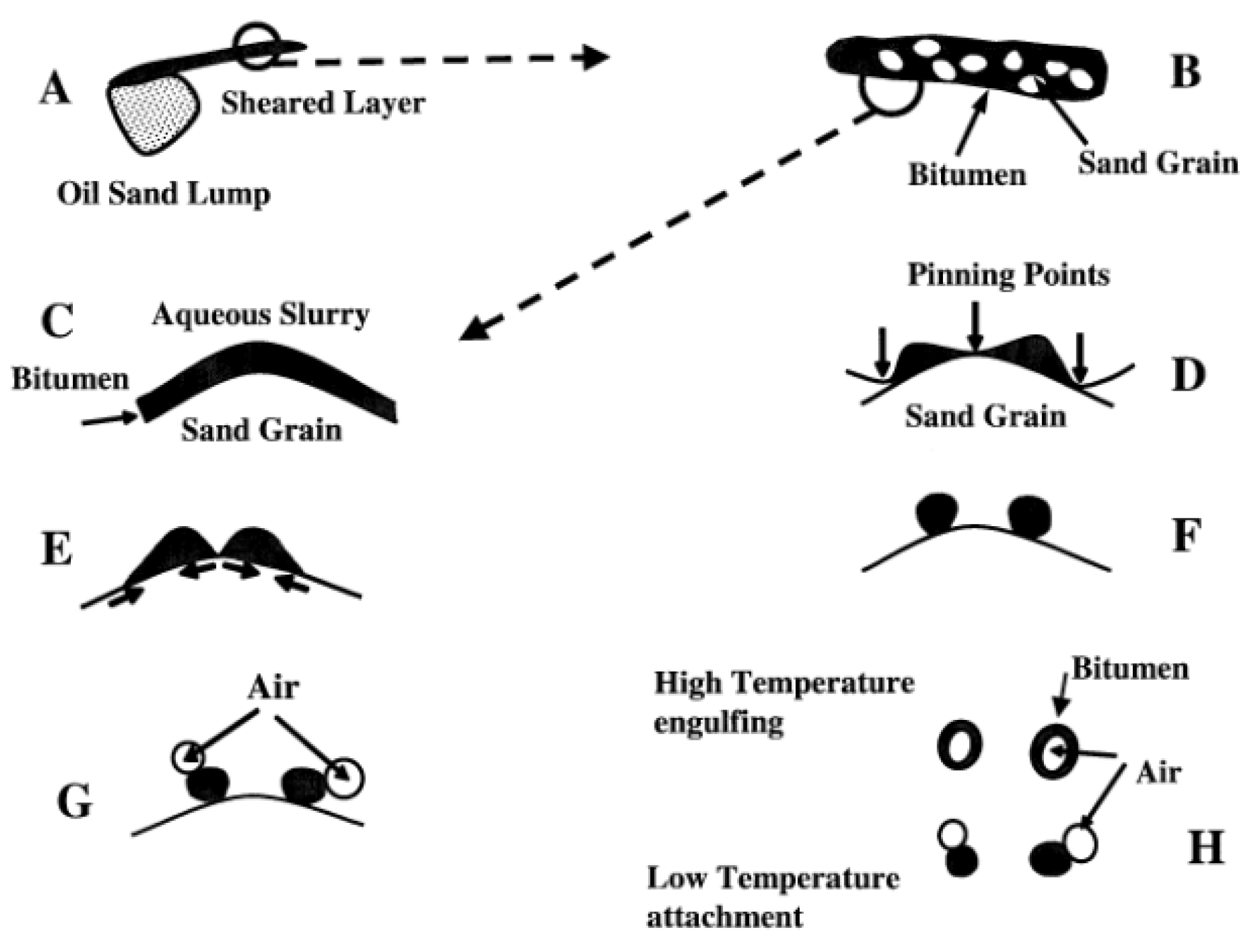
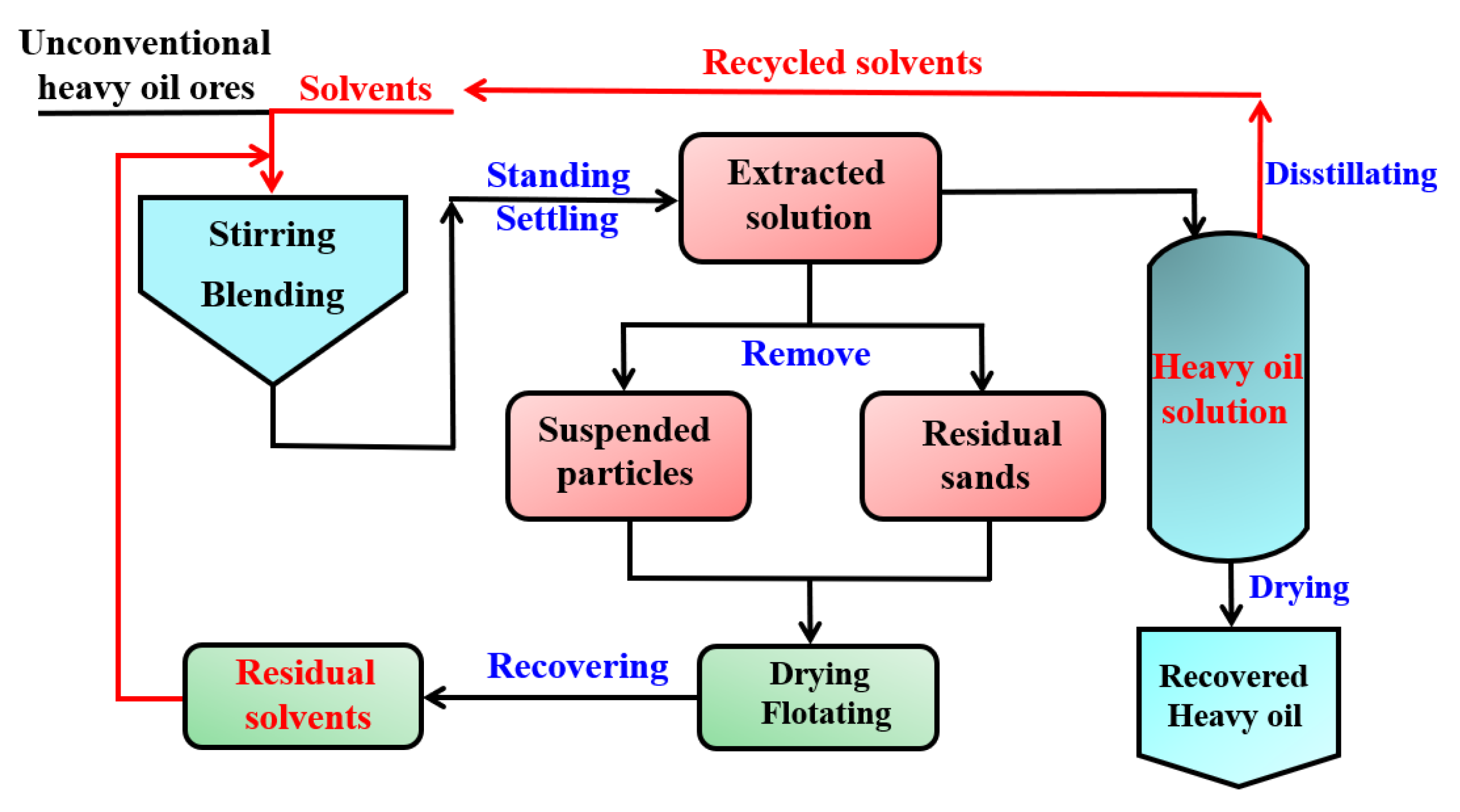
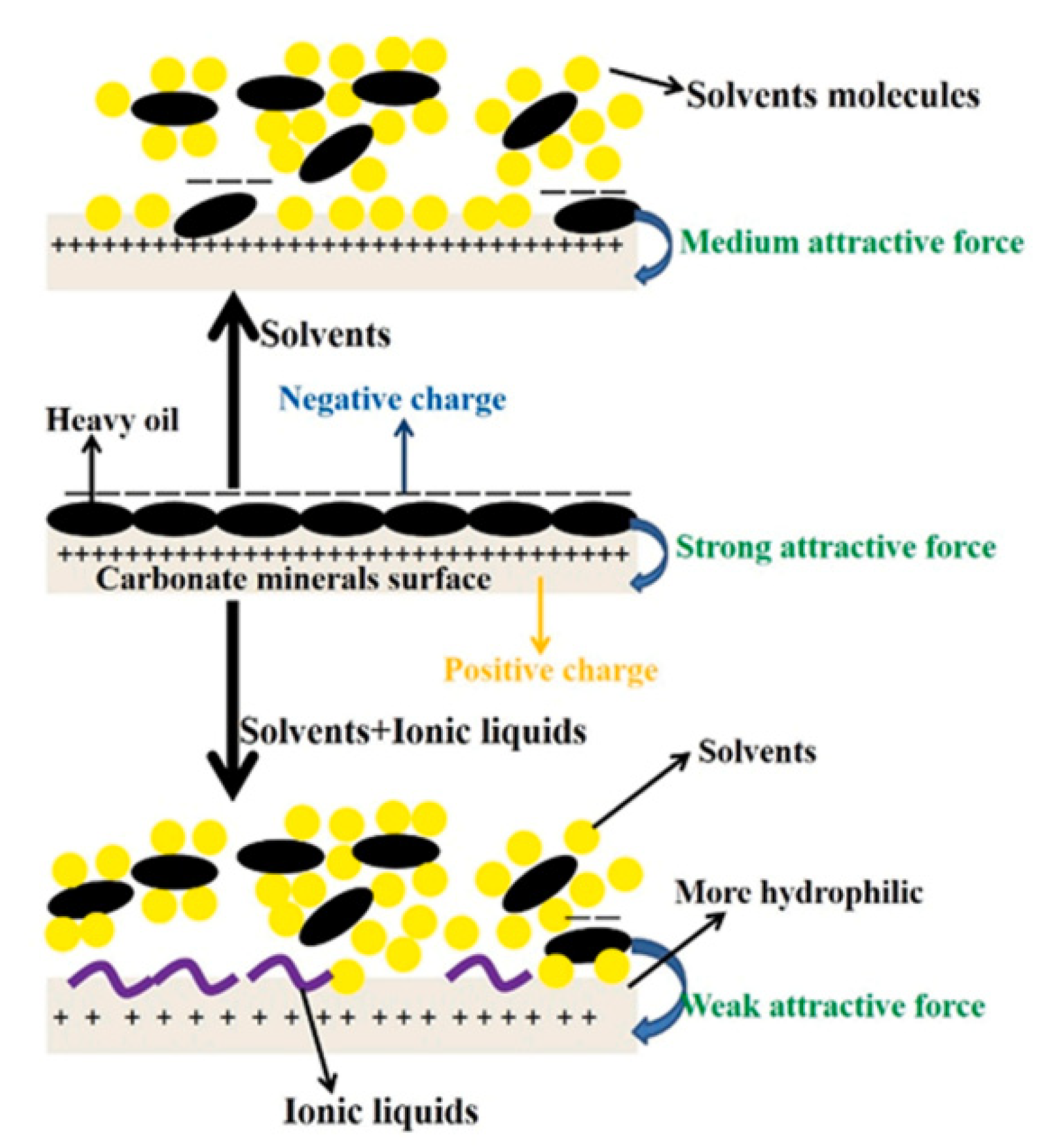
3.1. Oil–Solid Separation Mechanism
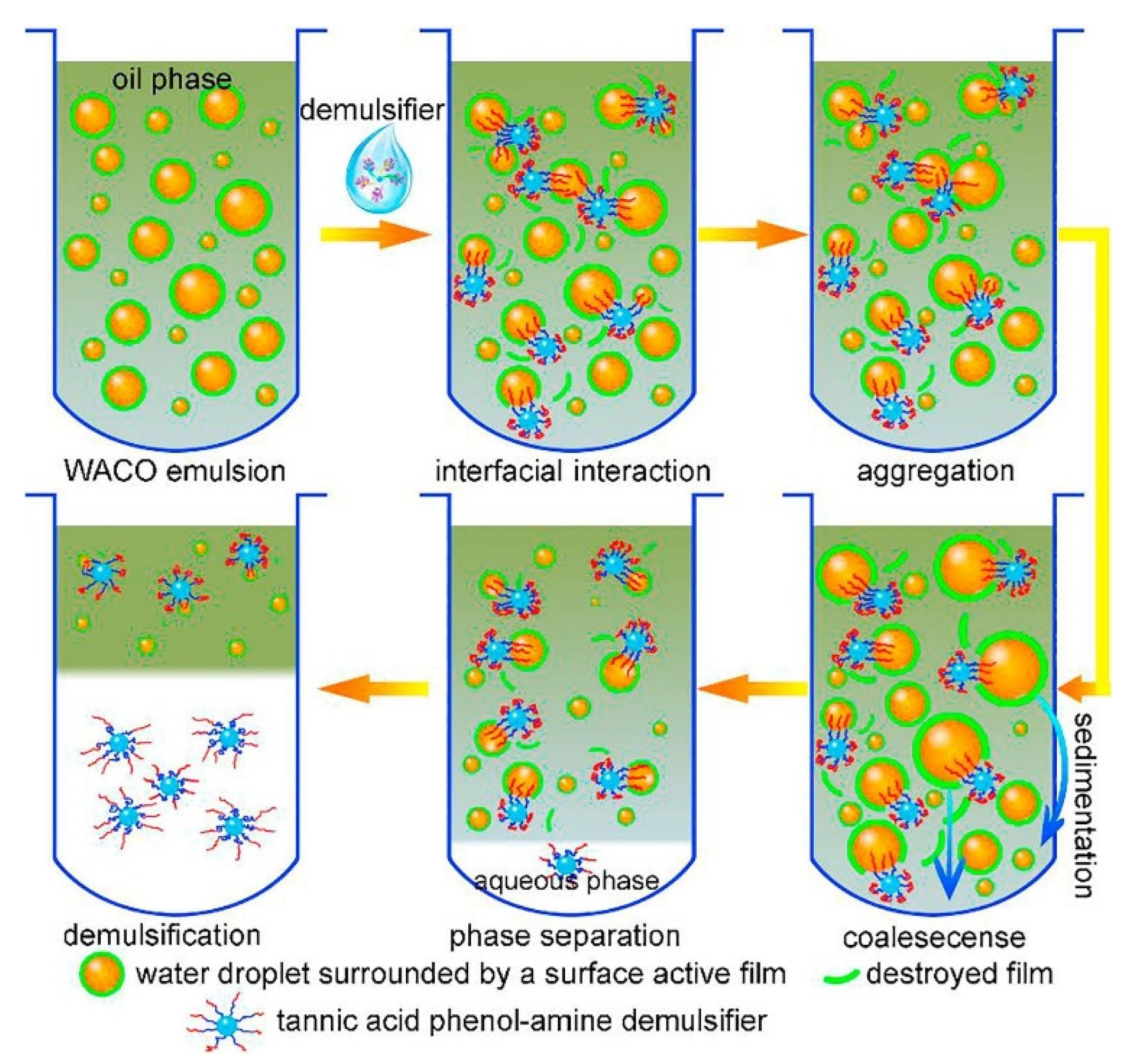
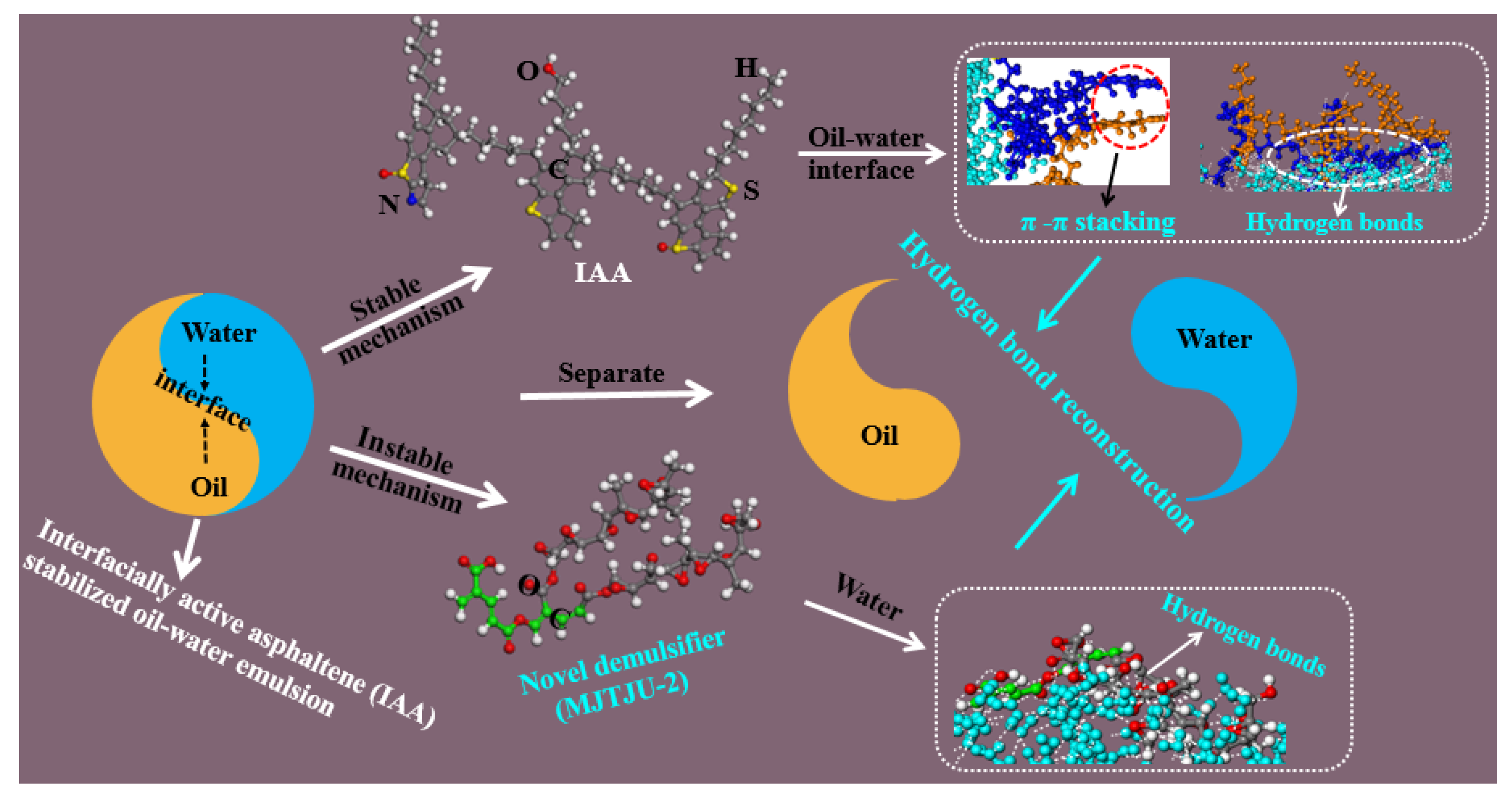
3.2. Oil–Water Separation Mechanism
4. Challenges and Perspectives
4.1. Construction of Oil–Solid Interaction Mechanism System
4.2. Construction of Oil–Water Interaction Mechanism System
4.3. Systematic Construction of Separation Mechanism of UHO
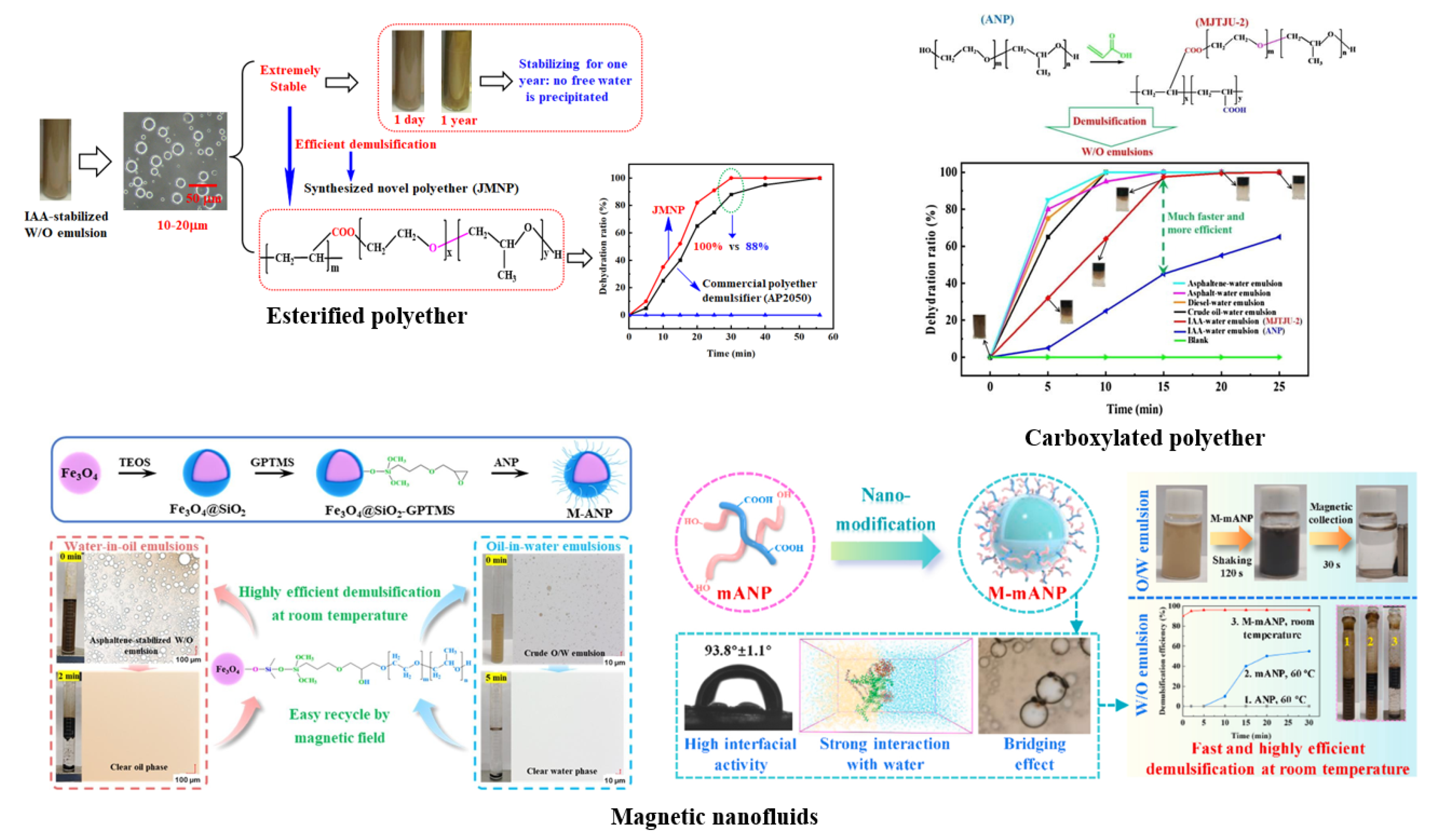
4.4. Research and Development of Novel Extractants and Demulsifiers
| Demulsifier | Concentration (ppm) | Demulsification Time (min) | Temperature (℃) | Dehydration Ratio (%) | References |
|---|---|---|---|---|---|
| SD31 | 100 | 60 | 60 | 85 | [236] |
| Biodegradable Ethylcellulose | 145 | 60 | 80 | 90 | [237] |
| TECA | 250 | 360 | 60 | 40 | [32] |
| Ethoxylated surfactant | 1000 | 30 | 70 | 80 | [238] |
| PEO/PPO (1) | 100 | 300 | 40 | 98.48 | [239] |
| TAPA | 100 | 90 | 70 | 91.7 | [29] |
| Star polymer | 1500 | 180 | 60 | 100 | [240] |
| PEO/PPO (2) | 2500 | 60 | 60 | <17 | [241] |
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Jie, X.; Gonzalez-Cortes, S.; Xiao, T.; Yao, B.; Wang, J.; Slocombe, D.R.; Fang, Y.; Miller, N.; Al-Megren, H.A.; Dilworth, J.R.; et al. The decarbonisation of petroleum and other fossil hydrocarbon fuels for the facile production and safe storage of hydrogen. Energy Environ. Sci. 2019, 12, 238–249. [Google Scholar] [CrossRef]
- Ma, J.; Yao, M.; Yang, Y.; Zhang, X. Comprehensive review on stability and demulsification of unconventional heavy oil–water emulsions. J. Mol. Liq. 2022, 350, 118510. [Google Scholar] [CrossRef]
- Lai, J.; Wang, G.; Fan, Q.; Pang, X.; Li, H.; Zhao, F.; Li, Y.; Zhao, X.; Zhao, Y.; Huang, Y.; et al. Geophysical Well-Log Evaluation in the Era of Unconventional Hydrocarbon Resources: A Review on Current Status and Prospects. Surv. Geophys. 2022, 43, 913–957. [Google Scholar] [CrossRef]
- Dong, X.; Liu, H.; Chen, Z.; Wu, K.; Lu, N.; Zhang, Q. Enhanced oil recovery techniques for heavy oil and oilsands reservoirs after steam injection. Appl. Energy 2019, 239, 1190–1211. [Google Scholar] [CrossRef]
- Taheri-Shakib, J.; Shekarifard, A.; Naderi, H. Experimental investigation of comparing electromagnetic and conventional heating effects on the unconventional oil (heavy oil) properties: Based on heating time and upgrading. Fuel 2018, 228, 243–253. [Google Scholar] [CrossRef]
- Klavers, K.; Atkins, L. Global Heavy Crude Oil Outlook to 2030. Proceedings of 20th World Petroleum Congress 2011, Doha, Qatar, 4–8 December 2011; p. WPC-20-0666. [Google Scholar]
- Santons, R.; Loh, W.; Bannwart, A.; Trevisan, O. An overview of heavy oil properties and its recovery and transportation methods. Braz. J. Chem. Eng. 2014, 31, 571–590. [Google Scholar] [CrossRef] [Green Version]
- Penner, S.S.; Benson, S.W.; Camp, F.W.; Clardy, J.; Deutch, J.; Kelley, A.E.; Lewis, A.E.; Mayer, F.X.; Oblad, A.G.; Sieg, R.P.; et al. Assessment of research needs for oil recovery from heavy-oil sources and tar sands. Energy 1982, 7, 567–602. [Google Scholar] [CrossRef]
- Zhou, H.; Ping, W.; Wang, Y.; Wang, Y.; Liu, K. China’s initial allocation of interprovincial carbon emission rights considering historical carbon transfers: Program design and efficiency evaluation. Ecol. Indic. 2021, 121, 106918. [Google Scholar] [CrossRef]
- Yang, Z.; Zou, C.; Wu, S.; Lin, S.; Pan, S.; Niu, X.; Men, G.; Tang, Z.; Li, G.; Zhao, J.; et al. Formation, distribution and resource potential of the “sweet areas (sections)” of continental shale oil in China. Mar. Pet. Geol. 2019, 102, 48–60. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Fan, X.; Li, X.; Gao, X. Insights into the synergetic effect for co-pyrolysis of oil sands and biomass using microwave irradiation. Fuel 2019, 239, 219–229. [Google Scholar] [CrossRef]
- Ding, M.; Zhang, Y.; Liu, J.; Jia, W.; Hu, B.; Ren, S. Application of microbial enhanced oil recovery technology in water-based bitumen extraction from weathered oil sands. AIChE J. 2014, 60, 2985–2993. [Google Scholar] [CrossRef]
- Sui, H.; Ma, G.; He, L.; Zhang, Z.; Li, X. Recovery of Heavy Hydrocarbons from Indonesian Carbonate Asphalt Rocks. Part 1: Solvent Extraction, Particle Sedimentation, and Solvent Recycling. Energy Fuels 2016, 30, 9242–9249. [Google Scholar] [CrossRef]
- Ramcharan, T.; Hosein, R. Radio Frequency Heating combined with Solvent Extraction- A method for oil recovery from surface oil sands. J. Pet. Sci. Eng. 2019, 179, 328–336. [Google Scholar] [CrossRef]
- Li, X.; Bian, R.; Wang, J.; Wang, X.; Ma, J.; Ma, G.; Sui, H.; He, L. Recovery of extra-heavy oil and minerals from carbonate asphalt rocks by reactive extraction. RSC Adv. 2019, 9, 14372–14381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Ridner, D.; Zhang, Z.; Li, X.; Li, H.; Sui, H.; Gao, X. Study on vacuum pyrolysis of oil sands by comparison with retorting and nitrogen sweeping pyrolysis. Fuel Process. Technol. 2017, 163, 51–59. [Google Scholar] [CrossRef]
- Zhu, Y.; Lu, Y.; Liu, Q.; Masliyah, J.; Xu, Z. Comprehensive study on cleaner production of heavy oil from Athabasca oil sands using chemical additives in biodiesel-assisted ambient-aqueous bitumen extraction process. J. Clean. Prod. 2020, 277, 122940. [Google Scholar] [CrossRef]
- Masliyah, J.; Zhou, Z.J.; Xu, Z.; Czarnecki, J.; Hamza, H. Understanding Water-Based Bitumen Extraction from Athabasca Oil Sands. Can. J. Chem. Eng. 2004, 82, 628–654. [Google Scholar] [CrossRef]
- Li, X.; Berg, S.; Castellanos-Diaz, O.; Wiegmann, A.; Verlaan, M. Solvent-dependent recovery characteristic and asphaltene deposition during solvent extraction of heavy oil. Fuel 2020, 263, 116716. [Google Scholar] [CrossRef]
- Ma, J.; Bian, R.; Ma, G.; Li, X.; Sui, H.; He, L. Separation of asphalt from carbonate ore surfaces by reactive extraction: Kinetics and modelling. Chem. Eng. Sci. 2020, 216, 115533. [Google Scholar] [CrossRef]
- Li, X.; Ma, J.; Bian, R.; Cheng, J.; Sui, H.; He, L. Novel Polyether for Efficient Demulsification of Interfacially Active Asphaltene-Stabilized Water-in-Oil Emulsions. Energy Fuels 2020, 34, 3591–3600. [Google Scholar] [CrossRef]
- Yao, X.; Hou, X.; Zhang, R. Flexible and mechanically robust polyimide foam membranes with adjustable structure for separation and recovery of oil–water emulsions and heavy oils. Polymer 2022, 250, 124889. [Google Scholar] [CrossRef]
- Li, X.; Sun, W.; Wu, G.; He, L.; Li, H.; Sui, H. Ionic Liquid Enhanced Solvent Extraction for Bitumen Recovery from Oil Sands. Energy Fuels 2011, 25, 5224–5231. [Google Scholar] [CrossRef]
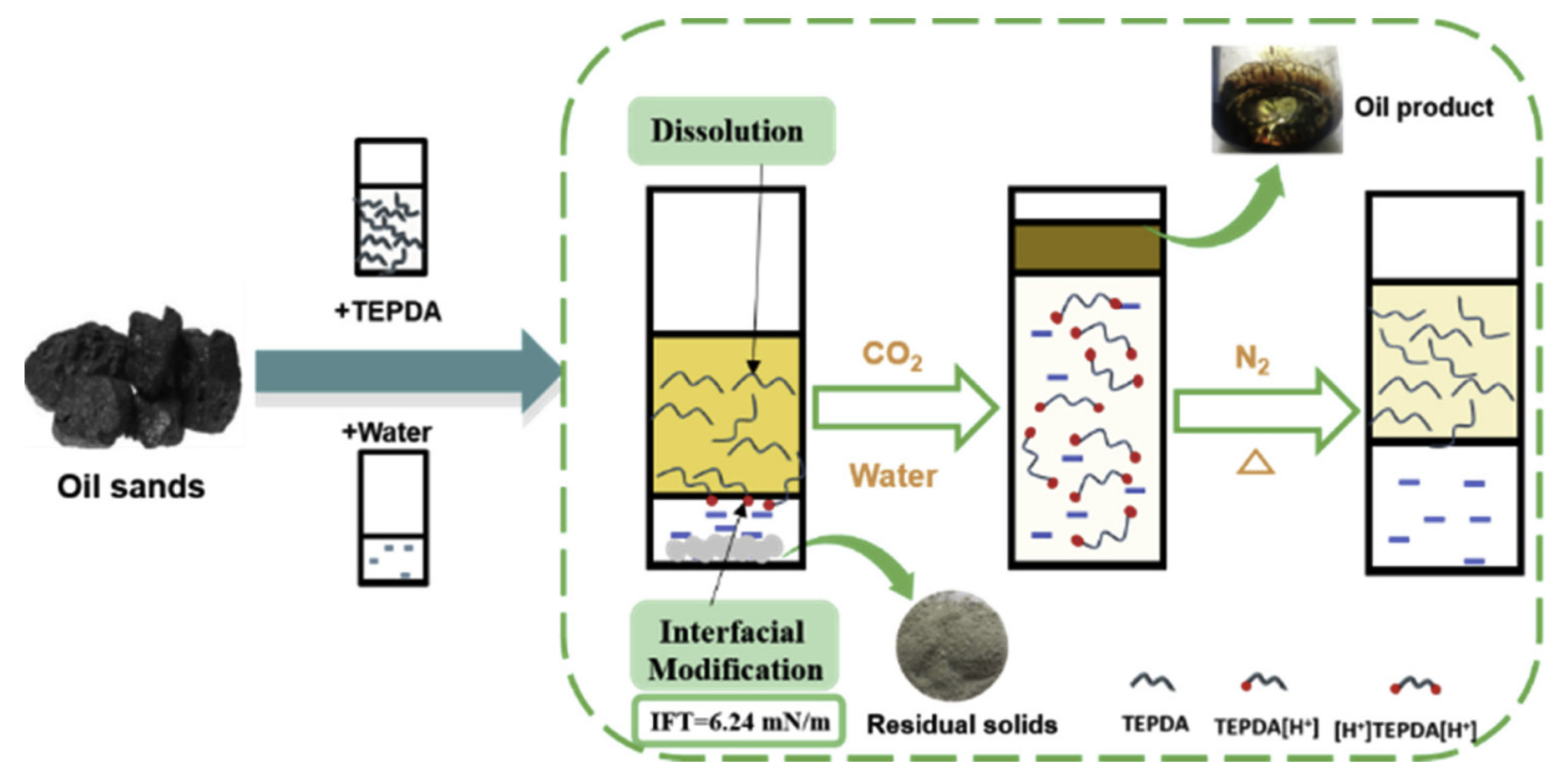
- Sui, H.; Xu, L.; Li, X.; He, L. Understanding the roles of switchable-hydrophilicity tertiary amines in recovering heavy hydrocarbons from oil sands. Chem. Eng. J. 2016, 290, 312–318. [Google Scholar] [CrossRef]
- Lu, Y.; Li, R.; Manica, R.; Liu, Q.; Xu, Z. Enhancing oil–solid and oil–water separation in heavy oil recovery by CO2-responsive surfactants. AIChE J. 2021, 67, e17033. [Google Scholar] [CrossRef]
- Abdulredha, M.M.; Hussain, S.A.; Abdullah, L.C. Optimization of the demulsification of water in oil emulsion via non-ionic surfactant by the response surface methods. J. Pet. Sci. Eng. 2020, 184, 106463. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; He, Q.; Wang, X.; Lu, H.; Guo, F.; Zhang, Y.; Wang, W. Multifunctional natural sepiolite nanofibre composite demulsifiers for efficient purification of oils and dyes in simulated and actual wastewater. Sep. Purif. Technol. 2022, 290, 120865. [Google Scholar] [CrossRef]
- Faizullayev, S.; Adilbekova, A.; Kujawski, W.; Mirzaeian, M. Recent demulsification methods of crude oil emulsions—Brief review. J. Pet. Sci. Eng. 2022, 215, 110643. [Google Scholar] [CrossRef]
- Li, Z.; Geng, H.; Wang, X.; Jing, B.; Liu, Y.; Tan, Y. Noval tannic acid-based polyether as an effective demulsifier for water-in-aging crude oil emulsions. Chem. Eng. J. 2018, 354, 1110–1119. [Google Scholar] [CrossRef]
- Mao, X.; Gong, L.; Xie, L.; Qian, H.; Wang, X.; Zeng, H. Novel Fe3O4 based superhydrophilic core-shell microspheres for breaking asphaltenes-stabilized water-in-oil emulsion. Chem. Eng. J. 2019, 358, 869–877. [Google Scholar] [CrossRef]
- Zhang, L.; Ying, H.; Yan, S.; Zhan, N.; Guo, Y.; Fang, W. Hyperbranched poly(amido amine) as an effective demulsifier for oil-in-water emulsions of microdroplets. Fuel 2018, 211, 197–205. [Google Scholar] [CrossRef]
- Atta, A.M.; Abdullah, M.M.S.; Al-Lohedan, H.A.; Ezzat, A.O. Demulsification of heavy crude oil using new nonionic cardanol surfactants. J. Mol. Liq. 2018, 252, 311–320. [Google Scholar] [CrossRef]
- Ma, J.; Li, X.; Zhang, X.; Sui, H.; He, L.; Wang, S. A novel oxygen-containing demulsifier for efficient breaking of water-in-oil emulsions. Chem. Eng. J. 2020, 385, 123826. [Google Scholar] [CrossRef]
- Wang, J.; Xu, W.; Liu, H.; Yu, F.; Wang, H. Extractant structures and their performance for palladium extraction and separation from chloride media: A review. Miner. Eng. 2021, 163, 106798. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, F.; Ouyang, J.; Zhang, H. The Development and Application of a Demulsifier Used for ASP Flooding–Produced Liquid from the Xing 2 Area of the Daqing Oilfield. Pet. Sci. Technol. 2011, 29, 69–78. [Google Scholar] [CrossRef]
- Fraga, A.K.; Souza, L.F.I.; Magalhães, J.R.; Mansur, C.R.E. Development and evaluation of oil in water nanoemulsions based on polyether silicone as demulsifier and antifoam agents for petroleum. J. Appl. Polym. Sci. 2014, 131, 40889. [Google Scholar] [CrossRef]
- Falode, O.; Adebanjo; Akinbuli, O. Development of Local Demulsifier For Water—In-Oil Emulsion Treatment. Int. J. Sci. Basic Appl. Res. 2015, 24, 301–320. [Google Scholar]
- Salakhov, R.K.; Khamidullin, R.F.; Mansurov, Z.; Bodykov, D.; Seitzhanova, M. Development of Demulsifier Compositions for the Destruction of Emulsions and Dehydration of Heavy Oils. Eurasian Chem. -Technol. J. 2018, 20, 81. [Google Scholar] [CrossRef]
- Liang, T.; Hou, J.-R.; Qu, M.; Xi, J.-X.; Raj, I. Application of nanomaterial for enhanced oil recovery. Pet. Sci. 2022, 19, 882–899. [Google Scholar] [CrossRef]
- Qu, M.; Liang, T.; Xiao, L.; Hou, J.; Qi, P.; Zhao, Y.; Song, C.; Li, J. Mechanism study of spontaneous imbibition with lower-phase nano-emulsion in tight reservoirs. J. Pet. Sci. Eng. 2022, 211, 110220. [Google Scholar] [CrossRef]
- Qu, M.; Liang, T.; Hou, J.; Liu, Z.; Yang, E.; Liu, X. Laboratory study and field application of amphiphilic molybdenum disulfide nanosheets for enhanced oil recovery. J. Pet. Sci. Eng. 2022, 208, 109695. [Google Scholar] [CrossRef]
- Li, X.; Yang, Z.; Sui, H.; Jain, A.; He, L. A hybrid process for oil–solid separation by a novel multifunctional switchable solvent. Fuel 2018, 221, 303–310. [Google Scholar] [CrossRef]
- Zhang, Z.; Kang, N.; Wang, J.; Sui, H.; He, L.; Li, X. Synthesis and application of amino acid ionic liquid-based deep eutectic solvents for oil-carbonate mineral separation. Chem. Eng. Sci. 2018, 181, 264–271. [Google Scholar] [CrossRef]
- Hou, J.; Du, J.; Sui, H.; Sun, L. Surfactants Enhanced Heavy Oil–solid separation from Carbonate Asphalt Rocks-Experiment and Molecular Dynamic Simulation. Nanomaterials 2021, 11, 1835. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Sun, L. Synergistic Effect of Nanofluids and Surfactants on Heavy Oil Recovery and Oil-Wet Calcite Wettability. Nanomaterials 2021, 11, 1849. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Li, C.; Wang, X.; Fang, S.; Xiong, Y.; Shi, P. Solid separation from the heavy oil sludge produced from Liaohe Oilfield. J. Pet. Sci. Eng. 2019, 172, 1112–1119. [Google Scholar] [CrossRef]
- De Gennes, P.G. Wetting: Statics and dynamics. Rev. Mod. Phys. 1985, 57, 827–863. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, Y. Contact angle hysteresis of a water droplet on a hydrophobic fuel cell surface. J. Colloid Interface Sci. 2019, 545, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.A.; Kundu, D. Ionic liquid promoted extraction of bitumen from oil sand: A review. J. Pet. Sci. Eng. 2021, 199, 108232. [Google Scholar] [CrossRef]
- Yue, L.; Pu, W.; Zhao, S.; Zhang, S.; Ren, F.; Xu, D. Insights into mechanism of low salinity water flooding in sandstone reservoir from interfacial features of oil/brine/rock via intermolecular forces. J. Mol. Liq. 2020, 313, 113435. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, L.; Cui, X.; Chen, J.; Zeng, H. Intermolecular and surface forces at solid/oil/water/gas interfaces in petroleum production. J. Colloid Interface Sci. 2019, 537, 505–519. [Google Scholar] [CrossRef]
- Sorci, M.; Woodcock, C.C.; Andersen, D.J.; Behzad, A.R.; Nunes, S.; Plawsky, J.; Belfort, G. Linking microstructure of membranes and performance. J. Membr. Sci. 2020, 594, 117419. [Google Scholar] [CrossRef]
- Zhou, Z.A.; Xu, Z.; Masliyah, J.; Czarnecki, J. Coagulation of bitumen with fine silica in model systems. Colloids Surf. A Physicochem. Eng. Asp. 1999, 148, 199–211. [Google Scholar] [CrossRef]
- Hogshead, C.G.; Manias, E.; Williams, P.; Lupinsky, A.; Painter, P. Studies of Bitumen−Silica and Oil−Silica Interactions in Ionic Liquids. Energy Fuels 2011, 25, 293–299. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Z.; Masliyah, J. Interaction forces in bitumen extraction from oil sands. J. Colloid Interface Sci. 2005, 287, 507–520. [Google Scholar] [CrossRef]
- Dai, Q.; Chung, K.H. Bitumen—Sand interaction in oil sand processing. Fuel 1995, 74, 1858–1864. [Google Scholar] [CrossRef]
- Dai, Q.; Chung, K.H. Hot water extraction process mechanism using model oil sands. Fuel 1996, 75, 220–226. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Z.; Masliyah, J. Studies on Bitumen−Silica Interaction in Aqueous Solutions by Atomic Force Microscopy. Langmuir 2003, 19, 3911–3920. [Google Scholar] [CrossRef]
- Liu, X.; Yan, W.; Stenby, E.H.; Thormann, E. Release of crude oil from silica and calcium carbonate surfaces: On the alternation of surface and molecular forces by high− and low−salinity aqueous salt solutions. Energy Fuels 2016, 30, 3986–3993. [Google Scholar] [CrossRef] [Green Version]
- Ren, S.; Dang-Vu, T.; Zhao, H.; Long, J.; Xu, Z.; Masliyah, J. Effect of Weathering on Surface Characteristics of Solids and Bitumen from Oil Sands. Energy Fuels 2009, 23, 334–341. [Google Scholar] [CrossRef]
- Ren, S.; Zhao, H.; Long, J.; Xu, Z.; Masliyah, J. Understanding weathering of oil sands ores by atomic force microscopy. AIChE J. 2009, 55, 3277–3285. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Z.; Masliyah, J. Role of fine clays in bitumen extraction from oil sands. AIChE J. 2004, 50, 1917–1927. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Z.; Masliyah, J. Interaction between Bitumen and Fines in Oil Sands Extraction System: Implication to Bitumen Recovery. Can. J. Chem. Eng. 2004, 82, 655–666. [Google Scholar] [CrossRef]
- Kumar, K.; Dao, E.K.; Mohanty, K.K. Atomic Force Microscopy Study of Wettability Alteration by Surfactants. SPE J. 2008, 13, 137–145. [Google Scholar] [CrossRef]
- Abraham, T.; Christendat, D.; Karan, K.; Xu, Z.; Masliyah, J. Asphaltene−Silica Interactions in Aqueous Solutions: Direct Force Measurements Combined with Electrokinetic Studies. Ind. Eng. Chem. Res. 2002, 41, 2170–2177. [Google Scholar] [CrossRef]
- Qi, Z.; Wang, Y.; He, H.; Li, D.; Xu, X. Wettability Alteration of the Quartz Surface in the Presence of Metal Cations. Energy Fuels 2013, 27, 7354–7359. [Google Scholar] [CrossRef]
- Wang, S.; Segin, N.; Wang, K.; Masliyah, J.H.; Xu, Z. Wettability Control Mechanism of Highly Contaminated Hydrophilic Silica/Alumina Surfaces by Ethyl Cellulose. J. Phys. Chem. C 2011, 115, 10576–10587. [Google Scholar] [CrossRef]
- Saad, M.A.; Kamil, M.; Abdurahman, N.H.; Yunus, R.M.; Awad, O.I. An Overview of Recent Advances in State-of-the-Art Techniques in the Demulsification of Crude Oil Emulsions. Processes 2019, 7, 470. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Hou, X.; Qi, G.; Zhang, R. Preparation of superhydrophobic polyimide fibrous membranes with controllable surface structure for high efficient oil–water emulsion and heavy oil separation. J. Environ. Chem. Eng. 2022, 10, 107470. [Google Scholar] [CrossRef]
- Martínez-Palou, R.; Mosqueira, M.D.L.; Zapata-Rendón, B.; Mar-Juárez, E.; Bernal-Huicochea, C.; de la Cruz Clavel-López, J.; Aburto, J. Transportation of heavy and extra-heavy crude oil by pipeline: A review. J. Pet. Sci. Eng. 2011, 75, 274–282. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, J.; He, C.; He, L.; Li, X.; Sui, H. The formation, stabilization and separation of oil–water emulsions: A Review. Processes 2022, 10, 738. [Google Scholar] [CrossRef]
- Yarranton, H.; Baydak, E.N. Effect of Solvents on the Stability of Water-in-Bitumen Emulsions. Energy Fuels 2022, 36, 6243–6260. [Google Scholar] [CrossRef]
- Ouyang, J.; Meng, Y. Quantitative effect of droplet size and emulsion viscosity on the storage stability of asphalt emulsion. Constr. Build. Mater. 2022, 342, 127994. [Google Scholar] [CrossRef]
- Tian, S.; Gao, W.; Liu, Y.; Kang, W. Study on the stability of heavy crude oil-in-water emulsions stabilized by two different hydrophobic amphiphilic polymers. Colloids Surf. A Physicochem. Eng. Asp. 2019, 572, 299–306. [Google Scholar] [CrossRef]
- Li, X.; Chi, P.; Guo, X.; Sun, Q. Effects of asphaltene concentration and asphaltene agglomeration on viscosity. Fuel 2019, 255, 115825. [Google Scholar] [CrossRef]
- Waldo, G.S.; Mullins, O.C.; Penner-Hahn, J.E.; Cramer, S.P. Determination of the chemical environment of sulfur in petroleum asphaltenes by X-ray absorption spectroscopy. Fuel 1992, 71, 53–57. [Google Scholar] [CrossRef] [Green Version]
- Gaweł, B.; Eftekhardadkhah, M.; Oye, G. Elemental Composition and Fourier Transform Infrared Spectroscopy Analysis of Crude Oils and Their Fractions. Energy Fuels 2014, 28, 997–1003. [Google Scholar] [CrossRef]
- Rogel, E.; Moir, M.; Witt, M. Atmospheric Pressure Photoionization and Laser Desorption Ionization Coupled to Fourier Transform Ion Cyclotron Resonance Mass Spectrometry to Characterize Asphaltene Solubility Fractions: Studying the Link between Molecular Composition and Physical Behavior. Energy Fuels 2015, 29, 4201–4209. [Google Scholar] [CrossRef]
- Ovalles, C.; Rogel, E.; Morazan, H.; Moir, M.E. Synthesis, characterization, and mechanism of asphaltene inhibition of phosphopropoxylated asphaltenes. Fuel 2016, 180, 20–26. [Google Scholar] [CrossRef]
- Salehzadeh, M.; Husein, M.M.; Ghotbi, C.; Dabir, B.; Taghikhani, V. In-depth characterization of light, medium and heavy oil asphaltenes as well as asphaltenes subfractions. Fuel 2022, 324, 124525. [Google Scholar] [CrossRef]
- Mullins, O.C.; Sabbah, H.; Eyssautier, J.; Pomerantz, A.E.; Barré, L.; Andrews, A.B.; Ruiz-Morales, Y.; Mostowfi, F.; McFarlane, R.; Goual, L.; et al. Advances in Asphaltene Science and the Yen–Mullins Model. Energy Fuels 2012, 26, 3986–4003. [Google Scholar] [CrossRef]
- Hemmati-Sarapardeh, A.; Dabir, B.; Ahmadi, M.; Mohammadi, A.H.; Husein, M.M. Toward mechanistic understanding of asphaltene aggregation behavior in toluene: The roles of asphaltene structure, aging time, temperature, and ultrasonic radiation. J. Mol. Liq. 2018, 264, 410–424. [Google Scholar] [CrossRef]
- Gray, M.R.; Chacón-Patiño, M.L.; Rodgers, R.P. Structure–Reactivity Relationships for Petroleum Asphaltenes. Energy Fuels 2022, 36, 4370–4380. [Google Scholar] [CrossRef]
- Moncayo-Riascos, I.; Rojas-Ruiz, F.A.; Orrego-Ruiz, J.A.; Cundar, C.; Torres, R.G.; Cañas-Marín, W. Reconstruction of a Synthetic Crude Oil Using Petroleomics and Molecular Dynamics Simulations: A Multistructural Approach to Understanding Asphaltene Aggregation Behavior. Energy Fuels 2022, 36, 837–850. [Google Scholar] [CrossRef]
- Zúñiga-Hinojosa, M.A.; Cosultchi, A.; Martinez-Martinez, M.T.; Cadena-Nava, R.D.; Ruiz-Garcia, J. Behavior comparison of films of Mexican bitumen and its asphaltene and maltenes fractions at interfaces. Fuel 2022, 307, 121852. [Google Scholar] [CrossRef]
- Chacón-Patiño, M.L.; Rowland, S.M.; Rodgers, R.P. Advances in Asphaltene Petroleomics. Part 1: Asphaltenes Are Composed of Abundant Island and Archipelago Structural Motifs. Energy Fuels 2017, 31, 13509–13518. [Google Scholar] [CrossRef]
- Chacón-Patiño, M.L.; Rowland, S.M.; Rodgers, R.P. Advances in Asphaltene Petroleomics. Part 2: Selective Separation Method That Reveals Fractions Enriched in Island and Archipelago Structural Motifs by Mass Spectrometry. Energy Fuels 2018, 32, 314–328. [Google Scholar] [CrossRef]
- Pradilla, D.; Simon, S.; Sjöblom, J.; Samaniuk, J.; Skrzypiec, M.; Vermant, J. Sorption and Interfacial Rheology Study of Model Asphaltene Compounds. Langmuir 2016, 32, 2900–2911. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zhao, Y.; Ren, S. Molecular Dynamics Simulation of Self-Aggregation of Asphaltenes at an Oil/Water Interface: Formation and Destruction of the Asphaltene Protective Film. Energy Fuels 2015, 29, 1233–1242. [Google Scholar] [CrossRef] [Green Version]
- Li, D.D.; Greenfield, M.L. High Internal Energies of Proposed Asphaltene Structures. Energy Fuels 2011, 25, 3698–3705. [Google Scholar] [CrossRef]
- Da Costa, L.M.; Stoyanov, S.R.; Gusarov, S.; Tan, X.; Gray, M.R.; Stryker, J.M.; Tykwinski, R.; de M. Carneiro, J.W.; Seidl, P.R.; Kovalenko, A. Density Functional Theory Investigation of the Contributions of π–π Stacking and Hydrogen-Bonding Interactions to the Aggregation of Model Asphaltene Compounds. Energy Fuels 2012, 26, 2727–2735. [Google Scholar] [CrossRef]
- Mullins, O.C. The Asphaltenes. Annu. Rev. Anal. Chem. 2011, 4, 393–418. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.R.; Tykwinski, R.R.; Stryker, J.M.; Tan, X. Supramolecular Assembly Model for Aggregation of Petroleum Asphaltenes. Energy Fuels 2011, 25, 3125–3134. [Google Scholar] [CrossRef]
- Speight, J.G. Chapter 2 Chemical and Physical Studies of Petroleum Asphaltenes. In Developments in Petroleum Science; Yen, T.F., Chilingarian, G.V., Eds.; Elsevier: Amsterdam, The Netherlands, 1994; Volume 40, pp. 7–65. [Google Scholar]
- Wu, J.; Prausnitz, J.M.; Firoozabadi, A. Molecular thermodynamics of asphaltene precipitation in reservoir fluids. AIChE J. 2000, 46, 197–209. [Google Scholar] [CrossRef]
- Petrova, L.M.; Abbakumova, N.A.; Foss, T.R.; Romanov, G.V. Structural features of asphaltene and petroleum resin fractions. Pet. Chem. 2011, 51, 252. [Google Scholar] [CrossRef]
- Morozova, A.V.; Volkova, G.I.; Krivtsov, E.B. Composition of Petroleum Resins Inhibiting the Precipitate Formation in an Ultrasonically Treated Solution of Petroleum Wax in Decane. Pet. Chem. 2022, 62, 161–168. [Google Scholar] [CrossRef]
- Gorbacheva, S.N.; Ilyin, S.O. Structure, rheology and possible application of water-in-oil emulsions stabilized by asphaltenes. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126442. [Google Scholar] [CrossRef]
- Kohli, K.; Prajapati, R.; Maity, S.K.; Sau, M.; Garg, M.O. Deactivation of hydrotreating catalyst by metals in resin and asphaltene parts of heavy oil and residues. Fuel 2016, 175, 264–273. [Google Scholar] [CrossRef]
- Murgich, J.; Rodriguez; Aray, Y. Molecular Recognition and Molecular Mechanics of Micelles of Some Model Asphaltenes and Resins. Energy Fuels 1996, 10, 68–76. [Google Scholar] [CrossRef]
- Yoon, S.; Bhatt, S.D.; Lee, W.; Lee, H.Y.; Jeong, S.Y.; Baeg, J.-O.; Lee, C.W. Separation and characterization of bitumen from Athabasca oil sand. Korean J. Chem. Eng. 2009, 26, 64–71. [Google Scholar] [CrossRef]
- He, L.; Li, X.; Wu, G.; Lin, F.; Sui, H. Distribution of Saturates, Aromatics, Resins, and Asphaltenes Fractions in the Bituminous Layer of Athabasca Oil Sands. Energy Fuels 2013, 27, 4677–4683. [Google Scholar] [CrossRef]
- Del Rio, L.F.; Hadwin, A.K.M.; Pinto, L.J.; MacKinnon, M.D.; Moore, M.M. Degradation of naphthenic acids by sediment micro-organisms. J. Appl. Microbiol. 2006, 101, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Naik, D.V.; Dutta, R.K.; Kanaujia, P.K. Biochars for the removal of naphthenic acids from water: A prospective approach towards remediation of petroleum refinery wastewater. J. Clean. Prod. 2020, 266, 121986. [Google Scholar] [CrossRef]
- Grewer, D.M.; Young, R.F.; Whittal, R.M.; Fedorak, P.M. Naphthenic acids and other acid-extractables in water samples from Alberta: What is being measured? Sci. Total Environ. 2010, 408, 5997–6010. [Google Scholar] [CrossRef] [PubMed]
- Headley, J.V.; Peru, K.M.; Barrow, M.P. Mass spectrometric characterization of naphthenic acids in environmental samples: A review. Mass Spectrom. Rev. 2009, 28, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Headley, J.V.; Peru, K.M.; Mohamed, M.H.; Frank, R.A.; Martin, J.W.; Hazewinkel, R.R.O.; Humphries, D.; Gurprasad, N.P.; Hewitt, L.M.; Muir, D.C.G.; et al. Chemical fingerprinting of naphthenic acids and oil sands process waters—A review of analytical methods for environmental samples. J. Environ. Sci. Health Part A 2013, 48, 1145–1163. [Google Scholar] [CrossRef]
- Fan, T.P. Characterization of naphthenic acids in petroleum by fast atom bombardment mass spectrometry. Energy Fuels 1991, 5, 371–375. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, G.; Serhan, M.; Koivu, G.; Yang, Z.; Hollebone, B.; Lambert, P.; Brown, C.E. Characterization of naphthenic acids in crude oils and refined petroleum products. Fuel 2019, 255, 115849. [Google Scholar] [CrossRef]
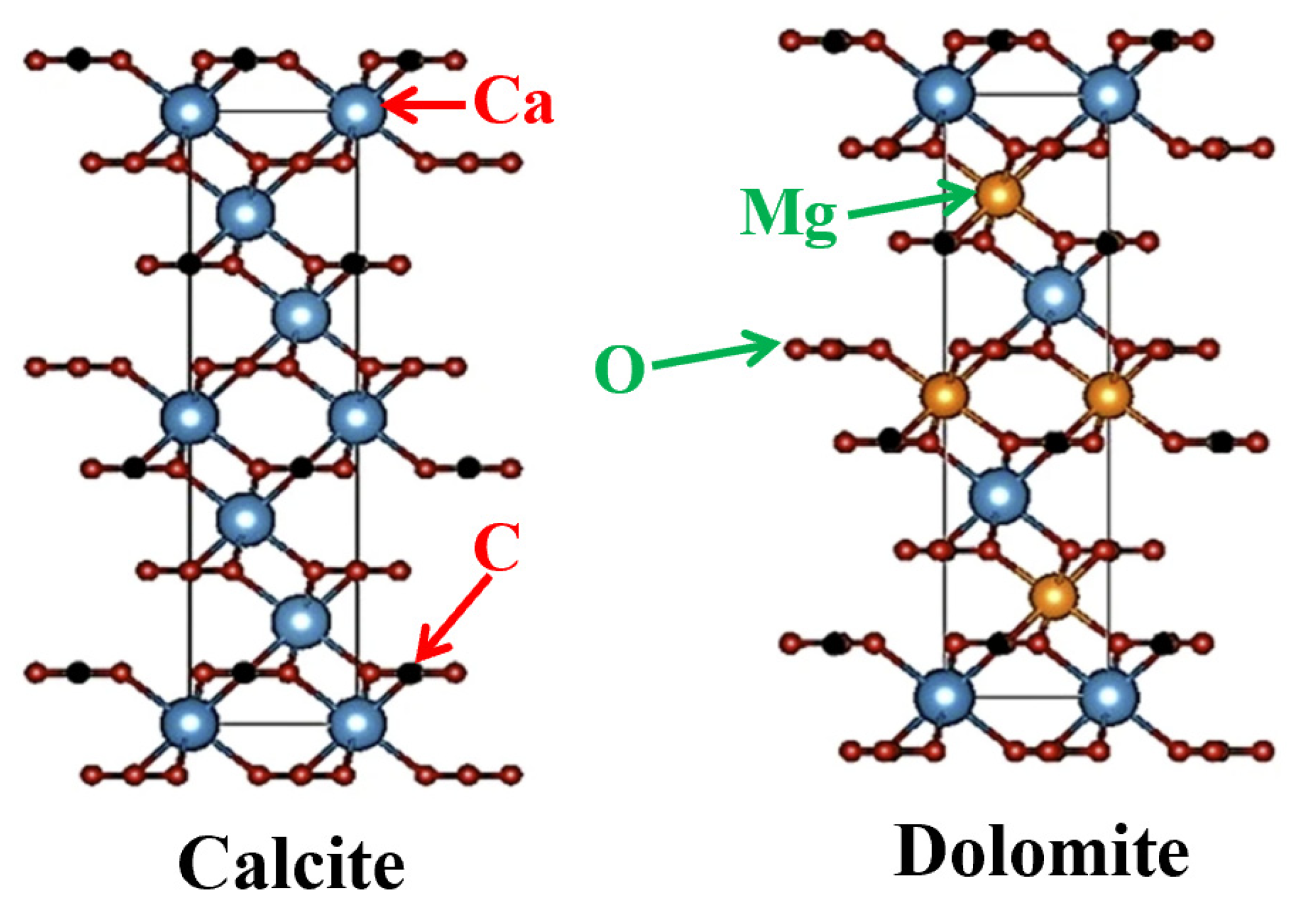
- Hsiao, Y.-H.; Wang, B.; La Plante, E.C.; Pignatelli, I.; Krishnan, N.M.A.; Le Pape, Y.; Neithalath, N.; Bauchy, M.; Sant, G. The effect of irradiation on the atomic structure and chemical durability of calcite and dolomite. NPJ Mater. Degrad. 2019, 3, 36. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Bertram, A.; Patey, G. Molecular Simulations of Feldspar Surfaces Interacting with Aqueous Inorganic Solutions: Interfacial Water/Ion Structure and Implications for Ice Nucleation. ACS Earth Space Chem. 2021, 5, 2169–2183. [Google Scholar] [CrossRef]
- Liang, J.; Tang, Z.; Jiang, W.; Liu, J.; Feng, G.; Lao, X.; Jiang, F.; Hu, Q.; Zhang, X.; Qi, F.; et al. Effects of magnesite addition on the properties and structure of foam ceramics. Ceram. Int. 2021, 47, 18584–18591. [Google Scholar] [CrossRef]
- He, L.; Lin, F.; Li, X.; Sui, H.; Xu, Z. Interfacial sciences in unconventional petroleum production: From fundamentals to applications. Chem. Soc. Rev. 2015, 44, 5446–5494. [Google Scholar] [CrossRef] [PubMed]
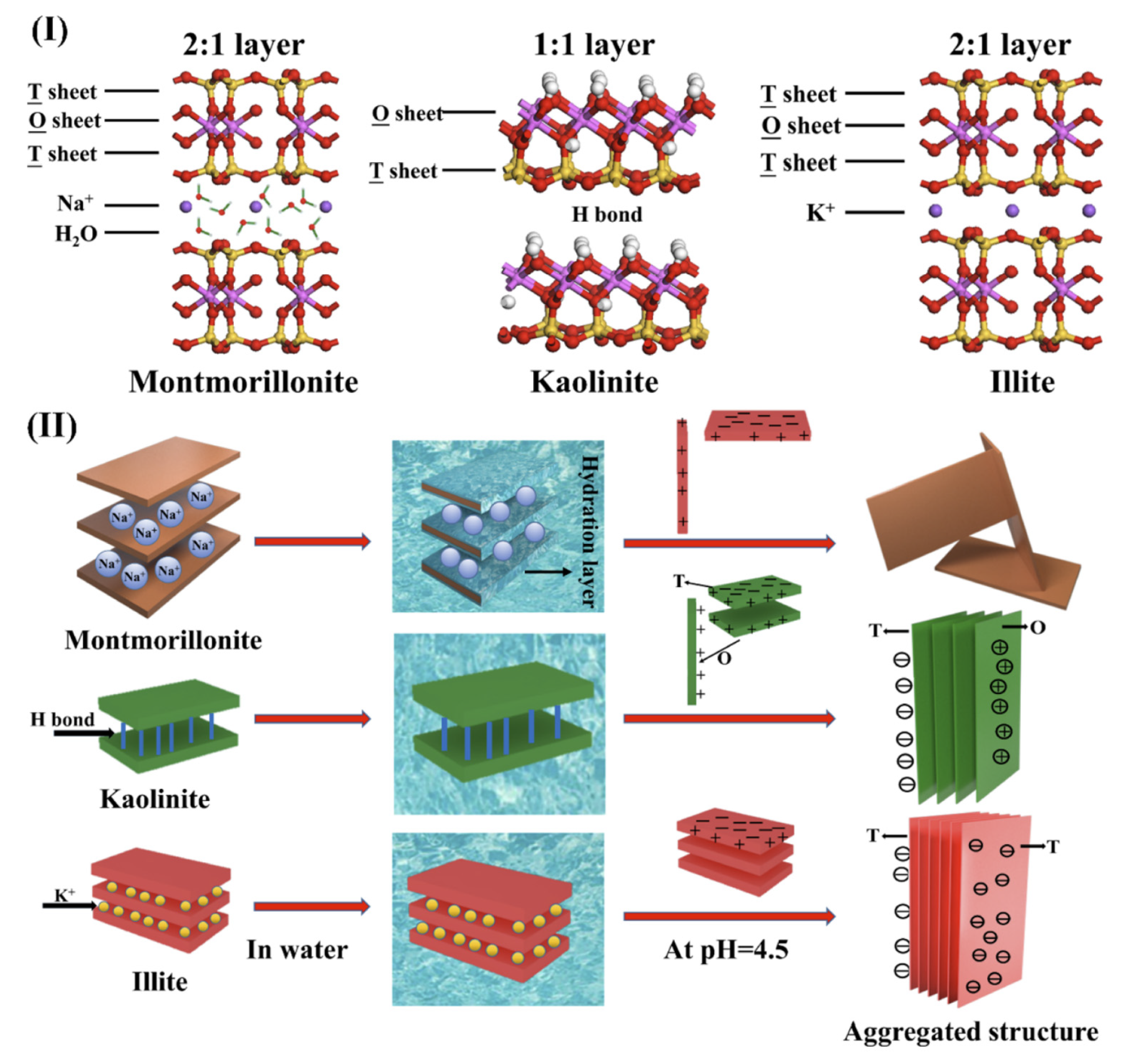
- Bian, Z.; Kawi, S. Preparation, characterization and catalytic application of phyllosilicate: A review. Catal. Today 2020, 339, 3–23. [Google Scholar] [CrossRef]
- Samantray, J.; Anand, A.; Dash, B.; Ghosh, M.K.; Behera, A.K. Silicate minerals—Potential source of potash—A review. Miner. Eng. 2022, 179, 107463. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, Y.; Bai, H.; Ai, Z.; Chen, P.; Hu, Y.; Song, S.; Komarneni, S. Role of Montmorillonite, Kaolinite, or Illite in Pyrite Flotation: Differences in Clay Behavior Based on Their Structures. Langmuir 2020, 36, 10860–10867. [Google Scholar] [CrossRef] [PubMed]
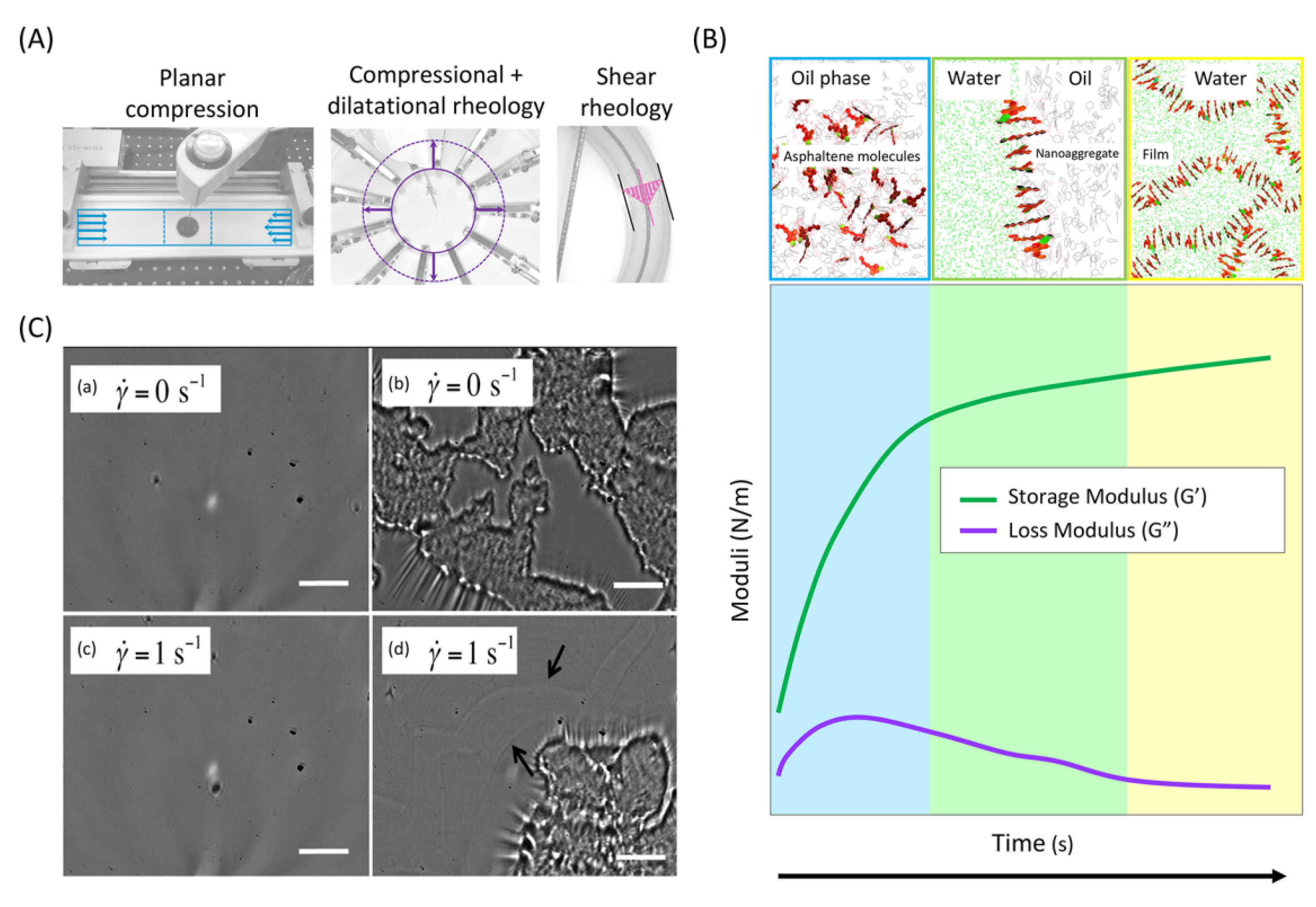
- Li, M.; Xu, M.; Ma, Y.; Wu, Z.; Christy, A.A. Interfacial film properties of asphaltenes and resins. Fuel 2002, 81, 1847–1853. [Google Scholar] [CrossRef]
- Rytting, B.M.; Harper, M.R.; Edmond, K.V.; Merchant, S.; Zhang, Y.; Kilpatrick, P.K. Interfacial Phenomena of Purified Petroporphyrins and Their Impact on Asphaltene Interfacial Film Formation. Energy Fuels 2020, 34, 5444–5456. [Google Scholar] [CrossRef]
- Sun, H.; Li, X.; Liu, D.; Li, X. Synergetic adsorption of asphaltenes and oil displacement surfactants on the oil-water interface: Insights on stabilization mechanism of the interfacial film. Chem. Eng. Sci. 2021, 245, 116850. [Google Scholar] [CrossRef]
- Chang, C.-C.; Williams, I.; Nowbahar, A.; Mansard, V.; Mecca, J.; Whitaker, K.A.; Schmitt, A.K.; Tucker, C.J.; Kalantar, T.H.; Kuo, T.-C.; et al. Effect of Ethylcellulose on the Rheology and Mechanical Heterogeneity of Asphaltene Films at the Oil–Water Interface. Langmuir 2019, 35, 9374–9381. [Google Scholar] [CrossRef]
- Ashoorian, S.; Javadi, A.; Hosseinpour, N.; Husein, M. Evolution of adsorbed layers of asphaltenes at oil-water interfaces: A novel experimental protocol. J. Colloid Interface Sci. 2021, 594, 80–91. [Google Scholar] [CrossRef]
- Zhao, X.; Xia, M.; Li, P.; Wang, J.; Xu, Y.; Guo, S. Effect of Polar Molecule Aggregation on the Stability of Crude Oil/Water Interface: A Molecular Simulation Study. Lithosphere 2021, 2021, 8465197. [Google Scholar] [CrossRef]
- Liu, D.; Li, C.; Yang, F.; Sun, G.; You, J.; Cui, K. Synergetic effect of resins and asphaltenes on water/oil interfacial properties and emulsion stability. Fuel 2019, 252, 581–588. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, H.; Li, C.; Zhang, H.; Yang, F.; Sun, G.; Zhao, Y. Study on the Interactive Effects of Solid Particles and Asphaltenes on the Interfacial Structure and Stability of a Water-in-Model Oil Emulsion. Langmuir 2021, 37, 10827–10837. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y.; Peng, K.; Zhao, X.; Xiong, Y.; Huang, X. A review of the interfacial stability mechanism of aging oily sludge: Heavy components, inorganic particles, and their synergism. J. Hazard. Mater. 2021, 415, 125624. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Gu, S.; Song, Z.; Xie, Z.; Chang, X.; Shen, P. The viscosifying behavior of W/O emulsion and its underlying mechanisms: Considering the interfacial adsorption of heavy components. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127794. [Google Scholar] [CrossRef]
- Gawrys, K.L.; Kilpatrick, P.K. Asphaltenic aggregates are polydisperse oblate cylinders. J. Colloid Interface Sci. 2005, 288, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Gawrys, K.L.; Blankenship, G.A.; Kilpatrick, P.K. Solvent Entrainment in and Flocculation of Asphaltenic Aggregates Probed by Small-Angle Neutron Scattering. Langmuir 2006, 22, 4487–4497. [Google Scholar] [CrossRef] [PubMed]
- Spiecker, P.M.; Gawrys, K.L.; Kilpatrick, P.K. Aggregation and solubility behavior of asphaltenes and their subfractions. J. Colloid Interface Sci. 2003, 267, 178–193. [Google Scholar] [CrossRef] [PubMed]
- Barrios, N.; Pereira, J.; Salas, C. Block copolymer penetration into an asphaltene film as a mechanism for water-in-crude oil emulsion break-up. Fuel 2021, 306, 121756. [Google Scholar] [CrossRef]
- Li, X.; Liu, D.; Sun, H.; Li, X. Effect of Oil-Displacing Agent Composition on Oil/Water Interface Stability of the Asphaltene-Rich ASP Flooding-Produced Water. Langmuir 2022, 38, 3329–3338. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, L.; Xie, L.; Lu, X.; Liu, Q.; He, J.; Mantilla, C.A.; Van den berg, F.G.A.; Zeng, H. Surface Interaction of Water-in-Oil Emulsion Droplets with Interfacially Active Asphaltenes. Langmuir 2017, 33, 1265–1274. [Google Scholar] [CrossRef]
- Verruto, V.J.; Le, R.K.; Kilpatrick, P.K. Adsorption and Molecular Rearrangement of Amphoteric Species at Oil−Water Interfaces. J. Phys. Chem. B 2009, 113, 13788–13799. [Google Scholar] [CrossRef] [PubMed]
- Yarranton, H.W.; Sztukowski, D.M.; Urrutia, P. Effect of interfacial rheology on model emulsion coalescence: I. Interfacial rheology. J. Colloid Interface Sci. 2007, 310, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Lowry, E.; Sedghi, M.; Goual, L. Polymers for asphaltene dispersion: Interaction mechanisms and molecular design considerations. J. Mol. Liq. 2017, 230, 589–599. [Google Scholar] [CrossRef] [Green Version]
- Durand, E.; Clemancey, M.; Lancelin, J.-M.; Verstraete, J.; Espinat, D.; Quoineaud, A.-A. Aggregation States of Asphaltenes: Evidence of Two Chemical Behaviors by 1H Diffusion-Ordered Spectroscopy Nuclear Magnetic Resonance. J. Phys. Chem. C 2009, 113, 16266–16276. [Google Scholar] [CrossRef]
- González, G.; Acevedo, S.; Castillo, J.; Villegas, O.; Ranaudo, M.A.; Guzmán, K.; Orea, M.; Bouyssiere, B. Study of Very High Molecular Weight Cluster Presence in THF Solution of Asphaltenes and Subfractions A1 and A2, by Gel Permeation Chromatography with Inductively Coupled Plasma Mass Spectrometry. Energy Fuels 2020, 34, 12535–12544. [Google Scholar] [CrossRef]
- Alimohammadi, S.; Sayyad Amin, J.; Nikkhah, S.; Soroush, M.; Zendehboudi, S. Development of a new scaling model for asphaltene precipitation in light, medium, and heavy crude oils. J. Mol. Liq. 2020, 312, 112974. [Google Scholar] [CrossRef]
- Schuler, B.; Zhang, Y.; Liu, F.; Pomerantz, A.E.; Andrews, A.B.; Gross, L.; Pauchard, V.; Banerjee, S.; Mullins, O.C. Overview of Asphaltene Nanostructures and Thermodynamic Applications. Energy Fuels 2020, 34, 15082–15105. [Google Scholar] [CrossRef]
- Rahham, Y.; Rane, K.; Goual, L. Characterization of the Interfacial Material in Asphaltenes Responsible for Oil/Water Emulsion Stability. Energy Fuels 2020, 34, 13871–13882. [Google Scholar] [CrossRef]
- Long, J.; Xu, Z.; Masliyah, J.H. Single Molecule Force Spectroscopy of Asphaltene Aggregates. Langmuir 2007, 23, 6182–6190. [Google Scholar] [CrossRef]
- Kabbach, C.B.; dos Santos, R.G. Effects of pH and Temperature on the Phase Behavior and Properties of Asphaltene Liquid Films. Energy Fuels 2018, 32, 2811–2818. [Google Scholar] [CrossRef]
- Jiguang, L.; Xin, G.; Haiping, S.; Xinheng, C.; Ming, D.; Huandi, H. The solubility of asphaltene in organic solvents and its relation to the molecular structure. J. Mol. Liq. 2021, 327, 114826. [Google Scholar] [CrossRef]
- Schuler, B.; Meyer, G.; Peña, D.; Mullins, O.C.; Gross, L. Unraveling the Molecular Structures of Asphaltenes by Atomic Force Microscopy. J. Am. Chem. Soc. 2015, 137, 9870–9876. [Google Scholar] [CrossRef] [PubMed]
- Poteau, S.; Argillier, J.-F.; Langevin, D.; Pincet, F.; Perez, E. Influence of pH on Stability and Dynamic Properties of Asphaltenes and Other Amphiphilic Molecules at the Oil−Water Interface. Energy Fuels 2005, 19, 1337–1341. [Google Scholar] [CrossRef]
- Lashkarbolooki, M.; Ayatollahi, S. The effects of pH, acidity, asphaltene and resin fraction on crude oil/water interfacial tension. J. Pet. Sci. Eng. 2018, 162, 341–347. [Google Scholar] [CrossRef]
- Wang, D.; Lin, M.; Dong, Z.; Li, L.; Jin, S.; Pan, D.; Yang, Z. Mechanism of High Stability of Water-in-Oil Emulsions at High Temperature. Energy Fuels 2016, 30, 1947–1957. [Google Scholar] [CrossRef]
- Jian, C.; Liu, Q.; Zeng, H.; Tang, T. A Molecular Dynamics Study of the Effect of Asphaltenes on Toluene/Water Interfacial Tension: Surfactant or Solute? Energy Fuels 2018, 32, 3225–3231. [Google Scholar] [CrossRef]
- Bochner de Araujo, S.; Merola, M.; Vlassopoulos, D.; Fuller, G.G. Droplet Coalescence and Spontaneous Emulsification in the Presence of Asphaltene Adsorption. Langmuir 2017, 33, 10501–10510. [Google Scholar] [CrossRef] [PubMed]
- Harbottle, D.; Chen, Q.; Moorthy, K.; Wang, L.; Xu, S.; Liu, Q.; Sjoblom, J.; Xu, Z. Problematic Stabilizing Films in Petroleum Emulsions: Shear Rheological Response of Viscoelastic Asphaltene Films and the Effect on Drop Coalescence. Langmuir 2014, 30, 6730–6738. [Google Scholar] [CrossRef]
- Sullivan, A.P.; Kilpatrick, P.K. The Effects of Inorganic Solid Particles on Water and Crude Oil Emulsion Stability. Ind. Eng. Chem. Res. 2002, 41, 3389–3404. [Google Scholar] [CrossRef]
- Kilpatrick, P.K. Water-in-Crude Oil Emulsion Stabilization: Review and Unanswered Questions. Energy Fuels 2012, 26, 4017–4026. [Google Scholar] [CrossRef]
- Pauchard, V.; Rane, J.P.; Banerjee, S. Asphaltene-Laden Interfaces Form Soft Glassy Layers in Contraction Experiments: A Mechanism for Coalescence Blocking. Langmuir 2014, 30, 12795–12803. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.A.; Baydak, E.N.; Yarranton, H.W. What Fraction of the Asphaltenes Stabilizes Water-in-Bitumen Emulsions? Energy Fuels 2018, 32, 1440–1450. [Google Scholar] [CrossRef]
- Pagán Pagán, N.M.; Zhang, Z.; Nguyen, T.V.; Marciel, A.B.; Biswal, S.L. Physicochemical Characterization of Asphaltenes Using Microfluidic Analysis. Chem. Rev. 2022, 122, 7205–7235. [Google Scholar] [CrossRef]
- McLean, J.D.; Kilpatrick, P.K. Effects of Asphaltene Aggregation in Model Heptane–Toluene Mixtures on Stability of Water-in-Oil Emulsions. J. Colloid Interface Sci. 1997, 196, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Kuznicki, T.; Masliyah, J.H.; Bhattacharjee, S. Molecular Dynamics Study of Model Molecules Resembling Asphaltene-Like Structures in Aqueous Organic Solvent Systems. Energy Fuels 2008, 22, 2379–2389. [Google Scholar] [CrossRef]
- Moud, A.A. Asphaltene induced changes in rheological properties: A review. Fuel 2022, 316, 123372. [Google Scholar] [CrossRef]
- Bi, J.; Yang, F.; Harbottle, D.; Pensini, E.; Tchoukov, P.; Simon, S.; Sjöblom, J.; Dabros, T.; Czarnecki, J.; Liu, Q.; et al. Interfacial Layer Properties of a Polyaromatic Compound and its Role in Stabilizing Water-in-Oil Emulsions. Langmuir 2015, 31, 10382–10391. [Google Scholar] [CrossRef]
- Yarranton, H.W.; Alboudwarej, H.; Jakher, R. Investigation of Asphaltene Association with Vapor Pressure Osmometry and Interfacial Tension Measurements. Ind. Eng. Chem. Res. 2000, 39, 2916–2924. [Google Scholar] [CrossRef]
- Rane, J.P.; Harbottle, D.; Pauchard, V.; Couzis, A.; Banerjee, S. Adsorption Kinetics of Asphaltenes at the Oil–Water Interface and Nanoaggregation in the Bulk. Langmuir 2012, 28, 9986–9995. [Google Scholar] [CrossRef]
- Jeribi, M.; Almir-Assad, B.; Langevin, D.; Hénaut, I.; Argillier, J.F. Adsorption Kinetics of Asphaltenes at Liquid Interfaces. J. Colloid Interface Sci. 2002, 256, 268–272. [Google Scholar] [CrossRef]
- Chang, C.-C.; Nowbahar, A.; Mansard, V.; Williams, I.; Mecca, J.; Schmitt, A.K.; Kalantar, T.H.; Kuo, T.-C.; Squires, T.M. Interfacial Rheology and Heterogeneity of Aging Asphaltene Layers at the Water–Oil Interface. Langmuir 2018, 34, 5409–5415. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, D.P.; Baydak, E.N.; Yarranton, H.W. Effect of surfactants on interfacial films and stability of water-in-oil emulsions stabilized by asphaltenes. J. Colloid Interface Sci. 2010, 351, 542–555. [Google Scholar] [CrossRef]
- Rahman, M.; Zhao, X.; Christopher, G.F. Two component model oils for interfacial shear characterization. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123780. [Google Scholar] [CrossRef]
- Feng, L.; Manica, R.; Lu, Y.; Liu, B.; Lu, H.; Liu, Q. Effect of sodium citrate on asphaltene film at the oil–water interface. J. Colloid Interface Sci. 2022, 625, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Samaniuk, J.R.; Hermans, E.; Verwijlen, T.; Pauchard, V.; Vermant, J. Soft-Glassy Rheology of Asphaltenes at Liquid Interfaces. J. Dispers. Sci. Technol. 2015, 36, 1444–1451. [Google Scholar] [CrossRef]
- Spiecker, P.M.; Kilpatrick, P.K. Interfacial Rheology of Petroleum Asphaltenes at the Oil−Water Interface. Langmuir 2004, 20, 4022–4032. [Google Scholar] [CrossRef] [PubMed]
- Kuznicki, T.; Masliyah, J.H.; Bhattacharjee, S. Aggregation and Partitioning of Model Asphaltenes at Toluene−Water Interfaces: Molecular Dynamics Simulations. Energy Fuels 2009, 23, 5027–5035. [Google Scholar] [CrossRef]
- Alicke, A.; Simon, S.; Sjöblom, J.; Vermant, J. Assessing the Interfacial Activity of Insoluble Asphaltene Layers: Interfacial Rheology versus Interfacial Tension. Langmuir 2020, 36, 14942–14959. [Google Scholar] [CrossRef]
- Quintero, C.G.; Noïk, C.; Dalmazzone, C.; Grossiord, J.L. Formation Kinetics and Viscoelastic Properties of Water/Crude Oil Interfacial Films. Oil Gas Sci. Technol.-Rev. IFP 2009, 64, 607–616. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Barman, S.; He, P.; Zhang, Z.; Christopher, G.F.; Biswal, S.L. Combined interfacial shear rheology and microstructure visualization of asphaltenes at air-water and oil-water interfaces. J. Rheol. 2017, 62, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.; Lin, S.; Zhang, M. Ionic-liquid-enhanced solvent extraction mechanism: A novel concept. J. Environ. Chem. Eng. 2022, 10, 107899. [Google Scholar] [CrossRef]
- Kang, W.; Guo, L.; Fan, H.; Meng, L.; Li, Y. Flocculation, coalescence and migration of dispersed phase droplets and oil–water separation in heavy oil emulsion. J. Pet. Sci. Eng. 2012, 81, 177–181. [Google Scholar] [CrossRef]
- Fajun, Z.; Zhexi, T.; Zhongqi, Y.; Hongzhi, S.; Yanping, W.; Yufei, Z. Research status and analysis of stabilization mechanisms and demulsification methods of heavy oil emulsions. Energy Sci. Eng. 2020, 8, 4158–4177. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, L.; Chao, M.; Jia, X.; Liu, C.; Shi, L. Synthesis and Study of a New Type of Nonanionic Demulsifier for Chemical Flooding Emulsion Demulsification. ACS Omega 2021, 6, 17709–17719. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Wang, Z.; Li, H.; Nørskov, J.K. Relations between Surface Oxygen Vacancies and Activity of Methanol Formation from CO2 Hydrogenation over In2O3 Surfaces. ACS Catal. 2021, 11, 1780–1786. [Google Scholar] [CrossRef]
- Husain, A.; Adewunmi, A.A.; Kamal, M.S.; Mahmoud, M.; Al-Harthi, M.A. Demulsification of Heavy Petroleum Emulsion Using Pyridinium Ionic Liquids with Distinct Anion Branching. Energy Fuels 2021, 35, 16527–16533. [Google Scholar] [CrossRef]
- Takahashi, Y.; Koizumi, N.; Kondo, Y. Demulsification of Redox-Active Emulsions by Chemical Oxidation. Langmuir 2016, 32, 7556–7563. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Q.; Li, X.; He, X. Novel polyether-polyquaternium copolymer as an effective reverse demulsifier for O/W emulsions: Demulsification performance and mechanism. Fuel 2020, 263, 116770. [Google Scholar] [CrossRef]
- Dezhi, S.; Shik Chung, J.; Xiaodong, D.; Ding, Z. Demulsification of water-in-oil emulsion by wetting coalescence materials in stirred- and packed-columns. Colloids Surf. A Physicochem. Eng. Asp. 1999, 150, 69–75. [Google Scholar] [CrossRef]
- Wang, D.; Qiao, C.; Zhao, Z.; Huang, Y.; Gao, S.; Yang, D.; Liu, Q.; Zeng, H. Effect of Charge Density of Reverse Emulsion Breaker on Demulsification Performance for Steam-Assisted Gravity Drainage (SAGD) Emulsions under High Temperature and High Pressure. Energy Fuels 2020, 34, 13893–13902. [Google Scholar] [CrossRef]
- Yuan, S.; Wang, Y.; Wang, X.; Wang, Y.; Liu, S.; Duan, M.; Fang, S. Efficient demulsification of cationic polyacrylate for oil-in-water emulsion: Synergistic effect of adsorption bridging and interfacial film breaking. Colloids Surf. A Physicochem. Eng. Asp. 2022, 640, 128393. [Google Scholar] [CrossRef]
- Xu, H.; Wang, J.; Ren, S. Removal of Oil from a Crude Oil-in-Water Emulsion by a Magnetically Recyclable Diatomite Demulsifier. Energy Fuels 2019, 33, 11574–11583. [Google Scholar] [CrossRef]
- Natarajan, A.; Kuznicki, N.; Harbottle, D.; Masliyah, J.; Zeng, H.; Xu, Z. Molecular Interactions between a Biodegradable Demulsifier and Asphaltenes in an Organic Solvent. Energy Fuels 2016, 30, 10179–10186. [Google Scholar] [CrossRef] [Green Version]
- Niu, Z.; Ma, X.; Manica, R.; Yue, T. Molecular Destabilization Mechanism of Asphaltene Model Compound C5Pe Interfacial Film by EO-PO Copolymer: Experiments and MD Simulation. J. Phys. Chem. C 2019, 123, 10501–10508. [Google Scholar] [CrossRef]
- Ye, F.; Jiang, X.; Liu, H.; Ai, G.; Shen, L.; Yang, Y.; Feng, X.; Yuan, H.; Zhang, Z.; Mi, Y.; et al. Amine functional cellulose derived from wastepaper toward oily wastewater treatment and its demulsification mechanism. J. Mol. Liq. 2022, 360, 119459. [Google Scholar] [CrossRef]
- Li, Z.; Chakraborty, A.; Fuentes, J.; Zamora, E.; Vázquez, F.; Xu, Z.; Liu, Q.; Flores, C.; McCaffrey, W.C. Study on demulsifier crude oil interactions at oil-water interface for crude oil dehydration. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127526. [Google Scholar] [CrossRef]
- Liu, W.; Fu, H.; Sun, X.; Bao, M.; Han, X.; Li, Y.; Lu, J. New insights into the interaction between asphaltene and hydrolyzed polyacrylamide at the oil-water interface based on emulsion stability. J. Pet. Sci. Eng. 2022, 215, 110628. [Google Scholar] [CrossRef]
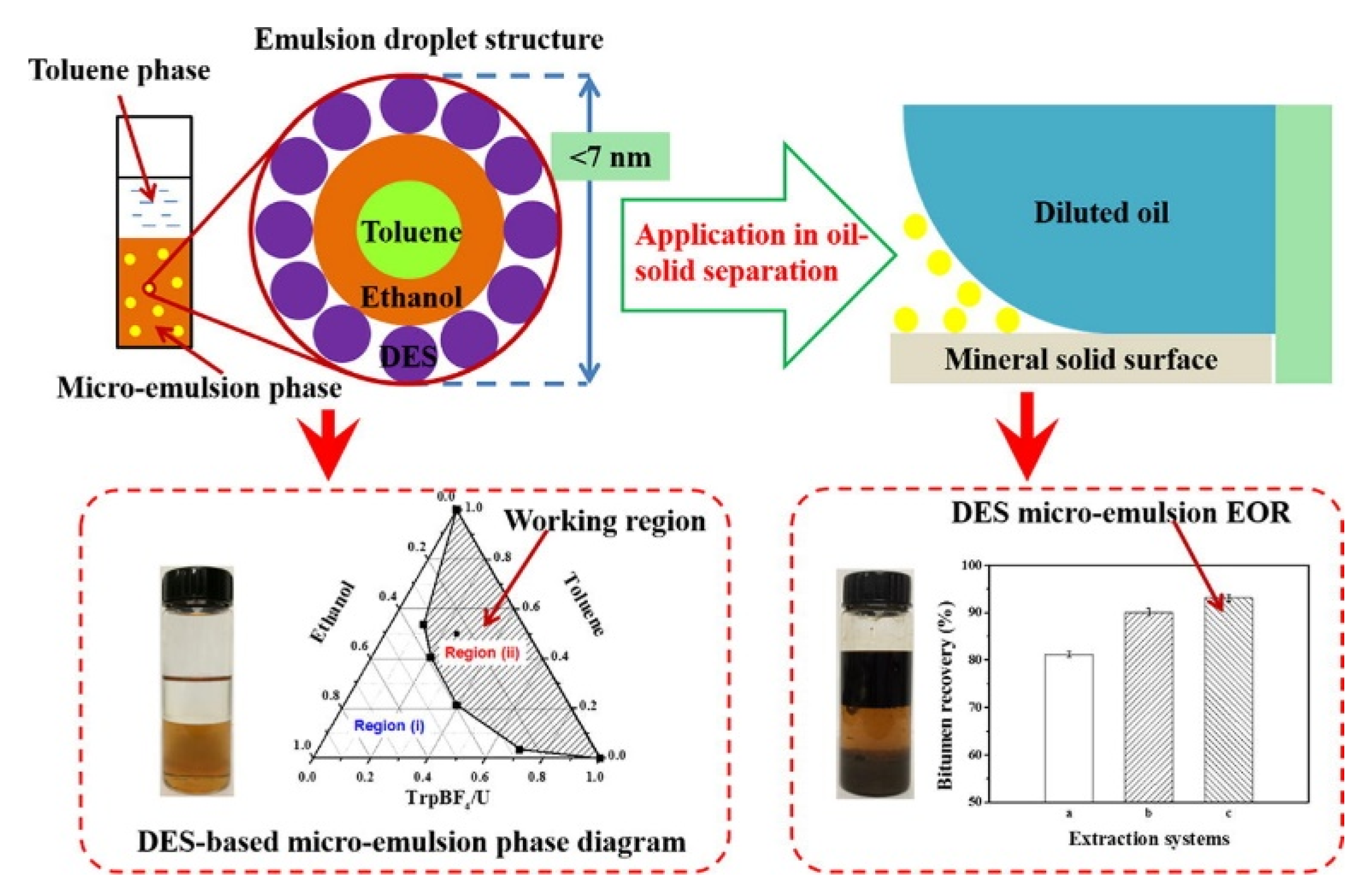
- Im, S.I.; Shin, S.; Park, J.W.; Yoon, H.J.; Go, K.S.; Nho, N.S.; Lee, K.B. Selective separation of solvent from deasphalted oil using CO2 for heavy oil upgrading process based on solvent deasphalting. Chem. Eng. J. 2018, 331, 389–394. [Google Scholar] [CrossRef]
- Ma, G.; Wang, J.; He, L.; Li, X.; Sui, H. The nature of the Indonesian carbonate asphalt rocks and its insights into the separation processes. J. Pet. Sci. Eng. 2020, 195, 107752. [Google Scholar] [CrossRef]
- Pripakhaylo, A.V.; Magomedov, R.N.; Maryutina, T.A. Separation of Heavy Oil into Narrow Fractions by Supercritical Fluid Extraction Using a CO2–Toluene Mixture. J. Anal. Chem. 2019, 74, 401–409. [Google Scholar] [CrossRef]
- Jena, N.K.; Lyne, Å.L.; Arul Murugan, N.; Ågren, H.; Birgisson, B. Atomic level simulations of the interaction of asphaltene with quartz surfaces: Role of chemical modifications and aqueous environment. Mater. Struct. 2016, 50, 99. [Google Scholar] [CrossRef]
- Kim, S.; Marcano, M.C.; Becker, U. Mechanistic Study of Wettability Changes on Calcite by Molecules Containing a Polar Hydroxyl Functional Group and Nonpolar Benzene Rings. Langmuir 2019, 35, 2527–2537. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Yi, J.; Feng, D.; Huang, Y.; Wang, D. Analysis of Adhesive Characteristics of Asphalt Based on Atomic Force Microscopy and Molecular Dynamics Simulation. ACS Appl. Mater. Interfaces 2016, 8, 12393–12403. [Google Scholar] [CrossRef] [PubMed]
- Raj, G.; Lesimple, A.; Whelan, J.; Naumov, P. Direct Observation of Asphaltene Nanoparticles on Model Mineral Substrates. Langmuir 2017, 33, 6248–6257. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Xie, L.; Zhang, L.; Lu, X.; Zeng, H. Probing the interaction mechanism between oil droplets with asphaltenes and solid surfaces using AFM. J. Colloid Interface Sci. 2020, 558, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bai, Y.; Sui, H.; He, L. Understanding the Liberation of Asphaltenes on the Muscovite Surface. Energy Fuels 2017, 31, 1174–1181. [Google Scholar] [CrossRef]
- Li, X.; Bai, Y.; Sui, H.; He, L. Understanding desorption of oil fractions from mineral surfaces. Fuel 2018, 232, 257–266. [Google Scholar] [CrossRef]
- Bai, Y.; Sui, H.; Liu, X.; He, L.; Li, X.; Thormann, E. Effects of the N, O, and S heteroatoms on the adsorption and desorption of asphaltenes on silica surface: A molecular dynamics simulation. Fuel 2019, 240, 252–261. [Google Scholar] [CrossRef]
- Pu, W.; He, M.; Yang, X.; Liu, R.; Shen, C. Experimental study on the key influencing factors of phase inversion and stability of heavy oil emulsion: Asphaltene, resin and petroleum acid. Fuel 2022, 311, 122631. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, J.; Lin, Y.-J.; Wang, X.; Biswal, S.L. Comparing the Coalescence Rate of Water-in-Oil Emulsions Stabilized with Asphaltenes and Asphaltene-like Molecules. Langmuir 2020, 36, 7894–7900. [Google Scholar] [CrossRef]
- Liu, D.; Li, C.; Zhang, X.; Yang, F.; Sun, G.; Yao, B.; Zhang, H. Polarity effects of asphaltene subfractions on the stability and interfacial properties of water-in-model oil emulsions. Fuel 2020, 269, 117450. [Google Scholar] [CrossRef]
- Moghadasi, R.; Kord, S.; Moghadasi, J.; Dashti, H. Mechanistic understanding of asphaltenes surface behavior at oil/water interface: An experimental study. J. Mol. Liq. 2019, 285, 562–571. [Google Scholar] [CrossRef]
- Yang, F.; Tchoukov, P.; Pensini, E.; Dabros, T.; Czarnecki, J.; Masliyah, J.; Xu, Z. Asphaltene Subfractions Responsible for Stabilizing Water-in-Crude Oil Emulsions. Part 1: Interfacial Behaviors. Energy Fuels 2014, 28, 6897–6904. [Google Scholar] [CrossRef]
- Yang, F.; Tchoukov, P.; Dettman, H.; Teklebrhan, R.B.; Liu, L.; Dabros, T.; Czarnecki, J.; Masliyah, J.; Xu, Z. Asphaltene Subfractions Responsible for Stabilizing Water-in-Crude Oil Emulsions. Part 2: Molecular Representations and Molecular Dynamics Simulations. Energy Fuels 2015, 29, 4783–4794. [Google Scholar] [CrossRef]
- Qiao, P.; Harbottle, D.; Tchoukov, P.; Wang, X.; Xu, Z. Asphaltene Subfractions Responsible for Stabilizing Water-in-Crude Oil Emulsions. Part 3. Effect of Solvent Aromaticity. Energy Fuels 2017, 31, 9179–9187. [Google Scholar] [CrossRef]
- Ma, J.; Yang, Y.; Li, X.; Sui, H.; He, L. Mechanisms on the stability and instability of water-in-oil emulsion stabilized by interfacially active asphaltenes: Role of hydrogen bonding reconstructing. Fuel 2021, 297, 120763. [Google Scholar] [CrossRef]
- Zhang, Z.; Kang, N.; Zhou, J.; Li, X.; He, L.; Sui, H. Novel Synthesis of Choline-Based Amino Acid Ionic Liquids and Their Applications for Separating Asphalt from Carbonate Rocks. Nanomaterials 2019, 9, 504. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Li, H.; Sui, H.; He, L.; Li, X. Synthesis and application of hydrophilically-modified Fe3O4 nanoparticles in oil sands separation. RSC Adv. 2018, 8, 15813–15824. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Sui, H.; Ma, J.; Li, X.; Al-Shiaani, N.H.A.; He, L. Fast demulsification of oil–water emulsions at room temperature by functionalized magnetic nanoparticles. Sep. Purif. Technol. 2021, 274, 118967. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, X.; He, L.; Sui, H.; Li, X. Nano-modification of carboxylated polyether for enhanced room temperature demulsification of oil–water emulsions: Synthesis, performance and mechanisms. J. Hazard. Mater. 2022, 439, 129654. [Google Scholar] [CrossRef]
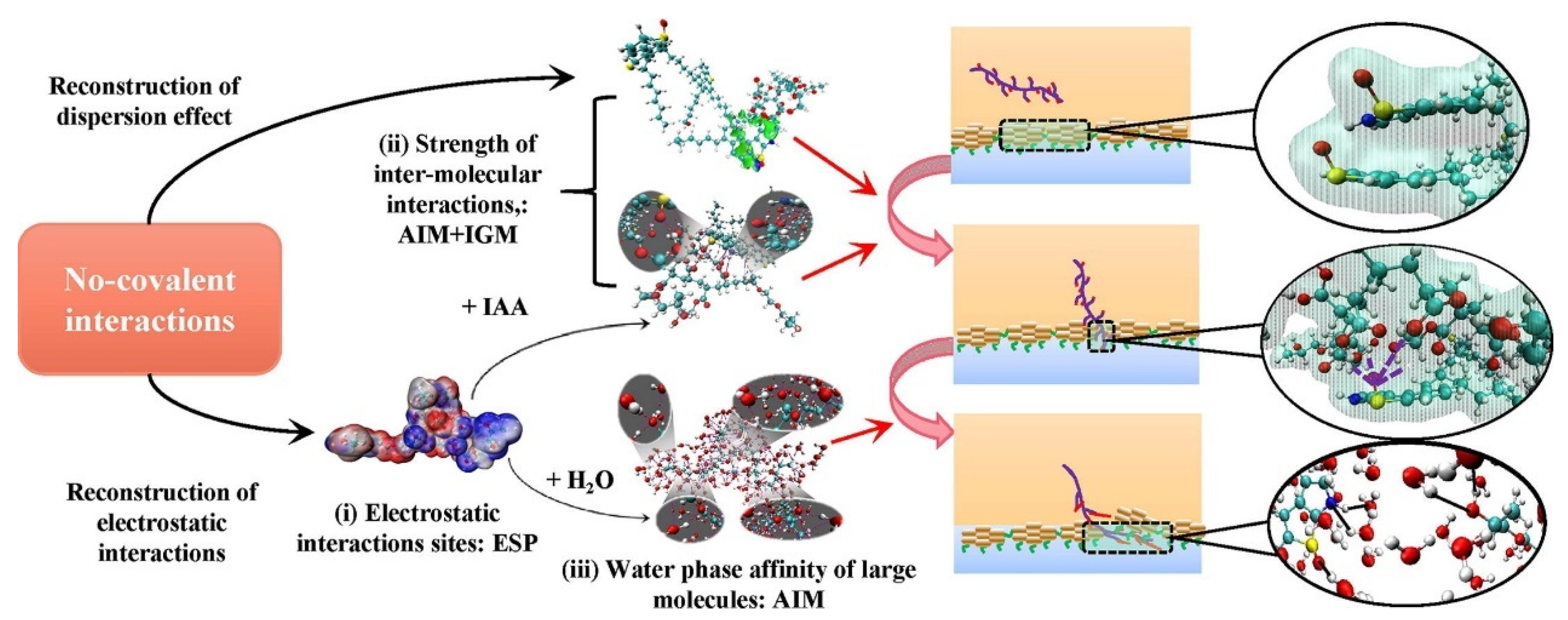
- He, C.; Zhang, X.; He, L.; Sui, H.; Li, X. Revealing the non-covalent interactions between oxygen-containing demulsifiers and interfacially active asphaltenes: A multi-level computational simulation. Fuel 2022, 329, 125375. [Google Scholar] [CrossRef]
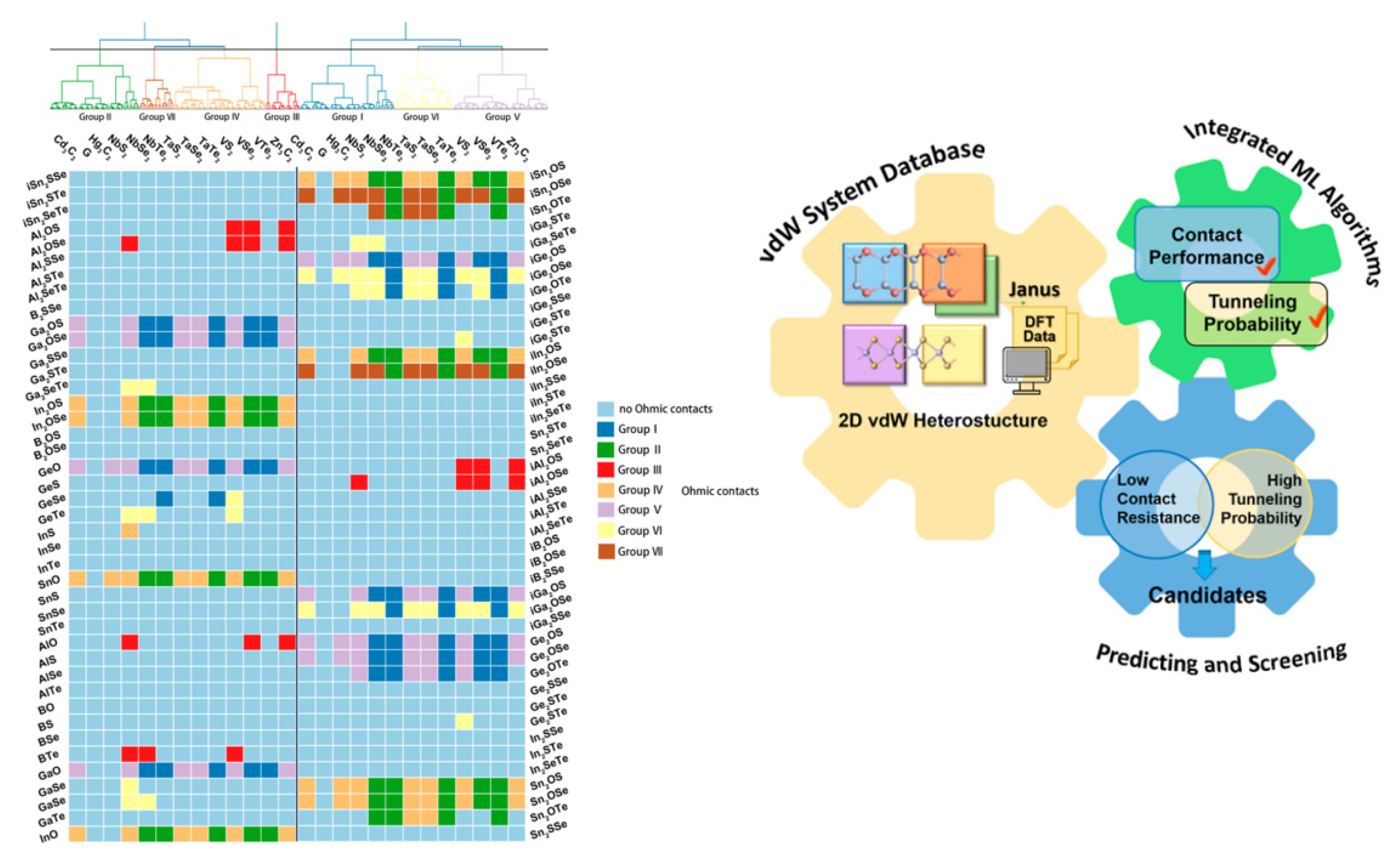
- Chen, A.; Wang, Z.; Zhang, X.; Chen, L.; Hu, X.; Han, Y.; Cai, J.; Zhou, Z.; Li, J. Accelerated Mining of 2D Van der Waals Heterojunctions by Integrating Supervised and Unsupervised Learning. Chem. Mater. 2022, 34, 5571–5583. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, C.; Zhao, R.; Zhu, C.; Yang, C.; Liu, C. Solvent Extraction of Bitumen from Oil Sands. Energy Fuels 2014, 28, 2297–2304. [Google Scholar] [CrossRef]
- Sui, H.; Zhang, J.; Yuan, Y.; He, L.; Bai, Y.; Li, X. Role of binary solvent and ionic liquid in bitumen recovery from oil sands. Can. J. Chem. Eng. 2016, 94, 1191–1196. [Google Scholar] [CrossRef]
- Lin, F.; Stoyanov, S.R.; Xu, Y. Recent Advances in Nonaqueous Extraction of Bitumen from Mineable Oil Sands: A Review. Org. Process Res. Dev. 2017, 21, 492–510. [Google Scholar] [CrossRef]
- Berton, P.; Manouchehr, S.; Wong, K.; Ahmadi, Z.; Abdelfatah, E.; Rogers, R.D.; Bryant, S.L. Ionic Liquids-Based Bitumen Extraction: Enabling Recovery with Environmental Footprint Comparable to Conventional Oil. ACS Sustain. Chem. Eng. 2020, 8, 632–641. [Google Scholar] [CrossRef]
- Yang, Z.; He, C.; Sui, H.; He, L.; Li, X. Recent advances of CO2-responsive materials in separations. J. CO2 Util. 2019, 30, 79–99. [Google Scholar] [CrossRef]
- Durelle, J.; Vanderveen, J.R.; Quan, Y.; Chalifoux, C.B.; Kostin, J.E.; Jessop, P.G. Extending the range of switchable-hydrophilicity solvents. Phys. Chem. Chem. Phys. 2015, 17, 5308–5313. [Google Scholar] [CrossRef]
- Alshamrani, A.K.; Vanderveen, J.R.; Jessop, P.G. A guide to the selection of switchable functional groups for CO2-switchable compounds. Phys. Chem. Chem. Phys. 2016, 18, 19276–19288. [Google Scholar] [CrossRef]
- Jessop, P.G.; Kozycz, L.; Rahami, Z.G.; Schoenmakers, D.; Boyd, A.R.; Wechsler, D.; Holland, A.M. Tertiary amine solvents having switchable hydrophilicity. Green Chem. 2011, 13, 619–623. [Google Scholar] [CrossRef]
- Samorì, C.; Pezzolesi, L.; Barreiro, D.L.; Galletti, P.; Pasteris, A.; Tagliavini, E. Synthesis of new polyethoxylated tertiary amines and their use as Switchable Hydrophilicity Solvents. RSC Adv. 2014, 4, 5999–6008. [Google Scholar] [CrossRef]
- Jessop, P.G.; Phan, L.; Carrier, A.; Robinson, S.; Dürr, C.J.; Harjani, J.R. A solvent having switchable hydrophilicity. Green Chem. 2010, 12, 809–814. [Google Scholar] [CrossRef]
- Jessop, P.G.; Mercer, S.M.; Heldebrant, D.J. CO2-triggered switchable solvents, surfactants, and other materials. Energy Environ. Sci. 2012, 5, 7240–7253. [Google Scholar] [CrossRef]
- Yang, H.-Y.; Lu, W.-J.; Chen, Y.-C.; Chen, K.-T.; Teng, J.-C.; Wan, H.-P. New algal lipid extraction procedure using an amphiphilic amine solvent and ionic liquid. Biomass Bioenergy 2017, 100, 108–115. [Google Scholar] [CrossRef]
- Kohno, Y.; Arai, H.; Ohno, H. Dual stimuli-responsive phase transition of an ionic liquid/water mixture. Chem. Commun. 2011, 47, 4772–4774. [Google Scholar] [CrossRef] [PubMed]
- Dionysiou, D.; Puma, G.L.; Korshin, G.; von Gunten, U. Catalytic processes and new materials and technologies in water/wastewater treatment. Water Res. 2015, 86, 1. [Google Scholar] [CrossRef]
- Kailey, I. Key Performance Indicators Reveal the Impact of Demulsifier Characteristics on Oil Sands Froth Treatment. Energy Fuels 2017, 31, 2636–2642. [Google Scholar] [CrossRef]
- Kailey, I.; Feng, X. Influence of Structural Variations of Demulsifiers on their Performance. Ind. Eng. Chem. Res. 2013, 52, 785–793. [Google Scholar] [CrossRef]
- Ezzat, A.O.; Atta, A.M.; Al-Lohedan, H.A.; Aldalbahi, A. New amphiphilic pyridinium ionic liquids for demulsification of water Arabic heavy crude oil emulsions. J. Mol. Liq. 2020, 312, 113407. [Google Scholar] [CrossRef]
- Zhang, Z.; Ai, G.; Zeng, G.; Yuan, H.; Yang, Y.; Shen, L.; Feng, X.; Ye, F.; Mi, Y. Demulsification of water-in-crude oil emulsion driven by a three-branch structure demulsifier. J. Mol. Liq. 2022, 354, 118822. [Google Scholar] [CrossRef]
- Feng, X.; Xu, Z.; Masliyah, J. Biodegradable Polymer for Demulsification of Water-in-Bitumen Emulsions. Energy Fuels 2009, 23, 451–456. [Google Scholar] [CrossRef]
- Zolfaghari, R.; Fakhru’l-Razi, A.; Abdullah, L.C.; Elnashaie, S.S.E.H.; Pendashteh, A. Demulsification techniques of water-in-oil and oil-in-water emulsions in petroleum industry. Sep. Purif. Technol. 2016, 170, 377–407. [Google Scholar] [CrossRef]
- Ma, S.; Liu, Z.-P. Machine Learning for Atomic Simulation and Activity Prediction in Heterogeneous Catalysis: Current Status and Future. ACS Catal. 2020, 10, 13213–13226. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, G.; Wang, F.; Dong, S.; Chen, Y. Demulsification by amphiphilic dendrimer copolymers. J. Colloid Interface Sci. 2005, 282, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, S.; Hou, J.; Wang, L.; Cepuch, C.; Masliyah, J.; Xu, Z. Effect of Hydroxyl Content and Molecular Weight of Biodegradable Ethylcellulose on Demulsification of Water-in-Diluted Bitumen Emulsions. Ind. Eng. Chem. Res. 2011, 50, 6347–6354. [Google Scholar] [CrossRef]
- Souza, A.V.; Mendes, M.T.; Souza, S.T.S.; Palermo, L.C.M.; Oliveira, P.F.; Mansur, C.R.E. Synthesis of Additives Based on Polyethylenimine Modified with Non-ionic Surfactants for Application in Phase Separation of Water-in-Oil Emulsions. Energy Fuels 2017, 31, 10612–10619. [Google Scholar] [CrossRef]
- Kang, W.; Yin, X.; Yang, H.; Zhao, Y.; Huang, Z.; Hou, X.; Sarsenbekuly, B.; Zhu, Z.; Wang, P.; Zhang, X.; et al. Demulsification performance, behavior and mechanism of different demulsifiers on the light crude oil emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2018, 545, 197–204. [Google Scholar] [CrossRef]
- El-Sharaky, E.-S.A.; El-Tabey, A.E.; Mishrif, M.R. Novel Star Polymeric Nonionic Surfactants as Crude Oil Emulsion Breakers. J. Surfactants Deterg. 2019, 22, 779–793. [Google Scholar] [CrossRef]
- Pradilla, D.; Ramírez, J.; Zanetti, F.; Álvarez, O. Demulsifier Performance and Dehydration Mechanisms in Colombian Heavy Crude Oil Emulsions. Energy Fuels 2017, 31, 10369–10377. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, X.; Ma, J.; Geng, S.; Liu, F.; Yao, M. A Review of Oil–Solid Separation and Oil–Water Separation in Unconventional Heavy Oil Production Process. Int. J. Mol. Sci. 2023, 24, 74. https://doi.org/10.3390/ijms24010074
Xia X, Ma J, Geng S, Liu F, Yao M. A Review of Oil–Solid Separation and Oil–Water Separation in Unconventional Heavy Oil Production Process. International Journal of Molecular Sciences. 2023; 24(1):74. https://doi.org/10.3390/ijms24010074
Chicago/Turabian StyleXia, Xiao, Jun Ma, Shuo Geng, Fei Liu, and Mengqin Yao. 2023. "A Review of Oil–Solid Separation and Oil–Water Separation in Unconventional Heavy Oil Production Process" International Journal of Molecular Sciences 24, no. 1: 74. https://doi.org/10.3390/ijms24010074
APA StyleXia, X., Ma, J., Geng, S., Liu, F., & Yao, M. (2023). A Review of Oil–Solid Separation and Oil–Water Separation in Unconventional Heavy Oil Production Process. International Journal of Molecular Sciences, 24(1), 74. https://doi.org/10.3390/ijms24010074








