Functional Correlates of Striatal Dopamine Transporter Cerebrospinal Fluid Levels in Alzheimer’s Disease: A Preliminary 18F-FDG PET/CT Study
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Subjects’ Enrolment
4.2. CSF Collection and Analysis
4.3. 18F-FDG PET Scanning Protocol
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Villemagne, V.L.; Burnham, S.; Bourgeat, P.; Brown, B.; Ellis, K.A.; Salvado, O.; Szoeke, C.; Macaulay, S.L.; Martins, R.; Maruff, P.; et al. Australian Imaging Biomarkers and Lifestyle (AIBL) Research Group. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: A prospective cohort study. Lancet Neurol. 2013, 12, 357–367. [Google Scholar] [CrossRef] [PubMed]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of Alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer, A.; Stelzmann, R.A.; Schnitzlein, H.N.; Murtagh, F.R. An English translation of Alzheimer’s 1907 paper, “Uber eine eigenartige Erkankung der Hirnrinde”. Clin. Anat. 1995, 8, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.; Revesz, T. The spread of neurodegenerative disease. N. Engl. J. Med. 2012, 366, 2126–2128. [Google Scholar] [CrossRef]
- D′Amelio, M.; Rossini, P.M. Brain excitability and connectivity of neuronal assemblies in Alzheimer’s disease: From animal models to human findings. Prog. Neurobiol. 2012, 99, 42–60. [Google Scholar] [CrossRef]
- Roy, D.S.; Arons, A.; Mitchell, T.I.; Pignatelli, M.; Ryan, T.J.; Tonegawa, S. Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature 2016, 531, 508–512. [Google Scholar] [CrossRef]
- Scheff, S.W.; Price, D.A.; Schmitt, F.A.; Mufson, E.J. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging 2006, 27, 1372–1384. [Google Scholar] [CrossRef]
- Zimmer, E.R.; Parent, M.J.; Souza, D.G.; Leuzy, A.; Lecrux, C.; Kim, H.I.; Gauthier, S.; Pellerin, L.; Hamel, E.; Rosa-Neto, P. [18F]FDG PET signal is driven by astroglial glutamate transport. Nat. Neurosci. 2017, 20, 393–395. [Google Scholar] [CrossRef]
- Frisoni, G.B.; Boccardi, M.; Barkhof, F.; Blennow, K.; Cappa, S.; Chiotis, K.; Démonet, J.F.; Garibotto, V.; Giannakopoulos, P.; Gietl, A.; et al. Strategic roadmap for an early diagnosis of Alzheimer’s disease based on biomarkers. Lancet Neurol. 2017, 16, 661–676. [Google Scholar] [CrossRef]
- Mosconi, L.; Mistur, R.; Switalski, R.; Tsui, W.H.; Glodzik, L.; Li, Y.; Pirraglia, E.; De Santi, S.; Reisberg, B.; Wisniewski, T.; et al. FDG-PET changes in brain glucose metabolism from normal cognition to pathologically verified Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 811–822. [Google Scholar] [CrossRef]
- Alexander, G.E.; Chen, K.; Pietrini, P.; Rapoport, S.I.; Reiman, E.M. Longitudinal PET Evaluation of Cerebral Metabolic Decline in Dementia: A Potential Outcome Measure in Alzheimer’s Disease Treatment Studies. Am. J. Psychiatry 2002, 159, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Minoshima, S.; Frey, K.A.; Koeppe, R.A.; Foster, N.L.; Kuhl, D.E. A diagnostic approach in Alzheimer’s disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J. Nucl. Med. 1995, 36, 1238–1248. [Google Scholar] [PubMed]
- Herholz, K.; Carter, S.F.; Jones, M. Positron emission tomography imaging in dementia. Br. J. Radiol. 2007, 80, S160–S167. [Google Scholar] [CrossRef]
- Henjum, K.; Watne, L.O.; Godang, K.; Halaas, N.B.; Eldholm, R.S.; Blennow, K.; Zetterberg, H.; Saltvedt, I.; Bollerslev, J.; Knapskog, A.B. Cerebrospinal fluid catecholamines in Alzheimer’s disease patients with and without biological disease. Transl. Psychiatry 2022, 12, 151. [Google Scholar] [CrossRef] [PubMed]
- Lopez, O.L.; Wisnieski, S.R.; Becker, J.T.; Boiler, F.; DeKosky, S.T. Extrapyramidal signs in patients with probable Alzheimer disease. Arch. Neurol. 1997, 54, 969–975. [Google Scholar] [CrossRef]
- Storga, D.; Vrecko, K.; Birkmayer, J.G.; Reibnegger, G. Monoaminergic neurotransmitters, their precursors and metabolites in brains of Alzheimer patients. Neurosci. Lett. 1996, 203, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Trillo, L.; Das, D.; Hsieh, W.; Medina, B.; Moghadam, S.; Lin, B.; Dang, V.; Sanchez, M.M.; De Miguel, Z.; Ashford, J.W.; et al. Ascending monoaminergic systems alterations in Alzheimer’s disease. Translating basic science into clinical care. Neurosci. Biobehav. Rev. 2013, 37, 1363–1379. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Di Lorenzo, F.; Bonnì, S.; Giacobbe, V.; Bozzali, M.; Caltagirone, C.; Martorana, A. Dopaminergic modulation of cortical plasticity in Alzheimer’s disease patients. Neuropsychopharmacology 2014, 39, 2654–2661. [Google Scholar] [CrossRef]
- Palmer, K.; Di Iulio, F.; Varsi, A.E.; Gianni, W.; Sancesario, G.; Caltagirone, C.; Spalletta, G. Neuropsychiatric predictors of progression from amnestic-mild cognitive impairment to Alzheimer’s disease: The role of depression and apathy. J. Alzheimers Dis. 2010, 20, 175–183. [Google Scholar] [CrossRef]
- Sala, A.; Caminiti, S.P.; Presotto, L.; Pilotto, A.; Liguori, C.; Chiaravalloti, A.; Garibotto, V.; Frisoni, G.B.; D’Amelio, M.; Paghera, B.; et al. In vivo human molecular neuroimaging of dopaminergic vulnerability along the Alzheimer’s disease phases. Alzheimers Res. Ther. 2021, 13, 187. [Google Scholar] [CrossRef]
- Martorana, A.; Koch, G. Is dopamine involved in Alzheimer’s disease? Front. Aging Neurosci. 2014, 6, 252. [Google Scholar] [CrossRef]
- Itoh, A.; Nitta, A.; Nadai, M.; Nishimura, K.; Hirose, M.; Hasegawa, T.; Nabeshima, T. Dysfunction of cholinergic and dopaminergic neuronal systems in beta-amyloid protein—infused rats. J. Neurochem. 1996, 66, 1113–1117. [Google Scholar] [CrossRef]
- Cheramy, A.; Leviel, V.; Glowinski, J. Dendritic release of dopamine in the substantia nigra. Nature 1981, 289, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Nissbrandt, H.; Sundström, E.; Jonsson, G.; Hjorth, S.; Carlsson, A. Synthesis and release of dopamine in rat brain: Comparison between substantia nigra pars compacts, pars reticulata, and striatum. J. Neurochem. 1989, 52, 1170–1182. [Google Scholar] [CrossRef] [PubMed]
- Uhl, G.R. Dopamine transporter: Basic science and human variation of a key molecule for dopaminergic function, locomotion, and parkinsonism. Mov. Disord. 2003, 18 Suppl. 7, S71–S80. [Google Scholar] [CrossRef] [PubMed]
- Sulzer, D.; Cragg, S.J.; Rice, M.E. Striatal dopamine neurotransmission: Regulation of release and uptake. Basal Ganglia 2016, 6, 123–148. [Google Scholar] [CrossRef] [PubMed]
- Palermo, G.; Giannoni, S.; Bellini, G.; Siciliano, G.; Ceravolo, R. Dopamine Transporter Imaging, Current Status of a Potential Biomarker: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 11234. [Google Scholar] [CrossRef]
- Haber, S.N.; Knutson, B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology 2010, 35, 4–26. [Google Scholar] [CrossRef]
- Giros, B.; Jaber, M.; Jones, S.R.; Wightman, R.M.; Caron, M.G. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature 1996, 379, 606–612. [Google Scholar] [CrossRef]
- Ma, S.Y.; Ciliax, B.J.; Stebbins, G.; Jaffar, S.; Joyce, J.N.; Cochran, E.J.; Kordower, J.H.; Mash, D.C.; Levey, A.I.; Mufson, E.J. Dopamine transporter-immunoreactive neurons decrease with age in the human substantia nigra. J. Comp. Neurol. 1999, 409, 25–37. [Google Scholar] [CrossRef]
- Murray, A.M.; Weihmueller, F.B.; Marshall, J.F.; Hurtig, H.I.; Gottleib, G.L.; Joyce, J.N. Damage to dopamine systems differs between Parkinson’s disease and Alzheimer’s disease with parkinsonism. Ann. Neurol. 1995, 37, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Joyce, J.N.; Meador-Woodruff, J.H. Linking the family of D2 receptors to neuronal circuits in human brain: Insights into schizophrenia. Neuropsychopharmacology 1997, 16, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Rinne, J.O.; Sahlberg, N.; Ruottinen, H.; Någren, K.; Lehikoinen, P. Striatal uptake of the dopamine reuptake ligand [11C]beta-CFT is reduced in Alzheimer’s disease assessed by positron emission tomography. Neurology 1998, 50, 152–156. [Google Scholar] [CrossRef]
- Lammel, S.; Ion, D.I.; Roeper, J.; Malenka, R.C. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron 2011, 70, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Bolam, J.P.; Pissadaki, E.K. Living on the edge with too many mouths to feed: Why dopamine neurons die. Mov. Disord. 2012, 27, 1478–1483. [Google Scholar] [CrossRef]
- Hodge, G.K.; Butcher, L.L. Pars compacta of the substantia nigra modulates motor activity but is not involved importantly in regulating food and water intake. Naunyn Schmiedebergs Arch. Pharmacol. 1980, 313, 51–67. [Google Scholar] [CrossRef]
- Sonne, J.; Reddy, V.; Beato, M.R. Neuroanatomy, Substantia Nigra. 2021 Oct 30. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Morris, J.C.; Drazner, M.; Fulling, K.; Grant, E.A.; Goldring, J. Clinical and pathological aspects of parkinsonism in Alzheimer’s disease. A role for extranigral factors? Arch. Neurol. 1989, 46, 651–657. [Google Scholar] [CrossRef]
- Kazee, A.M.; Cox, C.; Richfield, E.K. Substantia nigra lesions in Alzheimer disease and normal aging. Alzheimer Dis. Assoc. Disord. 1995, 9, 61–67. [Google Scholar] [CrossRef]
- Liu, Y.; Stern, Y.; Chun, M.R.; Jacobs, D.M.; Yau, P.; Goldman, J.E. Pathological correlates of extrapyramidal signs in Alzheimer’s disease. Ann. Neurol. 1997, 41, 368–374. [Google Scholar] [CrossRef]
- Joyce, J.N.; Smutzer, G.; Whitty, C.J.; Myers, A.; Bannon, M.J. Differential modification of dopamine transporter and tyrosine hydroxylase mRNAs in midbrain of subjects with Parkinson’s, Alzheimer’s with parkinsonism, and Alzheimer’s disease. Mov. Disord. 1997, 12, 885–897. [Google Scholar] [CrossRef]
- Gibb, W.R.; Mountjoy, C.Q.; Mann, D.M.; Lees, A.J. The substantia nigra and ventral tegmental area in Alzheimer’s disease and Down’s syndrome. J. Neurol. Neurosurg. Psychiatry 1989, 52, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Mann, D.M.; Yates, P.O.; Marcyniuk, B. Monoaminergic neurotransmitter systems in presenile Alzheimer’s disease and in senile dementia of Alzheimer type. Clin. Neuropathol. 1984, 3, 199–205. [Google Scholar] [PubMed]
- Attems, J.; Quass, M.; Jellinger, K.A. Tau and alpha-synuclein brainstem pathology in Alzheimer disease: Relation with extrapyramidal signs. Acta Neuropathol. 2007, 113, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.M.; Galvin, J.E.; Roe, C.M.; Morris, J.C.; McKeel, D.W. The pathology of the substantia nigra in Alzheimer disease with extrapyramidal signs. Neurology 2005, 64, 1397–1403. [Google Scholar] [CrossRef]
- Haber, S.N.; Fudge, J.L. The primate substantia nigra and VTA: Integrative circuitry and function. Crit. Rev. Neurobiol. 1997, 11, 323–342. [Google Scholar] [CrossRef]
- Rudelli, R.D.; Ambler, M.W.; Wisniewski, H.M. Morphology and distribution of Alzheimer neuritic (senile) and amyloid plaques in striatum and diencephalon. Acta Neuropathol. 1984, 64, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Alzheimer’s disease: Striatal amyloid deposits and neurofibrillary changes. J. Neuropathol. Exp. Neurol. 1990, 49, 215–224. [Google Scholar] [CrossRef]
- Selden, N.; Mesulam, M.M.; Geula, C. Human striatum: The distribution of neurofibrillary tangles in Alzheimer’s disease. Brain Res. 1994, 648, 327–331. [Google Scholar] [CrossRef]
- Roostaei, T.; Nazeri, A.; Felsky, D.; De Jager, P.L.; Schneider, J.A.; Pollock, B.G.; Bennett, D.A.; Voineskos, A.N. Alzheimer’s Disease Neuroimaging Initiative (ADNI). Genome-wide interaction study of brain beta-amyloid burden and cognitive impairment in Alzheimer’s disease. Mol. Psychiatry 2017, 22, 287–295. [Google Scholar] [CrossRef]
- Ditter, S.M.; Mirra, S.S. Neuropathologic and clinical features of Parkinson’s disease in Alzheimer’s disease patients. Neurology 1987, 37, 754–760. [Google Scholar] [CrossRef]
- Sibson, N.R.; Dhankhar, A.; Mason, G.F.; Rothman, D.L.; Behar, K.L.; Shulman, R.G. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc. Natl. Acad. Sci. USA 1998, 95, 316–321. [Google Scholar] [CrossRef]
- Sokoloff, L.; Reivich, M.; Kennedy, C.; Rosiers, M.D.; Patlak, C.S.; Pettigrew, K.E.A.; Sakurada, O.; Shinohara, M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: Theory, procedure, and normal values in the conscious and anesthetized albino rat. J. Neurochem. 1977, 28, 897–916. [Google Scholar] [CrossRef] [PubMed]
- Laforce, R., Jr.; Soucy, J.P.; Sellami, L.; Dallaire-Théroux, C.; Brunet, F.; Bergeron, D.; Miller, B.L.; Ossenkoppele, R. Molecular imaging in dementia: Past, present, and future. Alzheimers Dement. 2018, 14, 1522–1552. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Knopman, D.S.; Gunter, J.L.; Graff-Radford, J.; Vemuri, P.; Boeve, B.F.; Petersen, R.C.; Weiner, M.W.; Jack, C.R., Jr. Alzheimer’s Disease Neuroimaging Initiative. Cascading network failure across the Alzheimer’s disease spectrum. Brain 2016, 139 Pt 2, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E. The brain’s default mode network. Annu. Rev. Neurosci. 2015, 38, 433–447. [Google Scholar] [CrossRef]
- Buckner, R.L.; Snyder, A.Z.; Shannon, B.J.; LaRossa, G.; Sachs, R.; Fotenos, A.F.; Sheline, Y.I.; Klunk, W.E.; Mathis, C.A.; Morris, J.C.; et al. Molecular, structural, and functional characterization of Alzheimer’s disease: Evidence for a relationship between default activity, amyloid, and memory. J. Neurosci. 2005, 25, 7709–7717. [Google Scholar] [CrossRef]
- Herholz, K. Guidance for reading FDG PET scans in dementia patients. Q. J. Nucl. Med. Mol. Imaging 2014, 58, 332–343. [Google Scholar]
- Kato, T.; Inui, Y.; Nakamura, A.; Ito, K. Brain fluorodeoxyglucose (FDG) PET in dementia. Aging Res. Rev. 2016, 30, 73–84. [Google Scholar] [CrossRef]
- Foster, N.L.; Heidebrink, J.L.; Clark, C.M.; Jagust, W.J.; Arnold, S.E.; Barbas, N.R.; DeCarli, C.S.; Scott Turner, R.; Koeppe, R.A.; Higdon, R.; et al. FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer’s disease. Brain 2007, 130 Pt 10, 2616–2635. [Google Scholar] [CrossRef]
- Brown, R.K.; Bohnen, N.I.; Wong, K.K.; Minoshima, S.; Frey, K.A. Brain PET in suspected dementia: Patterns of altered FDG metabolism. Radiographics 2014, 34, 684–701. [Google Scholar] [CrossRef] [PubMed]
- Silverman, D.H.; Small, G.W.; Chang, C.Y.; Lu, C.S.; de Aburto, M.A.K.; Chen, W.; Czernin, J.; Rapoport, S.I.; Pietrini, P.; Alexander, G.E.; et al. Positron emission tomography in evaluation of dementia: Regional brain metabolism and long-term outcome. JAMA 2001, 286, 2120–2127. [Google Scholar] [CrossRef]
- Minoshima, S.; Giordani, B.; Berent, S.; Frey, K.A.; Foster, N.L.; Kuhl, D.E. Metabolic reduction in the posterior cingulate cortex in very early Alzheimer’s disease. Ann. Neurol. 1997, 42, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Cerami, C.; Della Rosa, P.A.; Magnani, G.; Santangelo, R.; Marcone, A.; Cappa, S.F.; Perani, D. Brain metabolic maps in Mild Cognitive Impairment predict heterogeneity of progression to dementia. Neuroimage Clin. 2014, 7, 187–194. [Google Scholar] [CrossRef]
- Jagust, W.; Gitcho, A.; Sun, F.; Kuczynski, B.; Mungas, D.; Haan, M. Brain imaging evidence of preclinical Alzheimer’s disease in normal aging. Ann. Neurol. 2006, 59, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Salmon, E.; Lekeu, F.; Garraux, G.; Guillaume, B.; Magis, D.; Luxen, A.; Moonen, G.; Collette, F. Metabolic correlates of clinical heterogeneity in questionable Alzheimer’s disease. Neurobiol. Aging 2008, 29, 1823–1829. [Google Scholar] [CrossRef]
- Drzezga, A.; Lautenschlager, N.; Siebner, H.; Riemenschneider, M.; Willoch, F.; Minoshima, S.; Schwaiger, M.; Kurz, A. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer’s disease: A PET follow-up study. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 1104–1113. [Google Scholar] [CrossRef]
- Silverman, D.H. Brain 18F-FDG PET in the diagnosis of neurodegenerative dementias: Comparison with perfusion SPECT and with clinical evaluations lacking nuclear imaging. J. Nucl. Med. 2004, 45, 594–607. [Google Scholar]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Ransohoff, R.M. How neuroinflammation contributes to neurodegeneration. Science 2016, 353, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Allen, N.J.; Lyons, D.A. Glia as architects of central nervous system formation and function. Science 2018, 362, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Luzi, F.; Savickas, V.; Taddei, C.; Hader, S.; Singh, N.; Gee, A.D.; Bongarzone, S. Radiolabeling of [11C]FPS-ZM1, a receptor for advanced glycation end products-targeting positron emission tomography radiotracer, using a [11C]CO2-to-[11C]CO chemical conversion. Future Med. Chem. 2020, 12, 511–521. [Google Scholar] [CrossRef]
- Xiang, X.; Wind, K.; Wiedemann, T.; Blume, T.; Shi, Y.; Briel, N.; Beyer, L.; Biechele, G.; Eckenweber, F.; Zatcepin, A.; et al. Microglial activation states drive glucose uptake and FDG-PET alterations in neurodegenerative diseases. Sci. Transl. Med. 2021, 13, eabe5640. [Google Scholar] [CrossRef]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Mazziotta, J.C.; Toga, A.W.; Evans, A.; Fox, P.; Lancaster, J. A probabilistic atlas of the human brain: Theory and rationale for its development. The International Consortium for Brain Mapping (ICBM). Neuroimage 1995, 2, 89–101. [Google Scholar] [CrossRef]
- Mazziotta, J.; Toga, A.; Evans, A.; Fox, P.; Lancaster, J.; Zilles, K.; Woods, R.; Paus, T.; Simpson, G.; Pike, B.; et al. A four-dimensional probabilistic atlas of the human brain. J. Am. Med. Inform. Assoc. 2001, 8, 401–430. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.M.; Wolford, G.L.; Miller, M.B. The principled control of false positives in neuroimaging. Soc. Cogn. Affect. Neurosci. 2009, 4, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Liguori, C.; Chiaravalloti, A.; Sancesario, G.; Stefani, A.; Sancesario, G.M.; Mercuri, N.B.; Schillaci, O.; Pierantozzi, M. Cerebrospinal fluid lactate levels and brain [18F]FDG PET hypometabolism within the default mode network in Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 2040–2049. [Google Scholar] [CrossRef]
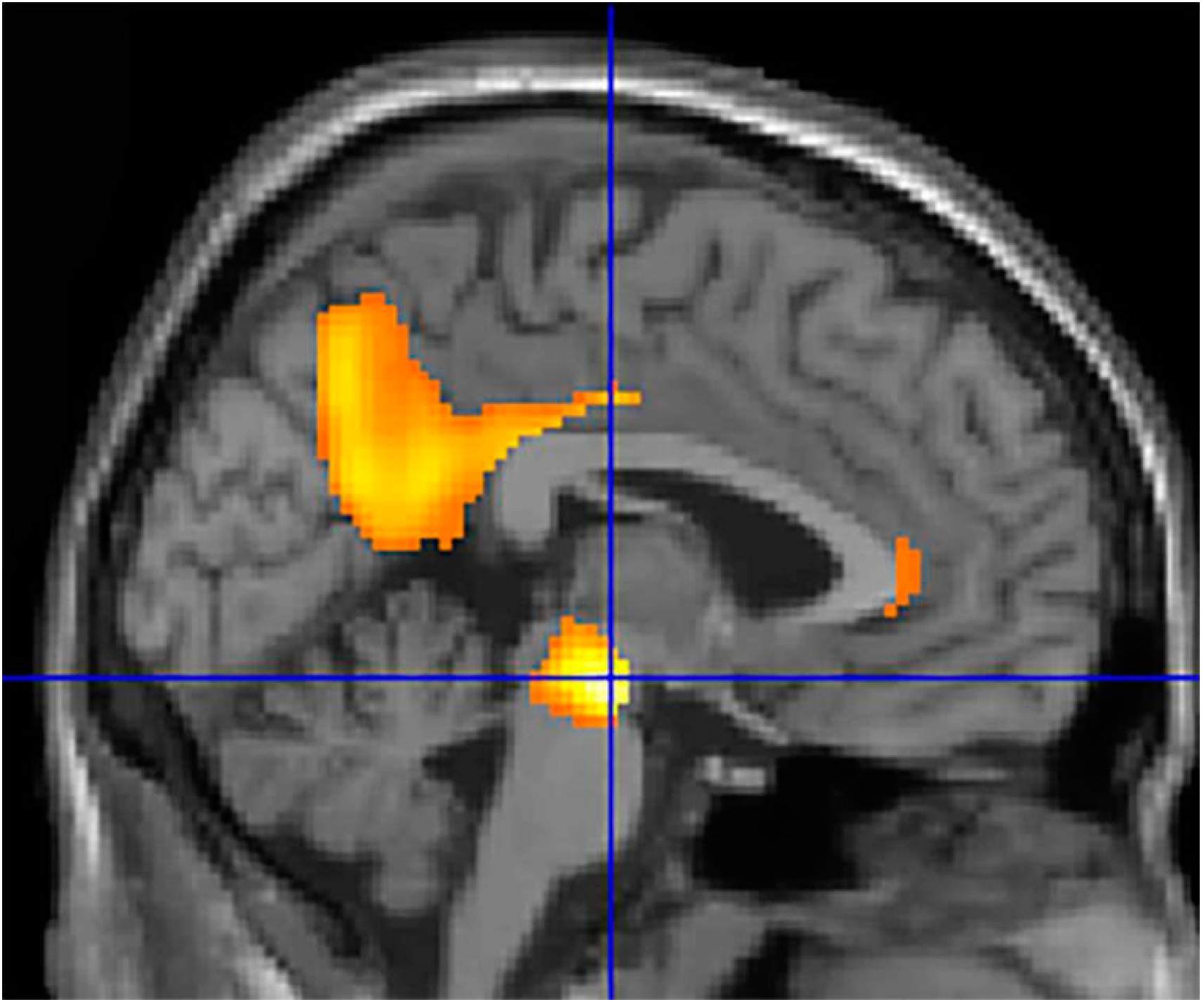
| Analysis | Cluster Level | Voxel Level | ||||||
|---|---|---|---|---|---|---|---|---|
| Cluster P | Cluster | Cluster | Cortical | Z Score of | Talairach | Cortical Region | BA | |
| (FWE-Corr) | p(FDR-Corr) | Extent | Region | Maximum | Coordinates | |||
| Positive correlation | 0.000 | 0.000 | 21,570 | Mid Brain | 3.65 | −6, −16, −14 | Substantia Nigra | |
| R Limbic | 3.43 | 16, −36, 40 | Posterior cingulate | 31 | ||||
| Study Cohort (n = 28) | |
|---|---|
| Age (yo) | 70.64 ± 6.16 |
| Sex (M:F) | 13:15 |
| CSF Aβ42 (pg/mL) | 379.07 ± 98.02 |
| CSF p-tau (pg/mL) | 68.29 ± 27–28 |
| CSF t-tau (pg/mL) | 501.61 ± 251.82 |
| CSF DAT (pg/mL) | 2.86 ± 1.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camedda, R.; Bonomi, C.G.; Di Donna, M.G.; Chiaravalloti, A. Functional Correlates of Striatal Dopamine Transporter Cerebrospinal Fluid Levels in Alzheimer’s Disease: A Preliminary 18F-FDG PET/CT Study. Int. J. Mol. Sci. 2023, 24, 751. https://doi.org/10.3390/ijms24010751
Camedda R, Bonomi CG, Di Donna MG, Chiaravalloti A. Functional Correlates of Striatal Dopamine Transporter Cerebrospinal Fluid Levels in Alzheimer’s Disease: A Preliminary 18F-FDG PET/CT Study. International Journal of Molecular Sciences. 2023; 24(1):751. https://doi.org/10.3390/ijms24010751
Chicago/Turabian StyleCamedda, Riccardo, Chiara Giuseppina Bonomi, Martina Gaia Di Donna, and Agostino Chiaravalloti. 2023. "Functional Correlates of Striatal Dopamine Transporter Cerebrospinal Fluid Levels in Alzheimer’s Disease: A Preliminary 18F-FDG PET/CT Study" International Journal of Molecular Sciences 24, no. 1: 751. https://doi.org/10.3390/ijms24010751
APA StyleCamedda, R., Bonomi, C. G., Di Donna, M. G., & Chiaravalloti, A. (2023). Functional Correlates of Striatal Dopamine Transporter Cerebrospinal Fluid Levels in Alzheimer’s Disease: A Preliminary 18F-FDG PET/CT Study. International Journal of Molecular Sciences, 24(1), 751. https://doi.org/10.3390/ijms24010751







