Non-Noble-Metal Mono and Bimetallic Composites for Efficient Electrocatalysis of Phosphine Oxide and Acetylene C-H/P-H Coupling under Mild Conditions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Nanocomposite Catalytic Systems
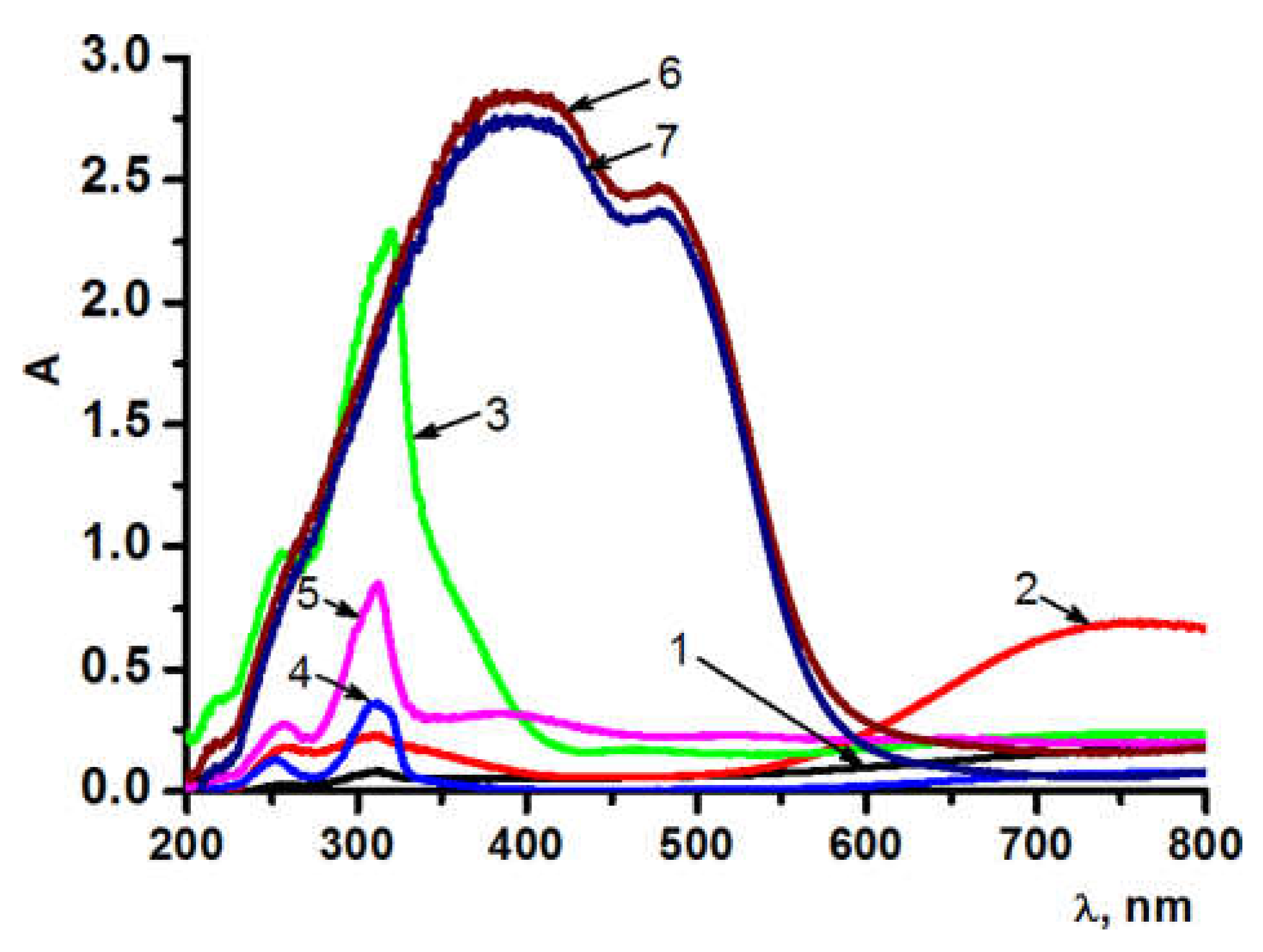
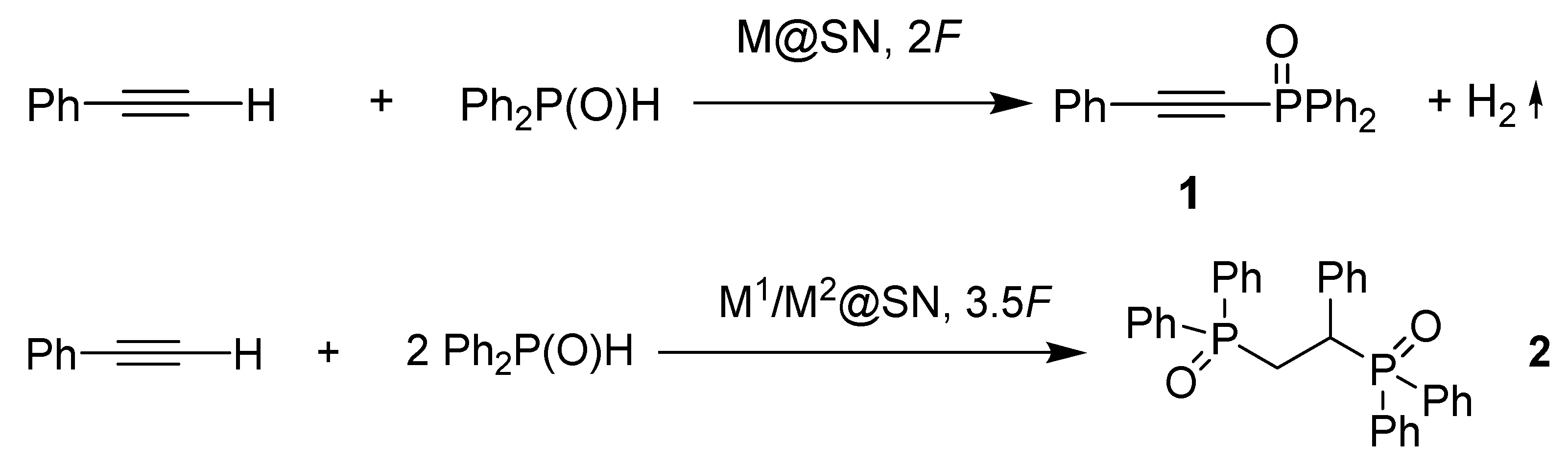
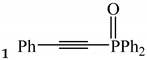
2.2. Electrocatalytic Phosphorylation of Phenylacetylene
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sheldon, R.A. Fundamentals of green chemistry: Efficiency in reaction design. Chem. Soc. Rev. 2012, 41, 1437–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huo, L.-Q.; Shi, L.-L.; Fu, J.-K. Iron–Copper Dual Catalysis Enabling C-C and C-X (X = N, B, P, S, Sn) Bond Formation. Eur. J. Org. Chem. 2022, 2022, e202200454. [Google Scholar] [CrossRef]
- Gandeepan, P.; Müller, T.; Zell, D.; Cera, G.; Warratz, S.; Ackermann, L. 3d Transition Metals for C–H Activation. Chem. Rev. 2019, 119, 2192–2452. [Google Scholar] [CrossRef] [PubMed]
- Nishad, R.C.; Kumar, S.; Rit, A. Hetero- and Homobimetallic Complexes Bridged by a Bis(NHC) Ligand: Synthesis via Selective Sequential Metalation and Catalytic Applications in Tandem Organic Transformations. Organometallics 2021, 40, 915–926. [Google Scholar] [CrossRef]
- Liu, X.; Wang, D.; Li, Y. Synthesis and catalytic properties of bimetallic nanomaterials with various architectures. Nano Today 2012, 7, 448–466. [Google Scholar] [CrossRef]
- Pérez-Temprano, M.H.; Casares, J.A.; Espinet, P. Bimetallic Catalysis using Transition and Group 11 Metals: An Emerging Tool for C-C Coupling and Other Reactions. Chem. Eur. J. 2012, 18, 1864–1884. [Google Scholar] [CrossRef]
- Kim, U.B.; Jung, D.J.; Jeon, H.J.; Rathwell, K.; Lee, S.-g. Synergistic Dual Transition Metal Catalysis. Chem. Rev. 2020, 120, 13382–13433. [Google Scholar] [CrossRef]
- Lorion, M.M.; Maindan, K.; Kapdi, A.R.; Ackermann, L. Heteromultimetallic catalysis for sustainable organic syntheses. Chem. Soc. Rev. 2017, 46, 7399–7420. [Google Scholar] [CrossRef]
- Loza, K.; Heggen, M.; Epple, M. Synthesis, Structure, Properties, and Applications of Bimetallic Nanoparticles of Noble Metals. Adv. Funct. Mater. 2020, 30, 1909260. [Google Scholar] [CrossRef] [Green Version]
- Kalidindi, S.B.; Jagirdar, B.R. Nanocatalysis and Prospects of Green Chemistry. ChemSusChem 2012, 5, 65–75. [Google Scholar] [CrossRef]
- Dang-Bao, T.; Pla, D.; Favier, I.; Gómez, M. Bimetallic Nanoparticles in Alternative Solvents for Catalytic Purposes. Catalysts 2017, 7, 207. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Muthurasu, A. Metal-organic framework–assisted bimetallic Ni@Cu microsphere for enzyme-free electrochemical sensing of glucose. J. Electroanal. Chem. 2020, 873, 114356. [Google Scholar] [CrossRef]
- Bochkova, O.; Khrizanforov, M.; Gubaidullin, A.; Gerasimova, T.; Nizameev, I.; Kholin, K.; Laskin, A.; Budnikova, Y.; Sinyashin, O.; Mustafina, A. Synthetic tuning of CoII-doped silica nanoarchitecture towards electro-chemical sensing ability. Nanomaterials 2020, 10, 1338. [Google Scholar] [CrossRef] [PubMed]
- Dudkina, Y.B.; Gryaznova, T.V.; Osin, Y.N.; Salnikov, V.V.; Davydov, N.D.; Fedorenko, S.V.; Mustafina, A.R.; Vicic, D.A.; Sinyashin, O.G.; Budnikova, Y.H. Nanoheterogeneous Catalysis in Electrochemically Induced Olefin Perfluoroalkylation. Dalton Trans. 2015, 44, 8833–8838. [Google Scholar] [CrossRef]
- Fedorenko, S.; Jilkin, M.; Nastapova, N.; Yanilkin, V.; Bochkova, O.; Buriliov, V.; Nizameev, I.; Nasretdinova, G.; Kadirov, M.; Mustafina, A.; et al. Surface decoration of silica nanoparticles by Pd(0) deposition for catalytic application in aqueous solutions. Colloids Surf. A 2015, 486, 185–191. [Google Scholar] [CrossRef]
- Khrizanforov, M.; Fedorenko, S.V.; Strekalova, S.O.; Kholin, K.V.; Mustafina, A.; Zhilkin, M.Y.; Khrizanforova, V.; Osin, Y.N.; Salnikov, V.V.; Gryaznova, T.; et al. Ni (III) Complex Stabilized by Silica Nanoparticles as an Efficient Nanoheterogeneous Catalyst for Oxidative C-H Fluoroalkylation. Dalton Trans. 2016, 45, 11976–11982. [Google Scholar] [CrossRef] [Green Version]
- Budnikova, Y. Group VIII Base Metal Nanocatalysts with Encapsulated Structures as an Area of Green Chemistry. Pet. Chem. 2017, 57, 1259–1276. [Google Scholar] [CrossRef]
- Fedorenko, S.V.; Jilkin, M.E.; Gryaznova, T.V.; Iurko, E.O.; Bochkova, O.D.; Mukhametshina, A.R.; Nizameev, I.R.; Kholin, K.V.; Mazzaro, R.; Morandi, V.; et al. Silica Nanospheres Coated by Ultrasmall Ag0 Nanoparticles for Oxidative Catalytic Application. Colloid Interface Sci. Commun. 2017, 21, 1–5. [Google Scholar] [CrossRef]
- Khrizanforov, M.N.; Fedorenko, S.V.; Mustafina, A.R.; Kholin, K.V.; Nizameev, I.R.; Strekalova, S.O.; Grinenko, V.V.; Gryaznova, T.V.; Zairov, R.R.; Mazzaro, R.; et al. Silica-Supported Silver Nanoparticles as an Efficient Catalyst for Aromatic C-H Alkylation and Fluoroalkylation. Dalton Trans. 2018, 47, 9608–9616. [Google Scholar] [CrossRef]
- Budnikova, Y.H.; Bochkova, O.; Khrizanforov, M.; Nizameev, I.; Kholin, K.; Gryaznova, T.; Laskin, A.; Dudkina, Y.; Strekalova, S.; Fedorenko, S.; et al. Selective C(sp2)-H Amination Catalyzed by High-Valent Cobalt(III)/(IV)-bpy Com-plex Immobilized on Silica Nanoparticles. Chemcatchem 2019, 11, 5615–5624. [Google Scholar] [CrossRef]
- Khrizanforov, M.N.; Fedorenko, S.V.; Mustafina, A.R.; Khrizanforova, V.V.; Kholin, K.V.; Nizameev, I.R.; Gryaznova, T.V.; Grinenko, V.V.; Budnikova, Y.H. Nano-architecture of silica nanoparticles as a tool to tune both electrochemical and catalytic behavior of NiII@SiO2. RSC Adv. 2019, 9, 22627–22635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, A.W.; Li, R.; Hua, X.; Zhang, R. Methanol electrooxidation on glassy carbon electrode modified with bimetallic Ni(II)Co(II)salen complexes encapsulated in mesoporous zeolite. Electrochim. Acta 2015, 163, 48–56. [Google Scholar] [CrossRef]
- Sun, H.; Lin, L.; Hua, W.; Xie, X.; Mu, Q.; Feng, K.; Zhong, J.; Lyu, F.; Deng, Z.; Peng, Y. Atomically dispersed Co–Cu alloy reconstructed from metal-organic framework to promote electrochemical CO2 methanation. Nano Res. 2022. [Google Scholar] [CrossRef]
- He, C.; Wang, S.; Jiang, X.; Hu, Q.; Yang, H.; He, C. Bimetallic Cobalt–Copper Nanoparticle-Decorated Hollow Carbon Nanofibers for Efficient CO2 Electroreduction. Front. Chem. 2022, 10, 904241. [Google Scholar] [CrossRef] [PubMed]
- Lecerclé, D.; Sawicki, M.; Taran, F. Phosphine-Catalyzed α-P-Addition on Activated Alkynes: A New Route to P–C–P Backbones. Org. Lett. 2006, 8, 4283–4285. [Google Scholar] [CrossRef]
- Tang, S.; Liu, Y.; Gao, X.; Wang, P.; Huang, P.; Lei, A. Multi-Metal-Catalyzed Oxidative Radical Alkynylation with Terminal Alkynes: A New Strategy for C(sp3)–C(sp) Bond Formation. J. Am. Chem. Soc. 2018, 140, 6006–6013. [Google Scholar] [CrossRef]
- Jiang, W.-Q.; Allan, G.; Chen, X.; Fiordeliso, J.J.; Linton, O.; Tannenbaum, P.; Xu, J.; Zhu, P.-F.; Gunnet, J.; Demarest, K.; et al. Novel phosphorus-containing 17β-side chain mifepristone analogues as progesterone receptor antagonists. Steroids 2006, 71, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Castoldi, L.; Rajkiewicz, A.A.; Olofsson, B. Transition metal-free and regioselective vinylation of phosphine oxides and H-phosphinates with VBX reagents. Chem. Commun. 2020, 56, 14389–14392. [Google Scholar] [CrossRef]
- Guo, H.-M.; Zhou, Q.-Q.; Jiang, X.; Shi, D.-Q.; Xiao, W.-J. Catalyst- and Oxidant-Free Desulfonative C-P Couplings for the Synthesis of Phosphine Oxides and Phosphonates. Adv. Synth. Catal. 2017, 359, 4141–4146. [Google Scholar] [CrossRef]
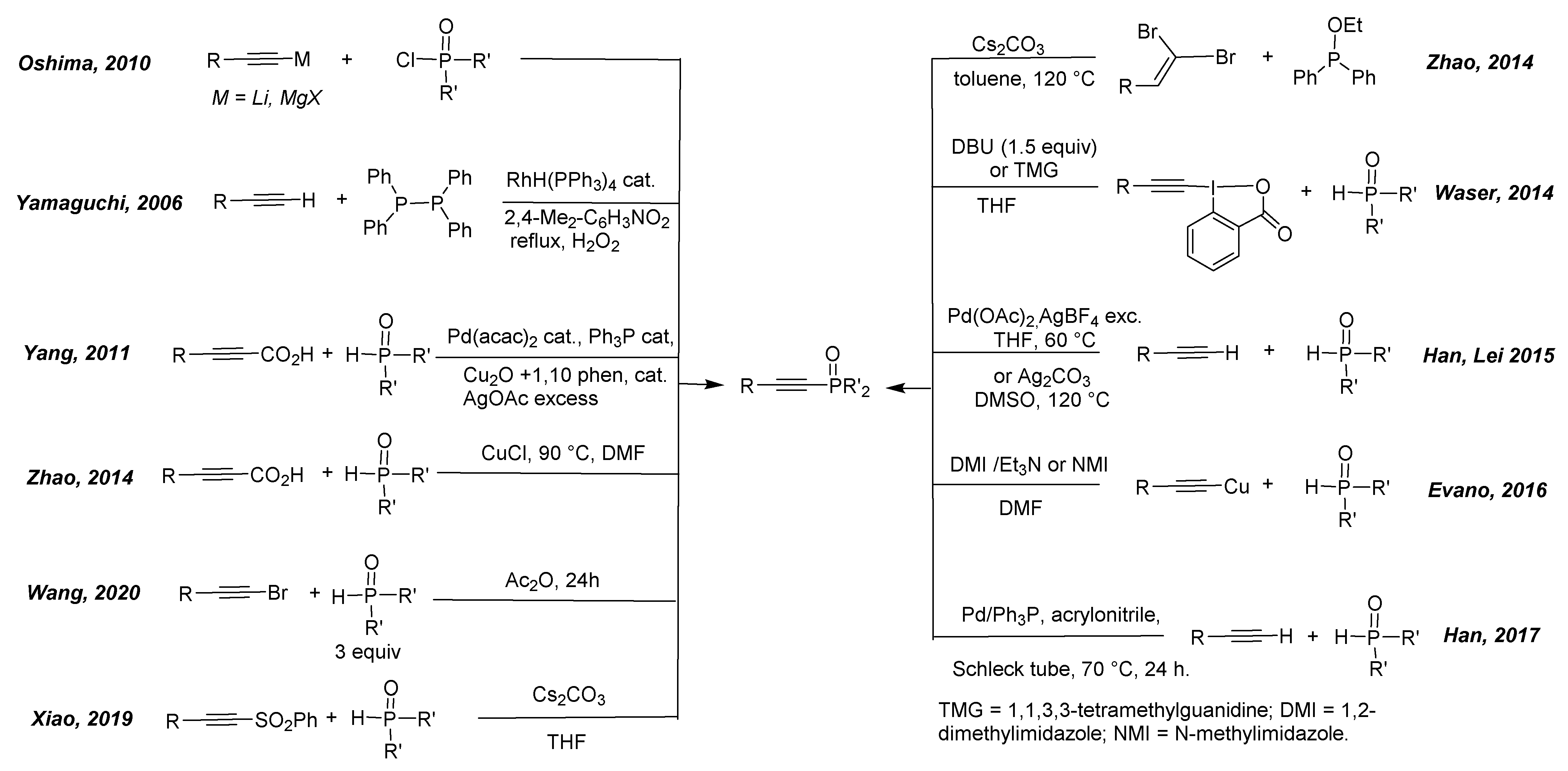
- Afanasiev, V.V.; Beletskaya, I.P.; Kazankova, M.A.; Efimova, I.V.; Antipin, M.U. A Convenient and Direct Route to Phosphinoalkynes via Copper-Catalyzed Cross-Coupling of Terminal Alkynes with Chlorophosphanes. Synthesis 2003, 18, 2835–2838. [Google Scholar] [CrossRef]
- Liu, L.; Wu, Y.; Wang, Z.; Zhu, J.; Zhao, Y. Mechanistic Insight into the Copper-Catalyzed Phosphorylation of Terminal Alkynes: A Combined Theoretical and Experimental Study. J. Org. Chem. 2014, 79, 6816–6822. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Waser, J. Room temperature alkynylation of H-phosphi(na)tes and secondary phosphine oxides with ethynylbenziodoxolone (EBX) reagents. Chem. Commun. 2014, 50, 12923–12926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondoh, A.; Yorimitsu, H.; Oshima, K. 1-Alkynylphosphines and Their Derivatives as Key Starting Materials in Creating New Phosphines. Chem. Asian J. 2010, 5, 398–409. [Google Scholar] [CrossRef]
- Arisawa, M.; Onoda, M.; Hori, C.; Yamaguchi, M. Copper-Catalyzed C–P Coupling of 1-alkynylphosphine oxides from 1-alkynes and tetraphenylbiphosphine. Tetrahedron Lett. 2006, 47, 5211–5213. [Google Scholar] [CrossRef]
- Hu, J.; Zhao, N.; Yang, B.; Wang, G.; Guo, L.-N.; Liang, Y.-M.; Yang, S.-D. Copper-Catalyzed C–P Coupling through Decarboxylation. Chem. Eur. J. 2011, 17, 5516–5521. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Gao, Y.; Zhao, Y. Copper-Catalyzed Decarboxylative C–P Cross-Coupling of Alkynyl Acids with H-Phosphine Oxides: A Facile and Selective Synthesis of (E)-1-Alkenylphosphine Oxides. Org. Lett. 2014, 16, 4464–4467. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.; Meng, L.-G.; Xu, H.; Liang, Y.; Wang, L. Additive-free coupling of bromoalkynes with secondary phosphine oxides to generate alkynylphosphine oxides in acetic anhydride. Org. Biomol. Chem. 2020, 18, 1087–1090. [Google Scholar] [CrossRef]
- Wang, Y.; Gan, J.; Liu, L.; Yuan, H.; Gao, Y.; Liu, Y.; Zhao, Y. Cs2CO3-Promoted One-Pot Synthesis of Alkynylphosphonates, -phosphinates, and -phosphine Oxides. J. Org. Chem. 2014, 79, 3678–3683. [Google Scholar] [CrossRef]
- Yang, J.; Chen, T.; Zhou, Y.; Yin, S.; Han, L.-B. Palladium-catalyzed dehydrogenative coupling of terminal alkynes with secondary phosphine oxides. Chem. Commun. 2015, 51, 3549–3551. [Google Scholar] [CrossRef]
- Wang, T.; Chen, S.; Shao, A.; Gao, M.; Huang, Y.; Lei, A. Silver-Mediated Selective Oxidative Cross-Coupling between C–H/P–H: A Strategy to Construct Alkynyl(diaryl)phosphine Oxide. Org. Lett. 2015, 17, 118–121. [Google Scholar] [CrossRef]
- Gérard, P.; Veillard, R.; Alayrac, C.; Gaumont, A.-C.; Evano, G. Room-Temperature Alkynylation of Phosphine Oxides with Copper Acetylides: Practical Synthesis of Alkynylphosphine Oxides. Eur. J. Org. Chem. 2016, 2016, 633–638. [Google Scholar] [CrossRef]
- Zhang, J.-Q.; Chen, T.; Zhang, J.-S.; Han, L.-B. Silver-Free Direct Synthesis of Alkynylphosphine Oxides via spC–H/P(O)–H Dehydrogenative Coupling Catalyzed by Palladium. Org. Lett. 2017, 19, 4692–4695. [Google Scholar] [CrossRef] [PubMed]
- King, A.K.; Gallagher, K.J.; Mahon, M.F.; Webster, R.L. Markovnikov versus anti-Markovnikov Hydrophosphination: Divergent Reactivity Using an Iron(II) b-Diketiminate Pre-Catalyst. Chem. Eur. J. 2017, 23, 9039–9043. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhao, C.-Q.; Han, L.-B. Hydrophosphorylation of Alkynes Catalyzed by Palladium: Generality and Mechanism. J. Am. Chem. Soc. 2018, 140, 3139–3155. [Google Scholar] [CrossRef]
- Ikeshita, D.; Masuda, Y.; Ishida, N.; Murakami, M. Photoinduced Hydrophosphination of Terminal Alkynes with Tri(o-tolyl)phosphine: Synthesis of Alkenylphosphonium Salts. Org. Lett. 2022, 24, 2504–2508. [Google Scholar] [CrossRef]
- Trostyanskaya, I.G.; Beletskaya, I.P. Copper (II)-catalyzed regio- and stereoselective addition of H/P(O)R2 to alkynes. Tetrahedron 2014, 70, 2556–2562. [Google Scholar] [CrossRef]
- Khemchyan, L.L.; Ivanova, J.V.; Zalesskiy, S.S.; Ananikov, V.P.; Beletskaya, I.P.; Starikova, Z.A. Unprecedented Control of Selectivity in Nickel-Catalyzed Hydrophosphorylation of Alkynes: Efficient Route to Mono and Bisphosphonates. Adv. Synth. Catal. 2014, 356, 771–780. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Chen, X.-L.; Qu, L.-B.; Chen, J.-Y.; Sun, K.; Zhao, Y.-F. A one-pot strategy to synthesize β-ketophosphonates: Silver/copper catalyzed direct oxyphosphorylation of alkynes with H-phosphonates and oxygen in the air. Chem. Commun. 2015, 51, 3846–3849. [Google Scholar] [CrossRef]
- Zhang, J.-S.; Zhang, J.-Q.; Chen, T.; Han, L.-B. t-BuOK-mediated reductive addition of P(O)–H compounds to terminal alkynes forming β-arylphosphine oxides. Org. Biomol. Chem. 2017, 15, 5462–5467. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, G.; Chen, L.; Xu, P.; Zhao, Y.; Zhou, Y.; Han, L.-B. Copper-Catalyzed Aerobic Oxidative Coupling of Terminal Alkynes with H-Phosphonates Leading to Alkynylphosphonates. J. Am. Chem. Soc. 2009, 131, 7956–7957. [Google Scholar] [CrossRef]
- Song, W.-Z.; Li, J.-H.; Li, M.; He, J.-N.; Dong, K.; Ullah, K.; Zheng, Y.-B. Copper-catalyzed one-pot synthesis of alkynylphophonates. Synth. Commun. 2019, 49, 697–703. [Google Scholar] [CrossRef]
- Shen, J.; Xiao, B.; Hou, Y.; Wang, X.; Li, G.-Z.; Chen, J.-C.; Wang, W.-L.; Cheng, J.-B.; Yang, B.; Yang, S.-D. Cobalt(II)-Catalyzed Bisfunctionalization of Alkenes with Diarylphosphine Oxide and Peroxide. Adv. Synth. Catal. 2019, 361, 5198–5209. [Google Scholar] [CrossRef]
- Mukhametshina, A.R.; Fedorenko, S.V.; Zueva, I.V.; Petrov, K.A.; Masson, P.; Nizameev, I.R.; Mustafina, A.R.; Sinyashin, O.G. Luminescent silica nanoparticles for sensing acetylcholinesterase-catalyzed hydrolysis of acetylcholine. Biosens. Bioelectron. 2016, 77, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Skripacheva, V.; Mustafina, A.; Davydov, N.; Burilov, V.; Konovalov, A.; Soloveva, S.; Antipin, I. Interfacial adsorption and stripping of ions as a reason of stimuli responsive luminescence of Tb-doped silica nanoparticles. Mater. Chem. Phys. 2012, 132, 488–493. [Google Scholar] [CrossRef]
- Ahkam, Q.M.; Khan, E.U.; Iqbal, J.; Murtaza, A.; Khan, M.T. Synthesis and characterization of cobalt-doped SiO2 nanoparticles. Phys. B Condens. Matter 2019, 572, 161–167. [Google Scholar] [CrossRef]
- Nicholls, D. Electronic spectra of transition-metal complexes. In Complexes and First-Row Transition Elements; Palgrave: London, UK, 1974; pp. 73–99. [Google Scholar] [CrossRef]
- Bhol, P.; Bhavya, M.B.; Swain, S.; Saxena, M.; Samal, A.K. Modern Chemical Routes for the Controlled Synthesis of Anisotropic Bimetallic Nanostructures and Their Application in Catalysis. Front. Chem. 2020, 8, 357. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Kumar, A.; Sharma, S.; Naushad, M.; Dwivedi, R.P.; ALOthman, Z.A.; Mola, G.T. Novel development of nanoparticles to bimetallic nanoparticles and their composites: A review. J. King Saud Univ.-Sci. 2019, 31, 257–269. [Google Scholar] [CrossRef]
- Li, K.; Li, Y.; Peng, W.; Zhang, G.; Zhang, F.; Fan, X. Bimetallic Iron–Cobalt Catalysts and Their Applications in Energy-Related Electrochemical Reactions. Catalysts 2019, 9, 762. [Google Scholar] [CrossRef] [Green Version]
- Rai, R.K.; Al Maksoud, W.; Morlanés, N.; Harb, M.; Ahmad, R.; Genovese, A.; Hedhili, M.N.; Cavallo, L.; Basset, J.-M. Iron−Cobalt-Based Materials: An Efficient Bimetallic Catalyst for Ammonia Synthesis at Low Temperatures. ACS Catal. 2022, 12, 587–599. [Google Scholar] [CrossRef]
- Xiang, Y.; Kruse, N. Cobalt–copper based catalysts for higher terminal alcohols synthesis via Fischer–Tropsch reaction. J. Energy Chem. 2016, 25, 895–906. [Google Scholar] [CrossRef]
- Ge, X.; Sun, H.; Dong, K.; Tao, Y.; Wang, Q.; Chen, Y.; Zhang, G.; Cui, P.; Wang, Y.; Zhang, Q. Copper–cobalt catalysts supported on mechanically mixed HZSM-5 and g-Al2O3 for higher alcohols synthesis via carbon monoxide hydrogenation. RSC Adv. 2019, 9, 14592–14598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, S.; Jadeja, G.C.; Parikh, J. A versatile bi-metallic copper–cobalt catalyst for liquid phase hydrogenation of furfural to 2-methylfuran. RSC Adv. 2016, 6, 1649–1658. [Google Scholar] [CrossRef]
- Zhang, Q.; Zuo, J.; Wang, L.; Peng, F.; Chen, S.; Liu, Z. Non Noble-Metal Copper−Cobalt Bimetallic Catalyst for Efficient Catalysis of the Hydrogenolysis of 5-Hydroxymethylfurfural to 2,5-Dimethylfuran under Mild Conditions. ACS Omega 2021, 6, 10910–10920. [Google Scholar] [CrossRef]
- Call, A.; Casadevall, C.; Acuña-Parés, F.; Casitas, A.; Lloret-Fillol, J. Dual cobalt–copper light-driven catalytic reduction of aldehydes and aromatic ketones in aqueous media. Chem. Sci. 2017, 8, 4739–4749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-X.; Zhang, M.; Zhang, S.-Z.; Hao, Z.-Q.; Zhan, Z.-H. Copper-decorated covalent organic framework as a heterogeneous photocatalyst for phosphorylation of terminal alkynes. Green Chemistry 2022, 24, 4071–4081. [Google Scholar] [CrossRef]
- Guo, H.; Yoshimura, A.; Chen, T.; Saga, Y.; Han, L.-B. Air-induced double addition of P(O)–H bonds to alkynes: A clean and practical method for the preparation of 1,2-bisphosphorylethanes. Green Chemistry 2017, 19, 1502–1506. [Google Scholar] [CrossRef]


| Sample | Si:Co:Cu/Fe | ζ, mV (±5%) |
|---|---|---|
| SN50 | - | −36 |
| SN120 | - | −35 |
| SN50-CuII | 1:0:0.0067 | −23 |
| SN120-CuII | 1:0:0.0282 | −27 |
| SN50-FeIII | 1:0:0.0250 | −30 |
| CoII@SN50 | 1:0.0068 | −33 |
| CoII@SN50-CuII | 1:0.0063:0.0028 | −31 |
| CoIII@SN50 | 1:0.0072 | −33 |
| CoIII@SN120 | 1:0.0043 | −44 |
| CoIII@SN50-CuII | 1:0.0056:0.0056 | −33 |
| CoIII@SN120-CuII | 1:0.0013:0.0286 | −23 |
| CoIII@SN50-FeIII | 1:0.0050:0.0820 | −20 |
| N | Catalysts | −Epred, V | Products, Yields Based on 31P Spectra | |
|---|---|---|---|---|
 | 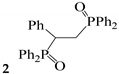 | |||
| 1 | CoIII-SN50 | −0.17, −1.37, −1.89 | 98 * | traces |
| 2 | CoIII-SN50-CuII | −0.9small, −1.41main, −2.35 | 80 * | 20 |
| 3 | CoII-SN50-CuII | −1.37, −2.56 | 85 | 15 |
| 4 | SN50-CuII | −1.23, −2.08 | 99 | traces |
| 5 | SN120-CuII | −1.23, −2.08 | 76 | 24 |
| 6 | SN50-FeIII | −1.85 | 97 | 3 |
| 7 | CoIII-SN50-FeIII | −0.88small, −1.45main, −2.51 | 80 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarasov, M.V.; Bochkova, O.D.; Gryaznova, T.V.; Mustafina, A.R.; Budnikova, Y.H. Non-Noble-Metal Mono and Bimetallic Composites for Efficient Electrocatalysis of Phosphine Oxide and Acetylene C-H/P-H Coupling under Mild Conditions. Int. J. Mol. Sci. 2023, 24, 765. https://doi.org/10.3390/ijms24010765
Tarasov MV, Bochkova OD, Gryaznova TV, Mustafina AR, Budnikova YH. Non-Noble-Metal Mono and Bimetallic Composites for Efficient Electrocatalysis of Phosphine Oxide and Acetylene C-H/P-H Coupling under Mild Conditions. International Journal of Molecular Sciences. 2023; 24(1):765. https://doi.org/10.3390/ijms24010765
Chicago/Turabian StyleTarasov, Maxim V., Olga D. Bochkova, Tatyana V. Gryaznova, Asiya R. Mustafina, and Yulia H. Budnikova. 2023. "Non-Noble-Metal Mono and Bimetallic Composites for Efficient Electrocatalysis of Phosphine Oxide and Acetylene C-H/P-H Coupling under Mild Conditions" International Journal of Molecular Sciences 24, no. 1: 765. https://doi.org/10.3390/ijms24010765
APA StyleTarasov, M. V., Bochkova, O. D., Gryaznova, T. V., Mustafina, A. R., & Budnikova, Y. H. (2023). Non-Noble-Metal Mono and Bimetallic Composites for Efficient Electrocatalysis of Phosphine Oxide and Acetylene C-H/P-H Coupling under Mild Conditions. International Journal of Molecular Sciences, 24(1), 765. https://doi.org/10.3390/ijms24010765







