Bacterial DNAemia in Alzheimer’s Disease and Mild Cognitive Impairment: Association with Cognitive Decline, Plasma BDNF Levels, and Inflammatory Response
Abstract
:1. Introduction
2. Results
2.1. Baseline Characteristics of EC, MCI and AD Patients
2.2. BB-DNA and Plasma BDNF Levels
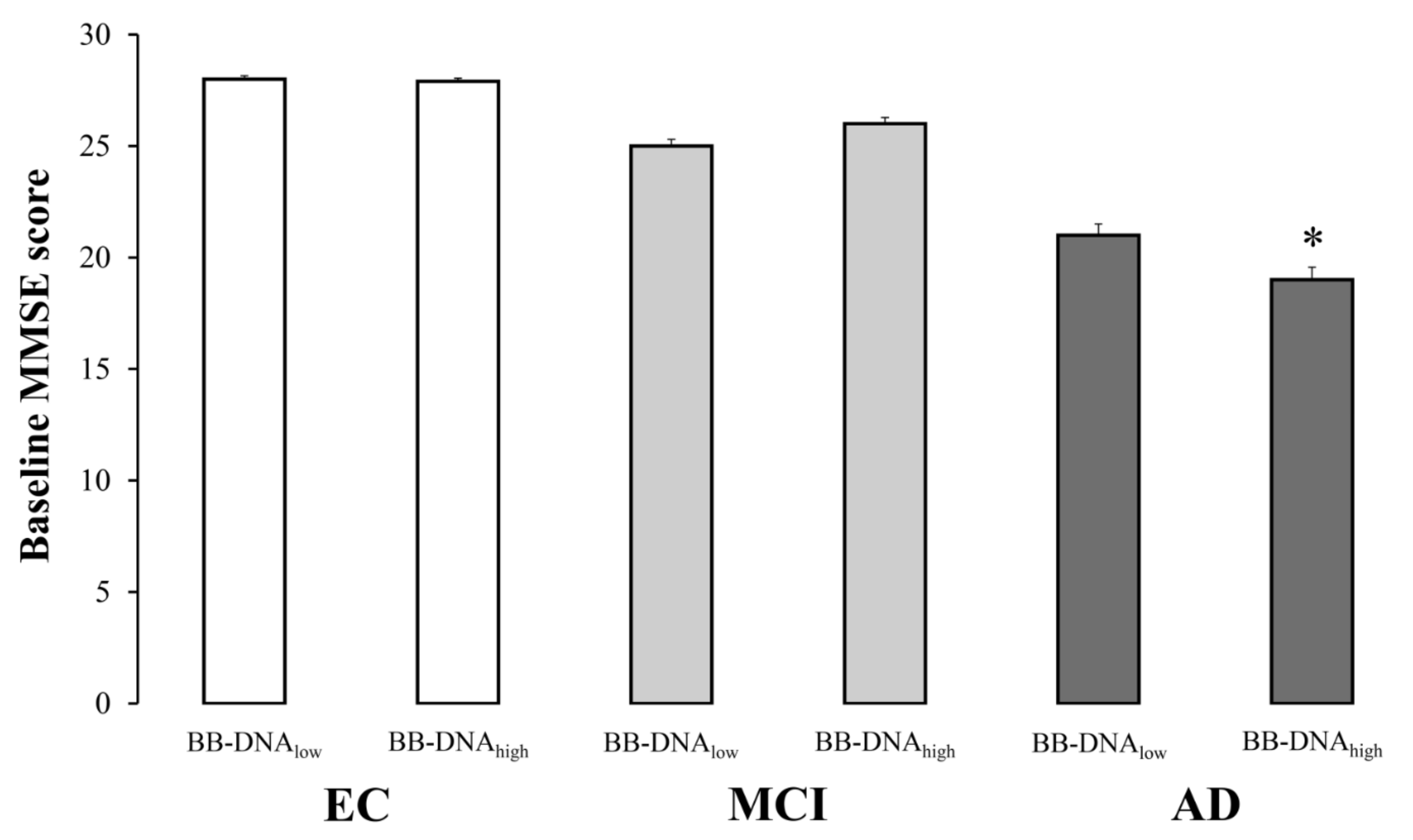
2.3. Cognitive Function in Relation to BB-DNA Percentiles
2.4. Plasma Cytokine Levels
2.5. Correlations between BB-DNA and Plasma Cytokines Levels at Baseline and after Two-Year Follow-Up Period
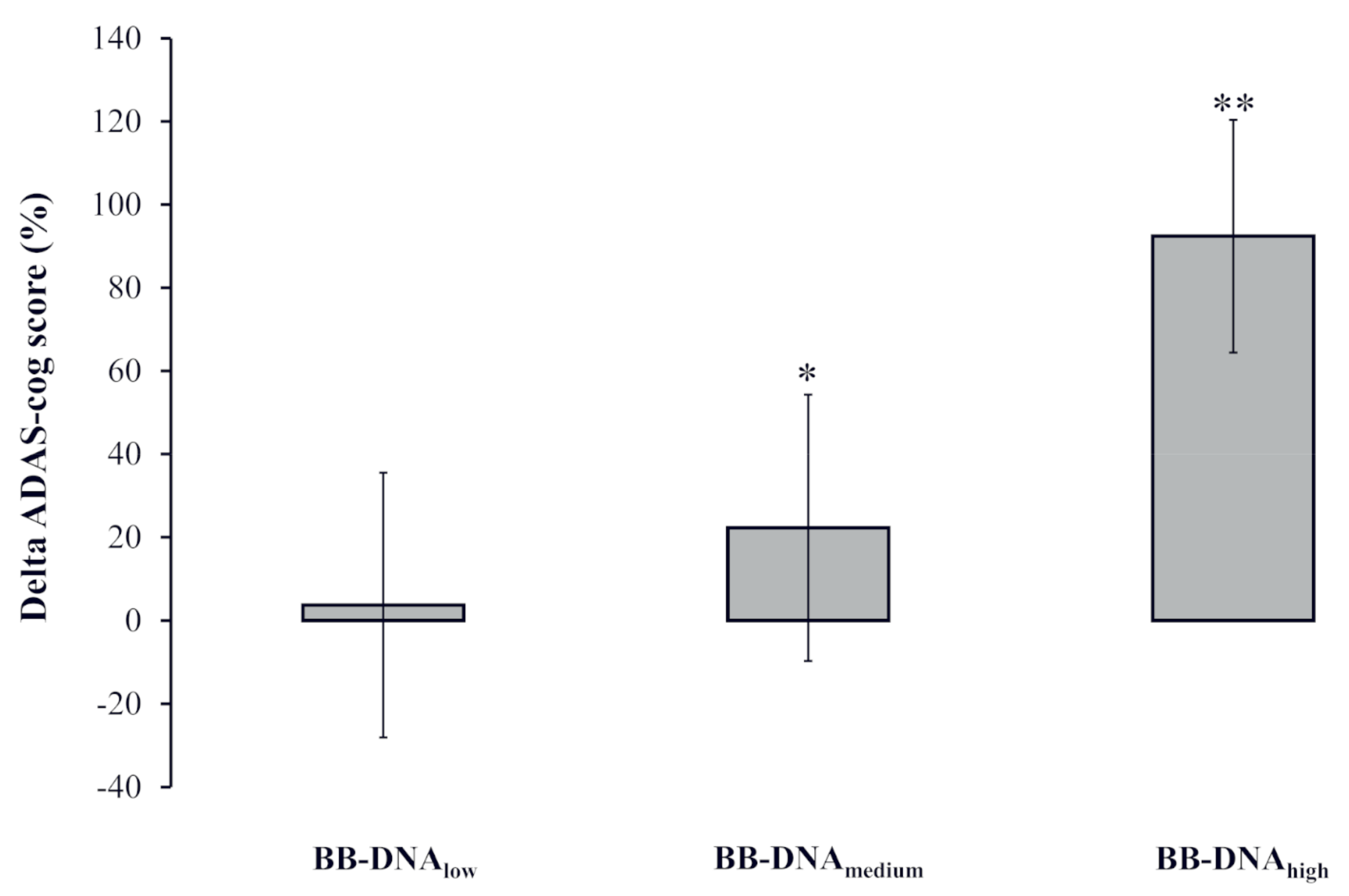
2.6. Change in AD Patients’ ADAS-Cog Score at Two-Year Follow-Up Based on BB-DNA Tertiles
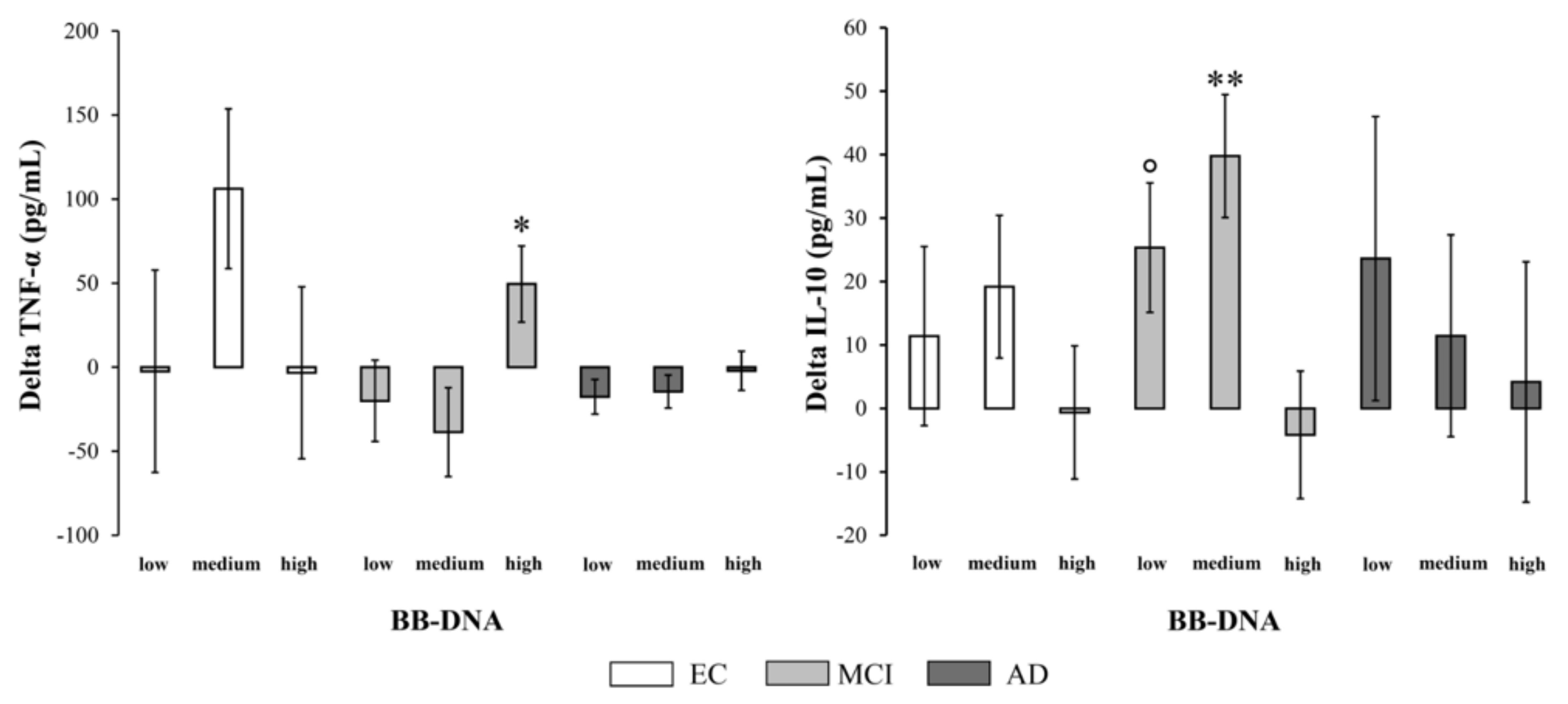
2.7. Changes in Plasma Cytokine and BDNF Levels at Two-Year Follow-Up in EC, MCI, and AD Patients Based on BB-DNA Tertiles
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Neuropsychological and Functional Assessment
4.3. Determination of BB-DNA
4.4. Determination of Plasma BDNF
4.5. Plasma TNF-α and IL-10 Measurements
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grobler, C.; van Tongeren, M.; Gettemans, J.; Kell, D.B.; Pretorius, E. Alzheimer’s Disease: A Systems View Provides a Unifying Explanation of Its Development. J. Alzheimer’s Dis. 2022, preprint. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Feng, X.; Sun, X.; Hou, N.; Han, F.; Liu, Y. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2019. Front. Aging Neurosci. 2022, 14, 937486. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, U.; Arshad, M.S.; Sameen, A.; Oh, D.H. Crosstalk between Gut and Brain in Alzheimer’s Disease: The Role of Gut Microbiota Modulation Strategies. Nutrients 2021, 13, 690. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, S.B.; Rathipriya, A.G.; Mahalakshmi, A.M.; Sharma, S.; Hediyal, T.A.; Ray, B.; Sunanda, T.; Rungratanawanich, W.; Kashyap, R.S.; Qoronfleh, M.W.; et al. The Influence of Gut Dysbiosis in the Pathogenesis and Management of Ischemic Stroke. Cells 2022, 11, 1239. [Google Scholar] [CrossRef]
- Païssé, S.; Valle, C.; Servant, F.; Courtney, M.; Burcelin, R.; Amar, J.; Lelouvier, B. Comprehensive description of blood microbiome from healthy donors assessed by 16S targeted metagenomic sequencing. Transfusion 2016, 56, 1138–1147. [Google Scholar] [CrossRef]
- Shah, N.B.; Nigwekar, S.U.; Kalim, S.; Lelouvier, B.; Servant, F.; Dalal, M.; Krinsky, S.; Fasano, A.; Tolkoff-Rubin, N.; Allegretti, A.S. The Gut and Blood Microbiome in IgA Nephropathy and Healthy Controls. Kidney360 2021, 2, 1261–1274. [Google Scholar] [CrossRef]
- Giacconi, R.; D’Aquila, P.; Malavolta, M.; Piacenza, F.; Bürkle, A.; Moreno Villanueva, M.; Dollé, M.E.T.; Jansen, E.; Grune, T.; Gonos, E.S.; et al. Bacterial DNAemia in older subjects and nonagenarian offspring and association with redox biomarkers: Results from MARK-AGE Study. J. Gerontol. Ser. A 2022. [Google Scholar] [CrossRef]
- Emery, D.C.; Cerajewska, T.L.; Seong, J.; Davies, M.; Paterson, A.; Allen-Birt, S.J.; West, N.X. Comparison of Blood Bacterial Communities in Periodontal Health and Periodontal Disease. Front. Cell. Infect. Microbiol. 2021, 10, 577485. [Google Scholar] [CrossRef]
- Dinakaran, V.; Rathinavel, A.; Pushpanathan, M.; Sivakumar, R.; Gunasekaran, P.; Rajendhran, J. Elevated levels of circulating DNA in cardiovascular disease patients: Metagenomic profiling of microbiome in the circulation. PLoS ONE 2014, 9, e105221. [Google Scholar] [CrossRef]
- Qiu, J.; Zhou, H.; Jing, Y.; Dong, C. Association between blood microbiome and type 2 diabetes mellitus: A nested case-control study. J. Clin. Lab. Anal. 2019, 33, e22842. [Google Scholar] [CrossRef]
- Shah, N.B.; Allegretti, A.S.; Nigwekar, S.U.; Kalim, S.; Zhao, S.; Lelouvier, B.; Servant, F.; Serena, G.; Thadhani, R.I.; Raj, D.S.; et al. Blood microbiome profile in CKD: A pilot study. Clin. J. Am. Soc. Nephrol. 2019, 14, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Tarawneh, R.; Penhos, E. The gut microbiome and Alzheimer’s disease: Complex and bidirectional interactions. Neurosci. Biobehav. Rev. 2022, 141, 104814. [Google Scholar] [CrossRef] [PubMed]
- Bello-Medina, P.C.; Corona-Cervantes, K.; Zavala Torres, N.G.; González, A.; Pérez-Morales, M.; González-Franco, D.A.; Gómez, A.; García-Mena, J.; Díaz-Cintra, S.; Pacheco-López, G. Chronic-Antibiotics Induced Gut Microbiota Dysbiosis Rescues Memory Impairment and Reduces β-Amyloid Aggregation in a Preclinical Alzheimer’s Disease Model. Int. J. Mol. Sci. 2022, 23, 8209. [Google Scholar] [CrossRef] [PubMed]
- Tauber, S.C.; Ebert, S.; Weishaupt, J.H.; Reich, A.; Nau, R.; Gerber, J. Stimulation of toll-like receptor 9 by chronic intraventricular unmethylated cytosine-guanine dna infusion causes neuroinflammation and impaired spatial memory. J. Neuropathol. Exp. Neurol. 2009, 68, 1116–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunt, V.E.; LaRocca, T.J.; Bazzoni, A.E.; Sapinsley, Z.J.; Miyamoto-Ditmon, J.; Gioscia-Ryan, R.A.; Neilson, A.P.; Link, C.D.; Seals, D.R. The gut microbiome–derived metabolite trimethylamine N-oxide modulates neuroinflammation and cognitive function with aging. GeroScience 2021, 43, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.C.H. Microbiota dysbiosis and barrier dysfunction in inflammatory bowel disease and colorectal cancers: Exploring a common ground hypothesis. J. Biomed. Sci. 2018, 25, 79. [Google Scholar] [CrossRef] [Green Version]
- Zhan, X.; Stamova, B.; Jin, L.W.; Decarli, C.; Phinney, B.; Sharp, F.R. Gram-negative bacterial molecules associate with Alzheimer disease pathology. Neurology 2016, 87, 2324–2332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Yang, X.; de Anda, J.; Huang, J.; Li, D.; Xu, H.; Shields, K.S.; Džunková, M.; Hansen, J.; Patel, I.J.; et al. Clostridioides difficile Toxin A Remodels Membranes and Mediates DNA Entry Into Cells to Activate Toll-Like Receptor 9 Signaling. Gastroenterology 2020, 159, 2181–2192.e1. [Google Scholar] [CrossRef]
- Tetz, G.; Tetz, V. Bacterial Extracellular DNA Promotes β-Amyloid Aggregation. Microorganisms 2021, 9, 1301. [Google Scholar] [CrossRef]
- Ma, X.; Shin, Y.J.; Jang, H.M.; Joo, M.K.; Yoo, J.W.; Kim, D.H. Lactobacillus rhamnosus and Bifidobacterium longum alleviate colitis and cognitive impairment in mice by regulating IFN-γ to IL-10 and TNF-α to IL-10 expression ratios. Sci. Rep. 2021, 11, 20659. [Google Scholar] [CrossRef]
- Thomas, A.J.; Hamilton, C.A.; Donaghy, P.C.; Martin-Ruiz, C.; Morris, C.M.; Barnett, N.; Olsen, K.; Taylor, J.P.; O’Brien, J.T. Prospective longitudinal evaluation of cytokines in mild cognitive impairment due to AD and Lewy body disease. Int. J. Geriatr. Psychiatry 2020, 35, 1250–1259. [Google Scholar] [CrossRef] [PubMed]
- Balietti, M.; Giuli, C.; Conti, F. Peripheral Blood Brain-Derived Neurotrophic Factor as a Biomarker of Alzheimer’s Disease: Are There Methodological Biases? Mol. Neurobiol. 2018, 55, 6661–6672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bercik, P.; Denou, E.; Collins, J.; Jackson, W.; Lu, J.; Jury, J.; Deng, Y.; Blennerhassett, P.; MacRi, J.; McCoy, K.D.; et al. The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 2011, 141, 599–609.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.L.; Li, M.M.; Zhou, M.F.; Xu, H.S.; Huan, F.; Liu, N.; Gao, R.; Wang, J.; Zhang, N.; Jiang, L. Links Between Gut Dysbiosis and Neurotransmitter Disturbance in Chronic Restraint Stress-Induced Depressive Behaviours: The Role of Inflammation. Inflammation 2021, 44, 2448–2462. [Google Scholar] [CrossRef] [PubMed]
- Princiotta Cariddi, L.; Mauri, M.; Cosentino, M.; Versino, M.; Marino, F. Alzheimer’s Disease: From Immune Homeostasis to Neuroinflammatory Condition. Int. J. Mol. Sci. 2022, 23, 13008. [Google Scholar] [CrossRef]
- Ismail, N.A.; Leong Abdullah, M.F.I.; Hami, R.; Ahmad Yusof, H. A narrative review of brain-derived neurotrophic factor (BDNF) on cognitive performance in Alzheimer’s disease. Growth Factors 2020, 38, 210–225. [Google Scholar] [CrossRef]
- Szandruk-Bender, M.; Wiatrak, B.; Szeląg, A. The Risk of Developing Alzheimer’s Disease and Parkinson’s Disease in Patients with Inflammatory Bowel Disease: A Meta-Analysis. J. Clin. Med. 2022, 11, 3704. [Google Scholar] [CrossRef]
- Khedr, E.M.; Omeran, N.; Karam-Allah Ramadan, H.; Ahmed, G.K.; Abdelwarith, A.M. Alteration of Gut Microbiota in Alzheimer’s Disease and Their Relation to the Cognitive Impairment. J. Alzheimer’s Dis. 2022, 88, 1103–1114. [Google Scholar] [CrossRef]
- Gargari, G.; Mantegazza, G.; Taverniti, V.; Del Bo’, C.; Bernardi, S.; Andres-Lacueva, C.; González-Domínguez, R.; Kroon, P.A.; Winterbone, M.S.; Cherubini, A.; et al. Bacterial DNAemia is associated with serum zonulin levels in older subjects. Sci. Rep. 2021, 11, 11054. [Google Scholar] [CrossRef]
- Dominy, S.S.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef]
- Dioguardi, M.; Crincoli, V.; Laino, L.; Alovisi, M.; Sovereto, D.; Mastrangelo, F.; Lo Russo, L.; Lo Muzio, L. The role of periodontitis and periodontal bacteria in the onset and progression of alzheimer’s disease: A systematic review. J. Clin. Med. 2020, 9, 495. [Google Scholar] [CrossRef] [Green Version]
- Tetz, G.; Pinho, M.; Pritzkow, S.; Mendez, N.; Soto, C.; Tetz, V. Bacterial DNA promotes Tau aggregation. Sci. Rep. 2020, 10, 2369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prykhodko, O.; Sandberg, J.; Burleigh, S.; Björck, I.; Nilsson, A.; Fåk Hållenius, F. Impact of Rye Kernel-Based Evening Meal on Microbiota Composition of Young Healthy Lean Volunteers with an Emphasis on Their Hormonal and Appetite Regulations, and Blood Levels of Brain-Derived Neurotrophic Factor. Front. Nutr. 2018, 5, 45. [Google Scholar] [CrossRef] [PubMed]
- Tauber, S.C.; Stadelmann, C.; Spreer, A.; Brück, W.; Nau, R.; Gerber, J. Increased expression of BDNF and proliferation of dentate granule cells after bacterial meningitis. J. Neuropathol. Exp. Neurol. 2005, 64, 806–815. [Google Scholar] [CrossRef] [Green Version]
- Shen, X.N.; Niu, L.D.; Wang, Y.J.; Cao, X.P.; Liu, Q.; Tan, L.; Zhang, C.; Yu, J.T. Inflammatory markers in Alzheimer’s disease and mild cognitive impairment: A meta-analysis and systematic review of 170 studies. J. Neurol. Neurosurg. Psychiatry 2019, 90, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Lee, K.J.; Kim, H. Serum tumour necrosis factor-α and interleukin-6 levels in Alzheimer’s disease and mild cognitive impairment. Psychogeriatrics 2017, 17, 224–230. [Google Scholar] [CrossRef]
- Wennberg, A.M.V.; Hagen, C.E.; Machulda, M.M.; Knopman, D.S.; Petersen, R.C.; Mielke, M.M. The Cross-sectional and longitudinal associations between il-6, il-10, and TNFα and cognitive outcomes in the mayo clinic study of aging. J. Gerontol.-Ser. A Biol. Sci. Med. Sci. 2019, 74, 1289–1295. [Google Scholar] [CrossRef]
- Hazen, J.; Vistnes, M.; Barca, M.L.; Eldholm, R.S.; Persson, K.; Brækhus, A.; Saltvedt, I.; Selbæk, G.; Engedal, K.; Knapskog, A.B. The Association between Circulating Inflammatory Markers and the Progression of Alzheimer Disease in Norwegian Memory Clinic Patients with Mild Cognitive Impairment or Dementia. Alzheimer Dis. Assoc. Disord. 2020, 34, 47–53. [Google Scholar] [CrossRef]
- Julian, A.; Rioux-Bilan, A.; Ragot, S.; Krolak-Salmon, P.; Berrut, G.; Dantoine, T.; Hommet, C.; Hanon, O.; Page, G.; Paccalin, M. Blood Inflammatory Mediators and Cognitive Decline in Alzheimer’s Disease: A Two Years Longitudinal Study. J. Alzheimer’s Dis. 2018, 63, 87–92. [Google Scholar] [CrossRef]
- Romero-Sevilla, R.; López-Espuela, F.; Fuentes, J.M.; de San Juan, B.D.; Portilla-Cuenca, J.C.; Hijon, C.C.; Casado-Naranjo, I. Role of Inflammatory Cytokines in the Conversion of Mild Cognitive Impairment to Dementia: A Prospective Study. Curr. Alzheimer Res. 2022, 19, 68–75. [Google Scholar] [CrossRef]
- Versele, R.; Sevin, E.; Gosselet, F.; Fenart, L.; Candela, P. TNF-α and IL-1β Modulate Blood-Brain Barrier Permeability and Decrease Amyloid-β Peptide Efflux in a Human Blood-Brain Barrier Model. Int. J. Mol. Sci. 2022, 23, 10235. [Google Scholar] [CrossRef] [PubMed]
- Iulita, M.F.; Ganesh, A.; Pentz, R.; Flores Aguilar, L.; Gubert, P.; Ducatenzeiler, A.; Christie, S.; Wilcock, G.K.; Cuello, A.C. Identification and Preliminary Validation of a Plasma Profile Associated with Cognitive Decline in Dementia and At-Risk Individuals: A Retrospective Cohort Analysis. J. Alzheimer’s Dis. 2019, 67, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Padhi, P.; Worth, C.; Zenitsky, G.; Jin, H.; Sambamurti, K.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Mechanistic Insights Into Gut Microbiome Dysbiosis-Mediated Neuroimmune Dysregulation and Protein Misfolding and Clearance in the Pathogenesis of Chronic Neurodegenerative Disorders. Front. Neurosci. 2022, 16, 836605. [Google Scholar] [CrossRef] [PubMed]
- Soto-Rojas, L.O.; Pacheco-Herrero, M.; Martínez-Gómez, P.A.; Campa-Córdoba, B.B.; Apátiga-Pérez, R.; Villegas-Rojas, M.M.; Harrington, C.R.; de la Cruz, F.; Garcés-Ramírez, L.; Luna-Muñoz, J. The Neurovascular Unit Dysfunction in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 2022. [Google Scholar] [CrossRef]
- Pagoni, P.; Korologou-Linden, R.S.; Howe, L.D.; Davey Smith, G.; Ben-Shlomo, Y.; Stergiakouli, E.; Anderson, E.L. Causal effects of circulating cytokine concentrations on risk of Alzheimer’s disease and cognitive function. Brain Behav. Immun. 2022, 104, 54–64. [Google Scholar] [CrossRef]
- Yeung, C.H.C.; Schooling, C.M. Systemic inflammatory regulators and risk of Alzheimer’s disease: A bidirectional Mendelian-randomization study. Int. J. Epidemiol. 2021, 50, 829–840. [Google Scholar] [CrossRef]
- Eldholm, R.S.; Barca, M.L.; Persson, K.; Knapskog, A.B.; Kersten, H.; Engedal, K.; Selbæk, G.; Brækhus, A.; Skovlund, E.; Saltvedt, I. Progression of Alzheimer’s disease: A longitudinal study in Norwegian memory clinics. J. Alzheimer’s Dis. 2018, 61, 1221–1232. [Google Scholar] [CrossRef] [Green Version]
- Giuli, C.; Fattoretti, P.; Gagliardi, C.; Mocchegiani, E.; Venarucci, D.; Balietti, M.; Casoli, T.; Costarelli, L.; Giacconi, R.; Malavolta, M.; et al. My Mind Project: The effects of cognitive training for elderly—The study protocol of a prospective randomized intervention study. Aging Clin. Exp. Res. 2017, 29, 353–360. [Google Scholar] [CrossRef] [Green Version]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, C.P.; Berg, L.; Danziger, W.L.; Coben, L.A.; Martin, R.L. A new clinical scale for the staging of dementia. Br. J. Psychiatry 1982, 140, 566–572. [Google Scholar] [CrossRef] [PubMed]
- D’Aquila, P.; Giacconi, R.; Malavolta, M.; Piacenza, F.; Bürkle, A.; Villanueva, M.M.; Dollé, M.E.T.; Jansen, E.; Grune, T.; Gonos, E.S.; et al. Microbiome in Blood Samples From the General Population Recruited in the MARK-AGE Project: A Pilot Study. Front. Microbiol. 2021, 12, 707515. [Google Scholar] [CrossRef] [PubMed]
- Balietti, M.; Giuli, C.; Fattoretti, P.; Fabbietti, P.; Papa, R.; Postacchini, D.; Conti, F. Effect of a Comprehensive Intervention on Plasma BDNF in Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 57, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Balietti, M.; Giuli, C.; Casoli, T.; Fabbietti, P.; Conti, F. Is blood brain-derived neurotrophic factor a useful biomarker to monitor mild cognitive impairment patients? Rejuvenation Res. 2020, 23, 411–419. [Google Scholar] [CrossRef]

| EC n = 94 | MCI n = 93 | AD n = 95 | |
|---|---|---|---|
| Age (years) | 72.7 ± 0.6 | 76.1 ± 0.6 * | 77.8 ± 0.5 * |
| Females | 79.6% | 63.4% § | 67.7% § |
| BMI | 26.5 ± 0.5 | 25.3 ± 0.4 | 25.8 ± 0.4 |
| MMSE | 28.1 ± 0.1 | 25.9 ± 0.2 */° | 20.2 ± 0.3 * |
| LSNS | 29.9 ± 0.9 | 29.2 ± 0.7 | 26.5 ± 0.7 ** |
| PASE | 110.6 ± 4.9 ° | 83.4 ± 4.5 °/* | 64.6 ± 4.6 |
| Smoking habits | |||
| never smoker | 58.5 % | 62.3 % | 56.8 % |
| former smoker | 28.7 % | 31.2 % | 30.5 % |
| current smoker | 12.8 % | 6.5 % | 12.7 % |
| GDS | 7.6 ± 0.5 | 8.7 ± 0.5 | 8.3 ± 0.3 |
| ADL | 5.90 ± 0.02 ° | 5.90 ± 0.04 ° | 5.20 ± 0.10 |
| IADL | 7.90 ± 0.03 ° | 6.90 ± 0.10 °/* | 3.30 ± 0.20 |
| Education (years) | 9.5 ± 0.5 ° | 5.9 ± 0.4 * | 5.0 ± 0.3 |
| Albumin (g/dL) | 4.28 ± 0.03 | 4.23 ± 0.03 | 4.20 ± 0.03 |
| CRP (pg/mL) | 0.29 ± 0.07 | 0.34 ± 0.05 | 0.35 ± 0.05 |
| COPD | 3.2% | 0.0% | 5.3% |
| Hypertension | 52.1% | 53.8% | 67.4% |
| Diabetes | 4.3% | 7.5% | 7.4% |
| CHD | 7.6% | 8.6% | 8.4% |
| Atrial fibrillation | 18.1% ° | 11.1% | 5.3% |
| CKD | 1.1% | 1.1% | 3.2% |
| Previous ictus | 1.1% | 7.5% | 10.5% § |
| PAD | 8.5% | 8.6% | 27.4% + |
| IBD | 3.2% | 1.1% | 0.0% |
| Peptic ulcers | 2.1% | 4.3% | 0.0% |
| Gastritis | 22.3% | 7.5% | 6.3% |
| Acetylcholinesterase inhibitors | 0.0% | 0.0% | 42.5% |
| Benzodiazepines | 24.7% | 9.5% § | 12.5% § |
| Antidepressants | 2.6% | 16.2% § | 18.8% § |
| Lipid-lowering medications | 14.3% | 21.6% | 18.8% |
| Anticoagulants/antiplatelets drugs | 37.7% | 51.4% | 57.5% § |
| NSAIDs | 26.0% | 27.0% | 41.3% |
| Antihypertensives | 51.9% | 55.4% | 57.5% |
| Corticosteroids | 1.3% | 0.0% | 0.0% |
| Variables | Unstandardized Coefficients | Standardized Coefficients | p Value | ||
|---|---|---|---|---|---|
| β | Std. Error | β | |||
| CDR1 | Sex | 25.263 | 11.974 | 0.368 | 0.043 |
| Age | 0.908 | 0.994 | 0.146 | 0.368 | |
| AChEIs | 14.495 | 10.931 | 0.219 | 0.195 | |
| Benzodiazepines | −3.584 | 13.775 | −0.041 | 0.796 | |
| Antidepressants | 26.822 | 17.021 | 0.264 | 0.125 | |
| Lipid lowering medications | −3.537 | 13.218 | −0.044 | 0.791 | |
| Plasma BDNF levels | 5.026 | 5.472 | 0.151 | 0.365 | |
| Alcohol consumption | 2.399 | 12.072 | 0.032 | 0.844 | |
| Smoking_habits | −0.231 | 8.113 | −0.005 | 0.977 | |
| PASE | −0.092 | 0.142 | −0.109 | 0.523 | |
| CDR2 | Sex | 17.329 | 15.439 | 0.231 | 0.272 |
| Age | 0.343 | 1.218 | 0.054 | 0.780 | |
| AChEIs | −8.667 | 12.175 | −0.119 | 0.483 | |
| Benzodiazepines | −21.675 | 23.614 | −0.178 | 0.367 | |
| Antidepressants | 0.344 | 12.037 | 0.005 | 0.977 | |
| Lipid lowering medications | −25.841 | 18.204 | −0.266 | 0.168 | |
| Plasma BDNF levels | 8.375 | 3.430 | 0.407 | 0.022 | |
| Alcohol consumption | −22.000 | 13.634 | −0.284 | 0.119 | |
| Smoking_habits | −13.065 | 10.939 | −0.251 | 0.243 | |
| PASE | −0.009 | 0.152 | −0.010 | 0.954 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giacconi, R.; D’Aquila, P.; Balietti, M.; Giuli, C.; Malavolta, M.; Piacenza, F.; Costarelli, L.; Postacchini, D.; Passarino, G.; Bellizzi, D.; et al. Bacterial DNAemia in Alzheimer’s Disease and Mild Cognitive Impairment: Association with Cognitive Decline, Plasma BDNF Levels, and Inflammatory Response. Int. J. Mol. Sci. 2023, 24, 78. https://doi.org/10.3390/ijms24010078
Giacconi R, D’Aquila P, Balietti M, Giuli C, Malavolta M, Piacenza F, Costarelli L, Postacchini D, Passarino G, Bellizzi D, et al. Bacterial DNAemia in Alzheimer’s Disease and Mild Cognitive Impairment: Association with Cognitive Decline, Plasma BDNF Levels, and Inflammatory Response. International Journal of Molecular Sciences. 2023; 24(1):78. https://doi.org/10.3390/ijms24010078
Chicago/Turabian StyleGiacconi, Robertina, Patrizia D’Aquila, Marta Balietti, Cinzia Giuli, Marco Malavolta, Francesco Piacenza, Laura Costarelli, Demetrio Postacchini, Giuseppe Passarino, Dina Bellizzi, and et al. 2023. "Bacterial DNAemia in Alzheimer’s Disease and Mild Cognitive Impairment: Association with Cognitive Decline, Plasma BDNF Levels, and Inflammatory Response" International Journal of Molecular Sciences 24, no. 1: 78. https://doi.org/10.3390/ijms24010078
APA StyleGiacconi, R., D’Aquila, P., Balietti, M., Giuli, C., Malavolta, M., Piacenza, F., Costarelli, L., Postacchini, D., Passarino, G., Bellizzi, D., & Provinciali, M. (2023). Bacterial DNAemia in Alzheimer’s Disease and Mild Cognitive Impairment: Association with Cognitive Decline, Plasma BDNF Levels, and Inflammatory Response. International Journal of Molecular Sciences, 24(1), 78. https://doi.org/10.3390/ijms24010078







