GmWAK1, Novel Wall-Associated Protein Kinase, Positively Regulates Response of Soybean to Phytophthora sojae Infection
Abstract
:1. Introduction
2. Results
2.1. GmWAK1 May Be Involved in Response to P. sojae Infection
2.2. Bioinformatics Analysis of GmWAK1
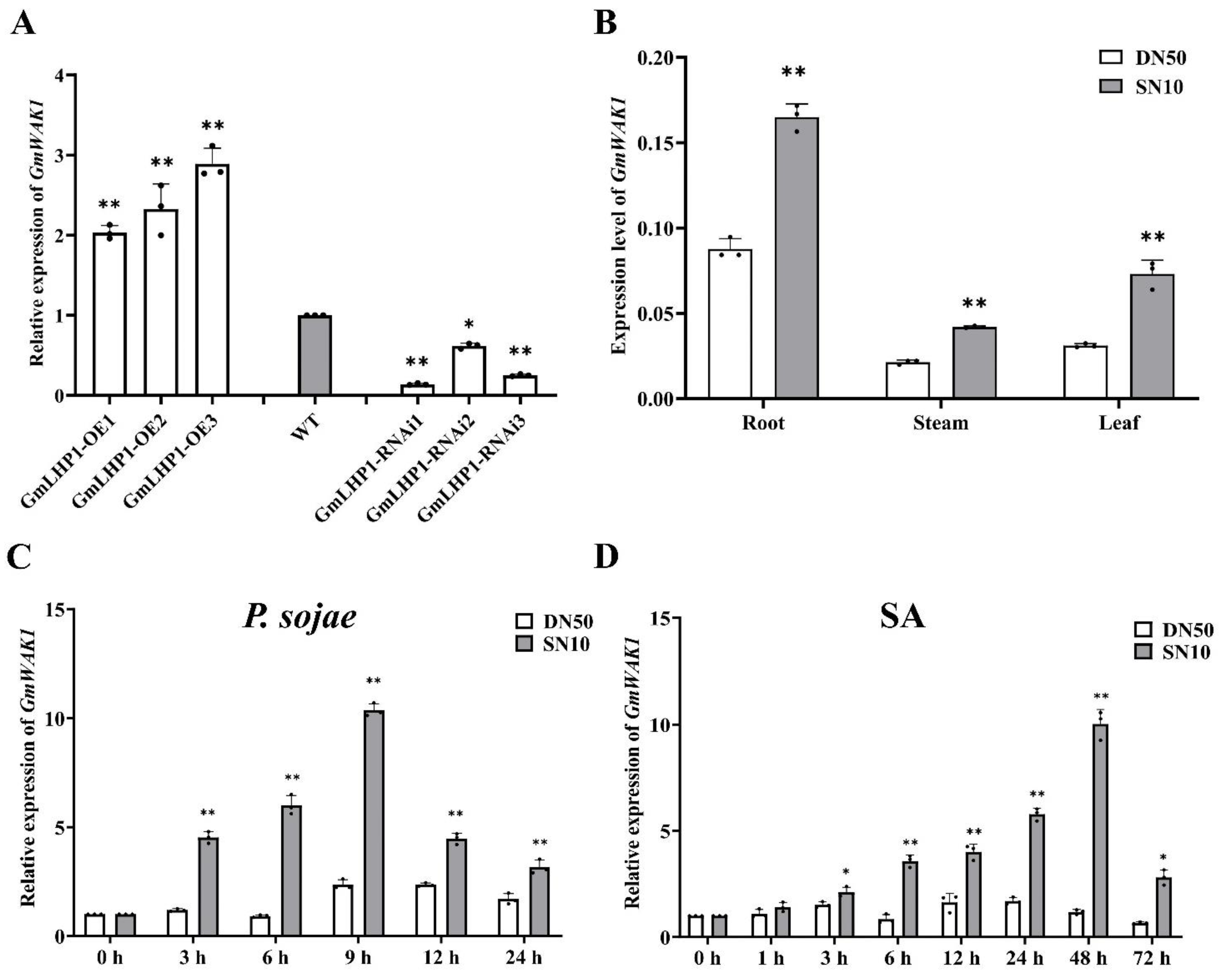
2.3. GmWAK1 Transcript Levels under Different Treatments
2.4. Subcellular Localization of the GmWAK1
2.5. GmWAK1 Is Positive Regulator in Soybean Resistance to P. sojae
2.6. GmWAK1-Dependent SA Signaling in Response to P. sojae
2.7. Soybean GmWAK1-Regulated Defense Response to P. sojae Involves Alleviating Oxidative Stress Damage
2.8. GmWAK1 Interacts with GmANNRJ4
2.9. GmANNRJ4 can Regulate Intracellular Ca2+ Concentrations and Promotes GmMPK6 Expression
2.10. GmANNRJ4 Enhances Resistance to P. sojae and Positively Regulates Expression of PR Genes in Hairy Roots of Transgenic Soybean
3. Discussion
4. Materials and Methods
4.1. Plant Materials, P. sojae Treatment, and Primers
4.2. Isolation and Sequence Analysis of GmWAK1
4.3. RT-PCR and qRT-PCR Analysis
4.4. Chromatin Immunoprecipitation (ChIP) qPCR Assay
4.5. Subcellular Localization of GmWAK1
4.6. Inducing the Expression of GmWAK1 Fusion Protein
4.7. Vector Construction and Transformation of Soybean
4.8. Assessment of Pathogen Resistance and the Disease Response
4.9. Bimolecular Fluorescence Complementation (BiFC) Assay
4.10. Pull-Down Assays
4.11. Determination of Plant Hormone and Antioxidant Enzyme Activity Levels
4.12. Intracellular Calcium Concentration Measurement
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wrather, J.A.; Anderson, T.R.; Arsyad, D.M.; Tan, Y.; Ploper, L.D.; PortaPuglia, A.; Ram, H.H.; Yorinori, J.T. Soybean disease loss estimates for the top 10 soybean producing countries in 1998. Plant Dis. 2001, 81, 107–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyler, B.M. Phytophthora sojae: Root rot pathogen of soybean and model oomycete. Mol. Plant Pathol. 2007, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Erwin, D.C. Phytophthora diseases worldwide. Plant Pathol. 1998, 47, 224–225. [Google Scholar] [CrossRef]
- Bernard, R.L.; Smith, P.E.; Kaufmann, M.J.; Schmitthenner, A.F. Inheritance of resistance to Phytophthora root and stem rot in soybean. Agron. J. 1957, 49, 391. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Xu, P.F.; Wu, J.J.; Xue, A.G.; Zhang, J.X.; Li, W.B.; Chen, C.; Chen, W.Y.; Lv, H.Y. Races of Phytophthora sojae and their virulences on soybean cultivars in Heilongjiang, China. Plant Dis. 2010, 94, 87–91. [Google Scholar] [CrossRef] [Green Version]
- Dixon, R.A.; Lamb, C.J. Molecular communication in interactions between plants and microbial pathogens. Annu. Rev. Plant Phys. 1990, 41, 339–367. [Google Scholar] [CrossRef]
- Walker, J.C.; Zhang, R. Relationship of a putative receptor protein kinase from maize to the S-locus glycoproteins of Brassica. Nature 1990, 345, 743–746. [Google Scholar] [CrossRef]
- Walker, J.C. Receptor-like protein kinase genes of Arabidopsis thaliana. Plant J. 1993, 3, 451–456. [Google Scholar] [CrossRef]
- Shiu, S.H.; Karlowski, W.M.; Pan, R.; Tzeng, Y.H.; Mayer, K.F.X.; Li, W.H. Comparative analysis of the receptor-like kinase family in arabidopsis and rice. Plant Cell 2004, 16, 1220–1234. [Google Scholar] [CrossRef] [Green Version]
- Alexandre, L.; Sabine, C.; Philippe, D.; Philippe, S.; Patrick, T.; Séverine, T. Involvement of a putative Lycopersicon esculentum wall-associated kinase in the early steps of tomato–Orobanche ramosa interaction. Physiol. Mol. Plant Pathol. 2006, 69, 3–12. [Google Scholar] [CrossRef]
- Verica, J.A.; He, Z.H. The cell wall-associated kinase (WAK) and WAKlike kinase gene family. Plant Physiol. 2002, 129, 455–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.X.; Stafford, L.; Julian, R.; Antony, B.; Kim, L.J. WAKL8 regulates Arabidopsis stem secondary wall development. Plants 2022, 11, 2297. [Google Scholar] [CrossRef]
- Shiu, S.H.; Bleecker, A.B. Plant receptor-like kinase gene family: Diversity, function, and signaling. Science’s STKE 2001, 2001, re22. [Google Scholar] [CrossRef] [PubMed]
- Becraft, P.W. Receptor kinase signaling in plant development. Annu. Rev. Plant Phys. 2002, 18, 163–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dievart, A.; Clark, S.E. Using mutant alleles to determine the structure and function of leucine-rich repeat receptor-like kinases. Curr. Opin. Plant. Biol. 2003, 6, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.P.; Wang, C.; He, J.N.; Liao, H.; Duan, Y.; Zhu, Z.Q.; Guo, Y.; Chen, Z.Z.; Gong, Z.Z. A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis. Plant Cell 2002, 24, 2546–2561. [Google Scholar] [CrossRef] [Green Version]
- He, Z.H.; Cheeseman, I.; He, D.; Kohorn, B.D. A cluster of five cell wall-associated receptor kinase genes, WAK1-5, are expressed in specific organs of Arabidopsis. Plant Mol. Biol. 1999, 39, 1189–1196. [Google Scholar] [CrossRef]
- He, Z.H.; Fujiki, M.; Kohorn, B.D. A cell wall associated receptor-like protein kinase. J. Biol. Chem. 1996, 271, 19789–19793. [Google Scholar] [CrossRef] [Green Version]
- Lally, D.; Ingmire, P.; Tong, H.Y.; He, Z.H. Antisense expression of a cell wall-associated protein kinase, WAK4, inhibits cell elongation and alters morphology. Plant Cell 2001, 13, 1317–1331. [Google Scholar] [CrossRef] [Green Version]
- Wagner, T.A.; Kohorn, B.D. Wall-associated kinases are expressed throughout plant development and are required for cell expansion. Plant Cell 2001, 13, 303–318. [Google Scholar] [CrossRef]
- Kohorn, B.D.; Kobayashi, M.; Johansen, S.; Riese, J.; Huan, L.F.; Koch, K.; Fu, S.; Dotson, A.; Byers, N. An Arabidopsis cell wall-associated kinase required for invertase activity and cell growth. Plant J. 2006, 46, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.M.; Wagner, T.A.; Perret, M.; He, Z.H.; He, D.; Kohorn, B.D. Waks: Cell wall-associated kinases linking the cytoplasm to the extracellular matrix. Plant Mol. Biol. 2001, 47, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Shiu, S.H.; Bleecker, A.B. Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc. Natl. Acad. Sci. USA 2001, 98, 10763–10768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verica, J.A.; Chae, L.; Tong, H.Y.; Ingmire, P.; Zheng, H.H. Tissue specific and developmentally regulated expression of a cluster of tandemly arrayed cell wall-associated kinase-like kinase genes in Arabidopsis. Plant Physiol. 2003, 133, 1732–1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Z.H.; He, D.; Kohorn, B.D. Requirement of the induced expression of a cell wall associated receptor kinase for survival during the pathogen response. Plant J. 1998, 14, 55–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maleck, K.; Levine, A.; Eulgem, T.; Morgan, A.; Schmid, J.; Lawton, K.A.; Dangl, J.L.; Dietrich, R.A. The transcriptome of Arabidopsis thaliana during systemic acquired resistance. Nat. Genet. 2000, 26, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Schenk, P.M.; Kazan, K.; Wilson, I.; Anderson, J.P.; Richmond, T.; Somerville, S.C.; Manners, J.M.; Kazan, K. Coordinated plant defense responses in Arabidopsis revealed by microarray analysis. Proc. Natl. Acad. Sci. USA 2000, 97, 11655–11660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosli, H.G.; Zheng, Y.; Pombo, M.A.; Zhong, S.; Bombarely, A.; Fei, Z. Transcriptomics-based screen for genes induced by flagellin and repressed by pathogen effectors identifies a cell wall-associated kinase involved in plant immunity. Genome Biol. 2013, 14, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.F.; Zhang, W.N.; Fan, Y.L.; Yang, X.Y.; Shi, M.F.; Zhang, R.Y.; Wang, Y.; Qin, S.H. Genome-wide identification and expression analysis of wall-associated kinase (WAK) gene family in potato (Solanum tuberosum L.). Plant Biotechnol. Rep. 2022, 16, 317–331. [Google Scholar] [CrossRef]
- Xia, X.; Zhang, X.; Zhang, Y.; Wang, L.; An, Q.; Tu, Q.; Wu, L.; Jiang, P.; Zhang, P.; Yu, L.; et al. Characterization of the WAK Gene Family Reveals Genes for FHB Resistance in Bread Wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2022, 23, 7157. [Google Scholar] [CrossRef]
- Delteil, A.; Gobbato, E.; Cayrol, B.; Estevan, J.; Michel-Romiti, C.; Dievart, A.; Kroj, T.; Morel, J.-B. Several wall-associated kinases participate positively and negatively in basal defense against rice blast fungus. BMC Plant Biol. 2016, 16, 17–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, K.M.; Cao, J.B.; Zhang, J.; Xia, F.; Ke, Y.G.; Zhang, H.T. Improvement of multiple agronomic traits by a disease resistance gene via cell wall reinforcement. Nat. Plants 2017, 3, 17009–17017. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.R.; Walker, J.C. Receptor-like protein kinases: The keys to response. Curr. Opin. Plant. Biol. 2003, 6, 339–342. [Google Scholar] [CrossRef]
- Hou, X.; Tong, H.; Selby, J.; Dewitt, J.; Peng, X.; He, Z.H. Involvement of a cell wall-associated kinase, WAKL4, in Arabidopsis mineral responses. Plant Physiol. 2005, 139, 1704–1716. [Google Scholar] [CrossRef] [Green Version]
- Sivaguru, M.; Ezaki, B.; He, Z.H.; Tong, H.; Osawa, H.; Baluska, F.; Hideaki, D.; Matsumoto, V. Aluminum induced gene expression and protein localization of a cell wall-associated receptor protein kinase in Arabidopsis thaliana. Plant Physiol. 2003, 132, 2256–2266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, A.; Cho, S.; Yun, U.; Jin, M.; Lee, S.; Sachetto-Martins, G.; Park, O.K. Interaction of the Arabidopsis receptor protein kinase Wak1 with a glycine-rich protein, AtGRP3. J. Biol. Chem. 2003, 276, 26688–26693. [Google Scholar] [CrossRef] [Green Version]
- Decreux, A.; Messiaen, J. Wall-associated kinase WAK1 interacts with cell wall pectins in a calcium-induced conformation. Plant Cell Physiol. 2005, 46, 268–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brutus, A.; Sicilia, F.; Macone, A.; Cervone, F.; Lorenzo, G.D. A domain swap approach reveals a role of the plant wall-associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proc. Natl. Acad. Sci. USA 2010, 107, 9452–9457. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.Z.; Cheng, Q.; Wang, H.Y.; Gao, H.; Fang, X.; Chen, X.; Zhao, M.; Wei, W.L.; Song, B.; Liu, S.S.; et al. GmBTB/POZ promotes the ubiquitination and degradation of LHP1 to regulate the response of soybean to Phytophthora sojae. Commun. Biol. 2021, 4, 372. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.Y.; He, S.F.; Meng, F.S.; Zhang, C.Z.; Fan, S.J.; Wu, J.J.; Xu, P.F.; Zhang, S.Z. GmSnRK1. 1, a sucrose non-fermenting-1 (SNF1)-related protein kinase, promotes soybean resistance to Phytophthora sojae. Front. Plant Sci. 2019, 10, 996. [Google Scholar] [CrossRef]
- Liu, Q.B.; Ding, Y.L.; Shi, Y.T.; Ma, L.; Yang, Y.; Song, C.P.; Wilkins, K.A.; Davies, J.M.; Knight, H.; Knight, M.R.; et al. The calcium transporter annexin1 mediates cold-induced calcium signaling and freezing tolerance in plants. EMBO J. 2020, 40, 2. [Google Scholar] [CrossRef] [PubMed]
- Mu, C.; Zhou, L.; Shan, L.B.; Li, F.J.; Li, Z.H. Phosphatase GhDsPTP3a interacts with annexin protein GhANN8b to reversely regulate salt tolerance in cotton (Gossypium spp.). N. Phytol. 2019, 223, 4. [Google Scholar] [CrossRef]
- Li, X.F.; Zhang, Q.; Yang, X.; Han, J.B.; Zhu, Z.G. OsANN3, a calcium-dependent lipid binding annexin is a positive regulator of aba-dependent stress tolerance in rice. Plant Sci. 2019, 284, 212–220. [Google Scholar] [CrossRef]
- Laohavisit, A.; Davies, J.M. Annexins. N. Phytol. 2011, 189, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Niebel, F.D.; Timmers, A.C.; Chabaud, M.; Defaux-Petras, A.; Barker, D.G. The nod factor-elicited annexin MtANN1 is preferentially localised at the nuclear periphery in symbiotically activated root tissues of Medicago truncatula. Plant J. 2002, 32, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Truman, W.; Bennett, M.H.; Kubigsteltig, I.; Turnbull, C.; Grant, M. Arabidpsis systemic immunity uses conserved defense signaling pathways and is mediated by jasmonates. Proc. Natl. Acad. Sci. USA 2007, 104, 1075–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandeputte, O.; Lowe, Y.O.; Burssens, S.; Raemdonck, D.V.; Hutin, D.; Boniver, D.; Geelen, D.; Jaziri, E.M.; Baucher, M. The tobacco Ntann12 gene, encoding an annexin, is induced upon Rhodoccocus fascians infection and during leafy gall development. Mol. Plant Pathol. 2007, 8, 185–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laohavisit, A.; Davies, J.M. Multifunctional annexins. Plant Sci. 2009, 177, 532–539. [Google Scholar] [CrossRef]
- Delmer, D.P.; Potikha, T.S. Structures and function of annexins in plants. Cell Mol. Life Sci. 1997, 53, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Laohavisit, A.; Mortimer, J.C.; Demidchik, V.; Coxon, K.M.; Stancombe, M.A.; Macpherson, N. Zea mays annexins modulate cytosolic free Ca2+ and generate a Ca2+-permeable conductance. Plant Cell 2009, 21, 479–493. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Gao, H.; Li, R.P.; Han, D.; Wang, L.; Wu, J.J.; Xu, P.F.; Zhang, S.Z. GmBTB/POZ, a novel BTB/POZ domain-containing nuclear protein, positively regulates the response of soybean to Phytophthora sojae infection. Mol. Plant Pathol. 2019, 20, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantu, D.; Vicente, A.R.; Labavitch, J.M.; Bennett, A.B.; Powell, A.L.T. Strangers in the matrix: Plant cell walls and pathogen susceptibility. Trends Plant Sci. 2008, 13, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.C. Structure and function of the receptor-like protein kinases of higher plants. Plant Mol. Biol. 1994, 26, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Moss, S.E.; Morgan, R.O. The annexins. Genome Biol. 2004, 5, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammad-Sidik, A.; Sun, J.; Shin, R.; Song, Z.; Ning, Y.; Matthus, E.; Wilkins, K.A.; Davies, J.M. Annexin 1 Is a Component of eATP-Induced Cytosolic Calcium Elevation in Arabidopsis thaliana Roots. Int. J. Mol. Sci. 2021, 22, 494. [Google Scholar] [CrossRef] [PubMed]
- Konopka-Postupolska, D.; Clark, G.; Goch, G.; Debski, J.; Floras, K.; Cantero, A.; Fijolek, B.; Roux, S.; Hennig, J. The role of annexin1 in drought stress in Arabidopsis. Plant Physiol. 2009, 150, 1394–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorantla, M.; Babu, P.R.; Vbr, L.; Feltus, F.A.; Reddy, A.R. Functional genomics of drought stress response in rice: Transcript mapping of annotated unigenes of an indica rice (Oryza sativa L. cv. Nagina22). Curr. Sci. 2005, 89, 496–514. Available online: https://www.jstor.org/stable/24110798 (accessed on 15 July 2022).
- Tuomainen, M.; Tervahauta, A.; Hassinen, V.; Schat, H.; Koistinen, K.M.; Lehesranta, S.; Rantalainen, K.; Jukka Ha yrinen, J.; Auriola, S.; Anttonen, M.; et al. Proteomics of Thlaspi caerulescens accessions and an inter-accession cross segregating for zinc accumulation. J. Exp. Bot. 2010, 61, 1075–1087. [Google Scholar] [CrossRef]
- Zhou, M.L.; Yang, X.B.; Zhang, Q.; Zhou, M.; Zhao, E.Z.; Tang, Y.X.; Zhu, X.M.; Shao, J.R.; Wu, Y.M. Induction of annexin by heavy metal and jasmonic acid in Zea mays. Funct. Integr. Genomic. 2013, 13, 241–251. [Google Scholar] [CrossRef]
- Qiao, B.; Zhang, Q.; Liu, D.L.; Wang, H.Q.; Yin, J.Y.; Wang, R.; He, M.; Cui, M.; Shang, Z.; Wang, D.; et al. A calcium-binding protein, rice annexin OsANN1, enhances heat stress tolerance by modulating the production of H2O2. J. Exp. Bot. 2015, 66, 5853–5866. [Google Scholar] [CrossRef] [Green Version]
- Jami, S.K.; Clark, G.B.; Turlapati, S.A.; Handley, C.; Roux, S.J.; Kirti, O.B. Ectopic expression of an annexin from Brassica juncea confers tolerance to abiotic and biotic stress treatments in transgenic tobacco. Plant Physiol. Bioch. 2008, 46, 1019–1030. [Google Scholar] [CrossRef]
- He, M.J.; Yang, X.L.; Cui, S.L.; Mu, G.J.; Hou, M.Y.; Chen, H.Y.; Liu, L.F. Molecular cloning and characterization of annexin genes in peanut (Arachis hypogaea L.). Gene 2015, 568, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.F.; Luo, Y.; Jiang, Z.R.; Wang, F.; Xu, Q.J. Cloning and expression characterization of four annexin genes during germination and abiotic stress in Brassica rapa subsp. rapa ‘Tsuda’. Plant Mol. Biol. Rep. 2015, 34, 467–482. [Google Scholar] [CrossRef]
- Szalonek, M.; Sierpien, B.; Rymaszewski, W.; Gieczewska, K.; Garstka, M.; Lichocka, M.; Sass, L.; Paul, K.; Vass, I.; Vankova, R.; et al. Potato annexin STANN1 promotes drought tolerance and mitigates light stress in transgenic Solanum tuberosum L. plants. PLoS ONE 2015, 10, e0132683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, H.; Jiang, L.; Du, B.; Ning, B.; Ding, X.; Zhang, C.; Song, B.; Liu, S.S.; Zhao, M.; Zhao, Y.X.; et al. GmMKK4-activated GmMPK6 stimulates GmERF113 to trigger resistance to Phytophthora sojae in soybean. Plant J. 2022, 111–112, 473–495. [Google Scholar] [CrossRef] [PubMed]
- Durrant, W.E.; Dong, X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef]
- Malamy, J.; Carr, J.P.; Klessig, D.F.; Raskin, I. Salicylic acid: A likely endogenous signal in the resistance response of tobacco to viral infection. Science 1990, 250, 1002–1004. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, L.; Courteaux, B.; Hubert, J.; Kauffmann, S.; Renault, J.H.; Clement, C.; Baillieul, F.; Dorey, S. Rhamnolipids elicit defense responses and induce disease resistance against biotrophic, hemibiotrophic, and necrotrophic pathogens that require different signaling pathways in arabidopsis and highlight a central role for salicylic acid. Plant Physiol. 2012, 160, 1630. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.X.; Klessig, D.F. Identification of a soluble salicylic acid-binding protein that may function in signal transduction in the plant disease-resistance response. Proc. Natl. Acad. Sci. USA 1991, 88, 8179–8183. [Google Scholar] [CrossRef] [Green Version]
- Dong, X. SA, JA, ethylene, and disease resistance in plants. Curr. Opin. Plant. Biol. 1998, 1, 316–323. [Google Scholar] [CrossRef]
- Qi, J.; Wang, J.; Gong, Z.; Zhou, J.M. Apoplastic ROS signaling in plant immunity. Curr. Opin. Plant. Biol. 2017, 38, 92–100. [Google Scholar] [CrossRef]
- Foyer, C.H.; Shigeoka, S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011, 155, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Hückelhoven, R.; Kogel, K.H. Reactive oxygen intermediates in plant-microbe interactions: Who is who in powdery mildew resistance? Planta 2003, 216, 891–902. [Google Scholar] [CrossRef]
- Shetty, N.P.; Kristensen, B.K.; Newman, M.A.; Moller, K.; Gregersen, P.L.; Jorgensen, H.J. Association of hydrogen peroxide with restriction of Septoria tritici in resistant wheat. Physiol. Mol. Plant Pathol. 2003, 62, 333–346. [Google Scholar] [CrossRef]
- Shetty, N.P.; Jorgensen, H.J.; Jensen, J.D.; Collinge, D.B.; Shetty, H.S. Roles of reactive oxygen species in interactions between plants and pathogens. Eur. J. Plant Pathol. 2008, 121, 267–280. [Google Scholar] [CrossRef]
- Du, X.M.; Yin, W.X.; Zhao, Y.X.; Zhang, H. The production and scavenging of reactive oxygen species in plants. Sheng Wu Gong Cheng Xue Bao (Chin. J. Biotechnol.) 2001, 17, 121–125. [Google Scholar] [PubMed]
- Quan, L.J.; Zhang, B.; Shi, W.W.; Li, H.Y. Hydrogen peroxide in plants: A versatile molecule of the reactive oxygen species network. J. Integr. Plant Biol. 2008, 50, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Moller, I.M. Plant mitochondria and oxidative stress: Electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu. Rev. Plant Physiol. Mol. Biol. 2001, 52, 561–591. [Google Scholar] [CrossRef] [Green Version]
- Hiraga, S.; Sasaki, K.; Ito, H.; Ohashi, Y.; Matsui, H. A large family of class III plant peroxidases. Plant Cell Physiol. 2001, 42, 462–468. [Google Scholar] [CrossRef] [Green Version]
- Passardi, F.; Cosio, C.; Penel, C.; Dunand, C. Peroxidases have more functions than a Swiss army knife. Plant Cell Rep. 2005, 24, 255–265. [Google Scholar] [CrossRef]
- Fehr, W.R.; Caviness, C.E.; Burmood, D.T.; Pennington, J. Stage of development descriptions for soybeans. Glycine max (L.) Merrill. Crop Sci. 1971, 11, 929–931. [Google Scholar] [CrossRef]
- Ward, E.W.B.; Lazarovits, G.; Unwin, C.H.; Buzzell, R.I. Hypocotyl reactions and glyceollin in soybeans inoculated with zoospores of Phytophthora megasperma var. sojae. Phytopathology 1979, 69, 951–955. [Google Scholar] [CrossRef]
- Zhang, Z.G.; Liu, X.; Wang, X.D.; Zhou, M.P.; Zhou, X.Y.; Ye, X.G.; Wei, X.N. An R2R3 MYB transcription factor in wheat, TaPIMP1, mediates host resistance to Bipolaris sorokiniana and drought stresses through regulation of defenseand stress-related genes. N. Phytol. 2012, 196, 1155–1170. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.; Alvarez-Venegas, R.; Avramova, Z. An effificient chromatin immunoprecipitation (ChIP) protocol for studying histone modififications in Arabidopsis plants. Nat. Protoc. 2008, 3, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Cho, Y.; Sheen, J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007, 2, 1565–1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerschen, A.; Napoli, C.A.; Jorgensen, R.A.; Müller, A.E. Effectiveness of RNA interference in transgenic plants. FEBS Lett. 2004, 566, 223–228. [Google Scholar] [CrossRef]
- Holsters, M.; De, W.D.; Depicker, A.; Messens, E.; Van, M.M.; Schell, J. Transfection and transformation of Agrobacterium tumefaciens. Mol. Gen. Genet. 1978, 163, 181–187. [Google Scholar] [CrossRef]
- Paz, M.M.; Shou, H.; Guo, Z.; Zhang, Z.; Banerjee, A.K.; Wang, K. Assessment of conditions affecting Agrobacterium-mediated soybean transformation using the cotyledonary node explant. Euphytica 2004, 136, 167–179. [Google Scholar] [CrossRef]
- Graham, T.L.; Graham, M.Y.; Subramanian, S.; Yu, O. RNAi silencing of genes for elicitation or biosynthesis of 5-deoxyisofavonoids suppresses race-specific resistance and HR cell death in Phytophthora sojae infected tissues. Plant Physiol. 2007, 144, 728–740. [Google Scholar] [CrossRef] [Green Version]
- Kereszt, A.; Li, D.; Indrasumunar, A.; Nguyen, C.D.; Nontachaiyapoom, S.; Kinkema, M.; Gresshoff, P.M. Agrobacterium rhizogenes-mediated transformation of soybean to study root biology. Nat. Protoc. 2007, 2, 948–952. [Google Scholar] [CrossRef]
- Morrison, R.H.; Thorne, J.C. Inoculation of detached cotyledons for screening soybeans against two races of Phytophthora megasperma Var. sojae 1. Crop Sci. 1978, 18, 1089–1091. [Google Scholar] [CrossRef]
- Dong, L.D.; Cheng, Y.X.; Wu, J.J.; Cheng, Q.; Li, W.B.; Fan, S.J.; Jiang, L.Y.; Xu, Z.L.; Kong, F.J.; Zhang, D.; et al. Overexpression of GmERF5, a new member of the soybean EAR-motifcontaining ERF transcription factor, enhances resistance to Phytophthora sojae in soybean. J. Exp. Bot. 2015, 66, 2635–2647. [Google Scholar] [CrossRef]
- Chacón, O.; González, M.; López, Y.; Portieles, R.; Pujol, M.; González, E.; Schoonbeek, H.J.; Métraux, J.P.; Borrás-Hidalgo, O. Over-expression of a protein kinase gene enhances the defense of tobacco against Rhizoctonia solani. Gene 2010, 452, 54–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Jiang, Y.; Wu, S.F.; Zhou, M.Y.; Wu, Y.L.; Chen, G.Q. CCAAT/enhancer-binding protein alpha antagonizes transcriptional activity of hypoxia-inducible factor 1 alpha with direct proteinprotein interaction. Carcinogenesis 2008, 29, 291–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, X.; Welti, R.; Wang, X. Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry. Nat. Protoc. 2010, 5, 986–992. [Google Scholar] [CrossRef]
- Wang, S.D.; Zhu, F.; Yuan, S.; Yang, H.; Xu, F.; Shang, J.; Jia, S.D.; Zhang, Z.W.; Wang, J.H.; Xi, D.H.; et al. The roles of ascorbic acid and glutathione in symptom alleviation to SA-deficient plants infected with RNA viruses. Planta 2011, 234, 171–181. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Qian, H.F.; Chen, W.; Sun, L.W.; Jin, Y.X.; Liu, W.P.; Fu, Z.W. Inhibitory effffects of paraquat on photosynthesis and the response to oxidative stress in Chlorella vulgaris. Ecotoxicology 2009, 18, 537–543. [Google Scholar] [CrossRef]
- Yu, R.; Hinkle, P.M. Rapid turnover of calcium in the endoplasmic reticulum during signaling: Studies with cameleon calcium indicators. J. Biol. Chem. 2000, 275, 23648–23653. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Li, N.; Chen, S.; Wu, J.; He, S.; Zhao, Y.; Wang, X.; Chen, X.; Zhang, C.; Fang, X.; et al. GmWAK1, Novel Wall-Associated Protein Kinase, Positively Regulates Response of Soybean to Phytophthora sojae Infection. Int. J. Mol. Sci. 2023, 24, 798. https://doi.org/10.3390/ijms24010798
Zhao M, Li N, Chen S, Wu J, He S, Zhao Y, Wang X, Chen X, Zhang C, Fang X, et al. GmWAK1, Novel Wall-Associated Protein Kinase, Positively Regulates Response of Soybean to Phytophthora sojae Infection. International Journal of Molecular Sciences. 2023; 24(1):798. https://doi.org/10.3390/ijms24010798
Chicago/Turabian StyleZhao, Ming, Ninghui Li, Simei Chen, Junjiang Wu, Shengfu He, Yuxin Zhao, Xiran Wang, Xiaoyu Chen, Chuanzhong Zhang, Xin Fang, and et al. 2023. "GmWAK1, Novel Wall-Associated Protein Kinase, Positively Regulates Response of Soybean to Phytophthora sojae Infection" International Journal of Molecular Sciences 24, no. 1: 798. https://doi.org/10.3390/ijms24010798



