Molecular Mechanisms of Scombroid Food Poisoning
Abstract
1. Introduction
2. Contributing Factors of Scombroid Food Poisoning
2.1. Fish Species
2.2. Bacteria Species
2.3. Environmental Conditions
3. Molecular Mechanisms of Scombroid Food Poisoning
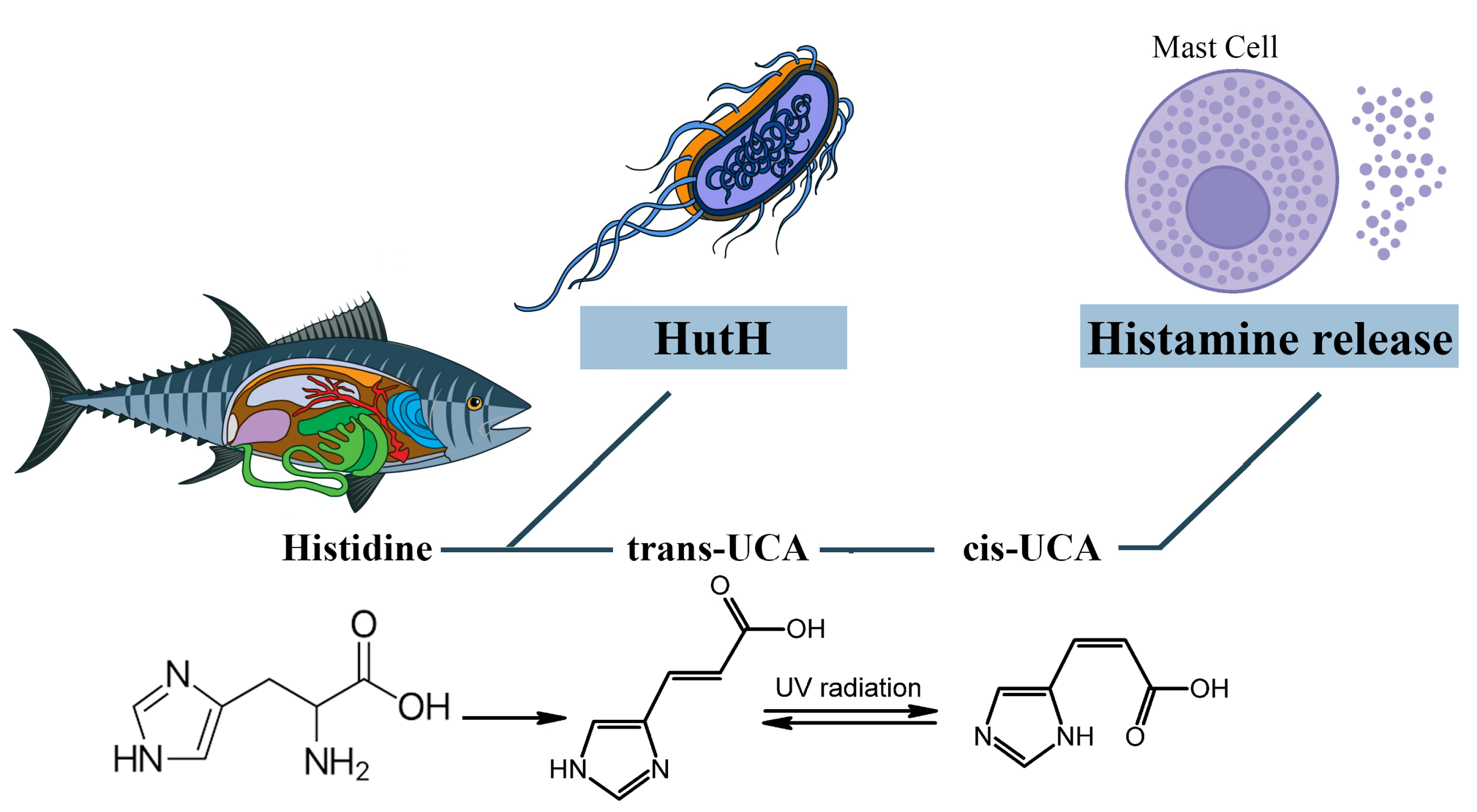
3.1. The Role of Exogenous Histamine and Its Metabolic Pathways
3.2. The Role of Other Biogenic Amines and Their Metabolic Pathways
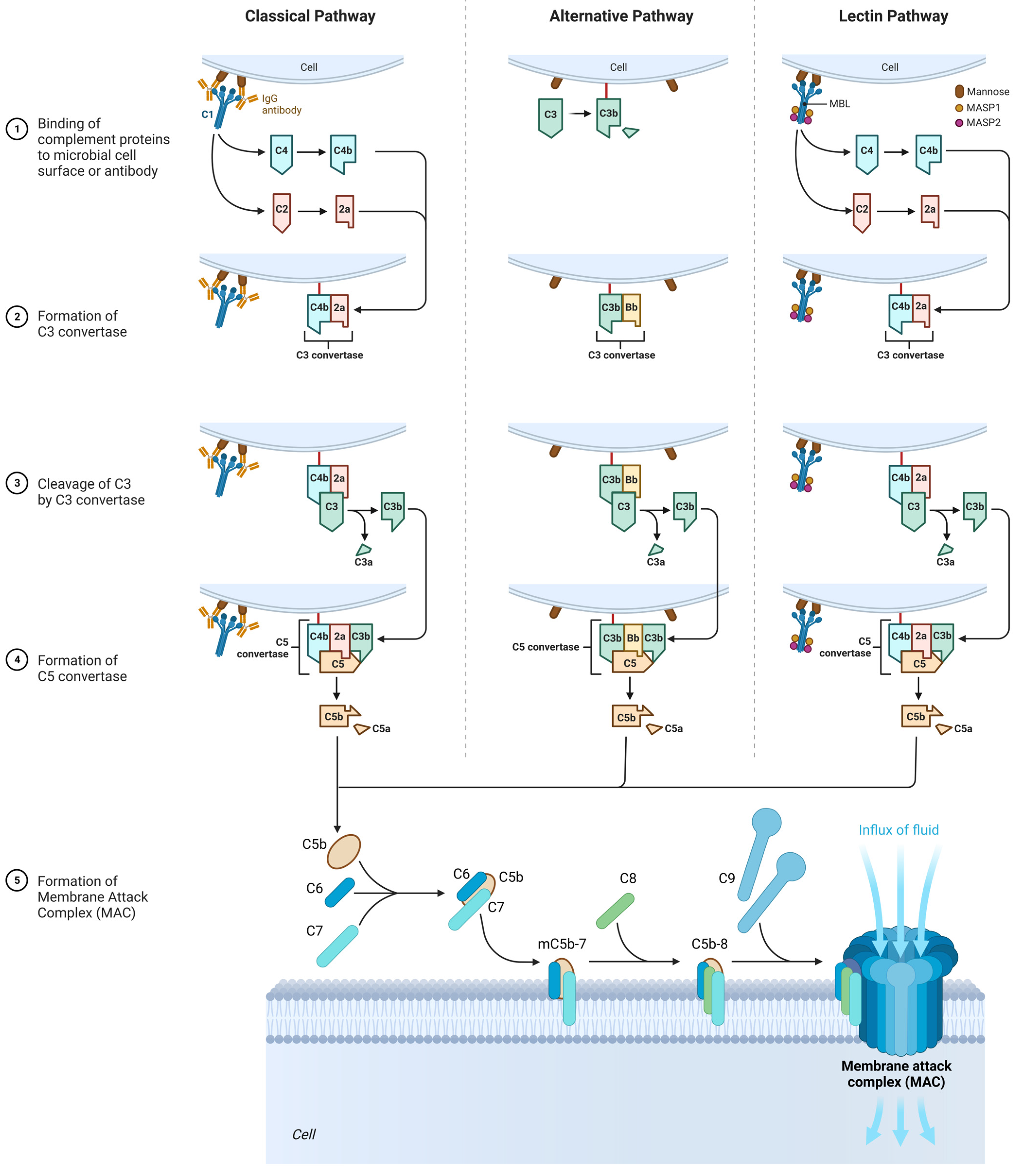
3.3. The Role of the Complement System
3.3.1. Classic Pathway
3.3.2. Alternative Pathway
3.3.3. Lectin Pathway
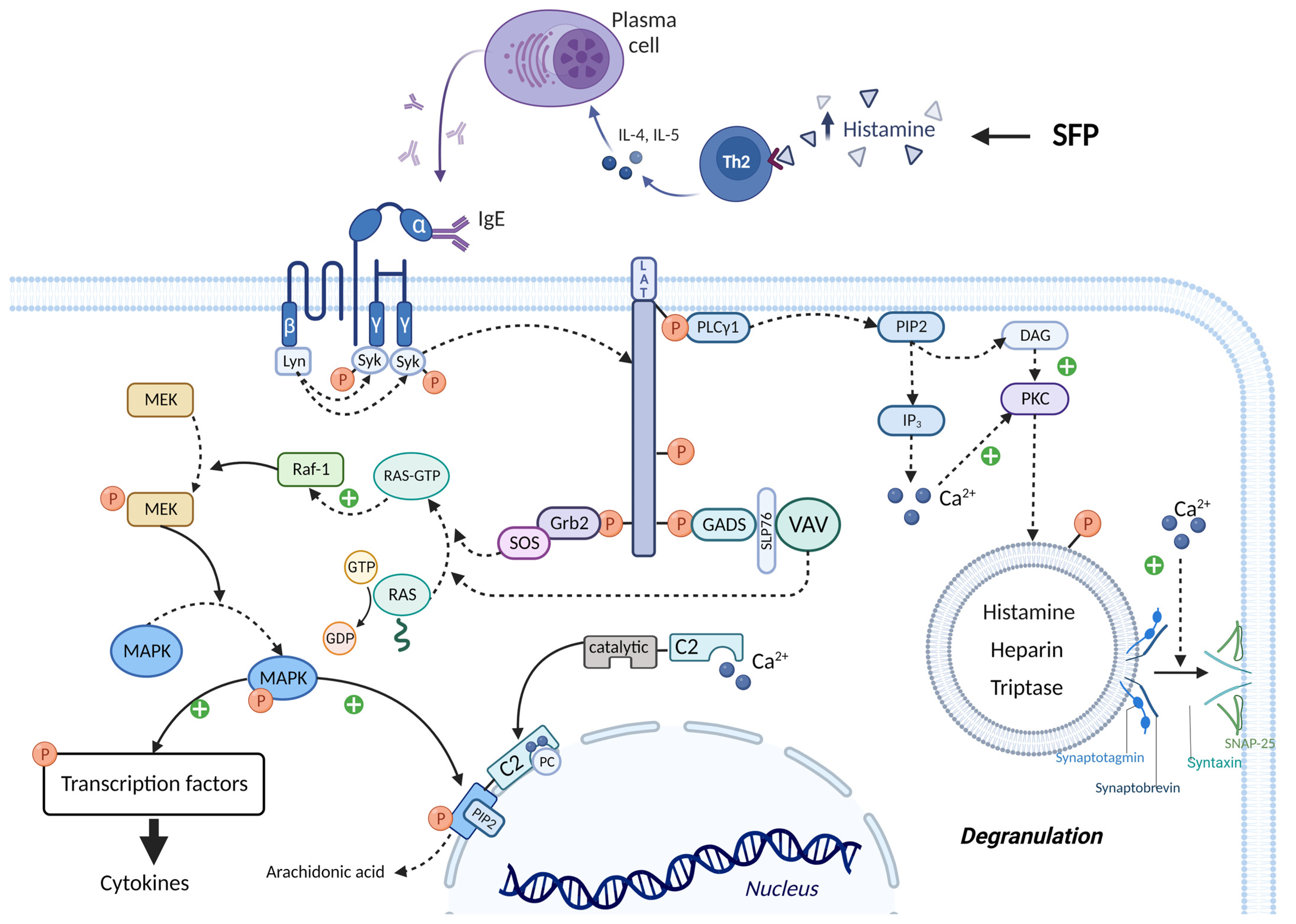
3.4. The Role of Histamine Liberators
3.5. The Role of Associated Diseases and Disorders in the SFP Mechanism
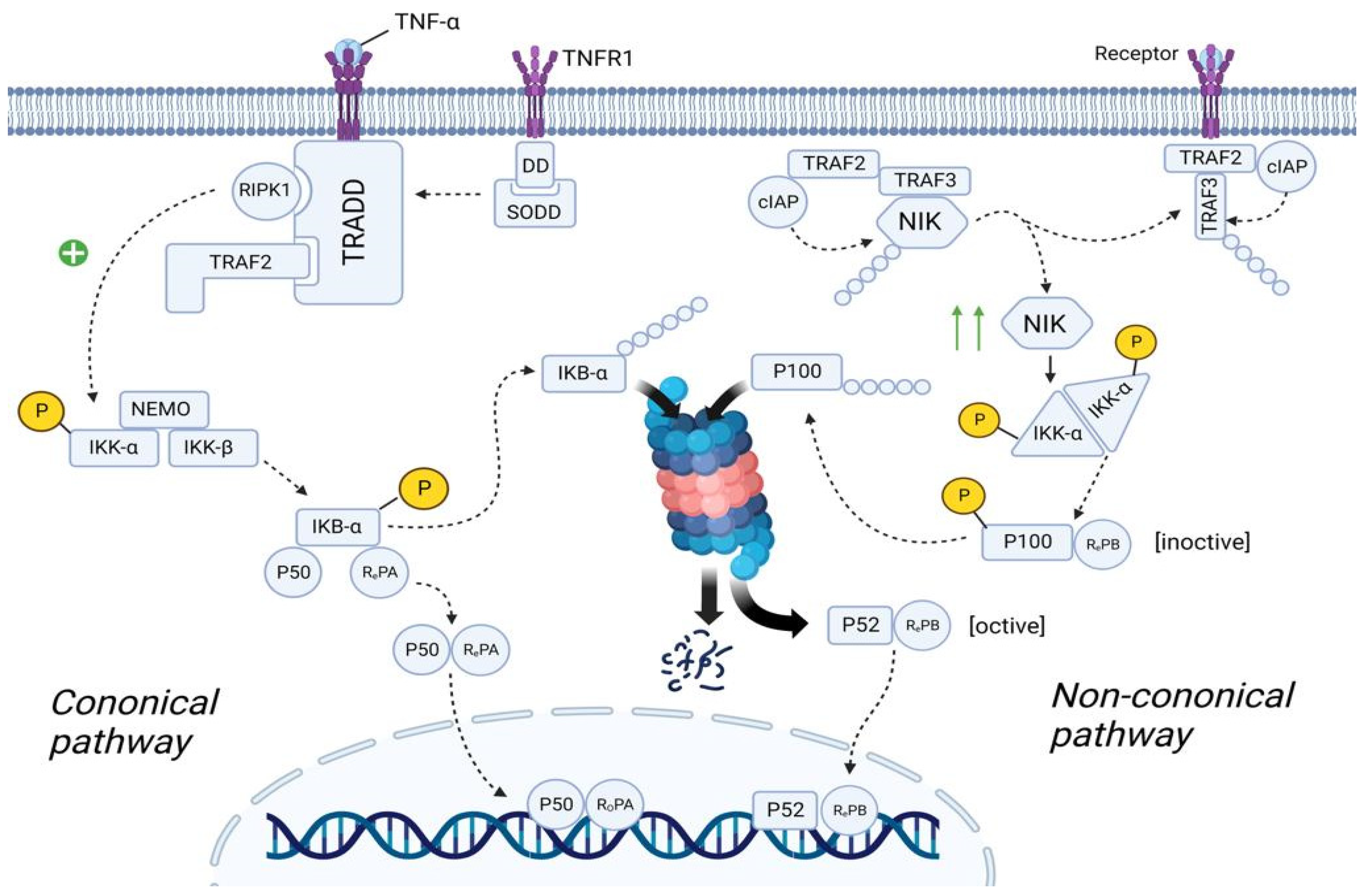
3.5.1. Canonical NF-κB Pathway
3.5.2. Non-Canonical NF-κB Pathway
4. Key Points in the Relief of the SFP
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhernov, Y.V.; Vysochanskaya, S.O.; Sukhov, V.A.; Zaostrovtseva, O.K.; Gorshenin, D.S.; Sidorova, E.A.; Mitrokhin, O.V. Molecular Mechanisms of Eosinophilic Esophagitis. Int. J. Mol. Sci. 2021, 22, 13183. [Google Scholar] [CrossRef] [PubMed]
- Moneret-Vautrin, D.A. False food allergies: Non-specific reactions to foodstuffs. Clin. React. Food 1983, 135–153. [Google Scholar] [CrossRef]
- Velázquez-Sámano, G.; Collado-Chagoya, R.; Cruz-Pantoja, R.A.; Velasco-Medina, A.A.; Rosales-Guevara, J. Reacciones de hipersensibilidad a aditivosalimentarios [Hypersensitivity reactions to food additives]. Revista alergia México 2019, 66, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Traylor, J.; Mathew, D. Histamine toxicity. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499871/ (accessed on 20 December 2022).
- Marcus, E.N. Scombroid (Histamine) Poisoning. Up to Date. [Literature Review Current through: April 2022.|This Topic Last Updated: 9 February 2022.]. Available online: http://www.uptodate.com/contents/scombroid-histamine-poisoning (accessed on 20 December 2022).
- Bulula, N.; Mugoyela, V.; Kaale, E. Investigation of Contributing Factors to Scombroid Fish Poisoning among Dar es Salaam City Residents in Tanzania. Open Access Libr. J. 2017, 4, 1–16. [Google Scholar] [CrossRef]
- Pinzer, T.C.; Tietz, E.; Waldmann, E.; Schink, M.; Neurath, M.F.; Zopf, Y. Circadian profiling reveals higher histamine plasma levels and lower diamine oxidase serum activities in 24% of patients with suspected histamine intolerance compared to food allergy and controls. Allergy 2018, 73, 949–957. [Google Scholar] [CrossRef]
- Gargano, D.; Appanna, R.; Santonicola, A.; De Bartolomeis, F.; Stellato, C.; Cianferoni, A.; Casolaro, V.; Iovino, P. Food Allergy and Intolerance: A Narrative Review on Nutritional Concerns. Nutrients 2021, 13, 1638. [Google Scholar] [CrossRef]
- Brock, I.; Eng, N.; Maitland, A. Adult-onset mast cell activation syndrome following scombroid poisoning: A case report and review of the literature. J. Med. Case Rep. 2021, 15, 620. [Google Scholar] [CrossRef]
- Lalmalani, R.M.; Gan Hs, J.; Stacey, S. Two Case Reports of Scombroid in Singapore: A Literature Review. Cureus 2022, 14, e22580. [Google Scholar] [CrossRef]
- Taylor, R.; Burg, J.; Opara, N. The Sea Was Angry That Day My Friends: An Inland Case of Acute Scombroid Poisoning with a Twist. Cureus 2021, 13, e18394. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, P.; Bian, L.; Hong, S. Rare Death via Histamine Poisoning Following Crab Consumption: A Case Report. J. Forensic Sci. 2018, 63, 980–982. [Google Scholar] [CrossRef]
- Hernandez Garcilazo, N.; Prasad, R.M.; Varghese, M.; Kemnic, T. Scombroid pancreatitis from mahi-mahi consumption. BMJ Case Rep. 2021, 14, e240261. [Google Scholar] [CrossRef] [PubMed]
- Van Hage, M.; Hamsten, C.; Valenta, R. ImmunoCAP assays: Pros and cons in allergology. J. Allergy Clin. Immunol. 2017, 140, 974–977. [Google Scholar] [CrossRef] [PubMed]
- Lupinek, C.; Wollmann, E.; Baar, A.; Banerjee, S.; Breiteneder, H.; Broecker, B.M.; Bublin, M.; Curin, M.; Flicker, S.; Garmatiuk, T.; et al. Advances in allergen-microarray technology for diagnosis and monitoring of allergy: The MeDALL allergen-chip. Methods 2014, 66, 106–119. [Google Scholar] [CrossRef]
- Garib, V.; Rigler, E.; Gastager, F.; Campana, R.; Dorofeeva, Y.; Gattinger, P.; Zhernov, Y.; Khaitov, M.; Valenta, R. Determination of IgE and IgG reactivity to more than 170 allergen molecules in paper-dried blood spots. J. Allergy Clin. Immunol. 2019, 143, 437–440. [Google Scholar] [CrossRef]
- Karsonova, A.; Riabova, K.; Villazala-Merino, S.; Campana, R.; Niederberger, V.; Eckl-Dorna, J.; Fröschl, R.; Perkmann, T.; Zhernov, Y.V.; Elisyutina, O.G.; et al. Highly sensitive ELISA-based assay for quantification of allergen-specific IgE antibody levels. Allergy 2020, 75, 2668–2670. [Google Scholar] [CrossRef] [PubMed]
- Joint Task Force on Practice Parameters; American Academy of Allergy, Asthma and Immunology; American College of Allergy, Asthma and Immunology; Joint Council of Allergy, Asthma and Immunology. Drug allergy: An updated practice parameter. Ann. Allergy Asthma Immunol. 2010, 105, 259–273. [Google Scholar] [CrossRef]
- Qiu, S.; Liu, Z.; Hou, L.; Li, Y.; Wang, J.; Wang, H.; Du, W.; Wang, W.; Qin, Y.; Liu, Z. Complement activation associated with polysorbate 80 in beagle dogs. Int. Immunopharmacol. 2013, 15, 144–149. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Q.; Shi, C.; Zhang, X. Drug-Induced Pseudoallergy: A Review of the Causes and Mechanisms. Pharmacology 2018, 101, 104–110. [Google Scholar] [CrossRef]
- Schirone, M.; Visciano, P.; Tofalo, R.; Suzzi, G. Histamine Food Poisoning. Handb. Exp. Pharmacol. 2017, 241, 217–235. [Google Scholar] [CrossRef]
- Maintz, L.; Novak, N. Histamine and histamine intolerance. Am. J. Clin. Nutr. 2007, 85, 1185–1196. [Google Scholar] [CrossRef]
- James, M. Hungerford, Histamine and Scombrotoxins. Toxicon 2021, 201, 115–126. [Google Scholar] [CrossRef]
- FAO/WHO [Food and Agriculture Organization of the United Nations/World Health Organization]. Public Health Risks of Histamine and other Biogenic Amines from Fish and Fishery Products. Meeting Report. 2013. Available online: https://www.who.int/publications/i/item/9789240691919 (accessed on 20 December 2022).
- Rodríguez-Caravaca, G.; Hijas-Gómez, A.I.; Tejedor-Alonso, M.Á.; Del-Moral-Luque, J.A.; Delgado-Iribarren, A.; Valverde-Cánovas, J.F.; Gil-de-Miguel, Á. Food poisoning caused by scombroids: A case-control study. J. Infect. Public Health 2019, 12, 591–593. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Joint FAO/WHO Literature Review: Histamine in Salmonids; Food & Agriculture Organization: Rome, Italy, 2018. [Google Scholar]
- Pereira, E.; Elliot, E.L.; Singleton, L.S.; Otto, M.; Tesfai, A.; Doyle, M.; Hawk, H.; Bloodgood, S.; Benner, R.A.; Ross, M.P.; et al. An Outbreak Investigation of Scombrotoxin Fish Poisoning Illnesses in the United States Linked to Yellowfin Tuna Imported from Vietnam-2019. J. Food Prot. 2021, 84, 962–972. [Google Scholar] [CrossRef]
- Zapata, R.; Acevedo, K.; Mella, M.; Mella, V.; Zapata, K. A clinical epidemiological onsite study of a massive outbreak of Scombroid fish poisoning after consumption of yellowtail kingfish in northern Chile. J. Food Safe Hyg. 2021, 6, 145–159. [Google Scholar] [CrossRef]
- Hwang, D.-F.; Chang, S.-H.; Shiau, C.-Y.; Cheng, C.-C. Biogenic Amines in the Flesh of Sailfish (Istiophorus plafypferus) Responsible for Scornbroid Poisoning. J. Food Sci. 1995, 60, 926–928. [Google Scholar] [CrossRef]
- James, M.J.; Martin, J.; Loessner, D.A. Golden Modern Food Microbiology, 7th ed.; Springer Science + Business Media, Inc.: New York, NY, USA, 2005; ISBN 0-387-23180-3. [Google Scholar]
- Dalgaard, P.; Madsen, H.L.; Samieian, N.; Emborg, J. Biogenic amine formation and microbial spoilage in chilled garfish (Belone belone belone)—Effect of modified atmosphere packaging and previous frozen storage. J. Appl. Microbiol. 2006, 101, 80–95. [Google Scholar] [CrossRef]
- Guergué-Díaz de Cerio, O.A.; Barrutia-Borque, J.; Gardeazabal-García, J. Scombroid Poisoning: A Practical Approach. Actas Dermo-Sifiliográficas (Engl. Ed.) 2016, 107, 567–571. [Google Scholar] [CrossRef]
- Chapter 3—Integrated Analytical Approaches for Food Safety and Environmental Sustainability. In Analytical Methods for Agricultural Contaminants; Maestroni, B., Ochoa, V., Cannavan, A., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 25–174. ISBN 9780128159408. [Google Scholar] [CrossRef]
- Bjornsdottir-Butler, K.; May, S.; Hayes, M.; Abraham, A.; Benner, R.A., Jr. Characterization of a novel enzyme from Photobacterium phosphoreum with histidine decarboxylase activity. Int. J. Food Microbiol. 2020, 334, 108815. [Google Scholar] [CrossRef] [PubMed]
- Kempkes, C.; Buddenkotte, J.; Cevikbas, F.; Buhl, T.; Steinhoff, M. 11 Role of PAR-2 in neuroimmune communication and itch. In Itch Mechanisms and Treatment; CRC Press: Boca Raton, FL, USA, 2014; Volume 193. [Google Scholar]
- Lin, C.-M.; Kung, H.-F.; Huang, Y.-L.; Huang, C.-Y.; Su, Y.-C.; Tsai, Y.-H. Histamine production by Raoultella ornithinolytica in canned tuna meat at various storage temperatures. Food Control 2012, 25, 723–727. [Google Scholar] [CrossRef]
- Kim, S.H.; Ben-Gigirey, B.; Barros-Velázquez, J.; Price, R.J.; An, H. Histamine and biogenic amine production by Morganella morganii isolated from temperature-abused albacore. J. Food Prot. 2000, 63, 244–251. [Google Scholar] [CrossRef]
- Kanki, M.; Yoda, T.; Tsukamoto, T.; Shibata, T. Klebsiella pneumoniae produces no histamine: Raoultella planticola and Raoultella ornithinolytica strains are histamine producers. Appl. Environ. Microbiol. 2002, 68, 3462–3466. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.L.; Guthertz, L.S.; Leatherwood, M.; Lieber, E.R. Histamine production by Klebsiella pneumoniae and an incident of scombroid fish poisoning. Appl. Environ. Microbiol. 1979, 37, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, D.H.; Frank, H.A. Histamine-producing bacteria in decomposing skipjack tuna (Katsuwonus pelamis). Appl. Environ. Microbiol. 1982, 44, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Bjornsdottir-Butler, K.; McCarthy, S.; Burkhardt, W., 3rd; Benner, R.A., Jr. Importance of histamine-producing Clostridium perfringens in scombrotoxin-forming fish. J. Food Prot. 2013, 76, 1283–1287. [Google Scholar] [CrossRef]
- Gullian Klanian, M.; Delgadillo Díaz, M.; Sánchez Solís, M.J. Molecular Characterization of Histamine-Producing Psychrotrophic Bacteria Isolated from Red Octopus (Octopus maya) in Refrigerated Storage. High Throughput 2018, 7, 25. [Google Scholar] [CrossRef]
- Behling, A.R.; Taylor, S.L. Bacterial histamine production as a function of temperature and time of incubation. J. Food Sci. 1982, 47, 1311–1314. [Google Scholar] [CrossRef]
- Margareta, G.; Ratnawati, S.E.; Puspita, I.D. Growth Rate and Histamine Production of Citrobacter freundii CK01 in Various Incubation Temperatures. E3S Web Conf. 2020, 147, 03018. [Google Scholar] [CrossRef]
- Food and Drug Administration. Fish and Fishery Products Hazards and Controls Guidance, 4th ed.; FDA: Silver Spring, MD, USA, 2020. Available online: https://www.fda.gov/media/80637/download (accessed on 20 December 2022).
- EU. Commission Regulation (EC) No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs. Off. J. Eur. Union 2005, 338, 1–25. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32005R2073 (accessed on 20 December 2022).
- Durak-Dados, A.; Michalski, M.; Osek, J. Histamine and Other Biogenic Amines in Food. J. Vet. Res. 2020, 64, 281–288. [Google Scholar] [CrossRef]
- Barrett, K.A.; Nakao, J.H.; Tyalor, E.V.; Eggerts, C.; Gould, L.H. Fish-associated foodborne disease outbreaks: United States, 1998–2015. Foodborne Pathog. Dis. 2017, 14, 537–543. [Google Scholar] [CrossRef]
- González, M.C.; Díaz, A.C.; Moncayo, J.G.; Marín, J.A. Scombroid poisoning secondary to tuna ingestion: A case report. Biomedica 2020, 40, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Scombroid (histamine) poisoning. Prepared for the Ministry of Health by ESR Ltd. Issued May 2001. Available online: https://www.mpi.govt.nz/dmsdocument/26078-Scombroid-Histamine-poisoning (accessed on 20 December 2022).
- Chung, B.Y.; Park, S.Y.; Byun, Y.S.; Son, J.H.; Choi, Y.W.; Cho, Y.S.; Kim, H.O.; Park, C.W. Effect of Different Cooking Methods on Histamine Levels in Selected Foods. Ann. Dermatol. 2017, 29, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Broczek, M.; Rautenstrauch, D.; Windyga, B.; Ścieżyńska, H.; Jędra, M.; Badowski, P.; Karłowska, B. The content of histamine and tyramine depending on the microbiological quality of salted herrings, stored at different temperatures. Roczn. PZH 2003, 54, 87–95. [Google Scholar]
- Buyuktiryaki, B.; Masini, M.; Mori, F.; Barni, S.; Liccioli, G.; Sarti, L.; Lodi, L.; Giovannini, M.; du Toit, G.; Lopata, A.L.; et al. IgE-Mediated Fish Allergy in Children. Medicina 2021, 57, 76. [Google Scholar] [CrossRef]
- Torres Borrego, J.; Martínez Cuevas, J.F.; Tejero García, J. Reactividad cruzada entre pescados y mariscos [Cross reactivity between fish and shellfish]. Allergol. Immunopathol. 2003, 31, 146–151. (In Spanish) [Google Scholar] [CrossRef]
- Szebeni, J. Complement activation-related pseudoallergy. In The Complement System; Szebeni, J., Ed.; Springer: Boston, MA, USA, 2004. [Google Scholar] [CrossRef]
- Mahdavinia, M. Food Allergy in Adults. Med. Clin. N. Am. 2019, 104, 145–155. [Google Scholar] [CrossRef]
- Thangam, E.B.; Jemima, E.A.; Singh, H.; Baig, M.S.; Khan, M.; Mathias, C.B.; Church, M.K.; Saluja, R. The Role of Histamine and Histamine Receptors in Mast Cell-Mediated Allergy and Inflammation: The Hunt for New Therapeutic Targets. Front. Immunol. 2018, 9, 1873. [Google Scholar] [CrossRef]
- Manjur, J.E.; Ciuffo, M.M.; Burdowski, J.; George, S.; Kalogeropoulos, A.; Chen, O. Scombroid Fish Poisoning Leading to Vasospastic Angina: A Rare Etiology of Chest Pain. JACC Case Rep. 2019, 1, 322–326. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Fish and Fishery Products Hazards and Controls Guide; U.S. Food and Drug Administration: Washington, DC, USA; Center for Food Safety and Applied Nutrition: Washington, DC, USA; Office of Seafood: Washington, DC, USA, 1998; pp. 245–248.
- Feldman, K.A.; Werner, S.B.; Cronan, S.; Hernandez, M.; Horvath, A.R.; Lea, C.S.; Au, A.M.; Vugia, D.J. A large outbreak of scombroid fish poisoning associated with eating escolar fish (Lepidocybium flavobrunneum). Epidemiol. Infect. 2005, 133, 29–33. [Google Scholar] [CrossRef]
- Feng, C.; Teuber, S.; Gershwin, M.E. Histamine (Scombroid) Fish Poisoning: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 50, 64–69. [Google Scholar] [CrossRef]
- Dessauer, C.W.; Ostrom, R.; Seifert, R.; Watts, V.J. Adenylyl cyclases (ACs) (version 2019.4) in the IUPHAR/BPS Guide to Pharmacology Database. IUPHAR/BPS Guide Pharmacol. CITE 2019, 4. [Google Scholar] [CrossRef]
- Álvarez-Santos, M.D.; Álvarez-González, M.; Estrada-Soto, S.; Bazán-Perkins, B. Regulation of Myosin Light-Chain Phosphatase Activity to Generate Airway Smooth Muscle Hypercontractility. Front. Physiol. 2020, 11, 701. [Google Scholar] [CrossRef] [PubMed]
- Raszewski, J.A.; Sharma, S. Physiology, Ryanodine Receptor. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538323/ (accessed on 20 December 2022).
- Frank, K.; Kranias, E.G. Phospholamban and cardiac contractility. Ann. Med. 2000, 32, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Primeau, J.O.; Armanious, G.P.; Fisher, M.E.; Young, H.S. The Sarco Endoplasmic Reticulum Calcium ATPase. Subcell Biochem. 2018, 87, 229–258. [Google Scholar] [CrossRef]
- Kania, E.; Roest, G.; Vervliet, T.; Parys, J.B.; Bultynck, G. IP3 Receptor-Mediated Calcium Signaling and Its Role in Autophagy in Cancer. Front. Oncol. 2017, 7, 140. [Google Scholar] [CrossRef]
- Fleming, I. Molecular mechanisms underlying the activation of eNOS. Pflug. Arch. 2010, 459, 793–806. [Google Scholar] [CrossRef]
- Kovalev, I.V.; Popov, A.G.; Panov, A.A.; Borodin, I.U.L.; Afinogenova, I.A.D.; Kapilevich, L.V.; Baskakov, M.B.; Medvedev, M.A. Issledovanie mekhanizmov NO-zavisimogo rasslableniia gladkikh myshts aorty krysy s pomoshch’iu nitrosoedineniĭ [Mechanisms of NO-dependent relaxation in smooth muscles of the rat aorta with nitro compounds]. Eksp. Klin. Farmakol. 2001, 64, 33–36. (In Russian) [Google Scholar]
- Schnittler, H. Contraction of endothelial cells: 40 years of research, but the debate still lives. Histochem. Cell Biol. 2016, 146, 651–656. [Google Scholar] [CrossRef]
- Hungerford, J.M. Scombroid poisoning: A review. Toxicon 2010, 56, 231–243. [Google Scholar] [CrossRef]
- Blackwell, B.; Mabbitt, L.A. Tyramine in Cheese Related to Hypertensive Crises after Monoamine-Oxidase Inhibition. Lancet 1965, 1, 938–940. [Google Scholar] [CrossRef]
- Staruszkiewiez, W.F.; Bond, J.F. Gas chromatographid determination of cadaverine, putrescine, and histamine in foods. J. Assoc. Off. Anal. Chem. 1981, 64, 584–591. [Google Scholar]
- Moret, S.; Smela, D.; Populin, T.; Conte, L.S. A survey on free biogenic amine content of fresh and preserved vegetables. Food Chem. 2005, 89, 355–361. [Google Scholar] [CrossRef]
- Stratton, J.E.; Hutkins, R.W.; Taylor, S.L. Biogenic Amines in Cheese and other Fermented Foods: A Review. J. Food Prot. 1991, 54, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Visciano, P.; Schirone, M.; Paparella, A. An Overview of Histamine and Other Biogenic Amines in Fish and Fish Products. Foods 2020, 9, 1795. [Google Scholar] [CrossRef] [PubMed]
- Prester, L. Biogenic amines in fish, fish products and shellfish: A review. Food Addit. Contam. Part A Chem. Anal. Control Expo Risk Assess. 2011, 28, 1547–1560. [Google Scholar] [CrossRef]
- Doeun, D.; Shin, H.S.; Chung, M.S. Effects of storage temperatures, vacuum packaging, and high hydrostatic pressure treatment on the formation of biogenic amines in Gwamegi. Appl. Biol. Chem. 2016, 59, 51–58. [Google Scholar] [CrossRef]
- Voigt, M.N.; Eitenmiller, R.R. Role of Histidine and Tyrosine Decarboxylases and Mono- and Diamine Oxidases in Amine Build-Up in Cheese. J. Food Prot. 1978, 41, 182–186. [Google Scholar] [CrossRef]
- Taylor, S.L. Histamine Poisoning Associated with Fish, Cheese and Other Foods; Report VPH/FOS/85.1; World Health Organization Press: Geneva, Switzerland, 1985; pp. 1–48.
- Bargossi, E.; Gardini, F.; Gatto, V.; Montanari, C.; Torriani, S.; Tabanelli, G. The Capability of Tyramine Production and Correlation between Phenotypic and Genetic Characteristics of Enterococcus faecium and Enterococcus faecalis Strains. Front. Microbiol. 2015, 6, 1371. [Google Scholar] [CrossRef]
- Burns, C.; Kidron, A. Biochemistry, Tyramine. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK563197/ (accessed on 20 December 2022).
- Szebeni, J. Complement activation-related pseudoallergy: A stress reaction in blood triggered by nanomedicines and biological. Mol. Immunol. 2014, 61, 163–173. [Google Scholar] [CrossRef]
- Macdougall, I.C.; Vernon, K. Complement Activation-Related Pseudo-Allergy: A Fresh Look at Hypersensitivity Reactions to Intravenous Iron. Am. J. Nephrol. 2017, 45, 60–62. [Google Scholar] [CrossRef]
- Neun, B.W.; Ilinskaya, A.N.; Dobrovolskaia, M.A. Analysis of Complement Activation by Nanoparticles. Methods Mol. Biol. 2018, 1682, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Nesargikar, P.N.; Spiller, B.; Chavez, R. The complement system: History, pathways, cascade and inhibitors. Eur. J. Microbiol. Immunol. 2012, 2, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Merle, N.S.; Church, S.E.; Fremeaux-Bacchi, V.; Roumenina, L.T. Complement System Part I—Molecular Mechanisms of Activation and Regulation. Front. Immunol. 2015, 6, 262. [Google Scholar] [CrossRef] [PubMed]
- Uvnas, B. The mechanism of histamine liberation. J. Pharm. Pharmacol. 1958, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Brunet, C.; Bédard, P.M.; Hébert, J. Analysis of compound 48/80-induced skin histamine release and leukotriene production in chronic urticaria. J. Allergy Clin. Immunol. 1988, 82 Pt 1, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Quan, P.L.; Sabaté-Brescó, M.; Guo, Y.; Martín, M.; Gastaminza, G. The Multifaceted Mas-Related G Protein-Coupled Receptor Member X2 in Allergic Diseases and Beyond. Int. J. Mol. Sci. 2021, 22, 4421. [Google Scholar] [CrossRef]
- Kumar, M.; Duraisamy, K.; Chow, B.K. Unlocking the Non-IgE-Mediated Pseudo-Allergic Reaction Puzzle with Mas-Related G-Protein Coupled Receptor Member X2 (MRGPRX2). Cells 2021, 10, 1033. [Google Scholar] [CrossRef]
- Wille, J.J.; Kydonieus, A.F.; Murphy, G.F. Cis-urocanic acid induces mast cell degranulation and release of preformed TNF-alpha: A possible mechanism linking UVB and cis-urocanic acid to immunosuppression of contact hypersensitivity. Skin Pharmacol. Appl. Skin Physiol. 1999, 12, 18–27. [Google Scholar] [CrossRef]
- Lehane, L.; Olley, J. Histamine fish poisoning revisited. Int. J. Food Microbiol. 2000, 58, 1–37. [Google Scholar] [CrossRef]
- Khalil, Z.; Townley, S.L.; Grimbaldeston, M.A.; Finlay-Jones, J.J.; Hart, P.H. Cis-Urocanic acid stimulates neuropeptide release from peripheral sensory nerves. J. Inves.t Dermatol. 2001, 117, 886–891. [Google Scholar] [CrossRef]
- Zare, D.; Muhammad, K.; Bejo, M.H.; Ghazali, H.M. Development and validation of an ion-pair chromatographic method for simultaneous determination of trans- and cis-urocanic acid in fish samples. J. Chromatogr. A 2012, 1256, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.H.; Grimbaldeston, M.A.; Finlay-Jones, J.J. Mast cells in UV-B-induced immunosuppression. J. Photochem. Photobiol. B 2000, 55, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Norval, M.; El-Ghorr, A.A. Studies to determine the immunomodulating effects of cis-urocanic acid. Methods 2002, 28, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Norval, M.; Gibbs, N.K.; Gilmour, J. The role of urocanic acid in UV-induced immunosuppression: Recent advances (1992–1994). Photochem. Photobiol. 1995, 62, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, S.E. 5.11—Skin immunology and immunotoxicity. In Comprehensive Toxicology, 2nd ed.; McQueen, C.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 217–234. ISBN 9780080468846. [Google Scholar] [CrossRef]
- Walterscheid, J.P.; Nghiem, D.X.; Kazimi, N.; Nutt, L.K.; McConkey, D.J.; Norval, M.; Ullrich, S.E. Cis-urocanic acid, a sunlight-induced immunosuppressive factor, activates immune suppression via the 5-HT2A receptor. Proc. Natl. Acad. Sci. USA 2006, 103, 17420–17425. [Google Scholar] [CrossRef]
- Zare, D.; Muhammad, K.; Ghazali, H.M. The manner of urocanic acid accumulation in fish by tracking histidine ammonia lyase activity during storage of vacuum-packed, eviscerated, and whole fish. J. Food Process Preserv. 2021, 45, e15288. [Google Scholar] [CrossRef]
- Wu, P.C.; Kroening, T.A.; White, P.J.; Kendrick, K.E. Histidine ammonia-lyase from Streptomyces griseus. Gene 1992, 115, 19–25. [Google Scholar] [CrossRef]
- Hug, D.H.; Dunkerson, D.D.; Hunter, J.K. The degradation of L-histidine and trans- and cis-urocanic acid by bacteria from skin and the role of bacterial cis-urocanic acid isomerase. J. Photochem. Photobiol. B 1999, 50, 66–73. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Elias, P.M.; Man, M.Q.; Hupe, M.; Selden, C.; Sundberg, J.P.; Tschachler, E.; Eckhart, L.; Mauro, T.M.; Feingold, K.R. Is the filaggrin-histidine-urocanic acid pathway essential for stratum corneum acidification? J. Invest. Dermatol. 2010, 130, 2141–2144. [Google Scholar] [CrossRef]
- Mackie, I.M.; Fernandez-Salguéro, J. Histidine metabolism in fish. Urocanic acid in mackerel (Scomber scombrus). J. Sci. Food Agric. 1977, 28, 935–940. [Google Scholar] [CrossRef]
- Zare, D.; Muhammad, K.; Bejo, M.H.; Ghazali, H.M. Determination of trans- and cis-urocanic acid in relation to histamine, putrescine, and cadaverine contents in tuna (Auxis Thazard) at different storage temperatures. J. Food Sci. 2015, 80, T479–T483. [Google Scholar] [CrossRef] [PubMed]
- Comas-Basté, O.; Sánchez-Pérez, S.; Veciana-Nogués, M.T.; Lator-re-Moratalla, M.; Vidal-Carou, M.D.C. Histamine Intolerance: The Current State of the Art. Biomolecules 2020, 10, 1181. [Google Scholar] [CrossRef]
- Petersen, J.; Drasche, A.; Raithel, M.; Schwelberger, H.G. Analysis of genetic polymorphisms of enzymes involved in histamine metabolism. Inflamm. Res. 2003, 52 (Suppl. S1), S69–S70. [Google Scholar] [CrossRef] [PubMed]
- Schwelberger, H.G.; Falus, A. Diamine oxidase (DAO) enzyme and gene. In Histamine: Biology and Medical Aspects; Spring Med Publishing: Budapest, Hungary, 2004; pp. 43–52. [Google Scholar]
- Palma, A.M.; Hanes, M.R.; Marshall, J.S. Mast Cell Modulation of B Cell Responses: An Under-Appreciated Partnership in Host Defence. Front. Immunol. 2021, 12, 718499. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, S.; Mehta, A. Review Article—Role of Cytokines in Pathophysiology of Asthma. Iran. J. Pharmacol. Ther. 2006, 5, 1–4. [Google Scholar]
- Packard, K.A.; Khan, M.M. Effects of histamine on Th1/Th2 cytokine balance. Int. Immunopharmacol. 2003, 3, 909–920. [Google Scholar] [CrossRef]
- Hsieh, F.H. Gastrointestinal Involvement in Mast Cell Activation Disorders. Immunol. Allergy Clin. N. Am. 2018, 38, 429–441. [Google Scholar] [CrossRef]
- Gilfillan, A.M.; Tkaczyk, C. Integrated signalling pathways for mast-cell activation. Nat. Rev. Immunol. 2006, 6, 218–230. [Google Scholar] [CrossRef]
- Colgan, J.; Rothman, P. Manipulation of signaling to control allergic inflammation. Curr. Opin. Allergy Clin. Immunol. 2007, 7, 51–56. [Google Scholar] [CrossRef]
- Rognlien, K.T.; Woodbury, D.J. Chapter 16—Reconstituting SNARE proteins into BLMs. In Membrane Science and Technology; Tien, H.T., Ottova-Leitmannova, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; Volume 7, pp. 479–488. ISBN 9780444509406. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, Y.; Zhang, J.; Hong, T. Allantoin Inhibits Compound 48/80-Induced Pseudoallergic Reactions In Vitro and In Vivo. Molecules 2022, 27, 3473. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Lin, L.; Zhang, Z.; Zhang, H.; Hu, H. Targeting NF-κB pathway for the therapy of diseases: Mechanism and clinical study. Signal Transduct. Target Ther. 2020, 5, 209. [Google Scholar] [CrossRef] [PubMed]
- Begalli, F.; Bennett, J.; Capece, D.; Verzella, D.; D’Andrea, D.; Tornatore, L.; Franzoso, G. Unlocking the NF-κB Conundrum: Embracing Complexity to Achieve Specificity. Biomedicines 2017, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Trask, O.J., Jr. Nuclear Factor Kappa B (NF-κB) Translocation assay development and validation for high content screening. In Assay Guidance Manual [Internet]; Markossian, S., Grossman, A., Brimacombe, K., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. Available online: https://www.ncbi.nlm.nih.gov/books/NBK100914/figure/nfkb.F2/ (accessed on 26 December 2022).
- Sun, S.C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Harmelin, Y.; Hubiche, T.; Pharaon, M.; Del Giudice, P. Three cases of scombroid poisoning. Ann. Dermatol. Venereol. 2018, 145, 29–32. [Google Scholar] [CrossRef] [PubMed]
- De Silva, D.; Geromi, M.; Panesar, S.S.; Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Cardona, V.; Dubois, A.E.; Halken, S.; et al. Acute and long-term management of food allergy: Systematic review. Allergy 2014, 69, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Beyer, K.; Bindslev-Jensen, C.; Cardona, V.; Dubois, A.; duToit, G.; Eigenmann, P.; et al. EAACI food allergy and anaphylaxis guidelines: Diagnosis and management of food allergy. Allergy 2014, 69, 1008–1025. [Google Scholar] [CrossRef] [PubMed]




| Bacteria | Conditions of Histamine Formation/ Food Source | Level of Histamine Forming | Reference |
|---|---|---|---|
| Morganella morganii | 15–37 °C, pH < 7/Fish, Tuna Salad | >5000 ppm | [34,37] |
| Photobacterium phosphoreum | 4–37 °C, pH < 7/Fish | 1188 ppm | [34] |
| Photobacterium kishitanii | 20–37 °C, pH < 7/Fish | 1545 ppm | [34] |
| Klebsiella pneumoniae | 37 °C, pH < 7/Fish, Swiss cheese | 442 ppm | [34,38,39] |
| Raoultella planticola (Synonym: Klebsiella pneumoniae strain T2 or Klebsiella planticola (ATCC 43176)) | 20–37 °C, pH < 7/Fish | between 2810 and 5250 mg/L | [38] |
| Raoultella ornithinolytica | 20–37 °C, pH < 7/Fish | between 2810 and 5250 mg/L | [38] |
| Clostridium perfringens | 20–37 °C, pH < 7/Fish | 19 ppm in tuna, 3 ppm in spanish mackerel | [40,41] |
| Hafnia alvei | 30–37 °C, pH < 7/Fish, Fish broth | >88.7 ppm (30 °C), 42.1 ppm (15 °C) | [42,43] |
| Enterobacter cloacae | 30–37 °C, pH < 7/Fish broth | >1000 ppm | [42] |
| Citrobacter freundii | 30–37 °C, pH < 7/Fish | >1600 ppm (37 °C), 474 ppm (30 °C) | [30,43,44] |
| Escherichia coli | 30–37 °C, pH < 7/Fish | Not detected <1 ppm, but they have the enzyme | [30,43] |
| Characteristic | IgE-Associated Allergy to Fish and Seafood | Scombroid Food Poisoning |
|---|---|---|
| Common mechanisms | ||
| Histamine release | Yes | |
| Activation of the complement system | Yes | |
| Atypical eicosanoid synthesis | Yes | |
| Inhibiting of bradykinin decomposition | Yes | |
| Different mechanisms | ||
| Dose dependence on antigen/allergen | Occasionally, depends on the molecular structure of the antigen | Always |
| Hidden sensibilization to the antigen | Yes | No |
| Elevation of non-specific IgE in serum | Often | Occasionally |
| Elevation of specific IgE | Always | Never |
| Concentration of the substance that induces the reaction | Low | High |
| Immunological stage | Yes | No |
| Formation of antigen-specific immune complexes | Yes | No |
| Influence on the mast cells | IgE-mediated influence | Direct influence with the substance |
| Mediators | Endogenous histamine, tryptase | Exogenous histamine, histamine liberators, serotonin liberators |
| DAO defect | Rare | Possible |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhernov, Y.V.; Simanduyev, M.Y.; Zaostrovtseva, O.K.; Semeniako, E.E.; Kolykhalova, K.I.; Fadeeva, I.A.; Kashutina, M.I.; Vysochanskaya, S.O.; Belova, E.V.; Shcherbakov, D.V.; et al. Molecular Mechanisms of Scombroid Food Poisoning. Int. J. Mol. Sci. 2023, 24, 809. https://doi.org/10.3390/ijms24010809
Zhernov YV, Simanduyev MY, Zaostrovtseva OK, Semeniako EE, Kolykhalova KI, Fadeeva IA, Kashutina MI, Vysochanskaya SO, Belova EV, Shcherbakov DV, et al. Molecular Mechanisms of Scombroid Food Poisoning. International Journal of Molecular Sciences. 2023; 24(1):809. https://doi.org/10.3390/ijms24010809
Chicago/Turabian StyleZhernov, Yury V., Mark Y. Simanduyev, Olga K. Zaostrovtseva, Ekaterina E. Semeniako, Kseniia I. Kolykhalova, Inna A. Fadeeva, Maria I. Kashutina, Sonya O. Vysochanskaya, Elena V. Belova, Denis V. Shcherbakov, and et al. 2023. "Molecular Mechanisms of Scombroid Food Poisoning" International Journal of Molecular Sciences 24, no. 1: 809. https://doi.org/10.3390/ijms24010809
APA StyleZhernov, Y. V., Simanduyev, M. Y., Zaostrovtseva, O. K., Semeniako, E. E., Kolykhalova, K. I., Fadeeva, I. A., Kashutina, M. I., Vysochanskaya, S. O., Belova, E. V., Shcherbakov, D. V., Sukhov, V. A., Sidorova, E. A., & Mitrokhin, O. V. (2023). Molecular Mechanisms of Scombroid Food Poisoning. International Journal of Molecular Sciences, 24(1), 809. https://doi.org/10.3390/ijms24010809










