Comparative Study on Epidermal Moisturizing Effects and Hydration Mechanisms of Rice-Derived Glucosylceramides and Ceramides
Abstract
:1. Introduction
2. Results
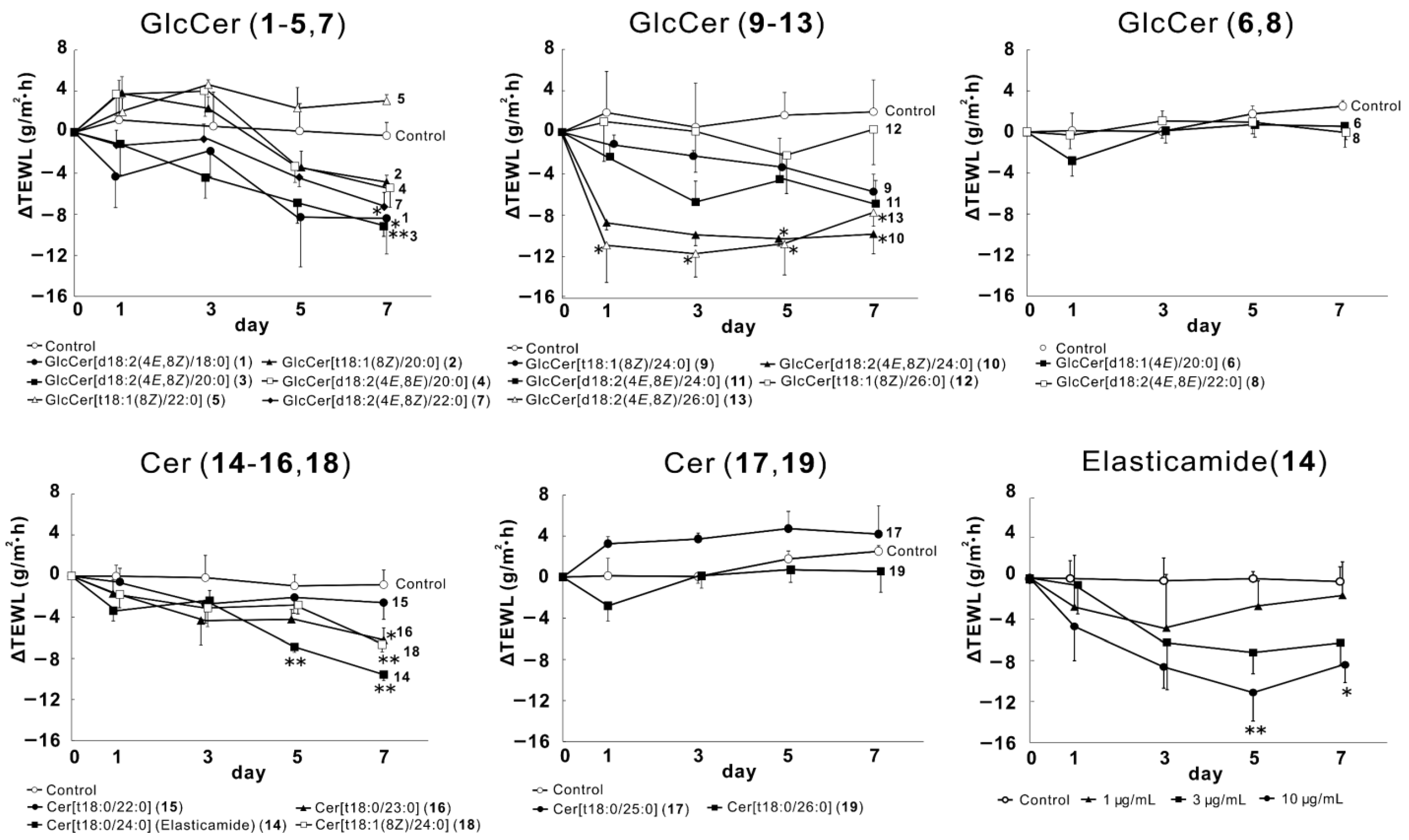
2.1. Effects of GlcCer (1–13) and Cer (14–19) on TEWL in the Reconstructed Human Epidermal Keratinization (RHEK) Model
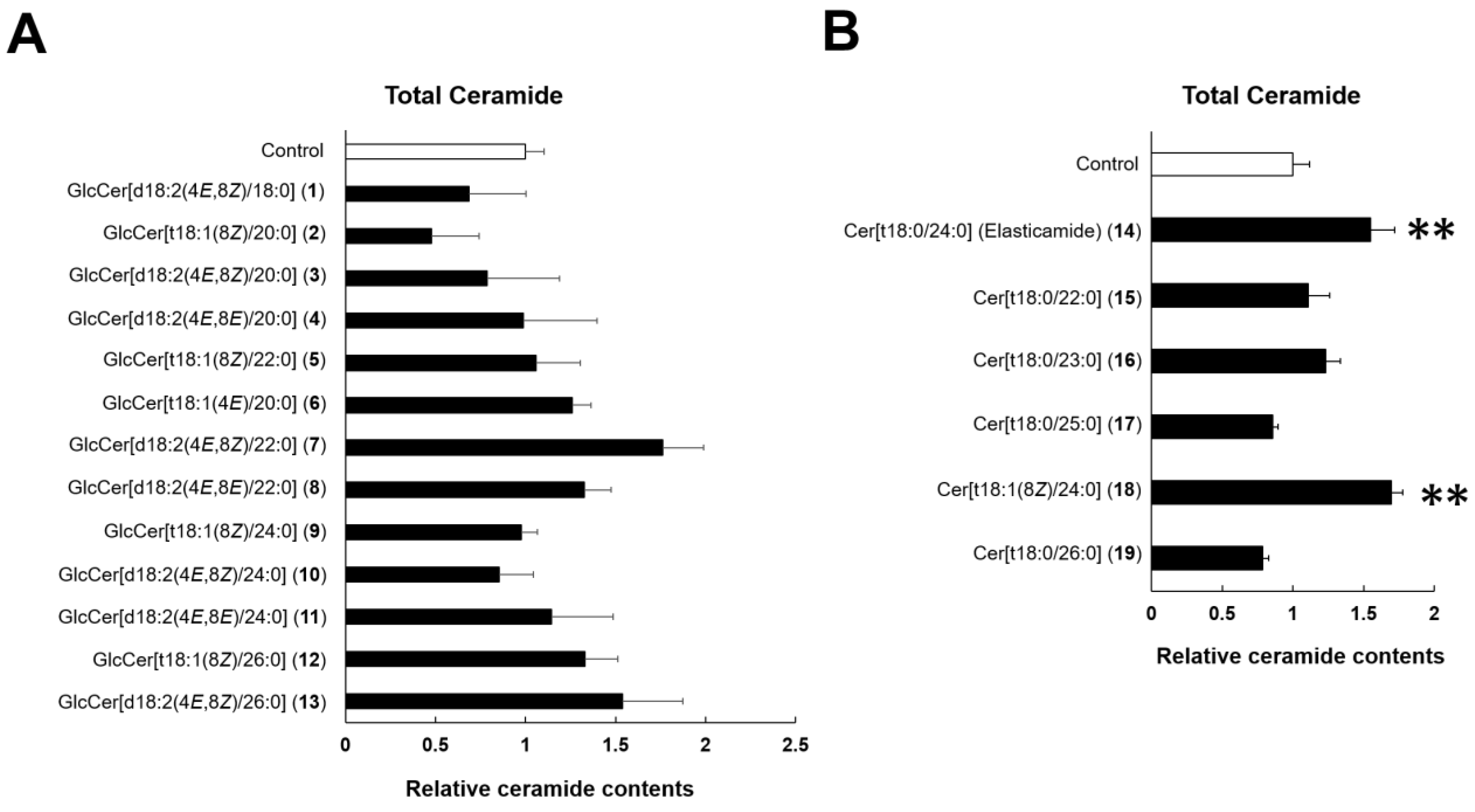
2.2. Effects of GlcCer (1–13) and Cer (14–19) on SC Cer contents in the RHEK Model
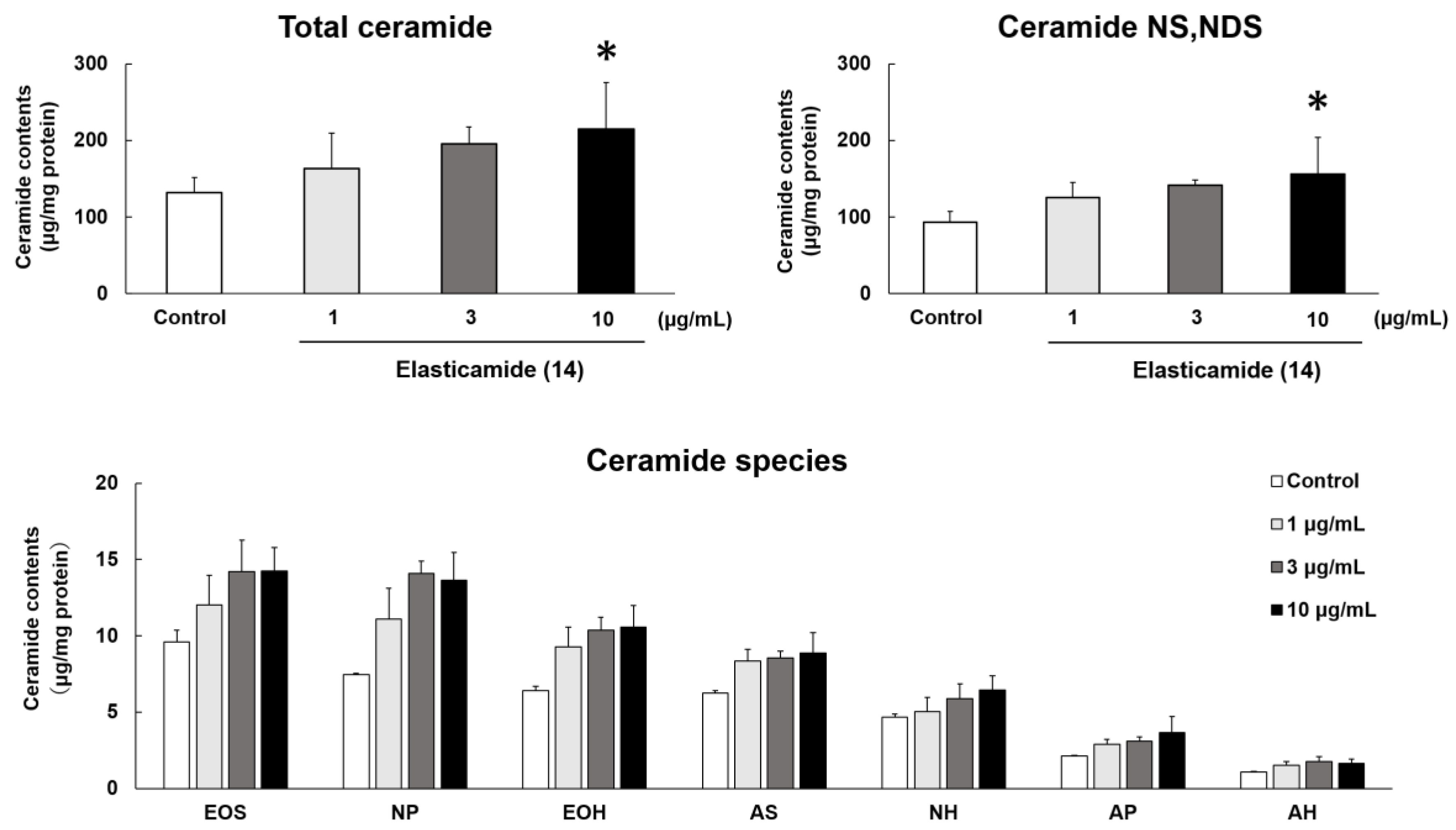
2.3. Effects of Elasticamide (14) on the Expression of Enzymes Related to SC Cer Synthesis in the RHEK Model
2.4. Effects of GlcCer[d18:2(4E,8Z)/26:0] (13) on Epidermal Hydration Factors in the RHEK Model
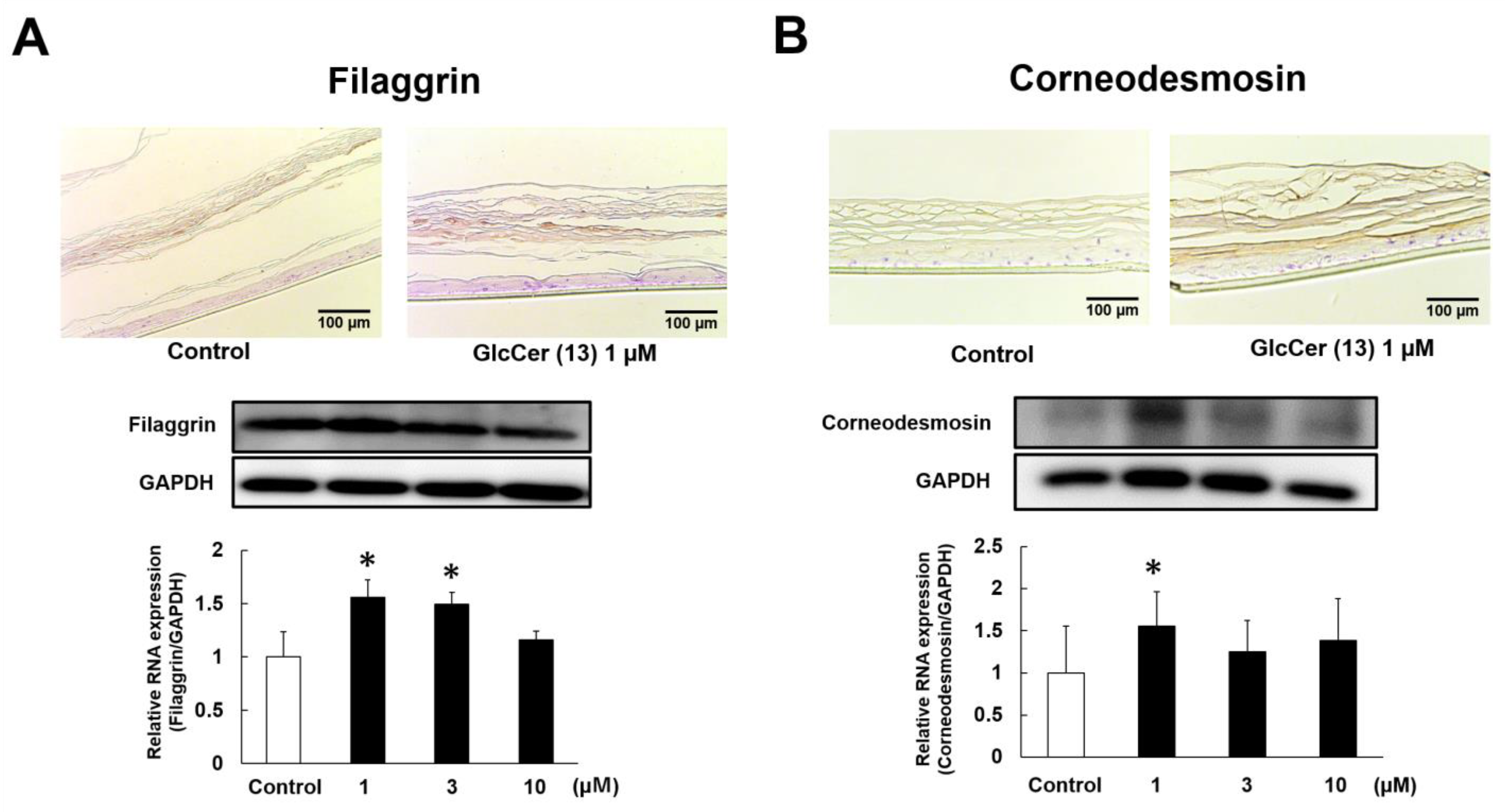
2.5. Effects of GlcCer[d18:2(4E,8Z)/26:0] (13) on the Protein Expression of Filaggrin and Corneodesmosin
3. Discussion
4. Conclusions
5. Materials and Methods
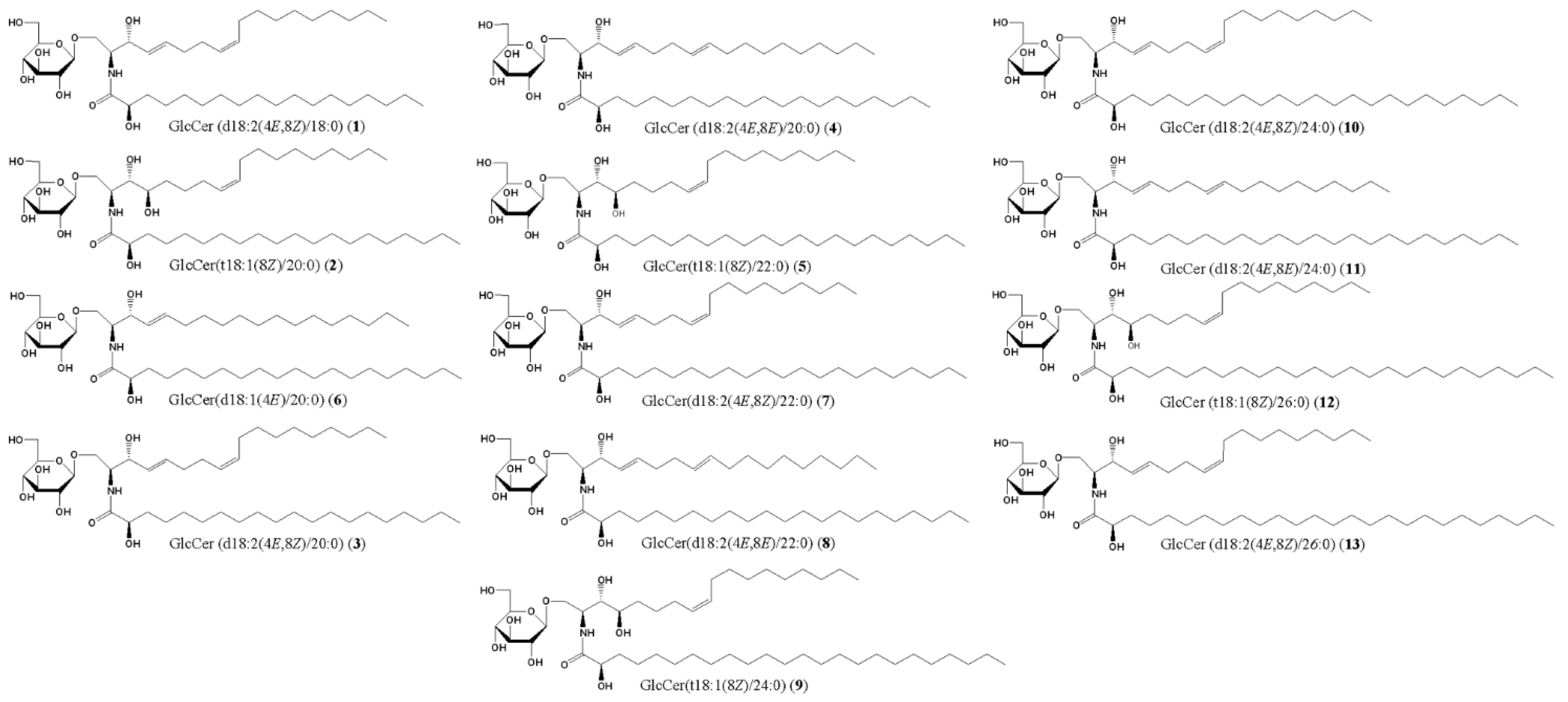
5.1. Preparation of GlcCer and Cer
5.2. Reagents
5.3. Culture of a Reconstructed Human Epidermal Keratinization (RHEK) Model
5.4. Measurement of TEWL in the RHEK Model
5.5. Lipid Extraction and Cer Determination
5.6. Real Time RT-PCR
5.7. Western Blotting Analysis
5.8. Microscopic Analysis and Immunostaining
5.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SC | stratum corneum |
| Cer | ceramide |
| GlcCer | glucosylceramide |
| SPT | serine palmitoyltransferase |
| CerS | ceramide synthase |
| GCS | glucosylceramide synthase |
| GBA | β-glucocerebrosidase |
| SMS | sphingomyelin synthase |
| ASM | acid sphingomyelinase |
| AD | atopic dermatitis |
| TEWL | transepidermal water loss |
| RHEK | reconstructed human epidermal keratinization |
| HPTLC | high performance thin-layer chromatography |
| PBS | phosphate buffered saline |
| DAB | 3,3′-diaminobenzidine |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| HRP | horseradish peroxidase |
| S.E. | standard error |
References
- Fluhr, J.W.; Kao, J.; Jain, M.; Ahn, S.K.; Feingold, K.R.; Elias, P.M. Generation of free fatty acids from phospholipids regulates stratum corneum acidification and integrity. J. Investig. Dermatol. 2001, 117, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Lim, K.M. Skin epidermis and barrier function. Int. J. Mol. Sci. 2021, 22, 3035. [Google Scholar] [CrossRef]
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef]
- Imokawa, G. Structure and function of intercellular lipis in the stratum corneum. J. Oleo. Sci. 1995, 44, 751–766. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.C.; Chang, T.M. Ceramide 1 and ceramide 3 act synergistically on skin hydration and the transepidermal water loss of sodium lauryl sulfate-irritated skin. Int. J. Dermatol. 2008, 47, 812–819. [Google Scholar] [CrossRef]
- Suzuki, M.; Ohno, Y.; Kihara, A. Whole picture of human stratum corneum ceramides, including the chain-length diversity of long-chain bases. J. Lipid. Res. 2022, 63, 100235. [Google Scholar] [CrossRef]
- Hanada, K. Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim. Biophys. Acta 2003, 1632, 16–30. [Google Scholar] [CrossRef]
- Levy, M.; Futerman, A.H. Mammalian ceramide synthases. IUBMB Life 2010, 62, 347–356. [Google Scholar] [CrossRef]
- Hamanaka, S.; Hara, M.; Nishio, H.; Otsuka, F.; Suzuki, A.; Uchida, Y. Human epidermal glucosylceramides are major precursors of stratum corneum ceramides. J. Investig. Dermatol. 2002, 119, 416–423. [Google Scholar] [CrossRef] [Green Version]
- Takagi, Y.; Kriehuber, E.; Imokawa, G.; Elias, P.M.; Holleran, W.M. β-Glucocerebrosidase activity in mammalian stratum corneum. J. Lipid. Res. 1999, 40, 861–869. [Google Scholar] [CrossRef]
- Tafesse, F.G.; Ternes, P.; Holthuis, J.C. The multigenic sphingomyelin synthase family. J. Biol. Chem. 2006, 281, 29421–29425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, R.W.; Canals, D.; Hannun, Y.A. Roles and regulation of secretory and lysosomal acid sphingomyelinase. Cell Signal 2009, 21, 836–846. [Google Scholar] [CrossRef] [Green Version]
- Imokawa, G.; Abe, A.; Jin, K.; Higaki, Y.; Kawashima, M.; Hidano, A. Decreased level of ceramides in stratum corneum of atopic dermatitis: An etiologic factor in atopic dry skin? J. Investig. Dermatol. 1991, 96, 523–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akimoto, K.; Yoshikawa, N.; Higaki, Y.; Kawashima, M.; Imokawa, G. Quantitative analysis of stratum corneum lipids in xerosis and asteatotic eczema. J. Dermatol. 1993, 20, 1–6. [Google Scholar] [CrossRef]
- Ishikawa, J.; Shimotoyodome, Y.; Ito, S.; Miyauchi, Y.; Fujimura, T.; Kitahara, T.; Hase, T. Variations in the ceramide profile in different seasons and regions of the body contribute to stratum corneum functions. Arch. Dermatol. Res. 2013, 305, 151–162. [Google Scholar] [CrossRef]
- Sugawara, T.; Aida, K.; Duan, J.; Hirata, T. Analysis of glucosylceramides from various sources by liquid chromatography-ion trap mass spectrometry. J. Oleo. Sci. 2010, 59, 387–394. [Google Scholar] [CrossRef] [Green Version]
- Shimoda, H.; Terazawa, S.; Hitoe, S.; Tanaka, J.; Nakamura, S.; Matsuda, H.; Yoshikawa, M. Changes in ceramides and glucosylceramides in mouse skin and human epidermal equivalents by rice-derived glucosylceramide. J. Med. Food. 2012, 15, 1064–1072. [Google Scholar] [CrossRef]
- Tsuji, K.; Mitsutake, S.; Ishikawa, J.; Takagi, Y.; Akiyama, M.; Shimizu, H.; Tomiyama, T.; Igarashi, Y. Dietary glucosylceramide improves skin barrier function in hairless mice. J. Dermatol. Sci. 2006, 44, 101–107. [Google Scholar] [CrossRef]
- Uchiyama, T.; Nakano, Y.; Ueda, O.; Mori, H.; Nakashima, M.; Noda, A.; Ishizaki, C.; Mizoguchi, M. Oral intake of glucosylceramide improves relatively higher level of transepidermal water loss in mice and healthy human subjects. J. Health Sci. 2008, 54, 559–566. [Google Scholar] [CrossRef] [Green Version]
- Kuwata, T.; Hashimoto, T.; Ohto, N.; Kuwahara, H.; Jae, W.L.; Bamba, T.; Mizuno, M. A metabolite of dietary glucosylceramide from pineapples improves the skin barrier function in hairless mice. J. Funct. Foods 2017, 30, 228–236. [Google Scholar] [CrossRef]
- Guillou, S.; Ghabri, S.; Jannot, C.; Gaillard, E.; Lamour, I.; Boisnic, S. The moisturizing effect of a wheat extract food supplement on women’s skin: A randomized, double-blind placebo-controlled trial. Int. J. Cosmet. Sci. 2011, 33, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Hirakawa, S.; Sato, A.; Hattori, Y.; Matsumoto, T.; Yokoyama, K.; Kanai, A. Dietary rice bran extract improves TEWL of whole body. Jpn. Pharmacol. Ther. 2013, 41, 1051–1059. [Google Scholar]
- Koikeda, T.; Tokudome, Y.; Okayasu, M.; Kobayashi, Y.; Kuroda, K.; Yamakawa, J.; Kiyosato, N.; Masuda, K.; Saito, M. Effects of peach (Prunus persica)-derived glucosylceramide on the human skin. Immunol. Endocr. Metab. Agents Med. Chem. 2017, 17, 56–70. [Google Scholar] [CrossRef] [Green Version]
- Mukai, K.; Shirai, H.; Koikeda, T.; Masuda, Y.; Saito, M. Skin symptom improvement test including skin moisturizing property by intake of konjac ceramide-containing food. Jpn. Pharmacol. Ther. 2018, 46, 781–799. [Google Scholar]
- Murakami, M.; Nishi, Y.; Harada, K.; Masuzaki, T.; Minemoto, Y.; Yanagisawa, T.; Shimizu, T.; Tsuboi, A.; Hamada, T.; Nishimura, M. Impact of oral intake of glucosylceramide extracted from pineapple on xerostomia: A double-blind randomized cross-over trial. Nutrients 2019, 11, 2020. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, S.; Iwasaki, T.; Nojima, J.; Ohto, N.; Kuwahara, H.; Nakata, S. Effect of glucosylceramide extracted from pineapple on healthy Japanese males and females with dullness and dry skin. Jpn. Pharmacol. Ther. 2015, 43, 1593–1599. [Google Scholar]
- Takara, T.; Yamamoto, K.; Suzuki, N.; Yamashita, S.; Iio, S.; Noguchi, H.; Kakinuma, T.; Baba, A.; Takeda, S.; Yamada, W.; et al. Oryza Ceramide®, a rice-derived extract consisting of glucosylceramides and β-sitosterol glucoside, improves facial skin dehydration in Japanese subjects. Funct. Foods Health Dis. 2021, 11, 385–407. [Google Scholar] [CrossRef]
- Miyasaka, K.; Manse, Y.; Yoneda, A.; Takeda, S.; Shimizu, N.; Yamada, W.; Morikawa, T.; Shimoda, H. Anti-melanogenic effects of glucosylceramides and elasticamide derived from rice oil by-products in melanoma cells, melanocytes and human skin. J. Food. Biochem. 2022, 46, e14353. [Google Scholar] [CrossRef]
- Fluhr, J.W.; Feingold, K.R.; Elias, P.M. Transepidermal water loss reflects permeability barrier status: Validation in human and rodent in vivo and ex vivo models. Exp. Dermatol. 2006, 15, 483–492. [Google Scholar] [CrossRef]
- Seidenari, S.; Giusti, G. Objective assessment of the skin of children affected by atopic dermatitis: A study of pH, capacitance and TEWL in eczematous and clinically uninvolved skin. Acta Derm. Venereol. 1995, 75, 429–433. [Google Scholar]
- Tezuka, T.; Fang, K.T.; Yamamura, T.; Masaki, H.; Sakon, K.; Suzuki, K. Changes of TEWL value at various skin diseases. Ski. Res. 1989, 31, 153–156. [Google Scholar]
- Takeda, S.; Miyasaka, K.; Shrestha, S.; Manse, Y.; Morikawa, T.; Shimoda, H. Lycoperoside H, a tomato seed saponin, improves epidermal dehydration by increasing ceramide in the stratum corneum and steroidal anti-inflammatory effect. Molecules 2021, 26, 5860. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Shimoda, H.; Takarada, T.; Imokawa, G. Strawberry seed extract and its major component, tiliroside, promote ceramide synthesis in the stratum corneum of human epidermal equivalents. PLoS ONE 2018, 13, e0205061. [Google Scholar] [CrossRef] [Green Version]
- Sandilands, A.; Sutherland, C.; Irvine, A.D.; McLean, W.H. Filaggrin in the frontline: Role in skin barrier function and disease. J. Cell Sci. 2009, 122, 1285–1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLean, W.H. Filaggrin failure—From ichthyosis vulgaris to atopic eczema and beyond. Br. J. Dermatol. 2016, 175, 4–7. [Google Scholar] [CrossRef]
- Kezic, S.; Jakasa, I. Filaggrin and skin barrier function. Curr. Probl. Dermatol. 2016, 49, 1–7. [Google Scholar]
- Sybert, V.P.; Dale, B.A.; Holbrook, K.A. Ichthyosis vulgaris: Identification of a defect in synthesis of filaggrin correlated with an absence of keratohyaline granules. J. Investig. Dermatol. 1985, 84, 191–194. [Google Scholar] [CrossRef] [Green Version]
- Fleckman, P.; Brumbaugh, S. Absence of the granular layer and keratohyalin define a morphologically distinct subset of individuals with ichthyosis vulgaris. Exp. Dermatol. 2002, 11, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Berika, M.; Garrod, D. Desmosomal adhesion in vivo. Cell Commun. Adhes. 2014, 21, 65–75. [Google Scholar] [CrossRef]
- Garrod, D.; Chidgey, M. Desmosome structure, composition and function. Biochim. Biophys. Acta 2008, 1778, 572–587. [Google Scholar] [CrossRef]
- Lefevre-Utile, A.; Braun, C.; Haftek, M.; Aubin, F. Five functional aspects of the epidermal barrier. Int. J. Mol. Sci. 2021, 22, 11676. [Google Scholar] [CrossRef] [PubMed]
- Jonca, N.; Leclerc, E.A.; Caubet, C.; Simon, M.; Guerrin, M.; Serre, G. Corneodesmosomes and corneodesmosin from the stratum corneum cohesion to the pathophysiology of genodermatoses. Eur. J. Dermatol. 2011, 21, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, E.A.; Huchenq, A.; Mattiuzzo, N.R.; Metzger, D.; Chambon, P.; Ghyselinck, N.B.; Serre, G.; Jonca, N.; Guerrin, M. Corneodesmosin gene ablation induces lethal skin-barrier disruption and hair-follicle degeneration related to desmosome dysfunction. J. Cell Sci. 2009, 122, 2699–2709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.M.; Zhang, A.L.; Chen, H.; Liu, J.K. Molecular species of ceramides from the ascomycete truffle Tuber indicum. Chem. Phys. Lipids. 2004, 131, 205–213. [Google Scholar] [CrossRef]
- Yaoita, Y.; Ishizuka, T.; Kakuda, R.; Machida, K.; Kikuchi, M. Structures of new ceramides from the fruit bodies of Grifola frondosa. Chem. Pharm. Bull. 2000, 48, 1356–1358. [Google Scholar] [CrossRef] [Green Version]
- Muralidhar, P.; Krishna, N.; Kumar, M.M.; Rao, C.B.; Rao, D.V. New sphingolipids from marine sponge Iotrochota baculifera. Chem. Pharm. Bull. 2003, 51, 1193–1195. [Google Scholar] [CrossRef]
- Zhu, Y.; Soroka, D.N.; Sang, S. Structure elucidation and chemical profile of sphingolipids in wheat bran and their cytotoxic effects against human colon cancer cells. J. Agric. Food Chem. 2013, 61, 866–874. [Google Scholar] [CrossRef]
- Huang, Q.; Tezuka, Y.; Kikuchi, T.; Nishi, A.; Tubaki, K.; Tanaka, K. Studies on metabolites of mycoparasitic fungi. II. Metabolites of Trichoderma koningii. Chem. Pharm. Bull. 1995, 43, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Mbosso Teinkela, J.E.; Noundou, X.S.; Nguemfo, E.L.; Meyer, F.; Djoukoue, A.; Van Antwerpen, P.; Ngouela, S.; Tsamo, E.; Mpondo Mpondo, E.A.; Vardamides, J.C.; et al. Identification of compounds with anti-proliferative activity from the wood of Ficus elastica Roxb. ex Hornem. aerial roots. Fitoterapia 2016, 112, 65–73. [Google Scholar] [CrossRef]
- Alexander, H.; Brown, S.; Danby, S.; Flohr, C. Research techniques made simple: Transepidermal water loss measurement as a research tool. J. Investig. Dermatol. 2018, 138, 2295–2300. [Google Scholar] [CrossRef] [Green Version]
- Joly-Tonetti, N.; Ondet, T.; Monshouwer, M.; Stamatas, G.N. EGFR inhibitors switch keratinocytes from a proliferative to a differentiative phenotype affecting epidermal development and barrier function. BMC Cancer 2021, 21, 5. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Terazawa, S.; Shimoda, H.; Imokawa, G. β-Sitosterol 3-O-D-glucoside increases ceramide levels in the stratum corneum via the up-regulated expression of ceramide synthase-3 and glucosylceramide synthase in a reconstructed human epidermal keratinization model. PLoS ONE 2021, 16, e0248150. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeda, S.; Yoneda, A.; Miyasaka, K.; Manse, Y.; Morikawa, T.; Shimoda, H. Comparative Study on Epidermal Moisturizing Effects and Hydration Mechanisms of Rice-Derived Glucosylceramides and Ceramides. Int. J. Mol. Sci. 2023, 24, 83. https://doi.org/10.3390/ijms24010083
Takeda S, Yoneda A, Miyasaka K, Manse Y, Morikawa T, Shimoda H. Comparative Study on Epidermal Moisturizing Effects and Hydration Mechanisms of Rice-Derived Glucosylceramides and Ceramides. International Journal of Molecular Sciences. 2023; 24(1):83. https://doi.org/10.3390/ijms24010083
Chicago/Turabian StyleTakeda, Shogo, Akari Yoneda, Kenchi Miyasaka, Yoshiaki Manse, Toshio Morikawa, and Hiroshi Shimoda. 2023. "Comparative Study on Epidermal Moisturizing Effects and Hydration Mechanisms of Rice-Derived Glucosylceramides and Ceramides" International Journal of Molecular Sciences 24, no. 1: 83. https://doi.org/10.3390/ijms24010083
APA StyleTakeda, S., Yoneda, A., Miyasaka, K., Manse, Y., Morikawa, T., & Shimoda, H. (2023). Comparative Study on Epidermal Moisturizing Effects and Hydration Mechanisms of Rice-Derived Glucosylceramides and Ceramides. International Journal of Molecular Sciences, 24(1), 83. https://doi.org/10.3390/ijms24010083






