Anti-Amnesic Effect of Synbiotic Supplementation Containing Corni fructus and Limosilactobacillus reuteri in DSS-Induced Colitis Mice
Abstract
:1. Introduction
2. Results
2.1. Effect of PRE, PRO, and SYN on Pathological Symptoms of Colitis
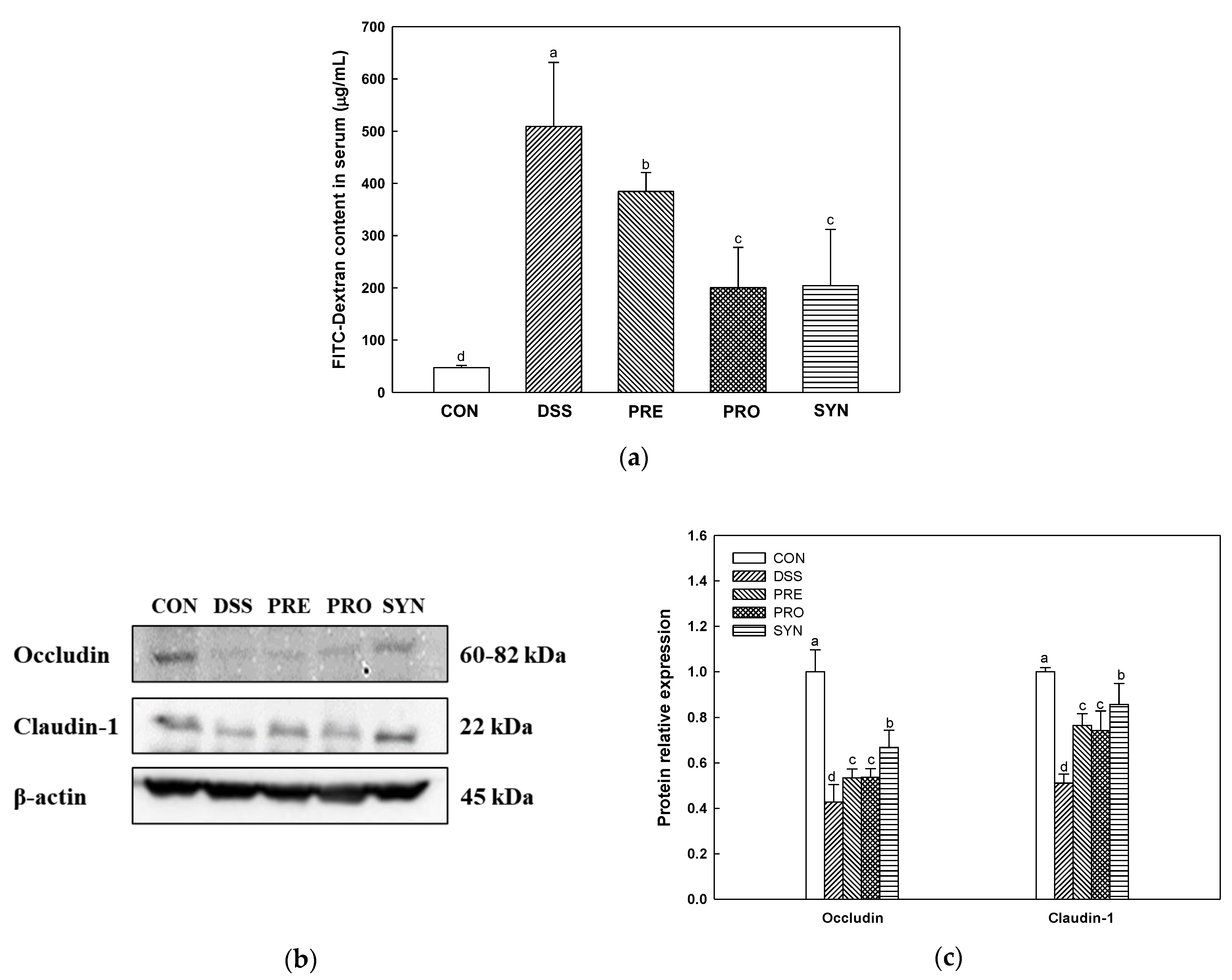
2.2. Effect of PRE, PRO and, SYN on Intestinal Permeability
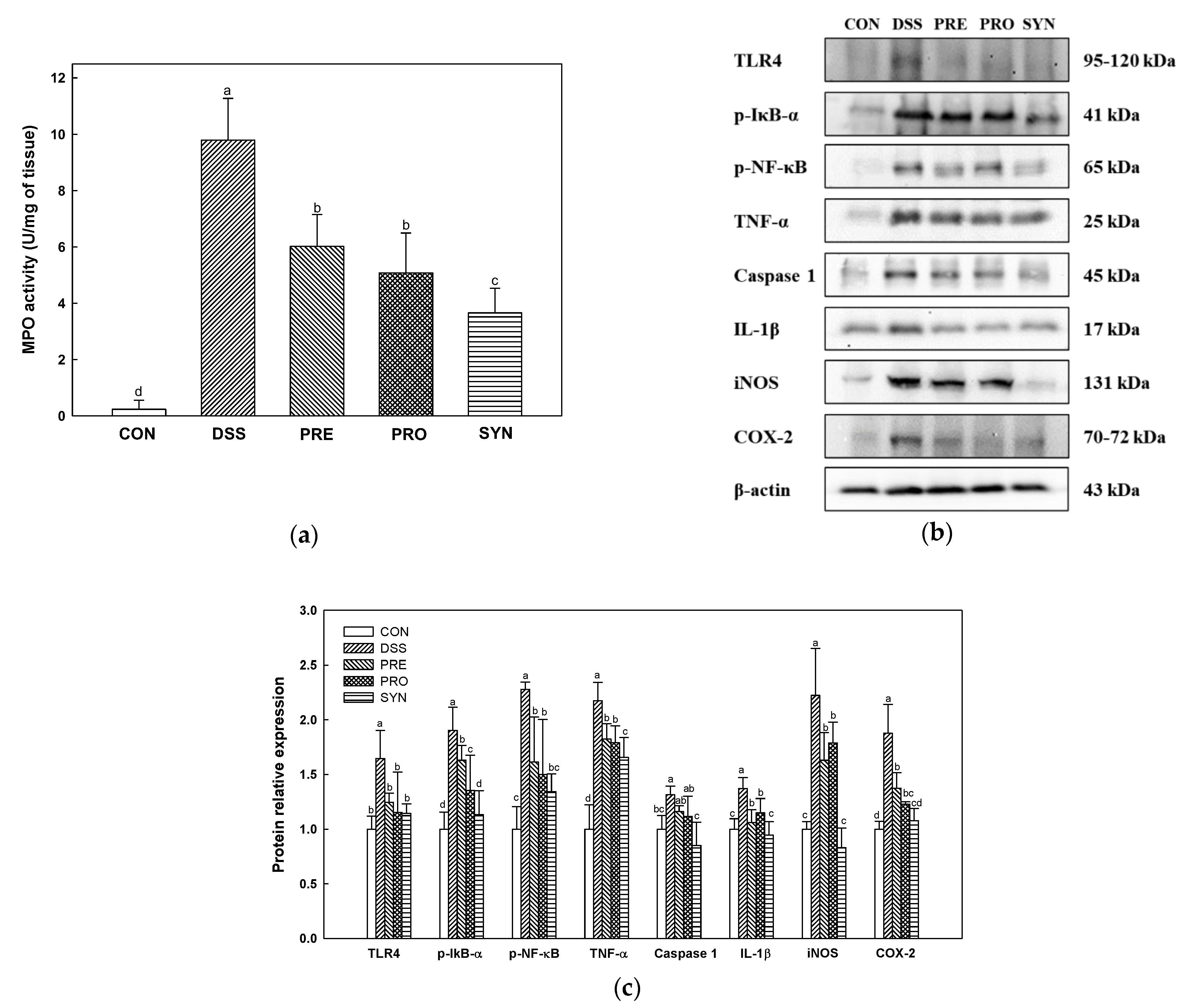
2.3. Effect of PRE, PRO, and SYN on Inflammatory Responses in Colon Tissue
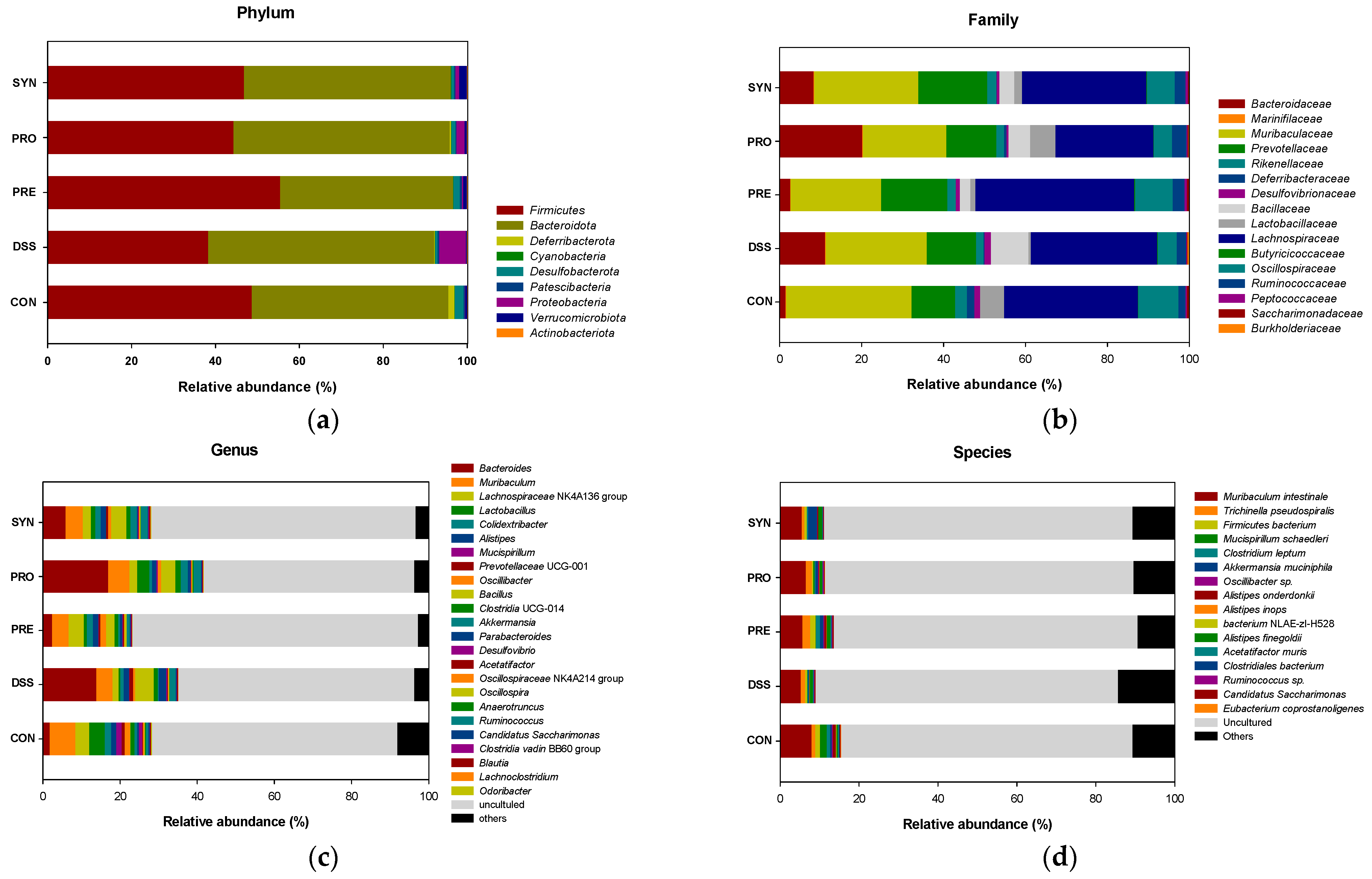
2.4. Effect of PRE, PRO, and SYN on Gut Microbiota Composition
2.5. Effect of PRE, PRO, and SYN on Concentration of Short-Chain Fatty Acids (SCFAs) in Fecal
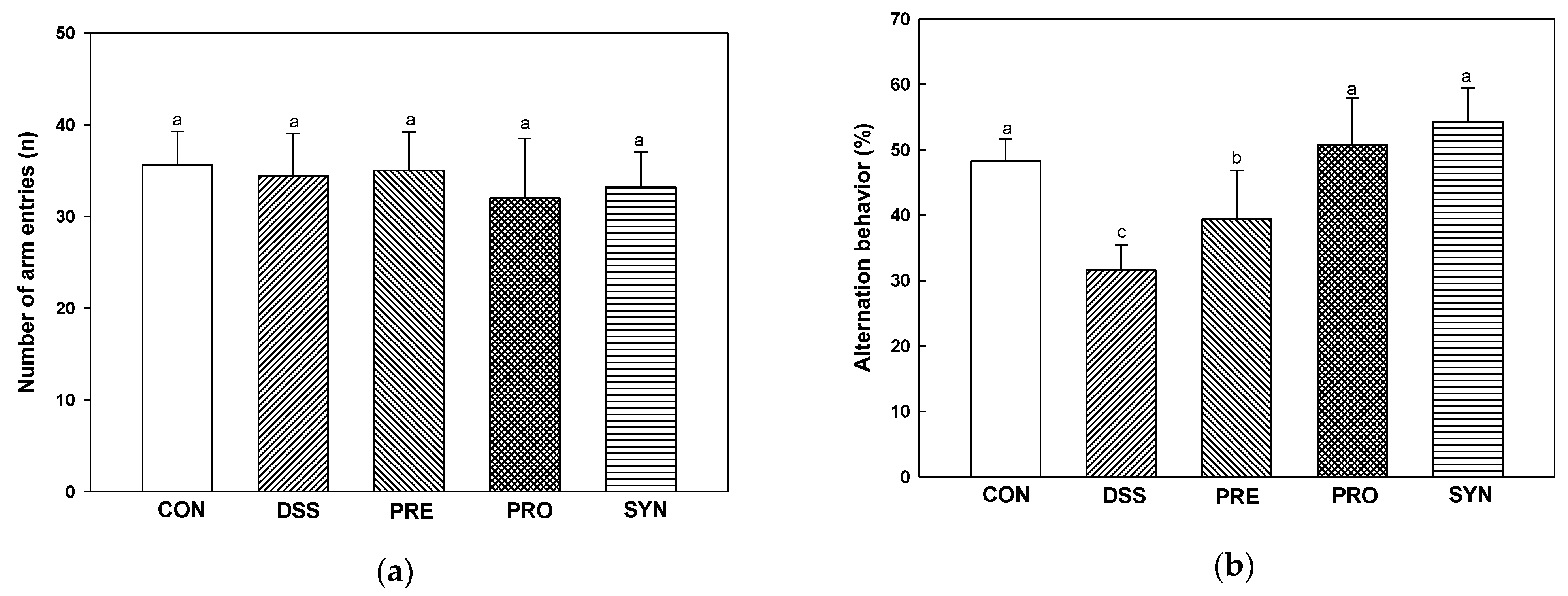
2.6. Effect of PRE, PRO, and SYN on Behavioral Disorder
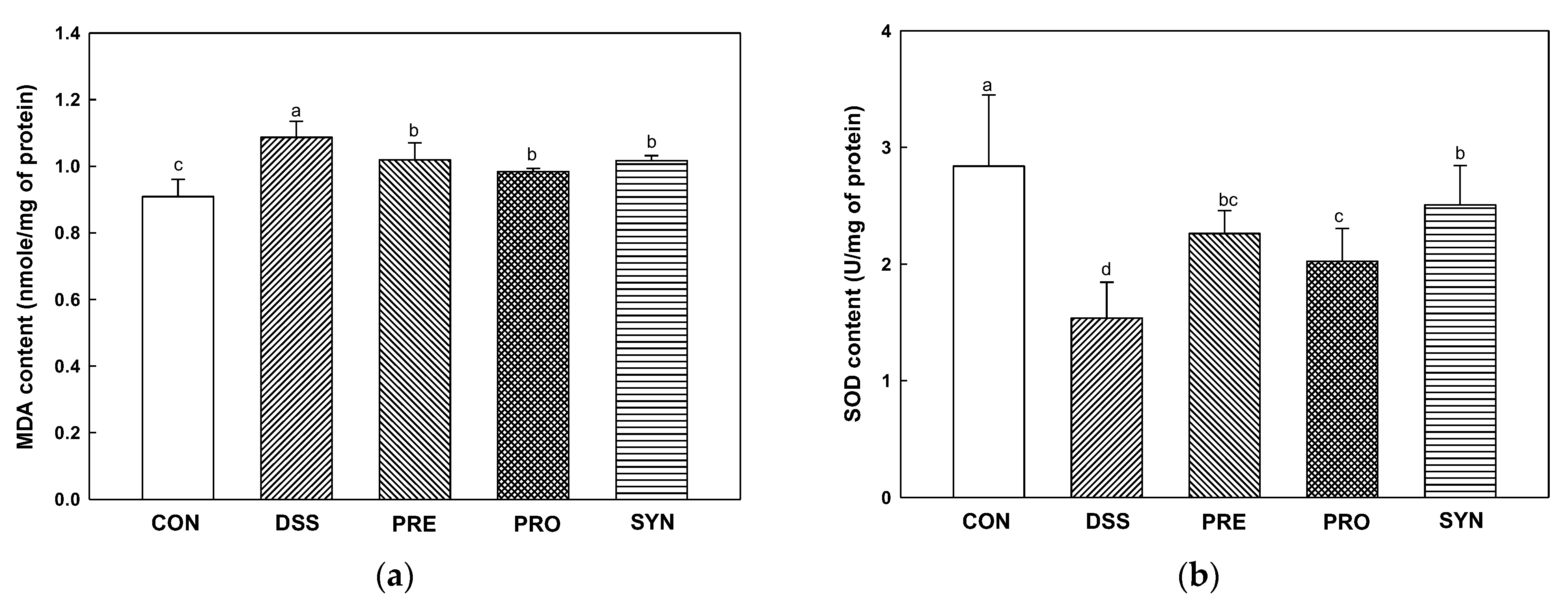
2.7. Effect of PRE, PRO, and SYN on Antioxidant Parameters in Brain Tissue
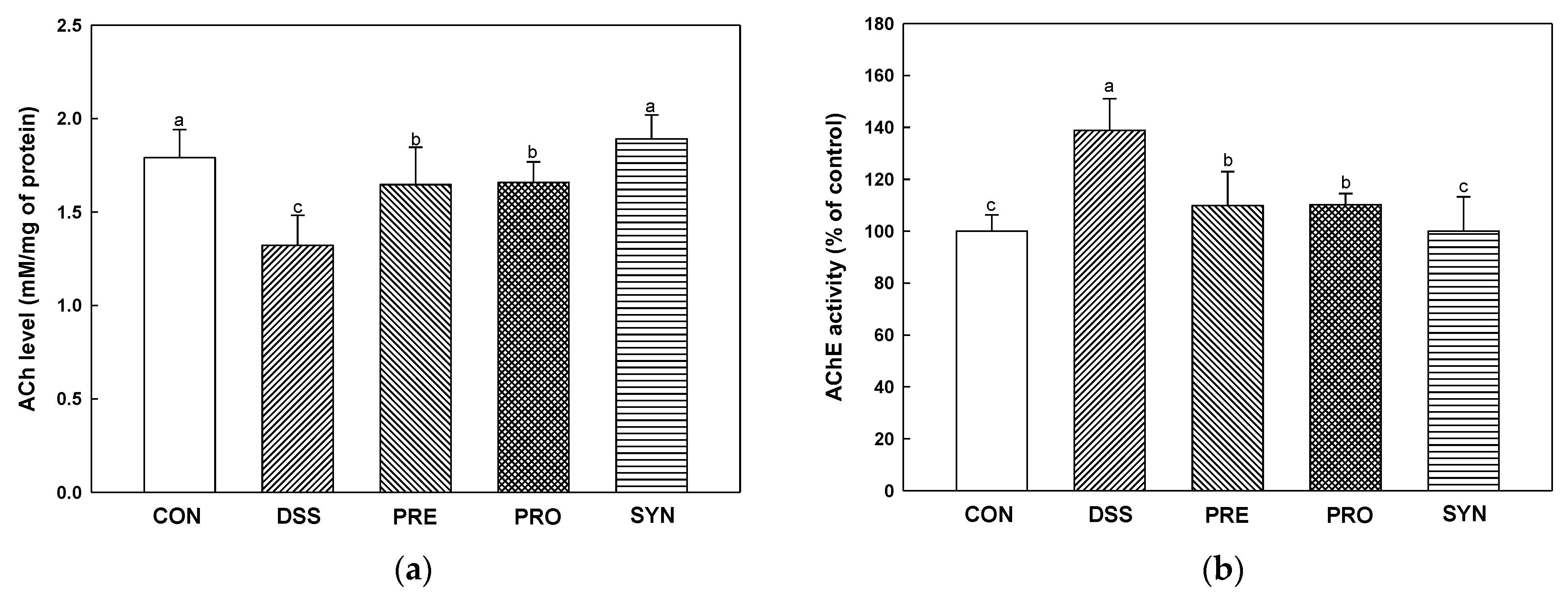
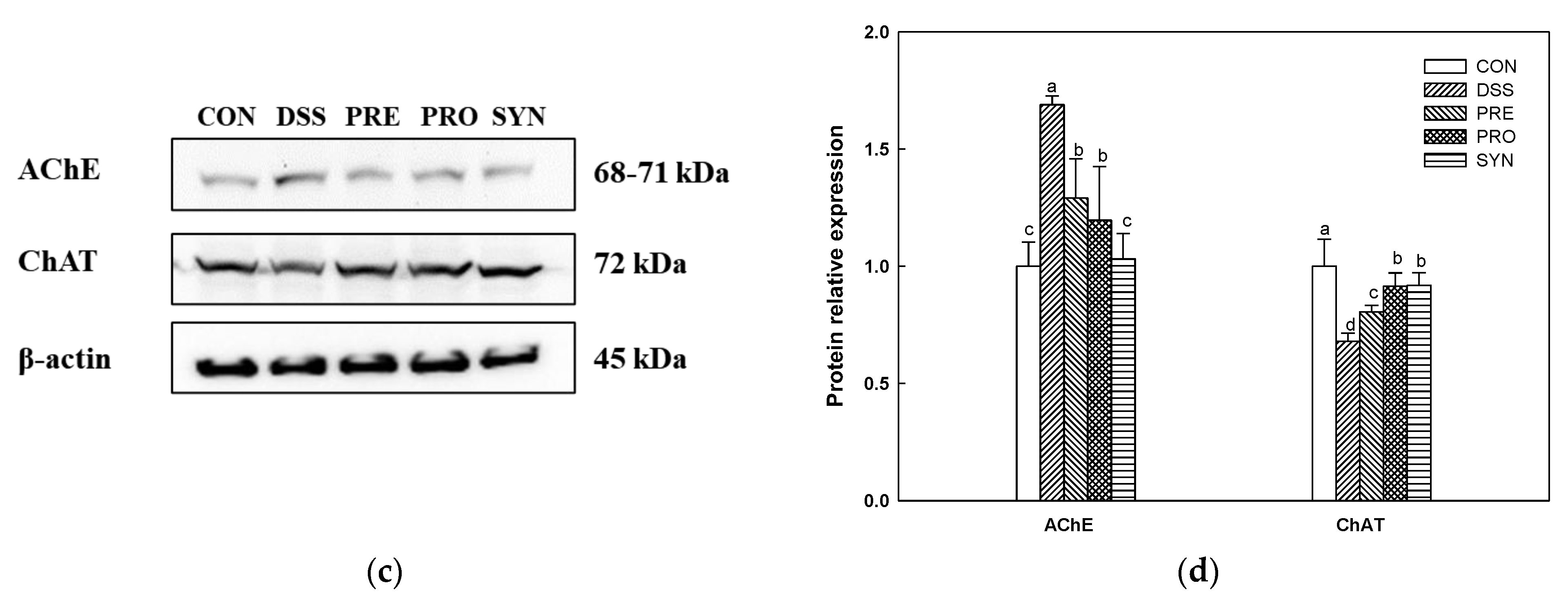
2.8. Effect of PRE, PRO, and SYN on Cholinergic System
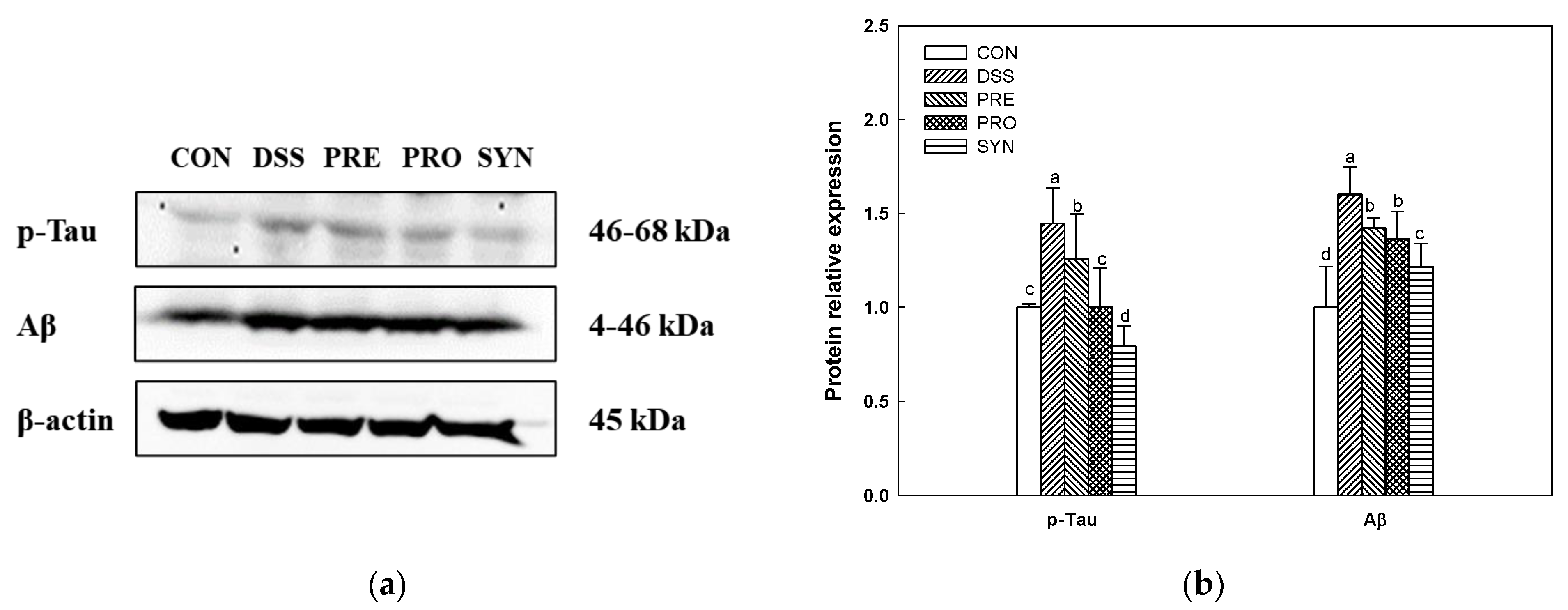
2.9. Effect of PRE, PRO, and SYN on Phosphorylated-Tau (p-Tau) and Amyloid β (Aβ) Protein Expression
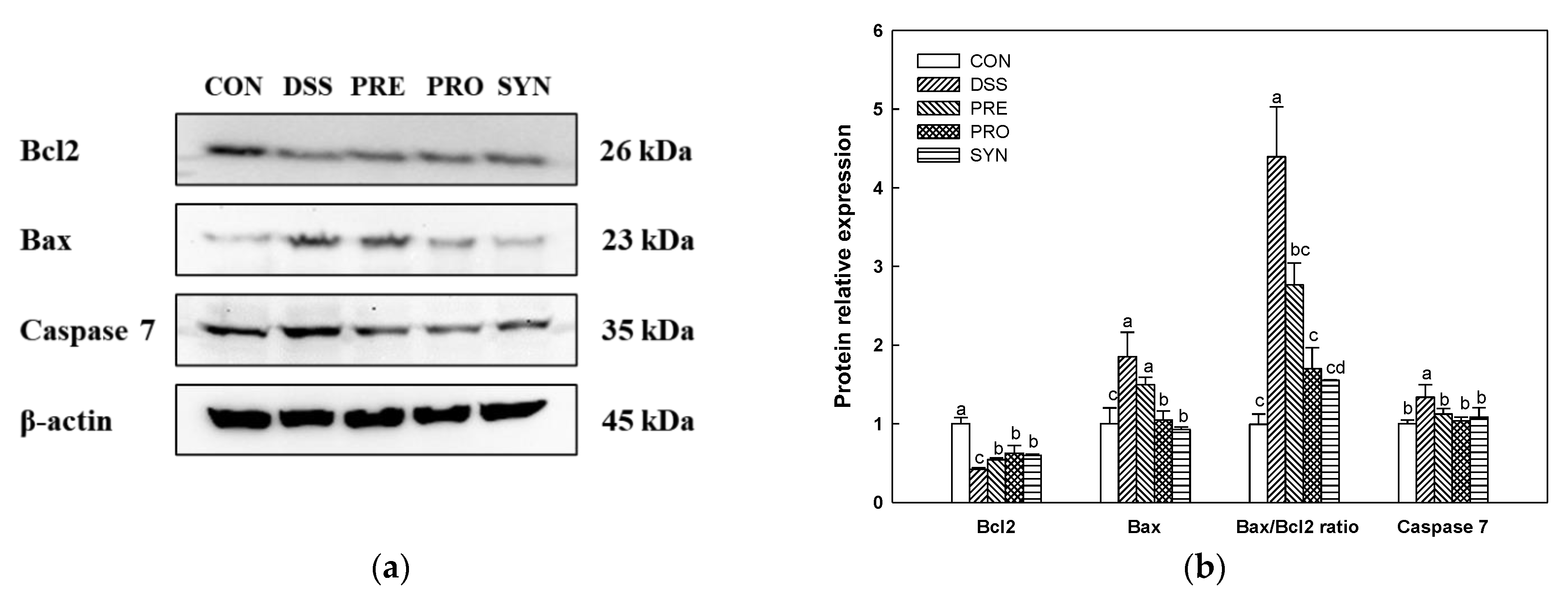
2.10. Effect of PRE, PRO, and SYN on Apoptosis Pathway in Brain Tissue
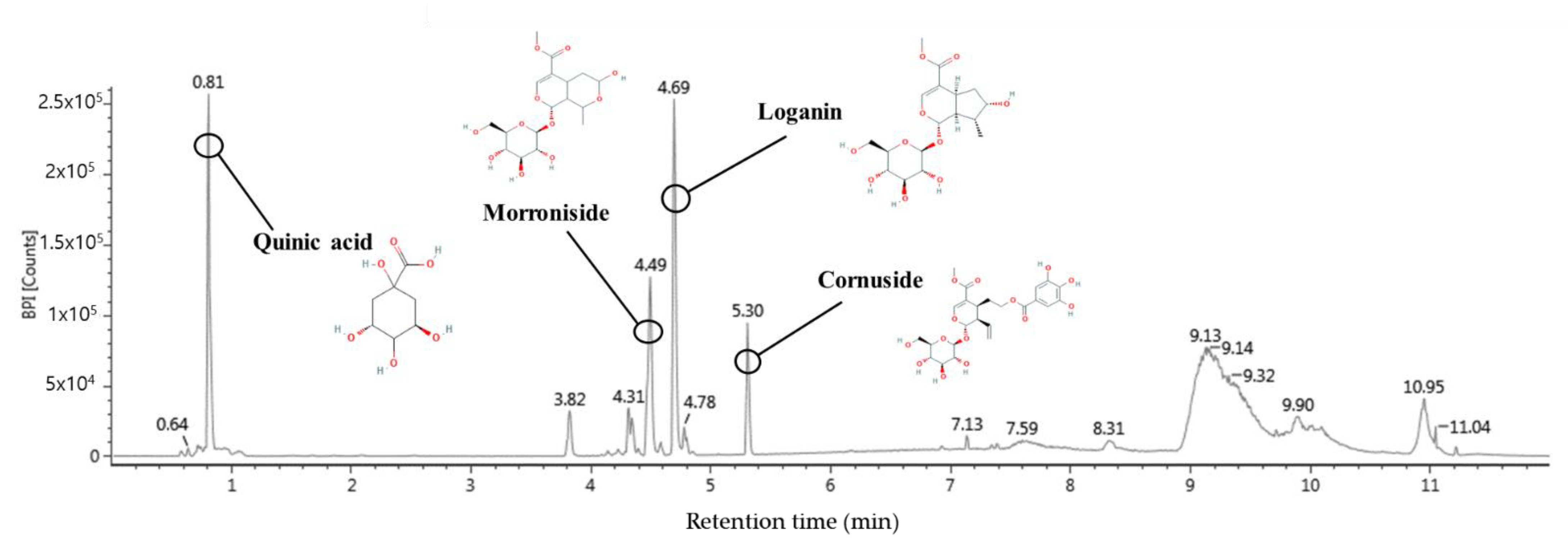
2.11. Bioactive Compounds of Water Extract of C. fructus (WCF)
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Sample Preparation
4.3. Animals and Experimental Design
4.4. Analysis of Serum Albumin
4.5. Intestinal Permeability Assay
4.6. Western Blot
4.7. MPO Activity in Colon Tissues
4.8. Behavioral Test
4.8.1. Y-Maze Test
4.8.2. Passive Avoidance Test
4.9. Antioxidant Pparameters in Brain Tissue
4.9.1. MDA Content
4.9.2. SOD Level
4.10. Cholinergic System Activity in Brain Tissue
4.10.1. ACh Level
4.10.2. AChE Activity
4.11. Determination of SCFAs
4.12. 16S rRNA Sequencing
4.13. Identification of Bioactive Compounds
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qin, X. Etiology of inflammatory bowel disease: A unified hypothesis. World J. Gastroenterol. 2012, 18, 1708–1722. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, G.G. The global burden of IBD: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Microbiol. 2009, 9, 799–809. [Google Scholar] [CrossRef]
- Kim, J.B.; Han, A.R.; Park, E.Y.; Kim, J.Y.; Cho, W.; Lee, J.; Lee, K.T. Inhibition of LPS-induced iNOS, COX-2 and cytokines expression by poncirin through the NF-κB inactivation in RAW 264.7 macrophage cells. Biol. Pharm. Bull. 2007, 30, 2345–2351. [Google Scholar] [CrossRef] [Green Version]
- Serra, D.; Almeida, L.M.; Dinis, T.C. The impact of chronic intestinal inflammation on brain disorders: The microbiota-gut-brain axis. Mol. Neurobiol. 2019, 56, 6941–6951. [Google Scholar] [CrossRef]
- Ghaisas, S.; Maher, J.; Kanthasamy, A. Gut microbiome in health and disease: Linking the microbiome–gut–brain axis and environmental factors in the pathogenesis of systemic and neurodegenerative diseases. Pharmacol. Ther. 2016, 158, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Banfi, D.; Moro, E.; Bosi, A.; Bistoletti, M.; Cerantola, S.; Crema, F.; Baj, A. Impact of microbial metabolites on microbiota–gut–brain axis in inflammatory bowel disease. Int. J. Mol. Sci. 2021, 22, 1623. [Google Scholar] [CrossRef]
- Mayer, E.A.; Nance, K.; Chen, S. The Gut–Brain Axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef]
- Pithadia, A.B.; Jain, S. Treatment of inflammatory bowel disease (IBD). Pharmacol. Res. 2011, 63, 629–642. [Google Scholar] [CrossRef]
- Mau, J.L.; Chen, C.P.; Hsieh, P.C. Antimicrobial effect of extracts from Chinese chive, cinnamon, and Corni fructus. J. Agric. Food Chem. 2001, 49, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, B.K.; Lee, Y.C. Effects of Corni fructus on ovalbumin-induced airway inflammation and airway hyper-responsiveness in a mouse model of allergic asthma. J. Inflamm. Res. 2012, 9, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Li, C.; Fan, W.; Yi, T.; Wei, A.; Ma, Y. Hypoglycemic and hypolipidemic effects of a polysaccharide from Fructus Corni in streptozotocin-induced diabetic rats. Int. J. Biol. Macromol. 2019, 133, 420–427. [Google Scholar] [CrossRef]
- Bhakta, H.K.; Park, C.H.; Yokozawa, T.; Min, B.S.; Jung, H.A.; Choi, J.S. Kinetics and molecular docking studies of loganin, morroniside and 7-O-galloyl-d-sedoheptulose derived from Corni fructus as cholinesterase and β-secretase 1 inhibitors. Arch. Pharm. Res. 2016, 39, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wu, H.Y.; Liu, H.; Gong, N.; Wang, Y.R.; Wang, Y.X. Morroniside, a secoiridoid glycoside from Cornus officinalis, attenuates neuropathic pain by activation of spinal glucagon-like peptide-1 receptors. Br. J. Pharmacol. 2017, 174, 580–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.; Feng, Z.L.; Chen, H.B.; Wang, F.S.; Lu, J.H. Corni Fructus: A review of chemical constituents and pharmacological activities. Chin. Med. 2018, 13, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in human health and diseases. Front. Microbiol. 2018, 9, 757. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Wang, J.; Qi, R.; Qiu, X.; Sun, Q.; Huang, J.; Wang, R. Effect of oral administration of Limosilactobacillus reuteri on intestinal barrier function and mucosal immunity of suckling piglets. Ital. J. Anim. Sci. 2022, 21, 612–623. [Google Scholar] [CrossRef]
- Greifová, G.; Májeková, H.; Greif, G.; Body, P.; Greifová, M.; Dubničková, M. Analysis of antimicrobial and immunomodulatory substances produced by heterofermentative Lactobacillus reuteri. Folia Microbiol. 2017, 62, 515–524. [Google Scholar] [CrossRef]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef]
- Dalile, B.; Van Oudenhove, L.; Vervliet, B.; Verbeke, K. The role of short-chain fatty acids in microbiota–gut–brain communication. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.; Lee, U.; Park, S.K.; Kang, J.Y.; Kim, J.M.; Kim, G.H.; Heo, H.J. Corni fructus extracts of prebiotics property and cell protective effect on LPS-induced human colon epithelial cell. J. Korean Soc. Food Sci. Nutr. 2021, 50, 445–455. [Google Scholar] [CrossRef]
- Flight, M.H. The gut-microbiome-brain connection. Nat. Rev. Neurol. 2014, 15, 65. [Google Scholar] [CrossRef]
- Mayer, E.A. Gut feelings: The emerging biology of gut–brain communication. Nat. Rev. Neurol. 2011, 12, 453–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Zhang, C. Alteration of gut microbiota in inflammatory bowel disease (IBD): Cause or consequence? IBD treatment targeting the gut microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef] [Green Version]
- Markowiak, P.; Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Roselli, M.; Finamore, A. Use of synbiotics for ulcerative colitis treatment. Curr. Clin. Pharmacol. 2020, 15, 174–182. [Google Scholar] [PubMed]
- Sánchez de Medina, F.; Romero-Calvo, I.; Mascaraque, C.; Martínez-Augustin, O. Intestinal inflammation and mucosal barrier function. Inflamm. Bowel Dis. 2014, 20, 2394–2404. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [Green Version]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Eckart, A.; Struja, T.; Kutz, A.; Baumgartner, A.; Baumgartner, T.; Zurfluh, S.; Schuetz, P. Relationship of nutritional status, inflammation, and serum albumin levels during acute illness: A prospective study. Am. J. Med. 2020, 133, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Izumi, H.; Minegishi, M.; Sato, Y.; Shimizu, T.; Sekine, K.; Takase, M. Bifidobacterium breve alters immune function and ameliorates DSS-induced inflammation in weanling rats. Pediatr. Res. 2015, 78, 407–416. [Google Scholar] [CrossRef]
- Ahl, D.; Liu, H.; Schreiber, O.; Roos, S.; Phillipson, M.; Holm, L. Lactobacillus reuteri increases mucus thickness and ameliorates dextran sulphate sodium-induced colitis in mice. Acta Physiol. 2016, 217, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.; Wang, L.; Xiong, Y.; Wang, Z.; Qiu, Y.; Wen, X.; Ma, X. Lactobacillus reuteri LR1 improved ex-pression of genes of tight junction proteins via the MLCK pathway in IPEC-1 cells during infection with enterotoxigenic Escherichia coli K88. Mediat. Inflamm. 2018, 2018, 6434910. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.; Cheng, W.; Zhang, G.; Ma, Q.; Li, X.; Zhang, B.; Song, G. Protective effects of iridoid glycosides on acute colitis via inhibition of the inflammatory response mediated by the STAT3/NF-κB pathway. Int. Immunopharmacol. 2020, 81, 106240. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, J.; Zhong, L. Hydroxytyrosol inhibits pro-inflammatory cytokines, iNOS, and COX-2 expression in human monocytic cells. Naunyn Schmiedebergs Arch. Pharmacol. 2009, 379, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Yamada, A.; Arakaki, R.; Saito, M.; Tsunematsu, T.; Kudo, Y.; Ishimaru, N. Role of regulatory T cell in the pathogenesis of inflammatory bowel disease. World J. Gastroenterol. 2016, 22, 2195. [Google Scholar] [CrossRef]
- Houser, M.C.; Tansey, M.G. The gut-brain axis: Is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Parkinson’s Dis. 2017, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Fatheree, N.Y.; Mangalat, N.; Rhoads, J.M. Human-derived probiotic Lactobacillus reuteri strains differentially reduce intestinal inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, 1087–1096. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.C.; Zhang, F.C.; Yin, X.; Cheng, B.J.; Zhao, C.H.; Wang, Y.L.; Ye, H.Q. Lactobacillus reuteri F-9-35 prevents DSS-Induced colitis by inhibiting proinflammatory gene expression and restoring the gut microbiota in mice. J. Food Sci. 2018, 83, 2645–2652. [Google Scholar] [CrossRef]
- Sung, Y.H.; Chang, H.K.; Kim, S.E.; Kim, Y.M.; Seo, J.H.; Shin, M.C.; Kim, C.J. Anti-inflammatory and analgesic effects of the aqueous extract of corni fructus in murine RAW 264.7 macrophage cells. J. Med. Food 2009, 12, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Jin, G.Y.; Li, G.Z.; Yan, G.H. Cornuside suppresses lipopolysaccharide-induced inflammatory mediators by inhibiting nuclear factor-kappa B activation in RAW 264.7 macrophages. Biol. Pharm. Bull. 2011, 34, 959–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, L.Y.; Ke, Y.S.; Zhao, H.H.; Wang, L.; Jia, C.; Liu, W.Z.; Li, S.C. Role of colonic microbiota in the pathogenesis of ulcerative colitis. BMC Gastroenterol. 2019, 19, 10. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Zheng, X.; Chen, J.; Luo, D.; Xie, J.; Su, Z.; Sun, Z. Protective effect of Bruguiera gymnorrhiza (L.) Lam. fruit on dextran sulfate sodium-induced ulcerative colitis in mice: Role of Keap1/Nrf2 pathway and gut microbiota. Front. Pharmacol. 2020, 10, 1602–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alipour, M.; Zaidi, D.; Valcheva, R.; Jovel, J.; Martínez, I.; Sergi, C.; Wine, E. Mucosal barrier depletion and loss of bacterial diversity are primary abnormalities in paediatric ulcerative colitis. J. Crohns. Colitis 2016, 10, 462–471. [Google Scholar] [CrossRef]
- Dziarski, R.; Park, S.Y.; Kashyap, D.R.; Dowd, S.E.; Gupta, D. Pglyrp-regulated gut microflora Prevotella falsenii, Parabacteroides distasonis and Bacteroides eggerthii enhance and Alistipes finegoldii attenuates colitis in mice. PLoS ONE 2016, 11, e0146162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, R.; Zhang, Y.; Chen, S.; Zeng, Y.; Fu, X.; Chen, T.; Zhang, X. The role of the probiotic Akkermansia muciniphila in brain functions: Insights underpinning therapeutic potential. Crit. Rev. Microbiol. 2022, 1–26. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.B.; Wang, P.Y.; Wang, X.; Wan, Y.L.; Liu, Y.C. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef]
- Ge, T.; Yao, X.; Zhao, H.; Yang, W.; Zou, X.; Peng, F.; Cui, R. Gut microbiota and neuropsychiatric disorders: Implications for neuroendocrine-immune regulation. Pharmacol. Res. 2021, 173, 105909. [Google Scholar] [CrossRef]
- Wang, J.; Ji, H.; Wang, S.; Liu, H.; Zhang, W.; Zhang, D.; Wang, Y. Probiotic Lactobacillus plantarum promotes intestinal barrier function by strengthening the epithelium and modulating gut microbiota. Front. Microbiol. 2018, 9, 1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, Q.; Feng, S.; Arjan, N.; Chen, W. A next generation probiotic, Akkermansia muciniphila. Crit. Rev. Food Sci. Nutr. 2019, 59, 3227–3236. [Google Scholar] [CrossRef] [PubMed]
- Abbring, S.; Engen, P.A.; Naqib, A.; Green, S.J.; Garssen, J.; Keshavarzian, A.; van Esch, B.C. Raw milk-induced protection against food allergic symptoms in mice is accompanied by shifts in microbial community structure. Int. J. Mol. Sci. 2021, 22, 3417. [Google Scholar] [CrossRef] [PubMed]
- He, X.F.; Li, L.L.; Xian, W.B.; Li, M.Y.; Zhang, L.Y.; Xu, J.H.; Hu, X.Q. Chronic colitis exacerbates NLRP3-dependent neuroinflammation and cognitive impairment in middle-aged brain. J. Neuroinflamm. 2021, 18, 153. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Lian, W.W.; He, J.; He, X.L.; Wang, Y.M.; Pan, C.H.; Xu, J.K. Cornuside ameliorates cognitive impairments in scopolamine induced AD mice: Involvement of neurotransmitter and oxidative stress. J. Ethnopharmacol. 2022, 293, 115252. [Google Scholar] [CrossRef]
- Jung, I.H.; Jung, M.A.; Kim, E.J.; Han, M.J.; Kim, D.H. Lactobacillus pentosus var. plantarum C29 protects scopolamine-induced memory deficit in mice. J. Appl. Microbiol. 2012, 113, 1498–1506. [Google Scholar] [CrossRef]
- Jang, H.M.; Lee, K.E.; Kim, D.H. The preventive and curative effects of Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98 on immobilization stress-induced anxiety/depression and colitis in mice. Nutrients 2019, 11, 819. [Google Scholar] [CrossRef] [Green Version]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Peng, Z.Y. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [Green Version]
- Ikonomidou, C.; Kaindl, A.M. Neuronal death and oxidative stress in the developing brain. Antioxid. Redox Signal. 2011, 14, 1535–1550. [Google Scholar] [CrossRef]
- Abdel Moneim, A.E. Oxidant/antioxidant imbalance and the risk of Alzheimer’s disease. Curr. Alzheimer Res. 2015, 12, 335–349. [Google Scholar] [CrossRef] [Green Version]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox. Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Leutner, S.; Eckert, A.; Müller, W.E. ROS generation, lipid peroxidation and antioxidant enzyme activities in the aging brain. J. Neural Transm. 2001, 108, 955–967. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Lauderback, C.M. Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: Potential causes and consequences involving amyloid β-peptide-associated free radical oxidative stress. Free Radic. Biol. Med. 2002, 32, 1050–1060. [Google Scholar] [CrossRef]
- Casado, A.; Encarnación López-Fernández, M.; Concepción Casado, M.; de La Torre, R. Lipid peroxidation and antioxidant enzyme activities in vascular and Alzheimer dementias. Neurochem. Res. 2008, 33, 450–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelova, P.R.; Esteras, N.; Abramov, A.Y. Mitochondria and lipid peroxidation in the mechanism of neurodegeneration: Finding ways for prevention. Med. Res. Rev. 2021, 41, 770–784. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, J.; Li, L.; Wang, P.; Ji, X.; Ai, H.; Li, L. Neuroprotective effect of morroniside on focal cerebral ischemia in rats. Brain Res. Bull. 2010, 83, 196–201. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Wang, Y.; Xu, H.; Mei, X.; Yu, D.; Li, W. Antioxidant properties of probiotic bacteria. Nutrients 2017, 9, 521. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Bai, X.; Jiang, Y.; Cheng, Y. Butyrate alleviates PTZ-induced mitochondrial dysfunction, oxidative stress and neuron apoptosis in mice via Keap1/Nrf2/HO-1 pathway. Brain Res. Bull. 2021, 168, 25–35. [Google Scholar] [CrossRef]
- Russo, I.; Luciani, A.; De Cicco, P.; Troncone, E.; Ciacci, C. Butyrate attenuates lipopolysaccharide-induced inflammation in intestinal cells and Crohn’s mucosa through modulation of antioxidant defense machinery. PLoS ONE 2012, 7, e32841. [Google Scholar] [CrossRef] [Green Version]
- Schliebs, R. Basal forebrain cholinergic dysfunction in Alzheimer’s disease–interrelationship with β-amyloid, inflammation and neurotrophin signaling. Neurochem. Res. 2005, 30, 895–908. [Google Scholar] [CrossRef]
- Deiana, S.; Platt, B.; Riedel, G. The cholinergic system and spatial learning. Behav. Brain Res. 2011, 221, 389–411. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Baker, G.B.; Dursun, S.M. The relationship between the gut microbiome-immune system-brain axis and major depressive disorder. Front. Neurol. 2021, 12, 721126. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of neurotransmitters by the gut microbiota and effects on cognition in neurological disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the cholinergic system. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [Green Version]
- Mattson, M.P. Pathways towards and away from Alzheimer’s disease. Nature 2004, 430, 631–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schliebs, R.; Arendt, T. The cholinergic system in aging and neuronal degeneration. Behav. Brain Res. 2011, 221, 555–563. [Google Scholar] [CrossRef]
- Nimgampalle, M.; Kuna, Y. Anti-Alzheimer properties of probiotic, Lactobacillus plantarum MTCC 1325 in Alzheimer’s disease induced albino rats. J. Clin. Diagn. Res. 2017, 11, KC01–KC05. [Google Scholar] [CrossRef]
- Shamsipour, S.; Sharifi, G.; Taghian, F. An 8-week administration of Bifidobacterium bifidum and Lactobacillus plantarum combined with exercise training alleviates neurotoxicity of Aβ and spatial learning via acetylcholine in Alzheimer rat model. J. Mol. Neurosci. 2021, 71, 1495–1505. [Google Scholar] [CrossRef]
- Shimohama, S. Apoptosis in Alzheimer’s disease—An update. Apoptosis 2000, 5, 9–16. [Google Scholar] [CrossRef]
- Yon, J.H.; Daniel-Johnson, J.; Carter, L.B.; Jevtovic-Todorovic, V. Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience 2005, 135, 815–827. [Google Scholar] [CrossRef]
- Jiang, W.L.; Chen, X.G.; Zhu, H.B.; Hou, J.; Tian, J.W. Cornuside attenuates apoptosis and ameliorates mitochondrial energy metabolism in rat cortical neurons. Pharmacology 2009, 84, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, F.; An, Y.; Ai, H.; Zhang, L.; Huang, W.; Li, L. Morroniside protects human neuroblastoma SH-SY5Y cells against hydrogen peroxide-induced cytotoxicity. Eur. J. Pharmacol. 2009, 613, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Zolfaghari, S.I.; Khorasgani, M.R.; Noorbakhshnia, M. The effects of lactobacilli (L. rhamnosus, L. reuteri, L. Plantarum) on LPS-induced memory impairment and changes in CaMKII-α and TNF-α genes expression in the hippocampus of rat. Physiol. Behav. 2021, 229, 113224. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Kim, Y.S.; Lim, J.Y.; Min, S.J.; Shin, J.H.; Ko, H.C.; Kim, Y. Sasa quelpaertensis leaf extract suppresses dextran sulfate sodium–induced colitis in mice by inhibiting the proinflammatory mediators and mitogen-activated protein kinase phosphorylation. Nutr. Res. 2014, 34, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Ning, Y.; Hu, J.; Wang, Z.; Chen, X.; Zhao, X. The Preventive Effect of Lactobacillus plantarum ZS62 on DSS-Induced IBD by Regulating Oxidative Stress and the Immune Response. Oxid. Med. Cell. Longev. 2021, 2021, 9416794. [Google Scholar] [CrossRef]
- Zor, T.; Selinger, Z. Linearization of the Bradford protein assay increases its sensitivity: Theoretical and experimental studies. Anal. Biochem. 1996, 236, 302–308. [Google Scholar] [CrossRef] [Green Version]
- Pulli, B.; Ali, M.; Forghani, R.; Schob, S.; Hsieh, K.L.; Wojtkiewicz, G.; Chen, J.W. Measuring myeloperoxidase activity in biological samples. PLoS ONE 2013, 8, e67976. [Google Scholar] [CrossRef] [Green Version]
- Heo, H.J.; Park, Y.J.; Suh, Y.M.; Choi, S.J.; Kim, M.J.; Cho, H.Y.; Shin, D.H. Effects of oleamide on choline acetyltransferase and cognitive activities. Biosci. Biotechnol. Biochem. 2003, 67, 1284–1291. [Google Scholar] [CrossRef] [Green Version]
- Ghani, M.A.; Barril, C.; Bedgood, D.R., Jr.; Prenzler, P.D. Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food Chem. 2017, 230, 195–207. [Google Scholar] [CrossRef]
- Wang, F.; Zeng, X.; Zhu, Y.; Ning, D.; Liu, J.; Liu, C.; Zhu, D. Effects of thyroxine and donepezil on hippocampal acetylcholine content, acetylcholinesterase activity, synaptotagmin-1 and SNAP-25 expression in hypothyroid adult rats. Mol. Med. Rep. 2015, 11, 775–782. [Google Scholar] [CrossRef]
- Akıncıoğlu, A.; Akıncıoğlu, H.; Gülçin, İ.; Durdagi, S.; Supuran, C.T.; Göksu, S. Discovery of potent carbonic anhydrase and acetylcholine esterase inhibitors: Novel sulfamoylcarbamates and sulfamides derived from acetophenones. Bioorg. Med. Chem. 2015, 23, 3592–3602. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Caporaso, J.G. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]


| CON | DSS | PRE | PRO | SYN | |
|---|---|---|---|---|---|
| Phylum | |||||
| Proteobacteria | 0.08 ± 0.01 b | 9.19 ± 2.87 a | 0.31 ± 0.01 b | 1.75 ± 0.40 b | 0.63 ± 0.25 b |
| Bacteroidota | 39.68 ± 3.30 b | 52.45 ± 3.51 a | 44.01 ± 3.49 ab | 43.43 ± 3.44 ab | 49.03 ± 6.64 a |
| Firmicutes | 40.05 ± 9.57 b | 37.15 ± 7.95 b | 59.62 ± 5.54 a | 37.27 ± 0.75 b | 46.63 ± 0.92 b |
| Firmicutes/Bacteroidota ratio | 1.39 ± 0.09 a | 0.61 ± 0.09 c | 1.36 ± 0.09 a | 0.90 ± 0.05 b | 1.04 ± 0.09 b |
| Family | |||||
| Bacillaceae | 0.06 ± 0.00 b | 6.93 ± 3.08 a | 3.63 ± 0.58 ab | 5.13 ± 1.28 a | 3.85 ± 2.24 ab |
| Lachnospiraceae | 26.68 ± 4.13 ab | 14.62 ± 5.70 b | 30.86 ± 2.46 a | 16.89 ± 6.95 ab | 29.19 ± 3.32 ab |
| Lactobacillaceae | 5.56 ± 2.82 a | 0.46 ± 0.04 b | 1.35 ± 0.71 b | 6.05 ± 4.05 a | 1.98 ± 1.81 b |
| Genus | |||||
| Bacillus | 0.06 ± 0.00 b | 6.14 ± 2.47 a | 3.46 ± 0.49 ab | 4.74 ± 0.99 a | 3.19 ± 1.53 b |
| Prevotellaceae UCG-001 | 1.01 ± 0.74 b | 2.31 ± 0.28 a | 0.81 ± 0.39 b | N.D. | 0.65 ± 0.09 b |
| Odoribacter | 0.31 ± 0.28 a | 0.04 ± 0.00 b | 0.10 ± 0.03 ab | 0.10 ± 0.02 ab | 0.11 ± 0.01 ab |
| Lactobacillus | 8.89 ± 4.58 a | 0.70 ± 0.23 b | 2.42 ± 1.36 ab | 7.41 ± 4.57 a | 2.77 ± 2.82 ab |
| Lactococcus | 0.53 ± 0.02 ab | 0.23 ± 0.24 b | 0.98 ± 0.02 a | 0.65 ± 0.12 ab | 0.87 ± 0.19 a |
| Lachnospiraceae NK4A136 group | 2.90 ± 0.80 ab | 1.43 ± 0.64 bc | 3.59 ± 0.75 a | 0.41 ± 0.03 c | 1.68 ± 0.76 abc |
| Anaerotruncus | 0.66 ± 0.16 a | 0.06 ± 0.04 b | 0.14 ± 0.00 b | 0.81 ± 0.27 a | 0.19 ± 0.10 b |
| Akkermansia | 0.43 ± 0.01 b | 0.21 ± 0.11 b | 0.97 ± 0.44 ab | 0.49 ± 0.13 b | 2.22 ± 0.93 a |
| Species | |||||
| Akkermansia muciniphila | 0.45 ± 0.04 d | 0.30 ± 0.16 e | 0.85 ± 0.40 b | 0.55 ± 0.18 c | 3.35 ± 0.19 a |
| Alistipes onderdonkii | 0.33 ± 0.02 ab | 0.02 ± 0.04 c | 0.34 ± 0.00 a | 0.19 ± 0.08 b | 0.28 ± 0.05 ab |
| CON | DSS | PRE | PRO | SYN | |
|---|---|---|---|---|---|
| Acetic acid | 182.93 ± 16.85 b | 81.76 ± 31.91 c | 216.39 ± 39.46 ab | 200.44 ± 42.16 b | 254.22 ± 39.50 a |
| Propionic acid | 18.44 ± 3.93 b | 8.85 ± 4.43 c | 28.04 ± 2.44 a | 25.79 ± 3.44 a | 30.12 ± 6.38 a |
| Butyric acid | 39.47 ± 12.68 a | 7.28 ± 3.13 d | 23.20 ± 2.31 c | 24.83 ± 5.42 bc | 38.18 ± 10.89 ab |
| Compound | Retention Time (min) | Formular | [M-H] (m/z) | Production (m/z) |
|---|---|---|---|---|
| Quinic acid | 0.81 | C7H12O6 | 191 | 85, 93, 109, 127, 191 |
| Morroniside | 4.49 | C17H26O11 | 451 | 101, 123, 141, 155, 243, 451 |
| Loganin | 4.69 | C17H26O10 | 435 | 101, 127, 209, 227, 435 |
| Cornuside | 5.30 | C24H30O14 | 541 | 125, 169, 347, 541 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.L.; Kim, J.M.; Moon, J.H.; Kim, M.J.; Jeong, H.R.; Go, M.J.; Kim, H.-J.; Eo, H.J.; Lee, U.; Heo, H.J. Anti-Amnesic Effect of Synbiotic Supplementation Containing Corni fructus and Limosilactobacillus reuteri in DSS-Induced Colitis Mice. Int. J. Mol. Sci. 2023, 24, 90. https://doi.org/10.3390/ijms24010090
Lee HL, Kim JM, Moon JH, Kim MJ, Jeong HR, Go MJ, Kim H-J, Eo HJ, Lee U, Heo HJ. Anti-Amnesic Effect of Synbiotic Supplementation Containing Corni fructus and Limosilactobacillus reuteri in DSS-Induced Colitis Mice. International Journal of Molecular Sciences. 2023; 24(1):90. https://doi.org/10.3390/ijms24010090
Chicago/Turabian StyleLee, Hyo Lim, Jong Min Kim, Jong Hyun Moon, Min Ji Kim, Hye Rin Jeong, Min Ji Go, Hyun-Jin Kim, Hyun Ji Eo, Uk Lee, and Ho Jin Heo. 2023. "Anti-Amnesic Effect of Synbiotic Supplementation Containing Corni fructus and Limosilactobacillus reuteri in DSS-Induced Colitis Mice" International Journal of Molecular Sciences 24, no. 1: 90. https://doi.org/10.3390/ijms24010090
APA StyleLee, H. L., Kim, J. M., Moon, J. H., Kim, M. J., Jeong, H. R., Go, M. J., Kim, H. -J., Eo, H. J., Lee, U., & Heo, H. J. (2023). Anti-Amnesic Effect of Synbiotic Supplementation Containing Corni fructus and Limosilactobacillus reuteri in DSS-Induced Colitis Mice. International Journal of Molecular Sciences, 24(1), 90. https://doi.org/10.3390/ijms24010090





