Natural Gallic Acid and Methyl Gallate Induces Apoptosis in Hela Cells through Regulation of Intrinsic and Extrinsic Protein Expression
Abstract
:1. Introduction
2. Results
2.1. Bioassay Guided Isolation of EAQI
2.2. Structure Elucidation
2.2.1. EAQI/FA3/B2 (Methylgallate, MG); Methyl 3,4,5-Trihydroxybenzoate
2.2.2. EAQI/FA3/B5 (Gallic Acid, GA); 3,4,5-Trihydroxybenzoic Acid
2.3. Antiproliferative Activity Gallic Acid and Methyl Gallate
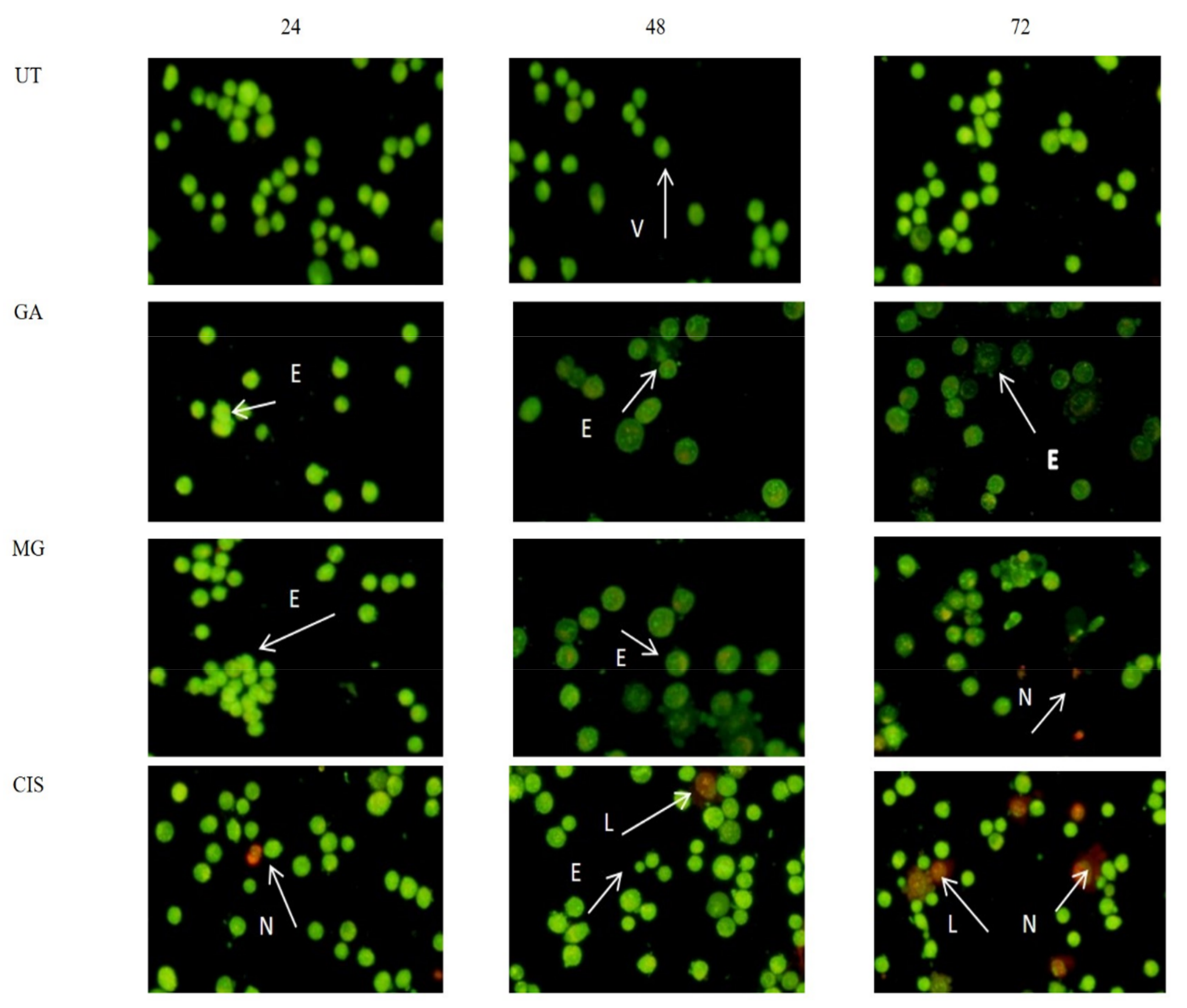
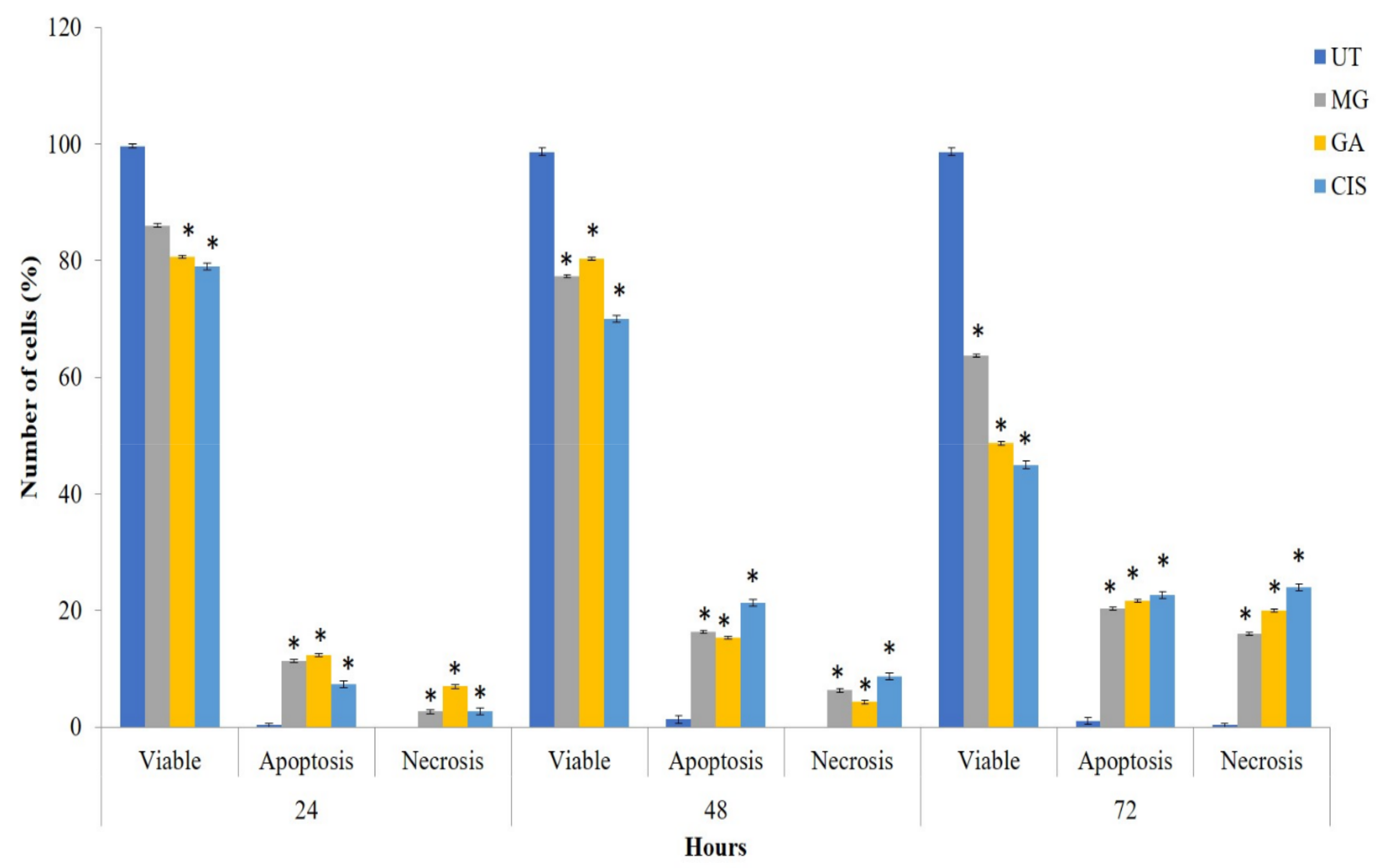
2.4. Mode of Cell Death in the Treated Cells
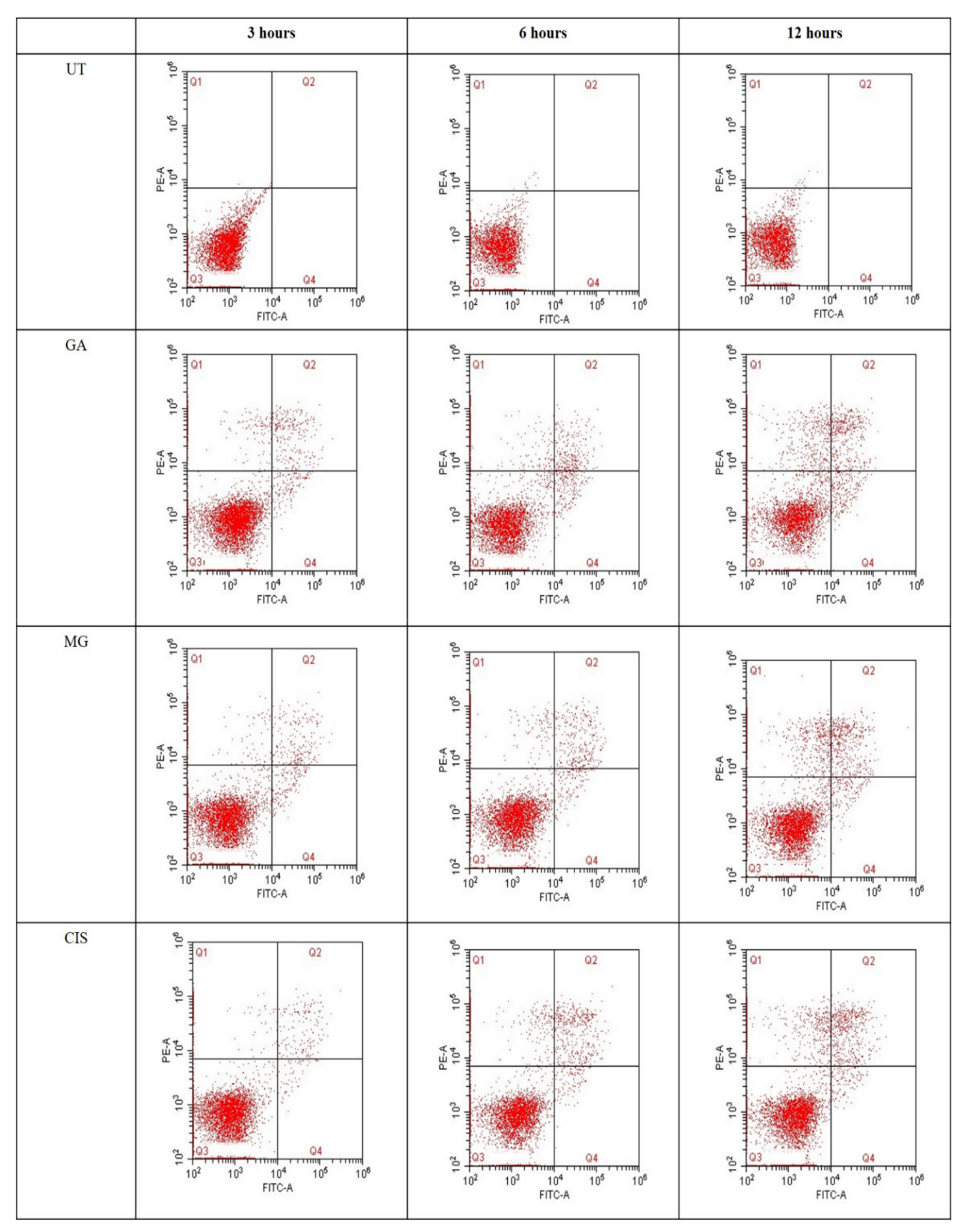
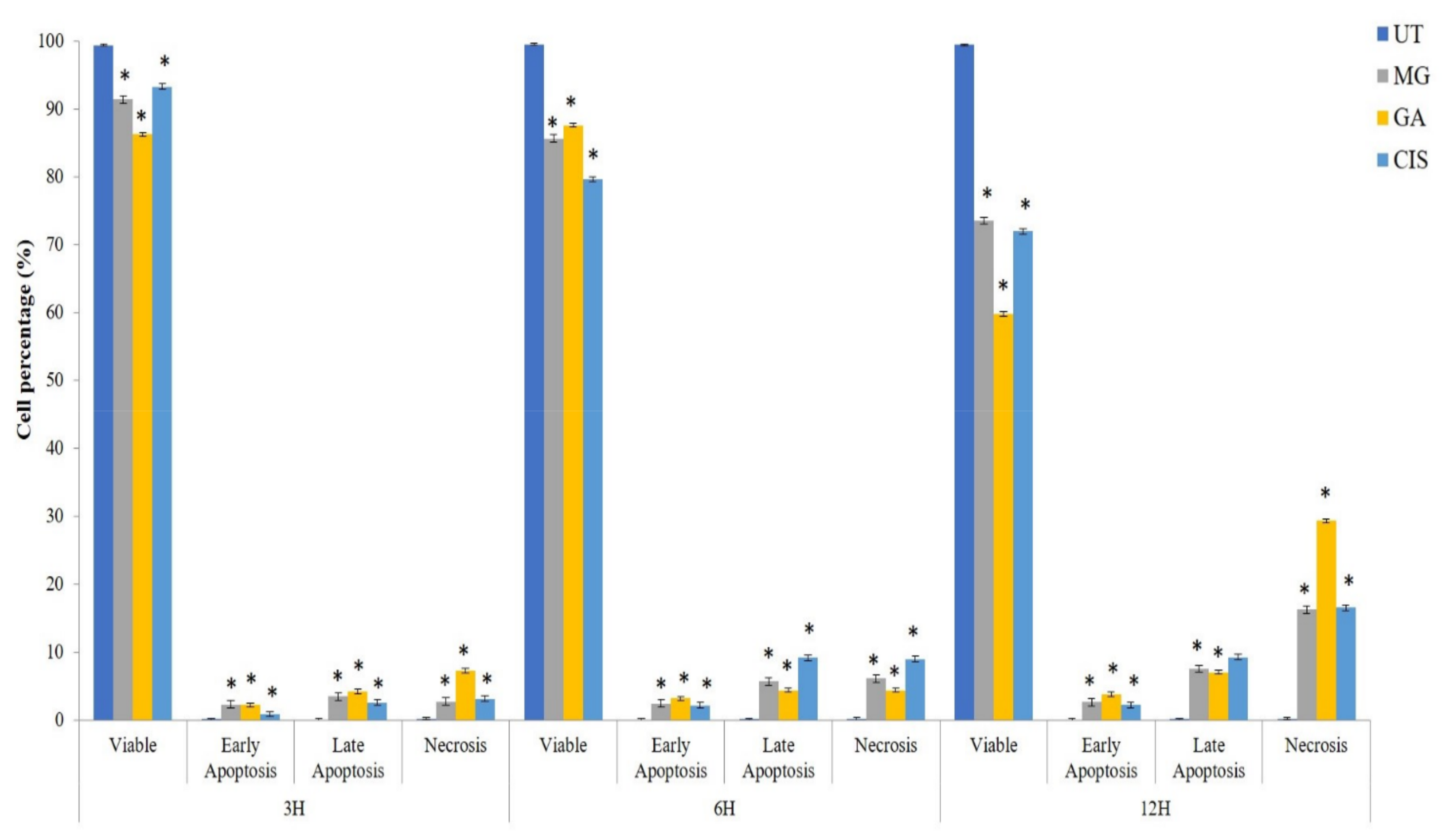
2.5. Detection of Phosphatidylserine (PS) Externalisation
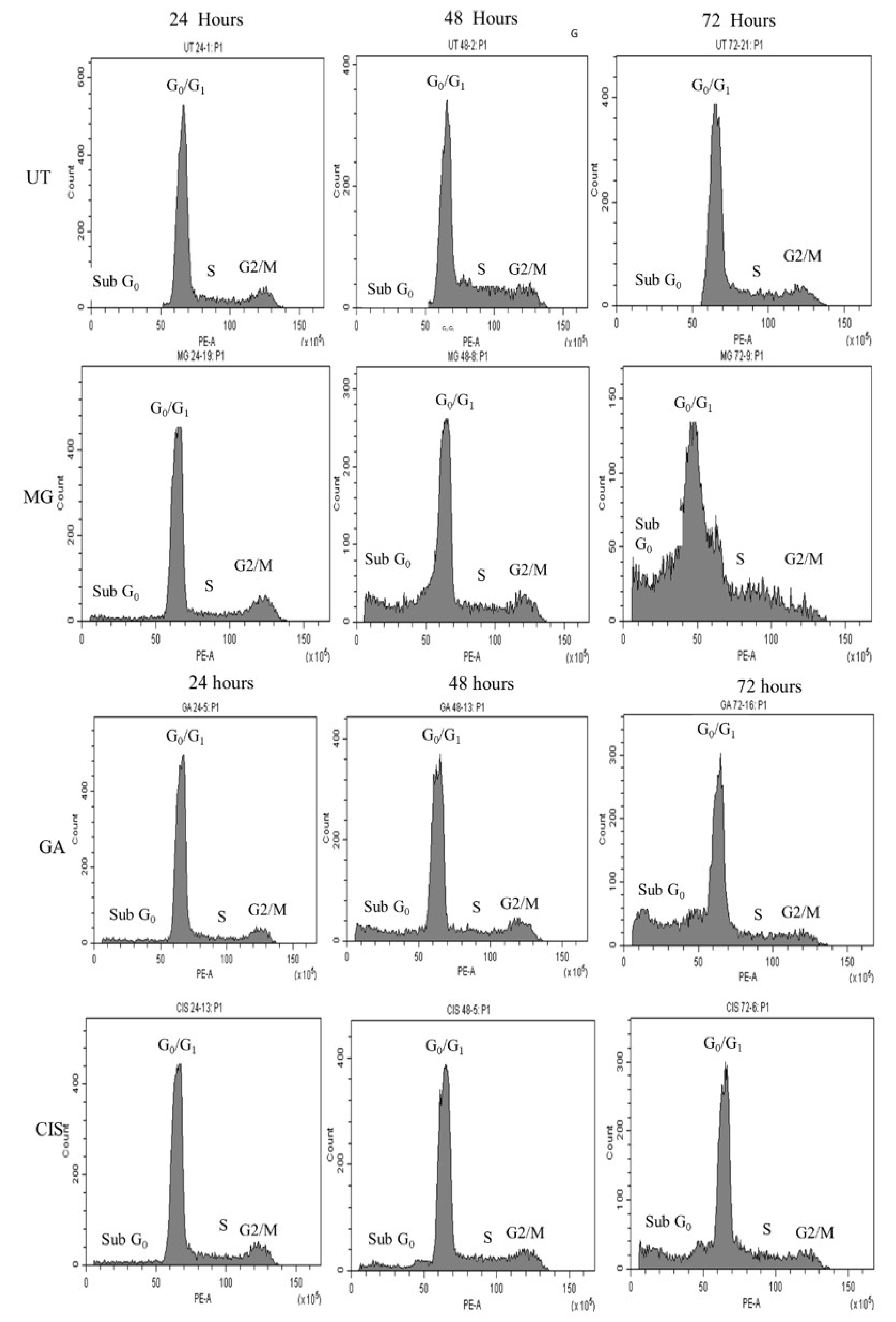
2.6. Cell Cycle Arrest
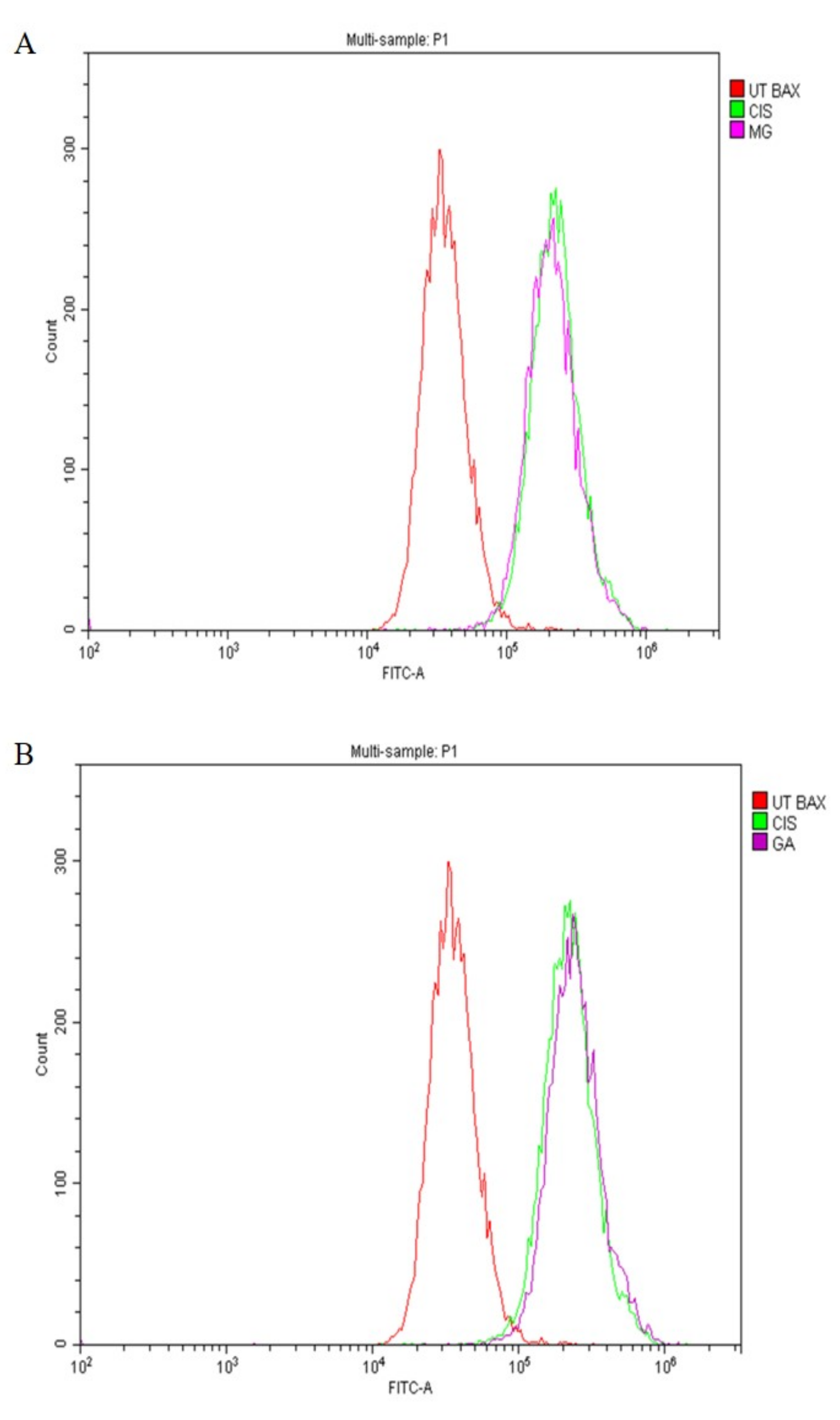
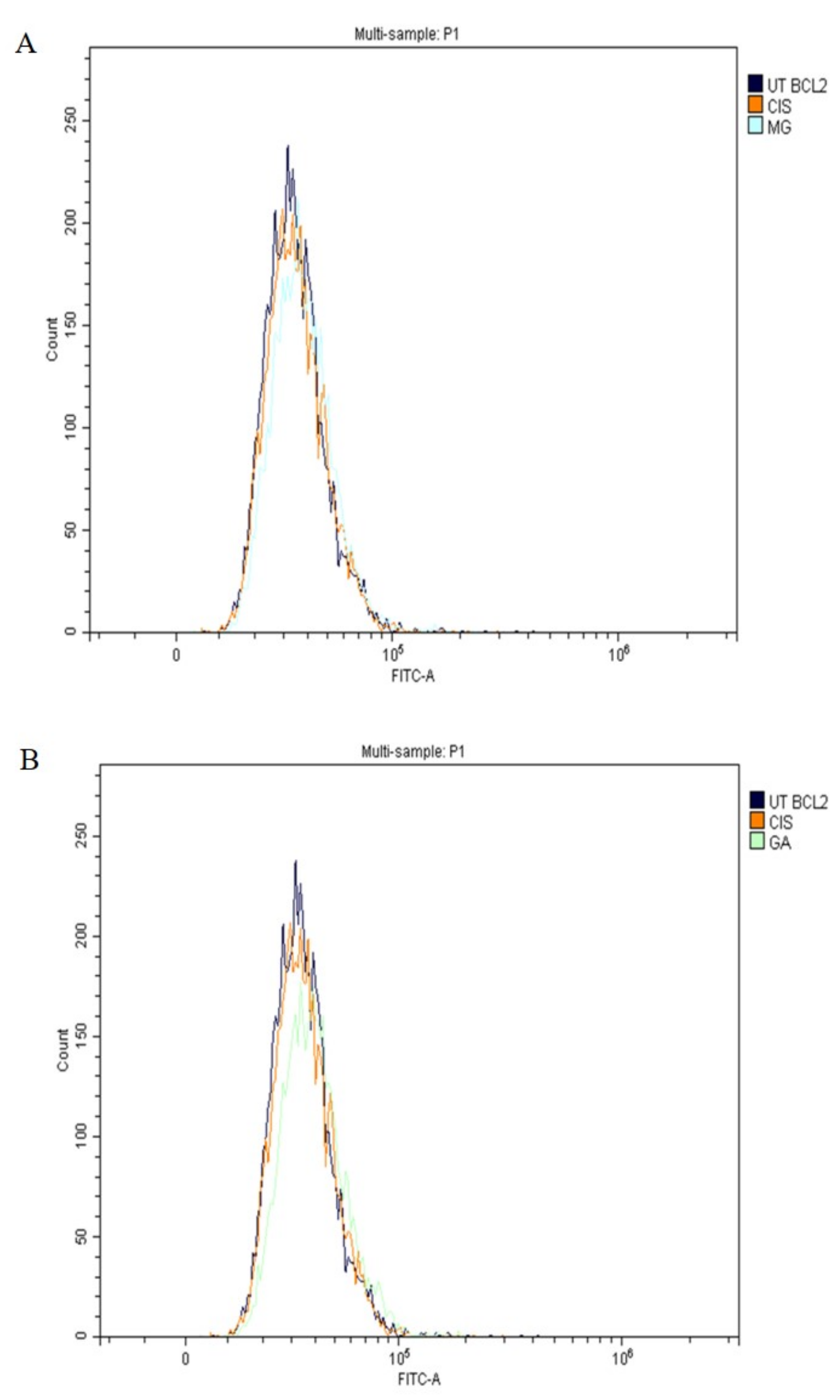
2.7. Expression of Apoptotic Protein
2.8. Caspase Analysis
3. Discussion
4. Materials and Methods
4.1. Gallic Acid and Methyl Gallic
4.2. Bioassay-Guided Isolation of Bioactive Compounds from Q. infectoria
4.3. Cell Culture
4.4. Antiproliferative Assay
4.5. Cell Morphology Analysis
4.6. Determination of Phosphatidylserine (PS) Externalisation
4.7. Cell Cycle Analysis
4.8. Determination of p53, Bax and Bcl-2 Expression
4.9. Caspases Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organisation (WHO). World Cancer Report 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organisation Regional Office for South-East Asia. Cancer Fact Sheet 2018; WHO South East Asia: New Delhi, India, 2018. [Google Scholar]
- Walboomers, J.M.; Jacobs, M.V.; Manos, M.M.; Bosch, F.X.; Kummer, J.A.; Shah, K.V.; Snijders, P.J.; Peto, J.; Meijer, C.J.; Muñoz, N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999, 189, 12–19. [Google Scholar] [CrossRef]
- Tarney, C.M.; Han, J. Postcoital Bleeding: A Review on Etiology, Diagnosis, and Management. Obstet. Gynecol. Int. 2014, 2014, 192087. [Google Scholar] [CrossRef] [PubMed]
- Nartthanarung, A.; Thanapprapasr, K.; Udomsubpayakul, U.; Thanapprapasr, D. Age and Survival of Cervical Cancer Patients with Bone Metastasis. Asian Pac. J. Cancer Prev. 2014, 15, 8401–8404. [Google Scholar] [CrossRef] [PubMed]
- Beddoe, A.M. Elimination of cervical cancer: Challenges for developing countries. Ecancermedicalscience 2019, 13, 975. [Google Scholar] [CrossRef]
- Šarenac, T.; Mikov, M. Cervical Cancer, Different Treatments and Importance of Bile Acids as Therapeutic Agents in This Disease. Front. Pharmacol. 2019, 10, 484. [Google Scholar] [CrossRef]
- Adhami, V.M.; Malik, A.; Zaman, N.; Sarfaraz, S.; Siddiqui, I.A.; Syed, D.N.; Afaq, F.; Pasha, F.S.; Saleem, M.; Mukhtar, H. Combined Inhibitory Effects of Green Tea Polyphenols and Selective Cyclooxygenase-2 Inhibitors on the Growth of Human Prostate Cancer Cells Both In vitro and In vivo. Clin. Cancer Res. 2007, 13, 1611–1619. [Google Scholar] [CrossRef]
- Aborehab, N.M.; Osama, N. Effect of Gallic acid in potentiating chemotherapeutic effect of Paclitaxel in HeLa cervical cancer cells. Cancer Cell Int. 2019, 19, 154. [Google Scholar] [CrossRef]
- American Cancer Society. Chemotherapy for Cervical Cancer. 2020. Available online: https://www.cancer.org/cancer/cervical-cancer/treating/chemotherapy.html (accessed on 31 January 2023).
- Jan, R.; Chaudhry, G.-E. Understanding Apoptosis and Apoptotic Pathways Targeted Cancer Therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Kaczanowski, S.; Sajid, M.; Reece, S.E. Evolution of apoptosis-like programmed cell death in unicellular protozoan parasites. Parasites Vectors 2011, 4, 44. [Google Scholar] [CrossRef]
- Plati, J.; Bucur, O.; Khosravi-Far, R. Apoptotic cell signaling in cancer progression and therapy. Integr. Biol. 2011, 3, 279–296. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Tajudin, T.-J.S.A.; Mat, N.; Siti-Aishah, A.B.; Yusran, A.A.M.; Alwi, A.; Ali, A.M. Cytotoxicity, Antiproliferative Effects, and Apoptosis Induction of Methanolic Extract of Cynometra cauliflora Linn. Whole Fruit on Human Promyelocytic Leukemia HL-60 Cells. Evid.-Based Complement. Altern. Med. 2012, 2012, 127373. [Google Scholar] [CrossRef]
- Khalili, R.M.A.; Noratiqah, J.M.; Norhaslinda, R.; Norhayati, A.H.; Amin, B.A.; Roslan, A.; Zubaidi, A.L.A. Cytotoxicity Effect and Morphological Study of Different Duku(Lansium domesticum corr.) Extract towards Human Colorectal Adenocarcinoma Cells Line (HT-29). Pharmacogn. J. 2017, 9, 757–761. [Google Scholar] [CrossRef]
- Chen, Y.; Hua, Y.; Li, X.; Arslan, I.M.; Zhang, W.; Meng, G. Distinct Types of Cell Death and the Implication in Diabetic Cardiomyopathy. Front. Pharmacol. 2020, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Jantamat, P.; Weerapreeyakul, N.; Puthongking, P. Cytotoxicity and Apoptosis Induction of Coumarins and Carbazole Alkaloids from Clausena Harmandiana. Molecules 2019, 24, 3385. [Google Scholar] [CrossRef]
- Moradi, M.-T.; Karimi, A.; Alidadi, S. In vitro antiproliferative and apoptosis-inducing activities of crude ethyle alcohole extract of Quercus brantii L. acorn and subsequent fractions. Chin. J. Nat. Med. 2016, 14, 196–202. [Google Scholar] [CrossRef]
- Cragg, G.M.; Pezzuto, J.M. Natural Products as a Vital Source for the Discovery of Cancer Chemotherapeutic and Chemopreventive Agents. Med. Princ. Pract. 2015, 25 (Suppl. S2), 41–59. [Google Scholar] [CrossRef]
- Gurib-Fakim, A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Asp. Med. 2006, 27, 1–93. [Google Scholar] [CrossRef]
- Kaur, M.; Velmurugan, B.; Rajamanickam, S.; Agarwal, R.; Agarwal, C. Gallic acid, an active constituent of grape seed extract, exhibits anti-proliferative, pro-apoptotic and antitumorigenic effects against prostate carcinoma xenograft growth in nude mice. Pharm Res. 2009, 26, 2133–2140. [Google Scholar] [CrossRef]
- Chang, Y.J.; Hsu, S.L.; Liu, Y.T.; Lin, Y.H.; Lin, M.H.; Huang, S.J.; Ho, J.A.; Wu, L.C. Gallic acid induces necroptosis via TNF-α signaling pathway in activated hepatic stellate cells. PLoS ONE 2015, 10, e120713. [Google Scholar] [CrossRef]
- Fernandes, F.H.A.; Salgado, H.R.N. Gallic Acid: Review of the Methods of Determination and Quantification. Crit. Rev. Anal. Chem. 2016, 46, 257–265. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Li, B.; Rankin, G.O.; Rojanasakul, Y.; Chen, Y.C. Selecting bioactive phenolic compounds as potential agents to inhibit proliferation and VEGF expression in human ovarian cancer cells. Oncol. Lett. 2015, 9, 1444–1450. [Google Scholar] [CrossRef] [PubMed]
- Habtemariam, S. Methyl-3-O-Methyl Gallate and Gallic Acid from the Leaves of Peltiphyllum peltatum: Isolation and Comparative Antioxidant, Prooxidant, and Cytotoxic Effects in Neuronal Cells. J. Med. Food 2011, 14, 1412–1418. [Google Scholar] [CrossRef]
- Hsieh, T.-J.; Liu, T.-Z.; Chia, Y.-C.; Chern, C.-L.; Lu, F.-J.; Chuang, M.-C.; Mau, S.-Y.; Chen, S.-H.; Syu, Y.-H.; Chen, C.-H. Protective effect of methyl gallate from Toona sinensis (Meliaceae) against hydrogen peroxide-induced oxidative stress and DNA damage in MDCK cells. Food Chem. Toxicol. 2004, 42, 843–850. [Google Scholar] [CrossRef]
- Choi, J.-G.; Kang, O.-H.; Lee, Y.-S.; Oh, Y.-C.; Chae, H.-S.; Jang, H.-J.; Shin, D.-W.; Kwon, D.-Y. Antibacterial Activity of Methyl Gallate Isolated from Galla Rhois or Carvacrol Combined with Nalidixic Acid Against Nalidixic Acid Resistant Bacteria. Molecules 2009, 14, 1773–1780. [Google Scholar] [CrossRef]
- Kang, M.-S.; Jang, H.-S.; Oh, J.-S.; Yang, K.-H.; Choi, N.-K.; Lim, H.-S.; Kim, S.-M. Effects of methyl gallate and gallic acid on the production of inflammatory mediators interleukin-6 and interleukin-8 by oral epithelial cells stimulated with Fusobacterium nucleatum. J. Microbiol. 2009, 47, 760–767. [Google Scholar] [CrossRef]
- Kamatham, S.; Kumar, N.; Gudipalli, P. Isolation and characterization of gallic acid and methyl gallate from the seed coats of Givotia rottleriformis Griff. and their anti-proliferative effect on human epidermoid carcinoma A431 cells. Toxicol. Rep. 2015, 2, 520–529. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, L.; Wu, P.; Li, W.; Li, T.; Gu, R.; Dan, X.; Li, Z.; Fan, X.; Xiao, Z. Gallic acid has anticancer activity and enhances the anticancer effects of cisplatin in non-small cell lung cancer A549 cells via the JAK/STAT3 signaling pathway. Oncol. Rep. 2019, 41, 1779–1788. [Google Scholar] [CrossRef]
- McChesney, J.D.; Venkataraman, S.K.; Henri, J.T. Plant natural products: Back to the future or into extinction? Phytochemistry 2007, 68, 2015–2022. [Google Scholar] [CrossRef]
- Badhani, A.; Rawat, S.; Bhatt, I.D.; Rawal, R.S. Variation in Chemical Constituents and Antioxidant Activity in Yellow Himalayan (Rubus ellipticus Smith) and Hill Raspberry (Rubus niveus Thunb.). J. Food Biochem. 2015, 39, 663–672. [Google Scholar] [CrossRef]
- Karamać, M.; Biskup, I.; Kulczyk, A. Fractionation of Buckwheat Seed Phenolics and Analysis of Their Antioxidant Activity. Pol. J. Food Nutr. Sci. 2015, 65, 243–249. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological effects of gallic acid in health and disease: A mechanistic review. Iran J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ibrahim, M.D.; Kntayya, S.B.; Ain, N.M.; Iori, R.; Ioannides, C.; Razis, A.F.A. Induction of Apoptosis and Cytotoxicity by Raphasatin in Human Breast Adenocarcinoma MCF-7 Cells. Molecules 2018, 23, 3092. [Google Scholar] [CrossRef] [PubMed]
- Shahruzaman, S.H.; Mustafa, M.F.; Ramli, S.; Maniam, S.; Fakurazi, S. The Cytotoxic Properties of Baeckea frutescens Branches Extracts in Eliminating Breast Cancer Cells. Evid.-Based Complement. Altern. Med. 2019, 2019, 9607590. [Google Scholar] [CrossRef]
- Kwan, Y.P.; Saito, T.; Ibrahim, D.; Al-Hassan, F.M.S.; Oon, C.E.; Chen, Y.; Jothy, S.L.; Kanwar, J.R.; Sasidharan, S. Evaluation of the cytotoxicity, cell-cycle arrest, and apoptotic induction by Euphorbia hirta in MCF-7 breast cancer cells. Pharm. Biol. 2015, 54, 1223–1236. [Google Scholar] [CrossRef]
- Li, X.; Luo, X.; He, J.; Wang, N.; Zhou, H.; Yang, P.; Zhang, T. Induction of apoptosis in human cervical carcinoma HeLa cells by active compounds from Hypericum ascyron L. Oncol. Lett. 2018, 15, 3944–3950. [Google Scholar] [CrossRef]
- Meli, M.; Shafie, N.H.; Loh, S.P.; Rahmat, A. Anti-proliferative and apoptosis-inducing effects of Morinda citrifolia L. shoot on breast, liver, and colorectal cancer cell lines. Mal. J. Med. Health Sci. 2019, 15, 129–135. [Google Scholar]
- Ameera, O.H.; Ghaidaa, J.M.; Mohammed, G.J.; Hameed, I.H. Phytochemical screening of methanolic dried galls extract of Quercus infectoria using gas chromatography-mass spectrometry (GC-MS) and Fourier transform-infrared (FT-IR). J. Pharmacogn. Phytotherapy 2016, 8, 49–59. [Google Scholar] [CrossRef]
- Burlacu, E.; Nisca, A.; Tanase, C. A Comprehensive Review of Phytochemistry and Biological Activities of Quercus Species. Forests 2020, 11, 904. [Google Scholar] [CrossRef]
- Gao, J.; Yang, X.; Yin, W.; Li, M. Gallnuts: A Potential Treasure in Anticancer Drug Discovery. Evid.-Based Complement. Altern. Med. 2018, 2018, 4930371. [Google Scholar] [CrossRef]
- Kheirandish, F.; Delfan, B.; Mahmoudvand, H.; Moradi, N.; Ezatpour, B.; Ebrahimzadeh, F.; Rashidipour, M. Antileishmanial, antioxidant, and cytotoxic activities of Quercus infectoria Olivier extract. Biomed. Pharmacother. 2016, 82, 208–215. [Google Scholar] [CrossRef]
- Yarani, R.; Mansouri, K.; Mohammadi-Motlagh, H.R.; Mahnam, A.; Aleagha, M.S.E. In vitro inhibition of angiogenesis by hydroalcoholic extract of oak (Quercus infectoria) acorn shell via suppressing VEGF, MMP-2, and MMP-9 secretion. Pharm. Biol. 2013, 51, 361–368. [Google Scholar] [CrossRef]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef]
- Mamat, N.; Abdullah, H.; Hapidin, H.; Mokhtar, N.F. Gallic Acid and Methyl Gallate Enhance Antiproliferative Effect of Cisplatin on Cervical Cancer (HeLa) Cells. Sains Malays. 2020, 49, 1107–1114. [Google Scholar] [CrossRef]
- Mandal, N.; Chaudhuri, D.; Ghate, N.B.; Singh, S.S. Methyl gallate isolated from Spondias pinnata exhibits anticancer activity against human glioblastoma by induction of apoptosis and sustained extracellular signal-regulated kinase 1/2 activation. Pharmacogn. Mag. 2015, 11, 269–276. [Google Scholar] [CrossRef]
- Rahim, A.C.; Abu Bakar, M.; Kassim, N.; Stanslas, J.; Zain, W.W.M. Selective Cytotoxic Activity of Methyl-3,4,5-trihydroxybenzoate Isolated from Kernel of Bambangan (Mangifera pajang). Asian J. Chem. 2018, 30, 2273–2276. [Google Scholar] [CrossRef]
- Taiwo, B.J.; Fatokun, A.A.; Olubiyi, O.O.; Bamigboye-Taiwo, O.T.; van Heerden, F.R.; Wright, C.W. Identification of compounds with cytotoxic activity from the leaf of the Nigerian medicinal plant, Anacardium occidentale L. (Anacardiaceae). Bioorg. Med. Chem. 2017, 25, 2327–2335. [Google Scholar] [CrossRef]
- Fiuza, S.; Gomes, C.; Teixeira, L.; da Cruz, M.T.G.; Cordeiro, M.N.D.S.; Milhazes, N.; Borges, F.; Marques, M. Phenolic acid derivatives with potential anticancer properties—A structure—Activity relationship study. Part 1: Methyl, propyl and octyl esters of caffeic and gallic acids. Bioorganic Med. Chem. 2004, 12, 3581–3589. [Google Scholar] [CrossRef]
- You, B.R.; Moon, H.J.; Han, Y.H.; Park, W.H. Gallic acid inhibits the growth of HeLa cervical cancer cells via apoptosis and/or necrosis. Food Chem. Toxicol. 2010, 48, 1334–1340. [Google Scholar] [CrossRef]
- Park, W.H. Gallic acid inhibits the growth of calf pulmonary arterial endothelial cells through cell death and glutathione depletion. Mol. Med. Rep. 2017, 16, 7805–7812. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Hu, M. Gallic acid reduces cell viability, proliferation, invasion and angiogenesis in human cervical cancer cells. Oncol. Lett. 2013, 6, 1749–1755. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, S.; Hamid, R.A.; Esa, N.M.; Ramachandran, V.; Aalam, G.T.F.; Etemad, A.; Ismail, P. Promotion of HepG2 cell apoptosis by flower of Allium atroviolaceum and the mechanism of action. BMC Complement. Altern. Med. 2017, 17, 104. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.-B.; Bustamam, A.; Sukari, M.A.; Abdelwahab, S.I.; Mohan, S.; Buckle, M.J.C.; Kamalidehghan, B.; Nadzri, N.M.; Anasamy, T.; Hadi, A.H.A.; et al. Induction of selective cytotoxicity and apoptosis in human T4-lymphoblastoid cell line (CEMss) by boesenbergin a isolated from boesenbergia rotunda rhizomes involves mitochondrial pathway, activation of caspase 3 and G2/M phase cell cycle arrest. BMC Complement. Altern. Med. 2013, 13, 41. [Google Scholar] [CrossRef]
- Allen, L.-A.H.; Aderem, A. Mechanisms of phagocytosis. Curr. Opin. Immunol. 1996, 8, 36–40. [Google Scholar] [CrossRef]
- Ziegler, U.; Groscurth, P. Morphological Features of Cell Death. Physiology 2004, 19, 124–128. [Google Scholar] [CrossRef]
- Nordin, N.; Yeap, S.K.; Rahman, H.S.; Zamberi, N.R.; Abu, N.; Mohamad, N.E.; How, C.W.; Masarudin, M.J.; Abdullah, R.; Alitheen, N.B. In vitro cytotoxicity and anticancer effects of citral nanostructured lipid carrier on MDA MBA-231 human breast cancer cells. Sci. Rep. 2019, 9, 1614. [Google Scholar] [CrossRef]
- Silva, M.T. Secondary necrosis: The natural outcome of the complete apoptotic program. FEBS Lett. 2010, 584, 4491–4499. [Google Scholar] [CrossRef]
- Wisitpongpun, P.; Suphrom, N.; Potup, P.; Nuengchamnong, N.; Calder, P.; Usuwanthim, K. In Vitro Bioassay-Guided Identification of Anticancer Properties from Moringa oleifera Lam. Leaf against the MDA-MB-231 Cell Line. Pharmaceuticals 2020, 13, 464. [Google Scholar] [CrossRef]
- Sourani, Z.; Pourgheysari, B.; Beshkar, P.; Shirzad, H.; Shirzad, M. Gallic Acid Inhibits Proliferation and Induces Apoptosis in Lymphoblastic Leukemia Cell Line (C121). Iran. J. Med. Sci. 2016, 41, 525–530. [Google Scholar]
- Sun, G.; Zhang, S.; Xie, Y.; Zhang, Z.; Zhao, W. Gallic acid as a selective anticancer agent that induces apoptosis in SMMC-7721 human hepatocellular carcinoma cells. Oncol. Lett. 2015, 11, 150–158. [Google Scholar] [CrossRef]
- Liang, W.; Li, X.; Li, Y.; Li, C.; Gao, B.; Gan, H.; Li, S.; Shen, J.; Kang, J.; Ding, S.; et al. Gallic acid induces apoptosis and inhibits cell migration by upregulating miR-518b in SW1353 human chondrosarcoma cells. Int. J. Oncol. 2014, 44, 91–98. [Google Scholar] [CrossRef]
- Liu, Z.; Li, D.; Yu, L.; Niu, F. Gallic Acid as a Cancer-Selective Agent Induces Apoptosis in Pancreatic Cancer Cells. Chemotherapy 2012, 58, 185–194. [Google Scholar] [CrossRef]
- Bendale, Y.; Bendale, V.; Paul, S. Evaluation of cytotoxic activity of platinum nanoparticles against normal and cancer cells and its anticancer potential through induction of apoptosis. Integr. Med. Res. 2017, 6, 141–148. [Google Scholar] [CrossRef]
- Beedessee, G.; Ramanjooloo, A.; Tiscornia, I.; Cresteil, T.; Raghothama, S.; Arya, D.; Rao, S.; Gowd, K.H.; Bollati-Fogolin, M.; Marie, D.E.P. Evaluation of hexane and ethyl acetate extracts of the sponge Jaspis diastra collected from Mauritius Waters on HeLa cells. J. Pharm. Pharmacol. 2014, 66, 1317–1327. [Google Scholar] [CrossRef]
- Kang, M.; Liu, W.Q.; Qin, Y.T.; Wei, Z.X.; Wang, R.S. Long-term efficacy of microwave hyperthermia combined with chemoradiotherapy in treatment of nasopharyngeal carcinoma with cervical lymph node metastases. Asian Pac. J. Cancer Prev. 2013, 14, 7395–7400. [Google Scholar] [CrossRef]
- Wong, Y.H.; Kadir, H.A.; Ling, S.K. Bioassay-Guided Isolation of Cytotoxic Cycloartane Triterpenoid Glycosides from the Traditionally Used Medicinal Plant Leea indica. Evid.-Based Complement. Altern. Med. 2011, 2012, 164689. [Google Scholar] [CrossRef]
- Kim, E.-J.; Kang, C.-W.; Kim, N.-H.; Seo, Y.B.; Nam, S.-W.; Kim, G.-D. Induction of Apoptotic Cell Death on Human Cervix Cancer HeLa cells by Extract from Loranthus yadoriki. Biotechnol. Bioprocess Eng. 2018, 23, 201–207. [Google Scholar] [CrossRef]
- Kntayya, S.B.; Ibrahim, M.D.; Mohd Ain, N.; Iori, R.; Ioannides, C.; Abdull Razis, A.F. Induction of Apoptosis and Cytotoxicity by Isothiocyanate Sulforaphene in Human Hepatocarcinoma HepG2 Cells. Nutrients 2018, 10, 718. [Google Scholar] [CrossRef]
- Ebrahimi Nigjeh, S.; Yusoff, F.M.; Mohamed Alitheen, N.B.; Rasoli, M.; Keong, Y.S.; Omar, A.R. Cytotoxic Effect of Ethanol Extract of Microalga, Chaetoceros calcitrans, and Its Mechanisms in Inducing Apoptosis in Human Breast Cancer Cell Line. BioMed Res. Int. 2013, 2013, 783690. [Google Scholar] [CrossRef]
- Surova, O.; Zhivotovsky, B. Various modes of cell death induced by DNA damage. Oncogene 2013, 32, 3789–3797. [Google Scholar] [CrossRef] [PubMed]
- Faried, A.; Kurnia, D.; Faried, L.S.; Usman, N.; Miyazaki, T.; Kato, H.; Kuwano, H. Anticancer effects of gallic acid isolated from Indonesian herbal medicine, Phaleria macrocarpa (Scheff.) Boerl, on human cancer cell lines. Int. J. Oncol. 2007, 30, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, G.; Sohn, S.-H.; Lee, C.; Kwak, J.W.; Bae, H. Immunotherapy with methyl gallate, an inhibitor of Treg cell migration, enhances the anti-cancer effect of cisplatin therapy. Korean J. Physiol. Pharmacol. 2016, 20, 261. [Google Scholar] [CrossRef] [PubMed]
- Ji, B.-C.; Hsu, W.-H.; Yang, J.-S.; Hsia, T.-C.; Lu, C.-C.; Chiang, J.-H.; Yang, J.-L.; Lin, C.-H.; Lin, J.-J.; Suen, L.-J.W.; et al. Gallic Acid Induces Apoptosis via Caspase-3 and Mitochondrion-Dependent Pathways in Vitro and Suppresses Lung Xenograft Tumor Growth in Vivo. J. Agric. Food Chem. 2009, 57, 7596–7604. [Google Scholar] [CrossRef]
- Madlener, S.; Illmer, C.; Horvath, Z.; Saiko, P.; Losert, A.; Herbacek, I.; Grusch, M.; Elford, H.L.; Krupitza, G.; Bernhaus, A.; et al. Gallic acid inhibits ribonucleotide reductase and cyclooxygenases in human HL-60 promyelocytic leukemia cells. Cancer Lett. 2007, 245, 156–162. [Google Scholar] [CrossRef]


| Compounds | IC50 (µg/mL) | |
|---|---|---|
| HeLa | Vero | |
| EAQI | 11.50 ± 0.50 * | >100 |
| Cisplatin (CIS) | 1.85 ± 0.15 | 14.33 ± 0.88 |
| Fractions/Subfractions | IC50 (µg/mL) | ||
|---|---|---|---|
| HeLa | Vero | ||
| 1st CC (Fractions) | EAQI/FA1 | >100 | >100 |
| EAQI/FA2 | >100 | >100 | |
| EAQI/FA3 | 16.67 ± 1.76 * | >100 | |
| EAQI/FA4 | 60.33 ± 1.45 | >100 | |
| Cisplatin | 3.33 ± 0.88 * | 13.67 ± 1.20 * | |
| 2nd CC (Subfractions) | EAQI/FA3/B1 | >100 | >100 |
| EAQI/FA3/B2 | 11.00 ± 0.58 * | >100 | |
| EAQI/FA3/B3 | 56.67 ± 1.76 | >100 | |
| EAQI/FA3/B4 | 51.33 ± 0.88 | >100 | |
| EAQI/FA3/B5 | 10.00 ± 1.06 * | >100 | |
| Cisplatin | 2.33 ± 0.33 * | 11.67 ± 0.88 * | |
| Compounds | IC50 (µg/mL) | |
|---|---|---|
| HeLa | Vero | |
| Methylgallate (Methyl 3,4,5-trihydroxybenzoate) | 11.00 ± 0.58 | >100 |
| Gallic acid (3,4,5-trihydroxybenzoic acid) | 10.00 ± 1.06 | >100 |
| Cisplatin | 1.85 ± 0.15 | 11.67 ± 0.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullah, H.; Ismail, I.; Suppian, R.; Zakaria, N.M. Natural Gallic Acid and Methyl Gallate Induces Apoptosis in Hela Cells through Regulation of Intrinsic and Extrinsic Protein Expression. Int. J. Mol. Sci. 2023, 24, 8495. https://doi.org/10.3390/ijms24108495
Abdullah H, Ismail I, Suppian R, Zakaria NM. Natural Gallic Acid and Methyl Gallate Induces Apoptosis in Hela Cells through Regulation of Intrinsic and Extrinsic Protein Expression. International Journal of Molecular Sciences. 2023; 24(10):8495. https://doi.org/10.3390/ijms24108495
Chicago/Turabian StyleAbdullah, Hasmah, Ilyana Ismail, Rapeah Suppian, and Nor Munirah Zakaria. 2023. "Natural Gallic Acid and Methyl Gallate Induces Apoptosis in Hela Cells through Regulation of Intrinsic and Extrinsic Protein Expression" International Journal of Molecular Sciences 24, no. 10: 8495. https://doi.org/10.3390/ijms24108495






