Probiotic Yoghurt Enriched with Mango Peel Powder: Biotransformation of Phenolics and Modulation of Metabolomic Outputs after In Vitro Digestion and Colonic Fermentation
Abstract
1. Introduction
2. Results and Discussions
2.1. Identification of Precursor Polyphenols and Phenolic Catabolites in MPP and MPP Fortified Yoghurts before and after In Vitro Colonic Fermentation
2.1.1. Phenolic Acids
2.1.2. Flavonoids
2.1.3. Other Polyphenols
2.2. Bioconversion of Phenolics during Colonic Fermentation of Mango-Based Yoghurts
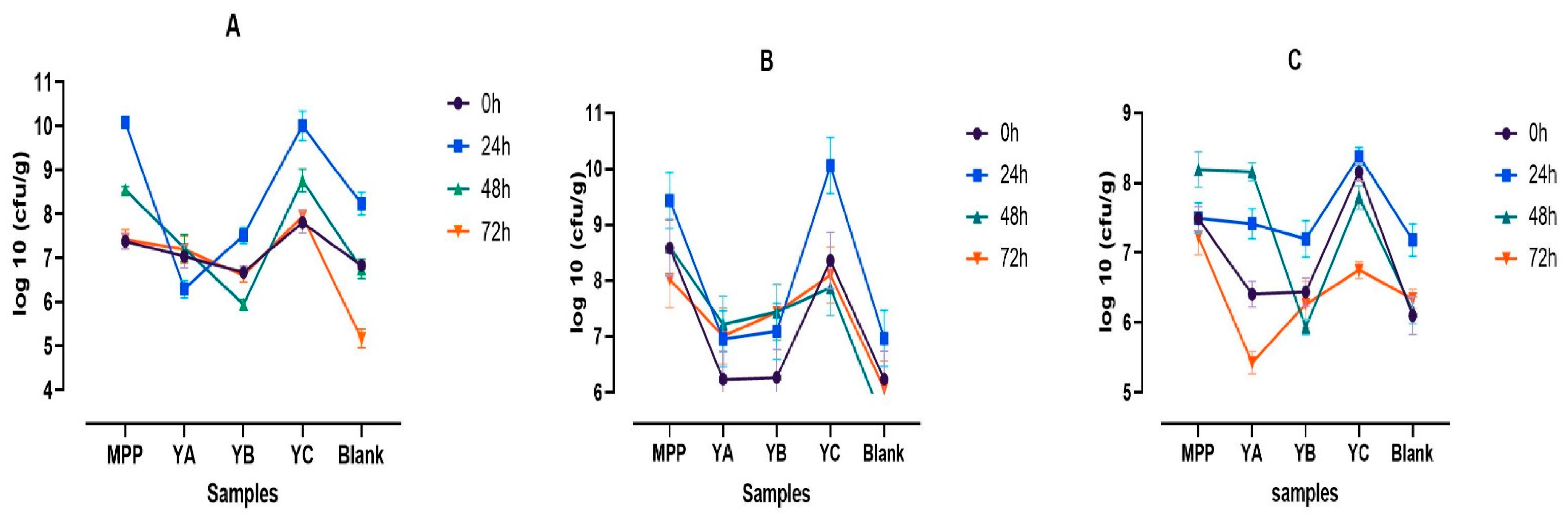
2.3. Variations in pH and Microbiological Population during Colonic Fermentation
2.3.1. pH
2.3.2. Microbial Population
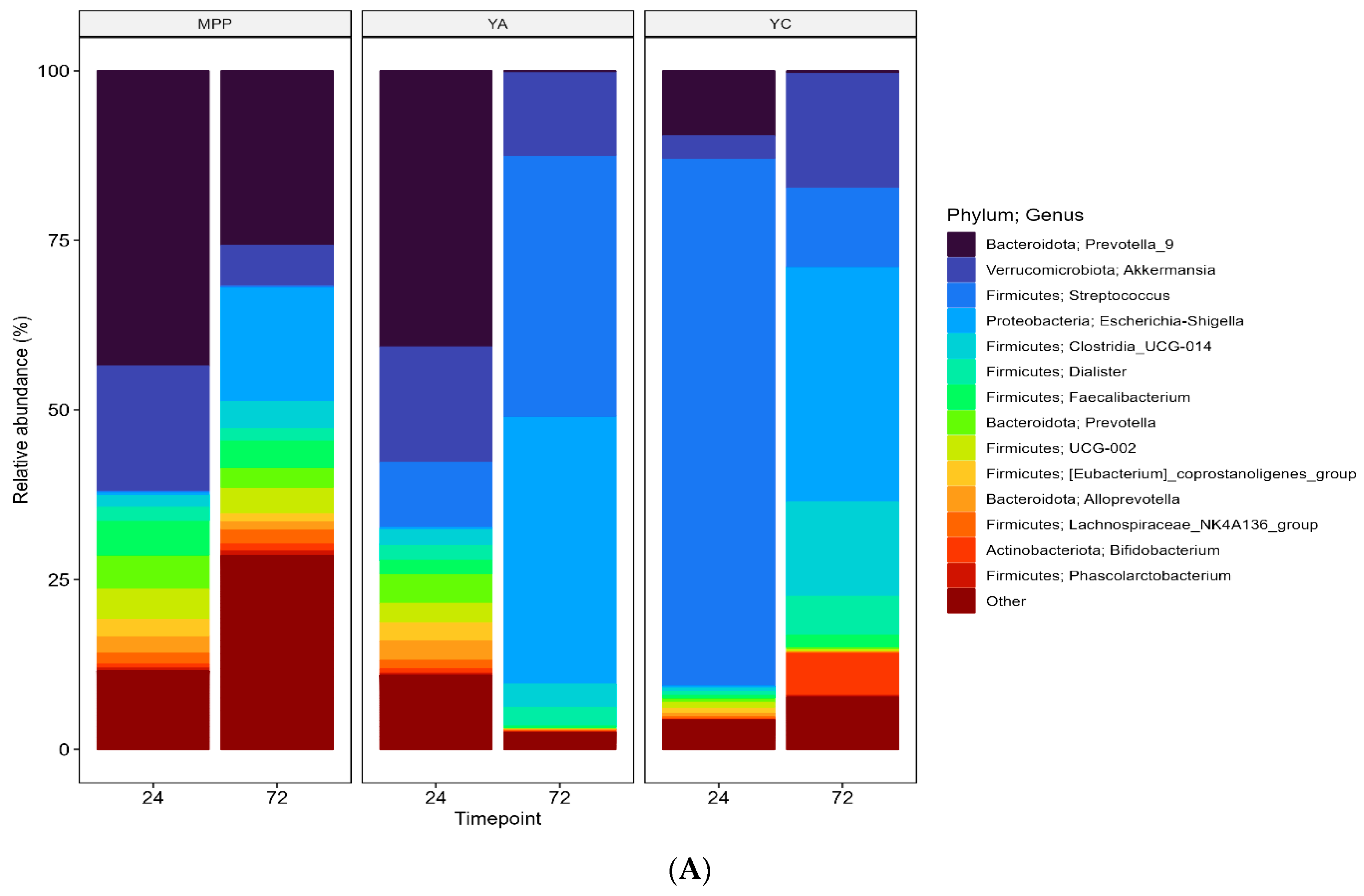
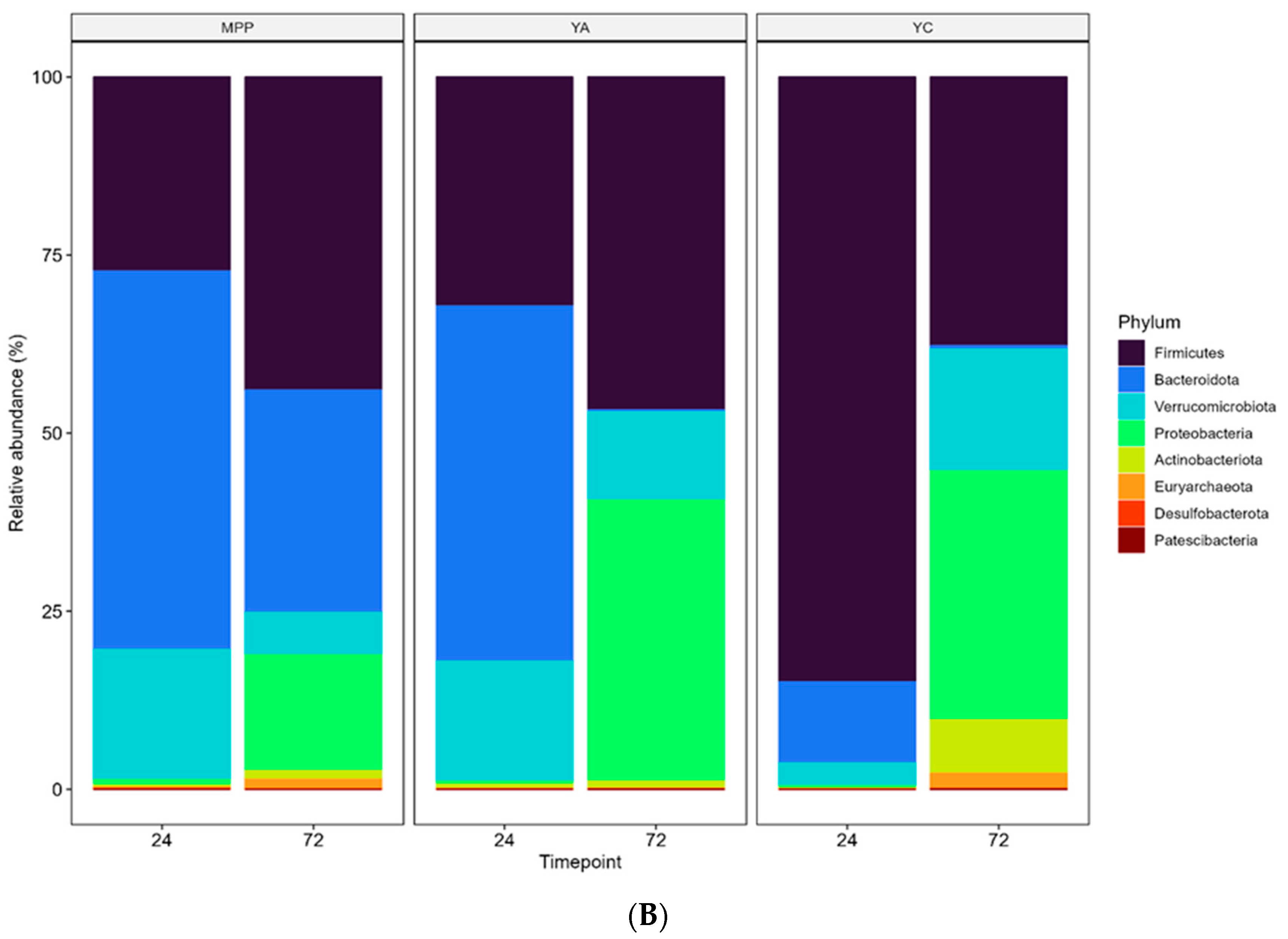
2.3.3. Changes in Faecal Microbial Diversity in the Presence of MPP
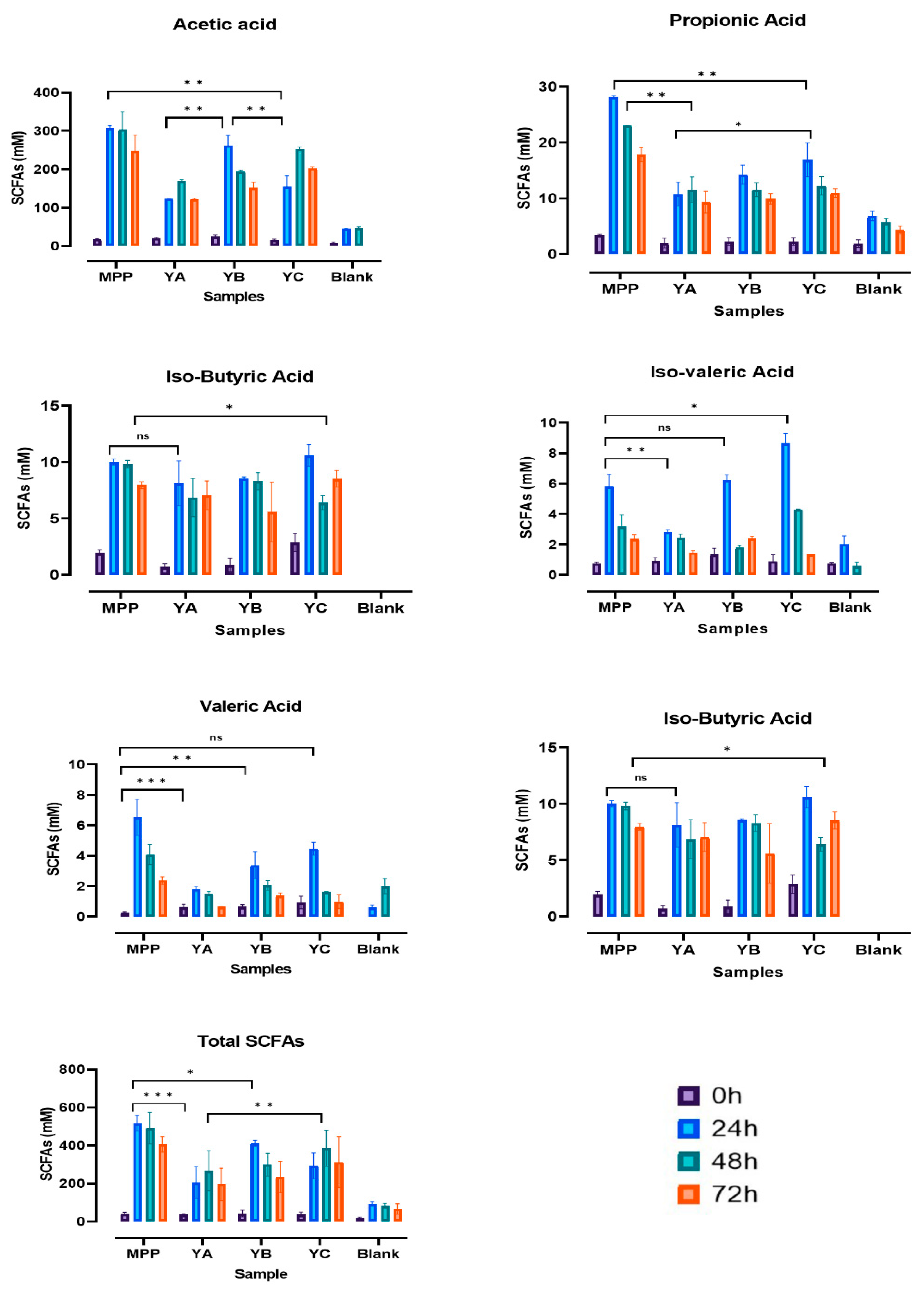
2.4. Short Chain Fatty Acids Production during In Vitro Colonic Fermentation of Yoghurt Enriched with Mango Peel Powder
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Preparation of Probiotic Cultures and Mango Peel Powder
3.2.2. Yoghurt Samples Preparation
3.2.3. In Vitro Gastrointestinal Digestion and Colonic Fermentation of MPP and MPP Fortified Yoghurts
3.2.4. Analysis of Phenolic Metabolites
Extraction of Phenolics
Qualitative Analysis of Polyphenols Using LC-ESI-QTOF-MS2
3.2.5. pH and Microbiological Analysis
3.2.6. 16S rRNA Sequencing Analysis
3.2.7. Determination of SCFA
3.2.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zahid, H.F.; Ranadheera, C.S.; Fang, Z.; Ajlouni, S. Healthy and Functional Ingredients from Fruit Processing Byproducts: A Review Focusing on Fruit Peels. Int. J. Agric. Environ. Biores. 2019, 4, 336–360. [Google Scholar] [CrossRef]
- Mohamed, S. Functional foods against metabolic syndrome (obesity, diabetes, hypertension and dyslipidemia) and cardiovasular disease. Trends Food Sci. Technol. 2014, 35, 114–128. [Google Scholar] [CrossRef]
- Gutiérrez-Sarmiento, W.; Sáyago-Ayerdi, S.G.; Goñi, I.; Gutiérrez-Miceli, F.A.; Abud-Archila, M.; Rejón-Orantes, J.d.C.; Rincón-Rosales, R.; Peña-Ocaña, B.A.; Ruíz-Valdiviezo, V.M. Changes in Intestinal Microbiota and Predicted Metabolic Pathways During Colonic Fermentation of Mango (Mangifera indica L.)—Based Bar Indigestible Fraction. Nutrients 2020, 12, 683. [Google Scholar] [CrossRef] [PubMed]
- Santhiravel, S.; Bekhit, A.E.D.A.; Mendis, E.; Jacobs, J.L.; Dunshea, F.R.; Rajapakse, N.; Ponnampalam, E.N. The impact of plant phytochemicals on the gut microbiota of humans for a balanced diet. Int. J. Mol. Sci. 2022, 23, 8124. [Google Scholar] [CrossRef] [PubMed]
- Leonard, W.; Zhang, P.; Ying, D.; Adhikari, B.; Fang, Z. Fermentation transforms the phenolic profiles and bioactivities of plant-based foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef] [PubMed]
- Quatrin, A.; Rampelotto, C.; Pauletto, R.; Maurer, L.H.; Nichelle, S.M.; Klein, B.; Rodrigues, R.F.; Maróstica Junior, M.R.; Fonseca, B.d.S.; de Menezes, C.R.; et al. Bioaccessibility and catabolism of phenolic compounds from jaboticaba (Myrciaria trunciflora) fruit peel during in vitro gastrointestinal digestion and colonic fermentation. J. Funct. Foods 2020, 65, 103714. [Google Scholar] [CrossRef]
- Hidalgo, M.; Oruna-Concha, M.J.; Kolida, S.; Walton, G.E.; Kallithraka, S.; Spencer, J.P.; de Pascual-Teresa, S. Metabolism of anthocyanins by human gut microflora and their influence on gut bacterial growth. J. Agric. Food Chem. 2012, 60, 3882–3890. [Google Scholar] [CrossRef]
- Sayago-Ayerdi, S.G.; Zamora-Gasga, V.M.; Venema, K. Prebiotic effect of predigested mango peel on gut microbiota assessed in a dynamic in vitro model of the human colon (TIM-2). Food Res. Int. 2019, 118, 89–95. [Google Scholar] [CrossRef]
- Zahid, H.F.; Ali, A.; Ranadheera, C.S.; Fang, Z.; Dunshea, F.R.; Ajlouni, S. In vitro bioaccessibility of phenolic compounds and alpha-glucosidase inhibition activity in yoghurts enriched with mango peel powder. Food Biosci. 2022, 50, 102011. [Google Scholar] [CrossRef]
- Peng, D.; Zahid, H.F.; Ajlouni, S.; Dunshea, F.R.; Suleria, H.A.R. LC-ESI-QTOF/MS Profiling of Australian Mango Peel By-Product Polyphenols and Their Potential Antioxidant Activities. Processes 2019, 7, 764. [Google Scholar] [CrossRef]
- Noto, A.; Fanos, V.; Barberini, L.; Grapov, D.; Fattuoni, C.; Zaffanello, M.; Casanova, A.; Fenu, G.; De Giacomo, A.; De Angelis, M. The urinary metabolomics profile of an Italian autistic children population and their unaffected siblings. J. Matern.-Fetal Neonatal Med. 2014, 27, 46–52. [Google Scholar] [CrossRef]
- Mosele, J.I.; Macià, A.; Romero, M.-P.; Motilva, M.-J. Stability and metabolism of Arbutus unedo bioactive compounds (phenolics and antioxidants) under in vitro digestion and colonic fermentation. Food Chem. 2016, 201, 120–130. [Google Scholar] [CrossRef]
- Hernandez-Maldonado, L.M.; Blancas-Benitez, F.J.; Zamora-Gasga, V.M.; Cardenas-Castro, A.P.; Tovar, J.; Sayago-Ayerdi, S.G. In Vitro Gastrointestinal Digestion and Colonic Fermentation of High Dietary Fiber and Antioxidant-Rich Mango (Mangifera indica L.) “Ataulfo”-Based Fruit Bars. Nutrients 2019, 11, 1564. [Google Scholar] [CrossRef] [PubMed]
- Pathak, G.; Singh, S.; Kumari, P.; Hussain, Y.; Raza, W.; Luqman, S.; Meena, A. Cirsilineol inhibits proliferation of lung squamous cell carcinoma by inducing ROS mediated apoptosis. Food Chem. Toxicol. 2020, 143, 111550. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Brown, N.M.; Lydeking-Olsen, E. The clinical importance of the metabolite equol—A clue to the effectiveness of soy and its isoflavones. J. Nutr. 2002, 132, 3577–3584. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Nájera, V.C.; Lugo-Cervantes, E.; Amaya-Delgado, L.; Madrigal-Pulido, J.A.; Rueda-Puente, E.O.; Borboa-Flores, J.; Del-Toro-Sánchez, C.L. Biotransformation of hesperidin from lime peel (Citrus limetta Risso) in solid fermentation by Aspergillus saitoi. CyTA-J. Food 2018, 16, 537–543. [Google Scholar] [CrossRef]
- Dong, R.; Liu, S.; Xie, J.; Chen, Y.; Zheng, Y.; Zhang, X.; Zhao, E.; Wang, Z.; Xu, H.; Yu, Q. The recovery, catabolism and potential bioactivity of polyphenols from carrot subjected to in vitro simulated digestion and colonic fermentation. Food Res. Int. 2021, 143, 110263. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Patán, F.; Cueva, C.; Monagas, M.; Walton, G.E.; Gibson, M.G.R.; Quintanilla-López, J.E.; Lebrón-Aguilar, R.; Martín-Álvarez, P.; Moreno-Arribas, M.V.; Bartolomé, B. In vitro fermentation of a red wine extract by human gut microbiota: Changes in microbial groups and formation of phenolic metabolites. J. Agric. Food Chem. 2012, 60, 2136–2147. [Google Scholar] [CrossRef]
- Almeida, A.F.; Borge, G.I.A.; Piskula, M.; Tudose, A.; Tudoreanu, L.; Valentova, K.; Williamson, G.; Santos, C.N. Bioavailability of Quercetin in humans with a focus on interindividual variation. Compr. Rev. Food Sci. Food Saf. 2018, 17, 714–731. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Villalba, R.; Vissenaekens, H.; Pitart, J.; Romo-Vaquero, M.; Espín, J.C.; Grootaert, C.; Selma, M.V.; Raes, K.; Smagghe, G.; Possemiers, S. Gastrointestinal simulation model TWIN-SHIME shows differences between human urolithin-metabotypes in gut microbiota composition, pomegranate polyphenol metabolism, and transport along the intestinal tract. J. Agric. Food Chem. 2017, 65, 5480–5493. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, X.; Lu, Q.; Zhang, L.; Wang, X.; Liu, R. C-ring cleavage metabolites of catechin and epicatechin enhanced antioxidant activities through intestinal microbiota. Food Res. Int. 2020, 135, 109271. [Google Scholar] [CrossRef]
- Monagas, M.; Urpi-Sarda, M.; Sánchez-Patán, F.; Llorach, R.; Garrido, I.; Gómez-Cordovés, C.; Andres-Lacueva, C.; Bartolomé, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef] [PubMed]
- Eeckhaut, E.; Struijs, K.; Possemiers, S.; Vincken, J.-P.; Keukeleire, D.D.; Verstraete, W. Metabolism of the lignan macromolecule into enterolignans in the gastrointestinal lumen as determined in the simulator of the human intestinal microbial ecosystem. J. Agric. Food Chem. 2008, 56, 4806–4812. [Google Scholar] [CrossRef]
- Shin, M.K.; Jeon, Y.D.; Jin, J.S. Apoptotic effect of enterodiol, the final metabolite of edible lignans, in colorectal cancer cells. J. Sci. Food Agric. 2019, 99, 2411–2419. [Google Scholar] [CrossRef]
- Zálešák, F.; Bon, D.J.-Y.D.; Pospíšil, J. Lignans and Neolignans: Plant secondary metabolites as a reservoir of biologically active substances. Pharmacol. Res. 2019, 146, 104284. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, M.; Yu, H.; Wang, W.; Han, L.; Chen, Q.; Ruan, J.; Wen, S.; Zhang, Y.; Wang, T. Mangiferin improves hepatic lipid metabolism mainly through its metabolite-norathyriol by modulating SIRT-1/AMPK/SREBP-1c signaling. Front. Pharmacol. 2018, 9, 201. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Yu, H.; Qi, M.; Yuan, X.; Ruan, Z.; Hu, C.; Xiao, M.; Xue, Y.; Yao, Y.; Liu, Q. Biotransformation of citrus fruits phenolic profiles by mixed probiotics in vitro anaerobic fermentation. LWT 2022, 160, 113087. [Google Scholar] [CrossRef]
- Burgos-Edwards, A.; Jimenez-Aspee, F.; Theoduloz, C.; Schmeda-Hirschmann, G. Colonic fermentation of polyphenols from Chilean currants (Ribes spp.) and its effect on antioxidant capacity and metabolic syndrome-associated enzymes. Food Chem. 2018, 258, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Boto-Ordonez, M.; Rothwell, J.A.; Andres-Lacueva, C.; Manach, C.; Scalbert, A.; Urpi-Sarda, M. Prediction of the wine polyphenol metabolic space: An application of the Phenol-Explorer database. Mol. Nutr. Food Res. 2013, 58, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; De Los Reyes-gavilán, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Freire, F.C.; Adorno, M.A.T.; Sakamoto, I.K.; Antoniassi, R.; Chaves, A.C.S.D.; Dos Santos, K.M.O.; Sivieri, K. Impact of multi-functional fermented goat milk beverage on gut microbiota in a dynamic colon model. Food Res. Int. 2017, 99, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Granado-Serrano, A.B.; Martín-Garí, M.; Sánchez, V.; Riart Solans, M.; Berdún, R.; Ludwig, I.A.; Rubió, L.; Vilaprinyó, E.; Portero-Otín, M.; Serrano, J. Faecal bacterial and short-chain fatty acids signature in hypercholesterolemia. Sci. Rep. 2019, 9, 1–13. [Google Scholar]
- Hossain, M.N.; Ranadheera, C.S.; Fang, Z.; Ajlouni, S. Production of short chain fatty acids and vitamin B12 during the in-vitro digestion and fermentation of probiotic chocolate. Food Biosci. 2022, 47, 101682. [Google Scholar] [CrossRef]
- Horiuchi, H.; Kamikado, K.; Aoki, R.; Suganuma, N.; Nishijima, T.; Nakatani, A.; Kimura, I. Bifidobacterium animalis subsp. lactis GCL2505 modulates host energy metabolism via the short-chain fatty acid receptor GPR43. Sci. Rep. 2020, 10, 4158. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Caro, G.; Oliver, C.M.; Weerakkody, R.; Singh, T.; Conlon, M.; Borges, G.; Sanguansri, L.; Lockett, T.; Roberts, S.A.; Crozier, A. Chronic administration of a microencapsulated probiotic enhances the bioavailability of orange juice flavanones in humans. Free Radic. Biol. Med. 2015, 84, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Alves-Santos, A.M.; Sugizaki, C.S.A.; Lima, G.C.; Naves, M.M.V. Prebiotic effect of dietary polyphenols: A systematic review. J. Funct. Foods 2020, 74, 104169. [Google Scholar] [CrossRef]
- Herrera-Cazares, L.A.; Ramírez-Jiménez, A.K.; Wall-Medrano, A.; Campos-Vega, R.; Loarca-Piña, G.; Reyes-Vega, M.; Vázquez-Landaverde, P.A.; Gaytán-Martínez, M. Untargeted metabolomic evaluation of mango bagasse and mango bagasse based confection under in vitro simulated colonic fermentation. J. Funct. Foods 2019, 54, 271–280. [Google Scholar] [CrossRef]
- Luzardo-Ocampo, I.; Loarca-Piña, G.; de Mejia, E.G. Gallic and butyric acids modulated NLRP3 inflammasome markers in a co-culture model of intestinal inflammation. Food Chem. Toxicol. 2020, 146, 111835. [Google Scholar] [CrossRef]
- Low, D.Y.; Williams, B.A.; D’Arcy, B.R.; Flanagan, B.M.; Gidley, M.J. In vitro fermentation of chewed mango and banana: Particle size, starch and vascular fibre effects. Food Funct. 2015, 6, 2464–2474. [Google Scholar] [CrossRef]
- Loo, Y.T.; Howell, K.; Suleria, H.; Zhang, P.; Gu, C.; Ng, K. Sugarcane polyphenol and fiber to affect production of short-chain fatty acids and microbiota composition using in vitro digestion and pig faecal fermentation model. Food Chem. 2022, 385, 132665. [Google Scholar] [CrossRef]
- Tamargo, A.; Cueva, C.; Taladrid, D.; Khoo, C.; Moreno-Arribas, M.V.; Bartolomé, B.; de Llano, D.G. Simulated gastrointestinal digestion of cranberry polyphenols under dynamic conditions. Impact on antiadhesive activity against uropathogenic bacteria. Food Chem. 2022, 368, 130871. [Google Scholar] [CrossRef] [PubMed]
- Zahid, H.F.; Ranadheera, C.S.; Fang, Z.; Ajlouni, S. Utilization of mango, apple and banana fruit peels as prebiotics and functional ingredients. Agriculture 2021, 11, 584. [Google Scholar] [CrossRef]
- Zahid, H.F.; Ranadheera, C.S.; Fang, Z.; Ajlouni, S. Functional and Healthy Yogurts Fortified with Probiotics and Fruit Peel Powders. Fermentation 2022, 8, 469. [Google Scholar] [CrossRef]
- Zahid, H.F.; Ali, A.; Ranadheera, C.S.; Fang, Z.; Ajlouni, S. Identification of Phenolics Profile in Freeze-Dried Apple Peel and Their Bioactivities during In Vitro Digestion and Colonic Fermentation. Int. J. Mol. Sci. 2023, 24, 1514. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Cottrell, J.J.; Dunshea, F.R. Identification and characterization of anthocyanins and non-anthocyanin phenolics from Australian native fruits and their antioxidant, antidiabetic, and anti-Alzheimer potential. Food Res. Int. 2022, 162, 111951. [Google Scholar] [CrossRef]
- Elhalis, H.; Cox, J.; Zhao, J. Ecological diversity, evolution and metabolism of microbial communities in the wet fermentation of Australian coffee beans. Int. J. Food Microbiol. 2020, 321, 108544. [Google Scholar] [CrossRef]
- Yu, Y.; Lee, C.; Kim, J.; Hwang, S. Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnol. Bioeng. 2005, 89, 670–679. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Sonnenburg, E.D.; Gardner, C.D.; Sonnenburg, J.L. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153. [Google Scholar] [CrossRef] [PubMed]



| Phenolic Classes | Proposed Compounds | Molecular Formula | RT (min) | Molecular Weight | Theoretical (m/z) | Observed (m/z) | Mass Error (ppm) | MS/MS Product Ions | Samples |
|---|---|---|---|---|---|---|---|---|---|
| Phenolic acids | |||||||||
| Hydroxybenzoic acids | |||||||||
| 1 | 4-Hydroxybenzoic acid | C7H6O3 | 4.295 | 138.0317 | 137.0244 | 137.0247 | 2.1894 | 93 | MPP-C1 |
| 2 | Gallic acid | C7H6O5 | 7.882 | 170.0215 | 169.0142 | 169.0135 | −4.1417 | 125 | MPP-I, YB-I, YC-I, MPP-C1 *, MPP-C2 *, YC-C1, YC-C2, YB-C1, YB-C2 |
| 3 | Protocatechuic acid | C7H6O4 | 12.235 | 154.0266 | 153.0193 | 153.0195 | 1.307 | 109 | MPP-I, MPP-C1, MPP-C2, MPP-C3 * |
| 4 | Gallic acid 3-O-gallate | C14H10O9 | 14.365 | 322.0325 | 321.0252 | 321.0258 | 1.869 | 303, 275, 169 | MPP-I, YB-I, YC-I |
| 5 | 3-O-Methylgallic acid | C8H8O5 | 15.105 | 184.0372 | 183.0299 | 183.0302 | 1.6391 | 123 | MPP-I, YB-I, YC-I |
| 6 | Ellagic acid | C14H6O8 | 20.059 | 302.0063 | 300.9991 | 300.9976 | −4.6512 | 257, 229 | MPP-I |
| 7 | Syringic acid | C9H10O5 | 20.168 | 198.0528 | 197.0455 | 197.0465 | 5.075 | 182, 153 | MPP-I, MPP-C1, MPP-C2, MPP-C3 |
| 8 | 2-Hydroxyhippuric acid | C9H9NO4 | 48.326 | 411.1717 | 410.1644 | 410.1615 | −7.0703 | 105, 77 | MPP-C1, YC-C1, YC-C2 |
| Hydroxycinnamic acids | |||||||||
| 9 | 3-Feruloylquinic acid | C17H20O9 | 4.104 | 368.1107 | 367.1034 | 367.1032 | −0.5448 | 298, 288, 192, 191 | MPP-C1, MPP-C2, MPP-C3, YC-C1, YC-C2 |
| 10 | 3-Sinapoylquinic acid | C18H22O10 | 4.104 | 398.1213 | 397.114 | 397.1134 | −1.5109 | 379, 351, 223 | MPP-C3 |
| 11 | Caffeoyl tartaric acid | C13H12O9 | 4.107 | 312.0481 | 311.0408 | 311.041 | 0.643 | 267, 247, 179 | YB-C1, YB-C2 |
| 12 | Ferulic acid | C10H10O4 | 4.124 | 194.0579 | 193.0506 | 193.0508 | 1.036 | 178, 149, 134 | MPP-I, MPP-C2, MPP-C3 |
| 13 | Caffeic acid 4-O-glucuronide | C15H16O10 | 9.134 | 356.0744 | 355.0671 | 355.0653 | −5.0695 | 179 | MPP-I, MPP-C1, MPP-C3 |
| 14 | 1,2-Disinapoylgentiobiose | C34H42O19 | 10.786 | 754.232 | 753.2247 | 753.228 | 4.3812 | 531, 369, 207, 175 | YB-I, YC-I, YC-C1, YB-C1 |
| 15 | p-Coumaric acid | C9H8O3 | 12.814 | 164.0473 | 163.04 | 163.0401 | 0.6133 | 195, 177, 145, 117 | MPP-I, YB-I, YB-C1, YB-C2, MPP-C1 |
| Hydroxyphenyl acetic acids | |||||||||
| 16 | Homovanillic acid | C9H10O4 | 6.081 | 182.0579 | 181.0506 | 181.0505 | −0.5523 | 137, 122 | MPP-C1, MPP-C2, MPP-C3, YC-C1, YC-C2, YB-C1, YB-C2 |
| Hydroxyphenylpentanoic acids | |||||||||
| 17 | 5-(3′,4′-dihydroxyphenyl)-valeric acid | C11H14O4 | 20.71 | 210.0892 | 209.0819 | 209.0828 | 4.3045 | 191, 165, 135 | MPP-C1, MPP-C2, MPP-C3, YC-C1, YC-C3, YB-C1, YB-C3 |
| 18 | 3-Hydroxyphenylvaleric acid | C11H14O3 | 30.542 | 194.0943 | 193.087 | 193.0869 | −0.5179 | 175, 149, 59 | MPP-C1, MPP-C2, MPP-C3, YC-C1, YC-C3, YB-C1, YB-C3 |
| Hydroxyphenylpropanoic acids | |||||||||
| 19 | Dihydroferulic acid 4-sulfate | C10H12O7S | 4.812 | 276.0304 | 275.0231 | 275.0239 | 2.9088 | 206 | MPP-I |
| 20 | Dihydroferuloylglycine | C12H15NO5 | 13.673 | 253.095 | 252.0877 | 252.0886 | 3.5702 | 149, 100 | YB-I, YC-I |
| 21 | 4-Hydroxyphenyl-2-propionic acid | C9H10O3 | 19.76 | 166.063 | 165.0557 | 165.0555 | −1.2117 | 121, 119, 93 | MPP-C1, MPP-C2, MP-C3, YC-C1, YB-C1 |
| Flavonoids | |||||||||
| Flavanols | |||||||||
| 22 | (-)-Epigallocatechin 3′-O-glucuronide | C21H22O13 | 4.16 | 482.106 | 481.0987 | 481.0979 | −1.6629 | 149, 121 | MPP-I, MPP-C1 |
| 23 | (-)-Epigallocatechin | C15H14O7 | 4.17 | 306.074 | 305.0667 | 305.0678 | 3.6058 | 261, 219 | MPP-C1 |
| 24 | 4′-O-Methylepigallocatechin | C16H16O7 | 4.206 | 320.0896 | 319.0823 | 319.0799 | −7.5216 | 181, 137, 125 | MPP-I, YB-I, YC-I |
| Flavones | |||||||||
| 25 | 7,4′-Dihydroxyflavone | C15H10O4 | 3.811 | 254.0579 | 253.0506 | 253.0516 | 3.9518 | 211, 135, 119 | MPP-C1 |
| 26 | 6-Hydroxyflavone | C15H10O3 | 4.11 | 238.063 | 237.0557 | 237.0559 | 0.8437 | 208, 193 | MPP-C1, YC-C1 |
| 27 | Cirsilineol | C18H16O7 | 19.171 | 344.0896 | 343.0823 | 343.0824 | 0.2915 | 328, 297 | MPP-C1, YC-C1, YC-C2, YC-C3, YB-C1 |
| 28 | 3,4′,7-Tetrahydroxyflavone | C15H10O6 | 36.661 | 286.0477 | 285.0404 | 285.0399 | −1.7541 | 287, 209 | MPP-I |
| Flavonols | |||||||||
| 29 | Kaempferide | C16H11O6 | 4.809 | 299.0556 | 298.0483 | 298.0491 | 2.6841 | 284, 255, 163, 107 | MPP-C1, MPP-C2 |
| 30 | Kaempferol 3-O-rhamnoside | C21H19O10 | 5.637 | 431.0978 | 430.0905 | 430.0906 | 0.2325 | 285 | YB-C3, YC-C3 |
| 31 | Quercetin | C15H10O7 | 31.9 | 302.0426 | 301.0353 | 301.034 | −4.3184 | 127, 285 | MPP-I, YC-I |
| 32 | Isorhamnetin | C16H12O7 | 39.883 | 316.0583 | 315.051 | 315.052 | 3.1741 | 300, 151, 107 | MPP-C1 |
| Isoflavonoids | |||||||||
| 33 | 5′-Methoxy-O-desmethylangolensin | C16H16O5 | 8.738 | 288.0998 | 287.0925 | 287.0918 | −2.4382 | 119 | MPP-I, MPP-C1 |
| 34 | Violanone | C17H16O6 | 20.207 | 316.0947 | 315.0874 | 315.0866 | −2.539 | 285, 135 | MPP-C2, MPP-C3 |
| 35 | 3′-Hydroxymelanettin | C16H12O6 | 36.548 | 300.0634 | 299.0561 | 299.0547 | −4.6814 | 284 | MPP-I |
| 36 | Hesperetin | C16H14O6 | 36.583 | 302.079 | 301.0717 | 301.0725 | 2.6572 | 283, 177 | MPP-C1, MPP-C2 |
| 37 | 2-Dehydro-O-desmethylangolensin | C15H12O4 | 43.652 | 256.0736 | 255.0663 | 255.0642 | −8.2332 | 227, 135 | MPP-C1 |
| Other polyphenols | |||||||||
| Hydroxycoumarins | |||||||||
| 38 | Urolithin A | C13H8O4 | 30.788 | 228.0423 | 227.0351 | 227.0349 | −0.4405 | 183 | MPP-C1, YC-C1, YC-C2 |
| 39 | Urolithin B | C13H8O3 | 38.902 | 212.0473 | 211.0472 | 211.0386 | −6.6338 | 215, 198, 187, 169 | YC-C1, YB-C1, YB-C2 |
| Hydroxybenzaldehydes | |||||||||
| 40 | p-Anisaldehyde | C8H8O2 | 12.814 | 136.0524 | 135.0451 | 135.0449 | −1.481 | 122, 109, 94 | MPP-I, YB-I, YC-I |
| Hydroxybenzoketones | |||||||||
| 41 | Norathyriol | C13H8O6 | 22.799 | 260.0321 | 259.0248 | 259.0235 | −5.0188 | 241, 231, 189, 109 | MPP-I, YB-I, YC-I, MPP-C1, MPP-C2, MPP-C3, YC-C1, YC-C2 |
| Alkylphenols | |||||||||
| 42 | 3-Methylcatechol | C7H8O2 | 12.654 | 124.0524 | 123.0451 | 123.0457 | 4.8763 | 281, 187, 165 | MPP-C1 |
| 43 | 4-Vinylphenol | C8H8O | 21.237 | 120.0575 | 119.0502 | 119.0501 | −0.84 | 93, 75, 65 | MPP-C1, MPP-C2, YC-C1, YC-C3 |
| Phenolic terpenes | |||||||||
| 44 | Rosmadial | C20H24O5 | 4.719 | 344.1624 | 343.1551 | 343.1545 | −1.7485 | 327, 297 | YC-C1, YB-C1 |
| 45 | Carvacrol | C10H14O | 57.007 | 150.1045 | 149.0972 | 149.0972 | 0 | 132, 108 | YB-C1 |
| Cyslitol | |||||||||
| 46 | Quinic Acid | C7H12O6 | 3.991 | 192.0634 | 191.0561 | 191.0559 | −1.0468 | 173, 127, 85 | MPP-C3, YC-C2, YC-C3 |
| Tyrosols | |||||||||
| 47 | Hydroxytyrosol | C8H10O3 | 14.391 | 154.063 | 153.0557 | 153.0544 | −8.4936 | 135, 123 | MPP-C1 |
| Xanthones | |||||||||
| 48 | Mangiferin | C19H18O11 | 13.992 | 422.0849 | 421.0776 | 421.0797 | 4.9872 | 331, 301, 259 | MPP-I, YB-I, YC-I |
| 49 | Mangiferin 6′-gallate | C26H22O15 | 16.163 | 574.0959 | 573.0886 | 573.0898 | 2.0939 | 421 | MPP-I, YB-I, YC-I |
| Other polyphenols | |||||||||
| 50 | Coumestrol | C15H8O5 | 7.895 | 268.0372 | 267.0299 | 267.0296 | −1.1235 | 266, 211 | MPP-C2, MPP-C3 |
| 51 | Phlorin | C12H16O8 | 8.923 | 288.0845 | 287.0772 | 287.0778 | 2.09 | 272, 237, 179 | MPP-I, MPP-C1, MPP-C2 |
| 52 | Pyrogallol | C6H6O3 | 10.231 | 126.0317 | 125.0244 | 125.0242 | −1.5997 | 97, 81 | MPP-I, YB-I, YC-I, MPP-C1, MPP-C2, YB-C1 |
| Stilbene | |||||||||
| 53 | 4-Hydroxy-3,5,4′-trimethoxystilbene | C17H18O4 | 25.451 | 286.1205 | 285.1132 | 285.1135 | 1.0522 | 269, 253, 227 | MPP-C1, MPP-C2, MPP-C3, YB-C1, YC-C1, YC-C2 |
| Lignans | |||||||||
| 54 | Lariciresinol | C20H24O6 | 4.163 | 360.1573 | 359.15 | 359.1494 | −1.6706 | 329 | MPP-I, YC-I, YC-C1 |
| 55 | Arctigenin | C21H24O6 | 4.444 | 372.1573 | 371.15 | 371.1473 | −7.2747 | 356, 312, 295 | MPP-I, YC-C1 |
| 56 | Schisandrin B | C23H28O6 | 10.646 | 400.1886 | 399.1813 | 399.1818 | 1.2526 | 385, 370, 330, 300 | MPP-I, YB-I, YC-I, YB-C1, YC-C1 |
| 57 | Secoisolariciresinol-sesquilignan | C30H38O10 | 15.697 | 558.2465 | 557.2392 | 557.2407 | 2.6918 | 539, 513, 361 | MPP-C1, MPP-C2, MPP-C3 |
| 58 | Enterodiol | C18H22O4 | 20.149 | 302.1518 | 301.1445 | 301.1435 | −3.3207 | 253 | MPP-I, YC-I |
| 59 | Dimethylmatairesinol | C22H26O6 | 23.989 | 386.1729 | 385.1656 | 385.167 | 3.6348 | 372, 369, 357, 329 | YB-I, YC-I, YC-C1, YC-C2, YB-C1, YB-C2 |
| 60 | Enterolactone | C18H18O4 | 36.442 | 298.1205 | 297.1132 | 297.1146 | 4.712 | 279, 131 | MPP-I, YB-I, YCI, MPP-C2, MPP-C3, YC-C1, YC-C3, YB-C1, YB-C2 |
| 61 | 7-Hydroxysecoisolariciresinol | C22H30O5 | 40.342 | 374.2093 | 373.202 | 373.2028 | 2.1436 | 357, 327 | MPP-C2, YB-C2 |
| 62 | Schisantherin A | C30H32O9 | 45.172 | 536.2046 | 535.1973 | 535.1999 | 4.858 | 519, 489, 415, 121 | YB-C1, YB-C2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahid, H.F.; Ali, A.; Legione, A.R.; Ranadheera, C.S.; Fang, Z.; Dunshea, F.R.; Ajlouni, S. Probiotic Yoghurt Enriched with Mango Peel Powder: Biotransformation of Phenolics and Modulation of Metabolomic Outputs after In Vitro Digestion and Colonic Fermentation. Int. J. Mol. Sci. 2023, 24, 8560. https://doi.org/10.3390/ijms24108560
Zahid HF, Ali A, Legione AR, Ranadheera CS, Fang Z, Dunshea FR, Ajlouni S. Probiotic Yoghurt Enriched with Mango Peel Powder: Biotransformation of Phenolics and Modulation of Metabolomic Outputs after In Vitro Digestion and Colonic Fermentation. International Journal of Molecular Sciences. 2023; 24(10):8560. https://doi.org/10.3390/ijms24108560
Chicago/Turabian StyleZahid, Hafza Fasiha, Akhtar Ali, Alistair R. Legione, Chaminda Senaka Ranadheera, Zhongxiang Fang, Frank R. Dunshea, and Said Ajlouni. 2023. "Probiotic Yoghurt Enriched with Mango Peel Powder: Biotransformation of Phenolics and Modulation of Metabolomic Outputs after In Vitro Digestion and Colonic Fermentation" International Journal of Molecular Sciences 24, no. 10: 8560. https://doi.org/10.3390/ijms24108560
APA StyleZahid, H. F., Ali, A., Legione, A. R., Ranadheera, C. S., Fang, Z., Dunshea, F. R., & Ajlouni, S. (2023). Probiotic Yoghurt Enriched with Mango Peel Powder: Biotransformation of Phenolics and Modulation of Metabolomic Outputs after In Vitro Digestion and Colonic Fermentation. International Journal of Molecular Sciences, 24(10), 8560. https://doi.org/10.3390/ijms24108560










