Neuronal Differentiation and Outgrowth Effect of Thymol in Trachyspermum ammi Seed Extract via BDNF/TrkB Signaling Pathway in Prenatal Maternal Supplementation and Primary Hippocampal Culture
Abstract
:1. Introduction
2. Results
2.1. Identification of Thymol in TASE
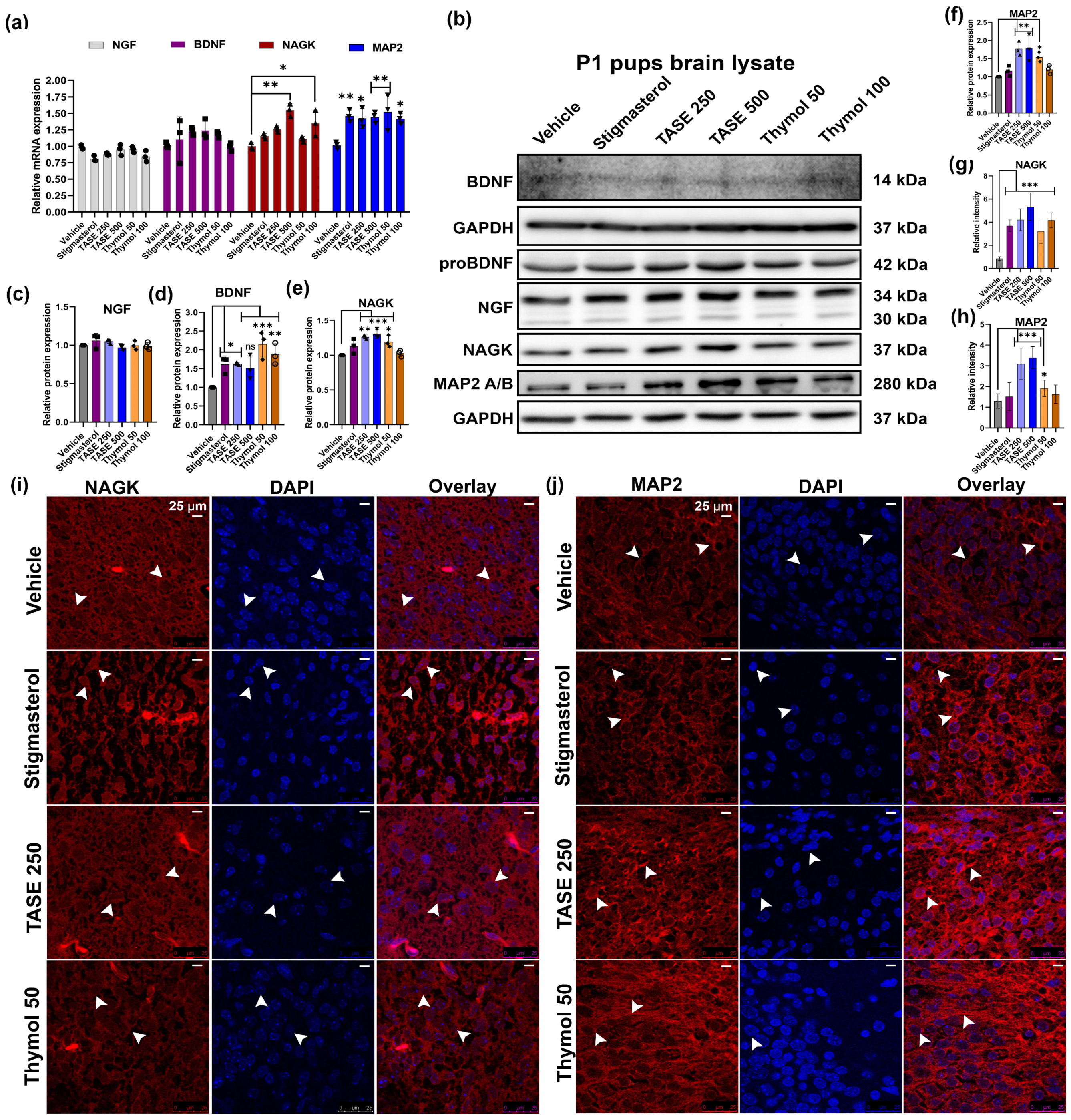
2.2. TASE and Thymol Treatment in the Pregnant Mice Upregulated the Neurite Growth Factors in the Pups In Vivo
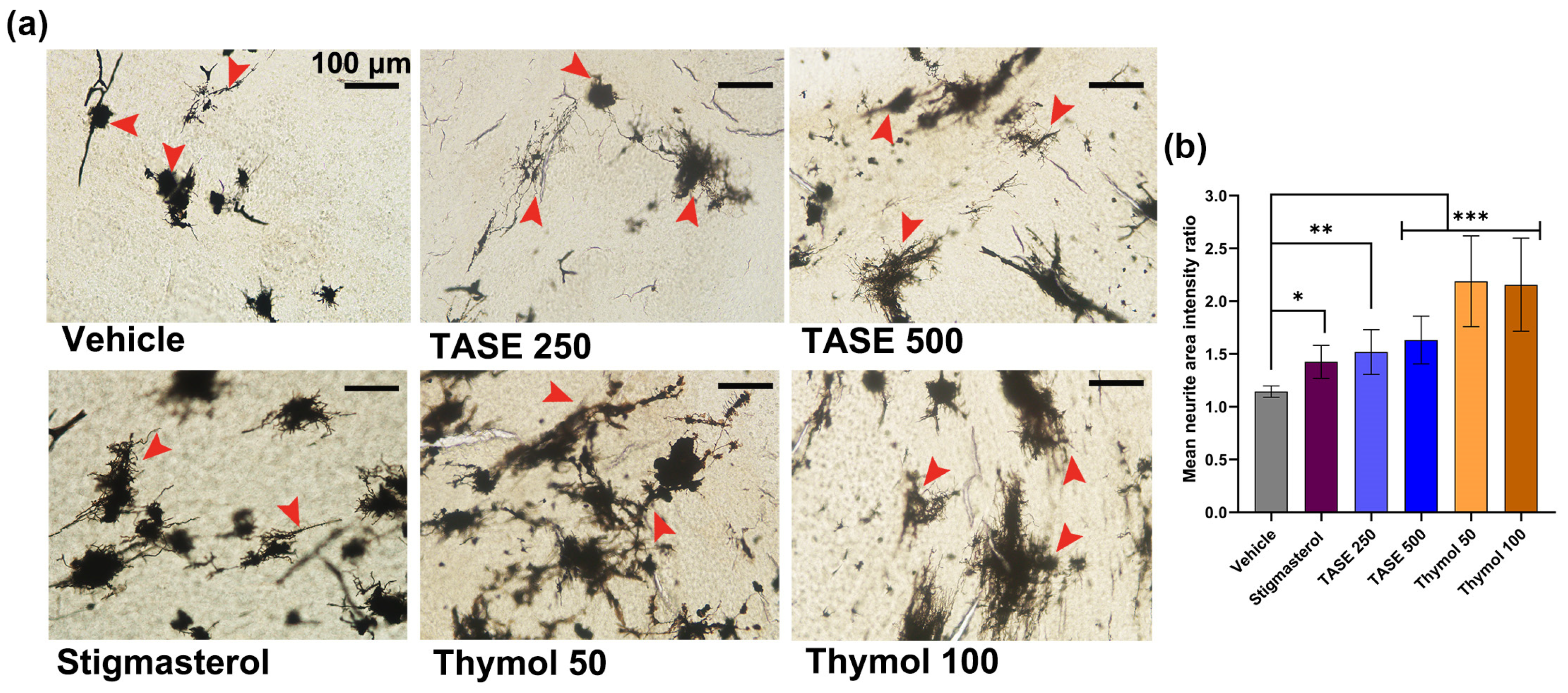
2.3. TASE and Thymol Promote Neurite Outgrowth in Prenatal Supplementation
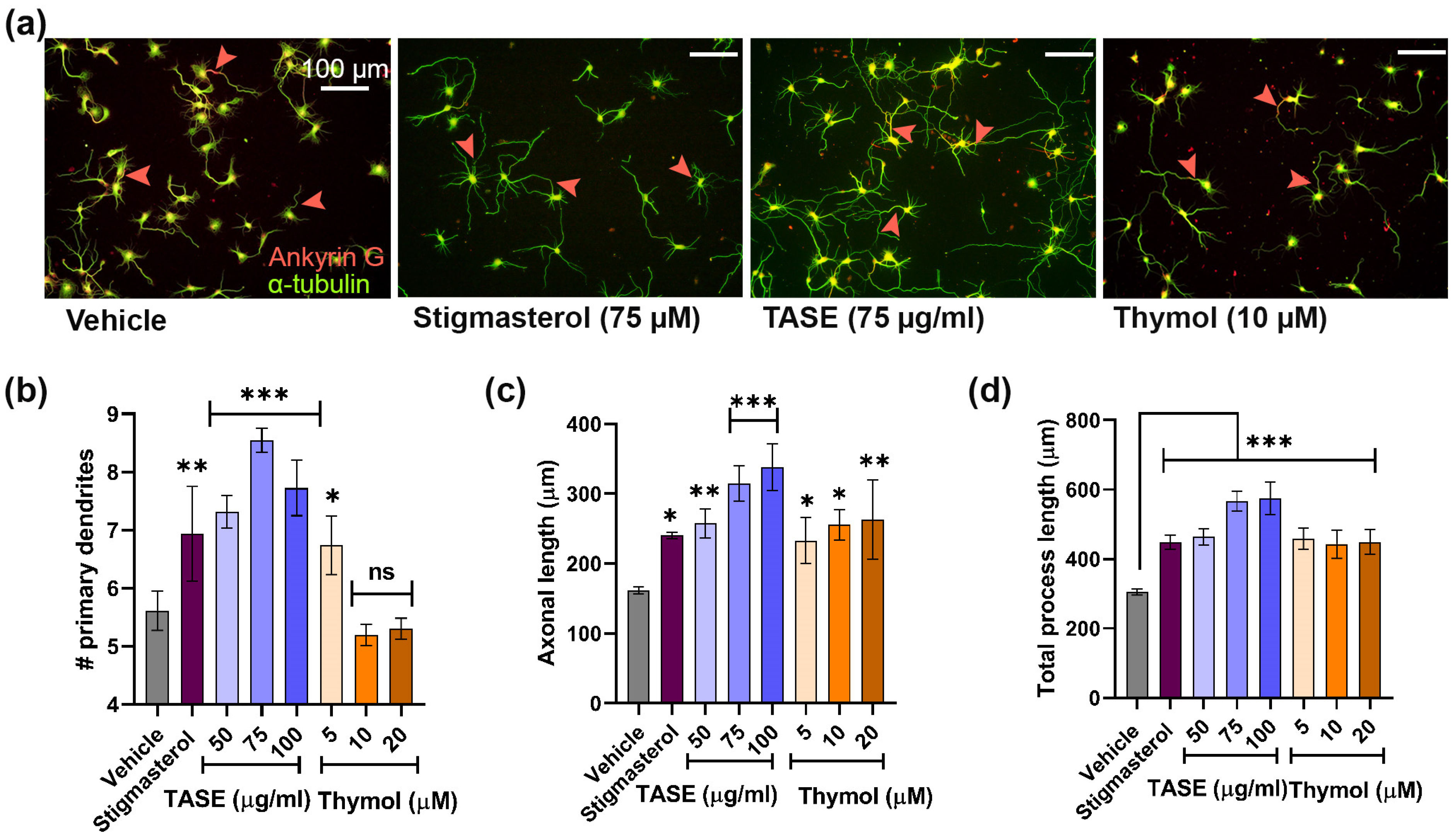
2.4. Determination of Optimal Dose for TASE and Thymol for Neurite Outgrowth Activity in Primary Hippocampal Culture
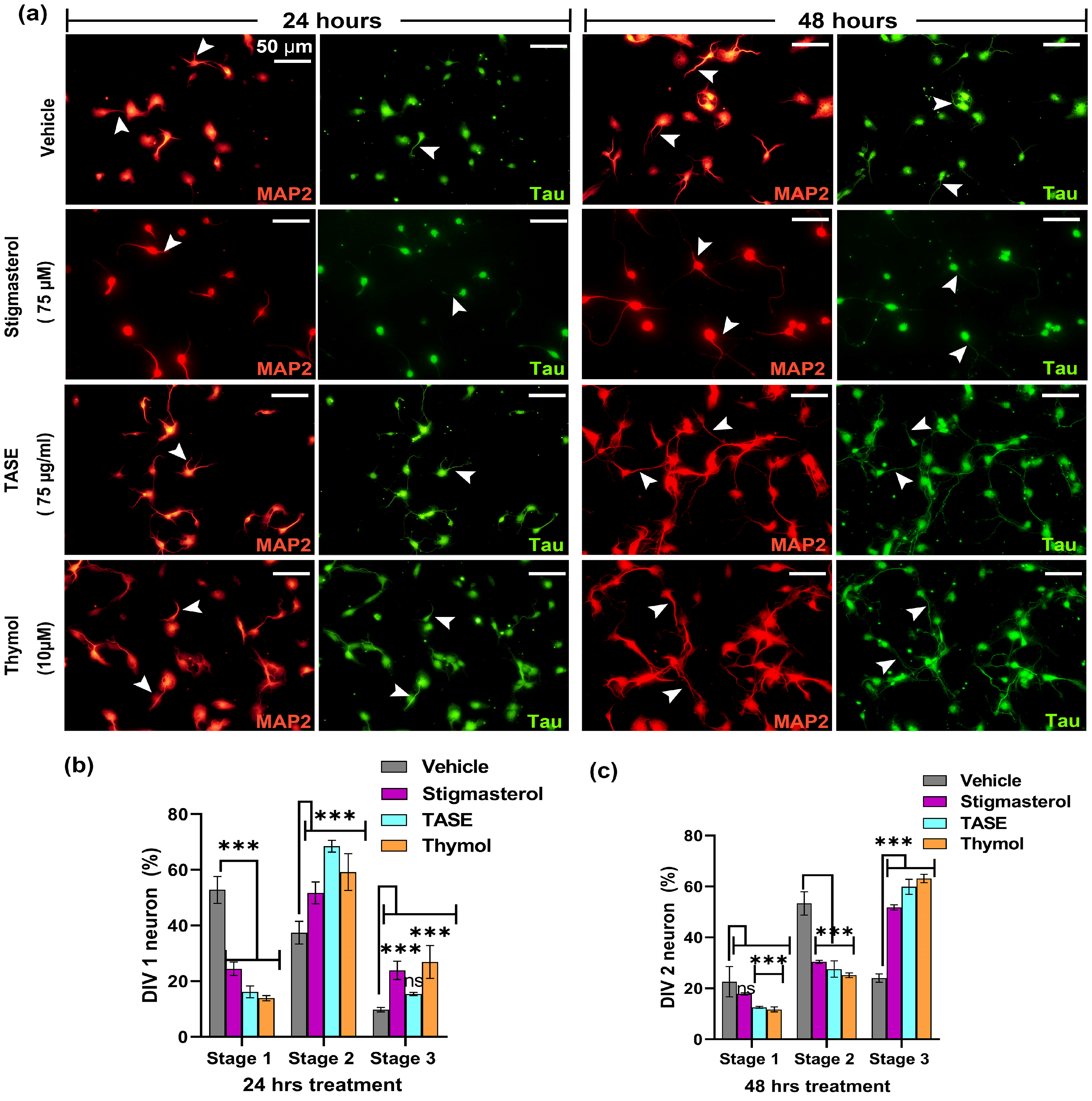
2.5. TASE and Its Active Constituent Thymol Promote Neuronal Differentiation
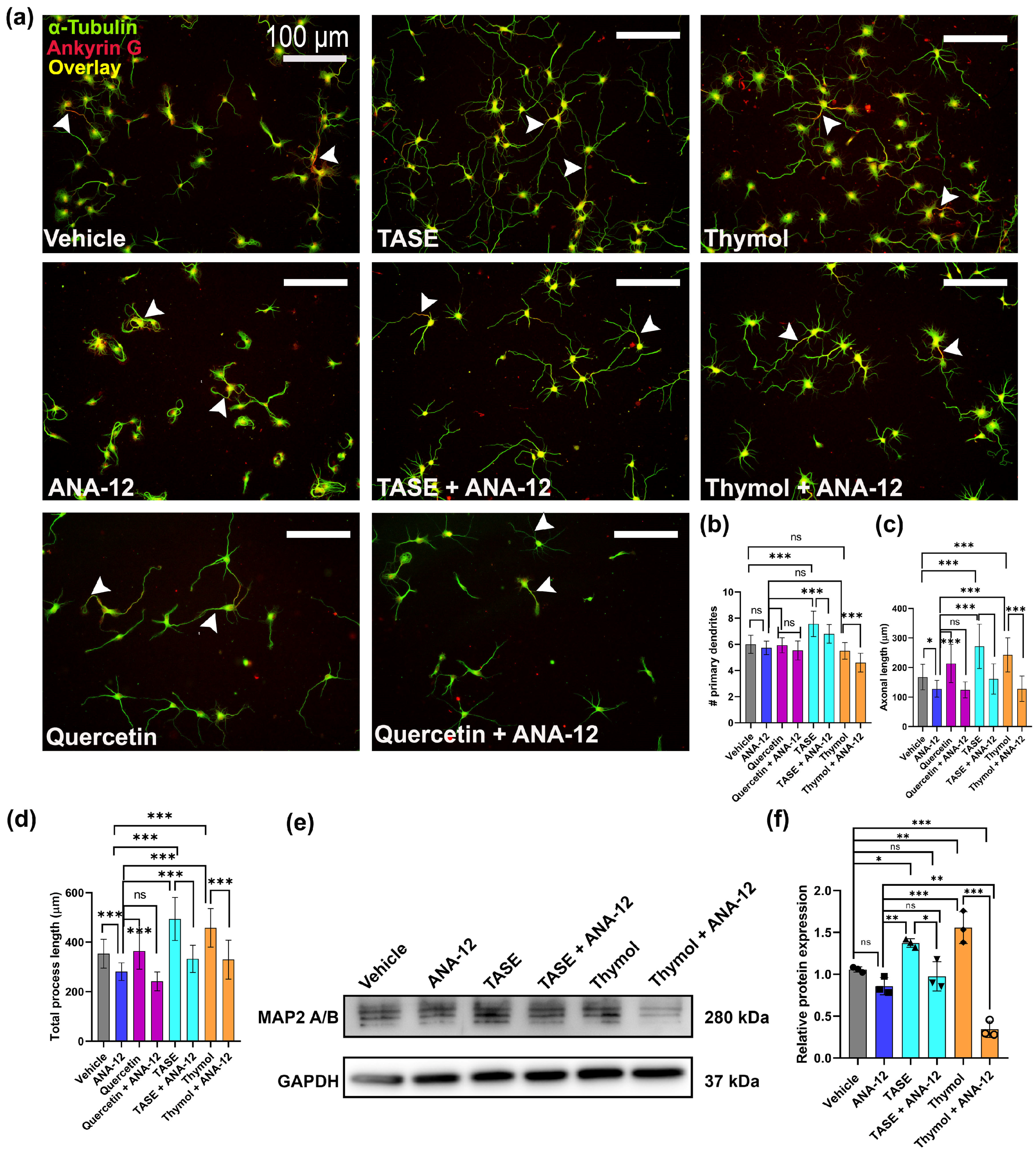
2.6. TASE and Thymol Exert Neurite Outgrowth Activity via the TrkB Signaling Pathway
2.7. TASE and Thymol Can Rescue the Nocodazole-Induced Microtubule Depolymerization
2.8. TASE and Thymol Promote Dendritic and Axonal Maturation
2.8.1. TASE and Thymol Promote Dendritic Arborization
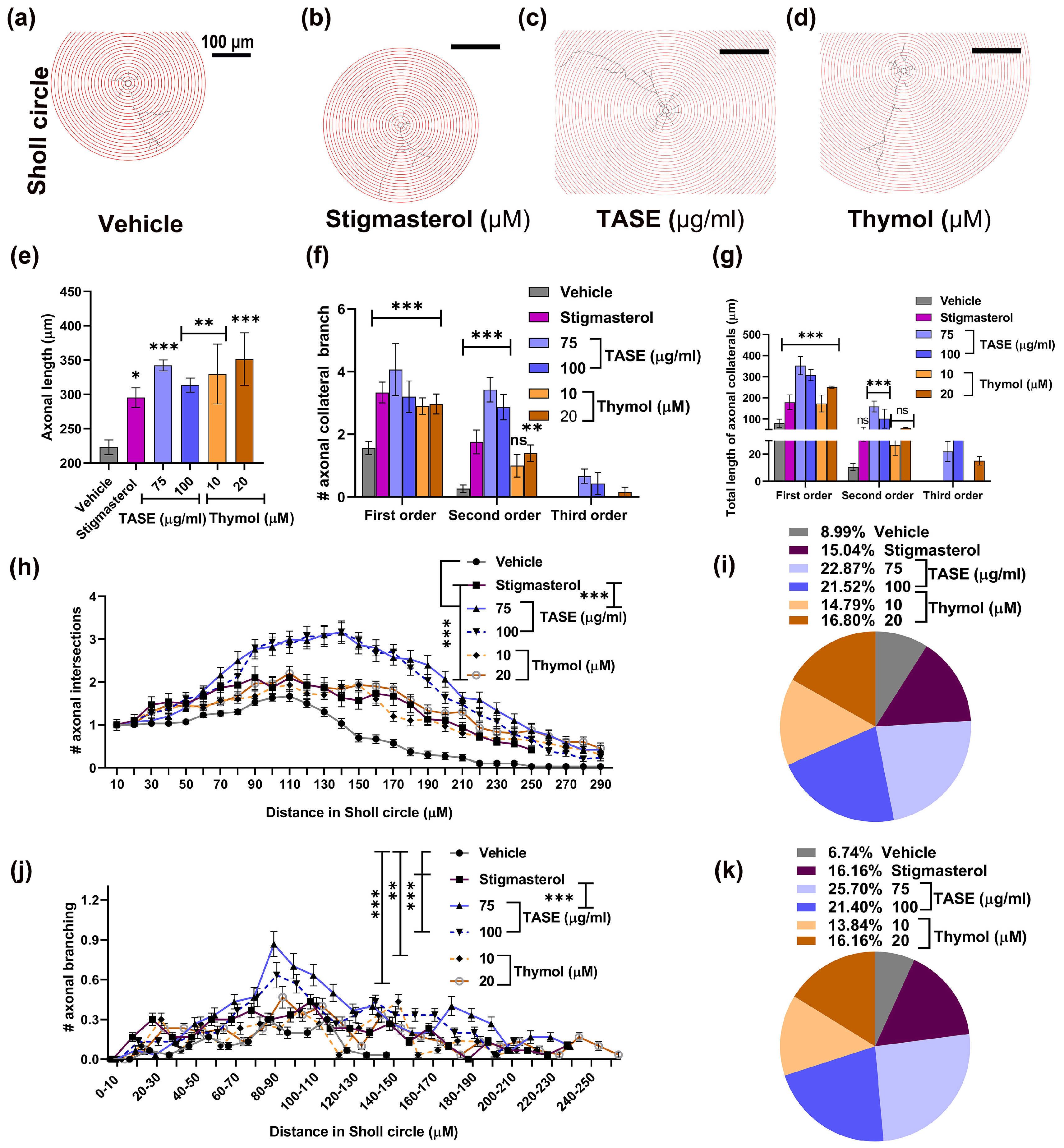
2.8.2. TASE and Thymol Promote Axonal Maturation
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Sample Collection and Extract Preparation
4.3. Primary Neuronal Culture and Extract/Compound Treatment
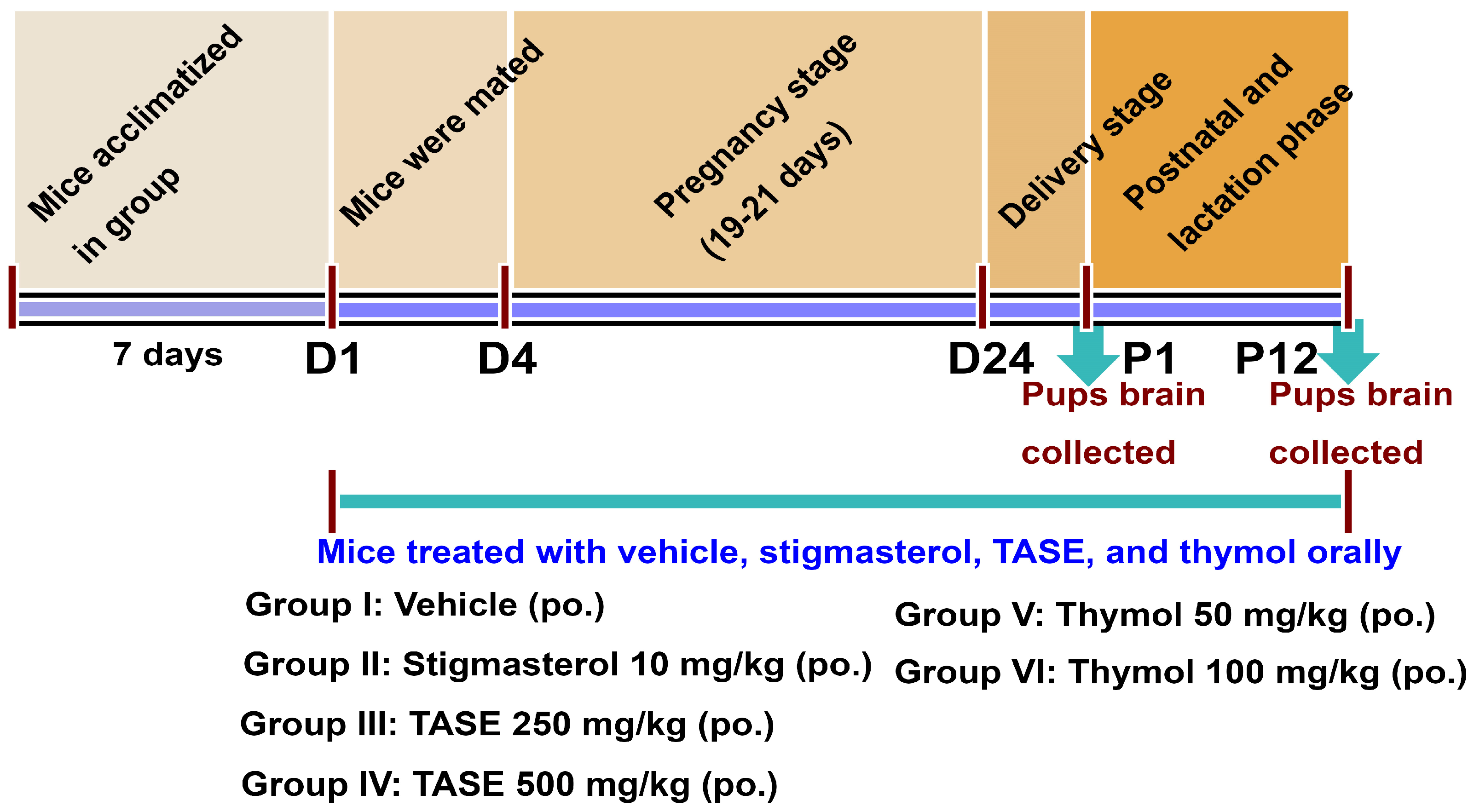
4.4. In Vivo Mice Experiments
4.5. RNA Isolation and Quantitative Real Time-PCR (RT-qPCR) Assay
4.6. Immunocytochemistry
4.7. Immunohistochemistry
4.8. Golgi-Cox Staining
4.9. Western Blotting
4.10. Microscopic Image Acquisition, Analysis, and Quantification
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lanni, C.; Stanga, S.; Racchi, M.; Govoni, S. The expanding universe of neurotrophic factors: Therapeutic potential in aging and age-associated disorders. Curr. Pharm. Des. 2010, 16, 698–717. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; O’Leary, D.D. Axon retraction and degeneration in development and disease. Annu. Rev. Neurosci. 2005, 28, 127–156. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, T.B.; Savall, A.S.; Gutierrez, M.E.Z.; Pinton, S. Neurotrophic factors in Alzheimer’s and Parkinson’s diseases: Implications for pathogenesis and therapy. Neural Regen. Res. 2017, 12, 549–557. [Google Scholar]
- Terenghi, G. Peripheral nerve regeneration and neurotrophic factors. J. Anat. 1999, 194, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chittka, A. Differential regulation of SC1/PRDM4 and PRMT5 mediated protein arginine methylation by the nerve growth factor and the epidermal growth factor in PC12 cells. Neurosci. Lett. 2013, 550, 87–92. [Google Scholar] [CrossRef]
- Gonzalez, A.; Moya-Alvarado, G.; Gonzalez-Billaut, C.; Bronfman, F.C. Cellular and molecular mechanisms regulating neuronal growth by brain-derived neurotrophic factor. Cytoskeleton 2016, 73, 612–628. [Google Scholar] [CrossRef]
- Naeve, G.S.; Ramakrishnan, M.; Kramer, R.; Hevroni, D.; Citri, Y.; Theill, L.E. Neuritin: A gene induced by neural activity and neurotrophins that promotes neuritogenesis. Proc. Natl. Acad. Sci. USA 1997, 94, 2648–2653. [Google Scholar] [CrossRef]
- Sánchez, C.; Díaz-Nido, J.; Avila, J. Phosphorylation of microtubule-associated protein 2 (MAP2) and its relevance for the regulation of the neuronal cytoskeleton function. Prog. Neurobiol. 2000, 61, 133–168. [Google Scholar] [CrossRef]
- DeGiosio, R.A.; Grubisha, M.J.; MacDonald, M.L.; McKinney, B.C.; Camacho, C.J.; Sweet, R.A. More than a marker: Potential pathogenic functions of MAP2. Front. Mol. Neurosci. 2022, 15, 974890. [Google Scholar] [CrossRef]
- Islam, M.A.; Sharif, S.R.; Lee, H.; Seog, D.-H.; Moon, I.S. N-acetyl-D-glucosamine kinase interacts with dynein light-chain roadblock type 1 at Golgi outposts in neuronal dendritic branch points. Exp. Mol. Med. 2015, 47, e177. [Google Scholar] [CrossRef]
- Lee, H.; Cho, S.-J.; Moon, I.S. The non-canonical effect of N-acetyl-D-glucosamine kinase on the formation of neuronal dendrites. Mol. Cells 2014, 37, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.N.; Mohibbullah, M.; Hong, Y.K.; Moon, I.S. Calotropis gigantea Promotes Neuritogenesis and Synaptogenesis through Activation of NGF-TrkA-Erk1/2 Signaling in Rat Hippocampal Neurons. Am. J. Chin. Med. 2018, 46, 1861–1877. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Haque, M.N.; Munni, Y.A.; Oktaviani, D.F.; Timalsina, B.; Dash, R.; Afrin, T.; Moon, I.S. Centella asiatica promotes early differentiation, axodendritic maturation and synaptic formation in primary hippocampal neurons. Neurochem. Int. 2021, 144, 104957. [Google Scholar] [CrossRef]
- Maqueshudul Haque Bhuiyan, M.; Mohibbullah, M.; Hannan, M.A.; Hong, Y.K.; Choi, J.S.; Choi, I.S.; Moon, I.S. Undaria pinnatifida Promotes Spinogenesis and Synaptogenesis and Potentiates Functional Presynaptic Plasticity in Hippocampal Neurons. Am. J. Chin. Med. 2015, 43, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Petramfar, P.; Moein, M.; Samani, S.M.; Tabatabaei, S.H.; Zarshenas, M.M. Trachyspermum ammi 10% topical cream versus placebo on neuropathic pain, a randomized, double-blind, placebo-controlled trial. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2016, 37, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Asif, H.M.; Sultana, S.; Akhtar, N. A panoramic view on phytochemical, nutritional, ethanobotanical uses and pharmacological values of Trachyspermum ammi Linn. Asian Pac. J. Trop. Biomed. 2014, 4, S545–S553. [Google Scholar] [CrossRef]
- Timalsina, B.; Haque, M.N.; Choi, H.J.; Dash, R.; Moon, I.S. Thymol in Trachyspermum ammi seed extract exhibits neuroprotection, learning, and memory enhancement in scopolamine-induced Alzheimer’s disease mouse model. Phytother. Res. 2023; early view. [Google Scholar] [CrossRef]
- Hajimehdipoor, H.; Shekarchi, M.; Khanavi, M.; Adib, N.; Amri, M. A validated high performance liquid chromatography method for the analysis of thymol and carvacrol in Thymus vulgaris L. volatile oil. Pharm. Mag 2010, 6, 154–158. [Google Scholar]
- Chiang, N.-N.; Lin, T.-H.; Teng, Y.-S.; Sun, Y.-C.; Chang, K.-H.; Lin, C.-Y.; Hsieh-Li, H.M.; Su, M.-T.; Chen, C.-M.; Lee-Chen, G.-J. Flavones 7,8-DHF, Quercetin, and Apigenin Against Tau Toxicity via Activation of TRKB Signaling in ΔK280 TauRD-DsRed SH-SY5Y Cells. Front. Aging Neurosci. 2021, 13, 758895. [Google Scholar] [CrossRef]
- Verstraelen, P.; Detrez, J.R.; Verschuuren, M.; Kuijlaars, J.; Nuydens, R.; Timmermans, J.-P.; De Vos, W.H. Dysregulation of Microtubule Stability Impairs Morphofunctional Connectivity in Primary Neuronal Networks. Front. Cell Neurosci. 2017, 11, 173. [Google Scholar] [CrossRef]
- Sholl, D.A. Dendritic organization in the neurons of the visual and motor cortices of the cat. J. Anat. 1953, 87, 387–406. [Google Scholar] [PubMed]
- Teter, B.; Ashford, J.W. Neuroplasticity in Alzheimer’s disease. J. Neurosci. Res. 2002, 70, 402–437. [Google Scholar] [CrossRef]
- Singh, G.; Maurya, S.; Catalan, C.; De Lampasona, M.P. Chemical constituents, antifungal and antioxidative effects of ajwain essential oil and its acetone extract. J. Agric. Food Chem. 2004, 52, 3292–3296. [Google Scholar] [CrossRef] [PubMed]
- Nagoor Meeran, M.F.; Javed, H.; Al Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological properties and molecular mechanisms of thymol: Prospects for its therapeutic potential and pharmaceutical development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef]
- Azizi, Z.; Ebrahimi, S.; Saadatfar, E.; Kamalinejad, M.; Majlessi, N. Cognitive-enhancing activity of thymol and carvacrol in two rat models of dementia. Behav. Pharmacol. 2012, 23, 241–249. [Google Scholar] [CrossRef]
- Bhandari, S.S.; Kabra, M.P. To evaluate anti-anxiety activity of thymol. J. Acute Dis. 2014, 3, 136–140. [Google Scholar] [CrossRef]
- Sancheti, J.; Shaikh, M.F.; Chaudhari, R.; Somani, G.; Patil, S.; Jain, P.; Sathaye, S. Characterization of anticonvulsant and antiepileptogenic potential of thymol in various experimental models. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2014, 387, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.-Y.; Li, H.-Y.; Chen, J.-J.; Li, R.-P.; Qu, R.; Fu, Q.; Ma, S.-P. Thymol produces an antidepressant-like effect in a chronic unpredictable mild stress model of depression in mice. Behav. Brain Res. 2015, 291, 12–19. [Google Scholar] [CrossRef]
- Anderson, R.C.; Krueger, N.A.; Genovese, K.J.; Stanton, T.B.; MacKinnon, K.M.; Harvey, R.B.; Edrington, T.S.; Callaway, T.R.; Nisbet, D.J. Effect of thymol or diphenyliodonium chloride on performance, gut fermentation characteristics, and Campylobacter colonization in growing swine. J. Food Prot. 2012, 75, 758–761. [Google Scholar] [CrossRef]
- Ogaly, H.A.; Abdel-Rahman, R.F.; Mohamed, M.A.; Ahmed-Farid, O.A.; Khattab, M.S.; Abd-Elsalam, R.M. Thymol ameliorated neurotoxicity and cognitive deterioration in a thioacetamide-induced hepatic encephalopathy rat model; involvement of the BDNF/CREB signaling pathway. Food Funct. 2022, 13, 6180–6194. [Google Scholar] [CrossRef]
- Youdim, K.A.; Deans, S.G. Effect of thyme oil and thymol dietary supplementation on the antioxidant status and fatty acid composition of the ageing rat brain. Br. J. Nutr. 2000, 83, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kim, D.H.; Jung, J.M.; Kim, J.M.; Cai, M.; Liu, X.; Hong, J.G.; Lee, C.H.; Lee, K.R.; Ryu, J.H. The ameliorating effects of stigmasterol on scopolamine-induced memory impairments in mice. Eur. J. Pharmacol. 2012, 676, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Dotti, C.G.; Sullivan, C.A.; Banker, G.A. The establishment of polarity by hippocampal neurons in culture. J. Neurosci. Off. J. Soc. Neurosci. 1988, 8, 1454–1468. [Google Scholar] [CrossRef] [PubMed]
- Mudd, A.T.; Dilger, R.N. Early-life nutrition and neurodevelopment: Use of the piglet as a translational model. Adv. Nutr. 2017, 8, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cong, P.; Wang, X.; Liu, Y.; Wu, L.; Li, H.; Xue, C.; Xu, J. Maternal diet with sea urchin gangliosides promotes neurodevelopment of young offspring via enhancing NGF and BDNF expression. Food Funct. 2020, 11, 9912–9923. [Google Scholar] [CrossRef]
- Pascal, L.E.; True, L.D.; Campbell, D.S.; Deutsch, E.W.; Risk, M.; Coleman, I.M.; Eichner, L.J.; Nelson, P.S.; Liu, A.Y. Correlation of mRNA and protein levels: Cell type-specific gene expression of cluster designation antigens in the prostate. BMC Genom. 2008, 9, 246. [Google Scholar] [CrossRef]
- Zhong, F.; Liu, L.; Wei, J.-L.; Dai, R.-P. Step by Step Golgi-Cox Staining for Cryosection. Front. Neuroanat. 2019, 13, 62. [Google Scholar] [CrossRef]
- Cheng, P.-L.; Song, A.-H.; Wong, Y.-H.; Wang, S.; Zhang, X.; Poo, M.-M. Self-amplifying autocrine actions of BDNF in axon development. Proc. Natl. Acad. Sci. USA 2011, 108, 18430–18435. [Google Scholar] [CrossRef]
- Crino, P.B.; Eberwine, J. Molecular characterization of the dendritic growth cone: Regulated mRNA transport and local protein synthesis. Neuron 1996, 17, 1173–1187. [Google Scholar] [CrossRef]
- Chapin, S.J.; Bulinski, J.C. Microtubule stabilization by assembly-promoting microtubule-associated proteins: A repeat performance. Cell Motil. Cytoskelet. 1992, 23, 236–243. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, X.-P. Dysfunction of microtubule-associated proteins of MAP2/tau family in Prion disease. Prion 2012, 6, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Kalcheva, N.; Rockwood, J.M.; Kress, Y.; Steiner, A.; Shafit-Zagardo, B. Molecular and functional characteristics of MAP-2a: Ability of MAP-2a versus MAP-2b to induce stable microtubules in COS cells. Cell Motil. Cytoskelet. 1998, 40, 272–285. [Google Scholar] [CrossRef]
- Freeze, H.H.; Elbein, A.D. Glycosylation precursors. In Essentials of Glycobiology, 2nd ed.; Cold Spring Harbor: New York, NY, USA, 2009. [Google Scholar]
- Hanover, J.A.; Krause, M.W.; Love, D.C. Linking metabolism to epigenetics through O-GlcNAcylation. Nat. Rev. Mol. Cell Biol. 2012, 13, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Hart, G.W.; Kreppel, L.K.; Comer, F.I.; Arnold, C.S.; Snow, D.M.; Ye, Z.; Cheng, X.; DellaManna, D.; Caine, D.S.; Earles, B.J. O-GlcNAcylation of key nuclear and cytoskeletal proteins: Reciprocity with O-phosphorylation and putative roles in protein multimerization. Glycobiology 1996, 6, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Rexach, J.E.; Clark, P.M.; Mason, D.E.; Neve, R.L.; Peters, E.C.; Hsieh-Wilson, L.C. Dynamic O-GlcNAc modification regulates CREB-mediated gene expression and memory formation. Nat. Chem. Biol. 2012, 8, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Vandré, D.D. High Molecular Weight Microtubule-associated Proteins Contain O-Linked N-Acetylglucosamine. J. Biol. Chem. 1996, 271, 12555–12561. [Google Scholar] [CrossRef]
- Hannan, M.A.; Kang, J.Y.; Hong, Y.K.; Lee, H.; Choi, J.S.; Choi, I.S.; Moon, I.S. The marine alga Gelidium amansii promotes the development and complexity of neuronal cytoarchitecture. Phytother. Res. 2013, 27, 21–29. [Google Scholar] [CrossRef]
- Kaech, S.; Banker, G. Culturing hippocampal neurons. Nat. Protoc. 2006, 1, 2406–2415. [Google Scholar] [CrossRef]
- Hannan, M.A.; Haque, M.N.; Dash, R.; Alam, M.; Moon, I.S. 3β, 6β-dichloro-5-hydroxy-5α-cholestane facilitates neuronal development through modulating TrkA signaling regulated proteins in primary hippocampal neuron. Sci. Rep. 2019, 9, 18919. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Il Soo, M.; Sun-Jung, C.; IngNyol, J.; Randall, W. A Simple Method for Combined Fluorescence In Situ Hybridization and Immunocytochemistry. Mol. Cells 2007, 24, 76–82. [Google Scholar]
- Du, F. Golgi-Cox Staining of Neuronal Dendrites and Dendritic Spines with FD Rapid GolgiStainTM Kit. Curr. Protoc. Neurosci. 2019, 88, e69. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.-Y.; Chao, C.-Y.; Huang, H.-L.; Chiu, T.-W.; Charoenkwan, P.; Hwang, E. NeurphologyJ: An automatic neuronal morphology quantification method and its application in pharmacological discovery. BMC Bioinform. 2011, 12, 230. [Google Scholar] [CrossRef]
- Strober, W. Trypan blue exclusion test of cell viability. Curr. Protoc. Immunol. 2001, 21, A.3B.1–A.3B.2. [Google Scholar] [CrossRef]
- Hannan, M.A.; Kang, J.Y.; Mohibbullah, M.; Hong, Y.K.; Lee, H.; Choi, J.S.; Choi, I.S.; Moon, I.S. Moringa oleifera with promising neuronal survival and neurite outgrowth promoting potentials. J. Ethnopharmacol. 2014, 152, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Du, S.; Chen, L.; Xu, X.; Wang, Y.; Ji, F. Hypomethylation of nerve growth factor (NGF) promotes binding of C/EBPα and contributes to inflammatory hyperalgesia in rats. J. Neuroinflamm. 2020, 17, 34. [Google Scholar] [CrossRef]
- Huang, T.; Krimm, R.F. Developmental expression of Bdnf, Ntf4/5, and TrkB in the mouse peripheral taste system. Dev. Dyn. 2010, 239, 2637–2646. [Google Scholar] [CrossRef]
- Campbell, S.; Mesaros, C.; Izzo, L.; Affronti, H.; Noji, M.; Schaffer, B.E.; Tsang, T.; Sun, K.; Trefely, S.; Kruijning, S. Glutamine deprivation triggers NAGK-dependent hexosamine salvage. Elife 2021, 10, e62644. [Google Scholar] [CrossRef]
- Bora, G.; Sucularli, C.; Hensel, N.; Claus, P.; Yurter, H.E. Investigations of microtubule-associated protein 2 gene expression in spinal muscular atrophy. J. Pediatr. Res. 2019, 6, 148–154. [Google Scholar] [CrossRef]
- Wan, K.; Mao, F.; Li, Q.; Wang, L.; Wei, Z.; Wang, P.; Liao, X.; Xu, M.; Huang, J.; Pan, Z. Neuritin-overexpressing transgenic mice demonstrate enhanced neuroregeneration capacity and improved spatial learning and memory recovery after ischemia-reperfusion injury. Aging 2021, 13, 2681. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Timalsina, B.; Haque, M.N.; Dash, R.; Choi, H.J.; Ghimire, N.; Moon, I.S. Neuronal Differentiation and Outgrowth Effect of Thymol in Trachyspermum ammi Seed Extract via BDNF/TrkB Signaling Pathway in Prenatal Maternal Supplementation and Primary Hippocampal Culture. Int. J. Mol. Sci. 2023, 24, 8565. https://doi.org/10.3390/ijms24108565
Timalsina B, Haque MN, Dash R, Choi HJ, Ghimire N, Moon IS. Neuronal Differentiation and Outgrowth Effect of Thymol in Trachyspermum ammi Seed Extract via BDNF/TrkB Signaling Pathway in Prenatal Maternal Supplementation and Primary Hippocampal Culture. International Journal of Molecular Sciences. 2023; 24(10):8565. https://doi.org/10.3390/ijms24108565
Chicago/Turabian StyleTimalsina, Binod, Md Nazmul Haque, Raju Dash, Ho Jin Choi, Nisha Ghimire, and Il Soo Moon. 2023. "Neuronal Differentiation and Outgrowth Effect of Thymol in Trachyspermum ammi Seed Extract via BDNF/TrkB Signaling Pathway in Prenatal Maternal Supplementation and Primary Hippocampal Culture" International Journal of Molecular Sciences 24, no. 10: 8565. https://doi.org/10.3390/ijms24108565
APA StyleTimalsina, B., Haque, M. N., Dash, R., Choi, H. J., Ghimire, N., & Moon, I. S. (2023). Neuronal Differentiation and Outgrowth Effect of Thymol in Trachyspermum ammi Seed Extract via BDNF/TrkB Signaling Pathway in Prenatal Maternal Supplementation and Primary Hippocampal Culture. International Journal of Molecular Sciences, 24(10), 8565. https://doi.org/10.3390/ijms24108565







