Chemical, Physical, and Toxicological Properties of V-Agents
Abstract
:1. Introduction
2. Methods
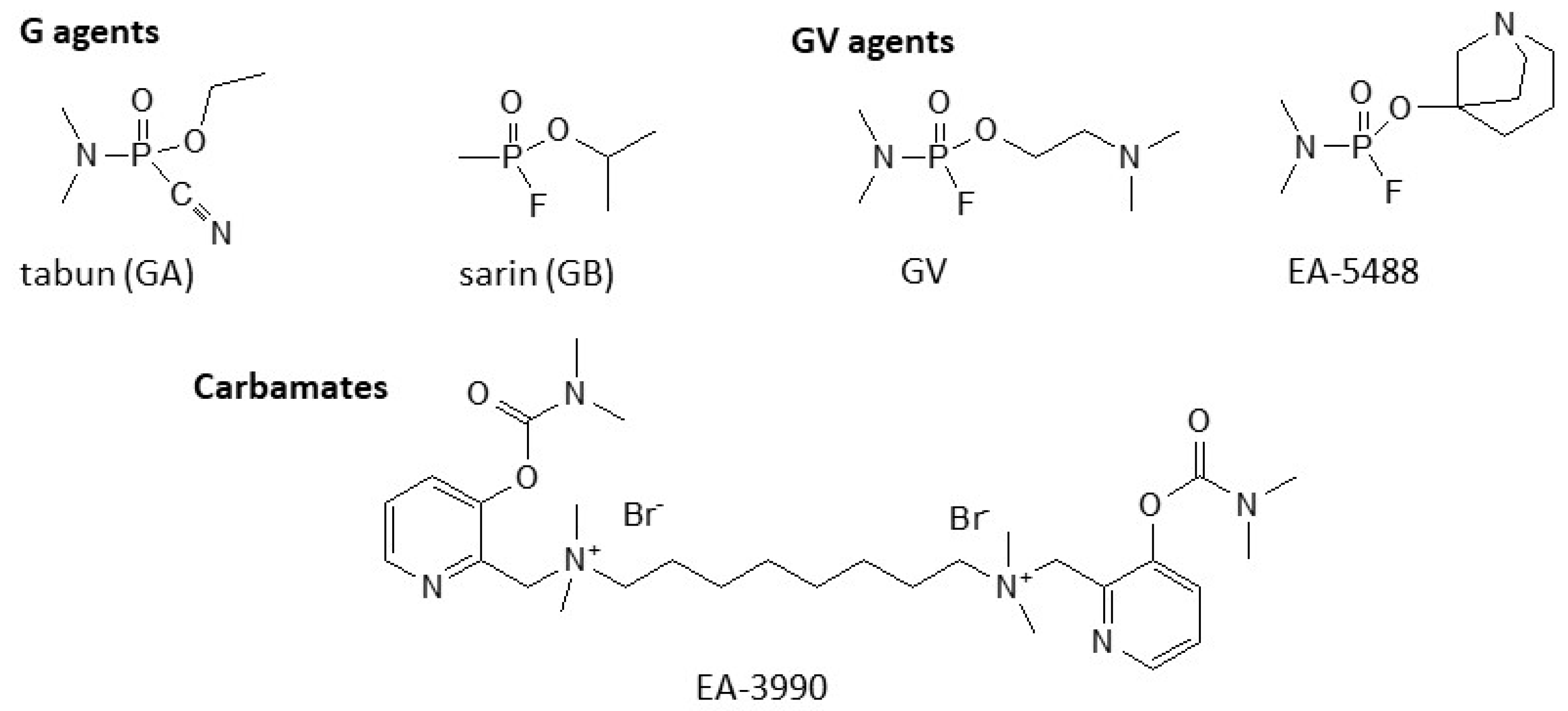
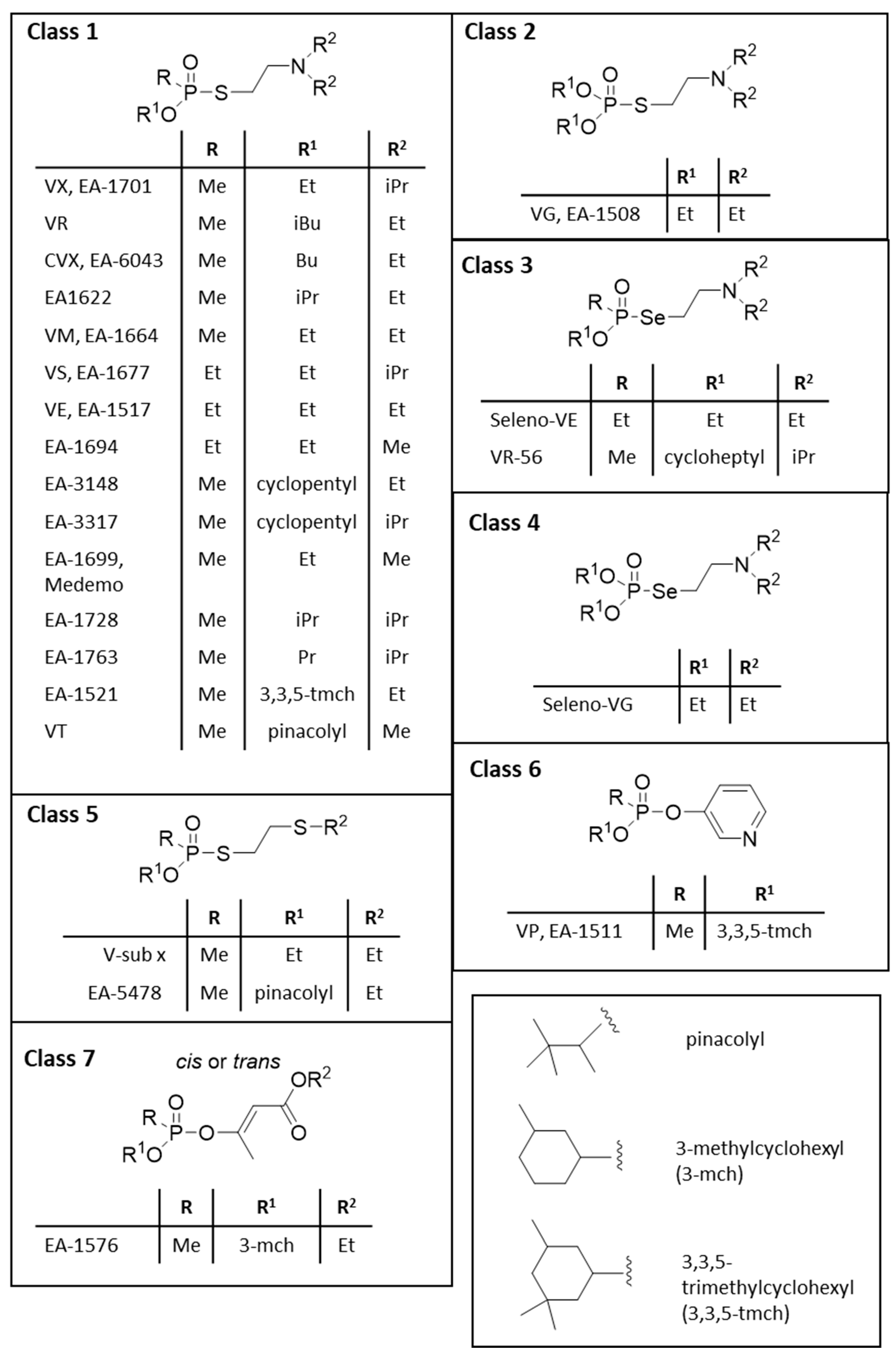
3. General Structures and (Sub)Categories of V-Agents
4. Physical Properties of V-Agents
5. Toxicity of V-Agents
6. Exposure to V-Agents
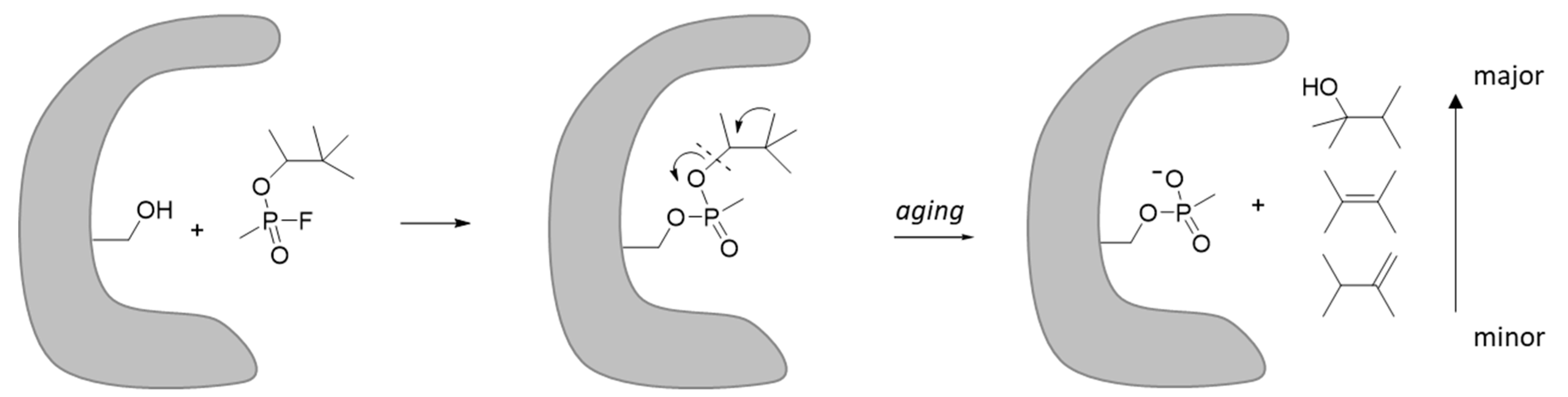
7. Stability of V-Agents during Storage
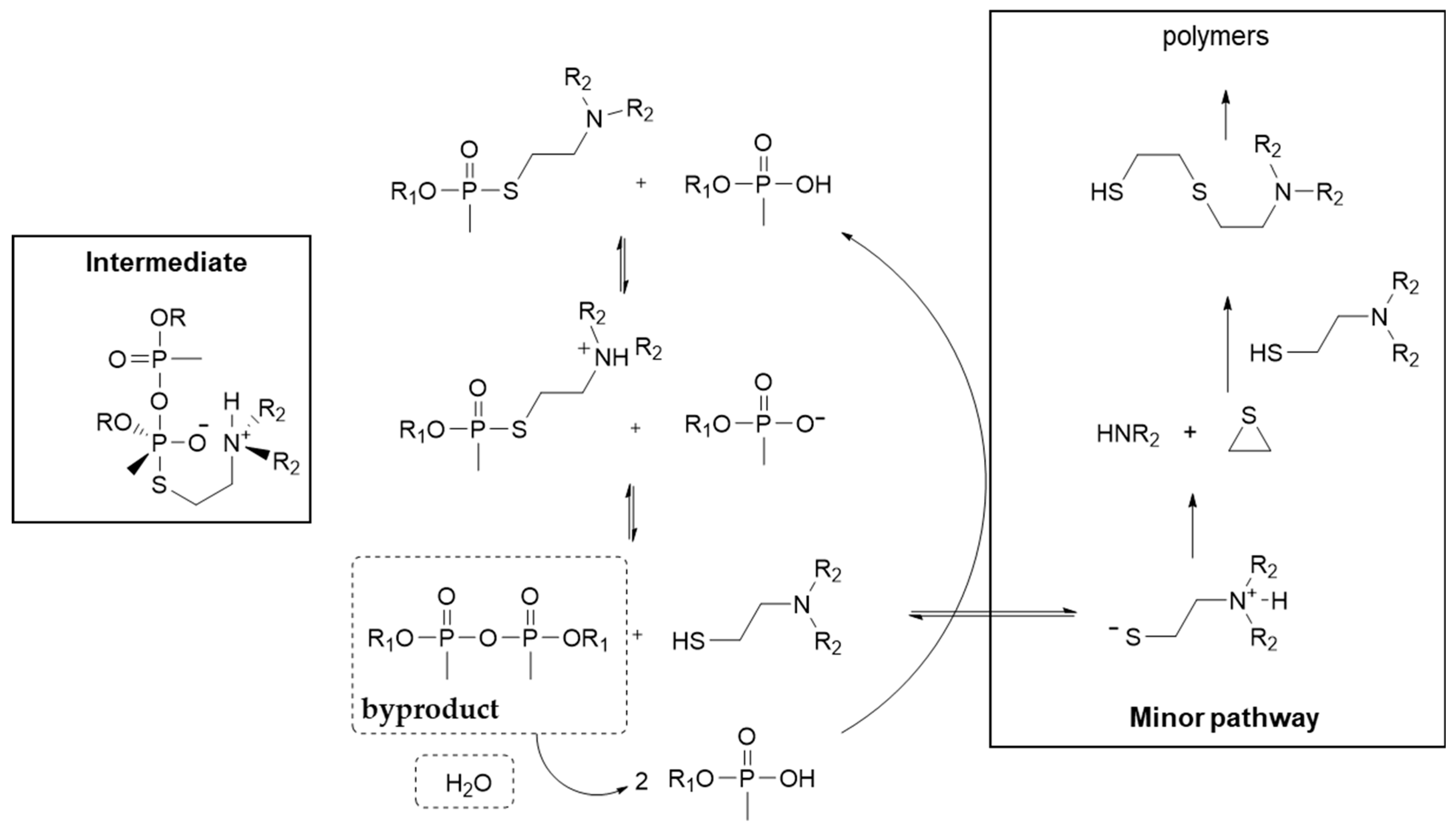
8. Unitary vs. Binary Formulations and Thickening of V-Agents
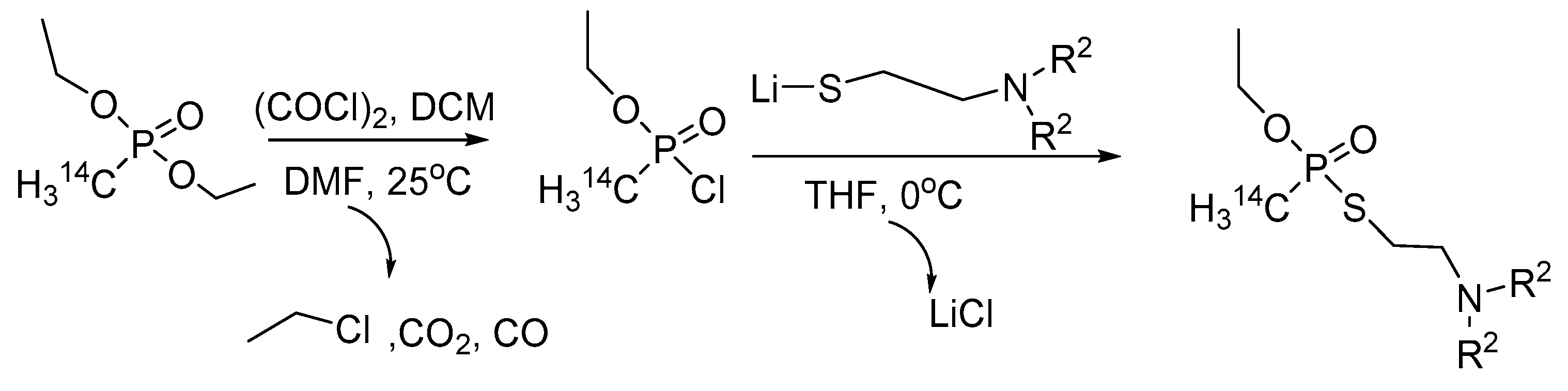
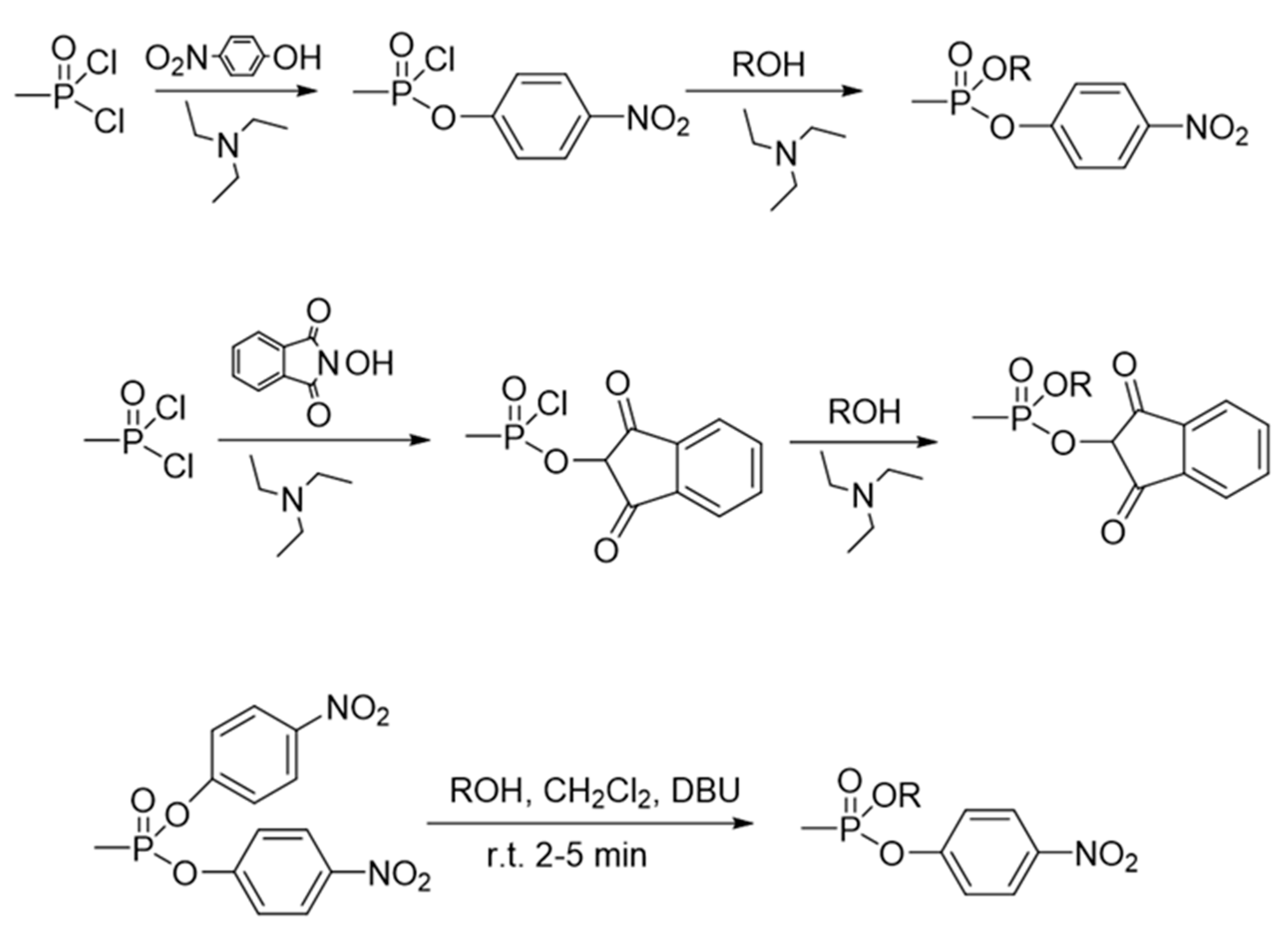
9. Chemical Synthesis
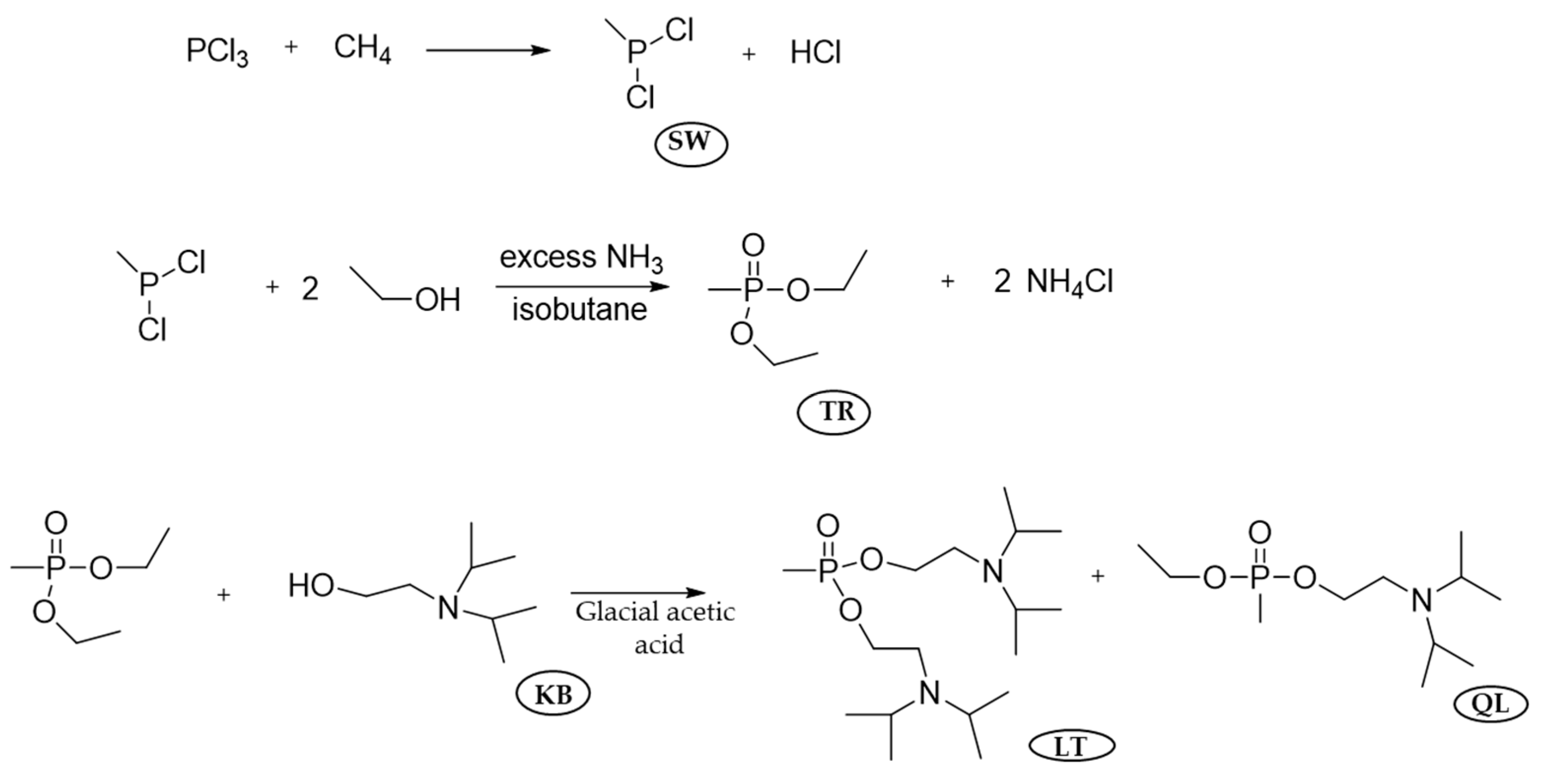
9.1. Major Industrial Production Process
9.2. The EMPTA Process
9.3. Production Process Based on O,O-Dialkyl Alkylphophonothionate Starting Material
9.4. Synthesis of EA-1576
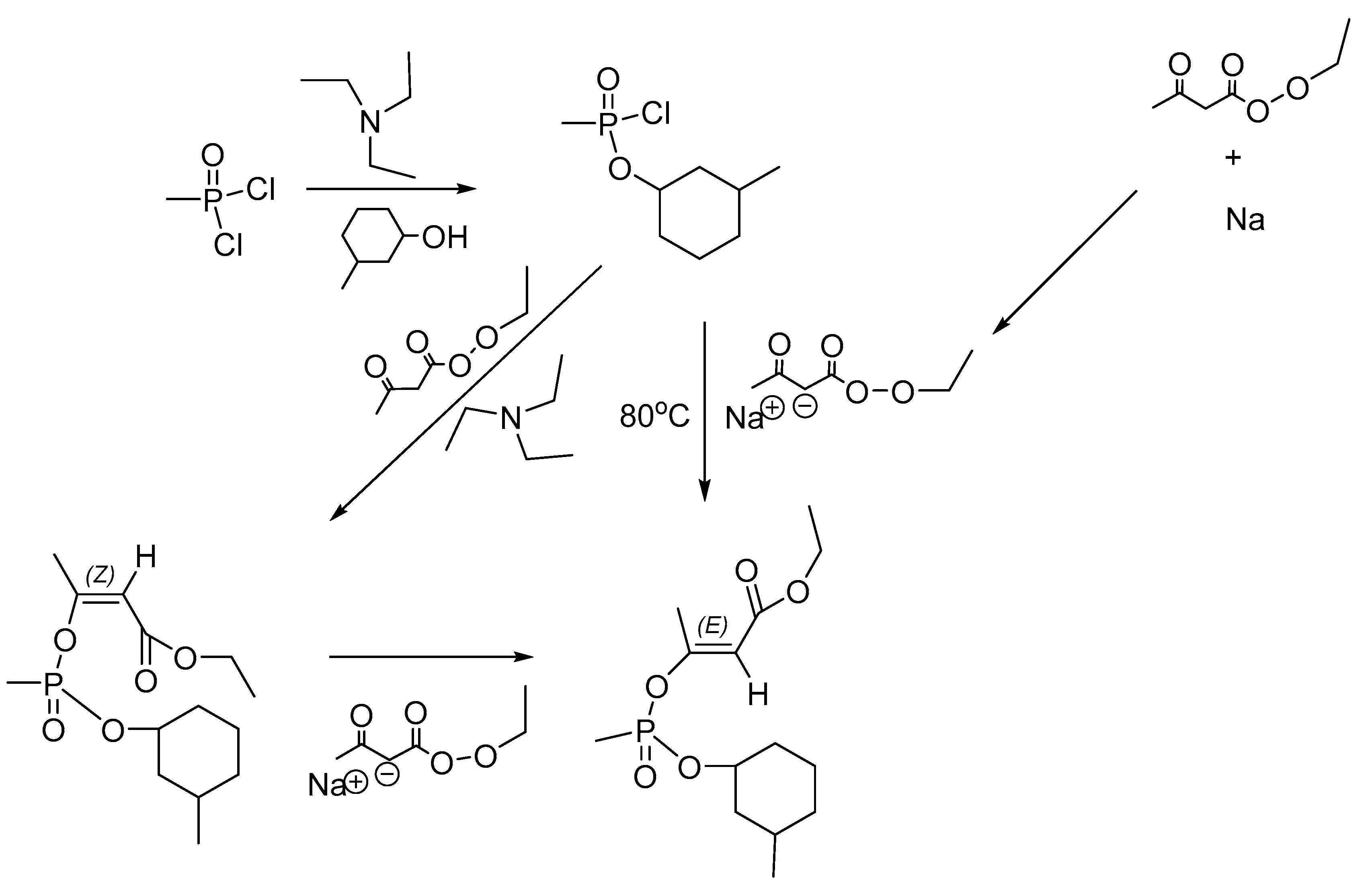
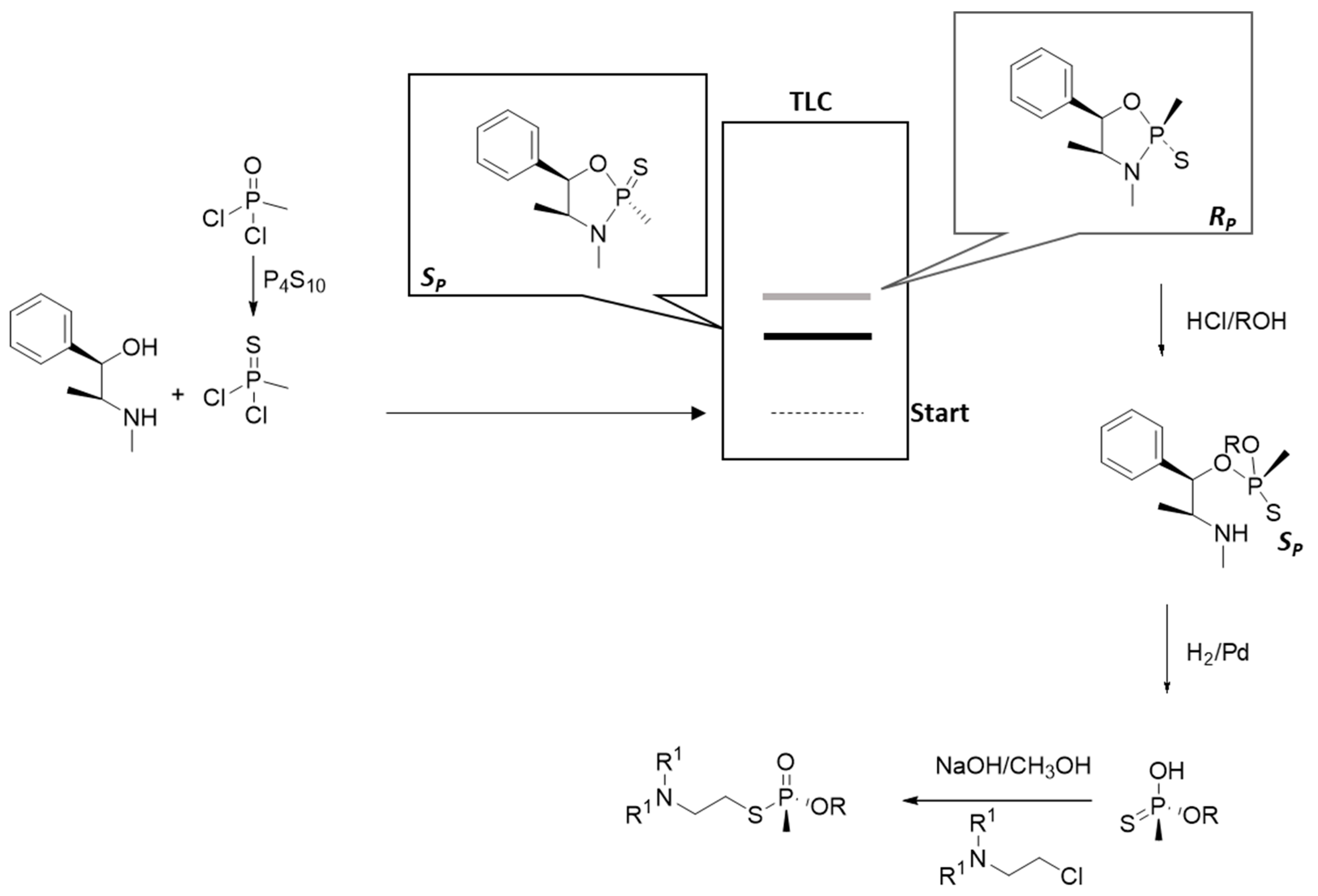
9.5. Small-Scale Synthesis for Specific Purposes
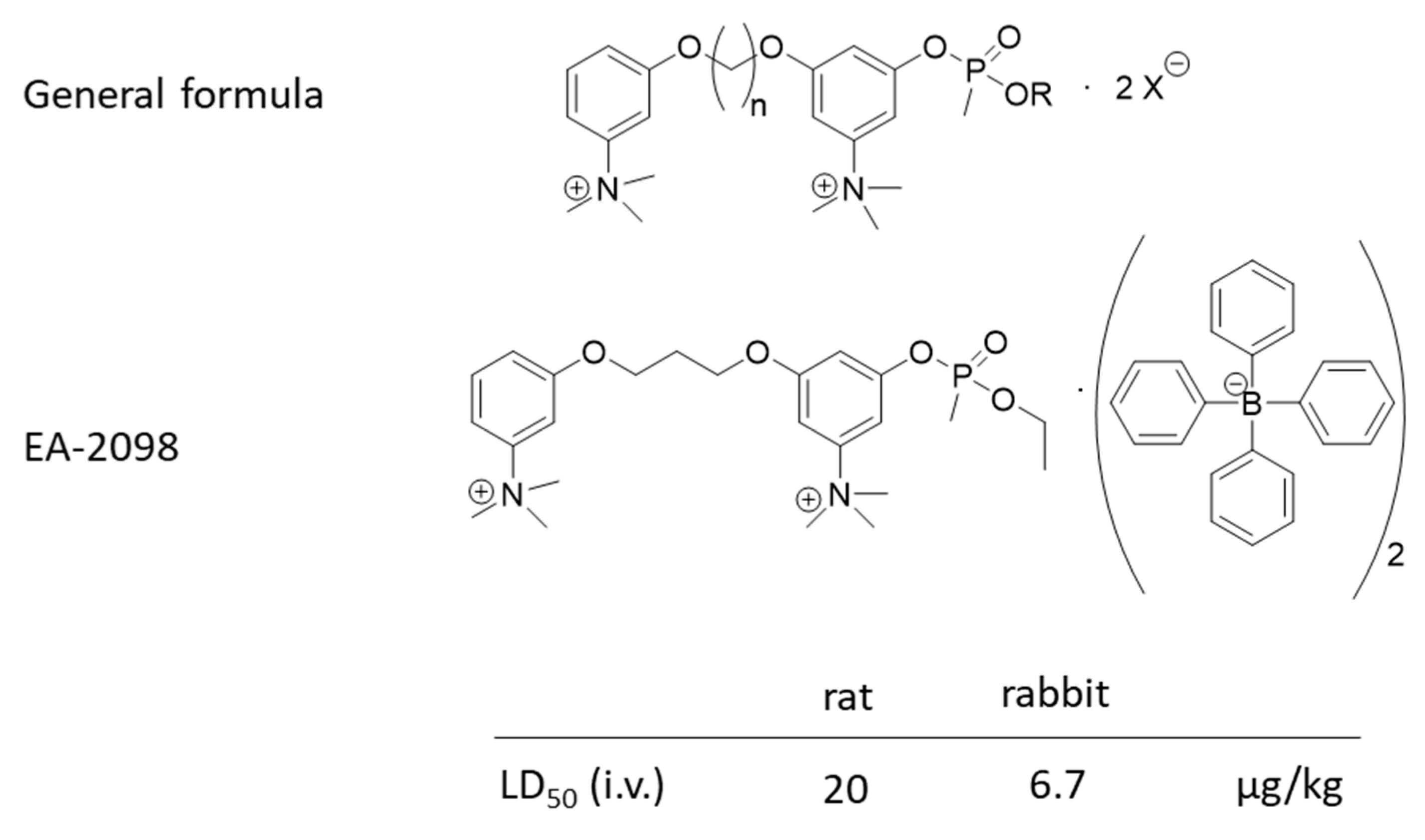
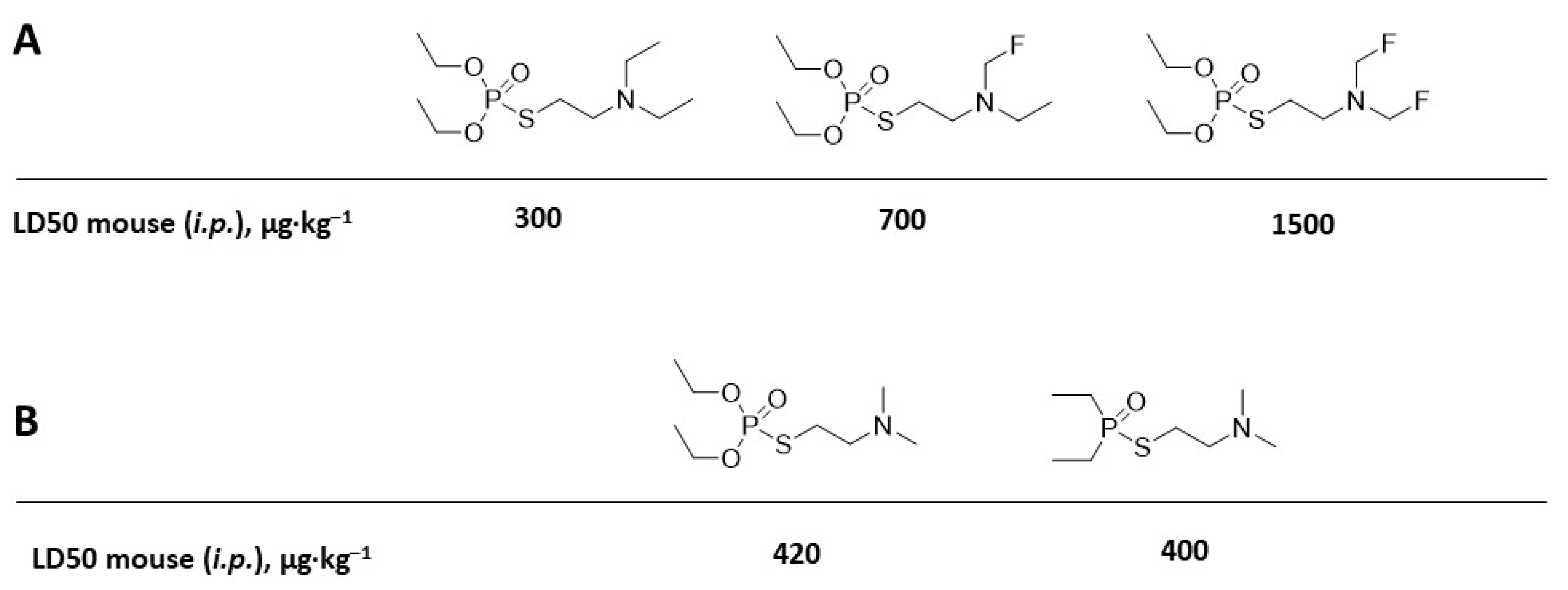
10. V-Agent Surrogates and Mimics
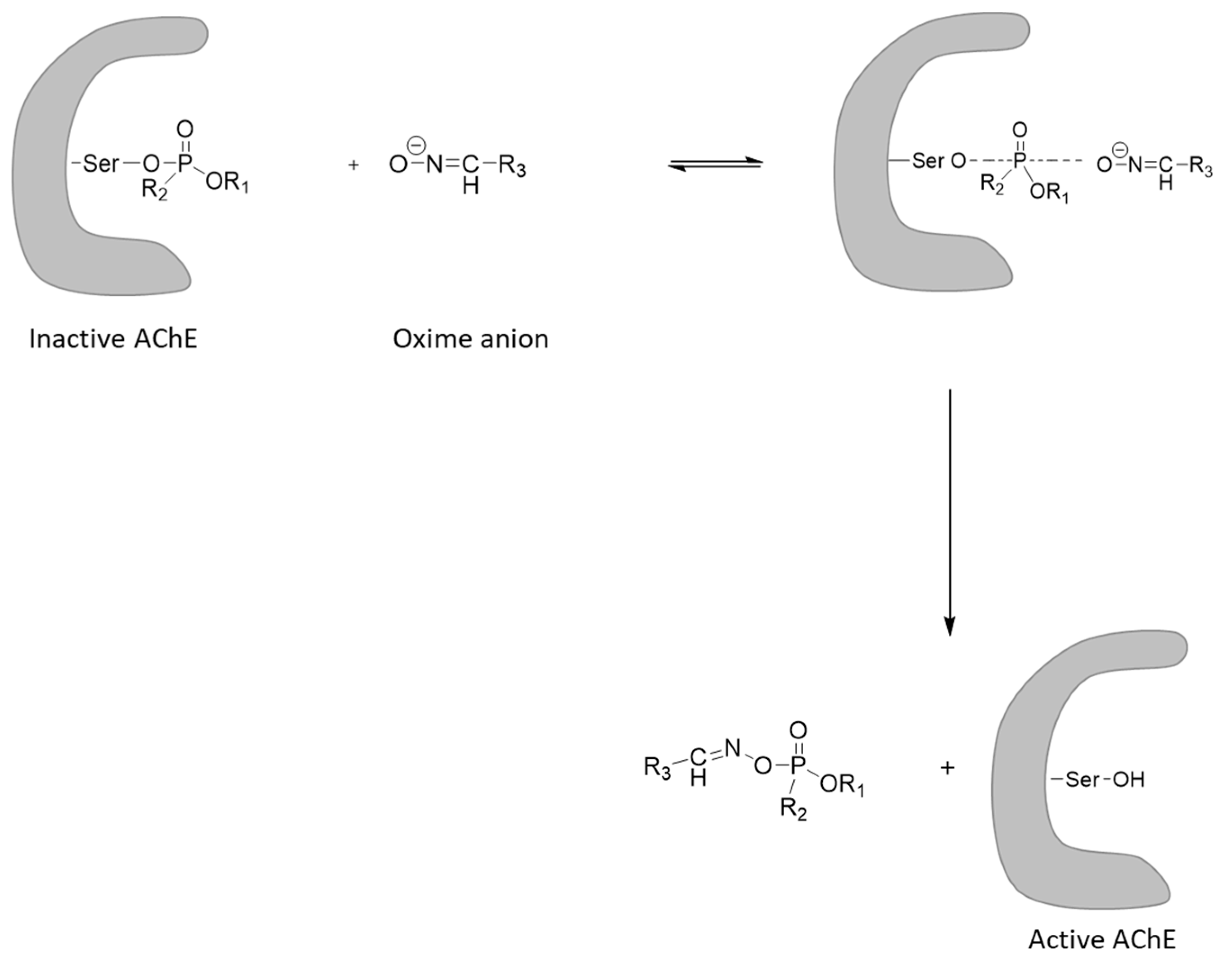
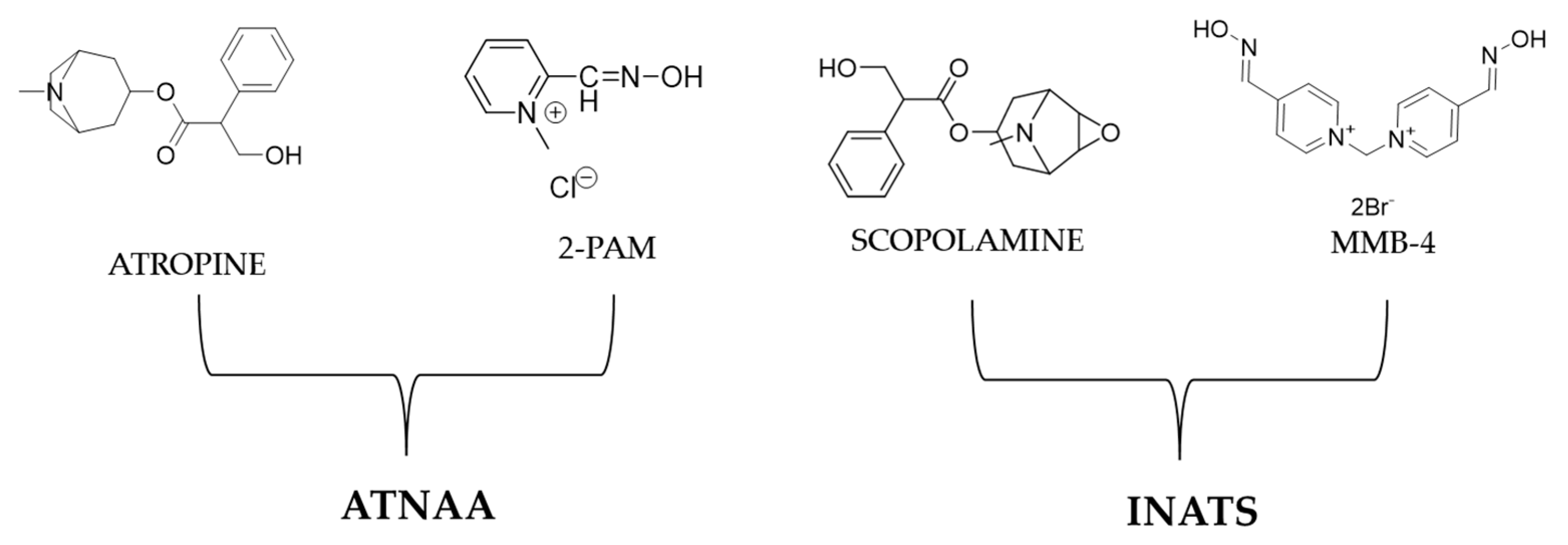
11. V-Agent Antidotes
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nakagawa, T.; Tu, T.A. Murders with VX: Aum Shinrikyo in Japan and the assassination of Kim Jong-Nam in Malaysia. Forensic Toxicol. 2018, 36, 542–544. [Google Scholar] [CrossRef]
- Aroniadou-Anderjaska, V.; Apland, J.P.; Figueiredo, T.H.; De Araujo Furtado, M.; Braga, M.F. Acetylcholinesterase inhibitors (nerve agents) as weapons of mass destruction: History, mechanism of action, and medical countermeasures. Neuropharmacology 2020, 181, 108298. [Google Scholar] [CrossRef] [PubMed]
- Bajgar, J.; Fusek, J.; Hrdina, V.; Patocka, J.; Vachek, J. Acute toxicities of 2-dialkylaminoalkyl-(dialkyamido)-fluoro-phosphates. Physiol. Res. 1992, 41, 399–402. [Google Scholar] [PubMed]
- Franca, T.C.C.; Kitagawa, D.A.S.; Cavalcante, S.F.A.; da Silva, J.A.V.; Nepovimova, E.; Kuca, K. Novichoks: The dangerous fourth generation of chemical weapons. Int. J. Mol. Sci. 2019, 20, 1222. [Google Scholar] [CrossRef]
- Chai, P.R.; Hayes, B.D.; Erickson, T.B.; Boyer, E.W. Novichok agents: A historical, current, and toxicological perspective. Toxicol. Commun. 2019, 2, 45–48. [Google Scholar] [CrossRef]
- Sommer, H.Z.; De Grace, H.; Krenzer, J.; Park, O.; Owens, O.O.; Miller, J.I. Chemical Agents. U.S. Patent 4,677,204, 30 June 1987. [Google Scholar]
- Tu, A.T. Aum Shinrikyo’s chemical and biological weapons: More than sarin. Forensic Sci. Rev. 2014, 26, 115–120. [Google Scholar]
- Alsaleh, O.I.; Elsafti Elsaeidy, A.M.; Saeed, S.; Alhallak, A.; Altelawi, M.A.; Van Berlear, G.; Hubloue, I. Acute health effects and outcome following sarin gas attacks in Khan Shaykhyn, Syria. Cureus 2022, 14, e22188. [Google Scholar]
- John, H.; van der Schans, M.; Koller, M.; Spruit, H.E.T.; Worek, F.; Thiermann, H.; Noort, D. Fatal sarin poisoning in Syria 2013: Forensic verification within an international laboratory network. Forensic Toxicol. 2018, 36, 61–71. [Google Scholar] [CrossRef]
- Haslam, J.D.; Russell, P.; Hill, S.; Emmett, S.R.; Blain, P.G. Chemical, biological, radiological, and nuclear mass casualty medicine: A review of lessons from the Salisbury and Amesbury novichok nerve agents incidents. Br. J. Anaesth 2022, 128, e200–e205. [Google Scholar] [CrossRef]
- Steindl, D.; Boehmerle, W.; Körner, R.; Praeger, D.; Haug, M.; Nee, J.; Schreiber, A.; Scheibe, F.; Demin, K.; Jacoby, P.; et al. Novichok nerve agent poisoning. Lancet 2021, 397, 249–252. [Google Scholar] [CrossRef]
- Akerfeldt, S.; Fagerlind, L. Selenophosphorus compounds as powerful cholinesterase inhibitors. J. Med. Chem. 1967, 10, 115–116. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.P.; Williamson, C.E. 3-Pyridyl Phosphonates. U.S. Patent 3,903,098, 2 September 1975. [Google Scholar]
- Dawson, T.P.; Williamson, C.E. Phosphonate Esters of Alkyl Acetoacetates. U.S. Patent 3,450,801, 17 June 1969. [Google Scholar]
- Natarelli, G.E.; Pinto, F.G.; Miller, J.I. Toxic Phosphorus Compounds. U.S. Patent 3,900,535, 19 August 1975. [Google Scholar]
- Jung, H.; Choi, S. VX evaporation and degradation from urban land surfaces. Environ. Eng. Sci. 2017, 35, 645–653. [Google Scholar] [CrossRef]
- Coulter, P.B.; Callahan, J.J.; Link, R.S. Physical Constants of Thirteen V Agents. CWLR 2346; US Army Chemical Center, Aberdeen Proving Ground: Aberdeen, MD, USA, 1959. [Google Scholar]
- Tevault, D.E.; Brozena, A.; Buchanan, J.H.; Abercrombie-Thomas, P.L.; Buettner, L.C. Thermophysical properties of VX and RVX. J. Chem. Eng. Data 2012, 57, 1970–1977. [Google Scholar] [CrossRef]
- Kuca, K.; Jun, D.; Cabal, J.; Hrabinova, M.; Bartosova, L.; Opletalova, V. Russian VX: Inhibition and reactivation of acetylcholinesterase compared with VX agent. Basic Clin. Pharmacol. Toxicol. 2006, 98, 389–394. [Google Scholar] [CrossRef]
- Buchanan, J.H.; Buettner, L.C.; Butrow, A.B.; Tevault, D.E. Vapor Pressure of VX; Edgewood Chemical Biological Center, ECBC-TR068, Aberdeen Proving Ground: Aberdeen, MD, USA, 1999.
- Ellison, H. Handbook of Chemical and Biological Warfare Agents, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Abercrombie-Thomas, P.L.; Brozena, A.; Buchanan, J.H.; Ellzy, M.W.; Berg, F.J.; Sumpter, K.B.; Wilcox, P.G. Thermophysical Properties and Spectral Characterization of EA 6043; US Army Research, Development and Engineering Command, Aberdeen Proving Ground: Aberdeen, MD, USA, 2014. [Google Scholar]
- Pulkrabkova, L.; Svobodova, B.; Konecny, J.; Kobrlova, T.; Muckova, L.; Janousek, J.; Pejchal, J.; Korabecny, J.; Soukup, O. Neurotoxicity evoked by organophosphates and available countermeasures. Arch. Toxicol. 2022, 97, 39–72. [Google Scholar] [CrossRef] [PubMed]
- Sejvar, J.J. Neurochemical and neurobiological weapons. Neurol. Clin. 2020, 38, 881–896. [Google Scholar] [CrossRef] [PubMed]
- Bajgar, J. Organophosphates/nerve agent poisoning: Mechanism of action, diagnosis, prophylaxis, and treatment. Adv. Clin. Chem. 2004, 38, 151–216. [Google Scholar]
- Misik, J.; Pavlikova, R.; Cabal, J.; Kuca, K. Acute toxicity of some nerve agents and pesticides in rats. Drug Chem. Toxicol. 2015, 38, 32–36. [Google Scholar] [CrossRef]
- Pubchem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/39793#section=Acute-Effects (accessed on 10 April 2023).
- Hall, C.R.; Inch, T.D.; Inns, R.H.; Muir, A.W.; Sellers, D.J.; Smith, A.P. Differences between some biological properties of enantiomers of alkyl S-alkyl methylphosphonothioates. J. Pharm. Pharmac. 1977, 29, 574–576. [Google Scholar] [CrossRef]
- Rice, H.; Dalton, C.H.; Price, M.E.; Graham, S.J.; Green, A.C.; Jenner, J.; Groombridge, H.J.; Timperley, C.M. Toxicity and medical countermeasure studies on the organophosphorus nerve agents VM and VX. Proc. R. Soc. A 2015, 471, 20140891. [Google Scholar] [CrossRef]
- Koplovitz, I.; Schulz, S.; Rousayne, C.; Smith, K.; Gray, R. Acute Toxicity and Efficacy of Current Medical Countermeasures against VM in Guinea Pigs: A Comparison to VX and VR; US Army Medical Research Institute of Chemical Defense, USAMRICD-TR-13-01, Aberdeen Proving Ground: Aberdeen, MD, USA, 2013. [Google Scholar]
- Marzulli, M.N. A Comparison of Toxic Properties of the V-Agents with GB, MLSR-75; Chemical Corps Medical Laboratories, Army Chemical Center: Aberdeen, MD, USA, 1955.
- Harris, L.; Broomfield, C.; Adams, N.; Stitcher, D. Detoxification of soman and O-cyclopentyl S-diethylaminoethyl methylphosphonothioate in vivo. Proc. West. Pharmacol. Soc. 1984, 27, 315–318. [Google Scholar] [PubMed]
- Bajgar, J. Comments to future Chemical Weapons Convention. Cs Farm 1989, 38, 239–240. [Google Scholar]
- Bajgar, J.; Fusek, J.; Patocka, J. Toxicities and rates of penetration of O-ethyl-S-(2-dimethylaminoethyl)-methylphosphonothioate into the blood following different routes of intoxication. Acta Biol. Med. Ger. 1978, 37, 633–636. [Google Scholar] [PubMed]
- Coleman, I.W.; Patton, G.E.; Bannard, R.A. Cholinolytics in the treatment of acetylcholinesterase poisoning. V. The effectiveness of Parpanit with oximes in the treatment of organophosphorus poisoning. Can. J. Physiol. Pharmacol. 1968, 46, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Vayron, P.; Renard, P.Y.; Taran, F.; Créminon, C.; Frobert, Y.; Grassi, J.; Mioskowski, C. Toward antibody-catalyzed hydrolysis of organophosphorus poisons. Proc. Natl. Acad. Sci. USA 2000, 97, 7058–7063. [Google Scholar] [CrossRef]
- Aquilonius, S.M.; Fredriksson, T.; Sundwall, A. Studies on phosphorylated thiocholine and choline derivatives I. General toxicology and pharmacology. Toxicol. Appl. Pharmacol. 1964, 6, 269–279. [Google Scholar] [CrossRef]
- Tammelin, E.L. Substrates and Inhibitors of Cholinesterases. Ph.D. Thesis, Stockholm University, Stockholm, Sweden, 1958. [Google Scholar]
- Amitai, G.; Ashani, Y.; Grunfeld, Y.; Kalir, A.; Cohen, S. Synthesis and properties of 2-S-[2’-(N,N-dialkylamino)ethyl]thio-1,3,2-dioxaphosphorinane 2-oxide and of the corresponding quaternary derivatives as potential nontoxic antiglaucoma agent. J. Med. Chem. 1976, 19, 810–813. [Google Scholar] [CrossRef]
- O’ Brien, R.D.; Hilton, B.D. The relation between basicity and selectivity in organophosphates. J. Agric. Food Chem. 1964, 12, 53–55. [Google Scholar] [CrossRef]
- Mager, P.P. Multidimensional Pharmacochemistry; Academic Press: Orlando, FL, USA, 1984. [Google Scholar]
- Urbina, F.; Lentzos, F.; Invernizzi, C.; Ekins, S. Dual use of artificial-intelligence-powered drug discovery. Nat. Mach. Intell. 2022, 4, 189–191. [Google Scholar] [CrossRef]
- Sim, V.M.; Stubbs, J.L. VX Percutaneous Studies in Man; US Army Chemical Research and Development Laboratories, CRDLR-3015: Army Chemical Center: Aberdeen, MD, USA, 1960. [Google Scholar]
- Sirin, G.S.; Zhou, Y.; Lior-Hoffmann, L.; Wang, S.; Zhang, Y. Aging mechanism of soman inhibited acetylcholinesterase. J. Phys. Chem. B 2012, 116, 12199–12207. [Google Scholar] [CrossRef]
- Shafferman, A.; Ordentlich, A.; Barak, D.; Stein, D.; Ariel, N.; Velan, B. Aging of phosphylated human acetylcholinesterase: Catalytic processes mediated by aromatic and polar residues of the active centre. Biochem. J. 1996, 318, 833–840. [Google Scholar] [CrossRef]
- Franjesevic, A.; Sillart, S.B.; Beck, J.M.; Vyas, S.; Callam, C.S.; Hadad, C.M. Resurrection and reactivation of acetylcholinesterase and butyrylcholinesterase. Chemistry 2019, 25, 5337–5371. [Google Scholar] [CrossRef] [PubMed]
- Duysen, E.G.; Stribley, J.A.; Fry, D.L.; Hinrichs, S.H.; Lockridge, O. Rescue of the acetylcholinesterase knockout mouse by feeding a liquid diet; phenotype of the adult acetylcholinesterase deficient mouse. Brain Res. Dev. Brain Res. 2002, 137, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Duysen, E.G.; Li, B.; Xie, W.; Schopfer, L.M.; Anderson, R.S.; Broomfield, C.A.; Lockridge, O. Evidence for nonacetylcholinesterase targets of organophosphorus nerve agent: Supersensitivity of acetylcholinesterase knockout mouse to VX lethality. J. Pharmacol. Exp. Ther. 2001, 299, 528–535. [Google Scholar] [PubMed]
- Richardson, R.J.; Fink, J.K.; Glynn, P.; Hufnagel, R.B.; Makhaeva, G.F.; Wijeyesakere, S.J. Neuropathy target esterase (NET/PNPLA6) and organophosphorus compound-induced delayed neurotoxicity (OPIDN). Adv. Neurotoxicol. 2020, 4, 1–78. [Google Scholar] [PubMed]
- Voorhees, J.R.; Rohlman, D.S.; Lein, P.J.; Pieper, A.A. Neurotoxicity in preclinical models of occupational exposure to organophosphorus compounds. Front. Neurosci. 2017, 10, 590. [Google Scholar] [CrossRef] [PubMed]
- Abou-Donia, M.; Siracuse, B.; Gupta, N.; Sokol, A.S. Sarin (GB, O-isopropyl methylphosphonofluoridate) neurotoxicity: Critical review. Crit. Rev. Toxicol. 2016, 46, 845–875. [Google Scholar] [CrossRef] [PubMed]
- Makhaeva, G.F.; Rubakova, E.V.; Hein, N.D.; Serebryakova, O.G.; Kovaleva, N.V.; Boltneva, N.P.; Fink, J.K.; Richardson, R.J. Further studies toward a mouse model for biochemical assessment of neuropathic potential of organophosphorus compounds. J. Appl. Toxicol. 2014, 34, 1426–1435. [Google Scholar] [CrossRef] [PubMed]
- Munro, N. Toxicity of the organophosphate chemical warfare agents GA, GB, and VX: Implications for public protection. Environ. Health Perspect 1994, 102, 18–38. [Google Scholar] [CrossRef]
- Sidell, F.R.; Groff, W.A.; Vocci, F. Effects of EA 3148 Administered Intravenously to Humans, CRDL TM 2-31; Edgewood: Aberdeen, MD, USA, 1965. [Google Scholar]
- Brown, M. Military chemical warfare agent human subjects testing: Part 1-History of six-decades of military experiments with chemical warfare agents. Mil. Med. 2009, 174, 1041. [Google Scholar] [CrossRef]
- Boffey, P.M. Nerve gas: Dugway accident linked to Utah sheep kill. Science 1968, 162, 1460–1464. [Google Scholar] [CrossRef] [PubMed]
- Tu, T.A. The use of VX as a terrorist agent: Action by Aum Shinrikyo of Japan and the death of Kim Yong-Nam in Malaysia: Four case studies. Glob. Secur. Health Sci. Policy 2020, 5, 48–56. [Google Scholar] [CrossRef]
- Zurer, P. Japanese cult used VX to slay member. Chem. Eng. News 1998, 76, 7. [Google Scholar] [CrossRef]
- Nozaki, H.; Aikawa, N.; Fujishima, S.; Suzuki, M.; Shinozawa, Y.; Hori, S.; Nogawa, S. A case of VX poisoning and the difference from sarin. Lancet 1995, 346, 698–699. [Google Scholar] [CrossRef]
- Kim, K.; Tsay, O.G.; Atwood, D.A.; Churchill, D.G. Destruction and detection of chemical warfare agents. Chem. Rev. 2011, 111, 5345–5403. [Google Scholar] [CrossRef]
- Groenewold, G.S. Degradation kinetics of VX. Main Group Chem. 2010, 9, 221–244. [Google Scholar] [CrossRef]
- Yang, Y.C.; Szafraniec, L.L.; Beaudry, W.T.; Rohrbaugh, D.K. Autocatalytic hydrolysis of V-type nerve agents. J. Org. Chem. 1996, 61, 8407–8413. [Google Scholar] [CrossRef]
- Buckles, L.C.; Lewis, S.M.; Lewis, F.E. S-(2-diisopropylamino-ethyl) O-ethyl Methylphosphonothioate Stabilized with Soluble Carbodiimides. U.S. Patent 4,012,464, 15 March 1977. [Google Scholar]
- Gross, D.; Reinhard, D.R. Production of QL at the Newport Army Ammunition Plant or Vertac Chemical Corporation, West Helena AR Plant Site: Environmental Impact Statement; Department of the Army, US Material Command: Alexandria, VA, USA, 1985. [Google Scholar]
- Koblin, A. Persistent Incapacitating Chemical Warfare Composition and Its Use. U.S. Patent 4,708,869, 24 November 1987. [Google Scholar]
- Cohen, L.; Coulter, P.B.; Zeffert, B.M. Thickened Phosphorus Esters. U.S. Patent 3868446, 25 February 1975. [Google Scholar]
- Pianfetti, J.A.; Quin, L.D. Formation of phosphonous dichlorides by alkylation of phosphorus trichloride with methane or ethane. J. Am. Chem. Soc. 1962, 84, 851–854. [Google Scholar] [CrossRef]
- Eckhaus, S.R.; Davis, B.M., Jr.; Moore, T.R. Preparation of Alkylphosphonothiolates. U.S. Patent 3,911,059, 7 October 1975. [Google Scholar]
- Ember, L. Detection of VX precursor in soil sample led U.S. to bomb Sudanese facility. Chem. Eng. News 1998, 76, 6. [Google Scholar] [CrossRef]
- Epstein, J.; Michel, H.O.; Plapinger, R.E.; Fleisher, J.H.; Callahan, J.J.; Jandorf, B.J. Process for Making Compounds Possessing Anticholinesterase Activity. U.S. Patent H346, 6 October 1987. [Google Scholar]
- Ford-Moore, A.H.; Bebbington, A. Preparation of O-Alkyl S-Dialkyl-Phosphonothiolates. U.S. Patent 3,781,387, 25 December 1973. [Google Scholar]
- Berman, H.A.; Leonard, K. Chiral reactions of acetylcholinesterase probed with enantiomeric methylphosphonothiolates. J. Biol. Chem. 1989, 264, 3942–3950. [Google Scholar] [CrossRef]
- Holmgren, K.H.; Valdez, C.A.; Magnusson, R.; Vu, A.K.; Lindberg, S.; Williams, A.M.; Alcaraz, A.; Åstot, C.; Hok, S.; Norlin, R. Part 1: Tracing Russian VX to its synthetic routes by multivariate statistics of chemical attribution signatures. Talanta 2018, 186, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Jansson, D.; Lindström, S.W.; Norlin, R.; Hok, S.; Valdez, C.A.; Williams, A.M.; Alcaraz, A.; Nilsson, C.; Åstot, C. Part 2: Forensic attribution profiling of Russian VX in food using liquid chromatography-mass spectrometry. Talanta 2018, 186, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, S.F.A.; Simas, A.B.C.; Kuca, K. Nerve agents’s surrogates: Invaluable tools for development of acetylcholinesterase reactivators. Curr. Org. Chem. 2019, 23, 1539–1559. [Google Scholar] [CrossRef]
- Wang, X.; Wang, X.; Feng, R.; Fu, T.; Jiang, Y.; Zhang, J.; Sun, X. Recent advances of chemosensors for nerve agents. Chem. Asian J. 2022, 17, e202200284. [Google Scholar] [CrossRef] [PubMed]
- Singer, B.C.; Hodgson, A.T.; Destaillats, H.; Hotchi, T.; Revzan, K.L.; Sextro, R.G. Indoor sorption of surrogates for sarin and related nerve agents. Environ. Sci. Technol. 2005, 39, 3203–3214. [Google Scholar] [CrossRef] [PubMed]
- Meek, E.; Chambers, H.W.; Coban, A.; Funck, K.E.; Pringle, R.B.; Ross, M.; Chambers, J.E. Synthesis and in vitro and in vivo inhibition potencies of highly relevant nerve agent surrogates. Toxicol. Sci. 2012, 126, 525–533. [Google Scholar] [CrossRef]
- Chao, C.K.; Balasubramanian, N.; Gerdes, J.M.; Thompson, C.M. The inhibition, reactivation and mechanism of VX-, sarin-, fluoro-VX and fluoro-sarin surrogates following their interaction with HuAChE and HuBuChE. Chem. Biol. Interact. 2018, 291, 220–227. [Google Scholar] [CrossRef]
- Dagnaw, F.W.; Cai, Y.P.; Song, Q.H. Rapid and sensitive detection of nerve agent mimics by meso-substituted BODIPY piperazines as fluorescent chemosensors. Dyes. Pigm. 2021, 189, 109257. [Google Scholar] [CrossRef]
- Kangas, M.J.; Ernest, A.; Lukowicz, R.; Mora, A.V.; Quossi, A.; Perez, M.; Kyes, N.; Holmes, A.E. The identification of seven chemical warfare mimics using a colorimetric array. Sensors 2018, 18, 4291. [Google Scholar] [CrossRef]
- Jokanovic, M.; Prostran, M. Pyridinium Oximes as Cholinesterase Reactivators. Structure-Activity Relationship and Efficacy in the Treatment of Poisoning with Organophosphorus Compound. Curr. Med. Chem. 2009, 16, 2177–2188. [Google Scholar] [CrossRef]
- Wong, L.; Radic, Z.; Bruggemann, R.J.M.; Hosea, N.; Berman, H.A.; Taylor, P. Mechanism of Oxime Reactivation of Acetylcholinesterase Analyzed by Chirality and Mutagenesis. Biochemistry 2000, 39, 5750–5757. [Google Scholar] [CrossRef] [PubMed]
- Lorke, D.E.; Petroianu, G.A. Reversible cholinesterase inhibitors as pretreatment for exposure to organophosphates. A review. J. Appl. Toxicol. 2019, 39, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Huang, Y.; Baldassarre, H.; Wang, B.; Lazaris, A.; Leduc, M.; Bilodeau, A.S.; Bellemare, A.; Côté, M.; Herskovits, P.; et al. Recombinant human butyrylcholinesterase from milk of transgenic animals to protect against organophosphate poisoning. Proc. Natl. Acad. Sci. USA 2007, 104, 13603–13608. [Google Scholar] [CrossRef] [PubMed]
- Mumford, H.; Troyer, J.K. Post-exposure therapy with recombinant human BuChE following percutaneous VX challenge in guinea-pigs. Toxicol. Lett. 2011, 206, 29–34. [Google Scholar] [CrossRef]
- Hanke, D.W.; Beckett, M.S.; Overton, M.A.; Burdick, C.K.; Lieske, C.N. Oxime-induced reactivation of acetylcholinesterase inhibited by organophosphates. J Appl Toxicol 1990, 10, 87–90. [Google Scholar] [CrossRef]
- Costanzi, S.; Machado, J.H.; Mitchell, M. Nerve agents. What they are, how they work, how to counter them. ACS Chem. Neurosci. 2018, 9, 873–885. [Google Scholar] [CrossRef]
- US Army Medical Research Institute of Chemical Defense. Available online: https://usamricd.health.mil/research/Pages/Products.aspx (accessed on 5 May 2023).
- Maxwell, D.M.; Koplovitz, I.; Worek, F.; Sweeney, R.E. A structure-activity analysis of the variation in oxime efficacy against nerve agents. Toxicol. Appl. Pharmacol. 2008, 231, 157–164. [Google Scholar] [CrossRef]
- Burback, B.L.; Cabell, L.A.; Johnson, J.D.; McDonough, J.A.; Osheroff, M.R. International journal of toxicology special editions volume of MMB4 DMS. Int. J. Toxicol. 2013, 32, 3S–4S. [Google Scholar] [CrossRef]
- Gorecki, L.; Korabecny, J.; Musilek, K.; Malinak, D.; Nepovimova, E.; Dolezal, R.; Jun, D.; Soukup, O.; Kuca, K. SAR study to find optimal cholinesterase reactivator against organophosphorus nerve agents and pesticides. Arch. Toxicol. 2016, 90, 2831–2859. [Google Scholar] [CrossRef]
- Cherny, I.; Greisen, P., Jr.; Ashani, Y.; Khare, S.D.; Oberdorfer, G.; Leader, H.; Baker, D.; Tawfic, D.S. Engineering V-type nerve agents detoxifying enzymes using computationally focused libraries. ACS Chem. Biol. 2018, 8, 2394–2403. [Google Scholar] [CrossRef]
- Mercey, G.; Verdelet, T.; Renou, J.; Kliachyna, M.; Baati, R.; Nachon, F.; Jean, L.; Renard, P.Y. Reactivators of acetylcholinesterase inhibited by organophosphorus nerve agents. Acc. Chem. Res. 2012, 45, 756–766. [Google Scholar] [CrossRef] [PubMed]




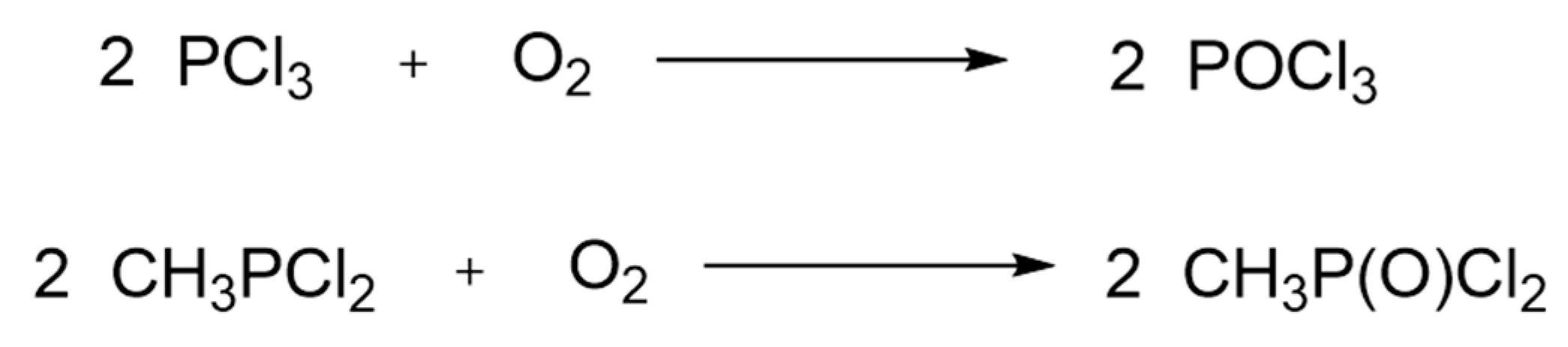
| @25 °C | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| V-Agent | Density, g/mL | Viscosity, cS | Surface Tension, mN/m | Vapor Pressure, mmHg | Melting Point, °C | Flash Point, °C | Lower Consolute T in Water, °C | Formula | MW |
| VG (EA-1508) | 1.0457 | 4.74 | 31.0 | ca. −51 | 25.5 | C10H24NO3PS | 269.34 | ||
| VP (EA-1511) | 1.023 | 29.6 | 30.4 | C15H24NO3P | 297.33 | ||||
| EA-1576 | 1.0829 | 23.3 | 32.4 | C14H25NO5P | 304.32 | ||||
| EA-1521 | 0.995 | C16H34NO2PS | 335.49 | ||||||
| EA-1622 # | 1.023 | 6.10 | 29.7 | 135 | C10H24NO2PS | 253.34 | |||
| EA-1699 # | 1.0600 | 5.31 | 32.0 | C7H18NO2PS | 211.26 | ||||
| VM (EA-1664) | 1.0311 | 5.85 | 31.2 | 74 | C9H22NO2PS | 239.32 | |||
| VR (RVX, EA-4243) | 1.0065 | 8.34 ## 8.58 | 26.9 ## | 0.00063 ## | 150 ### | C11H26NO2PS | 267.37 | ||
| EA-1694 # | 1.0453 | 4.92 | 31.5 | misc. 0–100 | C8H20NO2PS | 225.29 | |||
| VE (EA-1517) | 1.0180 | 5.44 | 29.5 | 157 | 41.4 | C10H24NO2PS | 253.34 | ||
| VS (EA-1677) | 1.0016 | 9.36 | 29.9 | ca. −35 | 168 | ca. −5 | C12H28NO2PS | 281.39 | |
| VX (EA-1701) | 1.0083 | 9.96 10.10 # 10.09 *** | 31.6 30.2 # | 0.000884 # 0.000878 * | 159 | 9.4 | C11H26NO2PS | 267.37 | |
| EA-1728 | 0.9899 | 11.4 | 29.2 | ca. −12 | 170 | −1.6 | C12H28NO2PS | 281.39 | |
| EA-1763 | 0.9973 | 11.3 | 30.2 | below 0 | C12H28NO2PS | 281.39 | |||
| EA-3148 | 1.05 ** | 1.96 ** | 0.0004 ** | C12H26NO2PS | 279.38 | ||||
| EA-3317 | 1.02 ** | 35.1 ** | 0.00014 ** | C14H30NO2PS | 307.43 | ||||
| CVX (EA-6043) | 1.0125 *** | 9.29 *** | 22.68 *** | 0.00025 *** | C11H26NO2PS | 267.37 | |||
| V-Agent | Mouse | Rat | Rabbit | Monkey | Cat | Guinea Pig | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| i.v. | i.m. | p.o. | s.c. | i.v. | s.c. | p.c. | i.p. | i.m. | p.o. | i.v. | p.c. | s.c. | i.v. | i.v. | p.c. | s.c. | |
| VX | 14.5 [25] | 26.8 [25] | 44.2 [25] | 22 [26] | 8.2 [25,26] 7 [27] | 11.9 [25,26] | 80 [25] | 45.6 [25,26] 37 [27] | 14 [25] | 85 [25] 122 [26] | 13.1 [28] | 6 [27] | 5 [27] | 613 [29] | 9 [29] | ||
| VR | 14.5 [25] 15.3 [26] | 15.9 [25,26] | 290 [25] | 63 [25,26] | 14.1 [25] | 20 [25] | 11.3 [29] | ||||||||||
| VM | 20 [25] | 212 [25] | 1289.9 [29] | 14.9 [30] | |||||||||||||
| VE (EA-1517) | 15.3 [31] | 40.7 [31] | |||||||||||||||
| VP (EA-1511) | 36.8 [31] | 81.8 [31] | |||||||||||||||
| EA-3148 | 3.1 [32] | 4.5 [32] | |||||||||||||||
| EA-1728 | 59.1 [33] | 46 [25] | 56 [25] | ||||||||||||||
| EA-1694 | 28.9 [25] | 121 [25] | |||||||||||||||
| iPr-Me * | 96 [25] | 874 [25] | |||||||||||||||
| EA-1699 | 17 [34] | 54.5 [34] | 23.6 [34] | 121.9 [34] | |||||||||||||
| VG (EA-1508) | 190 [35] | 150 [35] | 52.3 [31] | 167.3 [31] | 125 [35] | 80 [35] | |||||||||||
| Seleno-VG | 60 [12] | ||||||||||||||||
| Seleno-VE | 21 [12] | ||||||||||||||||
| Detection | |||
|---|---|---|---|
| Compound | 1st Woman | 2nd Woman | Victim |
| VX | x | x | |
| iPr2NCH2CH2Cl | x | x | |
| iPr2NCH2CH2SH | x | x | |
| CH3P(O)(OEt)SH | x | ||
| CH3P(O)(OEt)OH | x | x | |
| iPr2NCH2CH2SCH2CH2NiPr2 | x | ||
| iPr2NCH2CH2SSCH2CH2NiPr2 | x | x | |
| (CH3)2NCH2CH2OH | x | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pampalakis, G.; Kostoudi, S. Chemical, Physical, and Toxicological Properties of V-Agents. Int. J. Mol. Sci. 2023, 24, 8600. https://doi.org/10.3390/ijms24108600
Pampalakis G, Kostoudi S. Chemical, Physical, and Toxicological Properties of V-Agents. International Journal of Molecular Sciences. 2023; 24(10):8600. https://doi.org/10.3390/ijms24108600
Chicago/Turabian StylePampalakis, Georgios, and Stavroula Kostoudi. 2023. "Chemical, Physical, and Toxicological Properties of V-Agents" International Journal of Molecular Sciences 24, no. 10: 8600. https://doi.org/10.3390/ijms24108600





