TRPV3 Ion Channel: From Gene to Pharmacology
Abstract
1. Introduction
2. Molecular Characteristics of TRPV3
2.1. Gene and Evolution
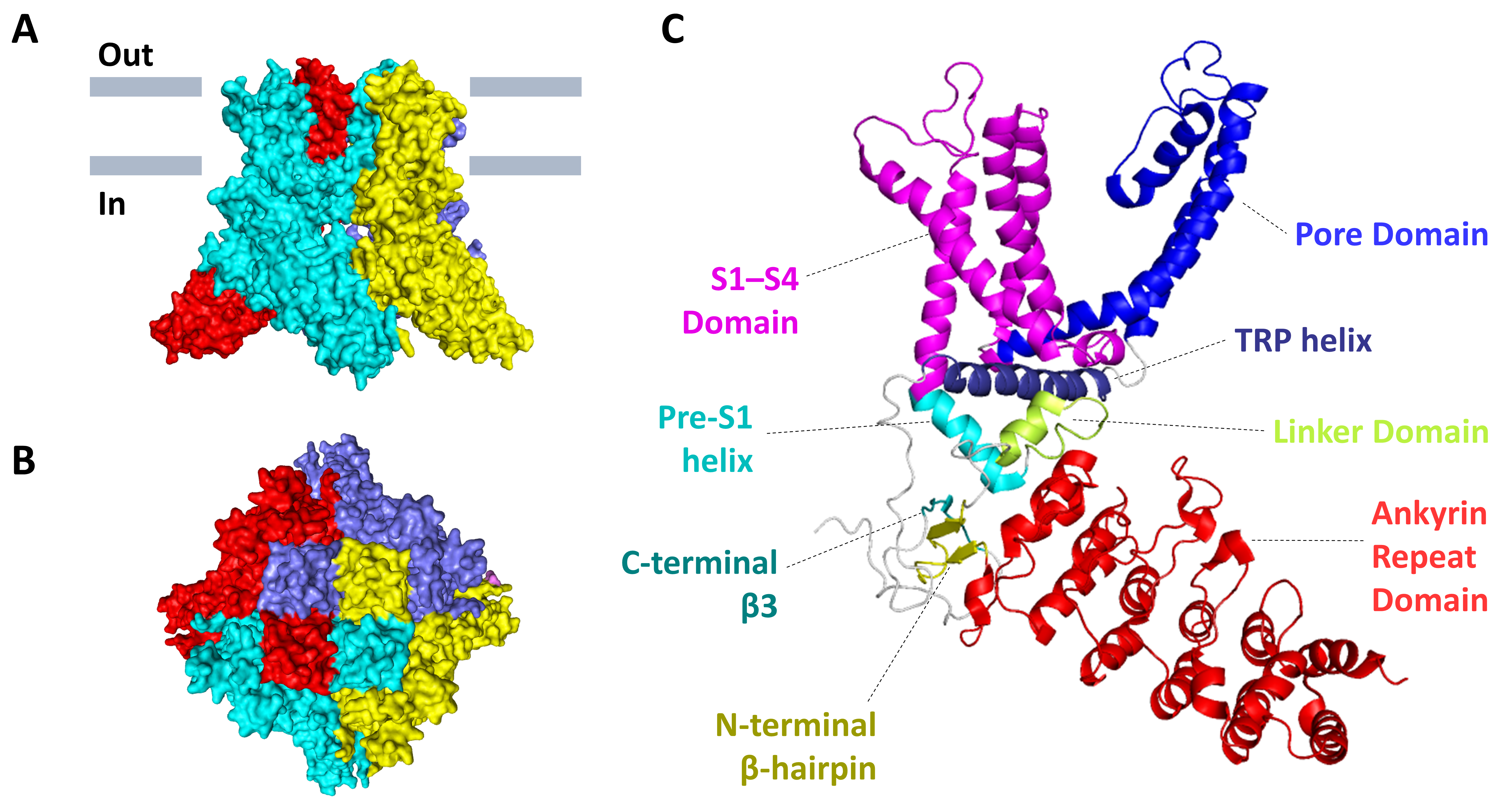
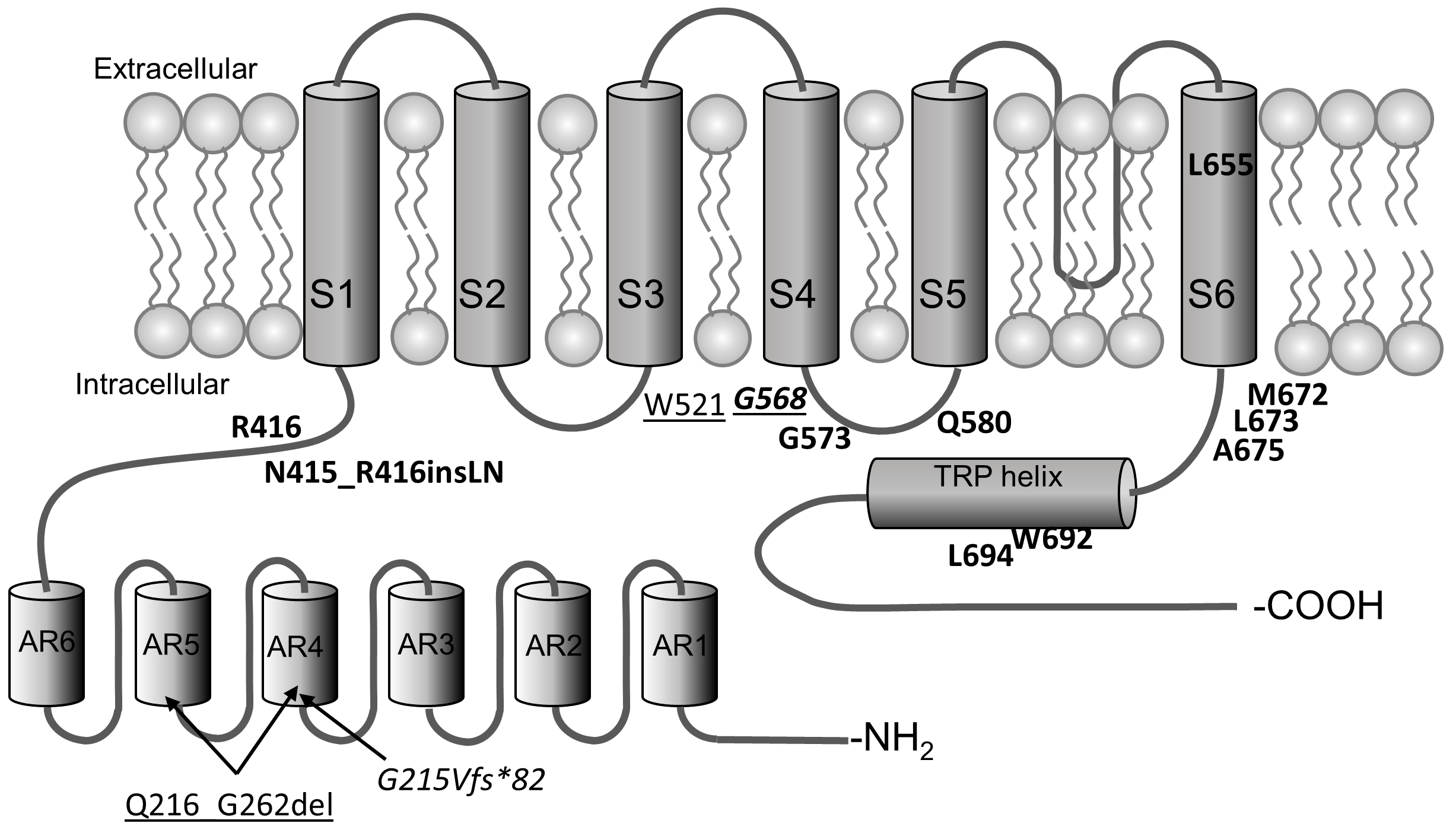
2.2. Protein
3. Ligands of TRPV3
3.1. Natural Agonists
| Compound | Potency | Confirmed by | Refs. | |
|---|---|---|---|---|
| Species | EC50, µM | |||
| Diphenyl-containing compounds | ||||
| 2-Aminoethoxydiphenyl borate | Mm | 28.3–165.8 | e/p * | [32,52] |
| Hs | 78 | c.i. ** | [53] | |
| Diphenylboronic anhydride | Mm | 64.1–85.1 | c.i., e/p | [52] |
| Drofenine | Hs | 207 | c.i., e/p | [53] |
| Other synthetic compounds | ||||
| KS0365 | Mm | 5.08 | c.i., e/p | [54] |
| Monoterpenes | ||||
| 6-tert-butyl-m-cresol | Mm | 370 | e/p | [44] |
| Carvacrol | Mm | 490 | e/p | [44] |
| Hs | 438 | c.i. | [53] | |
| Thymol | Mm | 860 | e/p | [44] |
| Citral | Mm | 926 | e/p | [55] |
| Dihydrocarveol | Mm | 2570 | e/p | [44] |
| (−)-Carveol | Mm | 3030 | e/p | [44] |
| (+)-Borneol | Mm | 3450 | e/p | [44] |
| Camphor | Mm | 6030 | e/p | [44] |
| (−)-Menthol | Mm | 20,000 | c.i., e/p | [56] |
| Sesquiterpenes | ||||
| Farnesyl pyrophosphate | Hs | 0.1311 | c.i., e/p | [45] |
| Diterpenes | ||||
| Serratol | Rn | 0.17 | c.i. | [48] |
| Incensole acetate | Mm | 16 | c.i., e/p | [47] |
| Bisandrographolide B | Mm | 40.5 | MST ***, e/p | [49] |
| Cannabinoids | ||||
| cannabidiol | Rn | 3.7 | c.i. | [51] |
| Δ9-tetrahydrocannabivarin | Rn | 3.8 | c.i. | [51] |
3.2. Synthetic Agonists
3.3. Natural Antagonists
3.4. Synthetic Antagonists
| Compound | Potency | Confirmed by | Refs. | |
|---|---|---|---|---|
| Species | IC50, µM | |||
| Endogenous compounds | ||||
| Isopentenyl pyrophosphate | Hs | 7.5 | c.i. *, e/p ** | [46] |
| 17(R)-resolvin D1 | Hs | 0.398 | c.i., e/p | [58] |
| Components of medicinal plants | ||||
| Osthole | Hs | 37 | c.i., e/p | [59] |
| Mm | 20.5 | c.i., e/p | [74] | |
| Forsythoside B | Hs | 6.7 | c.i., e/p | [75] |
| Verbascoside | Hs | 14.1 | e/p | [76] |
| Citrusinine-II | Mm | 12.43 | c.i., e/p | [61] |
| Isochlorogenic acid A | Hs | 2.7 | c.i., e/p | [62,77] |
| Isochlorogenic acid B | Hs | 0.9 | c.i., e/p | [62,77] |
| Scutellarein | Mm | 1.18 | e/p | [63] |
| Marine products | ||||
| Monanchomycalin B | Hs | 3.25 | c.i. | [68] |
| Echinochrome A | Hs | 2.11 | e/p | [72] |
| Clinical drugs | ||||
| Dyclonine | Mm | 3.2 | e/p | [78] |
| Bupivacaine | Hs | 170 | e/p | [79] |
| Ropivacaine | Hs | 280 | e/p | [79] |
| Other synthetic compounds | ||||
| 7c | Hs | 1.05 | e/p | [80] |
| 8c | Hs | 0.086 | e/p | [80] |
| 74a | Hs | 0.38 | c.i., e/p | [81] |
| Ruthenium red | Mm | - | c.i., e/p | [6] |
| Diphenyltetrahydrofuran | Mm | Biphasic: 6, 151.5 (−80 mV); 10, 226.7 (+80 mV) | c.i., e/p | [52] |
| 26E01 | Mm | 8.6 | c.i., e/p | [82] |
| PC5 | Mm | 2.63 | e/p | [83] |
| Trpvicin | Hs | 0.41 | e/p | [84] |
4. Structural Interactions of TRPV3 with the Ligands
5. TRPV3 Ligands in the Research of Channel’s Physiology
5.1. Chemo- and Thermosensation
5.2. Hair Growth
5.3. Brain Functions
6. TRPV3 Ligands in the Research and Correction of Channel’s Pathophysiology
6.1. Pain
6.2. Itch, Skin Inflammation, and Dermatitis
6.3. Olmsted Syndrome
6.4. Wound Healing and Barrier Functions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, H. TRP channel classification. In Advances in Experimental Medicine and Biology; Springer: Dordrecht, The Netherlands, 2017; Volume 976, pp. 1–8. [Google Scholar]
- Samanta, A.; Hughes, T.E.T.; Moiseenkova-Bell, V.Y. Transient receptor potential (TRP) channels. In Subcellular Biochemistry; Springer: Singapore, 2018; Volume 87, pp. 141–165. [Google Scholar]
- Kärki, T.; Tojkander, S. Trpv protein family—From mechanosensing to cancer invasion. Biomolecules 2021, 11, 1019. [Google Scholar] [CrossRef] [PubMed]
- Seebohm, G.; Schreiber, J.A. Beyond hot and spicy: TRPV channels and their pharmacological modulation. Cell. Physiol. Biochem. 2021, 22, 108–130. [Google Scholar] [CrossRef]
- Sasase, T.; Fatchiyah, F.; Ohta, T. Transient receptor potential vanilloid (TRPV) channels: Basal properties and physiological potential. Gen. Physiol. Biophys. 2022, 41, 165–190. [Google Scholar] [CrossRef] [PubMed]
- Peier, A.M.; Reeve, A.J.; Andersson, D.A.; Moqrich, A.; Earley, T.J.; Hergarden, A.C.; Story, G.M.; Colley, S.; Hogenesch, J.B.; McIntyre, P.; et al. A heat-sensitive TRP channel expressed in keratinocytes. Science 2002, 296, 2046–2049. [Google Scholar] [CrossRef]
- Xu, H.; Ramsey, I.S.; Kotecha, S.A.; Moran, M.M.; Chong, J.A.; Lawson, D.; Ge, P.; Lilly, J.; Silos-Santiago, I.; Xie, Y.; et al. TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 2002, 418, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.D.; Gunthorpe, M.J.; Kelsell, R.E.; Hayes, P.D.; Reilly, P.; Facer, P.; Wright, J.E.; Jerman, J.C.; Walhin, J.P.; Ooi, L.; et al. TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 2002, 418, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Broad, L.M.; Mogg, A.J.; Eberle, E.; Tolley, M.; Li, D.L.; Knopp, K.L. TRPV3 in drug development. Pharmaceuticals 2016, 9, 55. [Google Scholar] [CrossRef]
- Su, W.; Qiao, X.; Wang, W.; He, S.; Liang, K.; Hong, X. TRPV3: Structure, Diseases and Modulators. Molecules 2023, 28, 774. [Google Scholar] [CrossRef]
- Saito, S.; Shingai, R. Evolution of thermoTRP ion channel homologs in vertebrates. Physiol. Genom. 2006, 27, 219–230. [Google Scholar] [CrossRef]
- Saito, S.; Fukuta, N.; Shingai, R.; Tominaga, M. Evolution of Vertebrate Transient Receptor Potential Vanilloid 3 Channels: Opposite Temperature Sensitivity between Mammals and Western Clawed Frogs. PLoS Genet. 2011, 7, e1002041. [Google Scholar] [CrossRef]
- Yang, P.; Zhu, M.X. TRPV3. Handb. Exp. Pharmacol. 2014, 222, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, F.; Allen, J.E.; Allen, J.; Alvarez-Jarreta, J.; Amode, M.R.; Armean, I.M.; Austine-Orimoloye, O.; Azov, A.G.; Barnes, I.; Bennett, R.; et al. Ensembl 2022. Nucleic Acids Res. 2022, 50, D988–D995. [Google Scholar] [CrossRef] [PubMed]
- Morini, M.; Bergqvist, C.A.; Asturiano, J.F.; Larhammar, D.; Dufour, S. Dynamic evolution of transient receptor potential vanilloid (TRPV) ion channel family with numerous gene duplications and losses. Front. Endocrinol. 2022, 13, 1013868. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Jin, J.; Hu, L.; Shen, D.; Dong, X.P.; Samie, M.A.; Knoff, J.; Eisinger, B.; Liu, M.L.; Huang, S.M.; et al. TRP channel regulates EGFR signaling in hair morphogenesis and skin barrier formation. Cell 2010, 141, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Deme, L.; Zhang, Z.; Huang, X.; Xu, S.; Yang, G. Decay of TRPV3 as the genomic trace of epidermal structure changes in the land-to-sea transition of mammals. Ecol. Evol. 2022, 12, e8731. [Google Scholar] [CrossRef] [PubMed]
- Grandl, J.; Hu, H.; Bandell, M.; Bursulaya, B.; Schmidt, M.; Petrus, M.; Patapoutian, A. Pore region of TRPV3 ion channel is specifically required for heat activation. Nat. Neurosci. 2008, 11, 1007–1013. [Google Scholar] [CrossRef]
- Lynch, V.J.; Bedoya-Reina, O.C.; Ratan, A.; Sulak, M.; Drautz-Moses, D.I.; Perry, G.H.; Miller, W.; Schuster, S.C. Elephantid Genomes Reveal the Molecular Bases of Woolly Mammoth Adaptations to the Arctic. Cell Rep. 2015, 12, 217–228. [Google Scholar] [CrossRef]
- Price, E.; Gianfrancesco, O.; Harrison, P.T.; Frank, B.; Bubb, V.J.; Quinn, J.P. CRISPR Deletion of a SVA Retrotransposon Demonstrates Function as a cis-Regulatory Element at the TRPV1/TRPV3 Intergenic Region. Int. J. Mol. Sci. 2021, 22, 1911. [Google Scholar] [CrossRef]
- Singh, A.K.; McGoldrick, L.L.; Sobolevsky, A.I. Structure and gating mechanism of the transient receptor potential channel TRPV3. Nat. Struct. Mol. Biol. 2018, 25, 805–813. [Google Scholar] [CrossRef]
- Shi, D.J.; Ye, S.; Cao, X.; Zhang, R.; Wang, K.W. Crystal structure of the N-terminal ankyrin repeat domain of TRPV3 reveals unique conformation of finger 3 loop critical for channel function. Protein Cell 2013, 4, 942–950. [Google Scholar] [CrossRef]
- Phelps, C.B.; Wang, R.R.; Choo, S.S.; Gaudet, R. Differential regulation of TRPV1, TRPV3, and TRPV4 sensitivity through a conserved binding site on the ankyrin repeat domain. J. Biol. Chem. 2010, 285, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; McGoldrick, L.L.; Demirkhanyan, L.; Leslie, M.; Zakharian, E.; Sobolevsky, A.I. Structural basis of temperature sensation by the TRP channel TRPV3. Nat. Struct. Mol. Biol. 2019, 26, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Yelshanskaya, M.V.; Sobolevsky, A.I. Ligand-Binding Sites in Vanilloid-Subtype TRP Channels. Front. Pharmacol. 2022, 13, 900623. [Google Scholar] [CrossRef]
- Hellwig, N.; Albrecht, N.; Harteneck, C.; Schultz, G.; Schaefer, M. Homo- and heteromeric assembly of TRPV channel subunits. J. Cell Sci. 2005, 118, 917–928. [Google Scholar] [CrossRef]
- Cheng, W.; Yang, F.; Takanishi, C.L.; Zheng, J. Thermosensitive TRPV channel subunits coassemble into heteromeric channels with intermediate conductance and gating properties. J. Gen. Physiol. 2007, 129, 191–207. [Google Scholar] [CrossRef]
- Li, M.; Jiang, J.; Yue, L. Functional characterization of homo- and heteromeric channel kinases TRPM6 and TRPM7. J. Gen. Physiol. 2006, 127, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Strübing, C.; Krapivinsky, G.; Krapivinsky, L.; Clapham, D.E. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron 2001, 29, 645–655. [Google Scholar] [CrossRef]
- Hofmann, T.; Schaefer, M.; Schultz, G.; Gudermann, T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc. Natl. Acad. Sci. USA 2002, 99, 7461–7466. [Google Scholar] [CrossRef]
- Poteser, M.; Graziani, A.; Rosker, C.; Eder, P.; Derler, I.; Kahr, H.; Zhu, M.X.; Romanin, C.; Groschner, K. TRPC3 and TRPC4 associate to form a redox-sensitive cation channel. Evidence for expression of native TRPC3-TRPC4 heteromeric channels in endothelial cells. J. Biol. Chem. 2006, 281, 13588–13595. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, M. Homo- and heteromeric assembly of TRP channel subunits. Pflug. Arch. 2005, 451, 35–42. [Google Scholar] [CrossRef]
- Liebe, F.; Liebe, H.; Kaessmeyer, S.; Sponder, G.; Stumpff, F. The TRPV3 channel of the bovine rumen: Localization and functional characterization of a protein relevant for ruminal ammonia transport. Pflug. Arch. Eur. J. Physiol. 2020, 472, 693–710. [Google Scholar] [CrossRef] [PubMed]
- Liebe, F.; Liebe, H.; Sponder, G.; Mergler, S.; Stumpff, F. Effects of butyrate− on ruminal Ca2+ transport: Evidence for the involvement of apically expressed TRPV3 and TRPV4 channels. Pflug. Arch. Eur. J. Physiol. 2022, 474, 315–342. [Google Scholar] [CrossRef] [PubMed]
- Schrapers, K.T.; Sponder, G.; Liebe, F.; Liebe, H.; Stumpff, F. The bovine TRPV3 as a pathway for the uptake of Na+, Ca2+, and NH4+. PLoS ONE 2018, 13, e0193519. [Google Scholar] [CrossRef] [PubMed]
- Rosendahl, J.; Braun, H.S.; Schrapers, K.T.; Martens, H.; Stumpff, F. Evidence for the functional involvement of members of the TRP channel family in the uptake of Na+ and NH4+ by the ruminal epithelium. Pflug. Arch. Eur. J. Physiol. 2016, 468, 1333–1352. [Google Scholar] [CrossRef] [PubMed]
- Liebe, H.; Liebe, F.; Sponder, G.; Hedtrich, S.; Stumpff, F. Beyond Ca2+ signalling: The role of TRPV3 in the transport of NH4+. Pflug. Arch. Eur. J. Physiol. 2021, 473, 1859–1884. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Yang, F.; Liu, S.; Colton, C.K.; Wang, C.; Cui, Y.; Cao, X.; Zhu, M.X.; Sun, C.; Wang, K.W.; et al. Heteromeric heat-sensitive transient receptor potential channels exhibit distinct temperature and chemical response. J. Biol. Chem. 2012, 287, 7279–7288. [Google Scholar] [CrossRef]
- Chung, M.K.; Lee, H.; Mizuno, A.; Suzuki, M.; Caterina, M.J. 2-Aminoethoxydiphenyl borate activates and sensitizes the heat-gated ion channel TRPV3. J. Neurosci. 2004, 24, 5177–5182. [Google Scholar] [CrossRef]
- Xu, H.; Delling, M.; Jun, J.C.; Clapham, D.E. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat. Neurosci. 2006, 9, 628–635. [Google Scholar] [CrossRef]
- Xiao, R.; Tang, J.; Wang, C.; Colton, C.K.; Tian, J.; Zhu, M.X. Calcium plays a central role in the sensitization of TRPV3 channel to repetitive stimulations. J. Biol. Chem. 2008, 283, 6162–6174. [Google Scholar] [CrossRef]
- Liu, B.; Yao, J.; Zhu, M.X.; Qin, F. Hysteresis of gating underlines sensitization of TRPV3 channels. J. Gen. Physiol. 2011, 138, 509–520. [Google Scholar] [CrossRef]
- Nadezhdin, K.D.; Neuberger, A.; Trofimov, Y.A.; Krylov, N.A.; Sinica, V.; Kupko, N.; Vlachova, V.; Zakharian, E.; Efremov, R.G.; Sobolevsky, A.I. Structural mechanism of heat-induced opening of a temperature-sensitive TRP channel. Nat. Struct. Mol. Biol. 2021, 28, 564–572. [Google Scholar] [CrossRef]
- Vogt-Eisele, A.K.; Weber, K.; Sherkheli, M.A.; Vielhaber, G.; Panten, J.; Gisselmann, G.; Hatt, H. Monoterpenoid agonists of TRPV3. Br. J. Pharmacol. 2007, 151, 530–540. [Google Scholar] [CrossRef]
- Bang, S.; Yoo, S.; Yang, T.J.; Cho, H.; Hwang, S.W. Farnesyl pyrophosphate is a novel pain-producing molecule via specific activation of TRPV3. J. Biol. Chem. 2010, 285, 19362–19371. [Google Scholar] [CrossRef]
- Bang, S.; Yoo, S.; Yang, T.J.; Cho, H.; Hwang, S.W. Isopentenyl pyrophosphate is a novel antinociceptive substance that inhibits TRPV3 and TRPA1 ion channels. Pain 2011, 152, 1156–1164. [Google Scholar] [CrossRef]
- Moussaieff, A.; Rimmerman, N.; Bregman, T.; Straiker, A.; Felder, C.C.; Shoham, S.; Kashman, Y.; Huang, S.M.; Lee, H.; Shohami, E.; et al. Incensole acetate, an incense component, elicits psychoactivity by activating TRPV3 channels in the brain. FASEB J. 2008, 22, 3024–3034. [Google Scholar] [CrossRef] [PubMed]
- Pollastro, F.; Golin, S.; Chianese, G.; Putra, M.Y.; Schiano Moriello, A.; De Petrocellis, L.; García, V.; Munoz, E.; Taglialatela-Scafati, O.; Appendino, G. Neuroactive and Anti-inflammatory Frankincense Cembranes: A Structure-Activity Study. J. Nat. Prod. 2016, 79, 1762–1768. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wang, D.; Chai, H.; Xu, J.; Li, T.; Niu, Y.; Chen, X.; Qiu, F.; Li, Y.; Li, H.; et al. Unusual ent-Labdane Diterpenoid Dimers and their Selective Activation of TRPV Channels. J. Org. Chem. 2019, 84, 13595–13603. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid ligands targeting TRP channels. Front. Mol. Neurosci. 2019, 11, 487. [Google Scholar] [CrossRef] [PubMed]
- de Petrocellis, L.; Orlando, P.; Moriello, A.S.; Aviello, G.; Stott, C.; Izzo, A.A.; di Marzo, V. Cannabinoid actions at TRPV channels: Effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta Physiol. 2012, 204, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Güler, A.D.; Caterina, M.J. Biphasic Currents Evoked by Chemical or Thermal Activation of the Heat-Gated Ion Channel, TRPV3. J. Biol. Chem. 2005, 280, 15928–15941. [Google Scholar] [CrossRef]
- Deering-Rice, C.E.; Mitchell, V.K.; Romero, E.G.; Abdel Aziz, M.H.; Ryskamp, D.A.; Križaj, D.; Venkat, R.G.; Reilly, C.A. Drofenine: A 2-APB analog with improved selectivity for human TRPV3. Pharmacol. Res. Perspect. 2014, 2, e00062. [Google Scholar] [CrossRef]
- Maier, M.; Olthoff, S.; Hill, K.; Zosel, C.; Magauer, T.; Wein, L.A.; Schaefer, M. KS0365, a novel activator of the transient receptor potential vanilloid 3 (TRPV3) channel, accelerates keratinocyte migration. Br. J. Pharmacol. 2022, 179, 5290–5304. [Google Scholar] [CrossRef]
- Stotz, S.C.; Vriens, J.; Martyn, D.; Clardy, J.; Clapham, D.E. Citral Sensing by TRANSient Receptor Potential Channels in Dorsal Root Ganglion Neurons. PLoS ONE 2008, 3, e2082. [Google Scholar] [CrossRef]
- Macpherson, L.J.; Hwang, S.W.; Miyamoto, T.; Dubin, A.E.; Patapoutian, A.; Story, G.M. More than cool: Promiscuous relationships of menthol and other sensory compounds. Mol. Cell. Neurosci. 2006, 32, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Prakriya, M.; Lewis, R.S. Store-Operated Calcium Channels. Physiol. Rev. 2015, 95, 1383. [Google Scholar] [CrossRef]
- Bang, S.; Yoo, S.; Yang, T.J.; Cho, H.; Hwang, S. 17(R)-resolvin D1 specifically inhibits transient receptor potential ion channel vanilloid 3 leading to peripheral antinociception. Br. J. Pharmacol. 2012, 165, 683–692. [Google Scholar] [CrossRef]
- Sun, X.Y.; Sun, L.L.; Qi, H.; Gao, Q.; Wang, G.X.; Wei, N.N.; Wang, K.W. Antipruritic effect of natural coumarin osthole through selective inhibition of thermosensitive TRPV3 channel in the skin. Mol. Pharmacol. 2018, 94, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Georgiev, M.I.; Cao, H.; Nahar, L.; El-Seedi, H.R.; Sarker, S.D.; Xiao, J.; Lu, B. Therapeutic potential of phenylethanoid glycosides: A systematic review. Med. Res. Rev. 2020, 40, 2605–2649. [Google Scholar] [CrossRef]
- Han, Y.; Luo, A.; Kamau, P.M.; Takomthong, P.; Hu, J.; Boonyarat, C.; Luo, L.; Lai, R. A plant-derived TRPV3 inhibitor suppresses pain and itch. Br. J. Pharmacol. 2021, 178, 1669–1683. [Google Scholar] [CrossRef]
- Qi, H.; Shi, Y.; Wu, H.; Niu, C.; Sun, X.; Wang, K.W. Inhibition of temperature-sensitive TRPV3 channel by two natural isochlorogenic acid isomers for alleviation of dermatitis and chronic pruritus. Acta Pharm. Sin. B 2022, 12, 723–734. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, L.; Jiao, K.; Xue, C.; Tang, Q.; Jiang, S.; Ren, Y.; Chen, H.; El-Aziz, T.M.A.; Abdelazeem, K.N.M.; et al. Scutellarein attenuates atopic dermatitis by selectively inhibiting transient receptor potential vanilloid 3 channels. Br. J. Pharmacol. 2022, 179, 4792–4808. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.N.; Shi, H.; Yu, G.; Wang, C.M.; Zhu, C.; Yang, Y.; Yuan, X.L.; Tang, M.; Wang, Z.L.; Gegen, T.; et al. Osthole inhibits histamine-dependent itch via modulating TRPV1 activity. Sci. Rep. 2016, 6, 25657. [Google Scholar] [CrossRef]
- Torres, K.V.; Pantke, S.; Rudolf, D.; Eberhardt, M.M.; Leffler, A. The coumarin osthole is a non-electrophilic agonist of TRPA1. Neurosci. Lett. 2022, 789, 136878. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xu, L.N.; Liu, X.; He, C.Y.; Fang, R.Y.; Liu, J.; Hao, F.; Ma, T.H. Stimulation of airway and intestinal mucosal secretion by natural coumarin CFTR activators. Front. Pharmacol. 2011, 2, 52. [Google Scholar] [CrossRef] [PubMed]
- Leung, Y.M.; Kuo, Y.H.; Chao, C.C.; Tsou, Y.H.; Chou, C.H.; Lin, C.H.; Wong, K.L. Osthol is a use-dependent blocker of voltage-gated Na+ channels in mouse neuroblastoma N2A cells. Planta Med. 2010, 76, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Korolkova, Y.; Makarieva, T.; Tabakmakher, K.; Shubina, L.; Kudryashova, E.; Andreev, Y.; Mosharova, I.; Lee, H.S.; Lee, Y.J.; Kozlov, S. Marine cyclic guanidine alkaloids monanchomycalin B and urupocidin a act as inhibitors of TRPV1, TRPV2 and TRPV3, but not TRPA1 receptors. Mar. Drugs 2017, 15, 87. [Google Scholar] [CrossRef]
- Makarieva, T.N.; Ogurtsova, E.K.; Korolkova, Y.V.; Andreev, Y.A.; Mosharova, I.V.; Tabakmakher, K.M.; Guzii, A.G.; Denisenko, V.A.; Dmitrenok, P.S.; Lee, H.S.; et al. Pulchranins B and C, new acyclic guanidine alkaloids from the far-eastern marine sponge Monanchora pulchra. Nat. Prod. Commun. 2013, 8, 1229–1232. [Google Scholar] [CrossRef]
- Ogurtsova, E.K.; Makarieva, T.N.; Guzii, A.G.; Dmitrenok, P.S.; Denisenko, V.A.; Krasokhin, V.B.; Korolkova, Y.V.; Andreev, Y.A.; Mosharova, I.V.; Grishin, E.V. Inhibitory activity on trp receptors of pentacyclic alkaloids from the fungus Haliclona (Gellius) sp. Chem. Nat. Compd. 2015, 51, 404. [Google Scholar] [CrossRef]
- Ogurtsova, E.K.; Makarieva, T.N.; Korolkova, Y.V.; Andreev, Y.A.; Mosharova, I.V.; Denisenko, V.A.; Dmitrenok, P.S.; Lee, Y.J.; Grishin, E.V.; Stonik, V.A. New derivatives of natural acyclic guanidine alkaloids with TRPV receptor-regulating properties. Nat. Prod. Commun. 2015, 10, 1171–1173. [Google Scholar] [CrossRef]
- Kim, S.E.J.; Chung, E.D.S.; Vasileva, E.A.; Mishchenko, N.P.; Fedoreyev, S.A.; Stonik, V.A.; Kim, H.K.; Nam, J.H.; Kim, S.E.J. Multiple Effects of Echinochrome A on Selected Ion Channels Implicated in Skin Physiology. Mar. Drugs 2023, 21, 78. [Google Scholar] [CrossRef]
- Hu, H.Z.; Gu, Q.; Wang, C.; Colton, C.K.; Tang, J.; Kinoshita-Kawada, M.; Lee, L.Y.; Wood, J.D.; Zhu, M.X. 2-Aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J. Biol. Chem. 2004, 279, 35741–35748. [Google Scholar] [CrossRef]
- Neuberger, A.; Nadezhdin, K.D.; Zakharian, E.; Sobolevsky, A.I. Structural mechanism of TRPV3 channel inhibition by the plant-derived coumarin osthole. EMBO Rep. 2021, 22, e53233. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, X.; Qi, H.; Ma, Q.; Zhou, Q.; Wang, W.; Wang, K.W. Pharmacological inhibition of the temperature-sensitive and Ca2+-permeable transient receptor potential vanilloid TRPV3 channel by natural forsythoside B attenuates pruritus and cytotoxicity of keratinocytes. J. Pharmacol. Exp. Ther. 2019, 368, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Qi, H.; Wu, H.; Qu, Y.; Wang, K. Anti-pruritic and anti-inflammatory effects of natural verbascoside through selective inhibition of temperature-sensitive Ca2+-permeable TRPV3 channel. J. Dermatol. Sci. 2020, 97, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.W.; Wang, R.R.; Sun, X.Y.; Fan, J.H.; Qi, H.; Liu, Y.; Qin, G.Q.; Wang, W. Identification of Transient Receptor Potential Vanilloid 3 Antagonists from Achillea alpina L. And separation by liquid-liquid-refining extraction and high-speed counter-current chromatography. Molecules 2020, 25, 2025. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, J.; Wei, X.; Hu, J.; Ping, C.; Gao, Y.; Xie, C.; Wang, P.; Cao, P.; Cao, Z.; et al. Therapeutic inhibition of keratinocyte trpv3 sensory channel by local anesthetic dyclonine. eLife 2021, 10, e68128. [Google Scholar] [CrossRef]
- Horishita, R.; Ogata, Y.; Fukui, R.; Yamazaki, R.; Moriwaki, K.; Ueno, S.; Yanagihara, N.; Uezono, Y.; Yokoyama, Y.; Minami, K.; et al. Local Anesthetics Inhibit Transient Receptor Potential Vanilloid Subtype 3 Channel Function in Xenopus Oocytes. Anesth. Analg. 2021, 132, 1756–1767. [Google Scholar] [CrossRef]
- Lv, M.; Wu, H.; Qu, Y.; Wu, S.; Wang, J.; Wang, C.; Luan, Y.; Zhang, Z. The design and synthesis of transient receptor potential vanilloid 3 inhibitors with novel skeleton. Bioorg. Chem. 2021, 114, 105115. [Google Scholar] [CrossRef] [PubMed]
- Gomtsyan, A.; Schmidt, R.G.; Bayburt, E.K.; Gfesser, G.A.; Voight, E.A.; Daanen, J.F.; Schmidt, D.L.; Cowart, M.D.; Liu, H.; Altenbach, R.J.; et al. Synthesis and Pharmacology of (Pyridin-2-yl)methanol Derivatives as Novel and Selective Transient Receptor Potential Vanilloid 3 Antagonists. J. Med. Chem. 2016, 59, 4926–4947. [Google Scholar] [CrossRef]
- Bischof, M.; Olthoff, S.; Glas, C.; Thorn-Seshold, O.; Schaefer, M.; Hill, K. TRPV3 endogenously expressed in murine colonic epithelial cells is inhibited by the novel TRPV3 blocker 26E01. Cell Calcium 2020, 92, 102310. [Google Scholar] [CrossRef]
- Zhang, F.; Lin, Y.; Min, W.; Hou, Y.; Yuan, K.; Wang, J.; Yang, P. Computational discovery, structural optimization and biological evaluation of novel inhibitors targeting transient receptor potential vanilloid type 3 (TRPV3). Bioorg. Chem. 2021, 114, 105093. [Google Scholar] [CrossRef]
- Fan, J.; Hu, L.; Yue, Z.; Liao, D.; Guo, F.; Ke, H.; Jiang, D.; Yang, Y.; Lei, X. Structural basis of TRPV3 inhibition by an antagonist. Nat. Chem. Biol. 2023, 19, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Zubcevic, L.; Borschel, W.F.; Hsu, A.L.; Borgnia, M.J.; Lee, S.Y. Regulatory switch at the cytoplasmic interface controls TRPV channel gating. eLife 2019, 8, e47746. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Sun, X.; Hu, F.; Tang, X.; Wang, K.W. Molecular determinants for the chemical activation of the warmth-sensitive TRPV3 channel by the natural monoterpenoid carvacrol. J. Biol. Chem. 2022, 298, 101706. [Google Scholar] [CrossRef]
- Neuberger, A.; Nadezhdin, K.D.; Sobolevsky, A.I. Structural mechanism of TRPV3 channel inhibition by the anesthetic dyclonine. Nat. Commun. 2022, 13, 2795. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Chen, X.; Zhao, Q.; Stanic, V.; Lin, Z.; Yang, S.; Chen, T.; Chen, J.; Yang, Y. Hair Loss Caused by Gain-of-Function Mutant TRPV3 Is Associated with Premature Differentiation of Follicular Keratinocytes. J. Investig. Dermatol. 2021, 141, 1964–1974. [Google Scholar] [CrossRef] [PubMed]
- Asakawa, M.; Yoshioka, T.; Matsutani, T.; Hikita, I.; Suzuki, M.; Oshima, I.; Tsukahara, K.; Arimura, A.; Horikawa, T.; Hirasawa, T.; et al. Association of a mutation in TRPV3 with defective hair growth in rodents. J. Investig. Dermatol. 2006, 126, 2664–2672. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Sun, X.; Wang, G.; Liu, Y.; Wang, K.W. Pharmacological activation of thermo–transient receptor potential vanilloid 3 channels inhibits hair growth by inducing cell death of hair follicle outer root sheath. J. Pharmacol. Exp. Ther. 2019, 370, 299–307. [Google Scholar] [CrossRef]
- Borbíró, I.; Lisztes, E.; Tóth, B.I.; Czifra, G.; Oláh, A.; Szöllsi, A.G.; Szentandrássy, N.; Nánási, P.P.; Péter, Z.; Paus, R.; et al. Activation of transient receptor potential vanilloid-3 inhibits human hair growth. J. Investig. Dermatol. 2011, 131, 1605–1614. [Google Scholar] [CrossRef]
- Luo, J.; Stewart, R.; Berdeaux, R.; Hu, H. Tonic inhibition of TRPV3 by Mg2+ in mouse epidermal keratinocytes. J. Investig. Dermatol. 2012, 132, 2158–2165. [Google Scholar] [CrossRef]
- Facer, P.; Casula, M.A.; Smith, G.D.; Benham, C.D.; Chessell, I.P.; Bountra, C.; Sinisi, M.; Birch, R.; Anand, P. Differential expression of the capsaicin receptor TRPV1 and related novel receptors TRPV3, TRPV4 and TRPM8 in normal human tissues and changes in traumatic and diabetic neuropathy. BMC Neurol. 2007, 7, 11. [Google Scholar] [CrossRef]
- Hamamoto, T.; Takumida, M.; Hirakawa, K.; Takeno, S.; Tatsukawa, T. Localization of transient receptor potential channel vanilloid subfamilies in the mouse larynx. Acta Otolaryngol. 2008, 128, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto, T.; Takumida, M.; Hirakawa, K.; Tatsukawa, T.; Ishibashi, T. Localization of transient receptor potential vanilloid (TRPV) in the human larynx. Acta Otolaryngol. 2009, 129, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Sobhan, U.; Sato, M.; Shinomiya, T.; Okubo, M.; Tsumura, M.; Muramatsu, T.; Kawaguchi, M.; Tazaki, M.; Shibukawa, Y. Immunolocalization and distribution of functional temperature-sensitive TRP channels in salivary glands. Cell Tissue Res. 2013, 354, 507–519. [Google Scholar] [CrossRef]
- Moayedi, Y.; Michlig, S.; Park, M.; Koch, A.; Lumpkin, E.A. Localization of TRP Channels in Healthy Oral Mucosa from Human Donors. eNeuro 2022, 9, ENEURO.0328-21.2022. [Google Scholar] [CrossRef] [PubMed]
- Starobova, H.; Alshammari, A.; Winkler, I.G.; Vetter, I. The Role of the Neuronal Microenvironment in Sensory Function and Pain Pathophysiology. J. Neurochem. 2022, 1–24. [Google Scholar] [CrossRef]
- Chung, M.-K.; Lee, H.; Mizuno, A.; Suzuki, M.; Caterina, M.J. TRPV3 and TRPV4 mediate warmth-evoked currents in primary mouse keratinocytes. J. Biol. Chem. 2004, 279, 21569–21575. [Google Scholar] [CrossRef]
- Moqrich, A.; Hwang, S.W.; Earley, T.J.; Petrus, M.J.; Murray, A.N.; Spencer, K.S.R.R.; Andahazy, M.; Story, G.M.; Patapoutian, A. Impaired thermosensation in mice lacking TRPV3, a heat and camphor sensor in the skin. Science 2005, 307, 1468–1472. [Google Scholar] [CrossRef]
- Mandadi, S.; Sokabe, T.; Shibasaki, K.; Katanosaka, K.; Mizuno, A.; Moqrich, A.; Patapoutian, A.; Fukumi-Tominaga, T.; Mizumura, K.; Tominaga, M. TRPV3 in keratinocytes transmits temperature information to sensory neurons via ATP. Pflug. Arch. Eur. J. Physiol. 2009, 458, 1093–1102. [Google Scholar] [CrossRef]
- Gifford, J.R.; Heal, C.; Bridges, J.; Goldthorpe, S.; Mack, G.W. Changes in dermal interstitial ATP levels during local heating of human skin. J. Physiol. 2012, 590, 6403–6411. [Google Scholar] [CrossRef]
- Huang, S.M.; Li, X.; Yu, Y.; Wang, J.; Caterina, M.J. TRPV3 and TRPV4 ion channels are not major contributors to mouse heat sensation. Mol. Pain 2011, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Marics, I.; Malapert, P.; Reynders, A.; Gaillard, S.; Moqrich, A. Acute heat-evoked temperature sensation is impaired but not abolished in mice lacking TRPV1 and TRPV3 channels. PLoS ONE 2014, 9, e99828. [Google Scholar] [CrossRef] [PubMed]
- Karashima, Y.; Damann, N.; Prenen, J.; Talavera, K.; Segal, A.; Voets, T.; Nilius, B. Bimodal Action of Menthol on the Transient Receptor Potential Channel TRPA1. J. Neurosci. 2007, 27, 9874–9884. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Kim, S.E.; Lee, S.E.; Kim, S.C. Olmsted syndrome caused by a heterozygous p.Gly568val missense mutation in TRPV3 gene. Yonsei Med. J. 2018, 59, 341–344. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Chen, Q.; Lee, M.; Cao, X.; Zhang, J.; Ma, D.; Chen, L.; Hu, X.; Wang, H.; Wang, X.; et al. Exome sequencing reveals mutations in TRPV3 as a cause of Olmsted syndrome. Am. J. Hum. Genet. 2012, 90, 558–564. [Google Scholar] [CrossRef]
- Mecklenburg, L.; Nakamura, M.; Paus, R.; Mecklenburg, L.; Sundberg, J.P. The nude mouse skin phenotype: The role of Foxn1 in hair follicle development and cycling. Exp. Mol. Pathol. 2001, 71, 171–178. [Google Scholar] [CrossRef]
- Kaufman, C.K.; Zhou, P.; Pasolli, H.A.; Rendl, M.; Bolotin, D.; Lim, K.C.; Dai, X.; Alegre, M.L.; Fuchs, E. GATA-3: An unexpected regulator of cell lineage determination in skin. Genes Dev. 2003, 17, 2108–2122. [Google Scholar] [CrossRef]
- Imura, K.; Yoshioka, T.; Hikita, I.; Tsukahara, K.; Hirasawa, T.; Higashino, K.; Gahara, Y.; Arimura, A.; Sakata, T. Influence of TRPV3 mutation on hair growth cycle in mice. Biochem. Biophys. Res. Commun. 2007, 363, 479–483. [Google Scholar] [CrossRef]
- Schneider, M.R.; Werner, S.; Paus, R.; Wolf, E. Beyond wavy hairs: The epidermal growth factor receptor and its ligands in skin biology and pathology. Am. J. Pathol. 2008, 173, 14–24. [Google Scholar] [CrossRef]
- Luo, J.; Hu, H. Thermally Activated TRPV3 Channels. In Current Topics in Membranes; Academic Press Inc.: Cambridge, MA, USA, 2014; Volume 74, pp. 325–364. [Google Scholar]
- Kumar, S.; Singh, O.; Singh, U.; Goswami, C.; Singru, P.S. Transient receptor potential vanilloid 1-6 (Trpv1-6) gene expression in the mouse brain during estrous cycle. Brain Res. 2018, 1701, 161–170. [Google Scholar] [CrossRef]
- Singh, U.; Upadhya, M.; Basu, S.; Singh, O.; Kumar, S.; Kokare, D.M.; Singru, P.S. Transient Receptor Potential Vanilloid 3 (TRPV3) in the Cerebellum of Rat and Its Role in Motor Coordination. Neuroscience 2020, 424, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Voronova, I.P.; Tuzhikova, A.A.; Kozyreva, T.V. Thermosensitive TRP channels gene expression in hypothalamus of normal rats and rats adapted to cold. Ross. Fiziol. Zh. Im. I. M. Sechenova 2012, 98, 1101–1110. [Google Scholar] [PubMed]
- Brown, T.E.; Chirila, A.M.; Schrank, B.R.; Kauer, J.A. Loss of Interneuron LTD and Attenuated Pyramidal cell LTP in Trpv1 and Trpv3 KO mice. Hippocampus 2013, 23, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Moussaieff, A.; Yu, J.; Zhu, H.; Gattoni-Celli, S.; Shohami, E.; Kindy, M.S. Protective effects of incensole acetate on cerebral ischemic injury. Brain Res. 2012, 1443, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, J.; Wang, K.W. Inhibition of intracellular proton-sensitive Ca2+-permeable TRPV3 channels protects against ischemic brain injury. Acta Pharm. Sin. B 2022, 12, 2330–2347. [Google Scholar] [CrossRef]
- Wu, S.W.; Lindberg, J.E.M.; Peters, J.H. Genetic and pharmacological evidence for low-abundance TRPV3 expression in primary vagal afferent neurons. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2016, 310, R794–R805. [Google Scholar] [CrossRef]
- Ślęczkowska, M.; Almomani, R.; Marchi, M.; Salvi, E.; de Greef, B.T.A.; Sopacua, M.; Hoeijmakers, J.G.J.; Lindsey, P.; Waxman, S.G.; Lauria, G.; et al. Peripheral Ion Channel Genes Screening in Painful Small Fiber Neuropathy. Int. J. Mol. Sci. 2022, 23, 14095. [Google Scholar] [CrossRef]
- Carreño, O.; Corominas, R.; Fernández-Morales, J.; Camiña, M.; Sobrido, M.J.; Fernández-Fernández, J.M.; Pozo-Rosich, P.; Cormand, B.; Macaya, A. SNP variants within the vanilloid TRPV1 and TRPV3 receptor genes are associated with migraine in the Spanish population. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2012, 159B, 94–103. [Google Scholar] [CrossRef]
- Duchatelet, S.; Pruvost, S.; De Veer, S.; Fraitag, S.; Nitschké, P.; Bole-Feysot, C.; Bodemer, C.; Hovnanian, A. A new TRPV3 missense mutation in a patient with Olmsted syndrome and erythromelalgia. JAMA Dermatol. 2014, 150, 303–306. [Google Scholar] [CrossRef]
- Fatima, M.; Slade, H.; Horwitz, L.; Shi, A.; Liu, J.; McKinstry, D.; Villani, T.; Xu, H.; Duan, B. Abnormal Somatosensory Behaviors Associated with a Gain-of-Function Mutation in TRPV3 Channels. Front. Mol. Neurosci. 2022, 14, 790435. [Google Scholar] [CrossRef]
- McGaraughty, S.; Chu, K.L.; Xu, J.; Leys, L.; Radek, R.J.; Dart, M.J.; Gomtsyan, A.; Schmidt, R.G.; Kym, P.R.; Brederson, J.D. TRPV3 modulates nociceptive signaling through peripheral and supraspinal sites in rats. J. Neurophysiol. 2017, 118, 904–916. [Google Scholar] [CrossRef]
- Yamamoto-Kasai, E.; Imura, K.; Yasui, K.; Shichijou, M.; Oshima, I.; Hirasawa, T.; Sakata, T.; Yoshioka, T. TRPV3 as a therapeutic target for itch. J. Investig. Dermatol. 2012, 132, 2109–2112. [Google Scholar] [CrossRef]
- Nilius, B.; Bíró, T. TRPV3: A “more than skinny” channel. Exp. Dermatol. 2013, 22, 447–452. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, G.; Sun, X.; Wang, K.W. Inhibition of the warm temperature–activated Ca2+-permeable transient receptor potential vanilloid TRPV3 channel attenuates atopic dermatitis. Mol. Pharmacol. 2019, 96, 393–400. [Google Scholar] [CrossRef]
- Zhao, J.; Munanairi, A.; Liu, X.Y.; Zhang, J.; Hu, L.; Hu, M.; Bu, D.; Liu, L.; Xie, Z.; Kim, B.S.; et al. PAR2 Mediates Itch via TRPV3 Signaling in Keratinocytes. J. Investig. Dermatol. 2020, 140, 1524–1532. [Google Scholar] [CrossRef]
- Huang, S.M.; Lee, H.; Chung, M.K.; Park, U.; Yin, Y.Y.; Bradshaw, H.B.; Coulombe, P.A.; Walker, J.M.; Caterina, M.J. Overexpressed transient receptor potential vanilloid 3 ion channels in skin keratinocytes modulate pain sensitivity via prostaglandin E2. J. Neurosci. 2008, 28, 13727–13737. [Google Scholar] [CrossRef]
- Mandadi, S.; Tominaga, T.; Numazaki, M.; Murayama, N.; Saito, N.; Armati, P.J.; Roufogalis, B.D.; Tominaga, M. Increased sensitivity of desensitized TRPV1 by PMA occurs through PKCε-mediated phosphorylation at S800. Pain 2006, 123, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Logashina, Y.A.; Korolkova, Y.V.; Kozlov, S.A.; Andreev, Y.A. TRPA1 Channel as a Regulator of Neurogenic Inflammation and Pain: Structure, Function, Role in Pathophysiology, and Therapeutic Potential of Ligands. Biochemistry 2019, 84, 101–118. [Google Scholar] [CrossRef]
- Gladkikh, I.N.; Sintsova, O.V.; Leychenko, E.V.; Kozlov, S.A. TRPV1 Ion Channel: Structural Features, Activity Modulators, and Therapeutic Potential. Biochemistry 2021, 86, S50–S70. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Dong, X. Trp channels and itch. Semin. Immunopathol. 2016, 38, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Um, J.Y.; Kim, H.B.; Kim, J.C.; Park, J.S.; Lee, S.Y.; Chung, B.Y.; Park, C.W.; Kim, H.O. TRPV3 and Itch: The Role of TRPV3 in Chronic Pruritus according to Clinical and Experimental Evidence. Int. J. Mol. Sci. 2022, 23, 14962. [Google Scholar] [CrossRef]
- Yoshioka, T.; Imura, K.; Asakawa, M.; Suzuki, M.; Oshima, I.; Hirasawa, T.; Sakata, T.; Horikawa, T.; Arimura, A. Impact of the Gly573Ser substitution in TRPV3 on the development of allergic and pruritic dermatitis in mice. J. Investig. Dermatol. 2009, 129, 714–722. [Google Scholar] [CrossRef]
- Kim, H.O.; Cho, Y.S.; Park, S.Y.; Kwak, I.S.; Choi, M.G.; Chung, B.Y.; Park, C.W.; Lee, J.Y. Increased activity of TRPV3 in keratinocytes in hypertrophic burn scars with postburn pruritus. Wound Repair Regen. 2016, 24, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, H.; Xue, C.; Chen, H.; Xue, Y.; Zhao, F.; Zhu, M.X.; Cao, Z. TRPV3 enhances skin keratinocyte proliferation through EGFR-dependent signaling pathways. Cell Biol. Toxicol. 2021, 37, 313–330. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.T.; Wang, G.X.; Wei, N.N.; Wang, K.W. A pivotal role for the activation of TRPV3 channel in itch sensations induced by the natural skin sensitizer carvacrol. Acta Pharmacol. Sin. 2018, 39, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Caengprasath, N.; Theerapanon, T.; Porntaveetus, T.; Shotelersuk, V. MBTPS2, a membrane bound protease, underlying several distinct skin and bone disorders. J. Transl. Med. 2021, 19, 114. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, A.; Scott, C.A.; Poon, D.S.; Yaghoobi, R.; Saleh-Gohari, N.; Plagnol, V.; Kelsell, D.P. A missense mutation in the MBTPS2 gene underlies the X-linked form of Olmsted syndrome. J. Investig. Dermatol. 2013, 133, 571–573. [Google Scholar] [CrossRef]
- Duchatelet, S.; Boyden, L.M.; Ishida-Yamamoto, A.; Zhou, J.; Guibbal, L.; Hu, R.; Lim, Y.H.; Bole-Feysot, C.; Nitschké, P.; Santos-Simarro, F.; et al. Mutations in PERP Cause Dominant and Recessive Keratoderma. J. Investig. Dermatol. 2019, 139, 380–390. [Google Scholar] [CrossRef]
- Dai, S.; Sun, Z.; Lee, M.; Wang, H.; Yang, Y.; Lin, Z. Olmsted syndrome with alopecia universalis caused by heterozygous mutation in PERP. Br. J. Dermatol. 2020, 182, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Sahu, R.P.; Goswami, C. Olmsted syndrome causing point mutants of TRPV3 (G568C and G568D) show defects in intracellular Ca2+-mobilization and induce lysosomal defects. Biochem. Biophys. Res. Commun. 2022, 628, 32–39. [Google Scholar] [CrossRef]
- Zhong, W.; Hu, L.; Cao, X.; Zhao, J.; Zhang, X.; Lee, M.; Wang, H.; Zhang, J.; Chen, Q.; Feng, C.; et al. Genotype–Phenotype Correlation of TRPV3-Related Olmsted Syndrome. J. Investig. Dermatol. 2021, 141, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Duchatelet, S.; Hovnanian, A. Olmsted syndrome: Clinical, molecular and therapeutic aspects. Orphanet J. Rare Dis. 2015, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Goswami, C. TRPV3 mutants causing Olmsted Syndrome induce impaired cell adhesion and nonfunctional lysosomes. Channels 2017, 11, 196–208. [Google Scholar] [CrossRef]
- Greco, C.; Leclerc-Mercier, S.; Chaumon, S.; Doz, F.; Hadj-Rabia, S.; Molina, T.; Boucheix, C.; Bodemer, C. Use of Epidermal Growth Factor Receptor Inhibitor Erlotinib to Treat Palmoplantar Keratoderma in Patients with Olmsted Syndrome Caused by TRPV3 Mutations. JAMA Dermatol. 2020, 156, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Song, Y.; Liu, W.; Wang, T.; Ma, X.; Yu, Z. Novel Insights into the Role of Keratinocytes-Expressed TRPV3 in the Skin. Biomolecules 2023, 13, 513. [Google Scholar] [CrossRef]
- Zhang, A.; Duchatelet, S.; Lakdawala, N.; Tower, R.L.; Diamond, C.; Marathe, K.; Hill, I.; Richard, G.; Diab, Y.; Kirkorian, A.Y.; et al. Targeted Inhibition of the Epidermal Growth Factor Receptor and Mammalian Target of Rapamycin Signaling Pathways in Olmsted Syndrome. JAMA Dermatol. 2020, 156, 196–200. [Google Scholar] [CrossRef]
- Aijima, R.; Wang, B.; Takao, T.; Mihara, H.; Kashio, M.; Ohsaki, Y.; Zhang, J.Q.; Mizuno, A.; Suzuki, M.; Yamashita, Y.; et al. The thermosensitive TRPV3 channel contributes to rapid wound healing in oral epithelia. FASEB J. 2015, 29, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.P.; Goswami, C. Presence of TRPV3 in macrophage lysosomes helps in skin wound healing against bacterial infection. Exp. Dermatol. 2023, 32, 60–74. [Google Scholar] [CrossRef]
- Romano, B.; Pagano, E.; Orlando, P.; Capasso, R.; Cascio, M.G.; Pertwee, R.; Di Marzo, V.; Izzo, A.A.; Borrelli, F. Pure Δ9-tetrahydrocannabivarin and a Cannabis sativa extract with high content in Δ9-tetrahydrocannabivarin inhibit nitrite production in murine peritoneal macrophages. Pharmacol. Res. 2016, 113, 199–208. [Google Scholar] [CrossRef]
- Miyamoto, T.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. TRPV3 regulates nitric oxide synthase-independent nitric oxide synthesis in the skin. Nat. Commun. 2011, 2, 369. [Google Scholar] [CrossRef]
- Reilly, R.M.; Kym, P.R. Analgesic Potential of TRPV3 Antagonists. Curr. Top. Med. Chem. 2011, 11, 2210–2215. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, A.P.; Belvisi, M.G.; Gaudet, R.; Szallasi, A. Advances in TRP channel drug discovery: From target validation to clinical studies. Nat. Rev. Drug Discov. 2022, 21, 41–59. [Google Scholar] [CrossRef] [PubMed]

| Experimental Model | Tested Compound | Effective Dose/Concentration, Route of Administration | Ref. |
|---|---|---|---|
| Formalin-induced pain | Citrusinine-II | 2.9, 14.3 mg/kg, i.p. * | [61] |
| Acetic acid-induced writhing | Citrusinine-II | 2.9, 14.3 mg/kg, i.p. | [61] |
| Hot plate test | Citrusinine-II | 2.9, 14.3 mg/kg, i.p. | [61] |
| Tail flick test | Citrusinine-II | 1.5, 2.9, 14.3 mg/kg, i.p. | [61] |
| Complete Freund’s adjuvant-induced inflammation, heat pain threshold | 17(R)-resolvin D1 | 30 µM in 20 µL, intraplantar | [58] |
| Isopentenyl pyrophosphate | 1 mM, i.d.** | [46] | |
| Complete Freund’s adjuvant-induced inflammation, FPP-induced pain | 17(R)-resolvin D1 | 30 µM in 20 µL, intraplantar | [58] |
| Carrageenan-induced inflammation, FPP-induced pain | Isopentenyl pyrophosphate | 1 mM, i.d. | [46] |
| Chronic constriction injury | 74a | 30, 100 mg/kg, p.o. *** | [81] |
| Sciatic nerve ligation | 74a | 30, 100 mg/kg, p.o. | [81] |
| Reserpine-induced central pain | 74a | 10, 30, 100 mg/kg, p.o. | [81] |
| Experimental Model | Tested Compound | Effective Dose/Concentration, Route of Administration | Ref. |
|---|---|---|---|
| Histamine-induced pruritus | Forsythoside B | 0.3, 3, 30 µM, i.d. * | [75] |
| Osthole | 300 nM, i.d. | [59] | |
| Citrusinine-II | 10 µM, i.d., t.d. ** | [61] | |
| Carvacrol-induced pruritus | Forsythoside B | 30, 300 µM, i.d. | [75] |
| Verbascoside | 300 µM, i.d. | [76] | |
| Isochlorogenic acid A | 1 mM, t.d. | [62] | |
| Isochlorogenic acid B | 1 mM, t.d. | [62] | |
| Scutellarein | 0.2, 0.5 mg/kg/day, s.c. *** | [63] | |
| Dyclonine | 10, 50 µM, i.d. | [78] | |
| Acetone-ether-water-induced pruritus | Forsythoside B | 3, 30 µM, i.d. | [75] |
| Osthole | 30, 300 nM, i.d. | [59] | |
| Citrusinine-II | 5, 10 µM, i.d. | [61] | |
| 2,4-dinitrofluorobenzene-induced dermatitis and pruritus | Scutellarein | 0.2, 0.5 mg/kg/day, s.c. | [63] |
| SLIGRL-induced pruritus | Trpvicin | 10, 100 µM, i.d. | [84] |
| Calcipotriol-induced pruritus | Trpvicin | 100 mg/kg/day, p.o. **** | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalinovskii, A.P.; Utkina, L.L.; Korolkova, Y.V.; Andreev, Y.A. TRPV3 Ion Channel: From Gene to Pharmacology. Int. J. Mol. Sci. 2023, 24, 8601. https://doi.org/10.3390/ijms24108601
Kalinovskii AP, Utkina LL, Korolkova YV, Andreev YA. TRPV3 Ion Channel: From Gene to Pharmacology. International Journal of Molecular Sciences. 2023; 24(10):8601. https://doi.org/10.3390/ijms24108601
Chicago/Turabian StyleKalinovskii, Aleksandr P., Lyubov L. Utkina, Yuliya V. Korolkova, and Yaroslav A. Andreev. 2023. "TRPV3 Ion Channel: From Gene to Pharmacology" International Journal of Molecular Sciences 24, no. 10: 8601. https://doi.org/10.3390/ijms24108601
APA StyleKalinovskii, A. P., Utkina, L. L., Korolkova, Y. V., & Andreev, Y. A. (2023). TRPV3 Ion Channel: From Gene to Pharmacology. International Journal of Molecular Sciences, 24(10), 8601. https://doi.org/10.3390/ijms24108601





