Study on the Effect of EZH2 Inhibitor Combined with TIGIT Monoclonal Antibody against Multiple Myeloma Cells
Abstract
:1. Introduction
2. Results
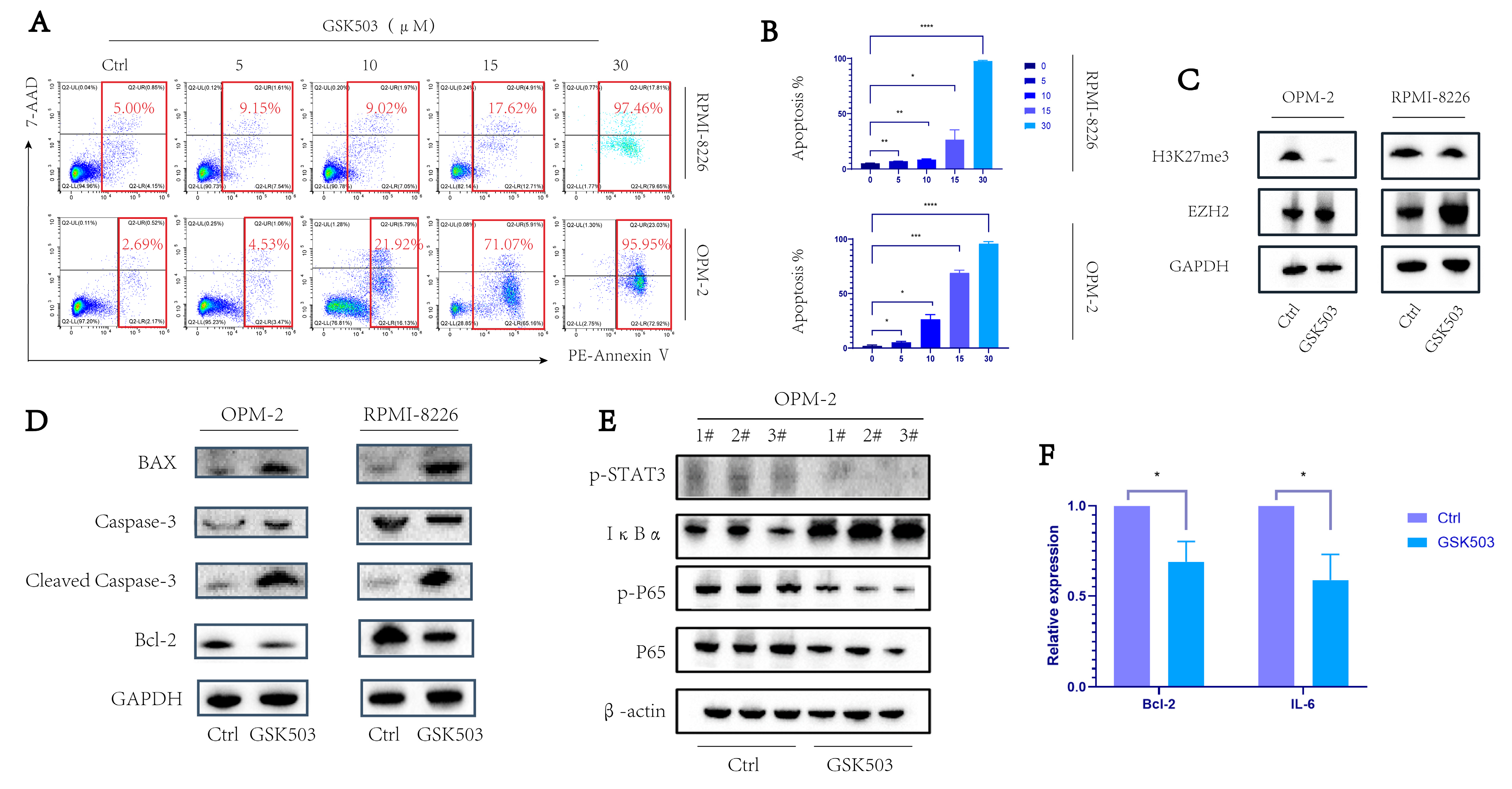
2.1. EZH2 Inhibitor GSK503 Enhanced the Apoptosis of MM Cell Line in a Concentration-Dependent Manner
2.2. GSK503 Inhibits the NF-κB Signaling Pathway
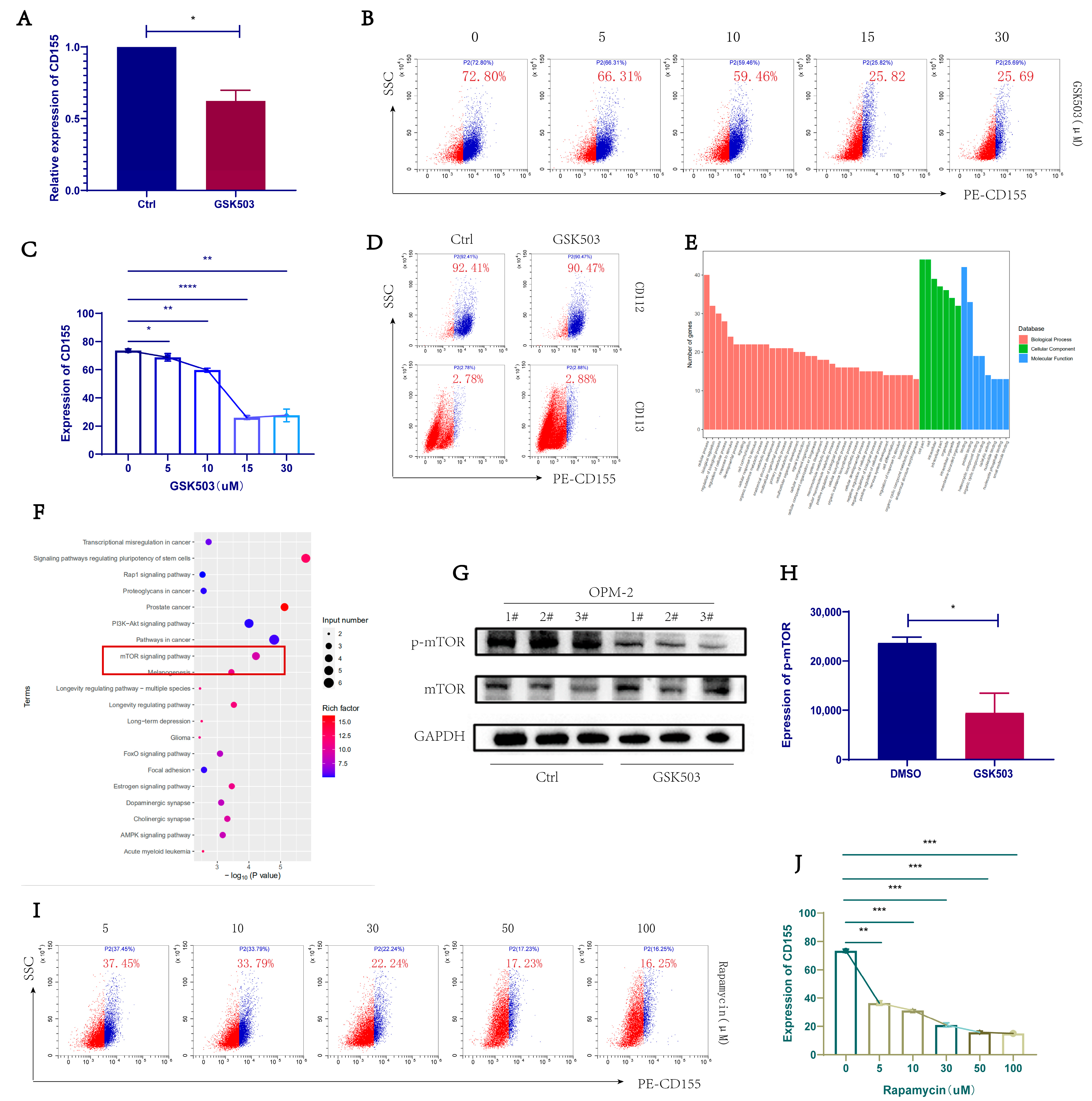
2.3. GSK503 Reduced the Expression of TIGIT Ligand CD155 on the Surface of MM Cell Line
2.4. EZH2 Inhibitor GSK503 Regulates MM Cell Surface CD155 Expression via the mTOR Signaling Pathway
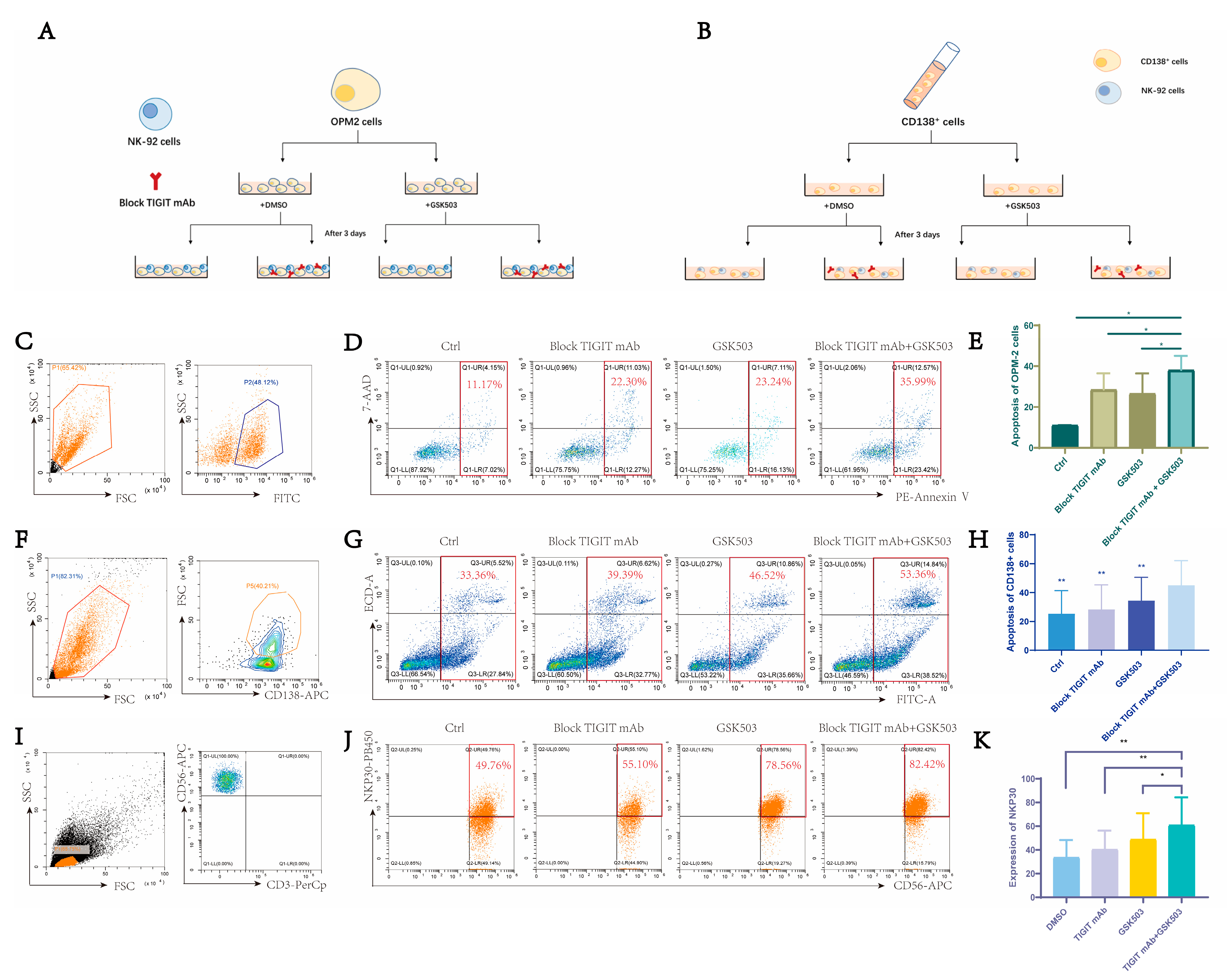
2.5. EZH2 Inhibitor GSK503 Enhances the Anti-Tumor Effect of TIGIT Monoclonal Antibody and NK Cell Function
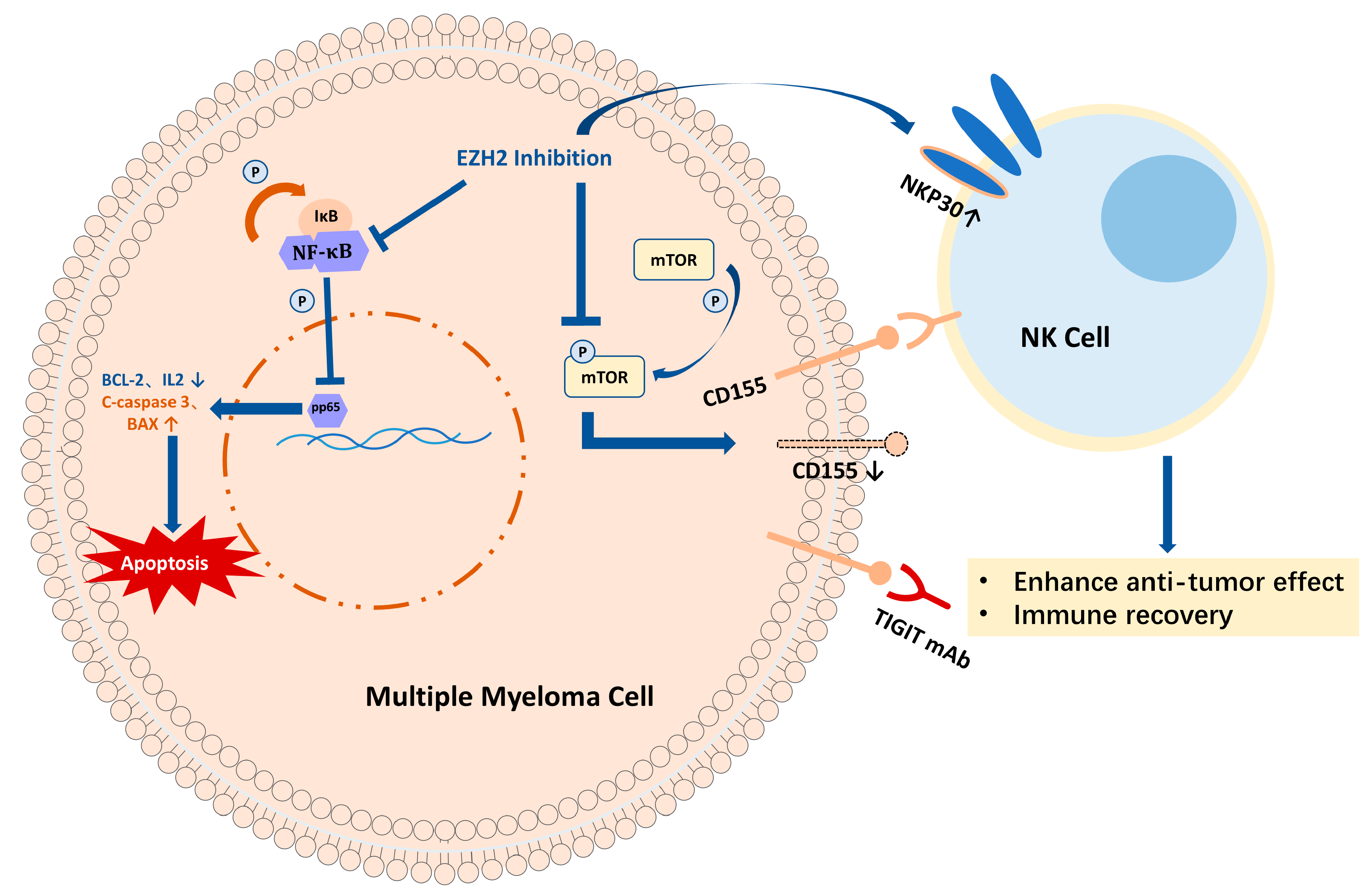
3. Discussion
4. Method
4.1. Patients
4.2. BMMNCs
4.3. Cells and Cell Culture
4.4. Western Blot
4.5. RNA Isolation and Real-Time Fluorescent Quantitative PCR (RT-PCR)
4.6. RNA Seq Analysis
4.7. Co-Culture MM Cells and NK-92 Cells
4.8. Annexin V/7-AAD Apoptosis Assay Kit to Detect Apoptosis
4.9. Flow Cytometry for NK-92 Cell Function
4.10. Statistics and Quantification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gan, L.; Yang, Y.; Li, Q.; Feng, Y.; Liu, T.; Guo, W. Epigenetic Regulation of Cancer Progression by EZH2: From Biological Insights to Therapeutic Potential. Biomark. Res. 2018, 6, 10. [Google Scholar] [CrossRef]
- Adamik, J.; Pulugulla, S.H.; Zhang, P.; Sun, Q.; Lontos, K.; Macar, D.A.; Auron, P.E.; Galson, D.L. EZH2 Supports Osteoclast Differentiation and Bone Resorption Via Epigenetic and Cytoplasmic Targets. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2020, 35, 181–195. [Google Scholar] [CrossRef]
- Nikoloski, G.; Langemeijer, S.M.C.; Kuiper, R.P.; Knops, R.; Massop, M.; Tönnissen, E.R.L.T.M.; van der Heijden, A.; Scheele, T.N.; Vandenberghe, P.; de Witte, T.; et al. Somatic Mutations of the Histone Methyltransferase Gene EZH2 in Myelodysplastic Syndromes. Nat. Genet. 2010, 42, 665–667. [Google Scholar] [CrossRef]
- Safaei, S.; Baradaran, B.; Hagh, M.F.; Alivand, M.R.; Talebi, M.; Gharibi, T.; Solali, S. Double Sword Role of EZH2 in Leukemia. Biomed. Pharmacother. Biomedecine Pharmacother. 2018, 98, 626–635. [Google Scholar] [CrossRef]
- Campbell, J.E.; Kuntz, K.W.; Knutson, S.K.; Warholic, N.M.; Keilhack, H.; Wigle, T.J.; Raimondi, A.; Klaus, C.R.; Rioux, N.; Yokoi, A.; et al. EPZ011989, A Potent, Orally-Available EZH2 Inhibitor with Robust in Vivo Activity. ACS Med. Chem. Lett. 2015, 6, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Derrien, J.; Guérin-Charbonnel, C.; Gaborit, V.; Campion, L.; Devic, M.; Douillard, E.; Roi, N.; Avet-Loiseau, H.; Decaux, O.; Facon, T.; et al. The DNA Methylation Landscape of Multiple Myeloma Shows Extensive Inter- and Intrapatient Heterogeneity That Fuels Transcriptomic Variability. Genome Med. 2021, 13, 127. [Google Scholar] [CrossRef]
- Zhan, F.; Hardin, J.; Kordsmeier, B.; Bumm, K.; Zheng, M.; Tian, E.; Sanderson, R.; Yang, Y.; Wilson, C.; Zangari, M.; et al. Global Gene Expression Profiling of Multiple Myeloma, Monoclonal Gammopathy of Undetermined Significance, and Normal Bone Marrow Plasma Cells. Blood 2002, 99, 1745–1757. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.A.; Boyle, E.M.; Wardell, C.P.; Murison, A.; Begum, D.B.; Dahir, N.M.; Proszek, P.Z.; Johnson, D.C.; Kaiser, M.F.; Melchor, L.; et al. Mutational Spectrum, Copy Number Changes, and Outcome: Results of a Sequencing Study of Patients With Newly Diagnosed Myeloma. J. Clin. Oncol. 2015, 33, 3911–3920. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-H.; Du, P.; Liu, W.; An, L.-K.; Li, J.; Zhu, W.-Y.; Yuan, S.; Wang, L.; Zang, L. LncRNA ANRIL Promotes Multiple Myeloma Progression and Bortezomib Resistance by EZH2-Mediated Epigenetically Silencing of PTEN. Neoplasma 2021, 68, 788–797. [Google Scholar] [CrossRef]
- Goldsmith, S.R.; Fiala, M.A.; O’Neal, J.; Souroullas, G.P.; Toama, W.; Vij, R.; Schroeder, M.A. EZH2 Overexpression in Multiple Myeloma: Prognostic Value, Correlation With Clinical Characteristics, and Possible Mechanisms. Clin. Lymphoma Myeloma Leuk. 2019, 19, 744–750. [Google Scholar] [CrossRef]
- Zeng, D.; Liu, M.; Pan, J. Blocking EZH2 Methylation Transferase Activity by GSK126 Decreases Stem Cell-like Myeloma Cells. Oncotarget 2017, 8, 3396–3411. [Google Scholar] [CrossRef]
- Rastgoo, N.; Pourabdollah, M.; Abdi, J.; Reece, D.; Chang, H. Dysregulation of EZH2/MiR-138 Axis Contributes to Drug Resistance in Multiple Myeloma by Downregulating RBPMS. Leukemia 2018, 32, 2471–2482. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Feng, J.; Liu, Y.; Liu, Q.; Fan, L.; Liu, Q.; She, X.; Liu, C.; Liu, T.; Zhao, C.; et al. Novel Therapy for Glioblastoma Multiforme by Restoring LRRC4 in Tumor Cells: LRRC4 Inhibits Tumor-Infitrating Regulatory T Cells by Cytokine and Programmed Cell Death 1-Containing Exosomes. Front. Immunol. 2017, 8, 1748. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Fujita, S.; Katsumoto, T.; Yamagata, K.; Ogawara, Y.; Hattori, A.; Kagiyama, Y.; Honma, D.; Araki, K.; Inoue, T.; et al. Dual Inhibition of Enhancer of Zeste Homolog 1/2 Overactivates WNT Signaling to Deplete Cancer Stem Cells in Multiple Myeloma. Cancer Sci. 2019, 110, 194–208. [Google Scholar] [CrossRef]
- Ansari-Pour, N.; Samur, M.; Flynt, E.; Gooding, S.; Towfic, F.; Stong, N.; Estevez, M.O.; Mavrommatis, K.; Walker, B.; Morgan, G.; et al. Whole-Genome Analysis Identifies Novel Drivers and High-Risk Double-Hit Events in Relapsed/Refractory Myeloma. Blood 2023, 141, 620–633. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, K.; Søgaard Helbo, A.; Fibiger Munch-Petersen, H.; Sjö, L.; Christensen, J.; Sommer Kristensen, L.; Asmar, F.; Hermansen, N.E.U.; O’Connel, C.; Gimsing, P.; et al. Dual Inhibition of DNMTs and EZH2 Can Overcome Both Intrinsic and Acquired Resistance of Myeloma Cells to IMiDs in a Cereblon-Independent Manner. Mol. Oncol. 2018, 12, 180–195. [Google Scholar] [CrossRef]
- Yu, T.; Du, C.; Ma, X.; Sui, W.; Yu, Z.; Liu, L.; Zhao, L.; Li, Z.; Xu, J.; Wei, X.; et al. Polycomb-like Protein 3 Induces Proliferation and Drug Resistance in Multiple Myeloma and Is Regulated by MiRNA-15a. Mol. Cancer Res. MCR 2020, 18, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Herviou, L.; Kassambara, A.; Boireau, S.; Robert, N.; Requirand, G.; Müller-Tidow, C.; Vincent, L.; Seckinger, A.; Goldschmidt, H.; Cartron, G.; et al. PRC2 Targeting Is a Therapeutic Strategy for EZ Score Defined High-Risk Multiple Myeloma Patients and Overcome Resistance to IMiDs. Clin. Epigenet. 2018, 10, 121. [Google Scholar] [CrossRef]
- Estrada, F.G.A.; Miccoli, S.; Aniceto, N.; García-Sosa, A.T.; Guedes, R.C. Exploring EZH2-Proteasome Dual-Targeting Drug Discovery through a Computational Strategy to Fight Multiple Myeloma. Mol. Basel Switz. 2021, 26, 5574. [Google Scholar] [CrossRef]
- Tremblay-LeMay, R.; Rastgoo, N.; Pourabdollah, M.; Chang, H. EZH2 as a Therapeutic Target for Multiple Myeloma and Other Haematological Malignancies. Biomark. Res. 2018, 6, 34. [Google Scholar] [CrossRef]
- Agarwal, P.; Alzrigat, M.; Párraga, A.A.; Enroth, S.; Singh, U.; Ungerstedt, J.; Österborg, A.; Brown, P.J.; Ma, A.; Jin, J.; et al. Genome-Wide Profiling of Histone H3 Lysine 27 and Lysine 4 Trimethylation in Multiple Myeloma Reveals the Importance of Polycomb Gene Targeting and Highlights EZH2 as a Potential Therapeutic Target. Oncotarget 2016, 7, 6809–6823. [Google Scholar] [CrossRef] [PubMed]
- Hernando, H.; Gelato, K.A.; Lesche, R.; Beckmann, G.; Koehr, S.; Otto, S.; Steigemann, P.; Stresemann, C. EZH2 Inhibition Blocks Multiple Myeloma Cell Growth through Upregulation of Epithelial Tumor Suppressor Genes. Mol. Cancer Ther. 2016, 15, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Ding, T.; Li, F.; Fan, J.; Fan, X.; Jia, R.; Zhang, H. Interruption of Aberrant Chromatin Looping Is Required for Regenerating RB1 Function and Suppressing Tumorigenesis. Commun. Biol. 2022, 5, 1036. [Google Scholar] [CrossRef]
- Béguelin, W.; Popovic, R.; Teater, M.; Jiang, Y.; Bunting, K.L.; Rosen, M.; Shen, H.; Yang, S.N.; Wang, L.; Ezponda, T.; et al. EZH2 Is Required for Germinal Center Formation and Somatic EZH2 Mutations Promote Lymphoid Transformation. Cancer Cell 2013, 23, 677–692. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Leavenworth, J.W.; Li, Y.; Luo, Q.; Xie, H.; Liu, X.; Huang, S.; Yan, H.; Fu, Z.; Zhang, L.Y.; et al. Ezh2 Regulates Differentiation and Function of Natural Killer Cells through Histone Methyltransferase Activity. Proc. Natl. Acad. Sci. USA 2015, 112, 15988–15993. [Google Scholar] [CrossRef]
- Yu, X.; Harden, K.; Gonzalez, L.C.; Francesco, M.; Chiang, E.; Irving, B.; Tom, I.; Ivelja, S.; Refino, C.J.; Clark, H.; et al. The Surface Protein TIGIT Suppresses T Cell Activation by Promoting the Generation of Mature Immunoregulatory Dendritic Cells. Nat. Immunol. 2009, 10, 48–57. [Google Scholar] [CrossRef]
- Stengel, K.F.; Harden-Bowles, K.; Yu, X.; Rouge, L.; Yin, J.; Comps-Agrar, L.; Wiesmann, C.; Bazan, J.F.; Eaton, D.L.; Grogan, J.L. Structure of TIGIT Immunoreceptor Bound to Poliovirus Receptor Reveals a Cell-Cell Adhesion and Signaling Mechanism That Requires Cis-Trans Receptor Clustering. Proc. Natl. Acad. Sci. USA 2012, 109, 5399–5404. [Google Scholar] [CrossRef]
- Pazina, T.; MacFarlane, A.W.; Bernabei, L.; Dulaimi, E.; Kotcher, R.; Yam, C.; Bezman, N.A.; Robbins, M.D.; Ross, E.A.; Campbell, K.S.; et al. Alterations of NK Cell Phenotype in the Disease Course of Multiple Myeloma. Cancers 2021, 13, 226. [Google Scholar] [CrossRef]
- Niu, J.; Maurice-Dror, C.; Lee, D.H.; Kim, D.-W.; Nagrial, A.; Voskoboynik, M.; Chung, H.C.; Mileham, K.; Vaishampayan, U.; Rasco, D.; et al. First-in-Human Phase 1 Study of the Anti-TIGIT Antibody Vibostolimab as Monotherapy or with Pembrolizumab for Advanced Solid Tumors, Including Non-Small-Cell Lung Cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2022, 33, 169–180. [Google Scholar] [CrossRef]
- Jeong, B.-S.; Nam, H.; Lee, J.; Park, H.-Y.; Cho, K.J.; Sheen, J.H.; Song, E.; Oh, M.; Lee, S.; Choi, H.; et al. Structural and Functional Characterization of a Monoclonal Antibody Blocking TIGIT. mAbs 2022, 14, 2013750. [Google Scholar] [CrossRef]
- Isola, I.; Brasó-Maristany, F.; Moreno, D.F.; Mena, M.-P.; Oliver-Calders, A.; Paré, L.; Rodríguez-Lobato, L.G.; Martin-Antonio, B.; Cibeira, M.T.; Bladé, J.; et al. Gene Expression Analysis of the Bone Marrow Microenvironment Reveals Distinct Immunotypes in Smoldering Multiple Myeloma Associated to Progression to Symptomatic Disease. Front. Immunol. 2021, 12, 792609. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Tao, S.-H.; Guo, F.; Li, L.-Z.; Yang, H.-L.; Liang, Y.; Zhang, L.-D.; Ma, L.; Fu, P. Selective EZH2 Inhibitor Zld1039 Alleviates Inflammation in Cisplatin-Induced Acute Kidney Injury Partially by Enhancing RKIP and Suppressing NF-ΚB P65 Pathway. Acta Pharmacol. Sin. 2022, 43, 2067–2080. [Google Scholar] [CrossRef]
- Scuderi, S.A.; Filippone, A.; Basilotta, R.; Mannino, D.; Casili, G.; Capra, A.P.; Chisari, G.; Colarossi, L.; Sava, S.; Campolo, M.; et al. GSK343, an Inhibitor of Enhancer of Zeste Homolog 2, Reduces Glioblastoma Progression through Inflammatory Process Modulation: Focus on Canonical and Non-Canonical NF-ΚB/IκBα Pathways. Int. J. Mol. Sci. 2022, 23, 13915. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.J.; Ósvaldsdóttir, Á.B.; Atzinger, B.; Traustadóttir, G.Á.; Jensen, K.N.; Lárusdóttir, A.E.; Bergthórsson, J.T.; Hardardóttir, I.; Magnúsdóttir, E. The BLIMP1-EZH2 Nexus in a Non-Hodgkin Lymphoma. Oncogene 2020, 39, 5138–5151. [Google Scholar] [CrossRef]
- Lu, C.; Han, H.D.; Mangala, L.S.; Ali-Fehmi, R.; Newton, C.S.; Ozbun, L.; Armaiz-Pena, G.N.; Hu, W.; Stone, R.L.; Munkarah, A.; et al. Regulation of Tumor Angiogenesis by EZH2. Cancer Cell 2010, 18, 185–197. [Google Scholar] [CrossRef]
- Ozmadenci, D.; Shankara Narayanan, J.S.; Andrew, J.; Ojalill, M.; Barrie, A.M.; Jiang, S.; Iyer, S.; Chen, X.L.; Rose, M.; Estrada, V.; et al. Tumor FAK Orchestrates Immunosuppression in Ovarian Cancer via the CD155/TIGIT Axis. Proc. Natl. Acad. Sci. USA 2022, 119, e2117065119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhu, Z.; Lv, H.; Li, F.; Sun, S.; Li, J.; Lee, C.-S. Immune Checkpoint Blockade Mediated by a Small-Molecule Nanoinhibitor Targeting the PD-1/PD-L1 Pathway Synergizes with Photodynamic Therapy to Elicit Antitumor Immunity and Antimetastatic Effects on Breast Cancer. Small Weinh. Bergstr. Ger. 2019, 15, e1903881. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, T.; Tian, X.; Li, W.; Zhao, R.; Yang, W.; Gao, Q.; Li, T.; Shim, J.-H.; Zhang, C.; et al. A Small Molecule Antagonist of PD-1/PD-L1 Interactions Acts as an Immune Checkpoint Inhibitor for NSCLC and Melanoma Immunotherapy. Front. Immunol. 2021, 12, 654463. [Google Scholar] [CrossRef]
- Ye, Y.; Kuang, X.; Xie, Z.; Liang, L.; Zhang, Z.; Zhang, Y.; Ma, F.; Gao, Q.; Chang, R.; Lee, H.-H.; et al. Small-Molecule MMP2/MMP9 Inhibitor SB-3CT Modulates Tumor Immune Surveillance by Regulating PD-L1. Genome Med. 2020, 12, 83. [Google Scholar] [CrossRef]
- Liang, J.; Wang, B.; Yang, Y.; Liu, B.; Jin, Y. Approaching the Dimerization Mechanism of Small Molecule Inhibitors Targeting PD-L1 with Molecular Simulation. Int. J. Mol. Sci. 2023, 24, 1280. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Guo, Z.; Wang, X.; Liao, H.; Chai, Y.; Wang, Z.; Wang, Z. Inhibition of EZH2 Ameliorates Sepsis Acute Lung Injury (SALI) and Non-Small-Cell Lung Cancer (NSCLC) Proliferation through the PD-L1 Pathway. Cells 2022, 11, 3958. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, M.; Bruns, H.; Völkl, S.; Lu, J.; Chartomatsidou, E.; Papakonstantinou, N.; Mentz, K.; Büttner-Herold, M.; Zenz, T.; Herling, M.; et al. Control of PD-L1 Expression in CLL-Cells by Stromal Triggering of the Notch-c-Myc-EZH2 Oncogenic Signaling Axis. J. Immunother. Cancer 2021, 9, e001889. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-Y.; Deng, L.; Jia, Y.; Liu, H.; Ding, K.; Wang, W.; Zhang, H.; Fu, R. CD155/TIGIT Signalling Plays a Vital Role in the Regulation of Bone Marrow Mesenchymal Stem Cell-Induced Natural Killer-Cell Exhaustion in Multiple Myeloma. Clin. Transl. Med. 2022, 12, e861. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Jia, Y.; Yang, C.; Liu, H.; Shen, H.; Wang, H.; Fu, R. Study on the Effect of EZH2 Inhibitor Combined with TIGIT Monoclonal Antibody against Multiple Myeloma Cells. Int. J. Mol. Sci. 2023, 24, 8603. https://doi.org/10.3390/ijms24108603
Liu Z, Jia Y, Yang C, Liu H, Shen H, Wang H, Fu R. Study on the Effect of EZH2 Inhibitor Combined with TIGIT Monoclonal Antibody against Multiple Myeloma Cells. International Journal of Molecular Sciences. 2023; 24(10):8603. https://doi.org/10.3390/ijms24108603
Chicago/Turabian StyleLiu, Zhaoyun, Yue Jia, Chun Yang, Hui Liu, Hongli Shen, Hao Wang, and Rong Fu. 2023. "Study on the Effect of EZH2 Inhibitor Combined with TIGIT Monoclonal Antibody against Multiple Myeloma Cells" International Journal of Molecular Sciences 24, no. 10: 8603. https://doi.org/10.3390/ijms24108603




