Identification of a Novel Linear B-Cell Epitope of HbpA Protein from Glaesserella parasuis Using Monoclonal Antibody
Abstract
:1. Introduction
2. Results
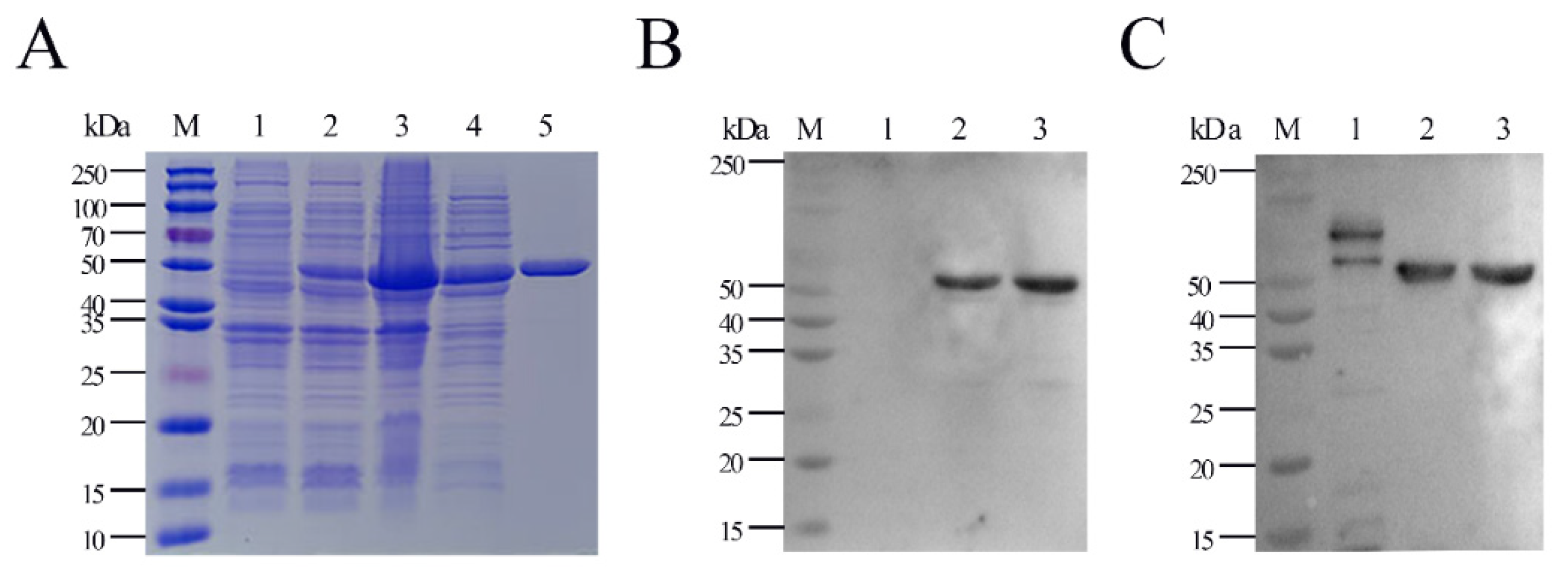
2.1. Expression, Purification, and Characterization of Full-Length G. parasuis Recombinant HbpA Protein (rHbpA)
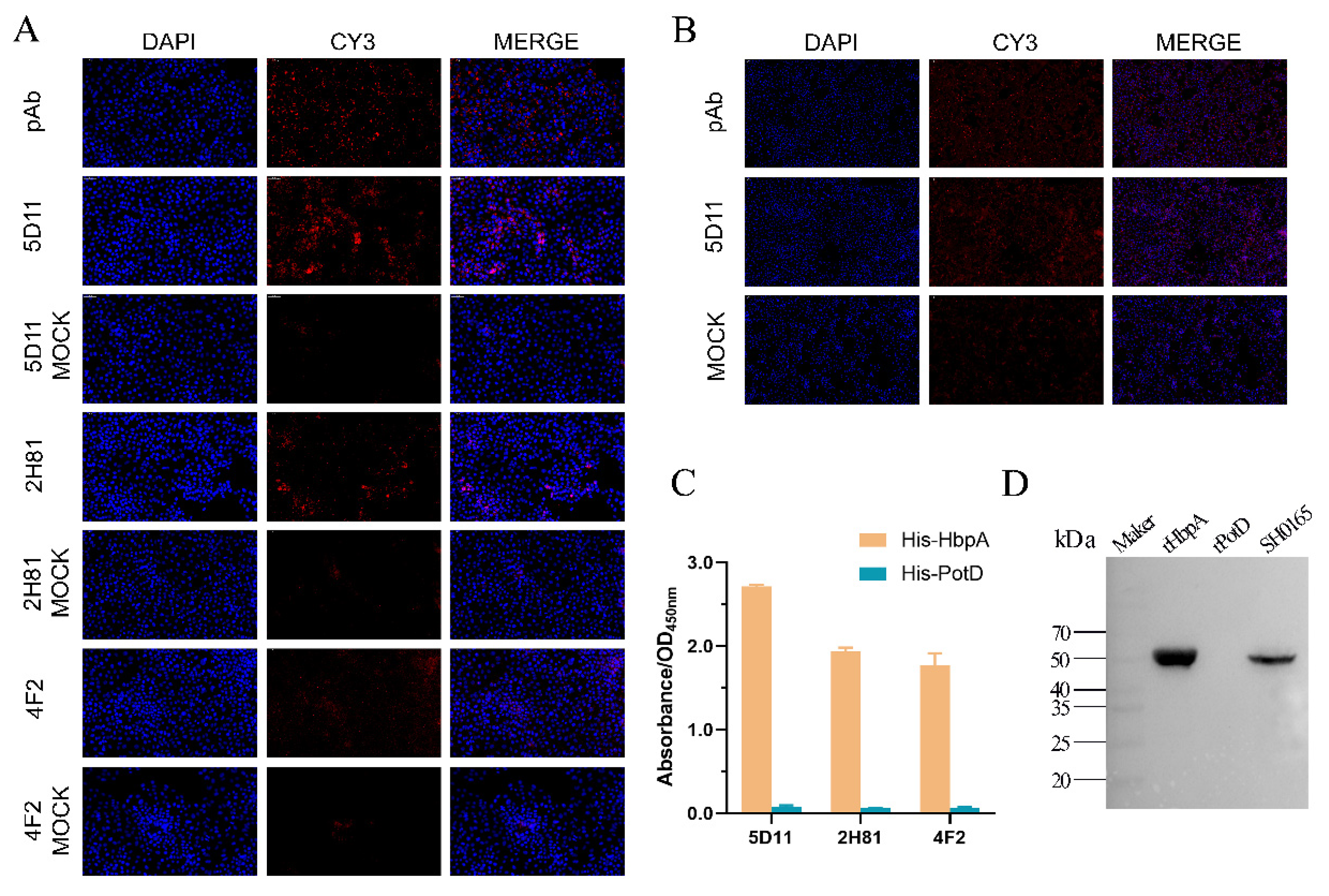
2.2. Production and Screening of G. parasuis rHbpA mAbs
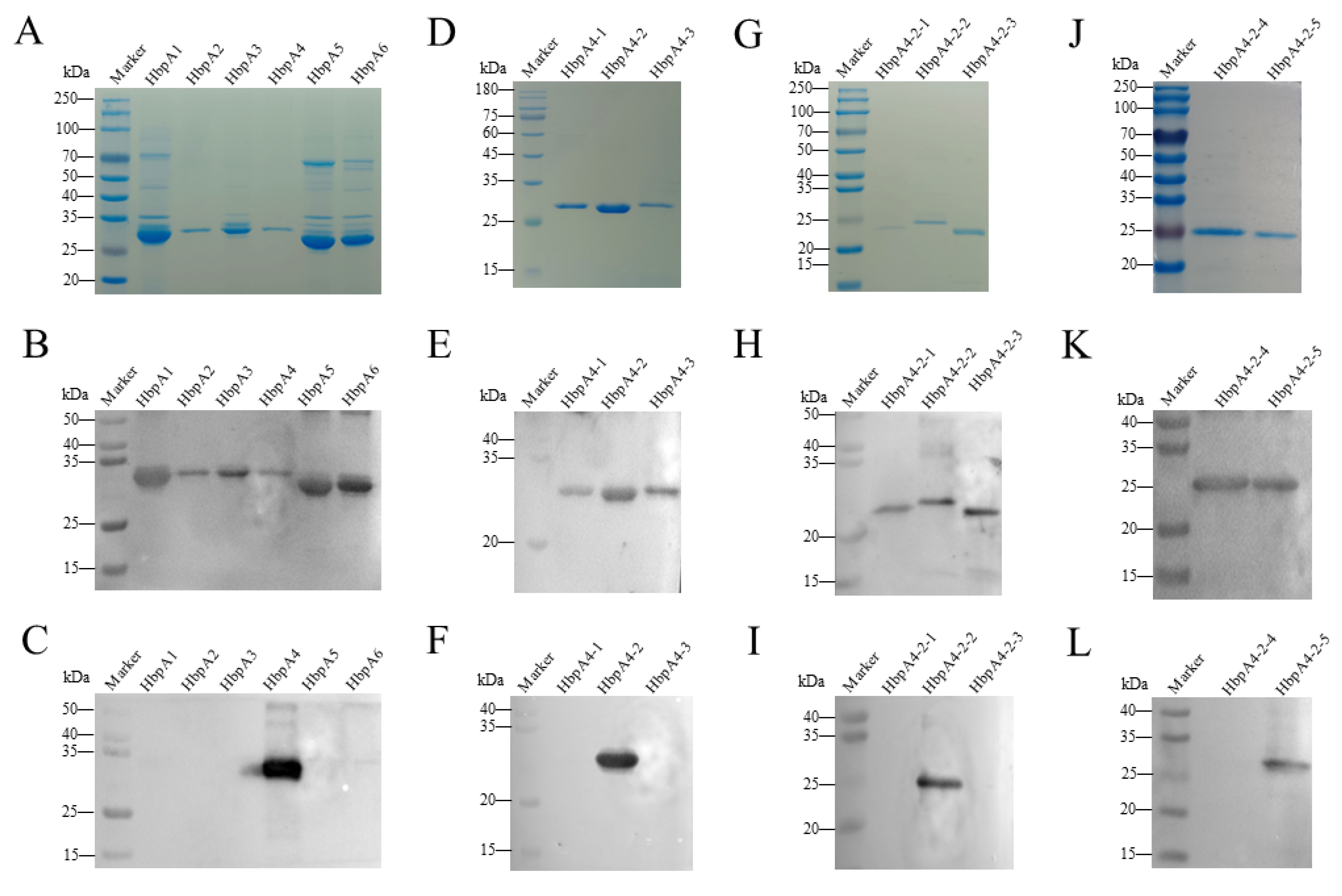
2.3. Epitope Mapping of G. parasuis rHbpA
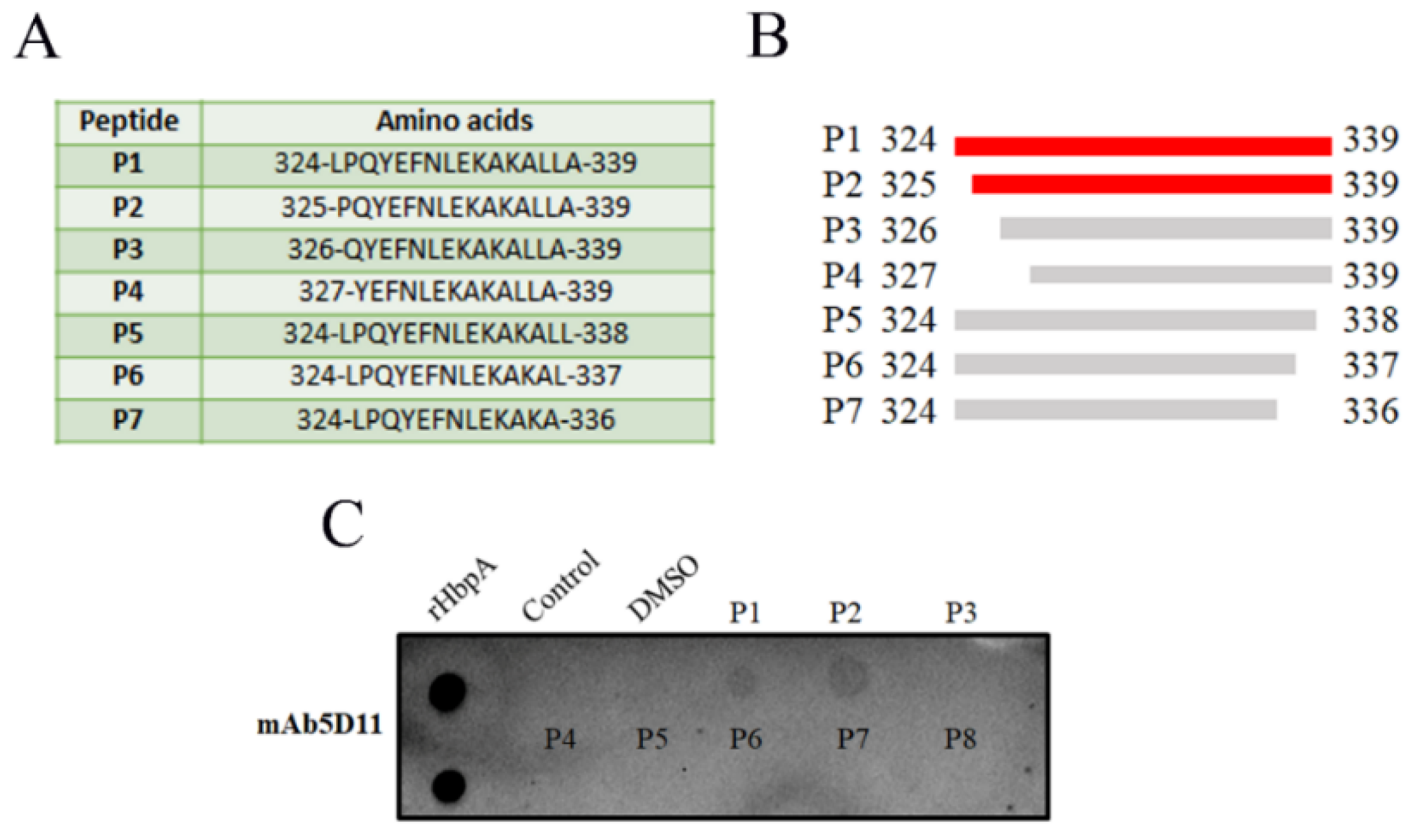
2.4. Identification of the Minimal Epitope
2.5. Cross-Reactivity Analysis
2.6. Alignment Analysis
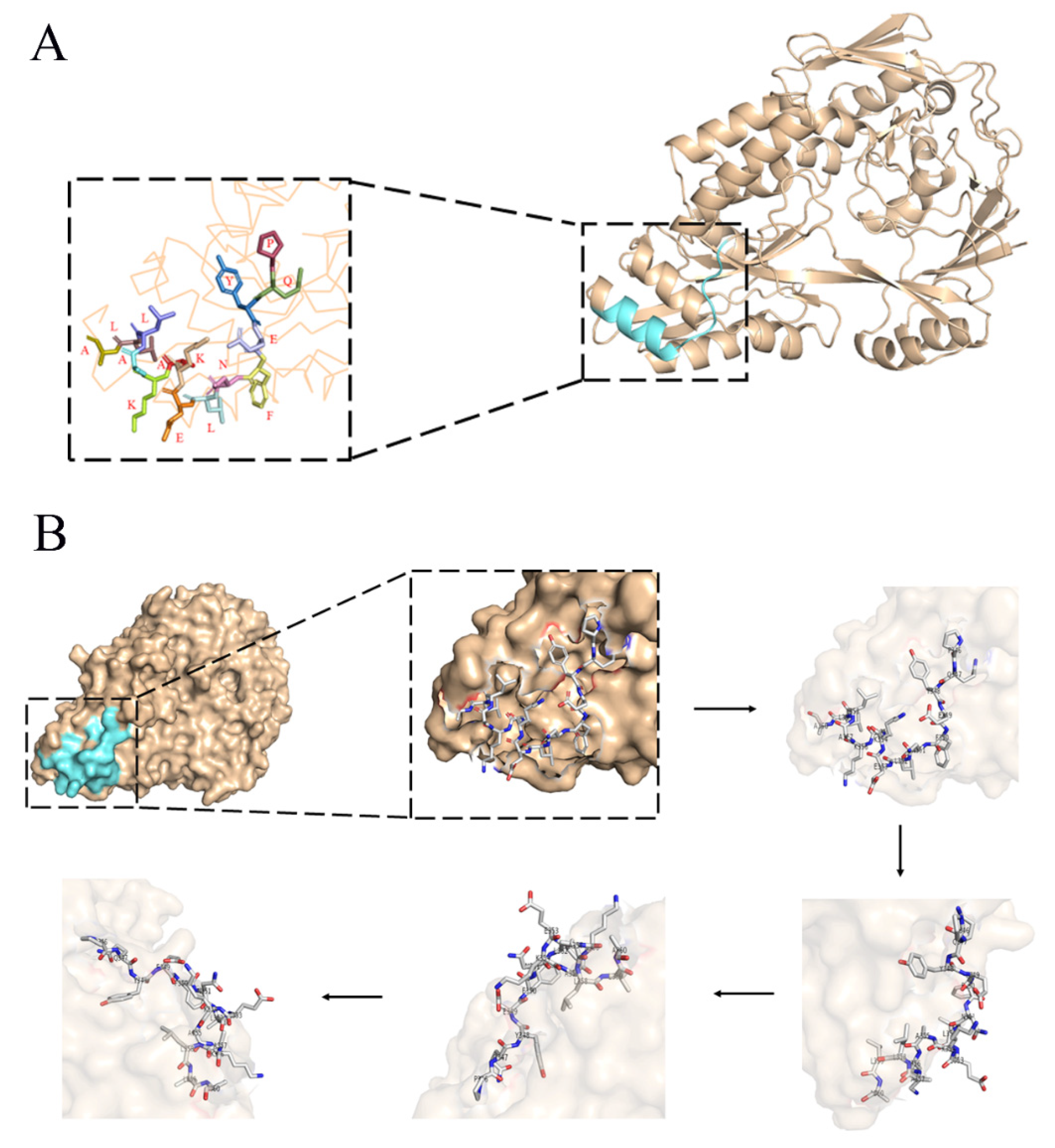
2.7. Three-Dimensional Structure Analysis of EP-5D11
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Strains, Bacterial Growth Conditions, Plasmids, Cells, and Primers
4.3. Expression/Purification of Full-Length and Truncated Recombinant HbpA Protein
4.4. Western Blot
4.5. Production and Subtype Identification of Anti-rHbpA Protein mAbs
4.6. Indirect ELISA
4.7. IFA
4.8. Identification of the Minimal 5D11 Epitope
4.9. Dot Blot Analysis
4.10. Alignment Analysis
4.11. Three-Dimensional Structure Analysis
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, Q.; Wang, J.; Li, R.; Han, Q.; Yuan, W.; Wang, J. Development of a recombinase polymerase amplification assay for rapid detection of Haemophilus parasuis in tissue samples. Vet. Med. Sci. 2020, 6, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Hao, C.; Yang, Q.; Zhang, W.; Li, G.; Liu, S.; Hua, X. Inhibition of Haemophilus parasuis by berberine and proteomic studies of its mechanism of action. Res. Vet. Sci. 2021, 138, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Dickerman, A.; Bandara, A.B.; Inzana, T.J. Phylogenomic analysis of Haemophilus parasuis and proposed reclassification to Glaesserella parasuis, gen. nov. comb. nov. Int. J. Syst. Evol. Microbiol. 2020, 70, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Ren, J.; Zhang, X.; Li, C.; Hu, Y.; Cao, H.; Zeng, W.; Li, Z.; He, Q. Deletion of the crp gene affects the virulence and the activation of the NF-kappaB and MAPK signaling pathways in PK-15 and iPAM cells derived from G. parasuis serovar 5. Vet. Microbiol. 2021, 261, 109198. [Google Scholar] [CrossRef] [PubMed]
- Jia, A.; Zhou, R.; Fan, H.; Yang, K.; Zhang, J.; Xu, Y.; Wang, G.; Liao, M. Development of Serotype-Specific PCR Assays for Typing of Haemophilus parasuis Isolates Circulating in Southern China. J. Clin. Microbiol. 2017, 55, 3249–3257. [Google Scholar] [CrossRef] [Green Version]
- Dai, K.; Yang, Z.; Ma, X.; Chang, Y.F.; Cao, S.; Zhao, Q.; Huang, X.; Wu, R.; Huang, Y.; Xia, J.; et al. Deletion of Polyamine Transport Protein PotD Exacerbates Virulence in Glaesserella (Haemophilus) parasuis in the Form of Non-biofilm-generated Bacteria in a Murine Acute Infection Model. Virulence 2021, 12, 520–546. [Google Scholar] [CrossRef]
- Liu, Y.; Du, Y.; Song, Y.; Tian, Y.; Qi, Y.; Zhang, Q.; He, Q.; Wang, X.; Chen, H.; Cai, X.; et al. Development and application of an antibody detection ELISA for Haemophilus parasuis based on a monomeric autotransporter passenger domain. BMC Vet. Res. 2019, 15, 436. [Google Scholar] [CrossRef]
- Zheng, N.; Chai, Z.; Fu, F.; Jiang, F.; Wang, X.; Zhang, X.; Wang, Z.; Li, X. Identification of a novel Haemophilus parasuis-specific B cell epitope using monoclonal antibody against the OppA protein. PLoS ONE 2014, 9, e84516. [Google Scholar] [CrossRef]
- Kong, N.; Meng, Q.; Jiao, Y.; Wu, Y.; Zuo, Y.; Wang, H.; Sun, D.; Dong, S.; Zhai, H.; Tong, W.; et al. Identification of a novel B-cell epitope in the spike protein of porcine epidemic diarrhea virus. Virol. J. 2020, 17, 46. [Google Scholar] [CrossRef] [Green Version]
- Zhu, K.; Yu, D.; An, J.; Li, Y. Characterization and protective activity of monoclonal antibodies directed against Fe (3+) ABC transporter substrate-binding protein of Glaesserella parasuis. Vet. Res. 2021, 52, 100. [Google Scholar] [CrossRef]
- Vergauwen, B.; Elegheert, J.; Dansercoer, A.; Devreese, B.; Savvides, S.N. Glutathione import in Haemophilus influenzae Rd is primed by the periplasmic heme-binding protein HbpA. Proc. Natl. Acad. Sci. USA 2010, 107, 13270–13275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanson, M.S.; Slaughter, C.; Hansen, E.J. The hbpA gene of Haemophilus influenzae type b encodes a heme-binding lipoprotein conserved among heme-dependent Haemophilus species. Infect. Immun. 1992, 60, 2257–2266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morton, D.J.; Madore, L.L.; Smith, A.; Vanwagoner, T.M.; Seale, T.W.; Whitby, P.W.; Stull, T.L. The heme-binding lipoprotein (HbpA) of Haemophilus influenzae: Role in heme utilization. FEMS Microbiol. Lett. 2005, 253, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morton, D.J.; Seale, T.W.; Bakaletz, L.O.; Jurcisek, J.A.; Smith, A.; VanWagoner, T.M.; Whitby, P.W.; Stull, T.L. The heme-binding protein (HbpA) of Haemophilus influenzae as a virulence determinant. Int. J. Med. Microbiol. 2009, 299, 479–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Xu, Z.; Li, L.; Chen, H.; Zhou, R. Identification of conserved surface proteins as novel antigenic vaccine candidates of Actinobacillus pleuropneumoniae. J. Microbiol. 2012, 50, 978–986. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, A.; Guo, Y.; Liao, Y.; Chen, H.; Jin, M. A comprehensive proteome map of the Haemophilus parasuis serovar 5. Proteomics 2009, 9, 2722–2739. [Google Scholar] [CrossRef]
- Zhang, L.; Wen, Y.; Li, Y.; Wei, X.; Yan, X.; Wen, X.; Wu, R.; Huang, X.; Huang, Y.; Yan, Q.; et al. Comparative proteomic analysis of the membrane proteins of two Haemophilus parasuis strains to identify proteins that may help in habitat adaptation and pathogenesis. Proteome Sci. 2014, 12, 38. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P. Studies on Immunoprotection of H. parasuis HAPS_RS00465 Protein and the Biological Characteristics of the Gene Deletion Strains. Master’s Thesis, Sichuan Agricultural University, Cheng’du, China, 2016. [Google Scholar]
- Ma, X.Y. Study on the Pro-Inflammatory Pathway of HbpA Protein of Haemophilus parahauis and Its Immunoprotective Effect in Mice. Master’s Thesis, Sichuan Agricultural University, Cheng’du, China, 2021. [Google Scholar]
- Payette, F.; Charlebois, A.; Fairbrother, J.H.; Beauchamp, G.; Leclere, M. Nicoletella semolina in the airways of healthy horses and horses with severe asthma. J. Vet. Intern. Med. 2021, 35, 1612–1619. [Google Scholar] [CrossRef]
- Kuhnert, P.; Brodard, I.; Schonecker, L.; Akarsu, H.; Christensen, H.; Bisgaard, M. Mannheimia pernigra sp. nov. isolated from bovine respiratory tract. Int. J. Syst. Evol. Microbiol. 2021, 71, 4643. [Google Scholar] [CrossRef]
- Confer, A.W.; Ayalew, S. Mannheimia haemolytica in bovine respiratory disease: Immunogens, potential immunogens, and vaccines. Anim. Health Res. Rev. 2018, 19, 79–99. [Google Scholar] [CrossRef]
- Johanne Hansen, M.; Strom Braaten, M.; Miki Bojesen, A.; Christensen, H.; Sonne, C.; Dietz, R.; Frost Bertelsen, M. Ursidibacter maritimus gen. nov. sp. nov. and Ursidibacter arcticus sp. nov. two new members of the family Pasteurellaceae isolated from the oral cavity of bears. Int. J. Syst. Evol. Microbiol. 2015, 65, 3683–3689. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, W.; Tan, Q.; Zhang, X.; Wang, M.; Duan, X.; Liu, Y.; Wu, Z.; Ma, J.; Song, B.; et al. Structural Features of a Conformation-dependent Antigen Epitope on ORFV-B2L Recognized by the 2E4 mAb. Sci. Rep. 2019, 9, 16094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, J.; Wei, Y.; Zhang, L.; Wang, X.; Yao, D.; Liu, D.; Liu, W.; Yu, S.; Yu, Y.; Wu, Z.; et al. Identification of a novel linear B-cell epitope as a vaccine candidate in the N2N3 subdomain of Staphylococcus aureus fibronectin-binding protein A. J. Med. Microbiol. 2018, 67, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Liu, Y.; Li, Y.; Tang, B.; Liang, X.; Yang, Y.; Liu, M.; Liu, X.; Zhou, Y. Rapid Quantum Dot Nanobead-mAb Probe-Based Immunochromatographic Assay for Antibody Monitoring of Trichinella spiralis Infection. Int. J. Nanomed. 2021, 16, 2477–2486. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Fu, F.; Li, X.; Chen, X.; Wang, W.; Lang, Y.; Cong, F.; Liu, C.; Tong, G.; Li, X. Identification of the immunogenic outer membrane protein A antigen of Haemophilus parasuis by a proteomics approach and passive immunization with monoclonal antibodies in mice. Clin. Vaccine Immunol. 2011, 18, 1695–1701. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Chen, R.; Hu, J.; Qu, H.; Zhao, Y.; Cao, S.; Wen, X.; Wen, Y.; Wu, R.; Zhao, Q.; et al. Identification of a Novel Linear B-Cell Epitope on the Nucleocapsid Protein of Porcine Deltacoronavirus. Int. J. Mol. Sci. 2020, 21, 648. [Google Scholar] [CrossRef] [Green Version]
- Bosse, J.T.; Li, Y.; Fernandez Crespo, R.; Angen, O.; Holden, M.T.G.; Weinert, L.A.; Maskell, D.J.; Tucker, A.W.; Wren, B.W.; Rycroft, A.N.; et al. Draft Genome Sequences of the Type Strains of Actinobacillus indolicus (46K2C) and Actinobacillus porcinus (NM319), Two NAD-Dependent Bacterial Species Found in the Respiratory Tract of Pigs. Microbiol. Resour. Announc. 2020, 9, e00716-19. [Google Scholar] [CrossRef] [Green Version]
- Loera-Muro, A.; Ramirez-Castillo, F.Y.; Moreno-Flores, A.C.; Martin, E.M.; Avelar-Gonzalez, F.J.; Guerrero-Barrera, A.L. Actinobacillus pleuropneumoniae Surviving on Environmental Multi-Species Biofilms in Swine Farms. Front. Vet. Sci. 2021, 8, 722683. [Google Scholar] [CrossRef]
- Liu, S.; Lin, L.; Yang, H.; Wu, W.; Guo, L.; Zhang, Y.; Wang, F.; Wang, X.; Song, W.; Hua, L.; et al. Pasteurella multocida capsular: Lipopolysaccharide types D:L6 and A:L3 remain to be the main epidemic genotypes of pigs in China. Anim. Dis. 2021, 1, 26. [Google Scholar] [CrossRef]
- Chen, C.W.; Chang, C.Y. Peptide Scanning-assisted Identification of a Monoclonal Antibody-recognized Linear B-cell Epitope. J. Vis. Exp. 2017, 24, e55417. [Google Scholar]



| Segment | Primers | Primer Sequences (5′→3′) | Positions (Amino Acids) |
|---|---|---|---|
| HbpA | HbpA-F | cagcaaatgggtcgcggatccGCACCGACAAATACATTGGTCA | 1–510 |
| HbpA-R | ctcgagtgcggccgcaagcttTTAAGGCTTCAGACTTACGCCAT | ||
| HbpA1 | HbpA1-F | gccatggctgatatcggatccGCACCGACAAATACATTGGTCA | 1–101 |
| HbpA1-R | ctcgagtgcggccgcaagcttGCGCTTGGCGTTGGAATGA | ||
| HbpA2 | HbpA2-F | gccatggctgatatcggatccGCCGATGATGTGGTGTTCTC | 90–197 |
| HbpA2-R | ctcgagtgcggccgcaagcttGGTGGTCGGTCTGATAAGTTTGG | ||
| HbpA3 | HbpA3-F | gccatggctgatatcggatccCAACCGATTGGAACGGGG | 181–286 |
| HbpA3-R | ctcgagtgcggccgcaagcttGTCGGCGAACTTTTACGTTGTTT | ||
| HbpA4 | HbpA4-F | gccatggctgatatcggatccGGGCTAAATACCACCAAACCTG | 271–380 |
| HbpA4-R | ctcgagtgcggccgcaagcttGCACGCCAATTTTAGCCCAG | ||
| HbpA5 | HbpA5-F | gccatggctgatatcggatccATTCAAGCAGACTGGGCTAAAA | 317–450 |
| HbpA5-R | ctcgagtgcggccgcaagcttGTTGTACCGCTGAGGCGAGTAG | ||
| HbpA6 | HbpA6-F | gccatggctgatatcggatccACCAATTACTCTCGCTGGACAG | 431–510 |
| HbpA6-R | ctcgagtgcggccgcaagcttGAGGCTTCAGACTTACGCCATAA | ||
| HbpA4-1 | HbpA4-1-F | gccatggctgatatcggatccGGGCTAAATACCACCAAACCTG | 271–318 |
| HbpA4-1-R | ctcgagtgcggccgcaagcttGTAATACTGCATCAGGGAACGGA | ||
| HbpA4-2 | HbpA4-2-F | gccatggctgatatcggatccGCAACCAATCCGTTCCCTG | 309–348 |
| HbpA4-2-R | ctcgagtgcggccgcaagcttGTTCAAAACCGTTTGGATAGCC | ||
| HbpA4-3 | HbpA4-3-F | gccatggctgatatcggatccAAAGCATTATTGGCAGAAGCTG | 335–380 |
| HbpA4-3-R | ctcgagtgcggccgcaagcttGCACGCCAATTTTAGCCCAG | ||
| HbpA4-2-1 | HbpA4-2-1-F | gccatggctgatatcggatccGCAACCAATCCGTTCCCTG | 309–329 |
| HbpA4-2-1-R | ctcgagtgcggccgcaagcttGAAATTCATATTGTGGCAAATGC | ||
| HbpA4-2-2 | HbpA4-2-2-F | gccatggctgatatcggatccTATAACCCGCATTTGCCACA | 320–339 |
| HbpA4-2-2-R | ctcgagtgcggccgcaagcttGTGCCAATAATGCTTTTGCTTTT | ||
| HbpA4-2-3 | HbpA4-2-3-F | gccatggctgatatcggatccAACTTGGAAAAAGCAAAAGCATT | 330–348 |
| HbpA4-2-3-R | ctcgagtgcggccgcaagcttGTTCAAAACCGTTTGGATAGCC | ||
| HbpA4-2-4 | HbpA4-2-4-F | gccatggctgatatcggatccGCAACCAATCCGTTCCCTG | 309–334 |
| HbpA4-2-4-R | ctcgagtgcggccgcaagcttGTGCTTTTTCCAAGTTAAATTCATAT | ||
| HbpA4-2-5 | HbpA4-2-5-F | gccatggctgatatcggatccTTGCCACAATATGAATTTAACTTGG | 324–348 |
| HbpA4-2-5-R | ctcgagtgcggccgcaagcttGTTCAAAACCGTTTGGATAGCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Wang, K.; Yang, Z.; Tang, X.; Chang, Y.-F.; Dai, K.; Tang, X.; Hu, B.; Zhang, Y.; Cao, S.; et al. Identification of a Novel Linear B-Cell Epitope of HbpA Protein from Glaesserella parasuis Using Monoclonal Antibody. Int. J. Mol. Sci. 2023, 24, 8638. https://doi.org/10.3390/ijms24108638
Liu G, Wang K, Yang Z, Tang X, Chang Y-F, Dai K, Tang X, Hu B, Zhang Y, Cao S, et al. Identification of a Novel Linear B-Cell Epitope of HbpA Protein from Glaesserella parasuis Using Monoclonal Antibody. International Journal of Molecular Sciences. 2023; 24(10):8638. https://doi.org/10.3390/ijms24108638
Chicago/Turabian StyleLiu, Geyan, Kang Wang, Zhen Yang, Xiaoyu Tang, Yung-Fu Chang, Ke Dai, Xinwei Tang, Bangdi Hu, Yiwen Zhang, Sanjie Cao, and et al. 2023. "Identification of a Novel Linear B-Cell Epitope of HbpA Protein from Glaesserella parasuis Using Monoclonal Antibody" International Journal of Molecular Sciences 24, no. 10: 8638. https://doi.org/10.3390/ijms24108638





