Preparation of Symmetrical Capacitors from Lignin-Derived Phenol and PANI Composites with Good Electrical Conductivity
Abstract
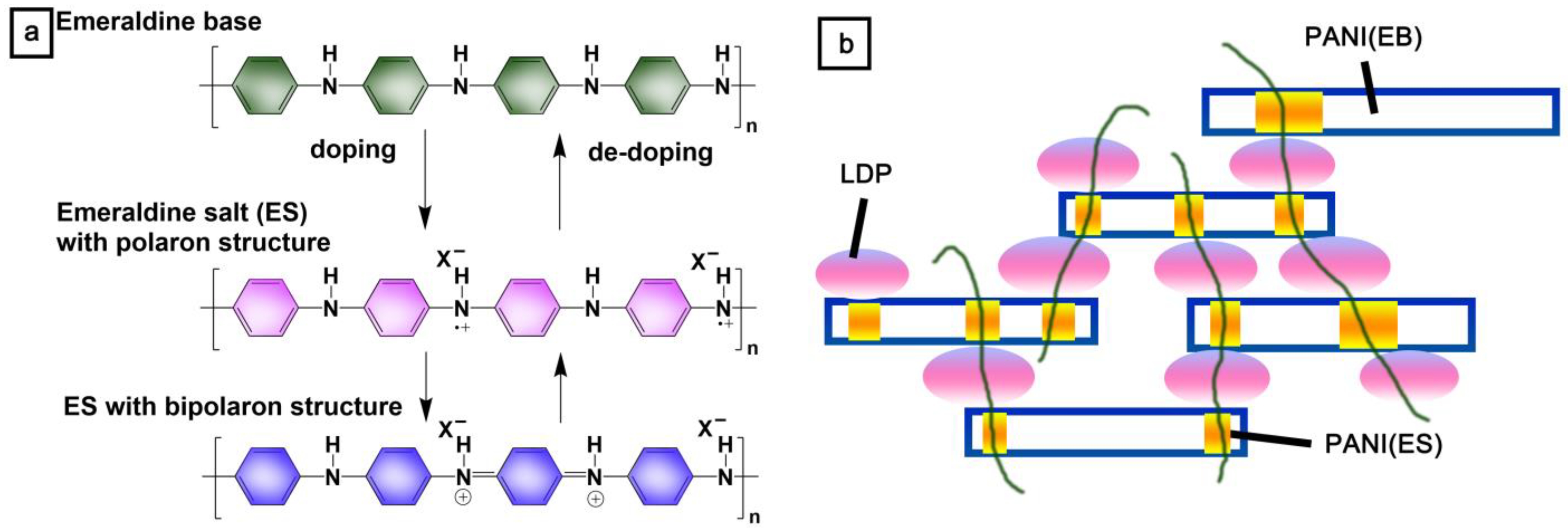
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Raw Materials and Reagents
3.2. Preparation of Lignin Degradation Product/Polyaniline (LDP/PANI) Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, D.W.; Jin, M.H.; Park, J.H.; Lee, Y.J.; Choi, Y.C. Flexible synthetic strategies for lignin-derived hierarchically porous carbon materials. ACS Sustain. Chem. Eng. 2018, 6, 10454–10462. [Google Scholar] [CrossRef]
- Wang, Z.; Hao, M.; Li, X.; Zhang, B.; Jiao, M.; Chen, B.Z. Promising and efficient lignin degradation versatile strategy based on DFT calculations. iScience 2022, 25, 103755. [Google Scholar] [CrossRef] [PubMed]
- Li, P.H.; Ren, J.P.; Jiang, Z.W.; Huang, L.J.; Wu, C.W.; Wu, W.J. Review on the preparation of fuels and chemicals based on lignin. RSC Adv. 2022, 12, 10289–10305. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, Z.; Xi, X.; Liu, B.; Cao, Y.; Xu, H.; Hu, Y. A bifunctional brønsted acidic deep eutectic solvent to dissolve and catalyze the depolymerization of alkali lignin. J. Renew. Mater. 2021, 9, 219–235. [Google Scholar] [CrossRef]
- Jung, J.W.; Son, S.H.; Choi, J. Polyaniline/reduced graphene oxide composites for hole transporting layer of high-performance inverted perovskite solar cells. Polymers 2021, 13, 1281. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, R.; Chen, Y.; Bai, H.; Zhang, T.Y. Superacid-doped polyaniline as a soluble polymeric active electrolyte for supercapacitors. Soft Matter. 2020, 16, 7305–7311. [Google Scholar] [CrossRef]
- Zhu, Z.Z.; Wang, G.C.; Sun, M.Q.; Li, X.W.; Li, C.Z. Fabrication and electrochemical characterization of polyaniline nanorods modified with sulfonated carbon nanotubes for supercapacitor applications. Electrochim. Acta 2011, 56, 1366–1372. [Google Scholar] [CrossRef]
- Wang, L.; Li, X.; Wang, G. Construction of an electrochemical stable conductive network to improve the pseudocapacitance of polyaniline. Electrochim. Acta 2020, 331, 135279. [Google Scholar] [CrossRef]
- Ryosuke, K.; Goto, H. Polyaniline/lignin composite prepared by oxidative polymerization. Mater. Sci. 2017, 1, 96427818. [Google Scholar]
- MacDiarmid, A.G.; Epstein, A.J. The concept of secondary doping as applied to polyaniline. Synth. Met. 1994, 65, 103–116. [Google Scholar] [CrossRef]
- Jyothibasu, J.P.; Wang, R.H.; Tien, Y.C.; Kuo, C.C.; Lee, R.H. Lignin-derived quinone redox moieties for bio-based supercapacitors. Polymers 2022, 14, 3106. [Google Scholar] [CrossRef]
- Zhou, B.; Li, Z.; Liu, W.; Shao, Y.; Ren, X.; Lv, C.; Liu, Q. Hierarchical porous carbon/Kraft lignin composite with significantly improved superior pseudocapacitive behavior. Electrochim. Acta 2021, 398, 139307. [Google Scholar] [CrossRef]
- Admassie, S.; Ajjan, F.N.; Elfwing, A.; Inganäs, O. Biopolymer hybrid electrodes for scalable electricity storage. Mater. Horiz. 2016, 3, 174–185. [Google Scholar] [CrossRef]
- Liu, H.; Xu, T.; Liu, K.; Zhang, M.; Liu, W.; Li, H.; Du, H.; Si, C. Lignin-based electrodes for energy storage application. Ind. Crop. Prod. 2021, 165, 113425. [Google Scholar] [CrossRef]
- Ehsani, A.; Moftakhar, M.K.; Karimi, F. Lignin-derived carbon as a high efficient active material for enhancing pseudocapacitance performance of p-type conductive polymer. J. Energy Storage 2021, 35, 102291. [Google Scholar] [CrossRef]
- Gonçalves, R.V.; Zanini, M.L.; Malmonge, J.A.; Bonnaud, L.; de Basso, N.R.S. Pristine cardanol as biobased dopant for polyaniline. Mater. Lett. 2016, 185, 327–330. [Google Scholar] [CrossRef]
- Aoyagi, M.; Funaoka, M. Conductive composites of lignophenol and polyaniline. Trans. Mater. Res. Soc. Jpn. 2007, 32, 1115–1118. [Google Scholar] [CrossRef]
- Li, P.H.; Wei, Y.M.; Wu, C.W.; Yang, C.; Jiang, B.; Wu, W.J. Lignin-based composites for high-performance supercapacitor electrode materials. RSC Adv. 2022, 12, 19485–19494. [Google Scholar] [CrossRef]
- Kazzaz, A.E.; Fatehi, P. Fabrication of amphoteric lignin and its hydrophilicity/oleophilicity at oil/water interface. J. Colloid. Interfaces Sci. 2022, 561, 231–243. [Google Scholar] [CrossRef]
- Zengin, H.; Kalaycı, G. Synthesis and characterization of polyaniline/activated carbon composites and preparation of conductive films. Mater. Chem. Phys. 2010, 120, 46–53. [Google Scholar] [CrossRef]
- Balakrishnan, D.; Pragathiswaran, C.; Thanikasalam, K.; Mohanta, Y.K.; Saravanan, M.; Abdellattif, M.H. Molecular docking and in vitro inhibitory effect of polyaniline (PANI)/ZnO nanocomposite on the growth of struvite crystal: A step towards control of UTI. Appl. Biochem. Biotech. 2022, 194, 4462–4476. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, X.W.; Xu, H.L.; Wang, G.C. Construction of polyaniline/lignin composite with interpenetrating fibrous networks and its improved electrochemical capacitance performances. Synth. Met. 2019, 248, 40–46. [Google Scholar] [CrossRef]
- Yin, Z.X.; Zhou, H.H.; Fu, C.P.; Zhang, N.S.; Liu, D.; Kuang, Y.F. Synthesis of curly graphene nanoribbon/polyaniline/MnO2 composite and its application in supercapacitor. RSC Adv. 2016, 6, 41142–41150. [Google Scholar] [CrossRef]
- He, Z.W.; Lu, Q.F.; Zhang, J.Y. Facile preparation of hierarchical polyaniline-lignin composite with a reactive silver-ion adsorbability. ACS Appl. Mater. Inter. 2012, 4, 369–374. [Google Scholar] [CrossRef]
- Dong, J.Q.; Shen, Q. Comparison of polyanilines doped by lignosulfonates with three different ions. J. Appl. Pharm. Sci. 2012, 126, E10–E16. [Google Scholar] [CrossRef]
- Altinisik, H.; Getiren, B.; Ciplak, Z.; Soysal, F.; Yildiz, N. Energy storage performance of nitrogen doped reduced graphene oxide/co-doped polyaniline nanocomposites. J. Inorg. Organomet. Polym. Mater. 2022, 33, 353–367. [Google Scholar] [CrossRef]
- Dianat, N.; Rahmanifar, M.S.; Noori, A.; El-Kady, M.F.; Chang, X.Y.; Kaner, R.B.; Mousavi, M.F. Polyaniline-lignin interpenetrating network for supercapacitive energy storage. Nano Lett. 2022, 21, 9485–9493. [Google Scholar] [CrossRef]
- Seo, J.H.; Choi, C.S.; Bae, J.H.; Jeong, H.; Lee, S.H.; Kim, Y.S. Preparation of a lignin/polyaniline composite and its application in Cr(VI) removal from aqueous solutions. Bioresources 2019, 14, 9169–9182. [Google Scholar] [CrossRef]
- Pawar, S.G.; Patil, S.L.; Chougule, M.A.; Raut, B.T.; Sen, S.; Patil, V.B. Camphor sulfonic acid doped polyaniline-titanium dioxide nanocomposite: Synthesis, structural, morphological, and electrical properties. Int. J. Polym. Mater. Polym. Biomater. 2011, 60, 979–987. [Google Scholar] [CrossRef]
- Li, P.H.; Yang, C.; Wu, C.W.; Wei, Y.M.; Jiang, B.; Wu, W.J. Bio-based carbon materials for high-performance supercapacitors. Nanomaterials 2022, 17, 2931. [Google Scholar] [CrossRef]
- Xiong, C.Y.; Li, M.R.; Nie, S.X.; Dang, W.H.; Zhao, W.; Dai, L.; Ni, Y.H. Non-carbonized porous lignin-free wood as an effective scaffold to fabricate lignin-free wood@polyaniline supercapacitor material for renewable energy storage application. J. Power Sources 2020, 471, 228448. [Google Scholar] [CrossRef]
- Gu, D.W.; Ding, C.; Qin, Y.L.; Jiang, H.Y.; Wang, L.; Shen, L.J. Behavior of electrical charge storage/release in polyaniline electrodes of symmetric supercapacitor. Electrochim. Acta 2017, 245, 138–147. [Google Scholar] [CrossRef]
- Xi, X.; Liu, R.L.; Huang, T.; Xu, Y.; Wu, D.Q. Strongly coupled polyaniline/graphene hybrids with much enhanced capacitance performance. J. Colloid Interfaces Sci. 2016, 483, 34–40. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Li, Y.; Feng, Y.Y.; Feng, W. Electropolymerization of graphene oxide/polyaniline composite for high-performance supercapacitor. Electrochim. Acta 2013, 90, 95–100. [Google Scholar] [CrossRef]
- Hong, X.D.; Lu, Y.G.; Li, S.L.; Wang, X.L.; Wang, X.W.; Liang, J. Carbon foam@reduced graphene oxide scaffold grown with polyaniline nanofibers for high performance symmetric supercapacitor. Electrochim. Acta 2018, 294, 376–382. [Google Scholar] [CrossRef]
- Trung, N.B.; Tam, T.V.; Kim, H.R.; Hur, S.H.; Kim, E.J.; Choi, W.M. Three-dimensional hollow balls of graphene-polyaniline hybrids for supercapacitor applications. Chem. Eng. J. 2014, 255, 89–96. [Google Scholar] [CrossRef]
- Devarayan, K.; Lei, D.; Kim, H.; Kim, B. Flexible transparent electrode based on PANi nanowire/nylon nanofiber reinforced cellulose acetate thin film as supercapacitor. Chem. Eng. J. 2015, 273, 603–609. [Google Scholar] [CrossRef]
- Cho, S.H.; Shin, K.H.; Jang, J. Enhanced electrochemical performance of highly porous supercapacitor electrodes based on solution processed polyaniline thin films. ACS Appl. Mater. Interfaces 2013, 5, 9186–9193. [Google Scholar] [CrossRef]
- Du, P.C.; Lin, L.; Wang, H.X.; Liu, D.; Wei, W.L.; Li, J.G.; Liu, P. Fabrication of porous polyaniline modified MWNTs core-shell structure for high performance supercapacitors with high rate capability. Mater. Des. 2017, 127, 76–83. [Google Scholar] [CrossRef]
- Luo, J.; Ma, Q.; Gu, H.H.; Zheng, Y.; Liu, X.Y. Three-dimensional graphene-polyaniline hybrid hollow spheres by layer-by-layer assembly for application in supercapacitor. Electrochim. Acta 2015, 173, 184–192. [Google Scholar] [CrossRef]
- Li, Z.F.; Zhang, H.Y.; Liu, Q.; Liu, Y.D.; Stanciu, L.; Xie, J. Covalently-grafted polyaniline on graphene oxide sheets for high performance electrochemical supercapacitors. Carbon 2014, 71, 257–267. [Google Scholar] [CrossRef]
- Du, P.C.; Wei, W.L.; Liu, D.; Kang, H.X.; Liu, P. Fabrication of hierarchical carbon layer encapsulated polyaniline core-shell structure nanotubes and application in supercapacitors. Chem. Eng. J. 2018, 335, 373–383. [Google Scholar] [CrossRef]
- Miao, Y.E.; Fan, W.; Chen, D.; Liu, T.X. High-performance supercapacitors based on hollow polyaniline nanofibers by electrospinning. ACS Appl. Mater. Interfaces 2013, 5, 4423–4428. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Zhang, C.; Weei, T.W.; Pallathadka, P.K.; He, C.B.; Liu, T.X. Graphene-wrapped polyaniline hollow spheres as novel hybrid electrode materials for supercapacitor applications. ACS Appl. Mater. Interfaces 2013, 5, 3382–3391. [Google Scholar] [CrossRef]
- Liu, G.; Chen, X.; Liu, C.; Jiang, Q.; Jiang, F.; An, J.; Xu, J.; Liu, P. DMSO-treated flexible PEDOT:PSS/PANi fiber electrode for high performance supercapacitors. J. Mater. Sci. 2021, 56, 14632–14643. [Google Scholar] [CrossRef]








| Samples | Conductivity (S/cm) |
|---|---|
| Pure PANI | 0.18 |
| LDP0.5/PANI | 0.57 |
| LDP1.0/PANI | 1.10 |
| LDP3.0/PANI | 1.36 |
| Samples | Current Density (A/g) | Specific Capacitance (F/g) | Cycle Numbers | Capacitance Retention (%) | Ref. |
|---|---|---|---|---|---|
| Ligninsulfonate/PANI | 1 | 554 | 5000 | 55 | [18] |
| 3D hollow rGO/PANI ball | 1 | 331 | 500 | 86 | [36] |
| PANI nanowire/ NA/CA | 0.3 | 402 | 1000 | 61 | [37] |
| Porous PANI film | 0.25 | 361 | 500 | 72.3 | [38] |
| Porous PANI-CNTs | 0.5 | 407 | 2200 | 55 | [39] |
| GR-PANI hollow sphere | 0.5 | 456 | 1000 | 83 | [40] |
| PANI-GO composite | 1 | 442 | 2000 | 83 | [41] |
| PANI-C nanotubes | 0.5 | 483 | 2000 | 63 | [42] |
| Hollow PANI nanofiber | 1 | 601 | 500 | 62 | [43] |
| Hollow PANI/rGO sphere | 1 | 614 | 500 | 90 | [44] |
| DMSO-PEDOT:PSS/PANI | 1 | 340 | 5000 | 69 | [45] |
| LDP3.0/PANI | 1 | 417 | 5000 | 42 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Yu, J.; Wang, M.; Su, W.; Yang, C.; Jiang, B.; Wu, W. Preparation of Symmetrical Capacitors from Lignin-Derived Phenol and PANI Composites with Good Electrical Conductivity. Int. J. Mol. Sci. 2023, 24, 8661. https://doi.org/10.3390/ijms24108661
Li P, Yu J, Wang M, Su W, Yang C, Jiang B, Wu W. Preparation of Symmetrical Capacitors from Lignin-Derived Phenol and PANI Composites with Good Electrical Conductivity. International Journal of Molecular Sciences. 2023; 24(10):8661. https://doi.org/10.3390/ijms24108661
Chicago/Turabian StyleLi, Penghui, Jiangdong Yu, Mingkang Wang, Wanting Su, Chi Yang, Bo Jiang, and Wenjuan Wu. 2023. "Preparation of Symmetrical Capacitors from Lignin-Derived Phenol and PANI Composites with Good Electrical Conductivity" International Journal of Molecular Sciences 24, no. 10: 8661. https://doi.org/10.3390/ijms24108661
APA StyleLi, P., Yu, J., Wang, M., Su, W., Yang, C., Jiang, B., & Wu, W. (2023). Preparation of Symmetrical Capacitors from Lignin-Derived Phenol and PANI Composites with Good Electrical Conductivity. International Journal of Molecular Sciences, 24(10), 8661. https://doi.org/10.3390/ijms24108661








