Exosomes Derived from Adipose Tissue-Derived Mesenchymal Stromal Cells Prevent Medication-Related Osteonecrosis of the Jaw through IL-1RA
Abstract
:1. Introduction
2. Results
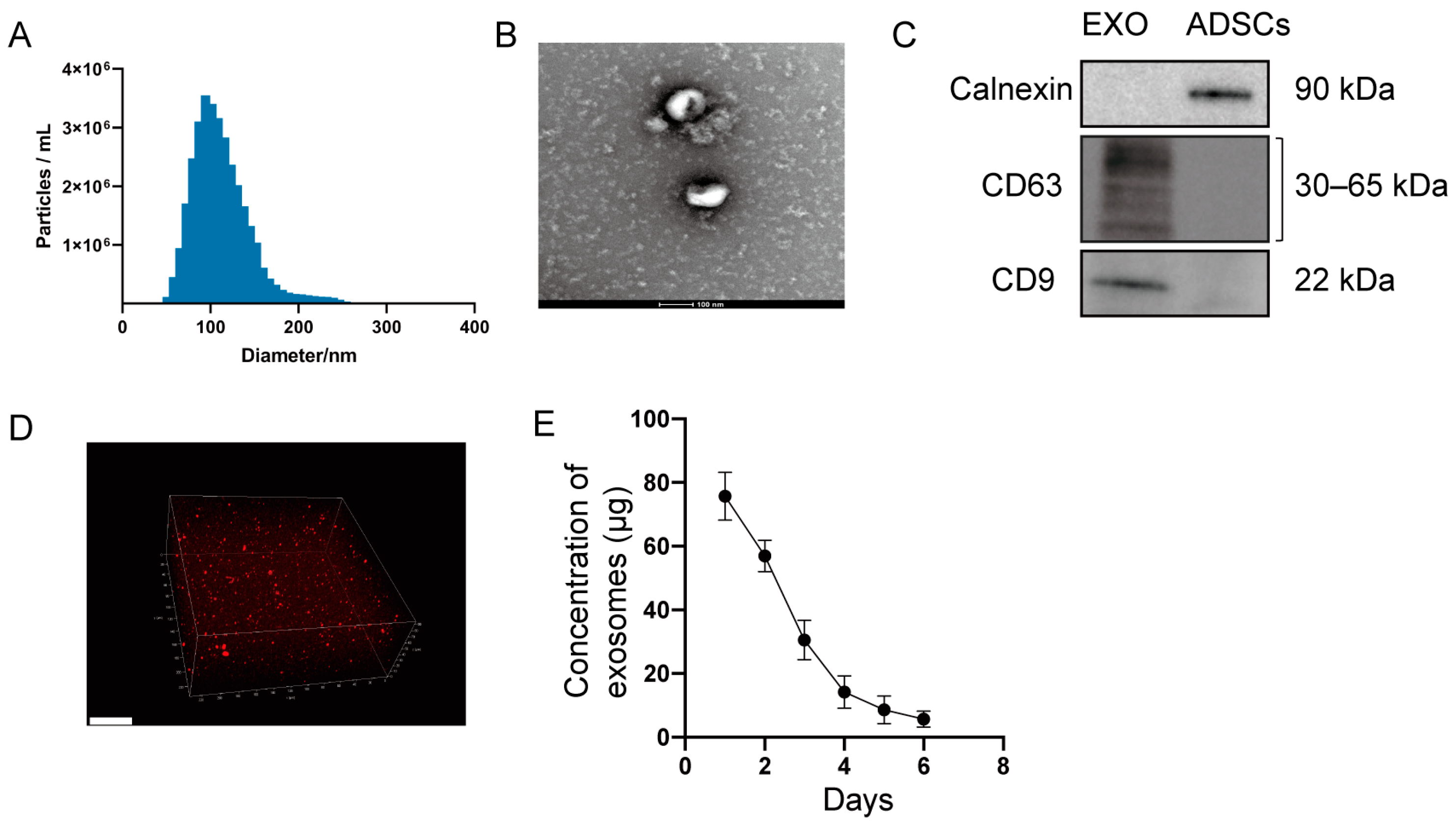
2.1. Characterization of Exosomes and Hydrogel Loaded with Exosomes
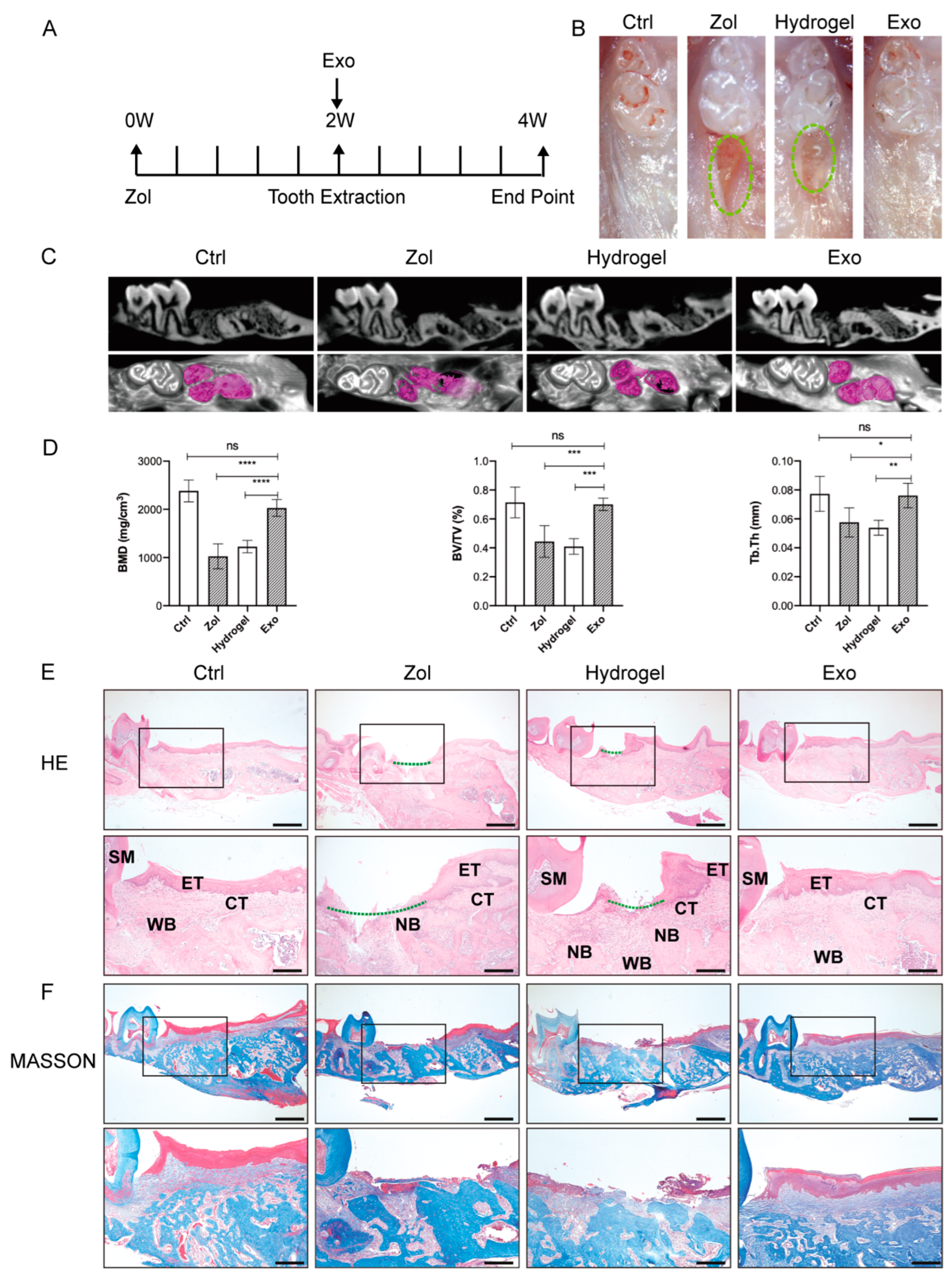
2.2. Exosomes Promote Gingival Wound Closure and Facilitate Socket Bone Regeneration in MRONJ-like Mice
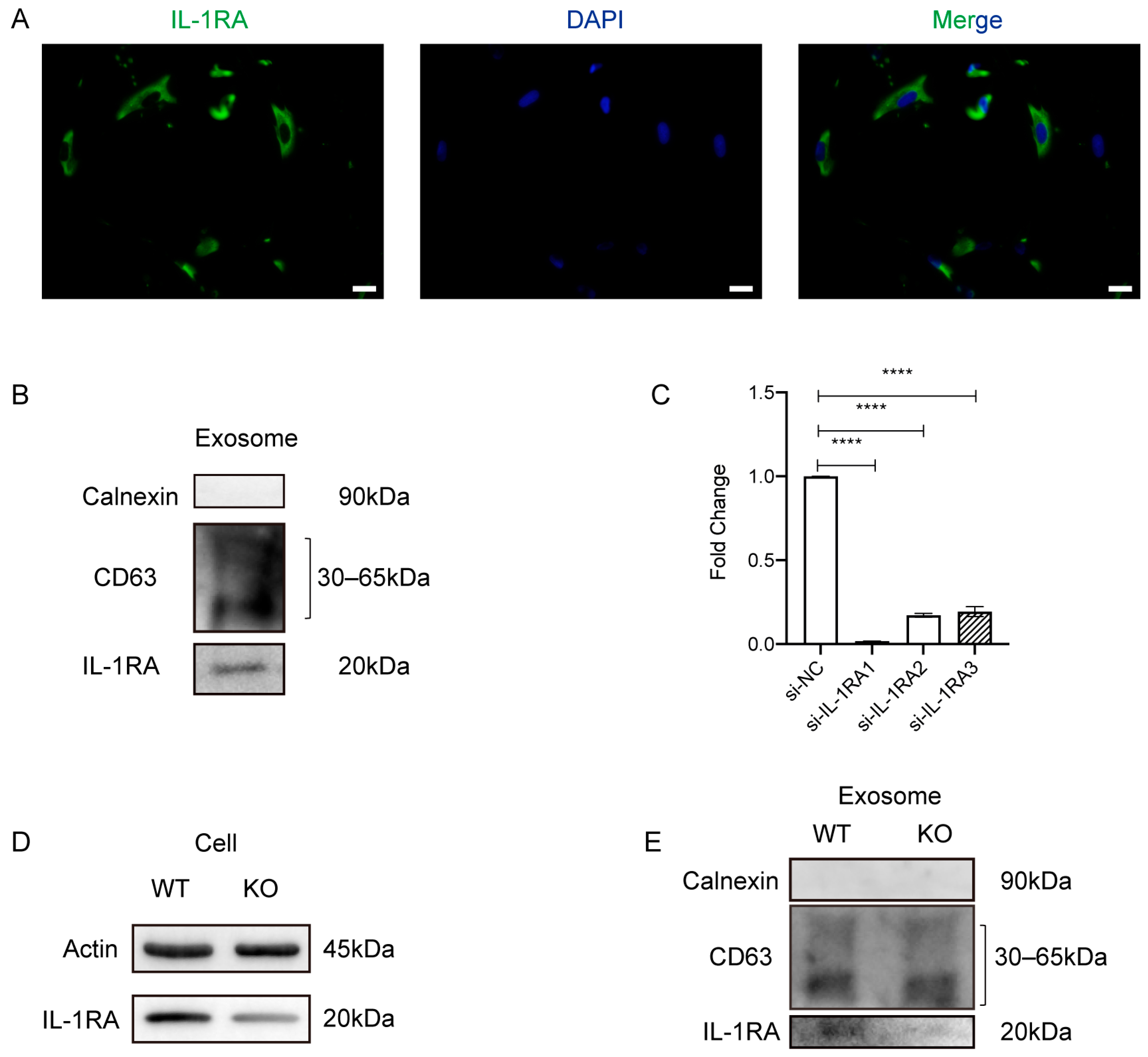
2.3. Exosomes Increase IL-1RA Expression and Decrease IL-1β and TNF-α Expression in the Gingival Tissue
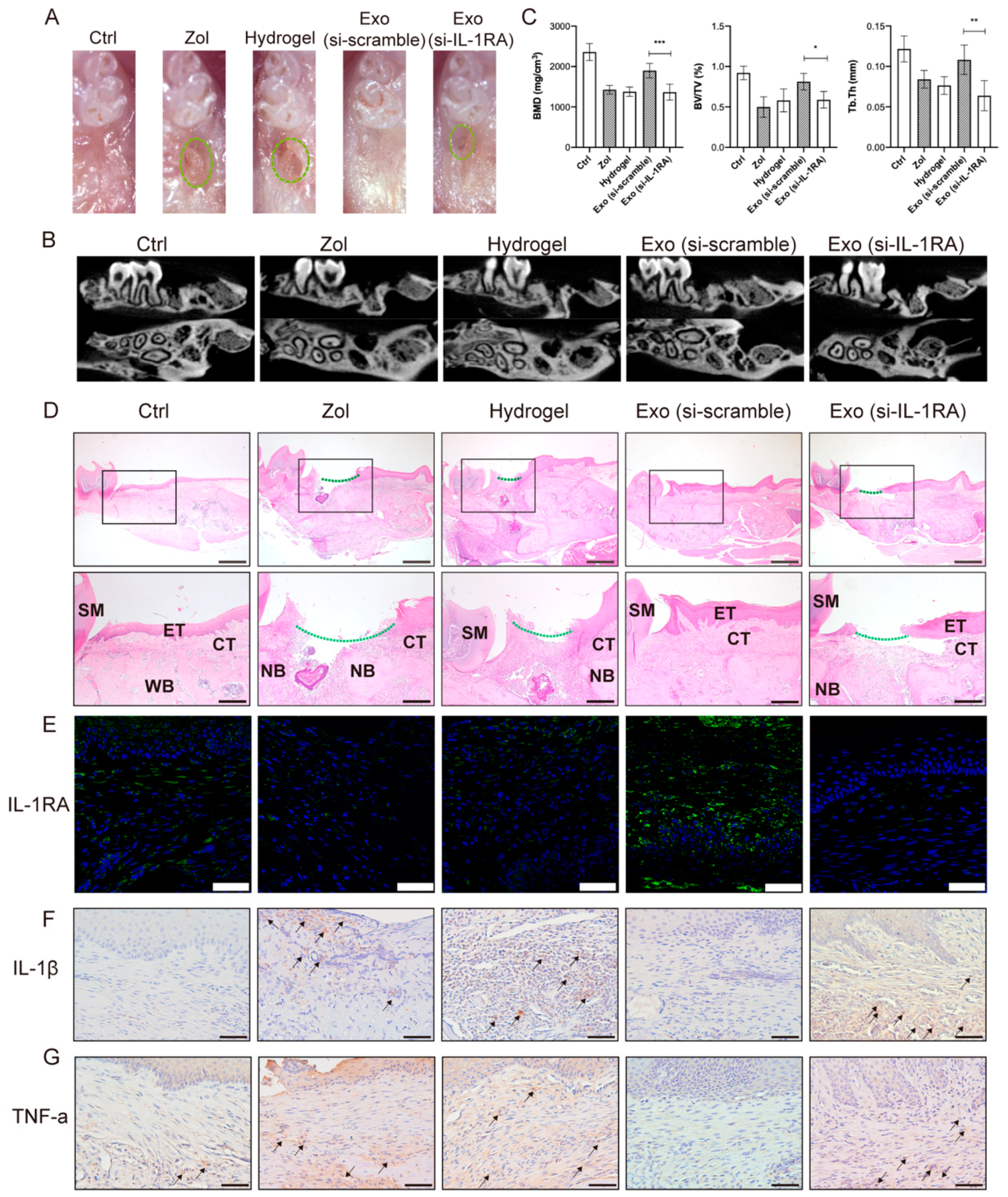
2.4. IL-1RA Deficiency Affects Exosomes in Promoting Gingival Wound Healing in MRONJ-like Mice
2.5. IL-1RA Deficiency in Exosomes Partially Inhibits the Migration and Collagen Synthesis of Gingival Fibroblasts
3. Discussion
4. Materials and Methods
4.1. Preparation and Identification of Exosomes
4.2. Isolation and Culture of Human Gingival Fibroblasts (HGFs)
4.3. Exosomes Uptake Assay
4.4. siRNA Transfection
4.5. Cell Viability
4.6. Cell Migration Assay
4.7. Cell Immunofluorescent Staining
4.8. Western Blot
4.9. Formation and Characterization of a Hydrogel Loaded with Exosomes
4.10. qRT-PCR
4.11. Animals
4.12. Induction of an MRONJ-like Mouse Model and Exosome Treatment
4.13. Patients
4.14. MicroCT Analysis
4.15. Histology
4.16. Tissue Immunochemistry Staining
4.17. Tissue Immunofluorescent Staining
4.18. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Drake, M.T.; Clarke, B.L.; Khosla, S. Bisphosphonates: Mechanism of action and role in clinical practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Dodson, T.B.; Aghaloo, T.; Carlson, E.R.; Ward, B.B.; Kademani, D. American Association of Oral and Maxillofacial Surgeons’ Position Paper on Medication-Related Osteonecrosis of the Jaws-2022 Update. J. Oral Maxillofac. Surg. 2022, 80, 920–943. [Google Scholar] [CrossRef]
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Hakam, A.E.; McCauley, L.K. Current Understanding of the Pathophysiology of Osteonecrosis of the Jaw. Curr. Osteoporos. Rep. 2018, 16, 584–595. [Google Scholar] [CrossRef] [PubMed]
- Tamari, T.; Elimelech, R.; Cohen, G.; Cohen, T.; Doppelt, O.; Eskander-Hashoul, L.; Zigdon-Giladi, H. Endothelial Progenitor Cells inhibit jaw osteonecrosis in a rat model: A major adverse effect of bisphosphonate therapy. Sci. Rep. 2019, 9, 18896. [Google Scholar] [CrossRef]
- Zang, X.; He, L.; Zhao, L.; He, Y.; Xiao, E.; Zhang, Y. Adipose-derived stem cells prevent the onset of bisphosphonate-related osteonecrosis of the jaw through transforming growth factor beta-1-mediated gingival wound healing. Stem Cell Res. Ther. 2019, 10, 169. [Google Scholar] [CrossRef]
- Hasegawa, T.; Kawakita, A.; Ueda, N.; Funahara, R.; Tachibana, A.; Kobayashi, M.; Kondou, E.; Takeda, D.; Kojima, Y.; Sato, S.; et al. A multicenter retrospective study of the risk factors associated with medication-related osteonecrosis of the jaw after tooth extraction in patients receiving oral bisphosphonate therapy: Can primary wound closure and a drug holiday really prevent MRONJ? Osteoporos. Int. 2017, 28, 2465–2473. [Google Scholar] [CrossRef]
- Ristow, O.; Rückschloß, T.; Moratin, J.; Müller, M.; Kühle, R.; Dominik, H.; Pilz, M.; Shavlokhova, V.; Otto, S.; Hoffmann, J.; et al. Wound closure and alveoplasty after preventive tooth extractions in patients with antiresorptive intake-A randomized pilot trial. Oral Dis. 2021, 27, 532–546. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Mao, L.; Liu, Y.; Gao, R.; Zheng, Z.; Chen, W.; Le, A.; Shi, S.; Wang, S. Allogeneic mesenchymal stem cell therapy for bisphosphonate-related jaw osteonecrosis in Swine. Stem Cells Dev. 2013, 22, 2047–2056. [Google Scholar] [CrossRef]
- Kikuiri, T.; Kim, I.; Yamaza, T.; Akiyama, K.; Zhang, Q.; Li, Y.; Chen, C.; Chen, W.; Wang, S.; Le, A.D.; et al. Cell-based immunotherapy with mesenchymal stem cells cures bisphosphonate-related osteonecrosis of the jaw-like disease in mice. J. Bone Miner. Res. 2010, 25, 1668–1679. [Google Scholar] [CrossRef]
- Kaibuchi, N.; Iwata, T.; Yamato, M.; Okano, T.; Ando, T. Multipotent mesenchymal stromal cell sheet therapy for bisphosphonate-related osteonecrosis of the jaw in a rat model. Acta Biomater. 2016, 42, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Kim, Y.N.; Kim, H.; Lee, B.K. Effect of Human Umbilical Cord Matrix-Derived Mesenchymal Stem Cells on Bisphosphonate-Related Osteonecrosis of the Jaw. Tissue Eng. Regen. Med. 2021, 18, 975–988. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; He, L.; Zang, X.; He, Y.; An, J.; Wu, B.; Liu, X.; Bi, H.; Zhang, Y.; Xiao, E. Adipose-Derived Stem Cells Promote Bone Coupling in Bisphosphonate-Related Osteonecrosis of the Jaw by TGF-beta1. Front. Cell Dev. Biol. 2021, 9, 639590. [Google Scholar] [CrossRef] [PubMed]
- Nifosì, G.; Nifosì, L.; Nifosì, A.F. Mesenchymal stem cells in the treatment of osteonecrosis of the jaw. J. Korean Assoc. Oral Maxillofac. Surg. 2021, 47, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Keshtkar, S.; Azarpira, N.; Ghahremani, M.H. Mesenchymal stem cell-derived extracellular vesicles: Novel frontiers in regenerative medicine. Stem Cell. Res. Ther. 2018, 9, 63. [Google Scholar] [CrossRef]
- Rani, S.; Ryan, A.E.; Griffin, M.D.; Ritter, T. Mesenchymal Stem Cell-derived Extracellular Vesicles: Toward Cell-free Therapeutic Applications. Mol. Ther. 2015, 23, 812–823. [Google Scholar] [CrossRef]
- Heidari, N.; Abbasi-Kenarsari, H.; Namaki, S.; Baghaei, K.; Zali, M.R.; Ghaffari Khaligh, S.; Hashemi, S.M. Adipose-derived mesenchymal stem cell-secreted exosome alleviates dextran sulfate sodium-induced acute colitis by Treg cell induction and inflammatory cytokine reduction. J. Cell Physiol. 2021, 236, 5906–5920. [Google Scholar] [CrossRef]
- Kourembanas, S. Exosomes: Vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu. Rev. Physiol. 2015, 77, 13–27. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, M.; Gong, A.; Zhang, X.; Wu, X.; Zhu, Y.; Shi, H.; Wu, L.; Zhu, W.; Qian, H.; et al. HucMSC-Exosome Mediated-Wnt4 Signaling Is Required for Cutaneous Wound Healing. Stem Cells 2015, 33, 2158–2168. [Google Scholar] [CrossRef]
- An, Y.; Lin, S.; Tan, X.; Zhu, S.; Nie, F.; Zhen, Y.; Gu, L.; Zhang, C.; Wang, B.; Wei, W.; et al. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif. 2021, 54, e12993. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, L.; Wang, S.; Han, Q.; Zhao, R.C. Exosomes secreted by mesenchymal stem cells promote endothelial cell angiogenesis by transferring miR-125a. J. Cell Sci. 2016, 129, 2182–2189. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tang, Y.; Liu, Y.; Zhang, P.; Lv, L.; Zhang, X.; Jia, L.; Zhou, Y. Exosomes derived from miR-375-overexpressing human adipose mesenchymal stem cells promote bone regeneration. Cell Prolif. 2019, 52, e12669. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Xu, X.; Chen, C.; Sanmillan, M.L.; Cai, T.; Zhou, Y.; Giraudo, C.; Le, A.; Shi, S. The Fas/Fap-1/Cav-1 complex regulates IL-1RA secretion in mesenchymal stem cells to accelerate wound healing. Sci. Transl. Med. 2018, 10, aai8524. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ma, C.; Gong, L.; Guo, Y.; Fu, K.; Zhang, Y.; Zhou, H.; Li, Y. Macrophage Polarization and Its Role in Liver Disease. Front. Immunol. 2021, 12, 803037. [Google Scholar] [CrossRef] [PubMed]
- Dayer, J.M.; Burger, D. Interleukin-1, tumor necrosis factor and their specific inhibitors. Eur. Cytokine Netw. 1994, 5, 563–571. [Google Scholar]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef]
- Ohsugi, Y.; Niimi, H.; Shimohira, T.; Hatasa, M.; Katagiri, S.; Aoki, A.; Iwata, T. In Vitro Cytological Responses against Laser Photobiomodulation for Periodontal Regeneration. Int. J. Mol. Sci. 2020, 21, 9002. [Google Scholar] [CrossRef]
- Yu, D.; Zhang, S.; Mo, W.; Jiang, Z.; Wang, M.; An, L.; Wang, Y.; Liu, Y.; Jiang, S.; Wu, A.; et al. Transplantation of the Stromal Vascular Fraction (SVF) Mitigates Severe Radiation-Induced Skin Injury. Radiat. Res. 2021, 196, 250–260. [Google Scholar] [CrossRef]
- Li, X.; Xie, X.; Lian, W.; Shi, R.; Han, S.; Zhang, H.; Lu, L.; Li, M. Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef]
- Shi, R.; Jin, Y.; Hu, W.; Lian, W.; Cao, C.; Han, S.; Zhao, S.; Yuan, H.; Yang, X.; Shi, J.; et al. Exosomes derived from mmu_circ_0000250-modified adipose-derived mesenchymal stem cells promote wound healing in diabetic mice by inducing miR-128-3p/SIRT1-mediated autophagy. Am. J. Physiol. Cell Physiol. 2020, 318, C848–C856. [Google Scholar] [CrossRef]
- Liu, Y.; Zhuang, X.; Yu, S.; Yang, N.; Zeng, J.; Liu, X.; Chen, X. Exosomes derived from stem cells from apical papilla promote craniofacial soft tissue regeneration by enhancing Cdc42-mediated vascularization. Stem Cell Res. Ther. 2021, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, L.; Tian, W. Small Extracellular Vesicles Derived from Adipose Tissue Prevent Bisphosphonate-Related Osteonecrosis of the Jaw by Promoting Angiogenesis. Int. J. Nanomed. 2021, 16, 3161–3172. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, J.; Sakai, K.; Urata, Y.; Toyama, N.; Nakamichi, E.; Hibi, H. Extracellular Vesicles of Stem Cells to Prevent BRONJ. J. Dent. Res. 2020, 99, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- Bainbridge, P. Wound healing and the role of fibroblasts. J. Wound Care 2013, 22, 407–412. [Google Scholar] [CrossRef]
- Eming, S.A.; Wynn, T.A.; Martin, P. Inflammation and metabolism in tissue repair and regeneration. Science 2017, 356, 1026–1030. [Google Scholar] [CrossRef]
- Lee, K.C.; Lin, H.C.; Huang, Y.H.; Hung, S.C. Allo-transplantation of mesenchymal stem cells attenuates hepatic injury through IL1Ra dependent macrophage switch in a mouse model of liver disease. J. Hepatol. 2015, 63, 1405–1412. [Google Scholar] [CrossRef]
- Luz-Crawford, P.; Djouad, F.; Toupet, K.; Bony, C.; Franquesa, M.; Hoogduijn, M.J.; Jorgensen, C.; Noel, D. Mesenchymal Stem Cell-Derived Interleukin 1 Receptor Antagonist Promotes Macrophage Polarization and Inhibits B Cell Differentiation. Stem Cells 2016, 34, 483–492. [Google Scholar] [CrossRef]
- Lee, K.; Park, N.; Jung, H.; Rim, Y.A.; Nam, Y.; Lee, J.; Park, S.H.; Ju, J.H. Mesenchymal stem cells ameliorate experimental arthritis via expression of interleukin-1 receptor antagonist. PLoS ONE 2018, 13, e0193086. [Google Scholar] [CrossRef]
- Harrell, C.R.; Markovic, B.S.; Fellabaum, C.; Arsenijevic, N.; Djonov, V.; Volarevic, V. The role of Interleukin 1 receptor antagonist in mesenchymal stem cell-based tissue repair and regeneration. Biofactors 2020, 46, 263–275. [Google Scholar] [CrossRef]
- Muzio, M.; Polentarutti, N.; Sironi, M.; Poli, G.; De Gioia, L.; Introna, M.; Mantovani, A.; Colotta, F. Cloning and characterization of a new isoform of the interleukin 1 receptor antagonist. J. Exp. Med. 1995, 182, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.L.; Lash, B.; Karami, R.; Nayer, B.; Lu, Y.Z.; Piotto, C.; Julier, Z.; Martino, M.M. Restoration of the healing microenvironment in diabetic wounds with matrix-binding IL-1 receptor antagonist. Commun. Biol. 2021, 4, 422. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yu, H.; Sun, M.; Yang, P.; Hu, X.; Ao, Y.; Cheng, J. The tissue origin effect of extracellular vesicles on cartilage and bone regeneration. Acta Biomater. 2021, 125, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Dong, X.; Chen, S.; He, Y.; An, J.; Liu, M.; He, L.; Zhang, Y. Low-level laser therapy prevents medication-related osteonecrosis of the jaw-like lesions via IL-1RA-mediated primary gingival wound healing. BMC Oral Health 2023, 23, 14. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Dong, X.; Wang, X.; Wang, J.; Chen, S.; He, Y.; An, J.; He, L.; Zhang, Y. Exosomes Derived from Adipose Tissue-Derived Mesenchymal Stromal Cells Prevent Medication-Related Osteonecrosis of the Jaw through IL-1RA. Int. J. Mol. Sci. 2023, 24, 8694. https://doi.org/10.3390/ijms24108694
Zheng Y, Dong X, Wang X, Wang J, Chen S, He Y, An J, He L, Zhang Y. Exosomes Derived from Adipose Tissue-Derived Mesenchymal Stromal Cells Prevent Medication-Related Osteonecrosis of the Jaw through IL-1RA. International Journal of Molecular Sciences. 2023; 24(10):8694. https://doi.org/10.3390/ijms24108694
Chicago/Turabian StyleZheng, Yi, Xian Dong, Xinyu Wang, Jie Wang, Shuo Chen, Yang He, Jingang An, Linhai He, and Yi Zhang. 2023. "Exosomes Derived from Adipose Tissue-Derived Mesenchymal Stromal Cells Prevent Medication-Related Osteonecrosis of the Jaw through IL-1RA" International Journal of Molecular Sciences 24, no. 10: 8694. https://doi.org/10.3390/ijms24108694



