Abstract
An imbalance of homeostasis in the retina leads to neuron loss and this eventually results in a deterioration of vision. If the stress threshold is exceeded, different protective/survival mechanisms are activated. Numerous key molecular actors contribute to prevalent metabolically induced retinal diseases—the three major challenges are age-related alterations, diabetic retinopathy and glaucoma. These diseases have complex dysregulation of glucose-, lipid-, amino acid or purine metabolism. In this review, we summarize current knowledge on possible ways of preventing or circumventing retinal degeneration by available methods. We intend to provide a unified background, common prevention and treatment rationale for these disorders and identify the mechanisms through which these actions protect the retina. We suggest a role for herbal medicines, internal neuroprotective substances and synthetic drugs targeting four processes: parainflammation and/or glial cell activation, ischemia and related reactive oxygen species and vascular endothelial growth factor accumulation, apoptosis and/or autophagy of nerve cells and an elevation of ocular perfusion pressure and/or intraocular pressure. We conclude that in order to achieve substantial preventive or therapeutic effects, at least two of the mentioned pathways should be targeted synergistically. A repositioning of some drugs is considered to use them for the cure of the other related conditions.
1. Introduction
The visual world is the most important environmental information source for humans. None of the other sensory signals reaches the brain in such variety and none is processed by as many cortical areas as the visual cues [1,2]. The first steps of visual processing however do not happen in the brain; these are performed by a thin sheath of neural tissue at the back of the eye, called the retina. After phototransduction by photoreceptors (PRs), light information is translated into neural signals. Visual signals to the brain are solely of retinal origin. They are generated by the retinal ganglion cells (RGC) in the form of spike trains [3]. Therefore, any damage to the retinal tissue immediately results in vision loss and, in the worst case, causes total blindness.
Sight-threatening neurodegenerative diseases of the retina fall into two broad categories; one group is caused by genetic deficits, e.g., retinitis pigmentosa and microphthalmia [4]. Other retinodegenerative disorders such as age-related degenerations (age-related macular degeneration—AMD), diabetic retinopathy (DR) or glaucoma are thought to be consequences of pathological metabolic processes [5]. These three conditions contribute strongly to blindness causes worldwide. Exposure to extremely strong light or UV radiation, changes in metabolic parameters or hormone secretion or eventually chronic high blood pressure (resulting in elevated intraocular pressure (IOP)) all may cause tissue metabolic changes. These processes induce elevated extracellular glutamate levels, provoking excitotoxic insults [6]. Besides apoptosis induced by the excitotoxic insults, other means of retinal deteriorations have also been studied in many laboratories. These include reactive oxygen species (ROS)-induced alterations [7,8,9], inflammatory/parainflammatory processes [10,11,12], vascular endothelial growth factor (VEGF)-mediated changes in retinal blood vessels [13] and a regulation of autophagic/necrotic events [14].
Neuronal viability of the retina is based on several fundamental homeostatic processes and discrete signaling pathways. Its neuronal complexity, high metabolic demand and limited regenerative capacity make it extremely vulnerable against a variety of intracellular and extracellular stress factors. If the stress threshold is exceeded, different protective/survival mechanisms are activated. Metabolic-related stresses could manifest at different levels and with diverse, severe consequences in the retina. Numerous key molecular actors contribute to the evolution of growingly prevalent metabolically induced retinal diseases. These diseases have a complex dysregulation of glucose-, lipid-, amino acid or purine metabolism in the molecular background of the pathology [15]. Several altered metabolic stress-related cellular pathways are induced such as oxidative stress [16], defects in autophagy and immune cascade, mitochondrial dysfunction, endoplasmatic stress, alterations in apoptotic pathways etc. Moreover, neuroinflammation, glial activation and glutamate excitotoxicity also could be main players in the development of the pathogenesis (Figure 1).
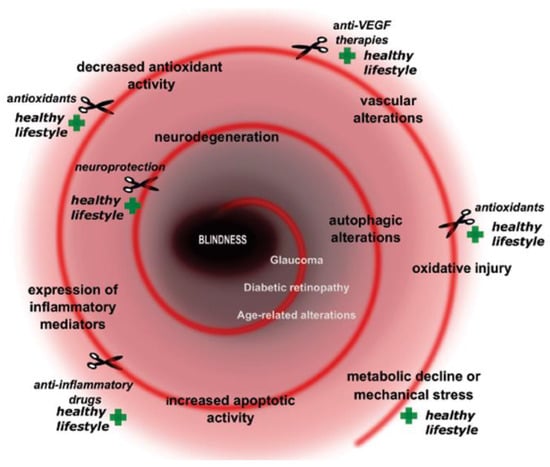
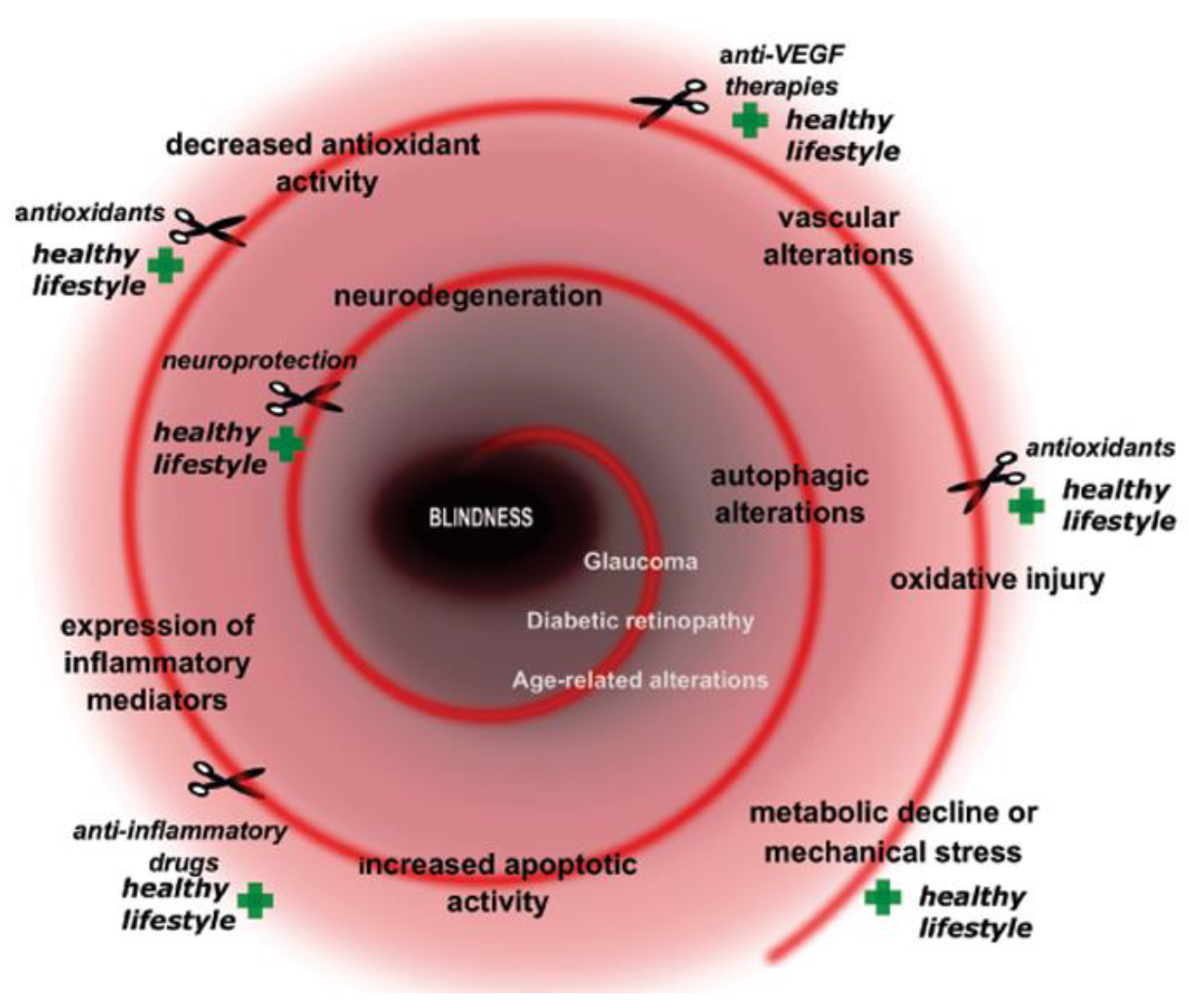
Figure 1.
Key elements and prevention/cure points in metabolically induced retinal diseases. Plus signs show prevention points, while scissor signs demonstrate cure or preventation possibilities.
Under physiological conditions, homeostatic parameters are in perfect balance. During the development of diseases, harmful or even toxic substances (see later) may be generated, which force the tissue to produce protective compounds. If the elements of the reina tissue (Müller cells, retinal vascular, endothel, microglia and even the retina nervous cells) react properly, the survival chances of retinal neurons increase [7,15].
The prevalence of vision loss will double by 2050 [17], from which the “big three”, AMD, DR and glaucoma, will have a substantial share; therefore, solutions for prevention that are widely available for patients are urgent. As indicated in the title above, we aimed at finding the common elements of the signalling pathways which cause age-related changes (most particularly AMD) and those disorders that may lead to blindness due to the deterioration of the retina: diabetes and glaucoma.
2. Aims
In this review, therefore, we summarise current knowledge on possible ways of preventing or circumventing retinal degeneration by available methods. We intend to provide a common treatment rationale for these disorders and to collect the possible mechanisms through which these actions protect the retina. To specify further, we shall consider (1) the possible role of natural products and complementary approaches to prevent the above disorders; (2) to review the endogenous substances in the service of retinal integrity and ways to use them for prevention and cure; and (3) to list synthetic drugs and other chemical means to protect the retinal circuitry. The successful efforts to use non-invasive or minimally invasive combination treatments (4) will also be considered. Finally, we shall summarise the common mechanisms (5) of the three disorders and point at the critical targets which can be attacked successfully in future therapeutic efforts.
3. Retinal Health: The Major Metabolic Threats to It and Ways to Avoid Damage
Since the 1900s, psychosomatic and mental factors have also been considered and confirmed to play a role in vision loss due to cataract and retinal disorders such as AMD, DR and glaucoma. These three disorders are responsible for the majority of blindness of retinal origin, and in the later parts of this paper, we shall call them the “big three”. Psychological and neurological aspects can be explained by the development of the fetal eye during embryogenesis: embryologically, the lens, corneal epithelium, neuroretina, brain and skin (eyelid as well) are ectodermal organs. Therefore, it is not surprising that almost all neurodevelopmental and psychiatric disorders affect the skin and eyes, becoming more multisystemic, explained by the same genetic factors (PAX6, SIX3, SEMA3A etc.) [18,19,20,21,22]. Mentioning a few relevant examples, neurofibromatosis is an inherited neuro-oculo-cutaneous syndrome affecting all parts of the ectoderm (nervous system, skin and the eye) [19]. Moreover, a higher incidence of mental illness and disabilities with behavioural abnormalities is also related to ocular structural anomalies [23]. One of the most surprising phenomena was the association between atopic dermatitis and ocular complications, including (subcapsular) cataract with an additional consequent retinal detachment. In the case of ocular symptoms, patients typically experience anxiety and a fear of becoming blind, which creates a psychological burden [24]. It is also known that a psychosocially stressful event contributes to an acute myopic shift in refraction in young adults, which suggests that acute mental stress factors may play a role in refractive errors [25]. A higher prevalence of depression was also found in AMD patients, and schizophrenic patients have significantly reduced retinal thickness and retinal blood flow [24,26]. Stress may play a greater role in younger patients with glaucoma, which is both an ocular and brain neurodegenerative disorder characterised by a progressive damage of the optic nerve. The glucocorticosteroid overexposure of the brain can become toxic to neurons and can even be toxic to the retinal tissues. Because of the feedback loops, glucocorticoid increase progressively damages the tissue, as a vicious cycle. Psychosocial stress can also activate inflammatory responses by neural activation of signaling pathways, resulting in an increased nuclear factor κB (NFκB) production. In glaucomatous and healthy eyes, mental stress is associated with IOP elevation and vascular dysregulation which are caused by the glucocorticoids, pro-inflammatory cytokines, and endothelin. Elevated levels of tumor necrosis factor (TNF)-α and interleukin (IL)-6 are found in the aqueous humour too [24]. These factors may all contribute to the loss of metabolic control. If the metabolic shift is slow, aging gains ground; if some of the changes are prominent, retinal diseases can be diagnosed. The most prominent ones worldwide are AMD, DR and glaucoma.
4. Aging
Over the past 50 years, rapid changes can be observed in the demographics of populations around the world due to increasing longevity. According to ‘The 2017 World Health Organization World report’, the number of people aged 60 years or older will be 1 billion in 2019. This number is expected to pass to 1.4 billion by 2030 and 2.1 billion by 2050, which at that time will represent close to 22% of the global population. Many of the consequences of population aging are common across human societies all around the world. Therefore, the prevalence of people with major eye conditions that cause vision impairment escalates sharply. There are currently at least 2.2 billion people who have a near or distance vision impairment including AMD (196 million), DR (146 million) and glaucoma (76 million) patients [27]. The incidence of early and late AMD was 4.1% and 2.3%, respectively, with the incidence of early and late AMD being highest in Caucasians (5.3% and 4.1%), intermediate in Asians (4.5% and 2.2%) and Hispanics (3.3% and 0.8%), and lowest in African Americans (1.6% and 0.4%). Adjusting for age and sex, African Americans had a 70% lower risk of developing early AMD than Caucasians, and this decreased only slightly to 67% lower risk after multivariable adjustments [28]. In 2009, the SEARCH Study for Diabetes in Youth determined a prevalence per 1000 for youths aged 0–19 years with type 1 diabetes from different ethnic groups in the United States. Type 1 diabetes remains a white-dominated disease, with rates of 2.00 in non-Hispanic white patients, 1.31 for African Americans, 0.99 for Hispanics, 0.94 for Native Americans and 0.52 for Asians and Pacific Islanders. For type 2 Diabetes, similar ratios were found [29]. The global prevalence of glaucoma was roughly 3.5% for people aged 40–80 years. The prevalence of primary open-angle glaucoma (POAG) was highest in Africa (4.2%), and primary angle-closure glaucoma was most prevalent in Asia (1.1%) [30].
4.1. Progression of Retinal Aging–Common Features in Model Species and Humans
The changes in visual function induced by aging have been commonly observed in several species such as mice, rats, sheep, degus, monkeys and humans [31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51]. Each component of the eye and, within it, the retina contribute differently to the overall decline in visual function with aging. Remarkable morphological changes occur that are primarily manifested by thinner retinal layers due to cell loss and/or synaptic connection alterations. Some studies have reported age-related ganglion cell loss [31,32,49,52,53,54] while others have not [34,39,44,55]. Nuclear densities declined more intensively in the outer nuclear layer than in inner nuclear layers [56]. Rod bipolar cell dendrites and horizontal cells sprouted into the PR layer most abundantly in the peripheral retina [35,57,58]. The generation of these aberrant processes is presumably driven by the PR death or loss of PR function. It is well established that PR density decreases with age: cones were more resistant than rods to destruction by age and ambient light [32,36,56,59]. Some studies demonstrate that the survival of cones depends on the presence of rods, even if these rods are not functional, as rods secrete cone survival factors [60,61,62,63]. However, at very advanced ages, a significant loss of central cones was also described [33,57,64]. The decline in rod density is supposed to be due to the decline of the neurons which are connected to them (such as the rod bipolar cells in the outer plexiform layer) [36]. Retinal pigment epithelium (RPE) neovascularization from retinal vessels appears also to be characteristic of most animal models undergoing PR cell degeneration [65]. In humans, age-related macular degeneration (AMD) is a complex eye disease. The overall prevalence of age-related macular degeneration is estimated to increase 7-fold, from 4.2% in those aged 45–49 years to 27.2% in those aged 80–85 years [66]. Based on the appearance of specific symptoms, we can speak about the more common non-neovascular (dry, nonexudative) AMD or the less prevalent neovascular (wet, exudative) types [67]. However, it is difficult to find the border between the changes caused by aging and the start of the pathology; several classifications or grading systems are known. The guidance of the National Institute for Health and Care Excellence (NICE) recommends distinguishing three states, depending on the presence, size and approximate number of drusen, the degree of neovascularization and pigmentary abnormalities. These are namely early, intermediate and advanced [68]. In 2008, Takahashi et al. described new classifications and diagnostic criteria for AMD therapy. According their suggestion, the prodominal stage can be divided into soft drusen and retinal pigment epithelial abnormalities, and AMD is classified into exudative, atrophic macular degeneration [69]. The Clinical Age-Related Maculopathy Staging System (CARMDS) classifies AMD into 1 of 5 mutually exclusive stages [70], while Sandberg’s system suggests four grades. In addition, the Age-Related Eye Disease Study (AREDS) recommends a nine- and a four-step scale for classifying the stage of the disease [71].
4.2. Cellular and Subcellular Changes
In the RPE, one other major marker of aging is the abnormal accumulation of lipofuscin that originates from the incomplete phagocytosis of the PR outer segments. Accumulation of the undegradable end product generates superoxide ions, singlet oxygen, hydrogen peroxide and lipid peroxides [72]. The lipofuscin fluorophore A2E mediates the apoptosis of RPE in a wavelength-dependent manner. Blue light (400–520 nm) generates more superoxide anions than red light (660–730 nm) or full white light [73]. In addition to the intracellular deposit of lipofuscin, an extracellular deposit also appears in the retina with age that is known as drusen. This debris is localized between the RPE basal lamina and the inner collagenous layer of the Bruch’s membrane. Drusen mainly contains proteins such as the serum amyloid P component, apolipoprotein E, vitronectin, immunoglobulin light chains, factor X, complement proteins and Alzheimer’s Ab-peptide [74]. Permeability of Bruch’s membrane and vascular basal membrane also decreases with age due to the accumulation of esterified cholesterol [75].
These morphological changes correlate strongly with functional disparities. Electroretinogram (ERG) analysis in the retina revealed the reduced amplitudes of rod- and cone-mediated responses in the elderly [35,76,77,78,79]. These studies also concluded that functional discrepancies measured by ERG could be due to a reduction of the number of PRs but even more, the mislocalization of PR synaptic terminals that caused a decline in PR transduction efficiency [79]. The amplitude of multifocal oscillatory potentials, which are thought to reflect inner retinal function and the rod–cone interaction, decreases linearly, whereas their latency increases with age [59,80]. Other investigations suggest an altered inflammatory response with age. Several inhibitory molecules keep retinal microglia and the complement system under tight control in normal physiological conditions.
4.3. Molecular Considerations
Just like cells in other organ systems, retinal cells suffer from accumulative oxidative load and metabolic stress in the aging process. Increased oxidative stress and the accumulation of damaged molecules lead to the dysfunction of various metabolic and signaling pathways which, in the retina, ultimately result in pathologies such as glaucoma or age-related macular degeneration, as well as the neuropathic complications of diabetes in the eye. The ROS particles originate mainly from mitochondria as part of the production of ATP by the classical glucose oxidation pathway, but mitochondria also regulate the intracellular pH, calcium concentration and contribute to apoptotic signaling pathways [81]. mtDNA mutations often associate with cytochrome c oxidase-deficient cones and ever more accumulate in PRs with age, mostly in the foveal region [82]. The partial loss of proteasomal function and expression are also typical phenomena in the retinae of elderly rats [80,83]. Proteins originated from drusen were also rich in post-translational oxidative modifications, such as carboxyethylpyrrole protein and advanced glycosylation end products (AGEs) [84]. Other investigations suggest an altered inflammatory response with age through the activation of the receptor of AGE [80]. Elevated levels of AGEs have also been found in retinal blood vessels, serum, and vitreous of diabetic patients. Several inhibitory molecules keep retinal microglia (certain interleukins) and the complement system under control in normal physiological conditions [85].
Besides the complex processes which occur during aging, aformentioned age-related structural and functional alterations show similarities with the initial phase of pathologies in many cases. To recognize the disparities and border between normal aging and the pathological state can be complicated but provides valuable information for the prevention or compensation of the processes of disease development. Two such major disorders are DR and glaucoma (see Section 5 and Section 6).
4.4. Preventive and Therapeutic Measures during Aging
The best characterized age-related retinal complication is AMD. A strong evidence-based empirically developed antioxidant formulation was introduced in the AREDS (Age-Related Eye Disease Studies). The effects were analysed in a blinded, randomized and controlled study on several thousand patients. The original AREDS formulation contained β-carotene, vitamin C, vitamin E and zinc, among other components. Further nutrients and nutraceuticals with antioxidant, anti-inflammatory and anti-apoptotic properties have been extensively studied to employ them for neuroprotection [86]. Immunostimulatory effects of some herbal compounds have been documented which include increased levels of cytokines (IL-2 and TNFα), resulting in retinal microglia activation. Pharmacophytones also interact with anti-apoptotic pathways and decrease proapoptotic pathways (e.g., NFκB, caspase activity), thereby preventing apoptosis and autophagy [87]. Herbal formulas and plant extracts may also exhibit anti-angiogenic effects by inhibiting VEGF signal transduction [88]. Thus, herbal extracts initiate various mechanisms to preserve the retina from neurodegeneration [87]. Table S1 in the Supplementary Materials collects herbs and active agents applied on humans and animals in connection with the therapy of retinal degenerations, such as AMD, DR and glaucoma [18,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133].
Epidemiological evidence has shown that patients with lower concentrations of macular pigment optical density measurements are at a higher risk of developing AMD [94]. Participants with the highest self-reported dietary intake of a mixture of vitamin A, vitamin C and vitamin E had a larger effect on the reduction of AMD risk than the individual vitamins [134].
Common pathophysiological pathways of ocular pathologies can be established due to metabolic stress that causes an elevated glutamate realisation and an insufficient supply of nutrients to the respective target structure, mainly on ganglion cells and a specific type of amacrine cells which contain ionotropic glutamate (N-methyl-D-aspartate, NMDA) receptors. Several potentially neuroprotective drugs have already been administered to reduce or prevent the death of retinal neurons. Table S2 in the Supplementary Materials [135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150] collects synthetic drugs applied on humans and animals in connection with the therapy of retinal degenerations, such as AMD, DR and glaucoma. The mode of action is mainly to block NMDA receptors or to interrupt the influx of calcium and sodium into cells to prevent the generation of free radicals linked to the formation of AGEs and/or advanced lipoxidation end products, as well as to avoid defects in the mitochondrial respiratory chain [151]. To evaluate current and new drugs that are neuroprotective, the following criteria must be met: there is a specific target in the retina/optic nerve; the drug must reach the retina at levels sufficient to activate its target; it must have a mechanism of action that enhances a neuron’s resistance to stress or suppresses toxic insults; and there should also be demonstrated activity in human clinical trials.
Recently, a transgenic mouse model has been developed where somatostatin (SST)—containing amacrine cells—have been tagged with the red td-tomato fluorescent marker. This way, the fate of these cells could be followed during the aging process. SST is an important marker of retinal health since it is downregulated in the diabetic eye, and SST-containing eye-drop treatment prevented ERG abnormalities, glial activation, apoptosis and a misbalance between pro-apoptotic and survival signaling in rats with DR [152]. If these animals received chronic pituitary adenylate cyclase-activating polypeptide (PACAP) injections every three months, the loss of SST cells from the aging retina could be reversed [153]. PACAP was known for a long time to inhibit the development of DR through increasing anti- and decreasing pro-apoptotic factors in different retinal and other injuries [154]. This mechanism involves also the upregulation of PAC1 receptors on dopaminergic amacrine cells of the retina [155]. Since the 27 amino acid forms of PACAP could be applied successfully in eye drops [156,157], the possibility of a convenient non-invasive preventive treatment option arises.
5. Diabetic Retinopathy
More than 536 million people were diagnosed with diabetes in 2021, and this will reach 783 million in 2045 [158]. Globally, an increasing number of patients with diabetes also predicts the rising prevalence of DR. DR is the leading cause of preventable vision-loss in the working population and affects one-third of diabetic patients [159], and 90% of patients will have DR within 25 years of diagnosis [160]. It is a complex, multifactorial neurovascular disease. Early and advanced stages can be distinguished during the disease development. In the advanced phase, the structural lesion is significant, and the visual acuity is also affected.
5.1. Risk Factors and Disease Progression
At the beginning of the pathomechanisms, hyperglycemia promotes microvascular degeneration and blood-retina-barrier (BRB) breakdown. Apoptosis of pericytes and endothelial cells, capillary occlusion and increased vascular permeability are also present at the early non-proliferative phases of DR. The microcirculatory pathology generates a hypoxic/ischemic environment in the retina. Due to the insufficient oxygen supply and hyperglycaemia, ROS production increases, and it accelerates several intracellular alterations such as oxidative stress, endoplasmic reticulum stress and mitochondrial dysfunction. Moreover, neuron-glia interaction changes and leads to a global neurovascular unit (NVU) impairment [161]. NVU consists of glial cells, neurons and a vascular network of the retina, and its damage causes the disruption of multiple retinal cell types. Oxidative stress aggravates the NVU impairment, and the expression of inflammatory mediators and VEGF level increases. The formation of neovascularization and vascular leakage means the beginning of the proliferative phasis. The BRB breakdown causes diabetic macular edema (DME) which is one of the leading causes of vision loss in DR besides neovascularization [161,162]. Vascular abnormalities (vascular leakage, neovascularization, venous dilatation, capillary hyperpermeability, reduced reperfusion etc.), an accumulation of subretinal or intraretinal fluid, decreased oxygen and nutrients supply and macula thickening are the main constituents of the DME pathomechanism [163,164]. Its classification is based on the angiographic pattern (focal or diffuse) [165,166] or on optical coherence tomography images (serous retinal detachment, diffuse thickening, cystoid macular edema) [167,168]. Their treatments are based on intravitreall pharmacological interventions and device-assisted operations (laser photocoagulation, victreoctomy) (see later in Section 7.1, ‘Device-assisted minimally invasive therapy plus drug application approach’ paragraph).
5.2. Cellular Stress Response: Oxidative Stress and Autophagy in DR
Chronic hyperglycaemia induces the alterations of the main molecular pathway networks such as polyol-, AGE-, hexosamine-, ROS-, PKC pathways. These are closely related to the dysregulation of autophagy, mitochondrial dysfunction, oxidative stress, glial activation and inflammation in the progression of DR [169,170]. These biochemical and cellular causative elements are responsible for neuronal alterations (neurodegeneration, neuronal apoptosis, altered neuronal communication, gliosis) and also for vascular degradation (BRB disruption, ischemia, neovascularization) [171]. Their causative role in the development of DR highlights the importance of research, which reveals the mechanisms pointing toward promising therapeutic targets.
Prolonged hyperglicemia, advanced glycation and hypoxia promotes the accumulation of ROS and causes endoplasmatic reticulum stress which are responsible for autophagy flux impairment and apoptosis [172,173]. Increased ROS production leads to an imbalance in redox homeostasis which results in neuronal cell loss, vascular abnormalities and the occurrence of advanced DR [16,174].
Autophagy belongs to the essential cellular homeostatic pathways which mediate degradation and recycling of intracellular compartments via lysosomal degradation. It has a meaningful role in the intracellular quality control by removing damaged or toxic cellular constituents and promotes the production of required metabolites. As a response to stress, dysregulation of autophagy was described in several retinal diseases and became an important therapeutic target in treatment strategies [171]. Triggered autophagy contributes to neovascularization, cell death, vascular damage and BRB breakdown [175,176]. The modulation of autophagy has a complex role in the pathomechanism of DR. On the one hand, it reduces apoptosis and neurovascularization but on the other, its pro-apoptotic effect has also been described. This dual phenomenon depends on the strength of cellular stress: it is pro-survival and anti-apoptotic in the early stage of DR but meanwhile, when the cellular stress is stronger, it could also provoke cell death in the advanced stage [177,178]. Lopes and his colleagues have described autophagy dysregulation, a higher VEGF expression and apoptotic rate in Müller cells under high glucose conditions; moreover, rapamicin helped to avoid those [179]. Autophagy has increased in RPE cell culture under high glucose conditions, and the inhibition of autophagy caused increased ROS levels [180]. Increased autophagy has also been described in induced diabetic mice retinas [181,182] and rats [183] and the blockade of autophagy has resulted in neuron protection.
5.3. Immune Response: Neuroinflammation and Glial Cell Activation in DR
The retina is immune privileged and usually, activation of retinal glial cells is the first reaction to harmful stimuli. Microglial cells are the main reactive immune cells and important homeostasis keepers in the retina. This type of cell monitors the retinal environment and responds effectively to different types of challenges by changing their activation status. They change their morphology, immunoreactivity and migration based on complex microglial–neuronal contact [184,185,186,187]. At the beginning, they try to maintain tissue integrity but as a consequence of prolonged stress, they become overly activated and express several inflammatory markers (chemokines, cytokines, cytotoxins). Hyperglycaemia, hypertension, oxidative stress, apoptosis, AGE and advanced lipoxidation end products production are together responsible for the induction of the inflammatory signaling in DR [188,189]. Inflammation is a nonspecific response of the immune system to harmful stimuli and one of the prominent factors in DR. This immune activation is responsible for structural and functional alterations in DR, which was identified as a chronic low-grade inflammatory disease of the retina [190,191,192]. Several inflammatory mediators, such as leukoctyes, cytokines [193], adhesion molecules [194,195], growth factors [196] and chemokines [197,198] contribute to the formation of the inflammatory milieu [199]. In a rat DR model, microglial cell status and morphology changed with the progression of diabetes [200,201]. Müller cells also express cytokines (IL-1β—Il-6, IL-8, TNFα), growth factors [202] and several other related factors (such as inducible nitric oxide synthase, prostaglandin E and glial fibrillary acidic protein (GFAP)) under high glucose conditions [190,203,204,205,206,207].
5.4. Therapeutic Approaches
Non-synthetic compounds of natural origin are a popular approach in treating diseases. In the PubMed database alone, there are close to one million entries for the isolation and use of such substances from antimicrobial purposes to cancer research. For DR alone, more than 200 extracts were tried with more or less success.
5.4.1. Flavonoids, Polyphenols and Other Natural Antioxidants (Table S1)
Flavonoids and polyphenols function as terminators of free radical chains and chelators of redox-active metal ions catalyzing lipid peroxidation. Baicalein (5,6,7-trihydroxy-2-phenyl-4H-1-banzopyran-4-one) is a flavonoid originally isolated from the roots of Scutellaria baicalensis. [208]. With its anti-inflammatory properties, this compound blocks high glucose- induced microglial and astroglial activation and VEGF overexpression, thereby preventing the secretion of inflammatory and/or cytotoxic factors, consequently protecting neurons and vasculature from damage in DR [190]. Eriodictyol is a strong antioxidative flavonoid extracted from Eriodictyon californicum. Treatment with eriodictyol reduces TNFα, VEGF, intercellular adhesion molecule 1 (ICAM-1) and endothelial nitric oxide synthase in DR in rats and also prevents BRB breakdown [209]. Similar properties were found for hesperodine which also prevents AGE accumulation [180]. Green tea extract is widely studied for its beneficial properties protecting against brain ischemia, and it is a rich source of polyphenols. After 12 weeks of oral green tea treatment, diabetic rats restored low GFAP, ROS, glutamine synthetase, glutamate transporter and receptor levels in the retina [210]. At the same time, the expression of proinflammatory parameters (TNFα and VEGF) was significantly inhibited [211]. Chlorogenic acid is a polyphenol found in coffee, beans, potatoes and apples. It has beneficial effects on glucose metabolism [212,213]. It reduces VEGF expression and decreases BRB breakdown [214].
Tinospora cordifolia has a long history of use in Ayurvedic medicine. It contains many pharmacologically active ingredients, such as alkaloids, glycosides and steroids. Users maintain that it reduces DR due to its anti-hyperglycemic, anti-angiogenic, anti-inflammatory and antioxidant properties and prevents cataract and vascular changes [215,216]. This effect may be due to octacosanol, which is reported to downregulate VEGF gene expression by inhibiting matrix metalloproteinases and nuclear translocation of NFκB and reducing its DNA binding activity. Both glutathione and catalase levels increased in the treated group as compared to the diabetic group [215]. It decreases the overexpression of VEGF and alleviates the effects of high glucose [202]. Further substances examined for their protective effects in DR include curcumin [217,218], Ginkgo biloba leaf extract [219], zeaxanthin [220], lutein [221], fenugreek (Trigonella foenum-graecum) and resveratrol [118], all resembling each other in their mode of action.
With a different molecular target, berberine significantly decreased peroxisome proliferator-activated receptor-γ expression in diabetic retina [222]. Ginseng plants (Araliaceae), including North American ginseng (Panax quinquefolius) root extracts, have multiple pharmacological actions because of their diverse phytochemical constituents. Ginsenosides are its major bioactive factors. Ginsenosides or panaxosides are the derivatives of protopanaxatriol, a class of steroid glycosides, and triterpene saponins [223]. Bioactives of ginseng possess antioxidant properties, quenching free radicals, protecting low-density lipoproteins from oxidation and inhibiting lipid peroxidation [224].
Limitations of herbal formulas are worth mentioning, since most of them are not well established in human clinical research. A majority of them have been tried and justified in animal experimental models or in vitro pilot studies, which were translated to possible human applications. However, only a few of them became evidence based or clinically tried. Due to inevitable environmental factors, some herbal formulae may even contain some heavy metal contaminations. Unlike in the case of synthetic drugs, the effective molecules cannot always be clearly identified in the case of herbal medicines. Further, according to Bürgi’s law (see below Section 7) and principles of herbalism, plant-derived remedies rather have combined synergistic effects than just one useful molecule, so they cannot be simplified to a single mechanism.
5.4.2. Endogenous Retinal Factors
There are several factors and processes associated with the neuron survival controlling oxidative status, autophagy and neuroinflammation in the retina [225]. These factors target different participants of the degeneration mechanisms and have already proved their therapeutic evidence in DR but are occasionally similar to those which play a role in glaucoma or age-related pathologies.
Erythropoietin (EPO) treatment reduced elevated IL-1 and TNFα expression in Müller cell culture and increased brain-derived neurotrophic factor (BDNF) expression in diabetic rat retinas [226,227]. Furthermore, EPO promotes ganglion cell survival in a rat model of glaucoma [228]. It ameliorates pro-survival signals involving Akt and reduces the loss of pericytes in the diabetic retina. EPO can reduce superoxide and other radicals, inducing glutathione peroxidase (GPX) and thus improving the balance between pro- and anti-oxidative factors and stimulating p-Akt. EPO treatment reduces pericyte dropout and also protects RPE cells against increased permeability. This effect is mediated by the downstream signaling of Janus kinase 2 and PI3K/Akt pathways [229].
Elevated pressure caused an increased pigment epithelium-derived factor (PEDF) and PEDF-R expression, which was related to Müller cells and ganglion cells in glaucoma. In addition, inhibition of PEDF expression resulted in increased apoptosis [230]. PEDF treatment has a reduced expression of several inflammation markers (such as IL-6, TNFα) in mouse models of DR [204]. Glucagon-like peptide 1 receptor agonist reduced microglia activation and TNFα expression in glaucoma models [231]. Furthermore, its topical administration was neuroprotective in a DR mouse model [232]. It also alleviates oxidative stress, apoptosis and autophagy in retinal cells of rats with induced diabetes [173].
Ciliary neurotrophic factor (CNTF) expression is altered in ganglion cells after glaucoma induction [233] and had a neuroprotective effect in glaucoma models of rat retinas [234,235]. Another similar compound, BDNF is expressed in RGCs and Müller glia [236] and causes delayed ganglion cell loss in glaucoma models [237] and attenuates abnormal microglial autophagy in DR [238]. Moreover, its role in AMD progression also has been investigated [239]. BDNF protects the neurons through (i) TrkB receptors, (ii) insulin responsive pathways and (iii) reduces systemic glucose levels locally in the retina. Inhibition of Müller cells and microglial connection attenuated microglia proliferation in a glaucoma model [240].
Autophagy flux decreased and apoptosis increased in ex vivo mouse retina explants under high glucose conditions, where application of the somatostatin analogue octreotide prevented cells from apoptosis. This protective effect of octreotide was demented by autophagy inhibition with chloroquine [241].
Besides different trophic factors, several endogenous antioxidant defence systems regulate the intracellular stress responses in the retina. When ROS production and antioxidants’ activity are imbalanced, oxidative injury and different retinal pathologies will occur [242]. ROS overproduction and decreased antioxidant defence engender damages of biochemical molecules and, if the repair mechanisms are inadequate, lead to apoptosis and neurodegeneration [242,243]. The main inducers of ROS production are hypoxia, metabolic defects, endoplasmatic reticulum stress and oncogenes and, on the other side, fundamental ROS scavengers are glutathione, NADPH, Nrf2, tumour suppressors and dietary antioxidants [244]. Antioxidants based on their origin could be exogenous (e.g., vitamin C, vitamin E, carotenoids) or endogenous. Depending on their activity, actions can be detected as enzymatic or nonenzymatic [245]. Furthermore, several studies have provided evidence for the lack of vitamin D triggering the pathogenesis of diabetes [246], including its accompanied syndrome DR [247]. This vitamin is a potent inhibitor of retinal neovascularization [247] through effectively decreasing VEGF levels [248].
Endogenous antioxidants are enzymes (e.g., superoxide dismutase (SOD), catalase, glutathione S-transferase, GPX, hem-oxygenase) or non-enzyme compounds (e.g., glutathione, melatonin, lipoic acid) and metal binding proteins (e.g., ferritin, transferrin) [249]. In the retina, the availability of different endogenous antioxidants decreases during metabolic retinal pathologies. In glaucoma, DR and aging models, increased ROS production [242,243,244,245,246,248,249,250,251,252,253] and decreased availability of different endogenous antioxidants has also been described in these pathologies [254,255,256,257,258,259].
Additionally, catalase, GPX, glutathione-reductase and copper-zinc SOD expression were downregulated in db/db mice [260]. Moreover, intravitreal SOD3 treatment prevented PEDF and parvalbumin expression and also reduced GFAP expression and ERG alteration [261]. Melatonin treatment reduced IL-6, TNFα, VEGF expression and apoptotic activity in STZ-induced diabetic retinopathy [262]. Photoreceptors showed lower catalase and higher lipid peroxidation in aging rat compared to younger rat retinas [263], and catalase-KO mice showed faster aging phenotype and impaired autophagy [264]. Decreased macroautophagy has been described in the aging retina. Furthermore, alterations in autophagy flux are also present in glaucoma and DR. Under high glucose conditions, autophagy is upregulated in Müller cells and rapamycin treatment could reset the autophagy and, as a consequence, VEGF expression remains at the baseline level [265].
5.4.3. Synthetic Drugs
To manage DR by synthetic drugs, the major options are synthetic corticosteroids and macular targeting agents (See in Table S2 [135,136,137,138,144,147,148,149,266,267,268,269,270,271,272,273]). While macular target agents interfere with one or more pathways involved in the pathogenesis of DR, corticosteroids affect the anti-inflammatory pathway. Intravitreal injection of corticosteroids such as triamcinolone acetonide could provide more beneficial therapy than anti-VEGF. Besides the suppression of VEGF, it also inhibits prostaglandin production. Various cytokines are suspected to be involved in DR pathogenesis and have anti-apoptotic effects on retinal neurons [138,268,274,275].
Today, the most common molecular agents are the VEGF-inhibitors. Several clinical studies have demonstrated the advantage of intravitreal anti-VEGF injections (aflibercept, bevacizumab and ranibizumab) in reducing retinal neovascularization in patients with DR [137,147,148]. Bevacizumab binds and inhibits all isoforms of VEGF, but with a lower affinity, and has a longer half-life than ranibizumab [138,268]. Use of VEGF inhibitors in combination provides more benefits to therapy than standalone ones (see Section 7.2). Anti-VEGF inhibitors (e.g., bevacizumab, pegaptanib, ranibizumab, aflibercept) are also first-line medical options in DME treatment [276,277].
NMDA antagonists (such as Memantine) delay or halt ganglion cell death effectively, but the clinical use of NMDA antagonists is limited by their side effect profile, whereas NMDA receptors are widely presented and involved in different processes in the central nervous system. However, the combined usage could provide a promising strategy for DR or glaucoma therapy [81,147].
As we mentioned earlier, the main risk factors of DR are hyperglycemia, hypertension and dyslipidemia. They have a critical role in evolving a retinal inflammation state via different processes, e.g., oxidative stress, nitric oxide synthase dysregulation, AGE formation, renin-angiotensin system activation or inhibition of endogenous anti-inflammatory pathways. It is known that elevated levels of AGEs are present in pericytes, retinal blood vessels causing a blood–retinal barrier dysfunction and disruption of microvascular homeostasis. Disrupting the AGE, AGE receptor interaction is an important way to reduce the unwanted effect of accumulated AGEs. Furthermore, AGEs activate different intracellular pathways, NFκB and NADPH oxidase, ERK1/2, Janus Kinase signaling pathways, to increase ROS production and apoptosis of retinal neurons. Lower levels of the anti-apoptotic pERK and an elevated level of the pro-apoptotic marker pJNK protein are characteristic in aging and diabetic animals [85,278,279].
It has also been proved by numerous investigations that inhibition of the key regulator elements (TNFα [145,280], NFκB [135], PARP [281,282] or cyclooxygenase (COX) [283]) in diabetic-induced inflammatory response is effectively usable for delaying DR-associated vascular complications. COX-1 and COX-2 are key enzymes in the conversion of arachidonic acid to prostaglandin that are involved in inflammation. Through an elevated expression of VEGF, they cause vascular leakage and neovascularization [271], so COX inhibitors (acetyl salicylic acid, nepafenac) block inflammation indirectly. In the early stage of diabetic rat retinas, inhibition of COX-2 (but not COX-1) provides an effective management to reduce enhanced prostaglandin secretion. They also inhibited the diabetes-induced apoptosis of endothelial cells and pericytes, or the degeneration of retinal capillaries [136].
6. Glaucoma
Glaucoma is a heterogeneous group of progressive optic neuropathies characterized by typical optic nerve head alterations and the loss of RGCs. Among eye disorders, it is the second major cause of vision loss behind cataract and was the most common cause of irreversible blindness throughout the world in 2022 [30,86,87,284,285]. Glaucoma is expected to affect approximately 112 million people between 40 and 80 years of age by 2040 [18,30]. The incidence among the Caucasian population in the 40-year-old age group is approx. 2–3.5%, which increases with age, and in the 70-year-old age group, it is already 10%. Some types of glaucoma show familial accumulation, although the exact genetic background is still under intensive research. Only few animal models are at service for research: elevated IOP [286,287,288] and monosodium glutamate administration in young ages [289].
6.1. Risk factors and Disease Progression
Several risk factor can be considered when underlying causes are listed for glaucoma. Ocular perfusion pressure and optic disc haemorrhage, systemic hypertension, type 2 diabetes mellitus, smoking and/or high myopia and lipid dysregulation may all increase the risk for primary open-angle glaucoma (POAG) [30,108,285]. The most prominent modifiable risk factor however is the IOP exerted by the aqueous humour produced in the eye. The IOP varies individually, between 12 and 21 mmHg. Primary glaucoma is defined if there is no other ophthalmic or systemic disease besides glaucoma. In secondary glaucoma, the disease develops for a known reason [285]. In cases of normotensive glaucoma, a significant number of patients have shown optic nerve damage and vision loss, but not all ocular hypertensive patients develop glaucoma [118]. In glaucoma, the degeneration of RGCs and their axons in the optic nerve fibre layer accelerates, which is associated with damage to the optic nerve and causes visual field deficits. As soon as optic nerve loss and/or visual field loss develops as a result, glaucomatous excavation is detected and the diagnosis is established [30,285]. Retinal nerve fibres do not regenerate, so the potential target in the treatment of glaucoma aims to slow or prevent the progressive disruption of RGCs and a loss of their axons.
The progression usually stops if the IOP is lowered by 30–50% from its baseline [30]. Despite being on a combination of eye drops, patients may fail to achieve goal IOP reduction, and laser or surgical interventions are considered. Although high IOP is a risk factor for glaucoma progression, glaucomatous optic neuropathy can occur in individuals with a normal IOP [116]. Thus, interventions that focus solely on IOP reduction may not be beneficial for some glaucoma patients [129]. However, it is difficult or impossible to completely stop the progression of glaucoma, and many glaucoma patients experience progressive neurodegeneration [284]. It is therefore a potential target for new neuroprotective, IOP-independent therapeutic approaches [86].
6.2. Cellular Mechanisms
The death of RGCs occurs via apoptosis. Glutamate is the most abundant excitatory neurotransmitter in the retina. Excessive glutamate releases and the subsequent influx of Ca2+ via NMDA receptors has been implicated as the underlying cause of RGC death [118]. Several molecular mechanisms including neurotrophic factor deprivation, or the inhibition of inflammatory cascade, oxidative stress, mitochondrial dysfunction, glutamate-excitotoxicity, autophagy dysregulation, protein misfolding, ischemia and hypoxia. Each of these pathways may contribute to the etiology and progression of the disease group [18,86]. Neuroprotection in glaucoma is similar in concept to the treatment of central nervous system diseases including cranial trauma, stroke, amyotrophic lateral sclerosis, Parkinson’s disease, Alzheimer’s disease etc. [284,285]. The progression of the optic nerve and retinal neurodegeneration and pathogenesis of glaucoma have several hypotheses, but the neurodegenerative process often extends along the visual axis affecting the lateral geniculate nucleus and visual cortex [86]. Furthermore, these molecular events should not be considered as isolated but rather as a sequence of interconnected mechanisms where sustained oxidative stress acts as a key factor. Indeed, the imbalance between reactive species production and endogenous antioxidant defences sustains a vicious cycle that promotes chronic inflammation and creates a hostile environment for neuronal survival [86]. Retinal ischemia has been studied extensively because it has been proposed to be involved in a number of optic neuropathies such as anterior ischemic optic neuropathy, glaucoma, retinal and choroidal vessel occlusions, retinopathy of prematurity, DR and traumatic optic neuropathy [87]. The most fundamental neuropathy of all forms of glaucoma, characterised by a loss of the neuroretinal rim and a widening of the cup in the optic disc, is an injury due to the strangulation of optic nerve fibres in the optic disc, especially in the lamina cribrosa (LC). The increased pressure difference across the LC causes stress and strain on this structure, resulting in eventual compression, deformation and remodelling and therefore impedes anterograde and retrograde axonal transport within the optic-nerve fibres. Secondary stretching and thinning of LC can be found in progressive high myopia in association with an elongation and thinning of the parapapillary tissues that could lead to pronounced changes in the biomechanics of the optic nerve head and an increase in glaucoma susceptibility [30]. Although the mechanism of glaucoma is still largely unknown, oxidative stress, optic nerve ischemia and neuroinflammation were found playing certain molecular roles in the development of glaucomatous optic nerve degeneration [129]. Furthermore, new evidence suggests that glaucoma could be an autoimmune disease with progressive retinal degeneration caused by T-cells [290].
6.3. Molecular Considerations
There is evidence that dying neural cells create a toxic internal milieu which can affect healthy cells surrounding it. The essence of neuroprotection involves protection afforded to these healthy cells from damage. Therefore, there is an urgent need to improve therapeutic approaches to RGC damage that can be applied worldwide [87]. Interestingly, retina has its own growth and protective factor agent: the retinal glial cells and the RPE [145]. Neurotrophic factors are known to enhance the cell survival mechanisms and hence exert neuroprotective effects towards injury and degeneration. Müller cells and astrocytes are the two types of glial cells that express GFAP in the retina. Excessive GFAP levels are markers of metabolic stress in the retinal tissue. Moreover, a glial cell line-derived neurotrophic factor is also produced which has been shown to promote the survival of RGCs when introduced in different retinal injury rat models. [132]. RPE is also an important layer of retina which provides nutrition to other retinal layers and is thus indispensable for proper growth and survival. It produces several peptides (bradykinin, cortistatin, orexin, urocortin), and most of them seem to be neuroprotective [288,291]. RPE damage due to oxidative stress is one of the various factors responsible for pathogenesis leading to AMD [87].
The retina is particularly susceptible to oxidative stress due to its high consumption of oxygen, and this susceptibility increases with aging due to the physiological decrease of antioxidant defence mechanisms [292]. Clinical and experimental studies have reported that there is a significant correlation in human trabecular meshwork (TM) between oxidative stress, inflammation, increased IOP and visual field defects in glaucoma patients. As confirmed by previous studies, ischemia results in a reduced flow of blood to the retina and elevated levels of free radicals such as superoxide anion, hydroxyl radical and hydrogen peroxide, along with a progressive depletion in endogenous antioxidant enzymes including SOD, catalase and GPX, or antioxidants such as GSH. Ischemia results in deprivation of oxygen to tissues and metabolic substrates, ultimately affecting waste recycling. These processes lead to a homeostatic imbalance, exacerbating injury. Retinal ischemia has been studied extensively because it has been proposed to be involved in a number of optic neuropathies such as AION, glaucoma, retinal and choroidal vessel occlusions, ROP, DR and traumatic optic neuropathy [87]. Oxidative stress leads to TM cell dysfunction and increasing aqueous outflow resistance [86,131]. High levels of oxidative stress markers have been found in the aqueous humour of patients with POAG and Primary Angle Closure Glaucoma (PACG) and in the serum of pseudoexfoliation syndrome (PEX) patients [86]. In a clinical study, patients with POAG exhibited a low level of circulating GSH, suggesting a general decrease of antioxidant capacity [118]. Excessive free radicals cause oxidative stress, damaging lipids, proteins and DNA and ultimately resulting in cell death. Therefore, ischemia results in deprivation of oxygen to tissues and metabolic substrates, ultimately affecting waste recycling. These processes lead to a homeostatic imbalance, exacerbating injury.
Some important protective factors, however, are produced by retinal neurons themselves. Brn3b is a major transcription factor that belongs to the mammalian POU family. It is a key regulatory marker of RGCs and is known to play an important role in RGC development and survival. BCL-2, an anti-apoptotic gene critical for the inhibition of programmed cell death [293,294,295], is known as an essential controller of PRs (rods, cones) and RGCs death in degenerated retinas [296,297,298].
6.4. Therapeutic Approaches
Currently, glaucoma cannot be cured, but with early detection and proper treatment of the disease, the rate of progression can be slowed and vision can be preserved [284,285]. Lowering IOP is the primary evidence-based therapeutic strategy including laser trabeculoplasty, glaucoma filtration surgery and antiglaucoma medications [30,284]. POAG is the most common type, and it differs from normal tension glaucoma in that in the former, elevated IOP occurs. Left uncontrolled, glaucoma has irreversible devastating visual consequences, therefore, it is called “the sneaky thief of sight” [134].
6.4.1. Some Herbal Remedies
Early pharmacological treatments of glaucoma relied on herbal remedies and diet, but the preparation of these medicines were empirical [129]. One of the first herbal-based active compounds used for glaucoma was pilocarpine—a parasympathomimetic alkaloid extracted from Pilocarpus jaborandi. It is still available for glaucoma management. It causes ciliary muscle contraction which facilitates the aqueous humour outflow through the TM [129]. Forskolin eye drops (1%) in India were applied in POAG to reduce IOP and visual field defects with efficient improvement of optic neuropathy. Hypothetically, Forskolin eye drops might be a safe alternative to β-blockers in glaucoma patients having concomitant asthma [89].
However, use of herbal medicines is not without potential side effects. Edington et al. explained in their case report that Hypericum perforatum L. may affect IOP control, because it is known to interact with several drugs, including β-blockers, as it induces the cytochrome P450 system and causes faster drug metabolism. Studies have previously confirmed high cytochrome P450-dependent monooxygenase activity in the ciliary body, iris and RPE in bovine and human eyes [30,299]. They reported the case of a POAG patient with suboptimal IOP control, which improved on discontinuation of the Hypericum perforatum product. It is, therefore, possible that the herbal compounds could have the same receptorial and enzymatic effect in the eye as it does elsewhere in the human body [299].
6.4.2. Synthetic Glaucoma Drugs
Besides herbal medicines, other pharmacological agents are in the toolset of preventing or circumventing glaucoma. Current treatment typically involves topical ocular hypotensives as first line therapy, and the six major drug classes are as follows: β-adrenergic blockers, α2-adrenergic agonists, prostaglandin analogues, carbonic anhydrase inhibitors, rho kinase inhibitors and cholinergic agonists (See in Table S2 [136,139,140,141,150,266,267,270,273,300,301,302,303,304,305,306,307,308,309,310,311,312,313,314,315,316,317,318,319,320,321,322,323,324,325,326,327,328,329]). These work to lower IOP by preventing aqueous humour build up in the anterior chamber via decreasing aqueous humour production from the ciliary body or increasing aqueous humour outflow through the trabecular meshwork or uveoscleral pathway [5,45]. For example, brimonidine is an α2-adrenergic agonist and is used to lower IOP and ocular hypertension in open-angle glaucoma. Several studies demonstrated that brimonidine could promote the survival of RGC [141,308,330]. Acheampong et al. proved that topically applied brimonidine widely distributes into the posterior segment of monkey and rabbit eyes after single and multiple dosing and that intraperitoneal administration of brimonidine also results in the significant availability of brimonidine in the posterior segment of rat eyes [330]. α2 receptors activate multiple pathways for instance the activation of intracellular kinases enhance cell survival, the induction of anti-apoptotic genes such as BCL-2, neuronal survival factors such as basic fibroblast growth factor and the inhibition of glutamate release and calcium influx into cells [141,308]. Another α2 adrenoreceptor agonist is apraclonidine which is used topically and can also decrease IOP in glaucoma patients by increasing trabecular outflow without cardiovascular side effects [267].
The β-blockers effectively lower IOP to decrease aqueous inflow and increase uveoscleral outflow during the day but not during the night [331]. Non-selective beta-adrenergic antagonists (carteolol, levobunolol, metipranolol, timolol) are mainly used in combination. It is important to know that they should not be given to patients with cardiac or pulmonary insufficiency [267,316,317]. Betaxolol is a β1-selective adrenergic antagonist, but it has less effects on cardiac and pulmonary functions than non-selective β-blockers. However, special attention has to be given when betaxolol is prescribed to patients with asthma.
Prostaglandin analogues are currently used as the most powerful agents to reduce IOP [307]. Several studies have demonstrated that the regulation of matrix metalloproteinase and remodelling of the extracellular matrix change the permeability and cause enhanced uveoscleral outflow with minor effects on trabecular outflow and aqueous flow [307]. However, remarkable effects on the cardiovascular system are characteristics for them [307,332]. An intracameral implant, brimatoprost is used to reduce IOP for up to four months after insertion in patients with glaucoma [333]. Preclinical and animal studies have demonstrated that tafluprost also promoted efficacy and safety in the treatment of glaucoma [322].
Another therapeutic approach is the application of carbonic anhydrase inhibitors to inhibit the activity of carbonic anhydrase 2 and HCO3− production in the non-pigmented ciliary epithelium, thus reducing aqueous humour formation. Acetazolamide and methazolamide are approved as oral medications, but topical eye drops are better tolerated, such as dorzolamide or brinzolamide [267,300,311,312,313,319]. In glaucoma, SOD, catalase, GPX and melatonin levels significantly decreased in rat retinas after 6 weeks of hyaluronic acid treatment [258]. GPX and catalase levels also decreased after sodium hyaluronate induction of ocular hypertension in rat retinas [259]. Furthermore, downregulation of glutathione was also described in glaucomatous eyes [261]. SOD and GPX levels decreased, and IL-6 and ICAM-1 levels increased in diabetic rat retinas [334]. Rapamycin treatment was neuroprotective by downregulating caspase-3 expression and inhibited TNFα, ROS and NO expression in different glaucoma models [335].
Rho(ρ)-kinase (Rho-associated coiled-coil containing protein kinase; ROCK), a member of the serine-threonine protein kinases, involve various physiological functions such as chemotaxis, neural growth and gene expression [336]. Most ROCK inhibitors reversibly compete with ATP, because their main target is the ATP-binding site of the kinase in its active conformation [337]. ROCK inhibitors (ripasudil, netarsudil, fasudil) have demonstrated efficacy in reducing IOP in animal models and humans [273,320,337,338,339,340,341,342]. Streptozotocin-induced diabetic models confirmed activation of the Rho/ROCK pathway in retinal microvessels, significantly reducing ICAM-1 expression and the number of damaged endothelial cells. The administration of oral ROCK inhibitor delayed RGC death [341,342]. The classic ROCK inhibitors, fasudil, is isoquinoline-derived and also inhibits other kinases including protein kinase C, protein kinase A and myosin light chain kinase [343]. Completed clinical trials verified the safety and effective application of 0.4% ripasudil to lower IOP [344,345,346]. However, usage with beta-blockers and prostaglandin analogues could provide more beneficial pressure-lowering effects [347].
7. Combination Treatments for the “Big Three”
Emil Bürgi, over 100 years ago, claimed that the combination of at least two different therapeutic substances or medications with equal or similar activities can lead to addition; on the other hand, when their points of action are different, they can produce greater synergy or coalism than the simple additive sum of their individual mechanisms (Bürgi’s law) [87,284]. It is important to remember that plant extracts used at that time for medications contained not a pure compound but other compounds that are present in order to have a therapeutic effect [88]. Experimentally proven pharmacological effects can be demonstrated by Curcuma longa L., which has limited oral absorption and systemic availability [90,91]. Piperine, a component of Piper nigrum L., is among the bioavailability enhancers used for this purpose. This agent, when used together with curcumin from C. longa, improves its bioavailability by 2000% [91].
In order to be effective in cures, the events which have to be avoided or prevented are (1) (para)inflammation and/or glial cell activation, (2) ischemia and related ROS and VEGF accumulation, (3) apoptosis and/or autophagy of nerve cells and (4) elevation of ocular perfusion pressure and/or IOP. Since the underlying causes in the case of the “big three” are similar, only the potential of the individual harming factors is different, and often the treatment options are also similar. Making things worse, in the case of aging, diagnosis is often difficult to tell apart from the other conditions, since patients may report vision changes in one of their eyes for a shorter or longer period of time, and characteristically, they describe central blurring in their vision and bending or waviness in straight lines and may report increasing difficulty reading print [348], which can be the hallmarks of the other two disorders at the beginning. Only detailed diagnostic examinations can confidently differentiate the disorders. In light of the above, we classified the combination treatments into the following categories: (i) device-assisted minimally invasive therapy plus drug application; (ii) antiangiogenic treatment plus drug application; and (iii) internal neuroprotective factors and drugs combined.
7.1. Device-Assisted Minimally Invasive Therapy plus Drug Application Approach
Since seriously invasive device-based therapies such as thermal laser photocoagulation have their limitations, therapies with other mechanisms of action are also solicited for. Possibilities for combination therapy are photodynamic therapy combined with anti-inflammatory drugs consisting of either corticosteroids or non-steroidal anti-inflammatory drugs. One of these treatments, photodynamic therapy combined with verteporfin administration, was recently evaluated by the Treatment of Age-Related Macular Degeneration with Photodynamic Therapy study group. The results showed that at 1 year, there was a 15% increase in eyes that retained better visual acuity than those that received a placebo. At 2 years, this ratio was 32% [349]. From the underlying causes listed above, this approach targets 3 and 1.
Adjunct to intravitreal pharmacotherapy (corticosteroids, anti-VEGF therapy etc.), other device-assistant evidence-based treatments are also available during the management of DR. These include laser photocoagulation and vitreoretinal surgeries [350,351,352]. Laser photocoagulation techniques (peripheral retinal laser photocoagulation, focal macular laser photocoagulation, grid photocoagulation) offer a reliable treatment option in proliferative retinopathy, macular oedema and retinal detachment. This type of laser surgery targets the newly formed and abnormal leaking vessels [353,354]. In clinical practice, another surgical technique is vitrectomy which serves for the treatment of vitreous haemorrhages, combined/tractional retinal detachment, macular heterotopia and fibrovascular proliferations [352,355].
In the case of glaucoma, the only proven and generally accepted treatment to reduce the risk of further progression of glaucomatous optic neuropathy is to lower IOP. Reduction of IOP is achieved by drug treatment, laser therapy or surgery. In eyes with an open anterior chamber angle, drug treatment could be augmented by, or in some cases replaced by, laser therapy (laser trabeculoplasty) to the trabecular meshwork, in particular if the target IOP is not achieved by use of drugs (particularly in poorly compliant patients). Independent of concurrent drug treatment, laser intervention can reduce the IOP by a few additional mm Hg. The good safety profile of laser trabeculoplasty is combined with its fairly low efficacy. If the IOP-lowering effect is not sufficient, incisional glaucoma surgery has to be done, usually under local anaesthetic but occasionally under topical anaesthesia. In patients with poor compliance or those intolerant to drug treatment, incisional surgery can also be conducted as the first step in the treatment of glaucoma. A panoply of surgical antiglaucomatous procedures have been developed in the past decade. Creating an additional outflow pathway from the eye for the aqueous humour, all surgical techniques (e.g., trabeculectomy) risk reduced long-term success secondary to fibrosis around the subconjunctival exit point of the fistula. During and after surgery, antimetabolites are applied to the surgical site to decrease the fibrotic response and to keep the fistula site open. Glaucoma implant drainage devices are another surgical option and act by channelling the aqueous humour through a tube out of the eye into the subconjunctival space. These devices are similarly effective in lowering IOP to trabeculectomy [356]. Minimally invasive glaucoma surgery, compared with standard trabeculectomy, has fewer side-effects but lower efficacy [357]. Similarly, trabeculectomy versus non-penetrating surgeries (e.g., deep sclerectomy, viscocanalostomy, and canaloplasty) is more effective at reducing the IOP but has a higher risk of complications. Treatment of congenital glaucomas is mainly surgical [30].
Recently, there has been increasing interest in the promising usage of stem cell therapy to achieve ocular tissue (RGC, RPE and photoreceptors) repair and/or regeneration. For the big three, only a limited number of studies based on stem cell-based therapies are currently completed worldwide (according to clinical trials.gov). Most of these trials are in the recruiting or unknown status. There are different methods of stem cell therapy. First, pluripotent stem cells can be used for the forward-moving development process to induce cell proliferation into the tissue of interest. Second, partially differentiated cells can be made backward in their developmental lineages and then redirect them onto the desired end tissue [358,359]. Their efficacy is dependent on the success of adult cells to return to the pluripotent stage, using molecular manipulation by retroviruses and lentiviruses to carry genes encoding transcription factors. This novel approach of glaucoma therapy is also represented in the recent scientific literature with the advances of the modified induced pluripotent stem cell technology. In cases of glaucoma that require tissue repair and regeneration of trabecular meshwork cells, RGCs are promising. Some modifications are required so that stem cells do not only have to differentiate into RGCs but also have to grow axons that are able to reach the retinal target areas in the central nervous system. The factors which guide the axons of the developing RGCs in order to reach the optic chiasm and stimulate synapse formation in the superior colliculus and the lateral geniculate nucleus still need to be better explored [360].
7.2. Anti-Angiogenic Injection Therapy and Drugs
Anti-VEGFs are classified as antibodies for VEGF and VEGF receptor-1 and -2 antibodies or alternatively, VEGF receptor tyrosine kinase inhibitors. Among combination therapies, anti-VEGF combination with sustained-release corticosteroids appears promising [87]. Additional candidates are natural products, but these may also serve as preventive measures. For each medication, there are several and severe adverse effects, but natural products have a potency as AMD, DR or glaucoma drugs, since they have been used as culinary materials and/or traditional medicines for a long time (see above in the relevant sections). In general, AMD drug candidates from natural products are more effective at treating early and intermediate AMD [361]. From the underlying causes, this approach fights 2 and 3.
It has been found that non-steroidal cyclooxygenase inhibitor nepafenac [193,362,363], salicylates [364] and reactive nitrogense species decomposition catalysts [365] inhibited RGC degeneration in DR. This offers an excellent opportunity to supplement bevacizumab treatment with drugs. In the case of patients with moderate visual loss or worse, aflibercept may be considered. Both the clinical trials and the laboratory investigations agree that besides the efficacy of the drug, the manner of administration may be the clue to treat retinal complications, since many of the drugs do not cross the BRB, but intraocular injection may be effective. This approach targets mechanisms 2 and 4.
In addition, the need for safe sustained delivery devices has emerged recently. The ultimate success of the delivery system will depend on efficacy relative to eye drop dosing, safety and patient acceptance. Cautious development efforts are warranted, considering prior failed approaches for sustained glaucoma drug delivery [366].
7.3. Internal Neuroprotective Factors and Drugs
Peptides acting through the insulin-like growth factor pathway attenuated the diabetes-induced apoptosis of retinal neurons and mitochondrial metamorphosis in RGCs. Consistent with these effects, it decreased cleaved caspase-3 and p-STAT3 levels. Rosiglitazone is an anti-diabetic drug in the thiazolidinedione class of drugs and works as an insulin sensitizer by binding to peroxisome proliferator-activated receptors in fat cells and making the cells more responsive to insulin. After treatment with rosiglitazone in experimental DR, the retina thickness and the RGC numbers were significantly greater than in control animals. Consequently, rosiglitazone might be used to prevent retinal neuronal damage in diabetes [367].
Targeting another pathway, with the combination of PACAP and the poly(ADP-ribose) polymerase inhibitors, good protection could be achieved in rat experimental DR models. This combination was useful in the case of diabetic animals with elevated systemic blood pressure (spontaneously hypertensive rats). Interestingly, this protection, provided by the distinct pathways 1, 3 and 4 listed above, seemed fully additive [368]. However, not all the combination treatments are successful. PACAP treatment alone and an enriched environment alone are neuroprotective, but their combination is not additive [369]. This situation may be due to overlapping intracellular protective pathways of the two protective mechanisms.
8. Conclusions
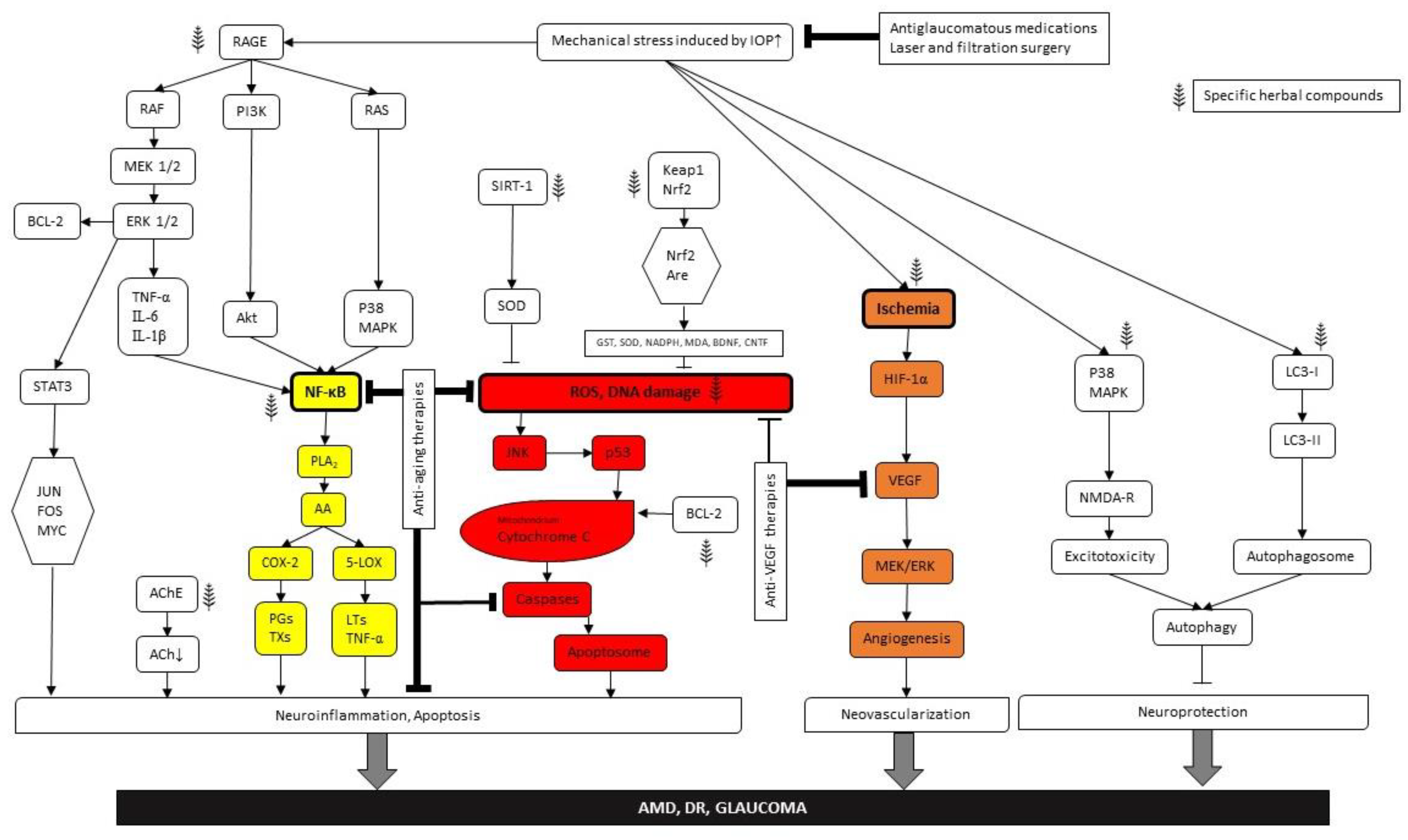
As for conclusions, we can state that there are common causes for diseases and common targets for protection in the case of the “big three”, age-related retinal degeneration, diabetic retinopathy and glaucoma. The intertwined molecular pathways are presented in Figure 2 as well as possible intervention possibilities. We identified three major nodes within the metabolic mesh at which effective interventions can be initiated: NFkB induction due to (para)inflammatory processes, ROS (DNA damage) reduction and anti-VEGF therapies. The first two lead to reduction of apoptotic damage, while the third one effectively inhabits neovascularisation, preventing retinal detachment and consequent vision loss. Besides direct targeting of the metabolic nodes of this network, we provided some proof that a healthy lifestyle can be an important factor in preventing metabolic retinal disorders. Furthermore, herbal medicines can be applied both for prevention and treatment purposes. We may also utilize the internal substances of our own body, particularly neuropeptides and trophic factors as well as synthetic drugs. There are a number of possibilities to combine these treatment options. However, it is important to note that we should target at least two of the four major harm-causing processes, namely (para)inflammation and/or glial cell activation, ischemia and related ROS and VEGF accumulation, apoptosis and/or autophagy of nerve cells and elevation of ocular perfusion pressure and/or IOP. This way, interventions maybe rather efficient. It might also be useful to seek repositioning of existing drugs and use them for the cure of the other related conditions. Targeting two distinct intracellular pathways may result in additive protection (Figure 2).
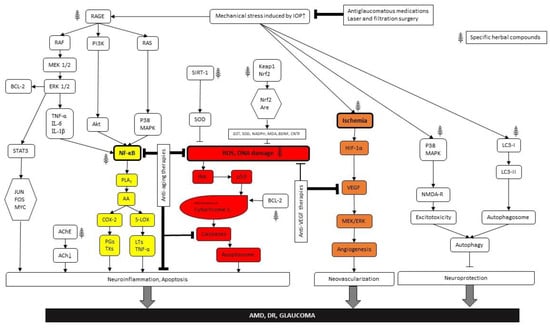
Figure 2.
Pathways presenting the evolution and therapeutic opportunities of the “big three” (AMD, DR, glaucoma). Arrows indicate the intracellular signal transduction pathways. Bold frames demonstrate the main nodes. Color coding means as follows: yellow—(para)inflammatory processes, red—DNA damage and orange—anti-VEGF therapies. Herbal signs demonstrate phitoremedial intervention sites.
Considering that vision loss threatens approximately 20% of the ever-growing and aging population of the world, safe, affordable and easily available therapies for the “big three” are badly needed. Some of the potential avenues to achieve these goals were outlined in this review.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24108728/s1.
Author Contributions
Conceptualization, R.G. and A.C.; writing—original draft preparation, A.K.-V., T.R., E.P. and R.G.; writing—review and editing, R.G., A.C., A.K.-V., T.R. and E.P.; visualization, T.R. and E.P.; supervision, R.G. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the European Union under the action of the ERA-NET COFUND (2019-2.1.7-ERANET-2021-00018) and was also funded by NN128293 and TKP2021-EGA-16.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analysed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors would like to acknowledge the Faculty of Sciences at the University of Pécs, especially the Biology and Sport Biology Ph.D. program.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
| AGEs | advanced glycation end products |
| AMD | age-related macular degeneration |
| AION | anterior ischemic optic neuropathy |
| AREDS | Age-Related Eye Disease Studies |
| BRB | blood–retinal barrier |
| BDNF | brain-derived neurotrofic factor |
| CNTF | ciliary neurotrophic factor |
| COX | cyclooxygenase |
| DR | diabetic retinopathy |
| DME | diabetic macular edema |
| ERG | electroretinogram |
| EPO | erythropoietin |
| GFAP | glial fibrillary acidic protein |
| GPX | gluthatione peroxidase |
| IL | interleukin |
| ICAM-1 | intercellular adhesion molecule 1 |
| IOP | intraocular pressure |
| NVU | neurovascularunit |
| NMDA | N-methyl-D-aspartate |
| NFκB | nuclear factor κB |
| PEDF | pigment epithelium-derived factor |
| PACAP | pituitary adenylate cyclase-activating polypeptide |
| PR | photoreceptor |
| POAG | primary open-angle glaucoma |
| ROS | reactive oxygen species |
| RGC | retinal ganglion cells |
| RPE | retinal pigment epithelium |
| ROP | retinopathy of prematurity |
| ROCK | Rho-associated coiled-coil containing protein kinase |
| PEX | serum of pseudoexfoliation syndrome |
| SST | somatostatin |
| SOD | superoxid dismutase |
| TM | trabecular meshwork |
| TNF | tumor necrosis factor |
| VEGF | vascular endothelial growth factor |
References
- Pollen, D.A. Explicit Neural Representations, Recursive Neural Networks and Conscious Visual Perception. Cereb. Cortex 2003, 13, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Victor, J.D. Analyzing Receptive Fields, Classification Images and Functional Images: Challenges with Opportunities for Synergy. Nat. Neurosci. 2005, 8, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Dowling, J.E. The Retina: An Approachable Part of the Brain; Belknap Press: Cambridge, MA, USA, 2012; p. 355. [Google Scholar]
- Pacione, L.R.; Szego, M.J.; Ikeda, S.; Nishina, P.M.; McInnes, R.R. Progress toward Understanding the Genetic and Biochemical Mechanisms of Inherited Photoreceptor Degenerations. Annu. Rev. Neurosci. 2003, 26, 657–700. [Google Scholar] [CrossRef]
- Osborne, N.N.; Casson, R.J.; Wood, J.P.M.; Chidlow, G.; Graham, M.; Melena, J. Retinal Ischemia: Mechanisms of Damage and Potential Therapeutic Strategies. Prog. Retin. Eye Res. 2004, 23, 91–147. [Google Scholar] [CrossRef]
- Atlasz, T.; Szabadfi, K.; Kiss, P.; Babai, N.; Koszegi, Z.; Tamas, A.; Reglodi, D.; Gabriel, R. PACAP-Mediated Neuroprotection of Neurochemically Identified Cell Types in MSG-Induced Retinal Degeneration. J. Mol. Neurosci. 2008, 36, 97–104. [Google Scholar] [CrossRef]
- Hernández, C.; Simó, R. Neuroprotection in Diabetic Retinopathy. Curr. Diab. Rep. 2012, 12, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Pinazo-Durán, M.D.; Gallego-Pinazo, R.; García-Medina, J.J.; Zanón-Moreno, V.; Nucci, C.; Dolz-Marco, R.; Martínez-Castillo, S.; Galbis-Estrada, C.; Marco-Ramírez, C.; López-Gálvez, M.I.; et al. Oxidative Stress and Its Downstream Signaling in Aging Eyes. Clin. Interv. Aging 2014, 9, 637–652. [Google Scholar] [CrossRef]
- Masmoudi-Kouki, O.; Douiri, S.; Hamdi, Y.; Kaddour, H.; Bahdoudi, S.; Vaudry, D.; Basille, M.; Leprince, J.; Fournier, A.; Vaudry, H.; et al. Pituitary Adenylate Cyclase-Activating Polypeptide Protects Astroglial Cells against Oxidative Stress-Induced Apoptosis. J. Neurochem. 2011, 117, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Steinle, J.J.; Sharma, S.; Smith, C.P.; McFayden-Ketchum, L.S. Normal Aging Involves Modulation of Specific Inflammatory Markers in the Rat Retina and Choroid. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 325–331. [Google Scholar] [CrossRef]
- Xu, H.; Chen, M.; Forrester, J.V. Para-Inflammation in the Aging Retina. Prog. Retin. Eye Res. 2009, 28, 348–368. [Google Scholar] [CrossRef]
- Chen, M.; Muckersie, E.; Forrester, J.V.; Xu, H. Immune Activation in Retinal Aging: A Gene Expression Study. Investig. Ophthalmol. Vis. Sci. 2010, 51, 5888–5896. [Google Scholar] [CrossRef]
- Hussain, R.M.; Shaukat, B.A.; Ciulla, L.M.; Berrocal, A.M.; Sridhar, J. Vascular Endothelial Growth Factor Antagonists: Promising Players in the Treatment of Neovascular Age-Related Macular Degeneration. Drug. Des. Devel. Ther. 2021, 15, 2653–2665. [Google Scholar] [CrossRef]
- Ientile, R.; Macaione, V.; Teletta, M.; Pedale, S.; Torre, V.; Macaione, S. Apoptosis and Necrosis Occurring in Excitotoxic Cell Death in Isolated Chick Embryo Retina. J. Neurochem. 2001, 79, 71–78. [Google Scholar] [CrossRef]
- Chen, Y.; Coorey, N.J.; Zhang, M.; Zeng, S.; Madigan, M.C.; Zhang, X.; Gillies, M.C.; Zhu, L.; Zhang, T. Metabolism Dysregulation in Retinal Diseases and Related Therapies. Antioxidants 2022, 11, 942. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Geng, Z.; Khattak, S.; Ji, X.; Wu, D.; Dang, Y. Role of Oxidative Stress in Retinal Disease and the Early Intervention Strategies: A Review. Oxid. Med. Cell. Longev. 2022, 2022, 7836828. [Google Scholar] [CrossRef] [PubMed]
- Projected Change in Vision Loss 2020 to 2050—The International Agency for the Prevention of Blindness. Available online: https://www.iapb.org/learn/vision-atlas/magnitude-and-projections/projected-change/ (accessed on 27 January 2023).
- Manthey, A.L.; Chiu, K.; So, K.F. Effects of Lycium Barbarum on the Visual System. Int. Rev. Neurobiol. 2017, 135, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.A.; Sood, G.C.; Raman, R. Ocular Neurofibromatosis. Indian. J. Ophthalmol. 1981, 29, 117–120. [Google Scholar] [CrossRef]
- Coles, R.S.; Laval, J. Retinal Detachments Occurring in Cataract Associated with Neurodermatitis. AMA Arch. Ophthalmol. 1952, 48, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Bair, B.; Dodd, J.; Heidelberg, K.; Krach, K. Cataracts in Atopic Dermatitis: A Case Presentation and Review of the Literature. Arch. Dermatol. 2011, 147, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic Dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Tian, C.; Duan, L.; Fu, C.; He, J.; Dai, J.; Zhu, G. Study on the Correlation Between Iris Characteristics and Schizophrenia. Neuropsychiatr. Dis. Treat. 2022, 18, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Sabel, B.A.; Wang, J.; Cárdenas-Morales, L.; Faiq, M.; Heim, C. Mental Stress as Consequence and Cause of Vision Loss: The Dawn of Psychosomatic Ophthalmology for Preventive and Personalized Medicine. EPMA J. 2018, 9, 133–160. [Google Scholar] [CrossRef]
- Edwards, M.J.M.; Hazi, A.; Crewther, S.G. Acute Psychosocial Stress Induces a Myopic Shift in Undergraduate Students. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2841. [Google Scholar]
- Tian, B.; Bilsbury, E.; Doherty, S.; Teebagy, S.; Wood, E.; Su, W.; Gao, G.; Lin, H. Ocular Drug Delivery: Advancements and Innovations. Pharmaceutics 2022, 14, 1931. [Google Scholar] [CrossRef] [PubMed]
- Nian, S.; Lo, A.C.Y.; Nian, S.; Lo, A.C.Y. Protecting the Aging Retina. Neuroprotection 2019, 5, 1–20. [Google Scholar] [CrossRef]
- Fisher, D.E.; Klein, B.E.K.; Wong, T.Y.; Rotter, J.I.; Li, X.; Shrager, S.; Burke, G.L.; Klein, R.; Cotch, M.F. Incidence of Age-Related Macular Degeneration in a Multi-Ethnic United States Population: The Multi-Ethnic Study of Atherosclerosis. Ophthalmology 2016, 123, 1297–1308. [Google Scholar] [CrossRef]
- Sivaprasad, S.; Gupta, B.; Crosby-Nwaobi, R.; Evans, J. Prevalence of Diabetic Retinopathy in Various Ethnic Groups: A Worldwide Perspective. Surv. Ophthalmol. 2012, 57, 347–370. [Google Scholar] [CrossRef]
- Jonas, J.B.; Aung, T.; Bourne, R.R.; Bron, A.M.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet 2017, 390, 2183–2193. [Google Scholar] [CrossRef]
- H Gao, J.G.H. Aging of the Human Retina. Differential Loss of Neurons and Retinal Pigment Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 1992, 33, 1–17. [Google Scholar]
- Curcio, C.A.; Drucker, D.N. Retinal Ganglion Cells in Alzheimer’s Disease and Aging. Ann. Neurol. 1993, 33, 248–257. [Google Scholar] [CrossRef]
- Panda-Jonas, S.; Jonas, J.B.; Jakobczyk-Zmija, M. Retinal Photoreceptor Density Decreases with Age. Ophthalmology 1995, 102, 1853–1859. [Google Scholar] [CrossRef]
- Kim, C.B.Y.; Tom, B.W.; Spear, P.D. Effects of Aging on the Densities, Numbers, and Sizes of Retinal Ganglion Cells in Rhesus Monkey. Neurobiol. Aging 1996, 17, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Liets, L.C.; Eliasieh, K.; Van Der List, D.A.; Chalupa, L.M. Dendrites of Rod Bipolar Cells Sprout in Normal Aging Retina. Proc. Natl. Acad. Sci. USA 2006, 103, 12156–12160. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Nag, T.C.; Wadhwa, S. Age-Related Decrease in Rod Bipolar Cell Density of the Human Retina: An Immunohistochemical Study. J. Biosci. 2007, 32, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Parikh, R.S.; Parikh, S.R.; Sekhar, G.C.; Prabakaran, S.; Babu, J.G.; Thomas, R. Normal Age-Related Decay of Retinal Nerve Fiber Layer Thickness. Ophthalmology 2007, 114, 921–926. [Google Scholar] [CrossRef]
- Freund, P.R.; Watson, J.; Gilmour, G.S.; Gaillard, F.; Sauvé, Y. Differential Changes in Retina Function with Normal Aging in Humans. Doc. Ophthalmol. 2011, 122, 177–190. [Google Scholar] [CrossRef]
- Samuel, M.A.; Zhang, Y.; Meister, M.; Sanes, J.R. Age-Related Alterations in Neurons of the Mouse Retina. J. Neurosci. 2011, 31, 16033–16044. [Google Scholar] [CrossRef]
- Cunea, A.; Powner, M.B.; Jeffery, G. Death by Color: Differential Cone Loss in the Aging Mouse Retina. Neurobiol. Aging 2014, 35, 2584–2591. [Google Scholar] [CrossRef]
- Cano, J.; Machado, A.; Reinoso-Suárez, F. Morphological Changes in the Retina of Ageing Rats. Arch. Gerontol. Geriatr. 1986, 5, 41–50. [Google Scholar] [CrossRef]
- Papazariri, P.; Podini, P.; Meldolesi, J.; Yamaguchi, T. Ageing Affects Cytosolic Ca2+ Binding Proteins and Synaptic Markers in the Retina but Not in Cerebral Cortex Neurons of the Rat. Neurosci. Lett. 1995, 186, 65–68. [Google Scholar] [CrossRef]
- Mansour, H.; Chamberlain, C.G.; Weible, M.W.; Hughes, S.; Chu, Y.; Chan-Ling, T. Aging-Related Changes in Astrocytes in the Rat Retina: Imbalance between Cell Proliferation and Cell Death Reduces Astrocyte Availability. Aging Cell 2008, 7, 526–540. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Nicolás, F.M.; Vidal-Sanz, M.; Agudo-Barriuso, M. The Aging Rat Retina: From Function to Anatomy. Neurobiol. Aging 2018, 61, 146–168. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.E.I.; El-Shaarawy, E.A.A.; Youakim, M.F.; Shuaib, D.M.A.; Ahmed, M.M. Aging Changes in the Retina of Male Albino Rat: A Histological, Ultrastructural and Immunohistochemical Study. Folia Morphol. 2019, 78, 237–258. [Google Scholar] [CrossRef] [PubMed]
- Atlasz, T.; Szabadfi, K.; Kiss, P.; Tamas, A.; Toth, G.; Reglodi, D.; Gabriel, R. Evaluation of the Protective Effects of PACAP with Cell-Specific Markers in Ischemia-Induced Retinal Degeneration. Brain Res. Bull. 2010, 81, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, N.; Pinilla, I.; Sauvé, Y.; Lund, R. Early Changes in Synaptic Connectivity Following Progressive Photoreceptor Degeneration in RCS Rats. Eur. J. Neurosci. 2005, 22, 1057–1072. [Google Scholar] [CrossRef]
- DiLoreto, D.A.; Luo, C.; Calkins, D.J.; Del Cerro, M. An Ultrastructural Study of the Pathology of the Retinal Pigment Epithelium, Bruch’s Membrane, and the Choriocapillaris in the Aged Fischer 344 Rat. Curr. Eye Res. 2006, 31, 749–763. [Google Scholar] [CrossRef]
- Harman, A.M.; MacDonald, A.; Meyer, P.; Ahmat, A. Numbers of Neurons in the Retinal Ganglion Cell Layer of the Rat Do Not Change throughout Life. Gerontology 2003, 49, 350–355. [Google Scholar] [CrossRef]
- Morrison, J.C.; Cork, L.C.; Dunkelberger, G.R.; Brown, A.; Quigley, H.A. Aging Changes of the Rhesus Monkey Optic Nerve. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1623–1627. [Google Scholar]
- Cavallotti, C.; Artico, M.; Pescosolido, N.; Tranquilli Leali, F.M.; Feher, J. Age-Related Changes in the Human Retina. Can. J. Ophthalmol. 2004, 39, 61–68. [Google Scholar] [CrossRef]
- Esquiva, G.; Lax, P.; Pérez-Santonja, J.J.; García-Fernández, J.M.; Cuenca, N. Loss of Melanopsin-Expressing Ganglion Cell Subtypes and Dendritic Degeneration in the Aging Human Retina. Front. Aging Neurosci. 2017, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.L.; Robison, W.G. Evidence of Cell Loss from the Rat Retina during Senescence. Exp. Eye Res. 1986, 42, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Danias, J.; Lee, K.C.; Zamora, M.F.; Chen, B.; Shen, F.; Filippopoulos, T.; Su, Y.; Goldblum, D.; Podos, S.M.; Mittag, T. Quantitative Analysis of Retinal Ganglion Cell (RGC) Loss in Aging DBA/2NNia Glaucomatous Mice: Comparison with RGC Loss in Aging C57/BL6 Mice. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5151–5162. [Google Scholar] [CrossRef]
- Feng, L.; Sun, Z.; Han, H.; Zhou, Y.; Zhang, M. No Age-Related Cell Loss in Three Retinal Nuclear Layers of the Long-Evans Rat. Vis. Neurosci. 2007, 24, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Weisse, I.; Loosen, H.; Peil, H. Age-Related Retinal Changes--Comparison between Albino and Pigmented Rats. Lens Eye Toxic. Res. 1990, 7, 717–739. [Google Scholar]
- Terzibasi, E.; Calamusa, M.; Novelli, E.; Domenici, L.; Strettoi, E.; Cellerino, A. Age-Dependent Remodelling of Retinal Circuitry. Neurobiol. Aging 2009, 30, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Eliasieh, K.; Liets, L.C.; Chalupa, L.M. Cellular Reorganization in the Human Retina during Normal Aging. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2824–2830. [Google Scholar] [CrossRef] [PubMed]
- Bonnel, S.; Mohand-Said, S.; Sahel, J.-A.A. The Aging of the Retina. Exp. Gerontol. 2003, 38, 825–831. [Google Scholar] [CrossRef]
- Mohand-Said, S.; Deudon-Combe, A.; Hicks, D.; Simonutti, M.; Forster, V.; Fintz, A.C.; Léveillard, T.; Dreyfus, H.; Sahel, J.A. Normal Retina Releases a Diffusible Factor Stimulating Cone Survival in the Retinal Degeneration Mouse. Proc. Natl. Acad. Sci. USA 1998, 95, 8357–8362. [Google Scholar] [CrossRef]
- Mohand-Said, S.; Hicks, D.; Dreyfus, H.; Sahel, J.A. Selective Transplantation of Rods Delays Cone Loss in a Retinitis Pigmentosa Model. Arch. Ophthalmol. 2000, 118, 807–811. [Google Scholar] [CrossRef]
- Mohand-Said, S.; Hicks, D.; Léveillard, T.; Picaud, S.; Porto, F.; Sahel, J.A. Rod-Cone Interactions: Developmental and Clinical Significance. Prog. Retin. Eye Res. 2001, 20, 451–467. [Google Scholar] [CrossRef]
- Fintz, A.C.; Audo, I.; Hicks, D.; Mohand-Said, S.; Léveillard, T.; Sahel, J. Partial Characterization of Retina-Derived Cone Neuroprotection in Two Culture Models of Photoreceptor Degeneration. Investig. Ophthalmol. Vis. Sci. 2003, 44, 818–825. [Google Scholar] [CrossRef] [PubMed]
- Feeney-Burns, L.; Burns, R.P.; Gao, C.L. Age-Related Macular Changes in Humans over 90 Years Old. Am. J. Ophthalmol. 1990, 109, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Lin, N.; Sheedlo, H.J.; Turner, J.E. Muller and RPE Cell Response to Photoreceptor Cell Degeneration in Aging Fischer Rats. Exp. Eye Res. 1996, 63, 9–18. [Google Scholar] [CrossRef]
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global Prevalence of Age-Related Macular Degeneration and Disease Burden Projection for 2020 and 2040: A Systematic Review and Meta-Analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.J.; Mirza, R.G.; Gill, M.K. Age-Related Macular Degeneration. Med. Clin. N. Am. 2021, 105, 473–491. [Google Scholar] [CrossRef] [PubMed]
- Age-Related Macular Degeneration: Diagnosis and Management. Br. Med. Bulletin. 2018, 85, 127–149.
- Takahashi, K.; Ishibashi, T.; Ogur, Y.; Yuzawa, M.; Working Group for Establishing Diagnostic Criteria for Age-Related Macular Degeneration. Classification and Diagnostic Criteria of Age-Related Macular Degeneration. Available online: https://pubmed.ncbi.nlm.nih.gov/19157028/ (accessed on 27 January 2023).
- Seddon, J.M.; Sharma, S.; Adelman, R.A. Evaluation of the Clinical Age-Related Maculopathy Staging System. Ophthalmology 2006, 113, 260–266. [Google Scholar] [CrossRef]
- The Age-Related Eye Disease Study System for Classifying Age-Related Macular Degeneration from Stereoscopic Color Fundus Photographs: The Age-Related Eye Disease Study Report Number 6. Am. J. Ophthalmol. 2001, 132, 668–681. [CrossRef] [PubMed]
- Wassell, J.; Davies, S.; Bardsley, W.; Boulton, M. The Photoreactivity of the Retinal Age Pigment Lipofuscin. J. Biol. Chem. 1999, 274, 23828–23832. [Google Scholar] [CrossRef]
- Boulton, M.; Dontsov, A.; Jarvis-Evans, J.; Ostrovsky, M.; Svistunenko, D. Lipofuscin Is a Photoinducible Free Radical Generator. J. Photochem. Photobiol. B 1993, 19, 201–204. [Google Scholar] [CrossRef]
- Mullins, R.F.; Russell, S.R.; Anderson, D.H.; Hageman, G.S. Drusen Associated with Aging and Age-Related Macular Degeneration Contain Proteins Common to Extracellular Deposits Associated with Atherosclerosis, Elastosis, Amyloidosis, and Dense Deposit Disease. FASEB J. 2000, 14, 835–846. [Google Scholar] [CrossRef]
- Curcio, C.A.; Millican, C.L.; Bailey, T.; Kruth, H.S. Accumulation of Cholesterol with Age in Human Bruch’s Membrane. Investig. Ophthalmol. Vis. Sci. 2001, 42, 265–274. [Google Scholar]
- Gresh, J.; Goletz, P.W.; Crouch, R.K.; Rohrer, B. Structure-Function Analysis of Rods and Cones in Juvenile, Adult, and Aged C57bl/6 and Balb/c Mice. Vis. Neurosci. 2003, 20, 211–220. [Google Scholar] [CrossRef]
- Williams, G.A.; Jacobs, G.H. Cone-Based Vision in the Aging Mouse. Vis. Res. 2007, 47, 2037–2046. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikov, A.V.; Fan, J.; Crouch, R.K.; Kefalov, V.J. Age-Related Deterioration of Rod Vision in Mice. J. Neurosci. 2010, 30, 11222–11231. [Google Scholar] [CrossRef] [PubMed]
- Birch, D.G.; Hood, D.C.; Locke, K.G.; Hoffman, D.R.; Tzekov, R.T. Quantitative Electroretinogram Measures of Phototransduction in Cone and Rod Photoreceptors: Normal Aging, Progression with Disease, and Test-Retest Variability. Arch. Ophthalmol. 2002, 120, 1045–1051. [Google Scholar] [CrossRef]
- Chen, M.; Luo, C.; Zhao, J.; Devarajan, G.; Xu, H. Immune Regulation in the Aging Retina. Prog. Retin. Eye Res. 2019, 69, 159–172. [Google Scholar] [CrossRef]
- Payne, A.J.; Kaja, S.; Naumchuk, Y.; Kunjukunju, N.; Koulen, P. Antioxidant Drug Therapy Approaches for Neuroprotection in Chronic Diseases of the Retina. Int. J. Mol. Sci. 2014, 15, 1865–1886. [Google Scholar] [CrossRef] [PubMed]
- Barron, M.J.; Johnson, M.A.; Andrews, R.M.; Clarke, M.P.; Griffiths, P.G.; Bristow, E.; He, L.; Durham, S.; Turnbull, D.M. Mitochondrial Abnormalities in Ageing Macular Photoreceptors. Investig. Ophthalmol. Vis. Sci. 2001, 42, 3016–3022. [Google Scholar]
- Louie, J.L.; Kapphahn, R.J.; Ferrington, D.A. Proteasome Function and Protein Oxidation in the Aged Retina. Exp. Eye Res. 2002, 75, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Crabb, J.W.; Miyagi, M.; Gu, X.; Shadrach, K.; West, K.A.; Sakaguchi, H.; Kamei, M.; Hasan, A.; Yan, L.; Rayborn, M.E.; et al. Drusen Proteome Analysis: An Approach to the Etiology of Age-Related Macular Degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 14682–14687. [Google Scholar] [CrossRef] [PubMed]
- Milne, R.; Brownstein, S. Advanced Glycation End Products and Diabetic Retinopathy. Amino Acids 2013, 44, 1397–1407. [Google Scholar] [CrossRef]
- Adornetto, A.; Rombolà, L.; Morrone, L.A.; Nucci, C.; Corasaniti, M.T.; Bagetta, G.; Russo, R. Natural Products: Evidence for Neuroprotection to Be Exploited in Glaucoma. Nutrients 2020, 12, 3158. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.; Modgil, S.; Sharma, V.L.; Shri, R.; Kaushik, S. Preserving Neural Retina through Re-Emerging Herbal Interventions. J. Cell. Biochem. 2014, 115, 1659–1668. [Google Scholar] [CrossRef] [PubMed]
- Tsang, J.K.W.; Wolf, S.A.; Pompoes, I.M.; Joussen, A.M.; Lam, W.C.; Yang, D.; Lo, A.C.Y. Potential Effects of Nutraceuticals in Retinopathy of Prematurity. Life 2021, 11, 79. [Google Scholar] [CrossRef]
- Pokkalath, A.S.; Sawant, A.; Sawarkar, S.P. Herbal Medicine for Ocular Diseases: An Age Old Therapy and Its Future Perspective. J. Drug Deliv. Sci. Technol. 2022, 68, 102979. [Google Scholar] [CrossRef]
- Nebbioso, M.; Franzone, F.; Greco, A.; Gharbiya, M.; Bonfiglio, V.; Polimeni, A. Recent Advances and Disputes About Curcumin in Retinal Diseases. Clin. Ophthalmol. 2021, 15, 2553–2571. [Google Scholar] [CrossRef]
- Radomska-Leśniewska, D.M.; Osiecka-Iwan, A.; Hyc, A.; Góźdź, A.; Dąbrowska, A.M.; Skopiński, P. Therapeutic Potential of Curcumin in Eye Diseases. Cent. J. Immunol. 2019, 44, 181–189. [Google Scholar] [CrossRef]
- Behl, T.; Kotwani, A. Chinese Herbal Drugs for the Treatment of Diabetic Retinopathy. J. Pharm. Pharmacol. 2017, 69, 223–235. [Google Scholar] [CrossRef]
- Heitmar, R.; Brown, J.; Kyrou, I. Saffron (Crocus sativus L.) in Ocular Diseases: A Narrative Review of the Existing Evidence from Clinical Studies. Nutrients 2019, 11, 649. [Google Scholar] [CrossRef]
- Camelo, S.; Latil, M.; Veillet, S.; Dilda, P.J.; Lafont, R. Beyond AREDS Formulations, What Is Next for Intermediate Age-Related Macular Degeneration (IAMD) Treatment? Potential Benefits of Antioxidant and Anti-Inflammatory Apocarotenoids as Neuroprotectors. Oxid. Med. Cell. Longev. 2020, 2020, 4984927. [Google Scholar] [CrossRef] [PubMed]
- Karhanová, M.; Eliášová, M.; Kuběna, T.; Pešková, H.; Mlčák, P.; Fryšák, Z.; Marešová, K.; Zapletalová, J. [ProVens® in the Therapy of Glaucoma and Ocular Hypertension]—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/26782917/ (accessed on 27 January 2023).
- Schönlau, F.; Rohdewald, P. Pycnogenol for Diabetic Retinopathy. A Review. Int. Ophthalmol. 2001, 24, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Alok, S.; Jain, S.K.; Verma, A.; Kumar, M.; Mahor, A.; Sabharwal, M. Herbal Antioxidant in Clinical Practice: A Review. Asian Pac. J. Trop. Biomed. 2014, 4, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.X.; Lin, F.J.; Li, H.; Li, H.B.; Wu, D.T.; Geng, F.; Ma, W.; Wang, Y.; Miao, B.H.; Gan, R.Y. Recent Advances in Bioactive Compounds, Health Functions, and Safety Concerns of Onion (Allium Cepa L.). Front. Nutr. 2021, 8, 669805. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.J.; Uddin, T.M.; Matin Zidan, B.M.R.; Mitra, S.; Das, R.; Nainu, F.; Dhama, K.; Roy, A.; Hossain, M.J.; Khusro, A.; et al. Allium Cepa: A Treasure of Bioactive Phytochemicals with Prospective Health Benefits. Evid. Based Complement. Alternat. Med. 2022, 2022, 4586318. [Google Scholar] [CrossRef]
- Silveira, M.A.D.; De Jong, D.; Berretta, A.A.; Galvão, E.B.d.S.; Ribeiro, J.C.; Cerqueira-Silva, T.; Amorim, T.C.; Conceição, L.F.M.R.d.; Gomes, M.M.D.; Teixeira, M.B.; et al. Efficacy of Brazilian Green Propolis (EPP-AF®) as an Adjunct Treatment for Hospitalized COVID-19 Patients: A Randomized, Controlled Clinical Trial. Biomed. Pharmacother. 2021, 138, 111526. [Google Scholar] [CrossRef]
- Wilkinson, J.T.; Fraunfelder, F.W. Use of Herbal Medicines and Nutritional Supplements in Ocular Disorders: An Evidence-Based Review. Drugs 2011, 71, 2421–2434. [Google Scholar] [CrossRef]
- Tomida, I.; Perlwee, R.G.; Azuara-Blanco, A. Cannabinoids and Glaucoma. Br. J. Ophthalmol. 2004, 88, 708–713. [Google Scholar] [CrossRef]
- Dheyab, N.; Ali, F.; Ismail, A.; Othman, F.B. Anti-Diabetic Retinopathy Potential of Noni: The Beneficial Effect and Possible Mechanism | Auctores. Available online: https://www.auctoresonline.org/article/anti-diabetic-retinopathy-potential-of-noni-the-beneficial-effect-and-possible-mechanism (accessed on 27 January 2023).
- Torres, M.A.O.; de Fátima Braga Magalhães, I.; Mondêgo-Oliveira, R.; de Sá, J.C.; Rocha, A.L.; Abreu-Silva, A.L. One Plant, Many Uses: A Review of the Pharmacological Applications of Morinda Citrifolia. Phytother. Res. 2017, 31, 971–979. [Google Scholar] [CrossRef]
- Rajabian, A.; Sadeghnia, H.R.; Hosseini, A.; Mousavi, S.H.; Boroushaki, M.T. 3-Acetyl-11-Keto-β-Boswellic Acid Attenuated Oxidative Glutamate Toxicity in Neuron-like Cell Lines by Apoptosis Inhibition. J. Cell. Biochem. 2020, 121, 1778–1789. [Google Scholar] [CrossRef]
- Siddiqui, A.; Shah, Z.; Jahan, R.N.; Othman, I.; Kumari, Y. Mechanistic Role of Boswellic Acids in Alzheimer’s Disease: Emphasis on Anti-Inflammatory Properties. Biomed. Pharmacother. 2021, 144, 112250. [Google Scholar] [CrossRef]
- Upadhayay, S.; Mehan, S.; Prajapati, A.; Sethi, P.; Suri, M.; Zawawi, A.; Almashjary, M.N.; Tabrez, S. Nrf2/HO-1 Signaling Stimulation through Acetyl-11-Keto-Beta-Boswellic Acid (AKBA) Provides Neuroprotection in Ethidium Bromide-Induced Experimental Model of Multiple Sclerosis. Genes 2022, 13, 1324. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.W.; Hsu, S.K.; Chen, J.Y.F.; Lin, I.L.; Chen, K.J.; Lee, P.Y.; Ng, H.S.; Chiu, C.C.; Cheng, K.C. Curcumin Metabolite Tetrahydrocurcumin in the Treatment of Eye Diseases. Int. J. Mol. Sci. 2020, 22, 212. [Google Scholar] [CrossRef]
- Lulli, M.; Cammalleri, M.; Fornaciari, I.; Casini, G.; Dal Monte, M. Acetyl-11-Keto-β-Boswellic Acid Reduces Retinal Angiogenesis in a Mouse Model of Oxygen-Induced Retinopathy. Exp. Eye Res. 2015, 135, 67–80. [Google Scholar] [CrossRef]
- Hong, S.C.; Ha, J.H.; Lee, J.K.; Jung, S.H.; Kim, J.C. In Vivo Anti-Inflammation Potential of Aster Koraiensis Extract for Dry Eye Syndrome by the Protection of Ocular Surface. Nutrients 2020, 12, 3245. [Google Scholar] [CrossRef]
- Zhang, L.; Park, J.Y.; Zhao, D.; Kwon, H.C.; Yang, H.O. Neuroprotective Effect of Astersaponin I against Parkinson’s Disease through Autophagy Induction. Biomol. Ther. 2021, 29, 615–629. [Google Scholar] [CrossRef]
- Kim, J.; Jo, K.; Kim, C.S.; Kim, J.S. Aster Koraiensis Extract Prevents Diabetes-Induced Retinal Vascular Dysfunction in Spontaneously Diabetic Torii Rats. BMC Complement. Altern. Med. 2017, 17, 497. [Google Scholar] [CrossRef]
- Lim, E.Y.; Kim, J.G.; Lee, J.; Lee, C.; Shim, J.; Kim, Y.T. Analgesic Effects of Cnidium Officinale Extracts on Postoperative, Neuropathic, and Menopausal Pain in Rat Models. Evid. Based. Complement. Alternat. Med. 2019, 2019, 9698727. [Google Scholar] [CrossRef] [PubMed]
- Sureshkumar, D.; Begum, S.; Johannah, N.M.; Maliakel, B.; Krishnakumar, I.M. Toxicological Evaluation of a Saponin-Rich Standardized Extract of Fenugreek Seeds (FenuSMART®): Acute, Sub-Chronic and Genotoxicity Studies. Toxicol. Rep. 2018, 5, 1060–1068. [Google Scholar] [CrossRef]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a Flavonoid with Potential for Cancer Prevention and Therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharifi-Rad, M.; Anil Kumar, N.V.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Curcumin: A Review of Clinical Trials. Eur. J. Med. Chem. 2019, 163, 527–545. [Google Scholar] [CrossRef]
- Kang, J.M.; Lin, S. Ginkgo Biloba and Its Potential Role in Glaucoma. Curr. Opin. Ophthalmol. 2018, 29, 116–120. [Google Scholar] [CrossRef]
- Kim, K.A.; Kim, S.M.; Kang, S.W.; Jeon, S.I.; Um, B.H.; Jung, S.H. Edible Seaweed, Eisenia Bicyclis, Protects Retinal Ganglion Cells Death Caused by Oxidative Stress. Mar. Biotechnol. 2012, 14, 383–395. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, Q.; Li, S.; Pu, L.; Yu, L.; Liu, Y.; Lai, X. Effects of Lycium Barbarum L. Polysaccharides on Vascular Retinopathy: An Insight Review. Molecules 2022, 27, 5628. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Choi, Y.R.; Shim, J.; Choi, Y.S.; Kim, Y.T.; Kim, M.K.; Kim, M.J. Suppressive Effect of Arctium Lappa L. Leaves on Retinal Damage Against A2E-Induced ARPE-19 Cells and Mice. Molecules 2020, 25, 1737. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.S.; Cheng, L.N.; Wu, J.H.; Chan, E.; Kwan, Y.W.; Lee, S.M.Y.; Leung, G.P.H.; Yu, P.H.F.; Chan, S.W. A Review of the Pharmacological Effects of Arctium Lappa (Burdock). Inflammopharmacology 2011, 19, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Varughese, R.S.; Lam, W.S.T.; Marican, A.A.; Bin, H.; Viganeshwari, S.H.; Bhave, A.S.; Syn, N.L.; Wang, J.; Wong, A.L.A.; Kumar, A.P.; et al. Biopharmacological Considerations for Accelerating Drug Development of Deguelin, a Rotenoid with Potent Chemotherapeutic and Chemopreventive Potential. Cancer 2019, 125, 1789–1798. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Ren, D.; Shen, Z.Y.; Qin, L.P.; Zhu, B. Pharmacology, Phytochemistry, and Traditional Uses of Scrophularia Ningpoensis Hemsl. J. Ethnopharmacol. 2021, 269, 113688. [Google Scholar] [CrossRef]
- Ong, C.P.; Lee, W.L.; Tang, Y.Q.; Yap, W.H. Honokiol: A Review of Its Anticancer Potential and Mechanisms. Cancers 2019, 12, 48. [Google Scholar] [CrossRef]
- Giannaccare, G.; Pellegrini, M.; Senni, C.; Bernabei, F.; Scorcia, V.; Cicero, A.F.G. Clinical Applications of Astaxanthin in the Treatment of Ocular Diseases: Emerging Insights. Mar. Drugs 2020, 18, 239. [Google Scholar] [CrossRef]
- Guo, X.; He, Q.; Qi, B.; Sun, C.; Lyu, D.; Zhang, H. A Poisoning Outbreak Caused by Anisodus Tanguticus—Maqin County, Qinghai Province, China, July 2021. China CDC Wkly. 2022, 4, 920–923. [Google Scholar] [PubMed]
- Rathnavelu, V.; Alitheen, N.B.; Sohila, S.; Kanagesan, S.; Ramesh, R. Potential Role of Bromelain in Clinical and Therapeutic Applications. Biomed. Rep. 2016, 5, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Ige, M.; Liu, J. Herbal Medicines in Glaucoma Treatment. Yale J. Biol. Med. 2020, 93, 347–353. [Google Scholar] [PubMed]
- Takeuchi, M.; Shieh, P.C.; Horng, C.T. Treatment of Symptomatic Vitreous Opacities with Pharmacologic Vitreolysis Using a Mixure of Bromelain, Papain and Ficin Supplement. Appl. Sci. 2020, 10, 5901. [Google Scholar] [CrossRef]
- Manabe, K.; Kaidzu, S.; Tsutsui, A.; Mochiji, M.; Matsuoka, Y.; Takagi, Y.; Miyamoto, E.; Tanito, M. Effects of French Maritime Pine Bark/Bilberry Fruit Extracts on Intraocular Pressure for Primary Open-Angle Glaucoma. J. Clin. Biochem. Nutr. 2021, 68, 67–72. [Google Scholar] [CrossRef]
- Kumar, S.; Modgil, S.; Bammidi, S.; Minhas, G.; Shri, R.; Kaushik, S.; Singh, V.; Anand, A. Allium Cepa Exerts Neuroprotective Effect on Retinal Ganglion Cells of Pterygopalatine Artery (PPA) Ligated Mice. J. Ayurveda Integr. Med. 2020, 11, 489–494. [Google Scholar] [CrossRef]
- Lee, Y.M.; Lee, Y.R.; Kim, C.S.; Jo, K.; Sohn, E.; Kim, J.S.; Kim, J. Cnidium Officinale Extract and Butylidenephthalide Inhibits Retinal Neovascularization in Vitro and in Vivo. BMC Complement. Altern. Med. 2016, 16, 231. [Google Scholar] [CrossRef]
- Ikonne, E.U.; Ikpeazu, V.O.; Ugbogu, E.A. Corrigendum to “The Potential Health Benefits of Dietary Natural Plant Products in Age Related Eye Diseases” [Heliyon 6 (7) (2020) E04408]. Heliyon 2021, 7, e07069. [Google Scholar] [CrossRef]
- Zheng, L.; Howell, S.J.; Hatala, D.A.; Huang, K.; Kern, T.S. Salicylate-Based Anti-Inflammatory Drugs Inhibit the Early Lesion of Diabetic Retinopathy. Diabetes 2007, 56, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Al-Zamil, W.M.; Yassin, S.A. Recent Developments in Age-Related Macular Degeneration: A Review. Clin. Interv. Aging 2017, 12, 1313. [Google Scholar] [CrossRef]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic Retinopathy: Current Understanding, Mechanisms, and Treatment Strategies. JCI Insight 2017, 2, e93751. [Google Scholar] [CrossRef] [PubMed]
- Iu, L.P.L.; Kwok, A.K.H. An Update of Treatment Options for Neovascular Age-Related Macular Degeneration. Hong Kong Med. J. 2007, 13, 460–470. [Google Scholar]
- Adkins, J.C.; Balfour, J.A. Brimonidine A Review of Its Pharmacological Properties and Clinical Potential in the Management of Open-Angle Glaucoma and Ocular Hypertension. Drugs Aging 1998, 12, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.M.; Knuckles, M.; Minni, J.P.; Johnson, S.M.; Belasco, K.T. The Role of Brimonidine Tartrate Gel in the Treatment of Rosacea. Clin. Cosmet. Investig. Dermatol. 2015, 8, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, L.A.; Lai, R.; Woldemussie, E. From the Lab to the Clinic: Activation of an Alpha-2 Agonist Pathway Is Neuroprotective in Models of Retinal and Optic Nerve Injury. Eur. J. Ophthalmol. 1999, 9 (Suppl. 1), S17–S21. [Google Scholar] [CrossRef] [PubMed]
- Dugel, P.U.; Jaffe, G.J.; Sallstig, P.; Warburton, J.; Weichselberger, A.; Wieland, M.; Singerman, L. Brolucizumab Versus Aflibercept in Participants with Neovascular Age-Related Macular Degeneration: A Randomized Trial. Ophthalmology 2017, 124, 1296–1304. [Google Scholar] [CrossRef]
- Motevasseli, T.; Mohammadi, S.; Abdi, F.; Freeman, W.R. Side Effects of Brolucizumab. J. Ophthalmic Vis. Res. 2021, 16, 670–675. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, Y.; Xie, J.; Li, D.; Hu, Q.; Li, X.; Zheng, W.; He, R. Conbercept for Patients with Age-Related Macular Degeneration: A Systematic Review. BMC Ophthalmol. 2018, 18, 142. [Google Scholar] [CrossRef]
- Theodossiadis, P.G.; Liarakos, V.S.; Sfikakis, P.P.; Vergados, I.A.; Theodossiadis, G.P. Intravitreal Administration of the Anti-Tumor Necrosis Factor Agent Infliximab for Neovascular Age-Related Macular Degeneration. Am. J. Ophthalmol. 2009, 147, 825–830.e1. [Google Scholar] [CrossRef]
- Vavvas, D.; D’Amico, D.J. Pegaptanib (Macugen): Treating Neovascular Age-Related Macular Degeneration and Current Role in Clinical Practice. Ophthalmol. Clin. N. Am. 2006, 19, 353–360. [Google Scholar]
- Gaudreault, J.; Fei, D.; Beyer, J.C.; Ryan, A.; Rangell, L.; Shiu, V.; Damico, L.A. Pharmacokinetics and Retinal Distribution of Ranibizumab, a Humanized Antibody Fragment Directed against VEGF-A, Following Intravitreal Administration in Rabbits. Retina 2007, 27, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Kourlas, H.; Abrams, P. Ranibizumab for the Treatment of Neovascular Age-Related Macular Degeneration: A Review. Clin. Ther. 2007, 29, 1850–1861. [Google Scholar] [CrossRef]
- Hasan, N.; Chawla, R.; Shaikh, N.; Kandasamy, S.; Azad, S.V.; Sundar, M.D. A Comprehensive Review of Intravitreal Immunosuppressants andbiologicals Used in Ophthalmology. Ther. Adv. Ophthalmol. 2022, 14, 251584142210974. [Google Scholar] [CrossRef]
- Kaszuba-Bartkowiak, K.; Nowak, S.; Jurowski, P. The Role of Trimetazidine in the Protection of the Retina. Arch. Med. Sci. 2007, 3, S65–S66. [Google Scholar]
- Schmidt, K.-G.; Bergert, H.; Funk, R. Neurodegenerative Diseases of the Retina and Potential for Protection and Recovery. Curr. Neuropharmacol. 2008, 6, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Hernández, C.; García-Ramírez, M.; Corraliza, L.; Fernández-Carneado, J.; Farrera-Sinfreu, J.; Ponsati, B.; González-Rodríguez, Á.; Valverde, Á.M.; Simó, R. Topical Administration of Somatostatin Prevents Retinal Neurodegeneration in Experimental Diabetes. Diabetes 2013, 62, 2569–2578. [Google Scholar] [CrossRef]
- Pöstyéni, E.; Kovács-Valasek, A.; Dénes, V.; Mester, A.; Sétáló, G.; Gábriel, R. PACAP for Retinal Health: Model for Cellular Aging and Rescue. Int. J. Mol. Sci. 2021, 22, 444. [Google Scholar] [CrossRef]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.C.C.; Hashimoto, H.; Galas, L.; et al. Pituitary Adenylate Cyclase-Activating Polypeptide and Its Receptors: 20 Years after the Discovery. Pharmacol. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef]
- Szabadfi, K.; Atlasz, T.; Kiss, P.; Reglodi, D.; Szabo, A.; Kovacs, K.; Szalontai, B.; Setalo, G.; Banki, E.; Csanaky, K.; et al. Protective Effects of the Neuropeptide PACAP in Diabetic Retinopathy. Cell Tissue Res. 2012, 348, 37–46. [Google Scholar] [CrossRef]
- Kovacs, A.K.; Atlasz, T.; Werling, D.; Szabo, E.; Reglodi, D.; Toth, G.K. Stability Test of PACAP in Eye Drops. J. Mol. Neurosci. 2021, 71, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Szabo, E.; Patko, E.; Vaczy, A.; Molitor, D.; Csutak, A.; Toth, G.; Reglodi, D.; Atlasz, T. Retinoprotective Effects of PACAP Eye Drops in Microbead-Induced Glaucoma Model in Rats. Int. J. Mol. Sci. 2021, 22, 8825. [Google Scholar] [CrossRef] [PubMed]
- IDF Diabetes Atlas | Tenth Edition. Available online: https://diabetesatlas.org/ (accessed on 27 January 2023).
- Yau, J.W.Y.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.J.; Dekker, J.M.; Fletcher, A.; Grauslund, J.; et al. Global Prevalence and Major Risk Factors of Diabetic Retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Holekamp, N.M. Review of Neovascular Age-Related Macular Degeneration Treatment Options. Am. J. Manag. Care 2019, 25, S172–S181. [Google Scholar]
- Nian, S.; Lo, A.C.Y.; Mi, Y.; Ren, K.; Yang, D. Neurovascular Unit in Diabetic Retinopathy: Pathophysiological Roles and Potential Therapeutical Targets. Eye Vis. 2021, 8, 15. [Google Scholar] [CrossRef]
- Wilkinson-Berka, J.L. Angiotensin and Diabetic Retinopathy. Int. J. Biochem. Cell Biol. 2006, 38, 752–765. [Google Scholar] [CrossRef]
- Musat, O.; Cernat, C.; Labib, M.; Gheorghe, A.; Toma, O.; Zamfir, M.; Boureanu, A.M. DIABETIC MACULAR EDEMA. Rom. J. Ophthalmol. 2015, 59, 133. [Google Scholar] [CrossRef]
- Browning, D.J.; Stewart, M.W.; Lee, C. Diabetic Macular Edema: Evidence-Based Management. Indian J. Ophthalmol. 2018, 66, 1736. [Google Scholar] [CrossRef]
- Meyer, C.H. Current Treatment Approaches in Diabetic Macular Edema. Ophthalmologica. 2007, 221, 118–131. [Google Scholar] [CrossRef]
- Chauhan, M.Z.; Rather, P.A.; Samarah, S.M.; Elhusseiny, A.M.; Sallam, A.B. Current and Novel Therapeutic Approaches for Treatment of Diabetic Macular Edema. Cells 2022, 11, 1950. [Google Scholar] [CrossRef]
- Chung, Y.R.; Kim, Y.H.; Ha, S.J.; Byeon, H.E.; Cho, C.H.; Kim, J.H.; Lee, K. Role of Inflammation in Classification of Diabetic Macular Edema by Optical Coherence Tomography. J. Diabetes Res. 2019, 2019, 8164250. [Google Scholar] [CrossRef] [PubMed]
- Otani, T.; Kishi, S.; Maruyama, Y. Patterns of Diabetic Macular Edema with Optical Coherence Tomography. Am. J. Ophthalmol. 1999, 127, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, M.; Wickremasinghe, S.; Osborne, A.; Van Wijngaarden, P.; Martin, K.R. Diabetic Retinopathy: A Complex Pathophysiology Requiring Novel Therapeutic Strategies. Expert Opin. Biol. Ther. 2018, 18, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Tarr, J.M.; Kaul, K.; Chopra, M.; Kohner, E.M.; Chibber, R. Pathophysiology of Diabetic Retinopathy. ISRN Ophthalmol. 2013, 2013, 343560. [Google Scholar] [CrossRef]
- Adornetto, A.; Gesualdo, C.; Laganà, M.L.; Trotta, M.C.; Rossi, S.; Russo, R. Autophagy: A Novel Pharmacological Target in Diabetic Retinopathy. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chuang, L.M. The Role of Oxidative Stress in the Pathogenesis of Type 2 Diabetes: From Molecular Mechanism to Clinical Implication. Am. J. Transl. Res. 2010, 2, 316–331. [Google Scholar]
- Cai, X.; Li, J.; Wang, M.; She, M.; Tang, Y.; Li, J.; Li, H.; Hui, H. GLP-1 Treatment Improves Diabetic Retinopathy by Alleviating Autophagy through GLP-1R-ERK1/2-HDAC6 Signaling Pathway. Int. J. Med. Sci. 2017, 14, 1203–1212. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Yang, C. Oxidative Stress and Diabetic Retinopathy: Molecular Mechanisms, Pathogenetic Role and Therapeutic Implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef]
- Gong, Q.; Wang, H.; Yu, P.; Qian, T.; Xu, X. Protective or Harmful: The Dual Roles of Autophagy in Diabetic Retinopathy. Front. Med. 2021, 8, 644121. [Google Scholar] [CrossRef]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular Death, Reactive Oxygen Species (ROS) and Diabetic Complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef]
- Fu, D.; Yu, J.Y.; Yang, S.; Wu, M.; Hammad, S.M.; Connell, A.R.; Du, M.; Chen, J.; Lyons, T.J. Survival or Death: A Dual Role for Autophagy in Stress-Induced Pericyte Loss in Diabetic Retinopathy. Diabetologia 2016, 59, 2251–2261. [Google Scholar] [CrossRef]
- Dehdashtian, E.; Mehrzadi, S.; Yousefi, B.; Hosseinzadeh, A.; Reiter, R.J.; Safa, M.; Ghaznavi, H.; Naseripour, M. Diabetic Retinopathy Pathogenesis and the Ameliorating Effects of Melatonin; Involvement of Autophagy, Inflammation and Oxidative Stress. Life Sci. 2018, 193, 20–33. [Google Scholar] [CrossRef]
- De Faria, J.M.L.; Duarte, D.A.; Montemurro, C.; Papadimitriou, A.; Consonni, S.R.; De Faria, J.B.L. Defective Autophagy in Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4356–4366. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Zhang, Z.; Wang, X.; Li, R.; Hou, W.; Bi, W.; Zhang, X. Inhibition of Autophagy Induces IL-1β Release from ARPE-19 Cells via ROS Mediated NLRP3 Inflammasome Activation under High Glucose Stress. Biochem. Biophys. Res. Commun. 2015, 463, 1071–1076. [Google Scholar] [CrossRef]
- Piano, I.; Novelli, E.; Della Santina, L.; Strettoi, E.; Cervetto, L.; Gargini, C. Involvement of Autophagic Pathway in the Progression of Retinal Degeneration in a Mouse Model of Diabetes. Front. Cell. Neurosci. 2016, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Madrakhimov, S.B.; Yang, J.Y.; Kim, J.H.; Han, J.W.; Park, T.K. MTOR-Dependent Dysregulation of Autophagy Contributes to the Retinal Ganglion Cell Loss in Streptozotocin-Induced Diabetic Retinopathy. Cell Commun. Signal. 2021, 19, 1–16. [Google Scholar] [CrossRef]
- Park, H.Y.L.; Kim, J.H.; Park, C.K. Different Contributions of Autophagy to Retinal Ganglion Cell Death in the Diabetic and Glaucomatous Retinas. Sci. Rep. 2018, 8, 13321. [Google Scholar] [CrossRef]
- Kreutzberg, G.W. Microglia: A Sensor for Pathological Events in the CNS. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef]
- Tambuyzer, B.R.; Ponsaerts, P.; Nouwen, E.J. Microglia: Gatekeepers of Central Nervous System Immunology. J. Leukoc. Biol. 2009, 85, 352–370. [Google Scholar] [CrossRef]
- Dick, A.D.; Carter, D.; Robertson, M.; Broderick, C.; Hughes, E.; Forrester, J.V.; Liversidge, J. Control of Myeloid Activity during Retinal Inflammation. J. Leukoc. Biol. 2003, 74, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Karlstetter, M.; Ebert, S.; Langmann, T. Microglia in the Healthy and Degenerating Retina: Insights from Novel Mouse Models. Immunobiology 2010, 215, 685–691. [Google Scholar] [CrossRef]
- Esposito, K.; Nappo, F.; Marfella, R.; Giugliano, G.; Giugliano, F.; Ciotola, M.; Quagliaro, L.; Ceriello, A.; Giugliano, D. Inflammatory Cytokine Concentrations Are Acutely Increased by Hyperglycemia in Humans: Role of Oxidative Stress. Circulation 2002, 106, 2067–2072. [Google Scholar] [CrossRef]
- Semeraro, F.; Cancarini, A.; Dell’Omo, R.; Rezzola, S.; Romano, M.R.; Costagliola, C. Diabetic Retinopathy: Vascular and Inflammatory Disease. J. Diabetes Res. 2015, 2015, 582060. [Google Scholar] [CrossRef]
- Yang, L.P.; Sun, H.L.; Wu, L.M.; Guo, X.J.; Dou, H.L.; Tso, M.O.M.; Zhao, L.; Li, S.M. Baicalein Reduces Inflammatory Process in a Rodent Model of Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2319–2327. [Google Scholar] [CrossRef]
- Tang, J.; Kern, T.S. Inflammation in Diabetic Retinopathy. Prog. Retin. Eye Res. 2011, 30, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, S.; McGuire, P.G.; Das, A. Diabetic Retinopathy and Inflammation: Novel Therapeutic Targets. Middle East Afr. J. Ophthalmol. 2012, 19, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Krady, J.K.; Basu, A.; Allen, C.M.; Xu, Y.; LaNoue, K.F.; Gardner, T.W.; Levison, S.W. Minocycline Reduces Proinflammatory Cytokine Expression, Microglial Activation, and Caspase-3 Activation in a Rodent Model of Diabetic Retinopathy. Diabetes 2005, 54, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Khalfaoui, T.; Lizard, G.; Ouertani-Meddeb, A. Adhesion Molecules (ICAM-1 and VCAM-1) and Diabetic Retinopathy in Type 2 Diabetes. J. Mol. Histol. 2008, 39, 243–249. [Google Scholar] [CrossRef]
- Joussen, A.M.; Poulaki, V.; Le, M.L.; Koizumi, K.; Esser, C.; Janicki, H.; Schraermeyer, U.; Kociok, N.; Fauser, S.; Kirchhof, B.; et al. A Central Role for Inflammation in the Pathogenesis of Diabetic Retinopathy. FASEB J. 2004, 18, 1450–1452. [Google Scholar] [CrossRef]
- Grant, M.B.; Afzal, A.; Spoerri, P.; Pan, H.; Shaw, L.C.; Mames, R.N. The Role of Growth Factors in the Pathogenesis of Diabetic Retinopathy. Expert Opin. Investig. Drugs 2004, 13, 1275–1293. [Google Scholar] [CrossRef]
- Rangasamy, S.; McGuire, P.G.; Nitta, C.F.; Monickaraj, F.; Oruganti, S.R.; Das, A. Chemokine Mediated Monocyte Trafficking into the Retina: Role of Inflammation in Alteration of the Blood-Retinal Barrier in Diabetic Retinopathy. PLoS ONE 2014, 9, e108508. [Google Scholar] [CrossRef]
- Maier, R.; Weger, M.; Haller-Schober, E.M.; El-Shabrawi, Y.; Wedrich, A.; Theisl, A.; Aigner, R.; Barth, A.; Haas, A. Multiplex Bead Analysis of Vitreous and Serum Concentrations of Inflammatory and Proangiogenic Factors in Diabetic Patients. Mol. Vis. 2008, 14, 637–643. [Google Scholar]
- QY, G.; GY, H.; SQ, Y.; TW, Q.; X, X. Comprehensive Assessment of Growth Factors, Inflammatory Mediators, and Cytokines in Vitreous from Patients with Proliferative Diabetic Retinopathy. Int. J. Ophthalmol. 2022, 15, 1736–1742. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, H.; Gong, Y.; Wei, S.; Zhang, M. Early Spatiotemporal Characterization of Microglial Activation in the Retinas of Rats with Streptozotocin-Induced Diabetes. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.X.; Ng, Y.K.; Ling, E.A. Neuronal and Microglial Response in the Retina of Streptozotocin-Induced Diabetic Rats. Vis. Neurosci. 2000, 17, 463–471. [Google Scholar] [CrossRef]
- Ke, M.; Hu, X.Q.; Ouyang, J.; Dai, B.; Xu, Y. The Effect of Astragalin on the VEGF Production of Cultured Müller Cells under High Glucose Conditions. Biomed. Mater. Eng. 2012, 22, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Dean, C.; Dunning, F.M.; Liu, H.; Bomba-Warczak, E.; Martens, H.; Bharat, V.; Ahmed, S.; Chapman, E.R. Axonal and Dendritic Synaptotagmin Isoforms Revealed by a PHluorin-Syt Functional Screen. Mol. Biol. Cell 2012, 23, 1715–1727. [Google Scholar] [CrossRef]
- Liu, Y.; Leo, L.F.; McGregor, C.; Grivitishvili, A.; Barnstable, C.J.; Tombran-Tink, J. Pigment Epithelium-Derived Factor (PEDF) Peptide Eye Drops Reduce Inflammation, Cell Death and Vascular Leakage in Diabetic Retinopathy in Ins2(Akita) Mice. Mol. Med. 2012, 18, 1387–1401. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xu, E.; Elliott, M.H.; Zhu, M.; Le, Y.Z. Müller Cell-Derived VEGF Is Essential for Diabetes-Induced Retinal Inflammation and Vascular Leakage. Diabetes 2010, 59, 2297–2305. [Google Scholar] [CrossRef]
- Zong, H.; Ward, M.; Madden, A.; Yong, P.H.; Limb, G.A.; Curtis, T.M.; Stitt, A.W. Hyperglycaemia-Induced pro-Inflammatory Responses by Retinal Müller Glia Are Regulated by the Receptor for Advanced Glycation End-Products (RAGE). Diabetologia 2010, 53, 2656–2666. [Google Scholar] [CrossRef]
- Coughlin, B.A.; Trombley, B.T.; Mohr, S. Interleukin-6 (IL-6) Mediates Protection against Glucose Toxicity in Human Müller Cells via Activation of VEGF-A Signaling. Biochem. Biophys. Res. Commun. 2019, 517, 227–232. [Google Scholar] [CrossRef]
- Huang, H.C.; Wang, H.R.; Hsieh, L.M. Antiproliferative Effect of Baicalein, a Flavonoid from a Chinese Herb, on Vascular Smooth Muscle Cell. Eur. J. Pharmacol. 1994, 251, 91–93. [Google Scholar] [CrossRef]
- Bucolo, C.; Leggio, G.M.; Drago, F.; Salomone, S. Eriodictyol Prevents Early Retinal and Plasma Abnormalities in Streptozotocin-Induced Diabetic Rats. Biochem. Pharmacol. 2012, 84, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Silva, K.C.; Rosales, M.A.B.; Hamassaki, D.E.; Saito, K.C.; Faria, A.M.; Ribeiro, P.A.O.; Lopes de Faria, J.B.; Lopes de Faria, J.M. Green Tea Is Neuroprotective in Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1325–1336. [Google Scholar] [CrossRef]
- Kumar, B.; Gupta, S.K.; Nag, T.C.; Srivastava, S.; Saxena, R. Green Tea Prevents Hyperglycemia-Induced Retinal Oxidative Stress and Inflammation in Streptozotocin-Induced Diabetic Rats. Ophthalmic Res. 2012, 47, 103–108. [Google Scholar] [CrossRef]
- Dos Santos, M.D.; Almeida, M.C.; Lopes, N.P.; De Souza, G.E.P. Evaluation of the Anti-Inflammatory, Analgesic and Antipyretic Activities of the Natural Polyphenol Chlorogenic Acid. Biol. Pharm. Bull. 2006, 29, 2236–2240. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Yu, H.G.; Sohn, J. The Anti-Angiogenic Effect of Chlorogenic Acid on Choroidal Neovascularization. Korean J. Ophthalmol. 2010, 24, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.Y.; Sohn, J.; Park, K.H. Chlorogenic Acid Decreases Retinal Vascular Hyperpermeability in Diabetic Rat Model. J. Korean Med. Sci. 2013, 28, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.S.; Naqvi, S.; Gupta, S.K.; Srivastava, S. Prevention and Management of Diabetic Retinopathy in STZ Diabetic Rats by Tinospora Cordifolia and Its Molecular Mechanisms. Food Chem. Toxicol. 2012, 50, 3126–3132. [Google Scholar] [CrossRef]
- Rathi, S.S.; Grover, J.K.; Vikrant, V.; Biswas, N.R. Prevention of Experimental Diabetic Cataract by Indian Ayurvedic Plant Extracts. Phytother. Res. 2002, 16, 774–777. [Google Scholar] [CrossRef]
- Gupta, S.K.; Kumar, B.; Nag, T.C.; Agrawal, S.S.; Agrawal, R.; Agrawal, P.; Saxena, R.; Srivastava, S. Curcumin Prevents Experimental Diabetic Retinopathy in Rats through Its Hypoglycemic, Antioxidant, and Anti-Inflammatory Mechanisms. J. Ocul. Pharmacol. Ther. 2011, 27, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z.F.; Zhang, Q.; Liu, X.Z. Protective Effects of Curcumin on Retinal Müller Cell in Early Diabetic Rats. Int. J. Ophthalmol. 2013, 6, 422–424. [Google Scholar] [PubMed]
- Ilieva, I.; Ohgami, K.; Shiratori, K.; Koyama, Y.; Yoshida, K.; Kase, S.; Kitamei, H.; Takemoto, Y.; Yazawa, K.; Ohno, S. The Effects of Ginkgo Biloba Extract on Lipopolysaccharide-Induced Inflammation in Vitro and in Vivo. Exp. Eye Res. 2004, 79, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A.; Menon, B.; Gierhart, D.L. Beneficial Effect of Zeaxanthin on Retinal Metabolic Abnormalities in Diabetic Rats. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Ozawa, Y.; Kurihara, T.; Kubota, S.; Yuki, K.; Noda, K.; Kobayashi, S.; Ishida, S.; Tsubota, K. Neurodegenerative Influence of Oxidative Stress in the Retina of a Murine Model of Diabetes. Diabetologia 2010, 53, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Y.; Zhou, S.W. Effect of Berberine on PPARalpha/Delta/Gamma Expression in Type 2 Diabetic Rat Retinae. Yao Xue Xue Bao 2007, 42, 1243–1249. [Google Scholar]
- Wang, C.Z.; Aung, H.H.; Zhang, B.; Sun, S.; Li, X.L.; He, H.; Xie, J.T.; He, T.C.; Du, W.; Yuan, C.S. Chemopreventive Effects of Heat-Processed Panax Quinquefolius Root on Human Breast Cancer Cells. Anticancer Res. 2008, 28, 2545–2551. [Google Scholar]
- Kim, S.H.; Park, K.S. Effects of Panax Ginseng Extract on Lipid Metabolism in Humans. Pharmacol. Res. 2003, 48, 511–513. [Google Scholar] [CrossRef]
- Pietrucha-Dutczak, M.; Amadio, M.; Govoni, S.; Lewin-Kowalik, J.; Smedowski, A. The Role of Endogenous Neuroprotective Mechanisms in the Prevention of Retinal Ganglion Cells Degeneration. Front. Neurosci. 2018, 12, 834. [Google Scholar] [CrossRef]
- Lei, X.; Zhang, J.; Shen, J.; Hu, L.M.; Wu, Y.; Mou, L.; Xu, G.; Li, W.; Xu, G.T. EPO Attenuates Inflammatory Cytokines by Muller Cells in Diabetic Retinopathy. Front. Biosci. 2011, 3, 201–211. [Google Scholar] [CrossRef]
- Wang, P.; Xia, F. EPO Protects Müller Cell under High Glucose State through BDNF/TrkB Pathway. Int. J. Clin. Exp. Pathol. 2015, 8, 8083–8090. [Google Scholar]
- Tsai, J.C.; Wu, L.; Worgul, B.; Forbes, M.; Cao, J. Intravitreal Administration of Erythropoietin and Preservation of Retinal Ganglion Cells in an Experimental Rat Model of Glaucoma. Curr. Eye Res. 2005, 30, 1025–1031. [Google Scholar] [CrossRef]
- Garcia-Ramírez, M.; Hernández, C.; Ruiz-Meana, M.; Villarroel, M.; Corraliza, L.; García-Dorado, D.; Simó, R. Erythropoietin Protects Retinal Pigment Epithelial Cells against the Increase of Permeability Induced by Diabetic Conditions: Essential Role of JAK2/ PI3K Signaling. Cell. Signal. 2011, 23, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Duncan, D.S.; Echevarria, F.; McLaughlin, W.M.; Hatcher, J.B.; Sappington, R.M. Pressure-Induced Alterations in PEDF and PEDF-R Expression: Implications for Neuroprotective Signaling in Glaucoma. J. Clin. Exp. Ophthalmol. 2015, 6, 491. [Google Scholar] [CrossRef]
- Sterling, J.K.; Adetunji, M.O.; Guttha, S.; Bargoud, A.R.; Uyhazi, K.E.; Ross, A.G.; Dunaief, J.L.; Cui, Q.N. GLP-1 Receptor Agonist NLY01 Reduces Retinal Inflammation and Neuron Death Secondary to Ocular Hypertension. Cell Rep. 2020, 33, 108271. [Google Scholar] [CrossRef]
- Hernández, C.; Dal Monte, M.; Simó, R.; Casini, G. Neuroprotection as a Therapeutic Target for Diabetic Retinopathy. J. Diabetes Res. 2016, 2016, 9508541. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, M.; Song, B.W.; Lu, B.; Hu, P. Expression of Ciliary Neurotrophic Factor after Induction of Ocular Hypertension in the Retina of Rats. Chin. Med. J. 2007, 120, 1825–1829. [Google Scholar] [CrossRef] [PubMed]
- Pease, M.E.; Zack, D.J.; Berlinicke, C.; Bloom, K.; Cone, F.; Wang, Y.; Klein, R.L.; Hauswirth, W.W.; Quigley, H.A. Effect of CNTF on Retinal Ganglion Cell Survival in Experimental Glaucoma. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.Z.; Elyaman, W.; Yip, H.K.; Lee, V.W.H.; Yick, L.W.; Hugon, J.; So, K.F. CNTF Promotes Survival of Retinal Ganglion Cells after Induction of Ocular Hypertension in Rats: The Possible Involvement of STAT3 Pathway. Eur. J. Neurosci. 2004, 19, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Seki, M.; Nawa, H.; Fukuchi, T.; Abe, H.; Takei, N. BDNF Is Upregulated by Postnatal Development and Visual Experience: Quantitative and Immunohistochemical Analyses of BDNF in the Rat Retina. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3211–3218. [Google Scholar] [CrossRef]
- Feng, L.; Chen, H.; Yi, J.; Troy, J.B.; Zhang, H.F.; Liu, X. Long-Term Protection of Retinal Ganglion Cells and Visual Function by Brain-Derived Neurotrophic Factor in Mice With Ocular Hypertension. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3793–3802. [Google Scholar] [CrossRef] [PubMed]
- Trotta, M.C.; Gesualdo, C.; Herman, H.; Gharbia, S.; Balta, C.; Lepre, C.C.; Russo, M.; Itro, A.; D’Amico, G.; Peluso, L.; et al. Systemic Beta-Hydroxybutyrate Affects BDNF and Autophagy into the Retina of Diabetic Mice. Int. J. Mol. Sci. 2022, 23, 10184. [Google Scholar] [CrossRef]
- Telegina, D.V.; Kolosova, N.G.; Kozhevnikova, O.S. Immunohistochemical Localization of NGF, BDNF, and Their Receptors in a Normal and AMD-like Rat Retina. BMC Med. Genom. 2019, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.X.; Zhao, G.L.; Hu, X.; Zhou, H.; Li, S.Y.; Li, F.; Miao, Y.; Lei, B.; Wang, Z. P2X7/P2X4 Receptors Mediate Proliferation and Migration of Retinal Microglia in Experimental Glaucoma in Mice. Neurosci. Bull. 2022, 38, 901–915. [Google Scholar] [CrossRef]
- Amato, R.; Catalani, E.; Dal Monte, M.; Cammalleri, M.; Di Renzo, I.; Perrotta, C.; Cervia, D.; Casini, G. Autophagy-Mediated Neuroprotection Induced by Octreotide in an Ex Vivo Model of Early Diabetic Retinopathy. Pharmacol. Res. 2018, 128, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Medina, J.J.; Rubio-Velazquez, E.; Foulquie-Moreno, E.; Casaroli-Marano, R.P.; Pinazo-Duran, M.D.; Zanon-Moreno, V.; Del-Rio-vellosillo, M. Update on the Effects of Antioxidants on Diabetic Retinopathy: In Vitro Experiments, Animal Studies and Clinical Trials. Antioxidants 2020, 9, 561. [Google Scholar] [CrossRef]
- Evans, Z.P.; Ellett, J.D.; Fariss, M.W.; Schnellmann, R.G.; Schmidt, M.G.; Chavin, K. Vitamin E Succinate Reduces Ischemia/Reperfusion Injury in Steatotic Livers. Transplant. Proc. 2008, 40, 3327–3329. [Google Scholar] [CrossRef]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal Prospects of Antioxidants: A Review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef] [PubMed]
- Young, I.S.; Woodside, J.V. Antioxidants in Health and Disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef]
- Takiishi, T.; Gysemans, C.; Bouillon, R.; Mathieu, C. Vitamin D and Diabetes. Endocrinol. Metab. Clin. N. Am. 2010, 39, 419–446. [Google Scholar] [CrossRef]
- Albert, D.M.; Scheef, E.A.; Wang, S.; Mehraein, F.; Darjatmoko, S.R.; Sorenson, C.M.; Sheibani, N. Calcitriol Is a Potent Inhibitor of Retinal Neovascularization. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2327–2334. [Google Scholar] [CrossRef]
- Ren, Z.; Li, W.; Zhao, Q.; Ma, L.; Zhu, J. The Impact of 1,25-Dihydroxy Vitamin D3 on the Expressions of Vascular Endothelial Growth Factor and Transforming Growth Factor-Β₁ in the Retinas of Rats with Diabetes. Diabetes Res. Clin. Pract. 2012, 98, 474–480. [Google Scholar] [CrossRef]
- Del Valle, L.G.; Noblet, M.C.; Martinez-Sanchez, G. Crosstalk between Oxidative Stress and Ocular Diseases. J. Clin. Res. Ophthalmol. 2020, 7, 037–047. [Google Scholar] [CrossRef]
- Ozawa, Y. Oxidative Stress in the Light-Exposed Retina and Its Implication in Age-Related Macular Degeneration. Redox Biol. 2020, 37, 101779. [Google Scholar] [CrossRef]
- Goyal, A.; Srivastava, A.; Sihota, R.; Kaur, J. Evaluation of Oxidative Stress Markers in Aqueous Humor of Primary Open Angle Glaucoma and Primary Angle Closure Glaucoma Patients. Curr. Eye Res. 2014, 39, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Gurler, B.; Vural, H.; Yilmaz, N.; Oguz, H.; Satici, A.; Aksoy, N. The Role of Oxidative Stress in Diabetic Retinopathy. Eye 2000, 14 Pt 5, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Terao, R.; Ahmed, T.; Suzumura, A.; Terasaki, H. Oxidative Stress-Induced Cellular Senescence in Aging Retina and Age-Related Macular Degeneration. Antioxidants 2022, 11, 2189. [Google Scholar] [CrossRef]
- Gherghel, D.; Mroczkowska, S.; Qin, L. Reduction in Blood Glutathione Levels Occurs Similarly in Patients with Primary-Open Angle or Normal Tension Glaucoma. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3333–3339. [Google Scholar] [CrossRef]
- Al-Dosari, D.I.; Ahmed, M.M.; Al-Rejaie, S.S.; Alhomida, A.S.; Ola, M.S. Flavonoid Naringenin Attenuates Oxidative Stress, Apoptosis and Improves Neurotrophic Effects in the Diabetic Rat Retina. Nutrients 2017, 9, 1161. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, E.S.M.; Abo-Salem, O.M.; Aly, H.A.; Mansour, A.M. Potential Antidiabetic and Hypolipidemic Effects of Propolis Extract in Streptozotocin-Induced Diabetic Rats. Pak. J. Pharm. Sci. 2009, 22, 168–174. [Google Scholar]
- Liles, M.R.; Newsome, D.A.; Oliver, P.D. Antioxidant Enzymes in the Aging Human Retinal Pigment Epithelium. Arch. Ophthalmol. 1991, 109, 1285–1288. [Google Scholar] [CrossRef]
- Moreno, M.C.; Campanelli, J.; Sande, P.; Sáenz, D.A.; Keller Sarmiento, M.I.; Rosenstein, R.E. Retinal Oxidative Stress Induced by High Intraocular Pressure. Free Radic. Biol. Med. 2004, 37, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, G.; Tolun, F.I.; Gul, M.; Imrek, S. Retinal Oxidative Stress Induced by Intraocular Hypertension in Rats May Be Ameliorated by Brimonidine Treatment and N-Acetyl Cysteine Supplementation. J. Glaucoma 2009, 18, 662–665. [Google Scholar] [CrossRef]
- Ramos, H.; Bogdanov, P.; Huerta, J.; Deàs-Just, A.; Hernández, C.; Simó, R. Antioxidant Effects of DPP-4 Inhibitors in Early Stages of Experimental Diabetic Retinopathy. Antioxidants 2022, 11, 1418. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, M.; Oh, S.B.; Kim, H.Y.; Kim, C.; Kim, T.Y.; Park, Y.H. Superoxide Dismutase 3 Prevents Early Stage Diabetic Retinopathy in Streptozotocin-Induced Diabetic Rat Model. PLoS ONE 2022, 17, e0262396. [Google Scholar] [CrossRef] [PubMed]
- Ferreira de Melo, I.M.; Martins Ferreira, C.G.; Lima da Silva Souza, E.H.; Almeida, L.L.; Bezerra de Sá, F.; Cavalcanti Lapa Neto, C.J.; Paz de Castro, M.V.; Teixeira, V.W.; Coelho Teixeira, Á.A. Melatonin Regulates the Expression of Inflammatory Cytokines, VEGF and Apoptosis in Diabetic Retinopathy in Rats. Chem. Biol. Interact. 2020, 327, 109183. [Google Scholar] [CrossRef] [PubMed]
- Ohia, S.E.; Bagchi, M.; Stohs, S.J. Age-Related Oxidative Damage in Long-Evans Rat Retina. Res. Commun. Mol. Pathol. Pharmacol. 1994, 85, 21–31. [Google Scholar]
- Dutta, R.K.; Lee, J.N.; Maharjan, Y.; Park, C.; Choe, S.K.; Ho, Y.S.; Kwon, H.M.; Park, R. Catalase-Deficient Mice Induce Aging Faster through Lysosomal Dysfunction. Cell. Commun. Signal. 2022, 20, 192. [Google Scholar] [CrossRef] [PubMed]
- Lopez, P.; Wagner, K.-D.; Hofman, P.; Van Obberghen, E. RNA Activation of the Vascular Endothelial Growth Factor Gene (VEGF) Promoter by Double-Stranded RNA and Hypoxia: Role of Noncoding VEGF Promoter Transcripts. Mol. Cell. Biol. 2016, 36, 1480–1493. [Google Scholar] [CrossRef]
- Koc, F.; Kansu, T.; Kavuncu, S.; Firat, E. Topical Apraclonidine Testing Discloses Pupillary Sympathetic Denervation in Diabetic Patients. J. Neuroophthalmol. 2006, 26, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Hoyng, P.F.J.; Van Beek, L.M. Pharmacological Therapy for Glaucoma: A Review. Drugs 2000, 59, 411–434. [Google Scholar] [CrossRef] [PubMed]
- Vaziri, K.; Schwartz, S.G.; Relhan, N.; Kishor, K.; Flynn, H.W. New Therapeutic Approaches in Diabetic Retinopathy. Rev. Diabet. Stud. 2015, 12, 196. [Google Scholar] [CrossRef]
- Boyer, D.S.; Yoon, Y.H.; Belfort, R.; Bandello, F.; Maturi, R.K.; Augustin, A.J.; Li, X.Y.; Cui, H.; Hashad, Y.; Whitcup, S.M. Three-Year, Randomized, Sham-Controlled Trial of Dexamethasone Intravitreal Implant in Patients with Diabetic Macular Edema. Ophthalmology 2014, 121, 1904–1914. [Google Scholar] [CrossRef]
- Kusari, J.; Zhou, S.; Padillo, E.; Clarke, K.G.; Gil, D.W. Effect of Memantine on Neuroretinal Function and Retinal Vascular Changes of Streptozotocin-Induced Diabetic Rats. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5152–5159. [Google Scholar] [CrossRef] [PubMed]
- Kern, T.S.; Miller, C.M.; Tang, J.; Du, Y.; Ball, S.L.; Berti-Matera, L. Comparison of Three Strains of Diabetic Rats with Respect to the Rate at Which Retinopathy and Tactile Allodynia Develop. Mol. Vis. 2010, 16, 1629–1639. [Google Scholar]
- Akiyode, O.; Dunkelly-Allen, N. Ranibizumab: A Review of Its Use in the Treatment of Diabetic Retinopathy in Patients With Diabetic Macular Edema. J. Pharm. Technol. 2016, 32, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Ohta, M.; Inoue, T.; Mizuno, K.; Isobe, T.; Tanabe, S.; Tanihara, H. Effects of K-115 (Ripasudil), a Novel ROCK Inhibitor, on Trabecular Meshwork and Schlemm’s Canal Endothelial Cells. Sci. Rep. 2016, 6, 19640. [Google Scholar] [CrossRef]
- Song, J.H.; Lee, J.J.; Lee, S.J. Comparison of the Short-Term Effects of Intravitreal Triamcinolone Acetonide and Bevacizumab Injection for Diabetic Macular Edema. Korean J. Ophthalmol. 2011, 25, 156–160. [Google Scholar] [CrossRef]
- Zhang, X.; Lai, D.; Bao, S.; Hambly, B.D.; Gillies, M.C. Triamcinolone Acetonide Inhibits P38MAPK Activation and Neuronal Apoptosis in Early Diabetic Retinopathy. Curr. Mol. Med. 2013, 13, 946–958. [Google Scholar] [CrossRef]
- Furino, C.; Boscia, F.; Reibaldi, M.; Alessio, G. Intravitreal Therapy for Diabetic Macular Edema: An Update. J. Ophthalmol. 2021, 2021, 6654168. [Google Scholar] [CrossRef]
- Boyer, D.S.; Hopkins, J.J.; Sorof, J.; Ehrlich, J.S. Anti-Vascular Endothelial Growth Factor Therapy for Diabetic Macular Edema. Ther. Adv. Endocrinol. Metab. 2013, 4, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Kovács-Valasek, A.; Szabadfi, K.; Dénes, V.; Szalontai, B.; Tamás, A.; Kiss, P.; Szabó, A.; Setalo, G.; Reglődi, D.; Gábriel, R. Accelerated Retinal Aging in PACAP Knock-out Mice. Neuroscience 2017, 348, 1–10. [Google Scholar] [CrossRef]
- Szabadfi, K.; Szabo, A.; Kiss, P.; Reglodi, D.; Setalo, G.; Kovacs, K.; Tamas, A.; Toth, G.; Gabriel, R. PACAP Promotes Neuron Survival in Early Experimental Diabetic Retinopathy. Neurochem. Int. 2014, 64, 84–91. [Google Scholar] [CrossRef]
- Huang, H.; Gandhi, J.K.; Zhong, X.; Wei, Y.; Gong, J.; Duh, E.J.; Vinores, S.A. TNFalpha Is Required for Late BRB Breakdown in Diabetic Retinopathy, and Its Inhibition Prevents Leukostasis and Protects Vessels and Neurons from Apoptosis. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1336–1344. [Google Scholar] [CrossRef]
- Zheng, L.; Szabó, C.; Kern, T.S. Poly(ADP-Ribose) Polymerase Is Involved in the Development of Diabetic Retinopathy via Regulation of Nuclear Factor-KappaB. Diabetes 2004, 53, 2960–2967. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, L.; Chen, R.; Lou, Q.; Wang, H. Poly(ADP-Ribose) Polymerase-1: An Update on Its Role in Diabetic Retinopathy. Discov. Med. 2021, 32, 13–22. [Google Scholar] [PubMed]
- Valverde, A.M.; Miranda, S.; García-Ramírez, M.; González-Rodriguez, Á.; Hernández, C.; Simó, R. Proapoptotic and Survival Signaling in the Neuroretina at Early Stages of Diabetic Retinopathy. Mol. Vis. 2013, 19, 47–53. [Google Scholar]
- Mi, X.S.; Zhong, J.X.; Chang, R.C.C.; So, K.F. Research Advances on the Usage of Traditional Chinese Medicine for Neuroprotection in Glaucoma. J. Integr. Med. 2013, 11, 233–240. [Google Scholar] [CrossRef]
- Andrea, H.; Roland, M.; Tibor, R.; Adrienne, C. Effect of Steroid Therapy on Intraocular Pressure. Orv. Hetil. 2022, 163, 1345–1352. [Google Scholar] [CrossRef]
- Pang, I.H.; Clark, A.F. Inducible Rodent Models of Glaucoma. Prog. Retin. Eye Res. 2020, 75, 100799. [Google Scholar] [CrossRef]
- Ficarrotta, K.R.; Mohamed, Y.H.; Passaglia, C.L. Experimental Glaucoma Model with Controllable Intraocular Pressure History. Sci. Rep. 2020, 10, 126. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Garcia-Herranz, D.; Subias, M.; Martinez-Rincón, T.; Mendez-Martínez, S.; Bravo-Osuna, I.; Carretero, A.; Ruberte, J.; Garcia-Feijoo, J.; Pablo, L.E.; et al. Chronic Glaucoma Using Biodegradable Microspheres to Induce Intraocular Pressure Elevation. Six-Month Follow-Up. Biomedicines 2021, 9, 682. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, H.; Sun, Y.; Li, Y.; Luo, B.; Cui, J.; Zhu, M.; Bi, F.; Chen, K.; Liu, Y. Monosodium Glutamate-Induced Mouse Model With Unique Diabetic Retinal Neuropathy Features and Artificial Intelligence Techniques for Quantitative Evaluation. Front. Immunol. 2022, 13, 862702. [Google Scholar] [CrossRef] [PubMed]
- Sankalp; Dada, T.; Yadav, R.K.; Sharma, H.B.; Netam, R.K.; Kochhar, K.P. Effect of Tratak (Yogic Ocular Exercises) on Intraocular Pressure in Glaucoma: An RCT. Int. J. Yoga 2022, 15, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Gábriel, R. Neuropeptides and Diabetic Retinopathy. Br. J. Clin. Pharmacol. 2013, 75, 1189–1201. [Google Scholar] [CrossRef]
- Pöstyéni, E.; Ganczer, A.; Kovács-Valasek, A.; Gabriel, R. Relevance of Peptide Homeostasis in Metabolic Retinal Degenerative Disorders: Curative Potential in Genetically Modified Mice. Front. Pharmacol. 2022, 12, 808315. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of Apoptosis in Health and Disease: The Balancing Act of BCL-2 Family Proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef]
- Yuan, Z.; Dewson, G.; Czabotar, P.E.; Birkinshaw, R.W. VDAC2 and the BCL-2 Family of Proteins. Biochem. Soc. Trans. 2021, 49, 2787–2795. [Google Scholar] [CrossRef]
- Roberts, A.W. Therapeutic Development and Current Uses of BCL-2 Inhibition. Hematol. Am. Soc. Hematol. Educ. Progr. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, Q.; Li, H.; Meng, Z.; Hao, M.; Ma, X.; Lin, W.; Kuang, H. Dapagliflozin Reduces Apoptosis of Diabetic Retina and Human Retinal Microvascular Endothelial Cells Through ERK1/2/CPLA2/AA/ROS Pathway Independent of Hypoglycemic. Front. Pharmacol. 2022, 13, 827896. [Google Scholar] [CrossRef]
- Huang, B.; Liang, J.J.; Zhuang, X.; Chen, S.W.; Ng, T.K.; Chen, H. Intravitreal Injection of Hydrogen Peroxide Induces Acute Retinal Degeneration, Apoptosis, and Oxidative Stress in Mice. Oxid. Med. Cell. Longev. 2018, 2018, 5489476. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, W.; Liu, A.; Tao, Y.; Wang, Q.; Yang, Y.; Wang, L.; Huang, Y. Protective Effect of Salvianolic Acid A against N-Methyl-N-Nitrosourea-Induced Retinal Degeneration. Evid. Based Complement. Alternat. Med. 2022, 2022, 1219789. [Google Scholar] [CrossRef] [PubMed]
- Edington, M.; Siempis, T.; Montgomery, D. Discontinuation of the Herbal Preparation Hypericum Perforatum, Also Known as St John’s Wort, Associated with Improved Intraocular Pressure Control in a Patient on Topical Beta-Blockers for Primary Open-Angle Glaucoma. Oman J. Ophthalmol. 2018, 11, 188–189. [Google Scholar] [PubMed]
- Mincione, F.; Scozzafava, A.; Supuran, C.T. The Development of Topically Acting Carbonic Anhydrase Inhibitors as Anti-Glaucoma Agents. Curr. Top. Med. Chem. 2007, 7, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Farzam, K.; Abdullah, M. Acetazolamide. Xpharm Compr. Pharmacol. Ref. 2022, 1–5. [Google Scholar] [CrossRef]
- Lesar, T.S. Comparison of Ophthalmic Beta-Blocking Agents. Clin. Pharm. 1987, 6, 451–463. [Google Scholar]
- Lim, K.S.; Nau, C.B.; O’Byrne, M.M.; Hodge, D.O.; Toris, C.B.; McLaren, J.W.; Johnson, D.H. Mechanism of Action of Bimatoprost, Latanoprost, and Travoprost in Healthy Subjects. A Crossover Study. Ophthalmology 2008, 115, 790–795.e4. [Google Scholar] [CrossRef]
- Brubaker, R.F. Mechanism of Action of Bimatoprost (LumiganTM). Surv. Ophthalmol. 2001, 45, S347–S351. [Google Scholar] [CrossRef]
- Easthope, S.E.; Perry, C.M. Topical Bimatoprost: A Review of Its Use in Open-Angle Glaucoma and Ocular Hypertension. Drugs Aging 2002, 19, 231–248. [Google Scholar] [CrossRef]
- Woodward, D.F.; Liang, Y.; Krauss, A.H.P. Prostamides (Prostaglandin-Ethanolamides) and Their Pharmacology. Br. J. Pharmacol. 2008, 153, 410–419. [Google Scholar] [CrossRef]
- Alm, A. Latanoprost in the Treatment of Glaucoma. Clin. Ophthalmol. 2014, 8, 1967–1985. [Google Scholar] [CrossRef]
- Wheeler, L.A.; Gil, D.W.; WoldeMussie, E. Role of Alpha-2 Adrenergic Receptors in Neuroprotection and Glaucoma. Surv. Ophthalmol. 2001, 45, S290–S294. [Google Scholar] [CrossRef] [PubMed]
- El-Kamel, A.; Al-Dosari, H.; Al-Jenoobi, F. Environmentally Responsive Ophthalmic Gel Formulation of Carteolol Hydrochloride. Drug Deliv. 2006, 13, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Arthur, S.; Cantor, L.B. Update on the Role of Alpha-Agonists in Glaucoma Management. Exp. Eye Res. 2011, 93, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Martens-Lobenhoffer, J.; Banditt, P. Clinical Pharmacokinetics of Dorzolamide. Clin. Pharmacokinet. 2002, 41, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Balfour, J.A.; Wilde, M.I. Dorzolamide. A Review of Its Pharmacology and Therapeutic Potential in the Management of Glaucoma and Ocular Hypertension. Drugs Aging 1997, 10, 384–403. [Google Scholar] [CrossRef]
- Loftsson, T.; Jansook, P.; Stefánsson, E. Topical Drug Delivery to the Eye: Dorzolamide. Acta Ophthalmol. 2012, 90, 603–608. [Google Scholar] [CrossRef]
- Schmidt, K.G.; Horowitz, Y.; Buckman, G.; Segev, E.; Levinger, E.; Geyer, O. Lowering of IOP by Echothiophate Iodide in Pseudophakic Eyes with Glaucoma. Curr. Eye Res. 2010, 35, 698–702. [Google Scholar] [CrossRef]
- Sjöquist, B.; Stjernschantz, J. Ocular and Systemic Pharmacokinetics of Latanoprost in Humans. Surv. Ophthalmol. 2002, 47, S6. [Google Scholar] [CrossRef]
- Gonzalez, J.P.; Clissold, S.P. Ocular Levobunolol. A Review of Its Pharmacodynamic and Pharmacokinetic Properties, and Therapeutic Efficacy. Drugs 1987, 34, 648–661. [Google Scholar] [CrossRef]
- Novack, G.D. Levobunolol for the Long-Term Treatment of Glaucoma. Gen. Pharmacol. 1986, 17, 373–377. [Google Scholar] [CrossRef]
- Ishibashi, T.; Yokoi, N.; Kinoshita, S. Comparison of the Effects of Topical Levobunolol and Timolol Solution on the Human Ocular Surface. Cornea 2003, 22, 709–715. [Google Scholar] [CrossRef]
- Skorobohach, B.J.; Ward, D.A.; Hendrix, D.V.H. Effects of Oral Administration of Methazolamide on Intraocular Pressure and Aqueous Humor Flow Rate in Clinically Normal Dogs. Am. J. Vet. Res. 2003, 64, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Li, G.; Le, T.D.; Kopczynski, C.; Stamer, W.D.; Gong, H. Netarsudil Increases Outflow Facility in Human Eyes Through Multiple Mechanisms. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6197–6209. [Google Scholar] [CrossRef] [PubMed]
- Papadia, M.; Bagnis, A.; Scotto, R.; Traverso, C.E. Tafluprost for Glaucoma. Expert Opin. Pharmacother. 2011, 12, 2393–2401. [Google Scholar] [CrossRef] [PubMed]
- Mina, B.P.; Leonard, K.S.; Nida, S.A.; Malik, Y.K. Tafluprost: A Novel Prostaglandin Analog for Treatment of Glaucoma. Adv. Ther. 2011, 28, 707–715. [Google Scholar] [CrossRef]
- Takagi, Y.; Nakajima, T.; Shimazaki, A.; Kageyama, M.; Matsugi, T.; Matsumura, Y.; Gabelt, B.T.; Kaufman, P.L.; Hara, H. Pharmacological Characteristics of AFP-168 (Tafluprost), a New Prostanoid FP Receptor Agonist, as an Ocular Hypotensive Drug. Exp. Eye Res. 2004, 78, 767–776. [Google Scholar] [CrossRef]
- Watanabe, K.; Chiou, G.C.Y. Action Mechanism of Timolol to Lower the Intraocular Pressure in Rabbits. Ophthalmic Res. 1983, 15, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Volotinen, M.; Hakkola, J.; Pelkonen, O.; Vapaatalo, H.; Mäenpää, J. Metabolism of Ophthalmic Timolol: New Aspects of an Old Drug. Basic Clin. Pharmacol. Toxicol. 2011, 108, 297–303. [Google Scholar] [CrossRef]
- Arranz-Marquez, E.; Teus, M.A. Prostanoids for the Management of Glaucoma. Expert Opin. Drug Saf. 2008, 7, 801–808. [Google Scholar] [CrossRef]
- Costagliola, C.; Dell’Omo, R.; Romano, M.R.; Rinaldi, M.; Zeppa, L.; Parmeggiani, F. Pharmacotherapy of Intraocular Pressure—Part II. Carbonic Anhydrase Inhibitors, Prostaglandin Analogues and Prostamides. Expert Opin. Pharmacother. 2009, 10, 2859–2870. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.; Su, W.; Zhang, Y.; Li, Z.; Deng, C.; Zhuo, Y. Trimetazidine Protects Retinal Ganglion Cells from Acute Glaucoma via the Nrf2/Ho-1 Pathway. Clin. Sci. 2017, 131, 2363–2375. [Google Scholar] [CrossRef] [PubMed]
- Fung, D.S.; Whitson, J.T. An Evidence-Based Review of Unoprostone Isopropyl Ophthalmic Solution 0.15% for Glaucoma: Place in Therapy. Clin. Ophthalmol. 2014, 8, 543. [Google Scholar] [CrossRef] [PubMed]
- Acheampong, A.A.; Shackleton, M.; John, B.; Burke, J.; Wheeler, L.; Tang-Liu, D. Distribution of Brimonidine into Anterior and Posterior Tissues of Monkey, Rabbit, and Rat Eyes. Drug Metab. Dispos. 2002, 30, 421–429. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, L.; Weinreb, R.N. Ophthalmic Drug Discovery: Novel Targets and Mechanisms for Retinal Diseases and Glaucoma. Nat. Rev. Drug Discov. 2012, 11, 541–559. [Google Scholar] [CrossRef]
- Costa, V.P.; Harris, A.; Stefánsson, E.; Flammer, J.; Krieglstein, G.K.; Orzalesi, N.; Heijl, A.; Renard, J.P.; Serra, L.M. The Effects of Antiglaucoma and Systemic Medications on Ocular Blood Flow. Prog. Retin. Eye Res. 2003, 22, 769–805. [Google Scholar] [CrossRef]
- Lewis, R.A.; Christie, W.C.; Day, D.G.; Craven, E.R.; Walters, T.; Bejanian, M.; Lee, S.S.; Goodkin, M.L.; Zhang, J.; Whitcup, S.M.; et al. Bimatoprost Sustained-Release Implants for Glaucoma Therapy: 6-Month Results From a Phase I/II Clinical Trial. Am. J. Ophthalmol. 2017, 175, 137–147. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, L.; Dai, Y.; Sheng, X.; Chen, S.; Xie, Q. Effects of Coconut Water on Retina in Diabetic Rats. Evid. Based Complement. Alternat. Med. 2020, 2020, 9450634. [Google Scholar] [CrossRef]
- Su, W.; Li, Z.; Jia, Y.; Zhuo, Y. Rapamycin Is Neuroprotective in a Rat Chronic Hypertensive Glaucoma Model. PLoS ONE 2014, 9, e99719. [Google Scholar] [CrossRef]
- Ishizaki, T.; Maekawa, M.; Fujisawa, K.; Okawa, K.; Iwamatsu, A.; Fujita, A.; Watanabe, N.; Saito, Y.; Kakizuka, A.; Morii, N.; et al. The Small GTP-Binding Protein Rho Binds to and Activates a 160 KDa Ser/Thr Protein Kinase Homologous to Myotonic Dystrophy Kinase. EMBO J. 1996, 15, 1885–1893. [Google Scholar] [CrossRef]
- Waki, M.; Yoshida, Y.; Oka, T.; Azuma, M. Reduction of Intraocular Pressure by Topical Administration of an Inhibitor of the Rho-Associated Protein Kinase. Curr. Eye Res. 2001, 22, 470–474. [Google Scholar] [CrossRef]
- Honjo, M.; Tanihara, H.; Inatani, M.; Kido, N.; Sawamura, T.; Yue, B.Y.J.T.; Narumiya, S.; Honda, Y. Effects of Rho-Associated Protein Kinase Inhibitor Y-27632 on Intraocular Pressure and Outflow Facility. Investig. Ophthalmol. Vis. Sci. 2001, 42, 137–144. [Google Scholar]
- Honjo, M.; Inatani, M.; Kido, N.; Sawamura, T.; Yue, B.Y.J.T.; Honda, Y.; Tanihara, H. Effects of Protein Kinase Inhibitor, HA1077, on Intraocular Pressure and Outflow Facility in Rabbit Eyes. Arch. Ophthalmol. 2001, 119, 1171–1178. [Google Scholar] [CrossRef]
- Lin, C.W.; Sherman, B.; Moore, L.A.; Laethem, C.L.; Lu, D.W.; Pattabiraman, P.P.; Rao, P.V.; Delong, M.A.; Kopczynski, C.C. Discovery and Preclinical Development of Netarsudil, a Novel Ocular Hypotensive Agent for the Treatment of Glaucoma. J. Ocul. Pharmacol. Ther. 2018, 34, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Arita, R.; Hata, Y.; Nakao, S.; Kita, T.; Miura, M.; Kawahara, S.; Zandi, S.; Almulki, L.; Tayyari, F.; Shimokawa, H.; et al. Rho Kinase Inhibition by Fasudil Ameliorates Diabetes-Induced Microvascular Damage. Diabetes 2009, 58, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Anwar, K.N.; Fazal, F.; Malik, A.B.; Rahman, A. RhoA/Rho-Associated Kinase Pathway Selectively Regulates Thrombin-Induced Intercellular Adhesion Molecule-1 Expression in Endothelial Cells via Activation of I Kappa B Kinase Beta and Phosphorylation of RelA/P65. J. Immunol. 2004, 173, 6965–6972. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Zhong, Y. Rho/Rho-Associated Kinase Pathway in Glaucoma (Review). Int. J. Oncol. 2013, 43, 1357–1367. [Google Scholar] [CrossRef]
- Tanihara, H.; Inoue, T.; Yamamoto, T.; Kuwayama, Y.; Abe, H.; Araie, M. Phase 1 Clinical Trials of a Selective Rho Kinase Inhibitor, K-115. JAMA Ophthalmol. 2013, 131, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Tanihara, H.; Inoue, T.; Yamamoto, T.; Kuwayama, Y.; Abe, H.; Araie, M. Phase 2 Randomized Clinical Study of a Rho Kinase Inhibitor, K-115, in Primary Open-Angle Glaucoma and Ocular Hypertension. Am. J. Ophthalmol. 2013, 156, 731–736.e2. [Google Scholar] [CrossRef]
- Tanihara, H.; Inoue, T.; Yamamoto, T.; Kuwayama, Y.; Abe, H.; Suganami, H.; Araie, M. Intra-Ocular Pressure-Lowering Effects of a Rho Kinase Inhibitor, Ripasudil (K-115), over 24 Hours in Primary Open-Angle Glaucoma and Ocular Hypertension: A Randomized, Open-Label, Crossover Study. Acta Ophthalmol. 2015, 93, e254–e260. [Google Scholar] [CrossRef]
- Tanihara, H.; Inoue, T.; Yamamoto, T.; Kuwayama, Y.; Abe, H.; Suganami, H.; Araie, M. Additive Intraocular Pressure-Lowering Effects of the Rho Kinase Inhibitor Ripasudil (K-115) Combined With Timolol or Latanoprost: A Report of 2 Randomized Clinical Trials. JAMA Ophthalmol. 2015, 133, 755–761. [Google Scholar] [CrossRef]
- Apte, R.S. Age-Related Macular Degeneration. N. Engl. J. Med. 2021, 385, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S. Update in Retina: Photodynamic Therapy for the Treatment of Subfoveal Choroidal Neovascularization Secondary to Age-Related Macular Degeneration. Can. J. Ophthalmol. 2001, 36, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.; Burgess, P.I.; Kayange, P. Management of Diabetic Retinopathy. Malawi Med. J. 2013, 25, 116–120. [Google Scholar] [PubMed]
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.J.; Jenkins, A.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.P.; Simó, R.; et al. The Progress in Understanding and Treatment of Diabetic Retinopathy. Prog. Retin. Eye Res. 2016, 51, 156–186. [Google Scholar] [CrossRef]
- Mounirou, B.A.; Adam, N.D.; Yakoura, A.K.; Aminou, M.S.; Liu, Y.T.; Tan, L.Y. Diabetic Retinopathy: An Overview of Treatments. Indian J. Endocrinol. Metab. 2022, 26, 111. [Google Scholar] [CrossRef]
- Pande, G.S.; Tidake, P. Laser Treatment Modalities for Diabetic Retinopathy. Cureus 2022, 14, e30024. [Google Scholar] [CrossRef] [PubMed]
- Ulbig, M.W.; Kollias, A.N. Diabetic Retinopathy: Early Diagnosis and Effective Treatment. Dtsch. Arztebl. Int. 2010, 107, 75–84. [Google Scholar] [CrossRef]
- Brănişteanu, D.C.; Bilha, A.; Moraru, A. Vitrectomy Surgery of Diabetic Retinopathy Complications. Rom. J. Ophthalmol. 2016, 60, 31. [Google Scholar] [PubMed]
- Gedde, S.; Anderson, D.; Budenz, D.; Del Calvo, M.; Fantes, F.; Greenfield, D.; Hodapp, E.; Lee, R.; Marcellino, A.; Palmberg, P.; et al. Treatment Outcomes in the Tube versus Trabeculectomy Study after One Year of Follow-Up. Am. J. Ophthalmol. 2007, 143, 22.e2. [Google Scholar] [CrossRef]
- Rulli, E.; Biagioli, E.; Riva, I.; Gambirasio, G.; De Simone, I.; Floriani, I.; Quaranta, L. Efficacy and Safety of Trabeculectomy vs Nonpenetrating Surgical Procedures: A Systematic Review and Meta-Analysis. JAMA Ophthalmol. 2013, 131, 1573–1582. [Google Scholar] [CrossRef]
- Rao, P.K. Stem Cell Therapies for Intraocular Disease. Mo. Med. 2022, 119, 65–68. [Google Scholar]
- Home—ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/ct2/home (accessed on 19 April 2023).
- Rizkiawan, D.E.; Evelyn, M.; Tjandra, K.C.; Setiawan, B. Utilization of Modified Induced Pluripotent Stem Cells as the Advance Therapy of Glaucoma: A Systematic Review. Clin. Ophthalmol. 2022, 16, 2851. [Google Scholar] [CrossRef]
- Cho, Y.K.; Park, D.H.; Jeon, I.C. Medication Trends for Age-Related Macular Degeneration. Int. J. Mol. Sci. 2021, 22, 11837. [Google Scholar] [CrossRef] [PubMed]
- Kern, T.S.; Miller, C.M.; Du, Y.; Zheng, L.; Mohr, S.; Ball, S.L.; Kim, M.; Jamison, J.A.; Bingaman, D.P. Topical Administration of Nepafenac Inhibits Diabetes-Induced Retinal Microvascular Disease and Underlying Abnormalities of Retinal Metabolism and Physiology. Diabetes 2007, 56, 373–379. [Google Scholar] [CrossRef]
- Vincent, J.A.; Mohr, S. Inhibition of Caspase-1/Interleukin-1beta Signaling Prevents Degeneration of Retinal Capillaries in Diabetes and Galactosemia. Diabetes 2007, 56, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Z.; Xi, X.; Gao, L.; Kern, T.S. Captopril Inhibits Capillary Degeneration in the Early Stages of Diabetic Retinopathy. Curr. Eye Res. 2007, 32, 883–889. [Google Scholar] [CrossRef] [PubMed]
- El-Remessy, A.B.; Bartoli, M.; Platt, D.H.; Fulton, D.; Caldwell, R.B. Oxidative Stress Inactivates VEGF Survival Signaling in Retinal Endothelial Cells via PI 3-Kinase Tyrosine Nitration. J. Cell Sci. 2016, 129, 3203. [Google Scholar] [CrossRef]
- Kompella, U.B.; Hartman, R.R.; Patil, M.A. Extraocular, Periocular, and Intraocular Routes for Sustained Drug Delivery for Glaucoma. Prog. Retin. Eye Res. 2021, 82, 100901. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xu, X.; Zheng, Z.; Zhu, B.; Shi, Y.; Liu, K. Protective Effects of Rosiglitazone on Retinal Neuronal Damage in Diabetic Rats. Curr. Eye Res. 2011, 36, 673–679. [Google Scholar] [CrossRef]
- Pöstyéni, E.; Szabadfi, K.; Sétáló, G.; Gabriel, R. A Promising Combination: Pacap and Parp Inhibitor Have Therapeutic Potential in Models of Diabetic and Hypertensive Retinopathies. Cells 2021, 10, 3470. [Google Scholar] [CrossRef] [PubMed]
- Kiss, P.; Atlasz, T.; Szabadfi, K.; Horvath, G.; Griecs, M.; Farkas, J.; Matkovits, A.; Toth, G.; Lubics, A.; Tamas, A.; et al. Comparison between PACAP- and Enriched Environment-Induced Retinal Protection in MSG-Treated Newborn Rats. Neurosci. Lett. 2011, 487, 400–405. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).