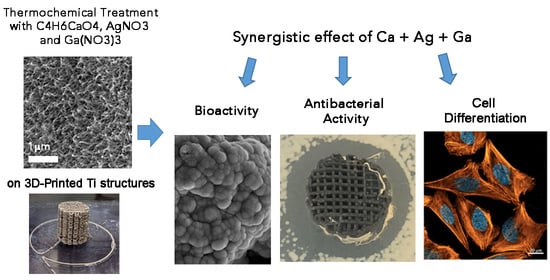
Dual-Action Effect of Gallium and Silver Providing Osseointegration and Antibacterial Properties to Calcium Titanate Coatings on Porous Titanium Implants
Abstract
:1. Introduction
2. Results and Discussion
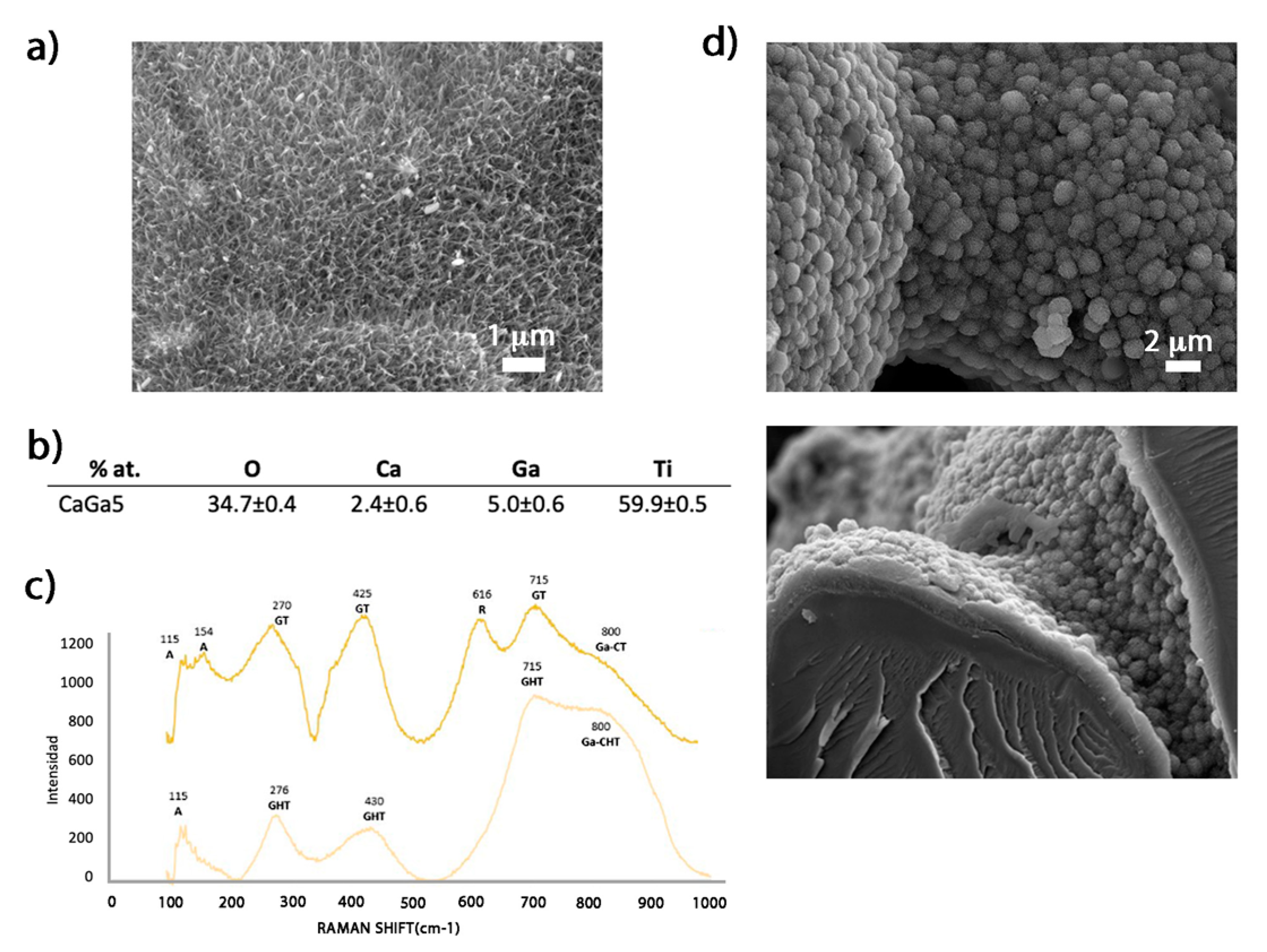
2.1. Generation of Bioactive Ga-Containing Calcium Titanate from Calcium Acetate
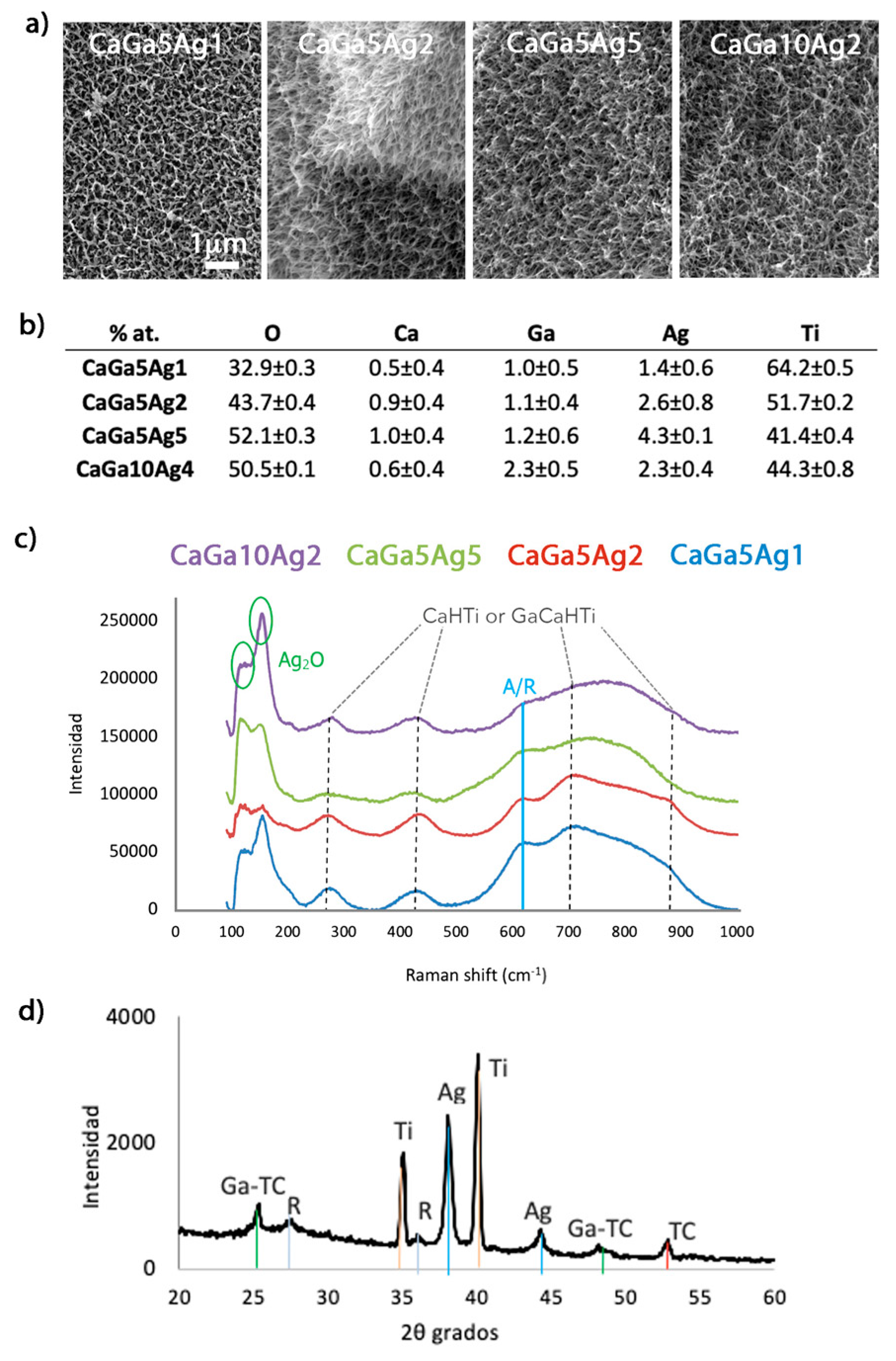
2.2. Reaction of Calcium Acetate with Different Concentrations of Gallium and Silver Nitrate
2.2.1. Surface Characterization
2.2.2. Bioactivity
2.3. Ion Release and Cytotoxicity
2.4. Antibacterial Effect
2.4.1. Bacterial Inhibition Halo
2.4.2. Bacterial Adhesion
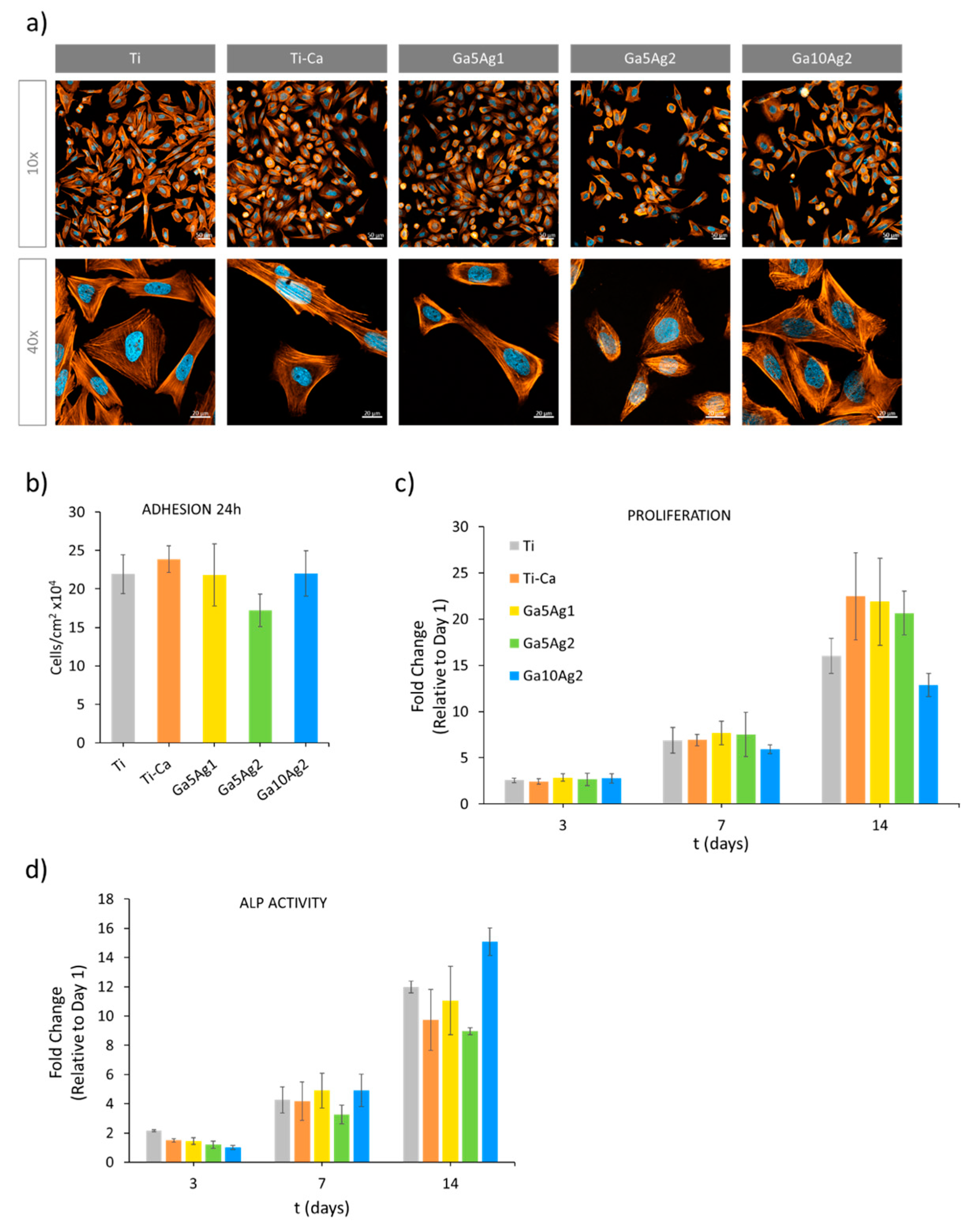
2.5. Cell Response
3. Materials and Methods
3.1. Titanium Porous Samples
3.2. Titanium Porous Samples
3.3. Bioactivity Characterization
3.4. Surface Characterization
3.4.1. Field Emission Scanning Electron Microscopy (FESEM)
3.4.2. Fourier Transform Confocal Laser Raman Spectrometry (Raman)
3.4.3. X-ray Diffraction (XRD)
3.5. Ion Release
3.6. Bacterial Characterization
3.6.1. Antibacterial Halo Assay
3.6.2. Bacterial Adhesion Assay
3.7. Cell Response
3.7.1. Cell Culture
3.7.2. Cytotoxicity
3.7.3. Cell Adhesion
3.7.4. Cell Proliferation
3.7.5. Cell Differentiation
3.8. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bozic, K.J.; Kamath, A.F.; Ong, K.; Lau, E.; Kurtz, S.; Chan, V.; Vail, T.P.; Rubash, H.; Berry, D.J. Comparative Epidemiology of Revision Arthroplasty: Failed THA Poses Greater Clinical and Economic Burdens Than Failed TKA. Clin. Orthop. Relat. Res. 2015, 473, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Sundfeldt, M.; Carlsson, L.V.; Johansson, C.B.; Thomsen, P.; Gretzer, C. Aseptic loosening, not only a question of wear: A review of different theories. Acta Orthop. 2006, 77, 177–197. [Google Scholar] [CrossRef]
- Geetha, M.; Asokamani, R.S.A.K.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar]
- Raphel, J.; Holodniy, M.; Goodman, S.B.; Heilshorn, S.C. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials 2016, 84, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Contreras, A.; Torres, D.; Guillem-Marti, J.; Sereno, P.; Ginebra, M.P.; Calero, J.A.; Manero, J.M.; Rupérez, E. Development of novel dual-action coatings with osteoinductive and antibacterial properties for 3D-printed titanium implants. Surf. Coatings Technol. 2020, 403, 126381. [Google Scholar] [CrossRef]
- León-Buitimea, A.; Garza-Cárdenas, C.R.; Garza-Cervantes, J.A.; Lerma-Escalera, J.A.; Morones-Ramírez, J.R. The Demand for New Antibiotics: Antimicrobial Peptides, Nanoparticles, and Combinatorial Therapies as Future Strategies in Antibacterial Agent Design. Front. Microbiol. 2020, 11, 1669. [Google Scholar] [CrossRef]
- Altun, E.; Aydogdu, M.O.; Chung, E.; Ren, G.; Homer-Vanniasinkam, S.; Edirisinghe, M. Metal-based nanoparticles for combating antibiotic resistance. Appl. Phys. Rev. 2021, 8, 41303. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, J.; Yan, T.; Han, Y. Fibroblast responses and antibacterial activity of Cu and Zn co-doped TiO2 for percutaneous implants. Appl. Surf. Sci. 2018, 434, 633–642. [Google Scholar] [CrossRef]
- Schuhladen, K.; Stich, L.; Schmidt, J.; Steinkasserer, A.; Boccaccini, A.R.; Zinser, E. Cu, Zn doped borate bioactive glasses: Antibacterial efficacy and dose-dependent in vitro modulation of murine dendritic cells. Biomater. Sci. 2020, 8, 2143–2155. [Google Scholar] [CrossRef]
- Kircheva, N.; Dudev, T. Gallium as an Antibacterial Agent: A DFT/SMD Study of the Ga3+/Fe3+ Competition for Binding Bacterial Siderophores. Inorg. Chem. 2020, 59, 6242–6254. [Google Scholar] [CrossRef]
- Nikolova, V.; Angelova, S.; Markova, N.; Dudev, T. Gallium as a Therapeutic Agent: A Thermodynamic Evaluation of the Competition between Ga3+ and Fe3+ Ions in Metalloproteins. J. Phys. Chem. B 2016, 120, 2241–2248. [Google Scholar] [CrossRef]
- Minandri, F.; Bonchi, C.; Frangipani, E.; Imperi, F.; Visca, P. Promises and failures of gallium as an antibacterial agent. Future Microbiol. 2014, 9, 379–397. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, Z.; He, H.; Cai, K.; Zhang, H.; Xu, L. Gallium and silicon synergistically promote osseointegration of dental implant in patients with osteoporosis. Med. Hypotheses 2017, 103, 35–38. [Google Scholar] [CrossRef]
- Chitambar, C.R. Gallium-containing anticancer compounds. Future Med. Chem. 2012, 4, 1257–1272. [Google Scholar] [CrossRef]
- Verron, E.; Verron, E.; Masson, M.; Masson, M.; Khoshniat, S.; Khoshniat, S.; Duplomb, L.; Duplomb, L.; Wittrant, Y.; Wittrant, Y.; et al. Gallium modulates osteoclastic bone resorption in vitro without affecting osteoblasts. Br. J. Pharmacol. 2010, 159, 1681–1692. [Google Scholar] [CrossRef]
- Yu, M.; Wang, Y.; Zhang, Y.; Cui, D.; Gu, G.; Zhao, D. Gallium ions promote osteoinduction of human and mouse osteoblasts via the TRPM7/Akt signaling pathway. Mol. Med. Rep. 2020, 22, 2741–2752. [Google Scholar] [CrossRef]
- Galdiero, S.; Falanga, A.; Vitiello, M.; Cantisani, M.; Marra, V.; Galdiero, M. Silver nanoparticles as potential antiviral agents. Molecules 2011, 16, 8894–8918. [Google Scholar] [CrossRef] [PubMed]
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831. [Google Scholar] [CrossRef]
- Madhavan, P.; Hong, P.-Y.; Sougrat, R.; Nunes, S.P. Silver-Enhanced Block Copolymer Membranes with Biocidal Activity. ACS Appl. Mater. Interfaces 2014, 6, 18497–18501. [Google Scholar] [CrossRef]
- Mulley, G.; Jenkins, A.T.A.; Waterfield, N.R. Inactivation of the antibacterial and cytotoxic properties of silver ions by biologically relevant compounds. PLoS ONE 2014, 9, e94409. [Google Scholar] [CrossRef] [PubMed]
- Sütterlin, S.; Dahlö, M.; Tellgren-Roth, C.; Schaal, W.; Melhus, Å. High frequency of silver resistance genes in invasive isolates of Enterobacter and Klebsiella species. J. Hosp. Infect. 2017, 96, 256–261. [Google Scholar] [CrossRef]
- Fischbach, M.A. Combination therapies for combating antimicrobial resistance. Curr. Opin. Microbiol. 2011, 14, 519–523. [Google Scholar] [CrossRef]
- Pormohammad, A.; Turner, R.J. Silver Antibacterial Synergism Activities with Eight Other Metal(loid)-Based Antimicrobials against Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus. Antibiotics 2020, 9, 853. [Google Scholar] [CrossRef]
- Rodríguez-Contreras, A.; Torres, D.; Rafik, B.; Ortiz-Hernandez, M.; Ginebra, M.P.; Calero, J.A.; Manero, J.M.; Ruperez, E. Bioactivity and antibacterial properties of calcium- and silver-doped coatings on 3D printed titanium scaffolds. Surf. Coatings Technol. 2021, 421, 127476. [Google Scholar] [CrossRef]
- Kim, H.M.; Miyaji, F.; Kokubo, T.; Nakamura, T. Preparation of bioactive Ti and its alloys via simple chemical surface treatment. J. Biomed. Mater. Res. 1996, 32, 409–417. [Google Scholar] [CrossRef]
- Kizuki, T.; Takadama, H.; Matsushita, T.; Nakamura, T.; Kokubo, T. Preparation of bioactive Ti metal surface enriched with calcium ions by chemical treatment. Acta Biomater. 2010, 6, 2836–2842. [Google Scholar] [CrossRef]
- Tite, T.; Popa, A.C.; Balescu, L.M.; Bogdan, I.M.; Pasuk, I.; Ferreira, J.M.; Stan, G.E. Cationic Substitutions in Hydroxyapatite: Current Status of the Derived Biofunctional Effects and Their In Vitro Interrogation Methods. Materials 2018, 11, 2081. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Nath, S.; Sugawara, Y.; Divakarla, K.; Das, T.; Manos, J.; Chrzanowski, W.; Matsushita, T.; Kokubo, T. Two-in-One Biointerfaces-Antimicrobial and Bioactive Nanoporous Gallium Titanate Layers for Titanium Implants. Nanomaterials 2017, 7, 229. [Google Scholar] [CrossRef] [PubMed]
- ISO 23317:2014; Implants for Surgery—In Vitro Evaluation for Apatite-Forming Ability of Implant Materials. ISO: New York, NY, USA, 2014.
- Raynaud, S.; Champion, E.; Bernache-Assollant, D.; Thomas, P. Calcium phosphate apatites with variable Ca/P atomic ratio I. Synthesis, characterisation and thermal stability of powders. Biomaterials 2002, 23, 1065–1072. [Google Scholar] [CrossRef]
- ISO 10993-12:2012; Biological Evaluation of Medical Devices-Part 12: Sample Preparation and Reference Materials. ISO: New York, NY, USA, 2012.
- Martina, I.; Wiesinger, R.; Jembrih-Simburger, D.; Schreiner, M. Micro-Raman characterization of silver corrosion products: Instrumental set-up and reference database. e-PS 2012, 9, 1–6. [Google Scholar]
- Kizuki, T.; Matsushita, T.; Kokubo, T. Antibacterial and bioactive calcium titanate layers formed on Ti metal and its alloys. J. Mater. Sci. Mater. Med. 2014, 25, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, C.; Hou, B.; Zhou, X. Room Temperature Synthesis and Catalytic Properties of Surfactant-Modified Ag Nanoparticles. Int. J. Spectrosc. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Valappil, S.P.; Coombes, M.; Wright, L.; Owens, G.J.; Lynch, R.J.; Hope, C.K.; Higham, S.M. Role of gallium and silver from phosphate-based glasses on in vitro dual species oral biofilm models of Porphyromonas gingivalis and Streptococcus gordonii. Acta Biomater. 2012, 8, 1957–1965. [Google Scholar] [CrossRef]
- Banin, E.; Lozinski, A.; Brady, K.M.; Berenshtein, E.; Butterfield, P.W.; Moshe, M.; Chevion, M.; Greenberg, E.P.; Banin, E. The potential of desferrioxamine-gallium as an anti-Pseudomonas therapeutic agent. Proc. Natl. Acad. Sci. USA 2008, 105, 16761–16766. [Google Scholar] [CrossRef]
- Moellering, R.C.J. Antimicrobial synergism—An elusive concept. J. Infect. Dis. 1979, 140, 639–641. [Google Scholar] [CrossRef]
- Brouqui, P.; Rousseau, M.C.; Stein, A.; Drancourt, M.; Raoult, D. Treatment of Pseudomonas aeruginosa-infected orthopedic prostheses with ceftazidime-ciprofloxacin antibiotic combination. Antimicrob. Agents Chemother. 1995, 39, 2423–2425. [Google Scholar] [CrossRef] [PubMed]
- Vidal, E.; Torres, D.; Guillem-Marti, J.; Scionti, G.; Manero, J.M.; Ginebra, M.-P.; Rodríguez, D.; Rupérez, E. Titanium scaffolds by direct ink writing: Fabrication and functionalization to guide osteoblast behavior. Metals 2020, 10, 1156. [Google Scholar] [CrossRef]
- ISO 14729:2001; Ophthalmic Optics. Contact Lens Care Products. Microbiological Requirements and Test Methods for Products and Regimens for Hygienic Management of Contact Lenses. ISO: New York, NY, USA, 2001.
- Brock, T.D.; Madigan, M.T.; Martinko, J.M.; Parker, J. Brock Biology of Microorganisms; Prentice-Hall: Upper Saddle River, NJ, USA, 2003. [Google Scholar]
- Nonsee, K.; Supitchaya, C.; Thawien, W. Antimicrobial activity and the properties of edible hydroxypropyl methylcellulose based films incorporated with encapsulated clove (Eugenia caryophyllata Thunb.) oil. Int. Food Res. J. 2011, 18, 1531–1541. [Google Scholar]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices-Part 5: Tests for In Vitro Cytotoxicity. ISO: New York, NY, USA, 2009.
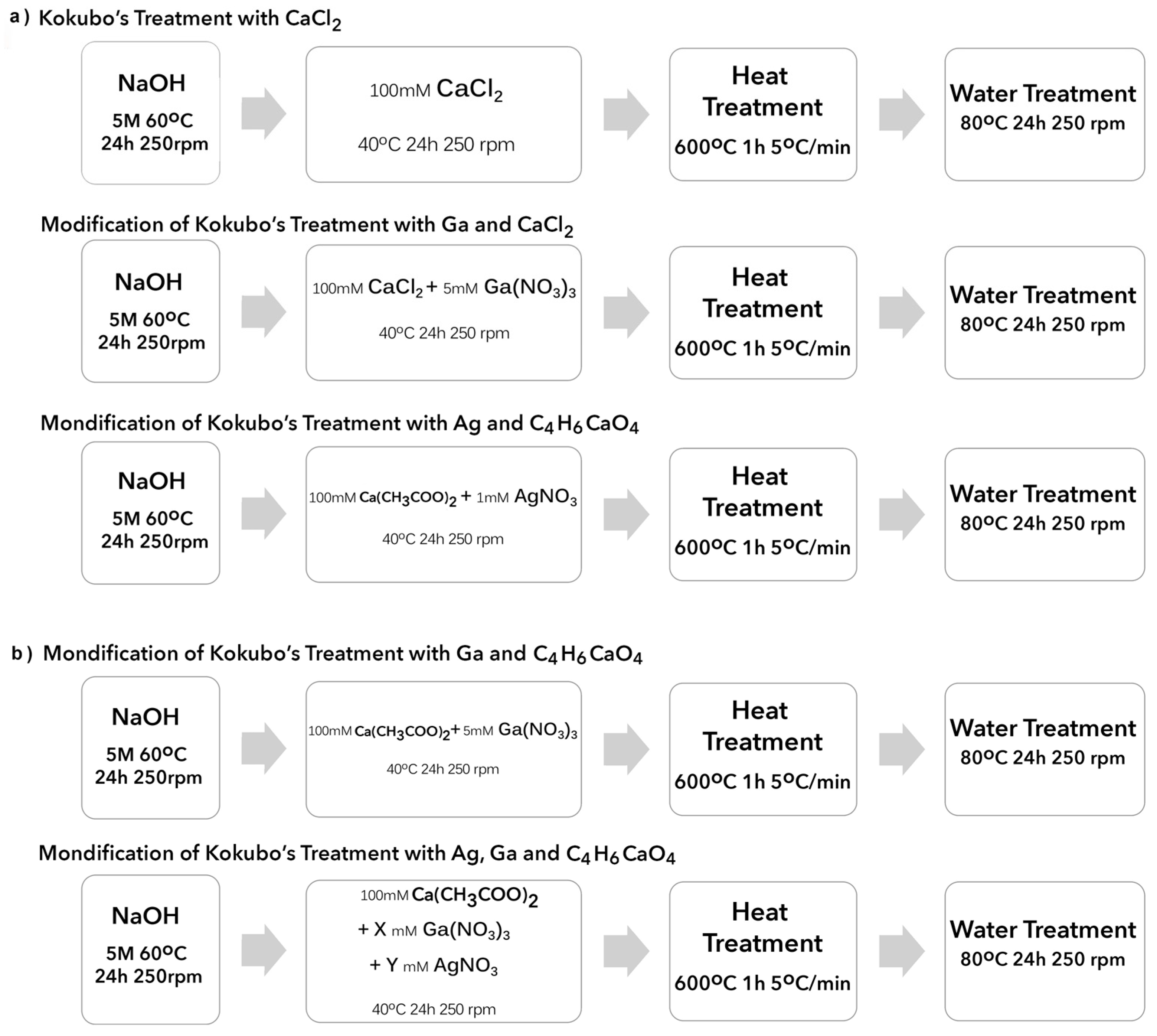


| Sample Reference | X Ga(NO3)3 (mM) | Y AgNO3 (mM) |
|---|---|---|
| CaGa5 | 5 | 0 |
| CaAg1 | 0 | 1 |
| CaGa5Ag1 | 5 | 1 |
| CaGa5Ag2 | 5 | 2 |
| CaGa5Ag5 | 5 | 5 |
| CaGa10Ag2 | 10 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Contreras, A.; Torres, D.; Piñera-Avellaneda, D.; Pérez-Palou, L.; Ortiz-Hernández, M.; Ginebra, M.P.; Calero, J.A.; Manero, J.M.; Rupérez, E. Dual-Action Effect of Gallium and Silver Providing Osseointegration and Antibacterial Properties to Calcium Titanate Coatings on Porous Titanium Implants. Int. J. Mol. Sci. 2023, 24, 8762. https://doi.org/10.3390/ijms24108762
Rodríguez-Contreras A, Torres D, Piñera-Avellaneda D, Pérez-Palou L, Ortiz-Hernández M, Ginebra MP, Calero JA, Manero JM, Rupérez E. Dual-Action Effect of Gallium and Silver Providing Osseointegration and Antibacterial Properties to Calcium Titanate Coatings on Porous Titanium Implants. International Journal of Molecular Sciences. 2023; 24(10):8762. https://doi.org/10.3390/ijms24108762
Chicago/Turabian StyleRodríguez-Contreras, Alejandra, Diego Torres, David Piñera-Avellaneda, Lluís Pérez-Palou, Mònica Ortiz-Hernández, María Pau Ginebra, José Antonio Calero, José María Manero, and Elisa Rupérez. 2023. "Dual-Action Effect of Gallium and Silver Providing Osseointegration and Antibacterial Properties to Calcium Titanate Coatings on Porous Titanium Implants" International Journal of Molecular Sciences 24, no. 10: 8762. https://doi.org/10.3390/ijms24108762