The Vanilloid (Capsaicin) Receptor TRPV1 in Blood Pressure Regulation: A Novel Therapeutic Target in Hypertension?
Abstract
1. Introduction
2. Animal Studies: The Biphasic Effect of Capsaicin on Blood Pressure
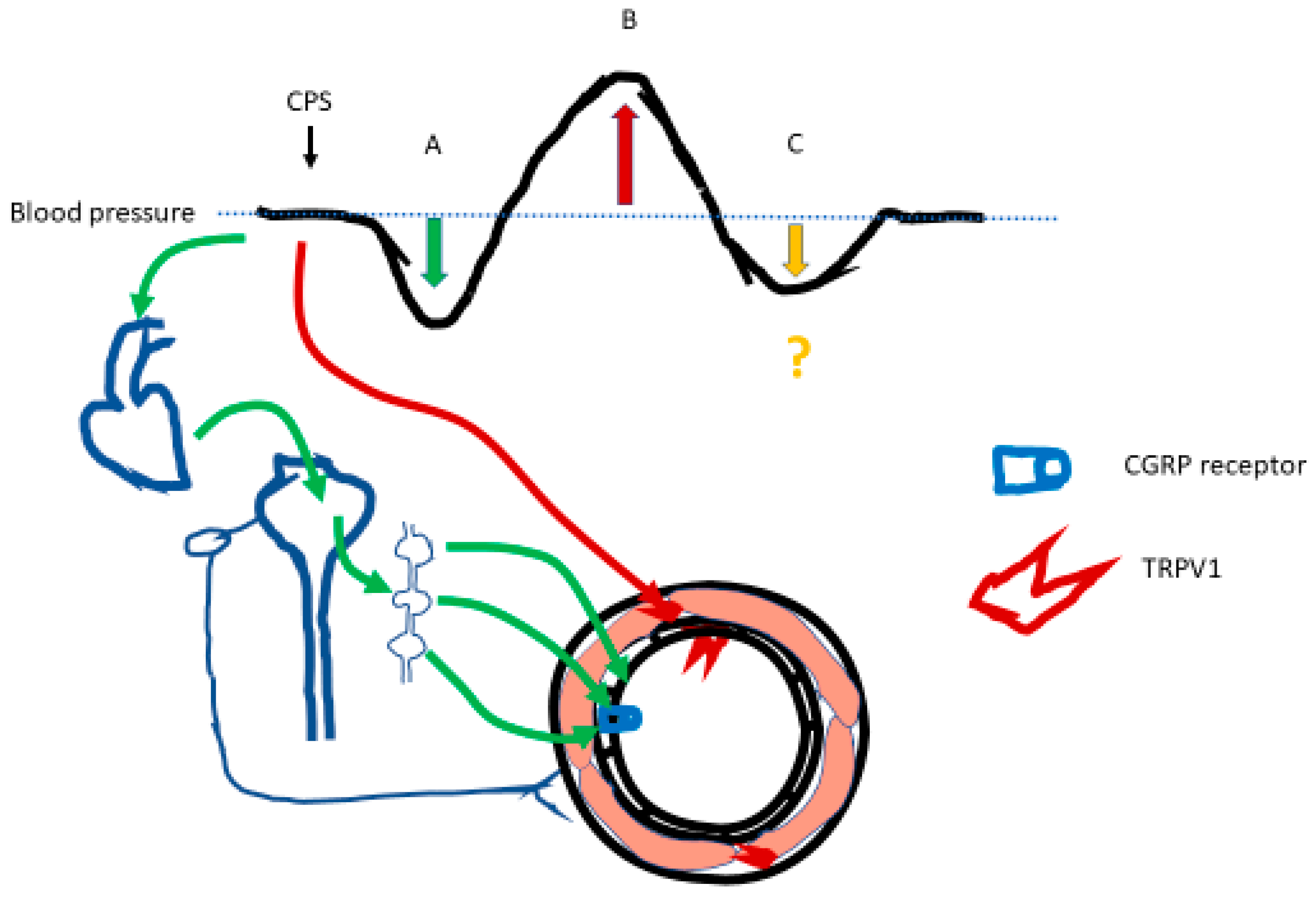
3. Capsaicin Causes Transient Hypotension by Triggering the Bezold–Jarisch Reflex
4. Capsaicin Evokes Hypertension by Activating TRPV1 Expressed on Vascular Smooth Muscle
5. Another Player Emerges: TRPV1 in Vascular Endothelium?
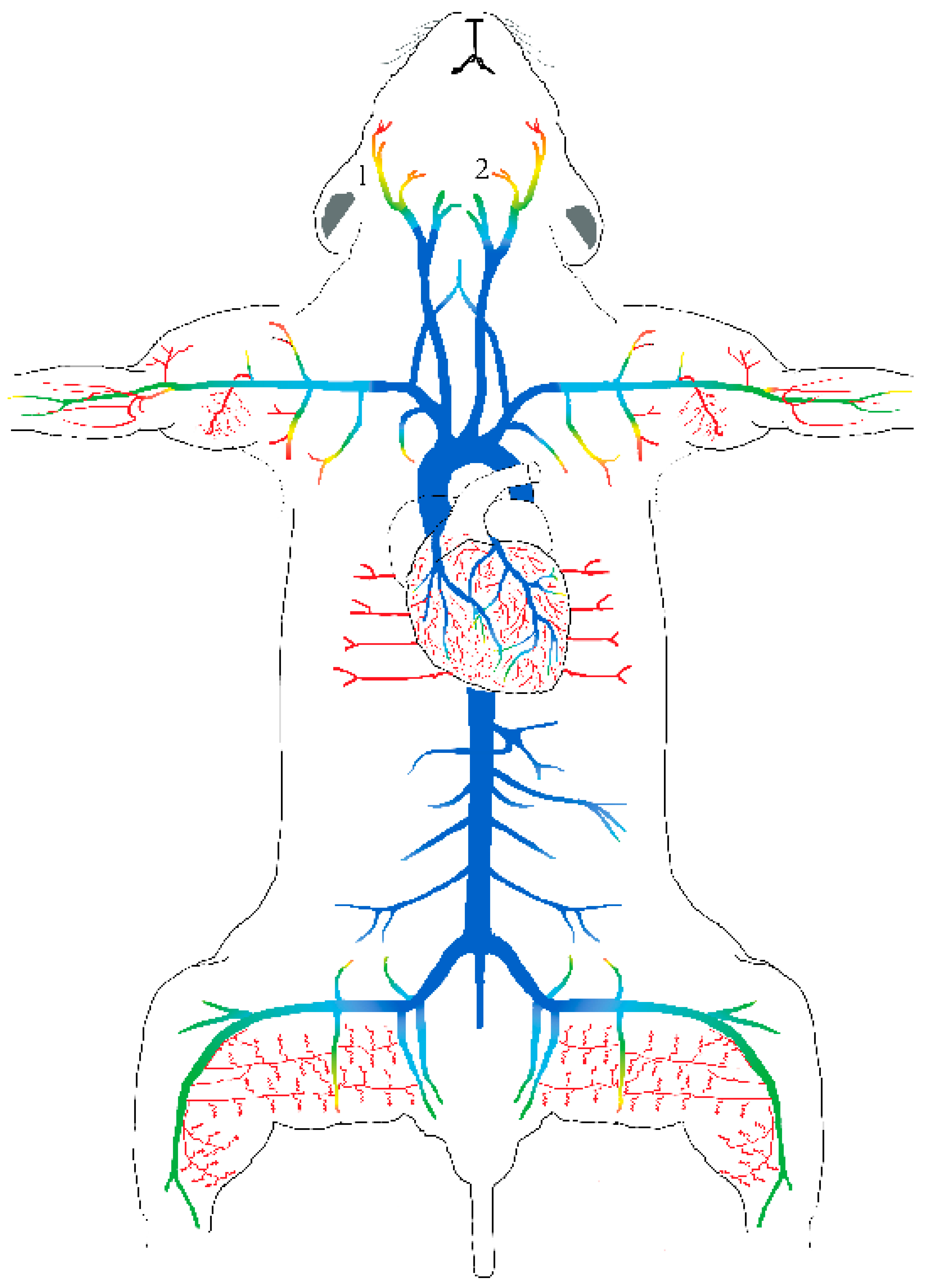
6. Do Capsaicin-Sensitive Afferents Play a Role in Physiological Blood Pressure Regulation?
7. Capsaicin Effects on Blood Pressure in Experimental Models of Hypertension
8. Dietary Capsaicin and Blood Pressure in Animal Studies
9. Human Observations
10. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, K.; Huang, S.; Feng, D.; Lang, X.; Wang, Q.; Liu, Y. Sedentary behavioral studies of young and middle-aged adults with hypertension in the framework of behavioral epidemiology: A scoping review. Int. J. Environ. Res. Public Health 2022, 19, 16796. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://cdc.gov/bloodpressure/facts.htm (accessed on 3 May 2023).
- McNiece, K.L.; Poffenbarger, T.S.; Turner, J.L.; Franco, K.D.; Sorof, J.M.; Portman, R.J. Prevalence of hypertension and pre-hypertension among adolescents. J. Pediatr. 2007, 6, 640–644. [Google Scholar] [CrossRef]
- Chandra, S.; Saklani, S.; Kumar, P.; Kim, B.; Coutinho, H.D.M. Nutraceuticals: Pharmacologically active potent dietary supplements. Biomed Res. Int. 2022, 2022, 2051017. [Google Scholar] [CrossRef] [PubMed]
- Borghi, C.; Tsioufis, K.; Agabiti-Rosei, E.; Burnier, M.; Cicero, A.F.G.; Clement, D.; Coca, A.; Desideri, G.; Grassi, G.; Lovic, D.; et al. Nutraceuticals and blood pressure control: A European Society of Hypertension position document. J. Hypertens. 2020, 38, 799–812. [Google Scholar] [CrossRef]
- Chopan, M.; Littenberg, B. The association of red hot chili pepper consumption and mortality. A large population-based cohort study. PLoS ONE 2017, 12, e0169876. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Qi, L.; Yu, C.; Yang, L.; Guo, Y.; Chen, Y.; Bian, Z.; Sun, D.; Du, J.; Ge, P. Consumption of spicy foods and total and case specific mortality: Population-based cohort study. Br. Med. J. 2015, 351, h3942. [Google Scholar] [CrossRef] [PubMed]
- Bonaccio, M.; Di Castelnuovo, A.; Costanzo, S.; Ruggiero, E.; De Curtis, A.; Persichillo, M.; Tabolacci, C.; Facchiano, F.; Cerletti, C.; Donati, M.B. Chili pepper consumption and mortality in Italian adults. J. Am. Coll. Cardiol. 2019, 74, 3139–3149. [Google Scholar] [CrossRef] [PubMed]
- Ofori-Asenso, R.; Mohsenpourn, M.A.; Nouri, M.; Faghih, S.; Liew, D.; Mazidi, M. Association of spicy food consumption with cardiovascular and all-cause mortality: A meta-analysis of prospective cohort studies. Angiology 2021, 72, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Szallasi, A. Dietary capsaicin: A spicy way to improve cardio-metabolic health? Biomolecules 2022, 12, 1783. [Google Scholar] [CrossRef] [PubMed]
- Szallasi, A. Capsaicin for weight control: “Exercise in a Pill” (or just another fad)? Pharmaceuticals 2022, 15, 851. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P. Capsaicin: Cellular targets, mechanism of action, and selectivity for thin sensory neurons. Pharmacol. Rev. 1991, 43, 143–201. [Google Scholar]
- Szolcsányi, J. Capsaicin and sensory neurons: A historical perspective. Prog. Drug Res. 2014, 68, 1–37. [Google Scholar] [PubMed]
- Fischer, M.J.M.; Ciotu, C.I.; Szallasi, A. The mysteries of capsaicin-sensitive afferents. Front. Physiol. 2020, 11, 554195. [Google Scholar] [CrossRef] [PubMed]
- Siebert, E.; Lee, S.-Y.; Pflugh Prescott, M. Chili pepper preference development and its impact on dietary intake: A narrative review. Front. Nutr. 2022, 9, 10392078. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Reeh, P.W.; Fischer, M.J.M. Nobel sensations and pain. Pflügers Arch. 2022, 474, 405–420. [Google Scholar] [CrossRef] [PubMed]
- Katz, B.; Zaguri, R.; Edvardson, S.; Maayan, C.; Elpeleg, O.; Lev, S.; Davidson, E.; Peters, M.; Kfir-Erenfeld, S.; Berger, E.; et al. Nociception and pain in humans lacking a functional TRPV1 channel. J. Clin. Investig. 2023, 133, e153558. [Google Scholar] [CrossRef]
- Amini, R.M.; Sheikhossein, F.; Bazshani, E.; Hajiaqaei, M.; Shafie, A.; Shahinfar, H.; Azizi, M.; Ghareghgeshlaghi, H.E.; Naghshi, S.; Fathipour, R.B.; et al. The effects of capsinoids and fermented red pepper paste supplementation on blood pressure: A systematic review and meta-analysis of randomized, controlled trials. Clin. Nutr. 2021, 40, 1767–1775. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Wang, M.; Tian, Z.; Zhang, J.; Liu, Y.; Zhang, Y.; Wang, P.; Xue, Y. Sex-dependent difference in the association between frequency of spicy food consumption and risk of hypertension in Chinese adults. Eur. J. Nutr. 2019, 58, 2449–2461. [Google Scholar] [CrossRef] [PubMed]
- Shirani, F.; Foshati, S.; Tavassoly, M.; Clark, C.C.T.; Rouhani, M.H. The effect of capsaicin/red pepper on blood pressure and heart rate: A systematic review and meta-analysis of clinical trials. Phytother. Res. 2021, 35, 6080–6088. [Google Scholar] [CrossRef] [PubMed]
- Hőgyes, E. Adatok a Capsicum annuum (paprika) alkatrèszeinek èlettani hatásához. Kolozsvári Orv.-Termèszettudományi Társulat Èrtesítő 1877, 2, 51–56. [Google Scholar]
- Hőgyes, E. Beiträge zur physiologischen Wirkung der Bestandtheile des Capsicum annuum (Spanischer Pfeffer). Arch. Exper. Path. Pharmac. 1878, 9, 117–130. [Google Scholar]
- Pórszász, J.; György, L.; Pórszász-Gibiszer, K. Cardiovascular and respiratory effects of capsaicin. Acta Physiol. Acad. Sci. Hung. 1955, 8, 61–76. [Google Scholar] [PubMed]
- Makara, G.B.; György, L.; Molnár, J. Circulatory and respiratory responses to capsaicin, 5-hydroxytryptamine and histamine in rats pretreated with capsaicin. Arch. Int. Pharmacodyn. 1967, 170, 39–45. [Google Scholar] [PubMed]
- Toda, N.; Usui, H.; Nishino, N.; Fujiwara, M. Cardiovascular effects of capsaicin in dogs and rabbits. J. Pharmacol. Exp. Ther. 1972, 181, 512–521. [Google Scholar]
- Jancsó, G.; Király, E.; Such, G.; Joó, F.; Nagy, A. Neurotoxic effect of capsaicin in mammals. Acta Physiol. Hung. 1987, 69, 295–313. [Google Scholar] [PubMed]
- Maggi, C.A.; Meli, A. The sensory-efferent function of capsaicin-sensitive sensory neurons. Gen. Pharmacol. 1988, 19, 1–43. [Google Scholar] [CrossRef]
- Bevan, S.; Szolcsányi, J. Sensory neuron-specific actions of capsaicin: Mechanisms and applications. Trends Pharmacol. Sci. 1990, 11, 330–333. [Google Scholar] [CrossRef]
- Jancsó, N.; Jancsó-Gábor, A.; Szolcsányi, J. Direct evidence for neurogenic inflammation and its prevention by denervation and pretreatment with capsaicin. Br. J. Pharmacol. 1967, 31, 138–151. [Google Scholar] [CrossRef]
- Jancsó, G.; Sántha, P. The foundation of sensory pharmacology: Nicholas (Miklós) Jancsó and the Szeged contribution. Temperature 2015, 2, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Szolcsányi, J. Forty years in capsaicin research for sensory pharmacology and physiology. Neuropeptides 2004, 38, 377–384. [Google Scholar] [CrossRef]
- Mark, A.L. The Bezold-Jarisch reflex revisited: Clinical implications of inhibitory reflexes originating in the heart. J. Am. Coll. Cardiol. 1983, 1, 90–102. [Google Scholar] [CrossRef]
- Warltier, D.C.; Campagna, J.A.; Carter, C. Clinical relevance of the Bezold-Jarisch reflex. Anesthesiol. 2003, 98, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- von Bezold, A.; Hirt, L. Über die physiologischen Wirkungen des essigsauren Veratrins. Untersuch. Physiol. Lab. Würzburg 1867, 1, 73–122. [Google Scholar]
- Jarisch, A.; Henze, C. Über Blutdruckenkung durch chemische Erregung depressorische Nerven. Naunyn-Schmiedebers Arch. Exper. Pathol. Pharmakol. 1937, 187, 706–730. [Google Scholar] [CrossRef]
- Robertson, R.M.; Robertson, D. The Bezold-Jarisch reflex: Possible role in limiting myocardial ischemia. Clin. Cardiol. 1981, 4, 75–79. [Google Scholar] [CrossRef]
- Oddby, E.; Hein, A.; Jakobsson, J.G. Circulatory collapse following epidural bolus for Caesarean section a profound vasovagal reaction? Int. J. Surg. Case Rep. 2016, 23, 74–76. [Google Scholar] [CrossRef]
- Thames, M.D.; Klopfenstein, H.S.; Abboud, F.M.; Mark, A.L.; Walker, J.L. Preferential distribution of inhibitory cardiac receptors with vagal afferents to the inferoposterior wall of the left ventricle activated during coronary occlusion in the dog. Circ. Res. 1978, 43, 512–519. [Google Scholar] [CrossRef]
- Pacher, P.; Bátkai, S.; Kunos, G. Haemodynamic profile and responsiveness to anandamide of TRPV1 receptor knock-out mice. J. Physiol. 2004, 558, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Larson, R.A.; Chapleau, M.W. Differential engagement of inhibitory and excitatory cardiopulmonary reflexes by capsaicin and phenylbiguanide in C57BL76 mice. Am. J. Physiol. Regul. Integr. Comp.Physiol. 2023, 324, R336–R344. [Google Scholar] [CrossRef]
- Harron, D.W.; Kobinger, W. Facilitation of the Bezold-Jarish re3flex by central stimulation of alpha2 adrenoreceptors in dogs. Naunyn-Schmiedebergs Arch. Pharmacol. 1984, 325, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Giles, T.D.; Sander, G.E. Comparative cardiovascular responses to intravenous capsaicin, phenyldiguanide, veratrum alkaloids and enkephalins in the conscious dog. J. Auton. Pharmacol. 1986, 6, 1–7. [Google Scholar] [CrossRef]
- Pórszász, R.; Szolcsányi, J. Circulatory and respiratory effects of capsaicin and resiniferatoxin on guinea pigs. Acta Biochim. Biophys. Hung. 1991, 26, 131–138. [Google Scholar] [PubMed]
- Szallasi, A.; Blumberg, P.M. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neuroscience 1989, 30, 515–520. [Google Scholar] [CrossRef]
- Szallasi, A.; Blumberg, P.M. Resiniferatoxin and its analogs provide novel insights into the pharmacology of the vanilloid (capsaicin) receptor. Life Sci. 1990, 47, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Szolcsányi, J.; Szallasi, A.; Szallasi, Z.; Joó, F.; Blumberg, P.M. Resiniferatoxin: An ultrapotent selective modulator of capsaicin-sensitive primary afferent neurons. J. Pharmacol. Exp. Ther. 1990, 255, 923–928. [Google Scholar]
- Kaczynska, K.; Szereda-Przestaszewska, M. Respiratory effects of capsaicin occur beyond the lung vagi in anesthesized rats. Acta Neurobiol. Exp. 2000, 60, 159–165. [Google Scholar]
- Szallasi, A.; Blumberg, P.M. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999, 51, 159–212. [Google Scholar]
- Pórszász, J.; Jancsó, N. Studies on the action potentials of sensory nerves in animals desensitized with capsaicine. Acta Physiol. Acad. Sci. Hung. 1959, 16, 299–306. [Google Scholar]
- Cimino Brown, D.; Iadarola, M.J.; Perkowski, S.Z.; Erin, H.; Shoer, F.; Karai, J.L.; Olah, Z.; Mannes, A.J. Physiologic and antinociceptive effects of intrathecal resiniferatoxin in a canine bone cancer model. J. Am. Soc. Anesthesiol. 2005, 103, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Molnár, J.; Makara, G.; György, L.; Unyi, G. The bronchoconstrictor action of capsaicin in the guinea pig. Acta Physiol. Acad. Sci. Hung. 1969, 36, 413–420. [Google Scholar] [PubMed]
- Szolcsányi, J.; Barthó, L. Capsaicin-sensitive non-cholinergic excitatory innervation of the guinea-pig tracheobronchial smooth muscle. Neurosci. Lett. 1982, 34, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.M.; Saria, A. Bronchial smooth muscle contraction induced by stimulation of capsaicin-sensitive sensory neurons. Acta Physiol. Scand. 1982, 116, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Szolcsányi, J.; Barthó, L. New type of nerve-mediated cholinergic contractions of the guinea-pig small intestine and its selective blockade by capsaicin. Naunyn-Schmiedebergs Arch. Pharmacol. 1978, 305, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Maggi, C.A.; Patacchini, P.; Santicioli, P.; Giuliani, S.; Turini, D.; Barbanti, G.; Beneforti, P.; Misuri, D.; Meli, A. Specific motor effects of capsaicin on human jejunum. Eur. J. Pharmacol. 1988, 149, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Scotland, R.S.; Chauhan, S.; Davis, C.; De Filipe, C.; Hunt, S.; Kabir, J.; Kotsonis, P.; Oh, U.; Ahluwalia, A. Vanilloid receptor TRPV1, sensory C-fibers, and vascular autoregulation: A novel mechanism involved in myogenic constriction. Circ. Res. 2004, 95, 1027–1034. [Google Scholar] [CrossRef]
- Björkroth, U. Inhibition of smooth muscle contractions induced by capsaicin and electrical transmural stimulation by a substance P antagonist. Acta Physiol. Scand. Suppl. 1983, 515, 11–16. [Google Scholar]
- Szolcsányi, J.; Oroszi, G.; Németh, J.; Szilvássy, Z.; Tósaki, A. Endothelin release by capsaicin in isolated working rat heart. Eur. J. Pharmacol. 1999, 376, 247–250. [Google Scholar] [CrossRef]
- Szolcsányi, J.; Oroszi, G.; Németh, J.; Szilvássy, Z.; Blasig, I.E.; Tósaki, A. Functional and biochemical evidence for capsaicin-induced neural endothelin release in isolated working rat heart. Eur. J. Pharmacol. 2001, 419, 215–221. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Kurihara, H.; Kimura, S.; Tomobe, Y.; Kobayashi, M.; Mitsui, Y.; Yazaki, Y.; Goto, K.; Masaki, T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988, 332, 411–415. [Google Scholar] [CrossRef]
- Goncalves de Oliveira, M.; Nadruz, W., Jr.; Mónica, F.Z. Endothelial and vascular smooth muscle dysfunction in hypertension. Biochem. Pharmacol. 2022, 205, 115263. [Google Scholar] [CrossRef] [PubMed]
- Lázár, Z.; Benkó, R.; Bölcskei, K.; Rumbus, Z.; Wolf, M.; Holzer, P.; Maggi, C.A.; Barthó, L. Actions of endothelin and corticotropin releasing factor in the guinea-pig ileum: No evidence for an interaction with capsaicin-sensitive neurons. Neuropeptides 2003, 37, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Duckles, S.P. Effects of capsaicin on vascular smooth muscle. Naunyn-Schmiedebergs Arch. Pharmacol. 1986, 333, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Pórszász, R.; Porkoláb, A.; Ferencz, A.; Pataki, T.; Szilvássy, Z.; Szolcsányi, J. Capsaicin-induced non-neural vasoconstriction in canine mesenteric arteries. Eur. J. Pharmacol. 2002, 441, 173–175. [Google Scholar] [CrossRef]
- Dux, M.; Sántha, P.; Jancsó, G. Capsaicin-sensitive neurogenic sensory vasodilation in the dura mater of the rat. J. Physiol. 2003, 552, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Monsereenusorn, Y.; Kongsamut, S. Inhibition of calcium uptake by capsaicin. Res. Commun. Chem. Pathol. Pharmacol. 1985, 47, 453–456. [Google Scholar]
- Jancsó, G.; Karcsú, S.; Király, E.; Szebeni, A.; Tóth, L.; Bácsy, E.; Joó, F.; Párducz, A. Neurotoxin induced nerve cell degeneration: Possible involvement of calcium. Brain Res. 1984, 295, 211–216. [Google Scholar] [CrossRef]
- Wood, J.N.; Winter, J.; James, I.F.; Rang, H.P.; Yeats, J.; Bevan, S. Capsaicin-induced ion fluxes in dorsal root ganglion cells in culture. J. Neurosci. 1988, 8, 3208–3220. [Google Scholar] [CrossRef] [PubMed]
- Blaźević, T.; Ciotu, C.I.; Gold-Binder, M.; Heiss, E.H.; Fischer, M.J.M.; Dirsch, V.M. Culture rat aortic smooth muscle cells do not express a functional TRPV1. PLoS ONE 2023, 18, e0281191. [Google Scholar] [CrossRef]
- Kark, T.; Bagi, Z.; Lizanecz, E.; Pásztor, E.T.; Erdei, N.; Czikora, A.; Papp, Z.; Ėdes, I.; Pórszász, R.; Tóth, A. Tissue-specific regulation of microvascular diameter: Opposite functional roles of neuronal and smooth muscle located vanilloid receptor-1. Mol. Pharmacol. 2008, 73, 1405–1412. [Google Scholar] [CrossRef]
- Czikora, A.; Lizanecz, E.; Bakó, P.; Rutkai, I.; Ruzsnavszky, F.; Magyar, J.; Pórszász, R.; Kark, T.; Facskó, A.; Papp, Z.; et al. Structure-activity relationships of vanilloid receptor agonists form arteriolar TRPV1. Br. J. Pharmacol. 2012, 165, 1801–1812. [Google Scholar] [CrossRef]
- Czikora, A.; Rutkai, I.; Pásztor, E.T.; Szalai, A.; Pórszász, R.; Boczán, J.; Ėdes, I.; Papp, Z.; Tóth, A. Different desensitization patterns for sensory and vascular TRPV1 populations in the rat: Expression, localization and functional consequences. PLoS ONE 2013, 8, e78184. [Google Scholar] [CrossRef] [PubMed]
- Tóth, A.; Czikora, A.; Pásztor, E.T.; Dienes, B.; Bai, P.; Csernoch, L.; Rutkai, I.; Csató, V.; Mányinè, I.S.; Pórszász, R.; et al. Vanilloid receptor-1 (TRPV1) expression and function in the vasculature of the rat. J. Histochem. Cytochem. 2014, 62, 129–144. [Google Scholar] [CrossRef]
- Cavanaugh, D.J.; Chesler, A.T.; Jackson, A.C.; Sigal, Y.M.; Yamanaka, H.; Grant, R.; O’Donnell, D.; Nicoll, R.A.; Shah, N.M.; Julius, D.; et al. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J. Neurosci. 2011, 31, 5067–5077. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.X.; Ton, H.T.; Gulyás, H.; Pórszász, R.; Tóth, A.; Russo, R.; Kay, M.W.; Sahibzaad, N.; Ahern, G.P. TRPV1 expressed throughout the arterial circulation regulates vasoconstriction and blood pressure. J. Physiol. 2020, 598, 5639–5659. [Google Scholar] [CrossRef]
- Phan, T.X.; Ton, H.T.; Gulyás, H.; Pórszász, R.; Tóth, A.; Russo, R.; Kay, M.W.; Sahibzaad, N.; Ahern, G.P. TRPV1 in arteries enables a rapid myogenic tone. J. Physiol. 2022, 600, 1651–1666. [Google Scholar] [CrossRef]
- Li, Q.; Garry, M.G. A murine model of the exercise pressor reflex. J. Physiol. 2020, 598, 3155–3171. [Google Scholar] [CrossRef]
- Martin, E.; Dahan, D.; Cardouat, G.; Gillibert-Duplantier, J.; Marthan, R.; Savineau, J.-P.; Ducret, T. Involvement of TRPV1 and TRPV4 channels in migration of rat pulmonary arterial smooth muscle cells. Pflüger’s Arch. 2012, 464, 261–272. [Google Scholar] [CrossRef]
- Ma, L.; Zhong, J.; Zhao, Z.; Luo, Z.; Ma, S.; Sun, J.; He, H.; Zhu, T.; Liu, D.; Zhu, Z.; et al. Activation of TRPV1 reduces vascular lipid accumulation and attenuates atherosclerosis. Cardiovasc. Res. 2011, 92, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Golech, S.A.; McCarron, R.M.; Chen, V.; Bembry, J.; Lenz, F.; Mechoulam, R.; Shohami, E.; Spatz, M. Human brain endothelium: Co-expression and function of vanilloid and endocannabinoid receptors. Brain Res. Mol. Brain Res. 2004, 132, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Dannert, M.T.; Alsasua, A.; Herradon, E.; Martin, M.I.; López-Miranda, V. Vasorelaxant effect of Win 55,212,2 in rat aorta: New mechanism involved. Vascul. Pharmacol. 2007, 46, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.M.; Franco-Cereceda, A.; Hua, X.; Hökfelt, T.; Fischer, J.A. Co-existence of substance P and calcitonin gene-related peptide-like immunoreactivities in sensory nerves in relation to cardiovascular and bronchoconstrictor effects of capsaicin. Eur. J. Pharmacol. 1985, 108, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Kee, Z.; Kodji, X.; Brain, S.D. The role of calcitonin gene-related peptide (CGRP) in neurogenic vasodilation and its cardioprotective effects. Front. Physiol. 2018, 9, 1249. [Google Scholar] [CrossRef] [PubMed]
- Herbert, M.K.; Holzer, P. Neurogenic inflammation. I. Basic mechanisms, physiology and pharmacology (in German). Anaesthesiol. Intensivmed. Notfallmed. Schmertzther. 2002, 37, 314–325. [Google Scholar]
- Luo, D.; Zhang, Y.-W.; Peng, W.-J.; Peng, J.; Chen, Q.-Q.; Li, D.; Deng, H.-W.; Li, Y.-J. Transient receptor potential vanilloid 1-mediated expression and secretion of endothelial cell-derived calcitonin gene-related peptide. Regul Pept. 2008, 150, 66–72. [Google Scholar] [CrossRef]
- Torres-Narvárez, J.C.; Del Valle Mondragón, L.; López, E.V.; Pèrez-Torres, I.; Díaz Juárez, J.A.; Suárez, J.; Pastelín Hernández, G. Role of the transient receptor potential vanilloid type 1 receptor and stretch-activated ion channels in nitric oxide release from endothelial cells of the aorta and heart of the rats. Exp. Clin. Cardiol. 2012, 17, 89–94. [Google Scholar]
- Chen, L.; Kassman, M.; Sendeski, M.; Tsvetkov, D.; Marko, L.; Michalik, L.; Riehle, M.; Liedtke, W.B.; Kuebler, W.M.; Harteneck, C.; et al. Functional transient receptor potential vanilloid 1 and transient receptor potential vanilloid 4 channels along different segments of the renal vasculature. Acta Physiol. 2015, 213, 481–491. [Google Scholar] [CrossRef]
- Donnerer, J.; Lembeck, F. Analysis of the effects of intravenously injected capsaicin in the rat. Naunyn-Schmiedebers Arch. Pharmacol. 1982, 320, 54–57. [Google Scholar] [CrossRef]
- Jancsó, G.; Király, E. Sensory neurotoxins: Chemically induced selective destruction of primary sensory neurons. Brain. Res. 1981, 210, 83–89. [Google Scholar] [CrossRef]
- Nagy, J.I.; Vincent, S.R.; Staines, W.A.; Fibigerm, H.C.; Reisine, T.D.; Yamamaura, H.I. Neurotoxic action of capsaicin on spinal substance P neurons. Brain Res. 1980, 186, 435–444. [Google Scholar] [CrossRef]
- Szallasi, A.; Szallasi, Z.; Blumberg, P.M. Permanent effects of neonatally administered resiniferatoxin in the rat. Brain Res. 1990, 537, 182–186. [Google Scholar] [CrossRef]
- Carobi, C. A quantitative investigation of the effects of neonatal capsaicin treatment on vagal afferent neurons in the rat. Cell Tissue Res. 1996, 283, 305–311. [Google Scholar] [CrossRef]
- Goso, C.; Piovacari, G.; Szallasi, A. Resiniferatoxin-induced loss of vanilloid receptors in reversible in the urinary bladder but not in the spinal cord of the rat. Neurosci. Lett. 1993, 162, 197–200. [Google Scholar] [CrossRef]
- Kennedy, W.R.; Vanhove, G.F.; Lu, S.-P.; Tobias, J.; Bley, K.R.; Walk, D.; Wendelschafer-Crabb, G.; Simone, D.A.; Selim, M.M. A randomized, controlled, open-label study of the long-term effects of NGX-4010, a high-concentration capsaicin patch, on epidermal nerve fiber density and sensory function in healthy volunteers. J. Pain 2010, 11, 579–587. [Google Scholar] [CrossRef]
- Bennett, T.; Gardiner, S.M. The influence of neonatal treatment with capsaicin on the control of blood pressure in adult rats in water-replate and water-deprived states. Br. J. Pharmacol. 1985, 85, 897–903. [Google Scholar] [CrossRef]
- McEwan, J.R.; Newman, C.; Wharton, J.; Polak, J.M.; MacDermot, J. Capsaicin induced afferent denervation and receptor-linked responses to CGRP in the rat. Regul. Pept. 1993, 44, 61–69. [Google Scholar] [CrossRef]
- Round, P.; Priestly, A.; Robinson, J. An investigation of the safety and pharmacokinetics of the novel TRPV1 antagonist XEN-D0501 in healthy subjects. Br. J. Clin, Pharmacol. 2011, 72, 921–931. [Google Scholar] [CrossRef]
- Szallasi, A.; Cortright, D.N.; Blum, C.A.; Eid, S. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat. Rev. Drug Discov. 2007, 6, 357–372. [Google Scholar] [CrossRef]
- Sun, H.; Li, D.-P.; Chen, S.-R.; Hittelman, W.N.; Pan, H.-L. Sensing blood pressure increase by transient receptor potential vanilloid 1 receptors on baroreceptors. J. Pharmacol. Exp. Ther. 2009, 331, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ballester, G.; Fernández-Carvajal, A.; Ferrer-Montiel, A. Progress in the structural basis of thermoTRP channel polymodal gating. Int. J. Mol. Sci. 2023, 24, 743. [Google Scholar] [CrossRef]
- Zhang, B.; Kario, K.; Yada, T.; Nakata, M. TRPV1-mediated sensing of sodium and osmotic pressure in POMC neurons in the arcuate nucleus of the hypothalamus. Nutrients 2022, 14, 2600. [Google Scholar] [CrossRef] [PubMed]
- Shuba, Y.M. Beyond neuronal heat sensing: Diversity of TRPV1 heat-capsaicin receptor channel functions. Front. Cell. Neurosci. 2021, 14, 612480. [Google Scholar] [CrossRef]
- Benítez-Angeles, M.; Morales-Lázaro, S.L.; Juárez-González, E.; Rosenbaum, T. TRPV1: Structure, endogenous agonists, and mechanisms. Int. J. Mol. Sci. 2020, 21, 3421. [Google Scholar] [CrossRef]
- Brenneis, C.; Kistner, K.; Puopolo, M.; Segal, D.; Robertson, D.; Sisignano, M.; Lobocha, S.; Ferreirós, N.; Strominger, A.; Cobos, E.J.; et al. Phenotyping the function of TRPV1-expressing sensory neurons by targeted axonal silencing. J. Neurosci. 2013, 33, 315–326. [Google Scholar] [CrossRef]
- Nagy, J.I.; van der Kooy, D. Effects of neonatal capsaicin treatment on nociceptive threshold in the rat. J. Neurosci. 1983, 3, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Bielefeldt, K.; Davis, B.M. Differential effects of ASIC3 and TRPV1 deletion on gastroesophageal sensation in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G130–G138. [Google Scholar] [CrossRef]
- Ducrocq, G.; Estrada, J.A.; Kim, J.S.; Kaufman, M.P. Blocking the transient receptor potential vanilloid-1 does not reduce the exercise pressor reflex in healthy rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2019, 317, R576–R587. [Google Scholar] [CrossRef]
- Weavil, J.C.; Kwon, O.S.; Hughen, R.W.; Zhang, J.; Light, A.R.; Amann, M. Gene and protein expression of dorsal root ganglion sensory receptors inn normotensive and hypertensive male rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2022, 323, R221–R226. [Google Scholar] [CrossRef]
- Virus, R.M.; Knuepfer, M.M.; McManus, D.Q.; Brody, M.J.; Gebhart, G.F. Capsaicin treatment in adult Wistar-Kyoto and spontaneously hypertensive rats: Effects on nociceptive behavior and cardiovascular regulation. Eur. J. Pharmacol. 1981, 72, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Virus, R.M.; McManus, D.Q.; Gebhart, G.F. Capsaicin treatment in adult Wistar-Kyoto and spontaneously hypertensive rats: Effects of substance P contents of peripheral and central nervous system. Eur. J. Pharmacol. 1982, 81, 67–73. [Google Scholar] [CrossRef]
- Girgis, S.I.; Macdonald, D.W.; Stevenson, J.C.; Bevis, P.J.; Lynch, C.; Wimalawansa, S.J.; Self, C.H.; Morris, H.R.; MacIntyre, I. Calcitonin gene-related peptide: A potent vasodilator and major product of the calcitonin gene. Lancet 1985, 2, 14–16. [Google Scholar] [CrossRef]
- Kawasaki, H.; Saito, A.; Takasaki, K. Age-related decrease of calcitonin gene-related peptide-containing vasodilator innervation in the mesenteric resistance vessel of the spontaneously hypertensive rat. Circ. Res. 1990, 67, 733–743. [Google Scholar] [CrossRef]
- Bigal, M.E.; Walter, S.; Bronson, M.; Alibhoy, A.; Escandon, R. Cardiovascular and hemodynamic parameters in women following prolonged CGRP inhibition using LBR-1, a monoclonal antibody against CGRP. Cephalalgia 2014, 34, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Tyshynsky, R.; Sensarma, S.; Riedl, M.; Bukowy, J.; Schramm, L.P.; Vulchanova, L.; Osborn, J.W. Periglomerular afferent innervation of the mouse renal cortex. Front. Neurosci. 2023, 17, 974197. [Google Scholar] [CrossRef]
- Burg, M.; Zahm, D.S.; Knuepfer, M.M. Intrathecal capsaicin enhances one-kidney renal warp hypertension in the rat. J. Auton. Nerv. System 1994, 2, 189–199. [Google Scholar] [CrossRef]
- Manzini, S.; Bacciarelli, C. Enhanced development of deoxycorticosterone-induced hypertension in neonatally capsaicin-treated rats. Regul. Pept. 1988, 22, 119–126. [Google Scholar] [CrossRef]
- Wang, Y.; Babánková, D.; Huang, J.; Swain, G.M.; Wang, D.H. Deletion of transient receptor potential vanilloid type 1 receptors exaggerates renal damage in deoxycorticosterone acetate salt-hypertension. Hypertension 2008, 52, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, D.H. Aggravated renal inflammatory responses in TRPV1 gene knock-out mice subjwected to DOCA salt-hypertension. Am. J. Physiol. Renal Physiol. 2009, 297, F1550–F1559. [Google Scholar] [CrossRef]
- Wang, D.H.; Li, J.; Qiu, J. Salt-sensitive hypertension induced by sensory denervation: Introduction of a new model. Hypertension 1998, 32, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.D.; Li, J. Antihypertensive mechanisms underlying a novel salt-sensitive hypertensive model induced by sensory nerve denervation. Hypertension 1999, 33, 499–503. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.H. A novel mechanism contributing to the development of Dahl salt-sensitive hypertension: A role of the transient receptor potential vanilloid type 1. Hypertension 2006, 47, 609–614. [Google Scholar] [CrossRef]
- Li, J.; Wang, D.H. Role of TRPV1 channels in renal haemodynamics and function in Dahl salt-sensitive hypertensive rats. Exp. Physiol. 2008, 93, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Marshall, N.J.; Liang, L.; Bodkin, J.; Dessapt-Baradez, C.; Nandi, M.; Collot-Teixeira, S.; Smillie, S.-J.; Lalgi, K.; Fernandez, E.S.; Gnudi, L.; et al. A role for TRPV1 in influencing the onset of cardiovascular disease in obesity. Hypertension 2013, 61, 246–252. [Google Scholar] [CrossRef]
- Bishnoi, M.; Kondepundi, K.; Gupta, A.; Karmase, A.; Boparai, R.K. Expression of multiple Transient Receptor Potential channel genes in murine 3T3-Li1 cell lines and adipose tissue. Pharmacol. Rep. 2013, 65, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.L.; Liu, D.Y.; Ma, L.Q.; Luo, D.Z.; Cao, T.B.; Zhong, J.; Yan, Z.C.; Wang, L.H.; Zhao, Z.G.; Zhu, S.J.; et al. Activation of transient receptor potential vanilloid type-1 channel prevents adipogenesis and obesity. Circ. Res. 2007, 100, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Feldman, H.M.; Golozoubova, V.; Cannon, B.; Nedergaard, J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 2009, 9, 203–209. [Google Scholar] [CrossRef]
- Li, L.; Ma, L.; Luo, Z.; Wei, X.; Zhao, Y.; Zhou, C.; Mou, A.; Lu, Z.; You, M.; He, C.; et al. Lack of TRPV1 aggravates obesity-associated hypertension through the disturbance of mitochondrial Ca2+ homeostasis in brown adipose tissue. Hypertens. Res. 2022, 45, 789–801. [Google Scholar] [CrossRef]
- Hao, X.; Chen, J.; Luo, Z.; He, H.; Yu, H.; Ma, L.; Ma, S.; Zhu, T.; Liu, D.; Zhu, Z. TRPV1 activation prevents high-salt diet-induced nocturnal hypertension in mice. Pflügers Arch. Eur. J. Physiol. 2011, 461, 345–353. [Google Scholar] [CrossRef]
- Li, l.; Wang, F.; Wei, X.; Liang, Y.; Cui, Y.; Gao, F.; Zhong, J.; Pu, Y.; Zhao, Y.; Yan, Z.; et al. Transient receptor potential vanilloid 1 activation by dietary capsaicin promotes urinary sedum excretion by inhibiting epithelial sodium channel α-subunit-mediated sodium reabsorption. Hypertension 2014, 64, 397–404. [Google Scholar] [CrossRef]
- Segawa, Y.; Hashimoto, H.; Maruyama, S.; Shintani, M.; Ohno, H.; Nakai, Y.; Osera, T.; Kurihara, N. Dietary capsaicin-mediated attenuation of hypertension in a rat model of renovascular hypertension. Clin. Exp. Hypertens. 2020, 42, 352–359. [Google Scholar] [CrossRef]
- Fingerhuth, K.A. Monographia Generis Capsici cum Tabulis X. coloratis (in Latin); Düsseldorff, MDCCCXXXII; NABU Press (Naturschutzbund Deutscland): Berlin, Germany, 2012. [Google Scholar]
- Winning, A.J.; Hamilton, R.D.; Shea, S.A.; Guz, A. Respiratory and cardiovascular effects of central and peripheral intravenous injections of capsaicin in man: Evidence for pulmonary chemosensitivity. Clin. Sci. 1986, 71, 519–526. [Google Scholar] [CrossRef]
- Silberberg, A.; Moelelr-Bertram, T.; Wallace, M.S. A randomized, double-blind, crossover study to evaluate the depth response relationship of intradermal capsaicin-induced pain and hyperalgesia in healthy volunteers. Pain Med. 2015, 16, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Bardo-Brouard, P.; Luizard, C.; Valeyrie-Allanore, L.; Goujon, C.; Do, B.; Colin, A.; Wolkenstein, P.; Paul, M. High-concentration topical capsaicin in the management of refractory neuropathic pain in patients with neurofibromatosis type 1: A case series. Curr. Med. Res. Opin. 2018, 34, 887–891. [Google Scholar] [CrossRef]
- Jones, V.M.; Moore, K.A.; Peterson, D.M. Capsaicin 8% topical patch (Qutenza)—A review of the evidence. J. Pain Palliat. Care Pharmacother. 2011, 25, 32–41. [Google Scholar] [CrossRef]
- Fragasso, C.; Palloshi, A.; Piatti, P.M.; Monti, L.; Rossetti, E.; Setola, E.; Montano, C.; Bassanelli, G.; Calori, G.; Margonato, A. Nitric-oxide mediated effects of transdermal capsaicin patches on the ischemic threshold in patients with stable coronary disease. J. Cardiovasc. Pharmacol. 2004, 44, 340–347. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, R.; Monga, M.; Fink, H.A.; Wilt, T.J. Neurotoxin treatments for urinary incontinence in subjects with spinal cord injury or multiple sclerosis: A systematic review of effectiveness and adverse effects. J. Spinal Cord Med. 2008, 31, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Igawa, Y.; Satoh, T.; Mizusawa, H.; Seki, S.; Kato, H.; Ishizuka, O.; Nishizawa, O. The role of capsaicin-sensitive afferents in autonomic dysreflexia in patients with spinal cord injury. BJU Int. 2003, 91, 637–641. [Google Scholar] [CrossRef]
- Arsenault, P.; Chiche, D.; Brown, W.; Miller, J.; Treister, R.; Leff, R.; Walker, P.; Katz, N. NEO06860, a modality-selective TRPV1 antagonist: A randomized, controlled, proof-of-concept trial in patients with osteoarthritis knee pain. Pain Rep. 2018, 3, e696. [Google Scholar] [CrossRef]
- Brown, W.; Leff, R.L.; Griffin, A.; Hossack, S.; Aubray, R.; Walker, P.; Chiche, D.A. Safety, pharmacokinetics, and pharmacodynamics study in healthy subjects of oral NEO06860, a modality selective Transient Receptor Potential Vanilloid Subtype 1 antagonist. J. Pain 2017, 18, 726–738. [Google Scholar] [CrossRef]
- Sogut, O.; Kaya, H.; Gokdemir, M.T.; Sezen, Y. Acute myocardial infarction and coronary vasospasm associated with ingestion of cayenne pepper in a 25-year-old male. Int. J. Emerg. Med. 2012, 5, 5. [Google Scholar] [CrossRef]
- Li, Q.; Cui, Y.; Jin, R.; Lang, H.; Yu, H.; Sun, F.; He, C.; Ma, T.; Li, Y.; Zhou, X.; et al. Enjoyment of spicy flavor enhances central salty-taste perception and reduces salt-intake and blood pressure. Hypertension 2017, 70, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szallasi, A. The Vanilloid (Capsaicin) Receptor TRPV1 in Blood Pressure Regulation: A Novel Therapeutic Target in Hypertension? Int. J. Mol. Sci. 2023, 24, 8769. https://doi.org/10.3390/ijms24108769
Szallasi A. The Vanilloid (Capsaicin) Receptor TRPV1 in Blood Pressure Regulation: A Novel Therapeutic Target in Hypertension? International Journal of Molecular Sciences. 2023; 24(10):8769. https://doi.org/10.3390/ijms24108769
Chicago/Turabian StyleSzallasi, Arpad. 2023. "The Vanilloid (Capsaicin) Receptor TRPV1 in Blood Pressure Regulation: A Novel Therapeutic Target in Hypertension?" International Journal of Molecular Sciences 24, no. 10: 8769. https://doi.org/10.3390/ijms24108769
APA StyleSzallasi, A. (2023). The Vanilloid (Capsaicin) Receptor TRPV1 in Blood Pressure Regulation: A Novel Therapeutic Target in Hypertension? International Journal of Molecular Sciences, 24(10), 8769. https://doi.org/10.3390/ijms24108769






