Calcium Homeostasis, Transporters, and Blockers in Health and Diseases of the Cardiovascular System
Abstract
1. Introduction
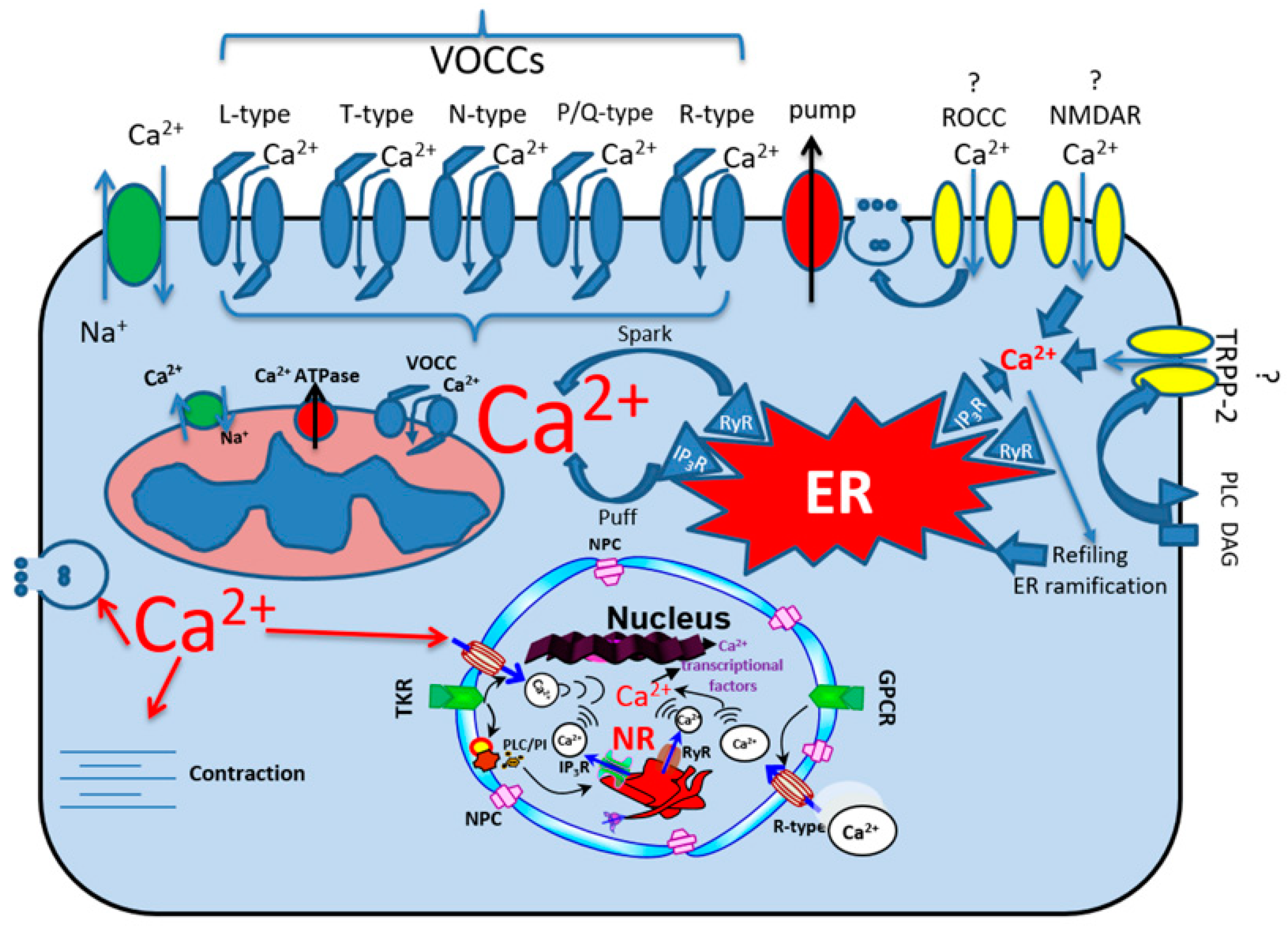
2. Calcium Ionic Transporters
2.1. Calcium Channels
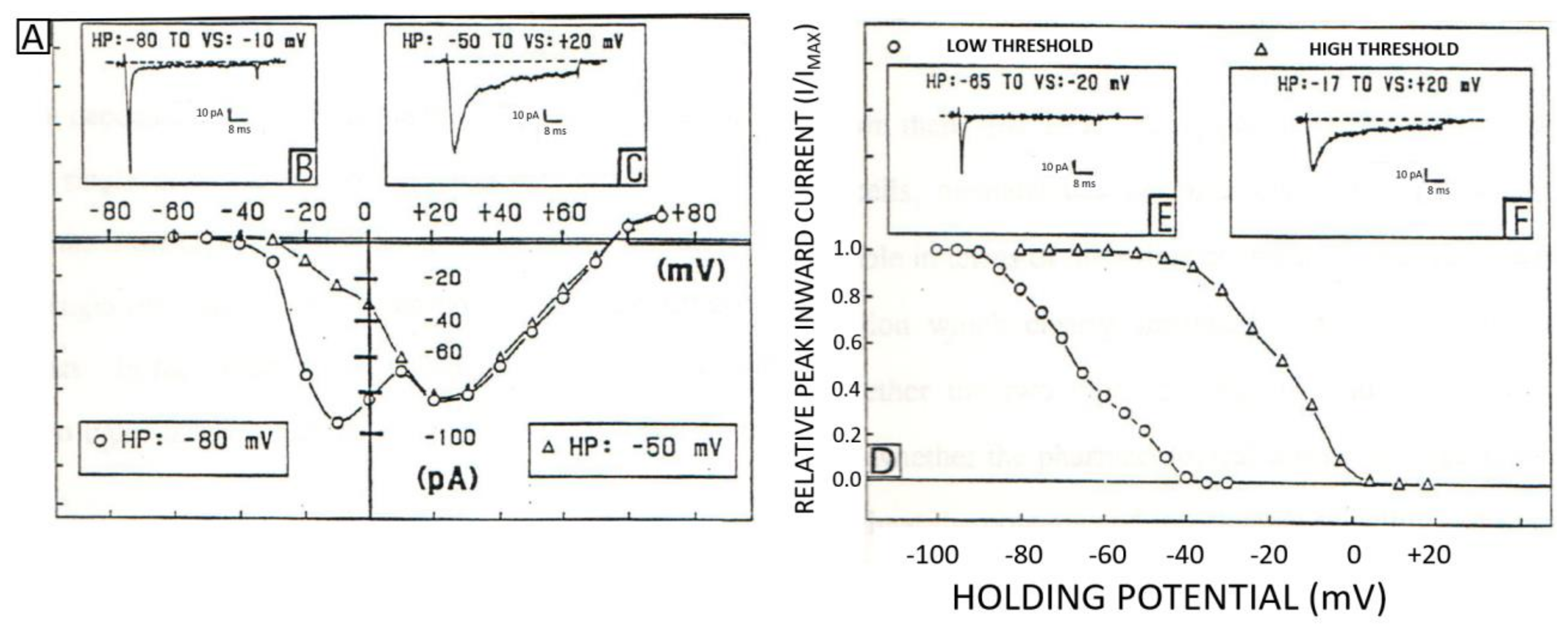
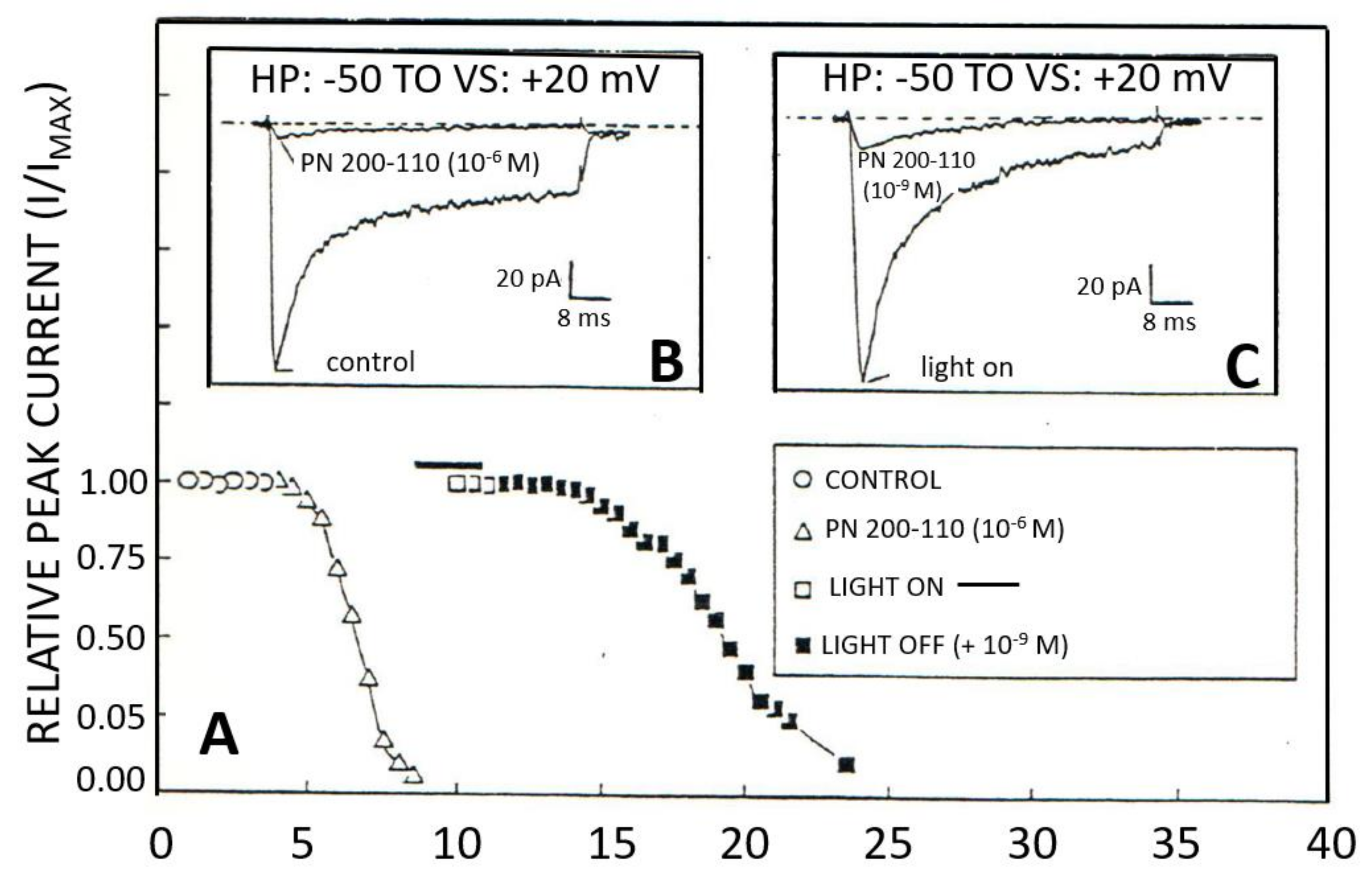
2.2. Classification, Function, and Pharmacology of VOCCs
2.3. The Receptor-Operated Calcium Channels (ROCCs)
2.4. ROCCs: TRPC1, TRPP2, NMDARs, and AMPARs
2.5. Sodium–Calcium Exchangers, Ca2+ Pumps and Ca2+ Release Channels
2.5.1. Sodium–Calcium Exchangers
2.5.2. Ca2+ Pumps
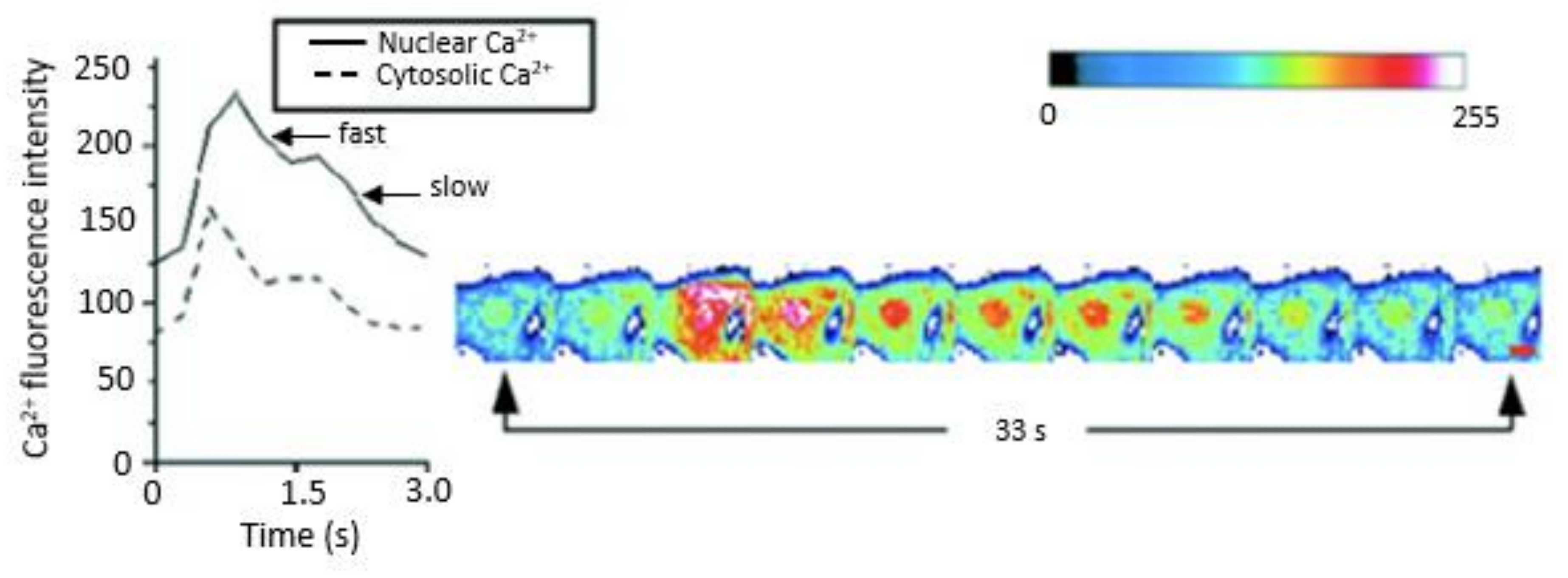
2.5.3. Ca2+ Release Channels
2.6. Organic and Inorganic Calcium Channel Blockers
2.7. Role of Ca2+ Channels, Exchangers, and Pumps in Cardiovascular Diseases
3. Discussion, Conclusions, and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bkaily, G.; Al-Khoury, J.; Simon, Y.; Jacques, D. Intracellular Free Calcium Measurement Using Confocal Imaging. Methods Mol. Biol. 2017, 1527, 177–187. [Google Scholar]
- Bkaily, G.; Avedanian, L.; Al-Khoury, J.; Chamoun, M.; Semaan, R.; Jubinville-Leblanc, C.; D’Orléans-Juste, P.; Jacques, D. Nuclear membrane R-type calcium channels mediate cytosolic ET-1-induced increase of nuclear calcium in human vascular smooth muscle cells. Can. J. Physiol. Pharmacol. 2015, 93, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Jacques, D.; D’Orleans-Juste, P.; Magder, S.; Bkaily, G. Neuropeptide Y and its receptors in ventricular endocardial endothelial cells. Can. J. Physiol. Pharmacol. 2017, 95, 1224–1229. [Google Scholar] [CrossRef]
- Jacques, D.B. Cardiovascular physiopathology of angiotensin II and its plasma and nuclear envelop membrane’s receptors. In The Renin Angiotensin System in Cardiovascular Disease; Advances in Biochemistry in Health and Disease; Dhalla, N.S., Bhullar, S.K., Shah, A.K., Eds.; Springer: Cham, Switzerland, 2023; pp. 63–80. [Google Scholar]
- Berridge, M.J.; Bootman, M.D.; Lipp, P. Calcium—A life and death signal. Nature 1998, 395, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.T. Intracellular calcium in the fertilization and development of mammalian eggs. Clin. Exp. Pharmacol. Physiol. 2007, 34, 1084–1089. [Google Scholar] [CrossRef]
- Bkaily, G.; Abou Abdallah, N.; Simon, Y.; Jazzar, A.; Jacques, D. Vascular smooth muscle remodeling in health and disease. Can. J. Physiol. Pharmacol. 2021, 99, 171–178. [Google Scholar] [CrossRef]
- Bkaily, G.; Gros-Louis, N.; Naik, R.; Jaalouk, D.; Pothier, P. Implication of the nucleus in excitation contraction coupling of heart cells. Mol. Cell. Biochem. 1996, 154, 113–121. [Google Scholar] [CrossRef]
- Keefe, J.A.; Moore, O.M.; Ho, K.S.; Wehrens, X.H.T. Role of Ca2+ in healthy and pathologic cardiac function: From normal excitation-contraction coupling to mutations that cause inherited arrhythmia. Arch. Toxicol. 2023, 97, 73–92. [Google Scholar] [CrossRef]
- Valentim, M.A.; Brahmbhatt, A.N.; Tupling, A.R. Skeletal and cardiac muscle calcium transport regulation in health and disease. Biosci. Rep. 2022, 42, BSR20211997. [Google Scholar] [CrossRef] [PubMed]
- Bkaily, G.; Jacques, D. Morphological and Functional Remodeling of Vascular Endothelium in Cardiovascular Diseases. Int. J. Mol. Sci. 2023, 24, 1998. [Google Scholar] [CrossRef]
- Borowiec, A.S.; Bidaux, G.; Pigat, N.; Goffin, V.; Bernichtein, S.; Capiod, T. Calcium channels, external calcium concentration and cell proliferation. Eur. J. Pharmacol. 2014, 739, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Munaron, L. Calcium signalling and control of cell proliferation by tyrosine kinase receptors (review). Int. J. Mol. Med. 2002, 10, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Munaron, L.; Fiorio Pla, A. Endothelial calcium machinery and angiogenesis: Understanding physiology to interfere with pathology. Curr. Med. Chem. 2009, 16, 4691–4703. [Google Scholar] [CrossRef] [PubMed]
- Antunes, F.T.T.; De Souza, A.H.; Figueira, J.; Binda, N.S.; Carvalho, V.P.R.; Vieira, L.B.; Gomez, M.V. Targeting N-type calcium channels in young-onset of some neurological diseases. Front. Cell Dev. Biol. 2022, 10, 1090765. [Google Scholar] [CrossRef]
- Avedanian, L.; Jacques, D.; Bkaily, G. Presence of tubular and reticular structures in the nucleus of human vascular smooth muscle cells. J. Mol. Cell Cardiol. 2011, 50, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Bkaily, G.; Avedanian, L.; Jacques, D. Nuclear membrane receptors and channels as targets for drug development in cardiovascular diseases. Can. J. Physiol. Pharmacol. 2009, 87, 108–119. [Google Scholar] [CrossRef]
- Berridge, M.J. Calcium microdomains: Organization and function. Cell Calcium 2006, 40, 405–412. [Google Scholar] [CrossRef]
- Curcic, S.; Erkan-Candag, H.; Pilic, J.; Malli, R.; Wiedner, P.; Tiapko, O.; Groschner, K. TRPC3 governs the spatiotemporal organization of cellular Ca2+ signatures by functional coupling to IP(3) receptors. Cell Calcium 2022, 108, 102670. [Google Scholar] [CrossRef]
- Ren, L.; Thai, P.N.; Gopireddy, R.R.; Timofeyev, V.; Ledford, H.A.; Woltz, R.L.; Park, S.; Puglisi, J.L.; Moreno, C.M.; Santana, L.F.; et al. Adenylyl cyclase isoform 1 contributes to sinoatrial node automaticity via functional microdomains. JCI Insight 2022, 7, e162602. [Google Scholar] [CrossRef]
- Serulle, Y.; Sugimori, M.; Llinás, R.R. Imaging synaptosomal calcium concentration microdomains and vesicle fusion by using total internal reflection fluorescent microscopy. Proc. Natl. Acad. Sci. USA 2007, 104, 1697–1702. [Google Scholar] [CrossRef]
- Toman, M.; Wade, J.J.; Verkhratsky, A.; Dallas, M.; Bithell, A.; Flanagan, B.; Harkin, J.; McDaid, L. The influence of astrocytic leaflet motility on ionic signalling and homeostasis at active synapses. Sci. Rep. 2023, 13, 3050. [Google Scholar] [CrossRef] [PubMed]
- Crowe, L.M.; Spargo, B.J.; Ioneda, T.; Beaman, B.L.; Crowe, J.H. Interaction of cord factor (alpha, alpha’-trehalose-6,6’-dimycolate) with phospholipids. Biochim. Biophys. Acta 1994, 1194, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Catacuzzeno, L.; Fioretti, B.; Franciolini, F. Modeling study of the effects of membrane surface charge on calcium microdomains and neurotransmitter release. Biophys. J. 2008, 95, 2160–2171. [Google Scholar] [CrossRef]
- Li, S.; Zhang, X.; Su, J. Surface charge density governs the ionic current rectification direction in asymmetric graphene oxide channels. Phys. Chem. Chem. Phys. 2023, 25, 7477–7486. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Lee, U.; Ryu, S.H.; Han, S.; Lee, S.Y.; Lee, J.S.; Ju, A.; Chang, S.; Lee, S.H.; Kim, S.H.; et al. L-type Ca2+ channels mediate regulation of glutamate release by subthreshold potential changes. Proc. Natl. Acad. Sci. USA 2023, 120, e2220649120. [Google Scholar] [CrossRef]
- Lacinová, L.; An, R.H.; Xia, J.; Ito, H.; Klugbauer, N.; Triggle, D.; Hofmann, F.; Kass, R.S. Distinctions in the molecular determinants of charged and neutral dihydropyridine block of L-type calcium channels. J. Pharmacol. Exp. Ther. 1999, 289, 1472–1479. [Google Scholar]
- Bolton, T.B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol. Rev. 1979, 59, 606–718. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, N.; Irisawa, H.; Kameyama, M. Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J. Physiol. 1988, 395, 233–253. [Google Scholar] [CrossRef]
- Hayashi, K.; Wakino, S.; Sugano, N.; Ozawa, Y.; Homma, K.; Saruta, T. Ca2+ channel subtypes and pharmacology in the kidney. Circ. Res. 2007, 100, 342–353. [Google Scholar] [CrossRef]
- Kochegarov, A.A. Pharmacological modulators of voltage-gated calcium channels and their therapeutical application. Cell Calcium 2003, 33, 145–162. [Google Scholar] [CrossRef]
- Reuter, H. A variety of calcium channels. Nature 1985, 316, 391. [Google Scholar] [CrossRef]
- Reuter, H. Calcium channel modulation by beta-adrenergic neurotransmitters in the heart. Experientia 1987, 43, 1173–1175. [Google Scholar] [CrossRef]
- Tsien, R.W. Calcium channels in excitable cell membranes. Annu. Rev. Physiol. 1983, 45, 341–358. [Google Scholar] [CrossRef]
- Tsien, R.W.; Bean, B.P.; Hess, P.; Lansman, J.B.; Nilius, B.; Nowycky, M.C. Mechanisms of calcium channel modulation by beta-adrenergic agents and dihydropyridine calcium agonists. J. Mol. Cell. Cardiol. 1986, 18, 691–710. [Google Scholar] [CrossRef] [PubMed]
- Tsien, R.W.; Hess, P.; McCleskey, E.W.; Rosenberg, R.L. Calcium channels: Mechanisms of selectivity, permeation, and block. Annu. Rev. Biophys. Biophys. Chem. 1987, 16, 265–290. [Google Scholar] [CrossRef] [PubMed]
- Tsien, R.W.; Lipscombe, D.; Madison, D.V.; Bley, K.R.; Fox, A.P. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988, 11, 431–438. [Google Scholar] [CrossRef]
- Van Breemen, C.; Aaronson, P.; Loutzenhiser, R. Sodium-calcium interactions in mammalian smooth muscle. Pharmacol. Rev. 1978, 30, 167–208. [Google Scholar]
- Wang, M.; Sun, Y.; Li, L.; Wu, P.; Dkw, O.; Shi, H. Calcium Channels: Noteworthy Regulators and Therapeutic Targets in Dermatological Diseases. Front. Pharmacol. 2021, 12, 702264. [Google Scholar] [CrossRef] [PubMed]
- Benke, T.; Traynelis, S.F. AMPA-Type Glutamate Receptor Conductance Changes and Plasticity: Still a Lot of Noise. Neurochem. Res. 2019, 44, 539–548. [Google Scholar] [CrossRef]
- Cauvin, C.; Loutzenhiser, R.; Van Breemen, C. Mechanisms of calcium antagonist-induced vasodilation. Annu. Rev. Pharmacol. Toxicol. 1983, 23, 373–396. [Google Scholar] [CrossRef]
- Högestätt, E.D. Characterization of two different calcium entry pathways in small mesenteric arteries from rat. Acta Physiol. Scand. 1984, 122, 483–495. [Google Scholar] [CrossRef]
- McFadzean, I.; Gibson, A. The developing relationship between receptor-operated and store-operated calcium channels in smooth muscle. Br. J. Pharmacol. 2002, 135, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bkaily, G. Single heart cells as models for studying cardiac toxicology. In In Vitro Methods in Toxicology; Jolles, G., Cordier, A., Eds.; Academic Press: London, UK, 1992; pp. 289–334. [Google Scholar]
- Black, J.L., III. The voltage-gated calcium channel gamma subunits: A review of the literature. J. Bioenerg. Biomembr. 2003, 35, 649–660. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Hess, P.; Lansman, J.B.; Tsien, R.W. A novel type of cardiac calcium channel in ventricular cells. Nature 1985, 316, 443–446. [Google Scholar] [CrossRef]
- Nowycky, M.C.; Fox, A.P.; Tsien, R.W. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature 1985, 316, 440–443. [Google Scholar] [CrossRef]
- Godfraind, T. Discovery and Development of Calcium Channel Blockers. Front. Pharmacol. 2017, 8, 286. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Boraso, A.; Condorelli, E.; De Giuli, F.; Pasini, E.; Cargnoni, A.; Agnoletti, G.; Ghielmi, S. Protective effects of gallopamil against ischemia and reperfusion damage. Z. Kardiol. 1989, 78 (Suppl. S5), 1–11. [Google Scholar]
- Wasserstrom, M.F.A. Mechanisms of action of antiarrhthmic drugs: A medical approach. In The Heart and Cardiovascular System; Fozzard, H.A., Haber, E., Jennings, R.B., Katz, A.M., Morgan, H.E., Eds.; Raven Press: New York, NY, USA, 1986; Volume 2, pp. 1259–1316. [Google Scholar]
- Kokilambigai, K.S.; Kavitha, J.; Seetharaman, R.; Lakshmi, K.S.; Sai Susmitha, A. Analytical and Bioanalytical Techniques for the Quantification of the Calcium Channel Blocker—Amlodipine: A Critical Review. Crit. Rev. Anal. Chem. 2021, 51, 754–786. [Google Scholar] [CrossRef]
- Torrente, A.G.; Mesirca, P.; Neco, P.; Rizzetto, R.; Dubel, S.; Barrere, C.; Sinegger-Brauns, M.; Striessnig, J.; Richard, S.; Nargeot, J.; et al. L-type Cav1.3 channels regulate ryanodine receptor-dependent Ca2+ release during sino-atrial node pacemaker activity. Cardiovasc. Res. 2016, 109, 451–461. [Google Scholar] [CrossRef]
- Kaku, T.; Lee, T.S.; Arita, M.; Hadama, T.; Ono, K. The gating and conductance properties of Cav3.2 low-voltage-activated T-type calcium channels. Jpn. J. Physiol. 2003, 53, 165–172. [Google Scholar] [CrossRef]
- Bankston, J.R.; Kass, R.S. Ion channels: The voltage-sensor quartet. Nature 2008, 456, 183–185. [Google Scholar] [CrossRef]
- Kamkin, A.G.; Kiseleva, I.S.; Kirishchuk, S.I.; Lozinskiĭ, I.T. Voltage-gated calcium channels. Usp Fiziol. Nauk. 2006, 37, 3–33. [Google Scholar] [PubMed]
- Palade, P.T.; Almers, W. Slow calcium and potassium currents in frog skeletal muscle: Their relationship and pharmacologic properties. Pflugers Arch. 1985, 405, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, A. Specific pharmacology of calcium in myocardium, cardiac pacemakers, and vascular smooth muscle. Annu. Rev. Pharmacol. Toxicol. 1977, 17, 149–166. [Google Scholar] [CrossRef] [PubMed]
- Godfraind, T.; Miller, R.; Wibo, M. Calcium antagonism and calcium entry blockade. Pharmacol. Rev. 1986, 38, 321–416. [Google Scholar]
- Harrison, P.J.; Husain, S.M.; Lee, H.; Los Angeles, A.; Colbourne, L.; Mould, A.; Hall, N.A.L.; Haerty, W.; Tunbridge, E.M. CACNA1C (Ca(V)1.2) and other L-type calcium channels in the pathophysiology and treatment of psychiatric disorders: Advances from functional genomics and pharmacoepidemiology. Neuropharmacology 2022, 220, 109262. [Google Scholar] [CrossRef]
- Hille, B. Ionic channels in excitable membranes. Current problems and biophysical approaches. Biophys. J. 1978, 22, 283–294. [Google Scholar] [CrossRef]
- Hisashi, K.; Nakagawa, T.; Yasuda, T.; Kimitsuki, T.; Komune, S.; Komiyama, S. Voltage-dependent Ca2+ channels in the spiral ganglion cells of guinea pig cochlea. Hearth Res. 1995, 91, 196–201. [Google Scholar] [CrossRef]
- Glossmann, H.; Striessnig, J. Molecular properties of calcium channels. Rev. Physiol. Biochem. Pharmacol. 1990, 114, 1–105. [Google Scholar]
- Porzig, H. Pharmacological modulation of voltage-dependent calcium channels in intact cells. Rev. Physiol. Biochem. Pharmacol. 1990, 114, 209–262. [Google Scholar]
- Bkaily, G.; El-Bizri, N.; Bui, M.; Sukarieh, R.; Jacques, D.; Fu, M.L. Modulation of intracellular Ca2+ via L-type calcium channels in heart cells by the autoantibody directed against the second extracellular loop of the alpha1-adrenoceptors. Can. J. Physiol. Pharmacol. 2003, 81, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Bkaily, G.; Sperelakis, N. Injection of protein kinase inhibitor into cultured heart cells blocks calcium slow channels. Am. J. Physiol. 1984, 246 Pt 2, H630–H634. [Google Scholar] [CrossRef]
- Bkaily, G.; Sperelakis, N. Injection of guanosine 5’-cyclic monophosphate into heart cells blocks calcium slow channels. Am. J. Physiol. 1985, 248 Pt 2, H745–H749. [Google Scholar] [CrossRef] [PubMed]
- Haddad, G.E.; Sperelakis, N.; Bkaily, G. Regulation of the calcium slow channel by cyclic GMP dependent protein kinase in chick heart cells. Mol. Cell Biochem. 1995, 148, 89–94. [Google Scholar] [CrossRef]
- Bkaily, G.; Sperelakis, N. Calmodulin is required for a full activation of the calcium slow channels in heart cells. J. Cyclic Nucleotide Protein Phosphor. Res. 1986, 11, 25–34. [Google Scholar]
- Perney, T.M.; Hirning, L.D.; Leeman, S.E.; Miller, R.J. Multiple calcium channels mediate neurotransmitter release from peripheral neurons. Proc. Natl. Acad. Sci. USA 1986, 83, 6656–6659. [Google Scholar] [CrossRef]
- Rane, S.G.; Holz, G.G.; Dunlap, K. Dihydropyridine inhibition of neuronal calcium current and substance P release. Pflugers Arch. 1987, 409, 361–366. [Google Scholar] [CrossRef]
- Burgess, D.E.; Crawford, O.; Delisle, B.P.; Satin, J. Mechanism of inactivation gating of human T-type (low-voltage activated) calcium channels. Biophys. J. 2002, 82, 1894–1906. [Google Scholar] [CrossRef]
- Catterall, W.A. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 2000, 16, 521–555. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.F.; Hess, P. Mechanism of gating of T-type calcium channels. J. Gen. Physiol. 1990, 96, 603–630. [Google Scholar] [CrossRef]
- Droogmans, G.; Nilius, B. Kinetic properties of the cardiac T-type calcium channel in the guinea-pig. J. Physiol. 1989, 419, 627–650. [Google Scholar] [CrossRef] [PubMed]
- Fry, C.H.; Sui, G.; Wu, C. T-type Ca2+ channels in non-vascular smooth muscles. Cell Calcium 2006, 40, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Nachshen, D.A. Regulation of cytosolic calcium concentration in presynaptic nerve endings isolated from rat brain. J. Physiol. 1985, 363, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Rahman, G.; Gorelik, J.; Bhargava, A. Voltage-Gated T-Type Calcium Channel Modulation by Kinases and Phosphatases: The Old Ones, the New Ones, and the Missing Ones. Cells 2023, 12, 461. [Google Scholar] [CrossRef]
- Li, B.; Tadross, M.R.; Tsien, R.W. Sequential ionic and conformational signaling by calcium channels drives neuronal gene expression. Science 2016, 351, 863–867. [Google Scholar] [CrossRef]
- Lux, H.D.C.; Zucker, H. Block of sodium currents through a neuronal calcium channel by external calcium and magnesium ions. In The Calcium Channel: Structure, Function and Implication; Springer: Berlin, Germany, 1988; pp. 128–137. [Google Scholar]
- Fox, A.P.; Nowycky, M.C.; Tsien, R.W. Single-channel recordings of three types of calcium channels in chick sensory neurones. J. Physiol. 1987, 394, 173–200. [Google Scholar] [CrossRef]
- Tang, C.M.; Presser, F.; Morad, M. Amiloride selectively blocks the low threshold (T) calcium channel. Science 1988, 240, 213–215. [Google Scholar] [CrossRef]
- Kuo, I.Y.; Wölfle, S.E.; Hill, C.E. T-type calcium channels and vascular function: The new kid on the block? J. Physiol. 2011, 589 Pt 4, 783–795. [Google Scholar] [CrossRef]
- Carmeliet, E. Pacemaking in cardiac tissue. From IK2 to a coupled-clock system. Physiol. Rep. 2019, 7, e13862. [Google Scholar] [CrossRef]
- Agler, H.L.; Evans, J.; Colecraft, H.M.; Yue, D.T. Custom distinctions in the interaction of G-protein beta subunits with N-type (CaV2.2) versus P/Q-type (CaV2.1) calcium channels. J. Gen. Physiol. 2003, 121, 495–510. [Google Scholar] [CrossRef]
- Hirning, L.D.; Fox, A.P.; McCleskey, E.W.; Olivera, B.M.; Thayer, S.A.; Miller, R.J.; Tsien, R.W. Dominant role of N-type Ca2+ channels in evoked release of norepinephrine from sympathetic neurons. Science 1988, 239, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Quastel, D.M.S.; Guan, Y.Y. Does the motor nerve terminal have only one transmitter release system and only one species of Ca2+ channel. Soc. Neurosci. Abstr. 1986, 12, 28. [Google Scholar]
- Gambardella, A.; Labate, A. The role of calcium channel mutations in human epilepsy. Prog. Brain Res. 2014, 213, 87–96. [Google Scholar] [PubMed]
- Zhang, G.; Liu, J.B.; Yuan, H.L.; Chen, S.Y.; Singer, J.H.; Ke, J.B. Multiple Calcium Channel Types with Unique Expression Patterns Mediate Retinal Signaling at Bipolar Cell Ribbon Synapses. J. Neurosci. 2022, 42, 6487–6505. [Google Scholar] [CrossRef]
- Bkaily, G. (Ed.) Regulation of Ca2+ channels in VSM by monocyte-released factors. In Ionic Channles in Vascular Smooth Muscle; Landes Company: Austin, TX, USA, 1994; pp. 53–64. [Google Scholar]
- Bkaily, G.; Naik, R.; D’Orléans-Juste, P.; Wang, S.; Fong, C.N. Endothelin-1 activates the R-type Ca2+ channel in vascular smooth-muscle cells. J. Cardiovasc. Pharmacol. 1995, 26 (Suppl. S3), S303–S306. [Google Scholar] [CrossRef] [PubMed]
- Bkaily, G.; Naik, R.; Jaalouk, D.; Jacques, D.; Economos, D.; D’Orléans-Juste, P.; Pothier, P. Endothelin-1 and insulin activate the steady-state voltage dependent R-type Ca2+ channel in aortic smooth muscle cells via a pertussis toxin and cholera toxin sensitive G-protein. Mol. Cell. Biochem. 1998, 183, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Bkaily, G.; Pothier, P.; D’Orléans-Juste, P.; Simaan, M.; Jacques, D.; Jaalouk, D.; Belzile, F.; Hassan, G.; Boutin, C.; Haddad, G.; et al. The use of confocal microscopy in the investigation of cell structure and function in the heart, vascular endothelium and smooth muscle cells. Mol. Cell. Biochem. 1997, 172, 171–194. [Google Scholar] [CrossRef]
- Akk, G.; Mennerick, S.; Steinbach, J.H. Actions of anesthetics on excitatory transmitter-gated channels. Handb. Exp. Pharmacol. 2008, 182, 53–84. [Google Scholar]
- Unwin, N. Nicotinic acetylcholine receptor and the structural basis of neuromuscular transmission: Insights from Torpedo postsynaptic membranes. Q. Rev. Biophys. 2013, 46, 283–322. [Google Scholar] [CrossRef] [PubMed]
- Changeux, J.P.; Devillers-Thiéry, A.; Chemouilli, P. Acetylcholine receptor: An allosteric protein. Science 1984, 225, 1335–1345. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ma, Y.; Song, X.; Wu, Y.; Jin, P.; Chen, G. PD-1: A new candidate target for analgesic peptide design. J. Pain, 2023; in press. [Google Scholar] [CrossRef] [PubMed]
- Mikami, Y.; Iizuka, M.; Onimaru, H.; Izumizaki, M. Glycine and GABAA receptors suppressively regulate the inspiratory-related calcium rise in the thoracic inspiratory cells of the neonatal rat. J. Physiol. Sci. 2022, 72, 24. [Google Scholar] [CrossRef] [PubMed]
- Rychkov, G.Y.; Litjens, T.; Roberts, M.L.; Barritt, G.J. ATP and vasopressin activate a single type of store-operated Ca2+ channel, identified by patch-clamp recording, in rat hepatocytes. Cell Calcium 2005, 37, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, D.T.; Hahn, T.J.; Iida-Klein, A.; Kleeman, C.R.; Muallem, S. Parathyroid hormone-activated calcium channels in an osteoblast-like clonal osteosarcoma cell line. cAMP-dependent and cAMP-independent calcium channels. J. Biol. Chem. 1987, 262, 7711–7718. [Google Scholar] [CrossRef]
- Nilius, B.; Owsianik, G. The transient receptor potential family of ion channels. Genome Biol. 2011, 12, 218. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Woolley, C.S. Mechanisms That Underlie Expression of Estradiol-Induced Excitatory Synaptic Potentiation in the Hippocampus Differ between Males and Females. J. Neurosci. 2023, 43, 1298–1309. [Google Scholar] [CrossRef]
- Soda, T.; Brunetti, V.; Berra-Romani, R.; Moccia, F. The Emerging Role of N-Methyl-D-Aspartate (NMDA) Receptors in the Cardiovascular System: Physiological Implications, Pathological Consequences, and Therapeutic Perspectives. Int. J. Mol. Sci. 2023, 24, 3914. [Google Scholar] [CrossRef]
- Du, J.; Fu, J.; Xia, X.M.; Shen, B. The functions of TRPP2 in the vascular system. Acta Pharmacol. Sin. 2016, 37, 13–18. [Google Scholar] [CrossRef]
- Anyatonwu, G.I.; Ehrlich, B.E. Organic cation permeation through the channel formed by polycystin-2. J. Biol. Chem. 2005, 280, 29488–29493. [Google Scholar] [CrossRef]
- Ćelić, A.S.; Petri, E.T.; Benbow, J.; Hodsdon, M.E.; Ehrlich, B.E.; Boggon, T.J. Calcium-induced conformational changes in C-terminal tail of polycystin-2 are necessary for channel gating. J. Biol. Chem. 2012, 287, 17232–17240. [Google Scholar] [CrossRef]
- Gonzalez-Perrett, S.; Batelli, M.; Kim, K.; Essafi, M.; Timpanaro, G.; Moltabetti, N.; Reisin, I.L.; Arnaout, M.A.; Cantiello, H.F. Voltage dependence and pH regulation of human polycystin-2-mediated cation channel activity. J. Biol. Chem. 2002, 277, 24959–24966. [Google Scholar] [CrossRef]
- Skopin, A.; Shalygin, A.; Vigont, V.; Zimina, O.; Glushankova, L.; Mozhayeva, G.N.; Kaznacheyeva, E. TRPC1 protein forms only one type of native store-operated channels in HEK293 cells. Biochimie 2013, 95, 347–353. [Google Scholar] [CrossRef]
- Vassilev, P.M.; Guo, L.; Chen, X.Z.; Segal, Y.; Peng, J.B.; Basora, N.; Babakhanlou, H.; Cruger, G.; Kanazirska, M.; Ye, C.; et al. Polycystin-2 is a novel cation channel implicated in defective intracellular Ca2+ homeostasis in polycystic kidney disease. Biochem. Biophys. Res. Commun. 2001, 282, 341–350. [Google Scholar] [CrossRef]
- McGee, M.A.; Abdel-Rahman, A.A. N-Methyl-D-Aspartate Receptor Signaling and Function in Cardiovascular Tissues. J. Cardiovasc. Pharmacol. 2016, 68, 97–105. [Google Scholar] [CrossRef]
- Arif Pavel, M.; Lv, C.; Ng, C.; Yang, L.; Kashyap, P.; Lam, C.; Valentino, V.; Fung, H.Y.; Campbell, T.; Møller, S.G.; et al. Function and regulation of TRPP2 ion channel revealed by a gain-of-function mutant. Proc. Natl. Acad. Sci. USA 2016, 113, e2363–e2372. [Google Scholar] [CrossRef] [PubMed]
- Aurélie, G.P. Activation Mechanisms and Functional Roles of TRPP2 Cation Channels. In Trp Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2007; pp. 189–202. [Google Scholar]
- Cai, Y.; Anyatonwu, G.; Okuhara, D.; Lee, K.B.; Yu, Z.; Onoe, T.; Mei, C.L.; Qian, Q.; Geng, L.; Wiztgall, R.; et al. Calcium dependence of polycystin-2 channel activity is modulated by phosphorylation at Ser812. J. Biol. Chem. 2004, 279, 19987–19995. [Google Scholar] [CrossRef]
- González-Perrett, S.; Kim, K.; Ibarra, C.; Damiano, A.E.; Zotta, E.; Batelli, M.; Harris, P.C.; Reisin, I.L.; Arnaout, M.A.; Cantiello, H.F. Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc. Natl. Acad. Sci. USA 2001, 98, 1182–1187. [Google Scholar] [CrossRef]
- Koulen, P.; Cai, Y.; Geng, L.; Maeda, Y.; Nishimura, S.; Witzgall, R.; Ehrlich, B.E.; Somlo, S. Polycystin-2 is an intracellular calcium release channel. Nat. Cell. Biol. 2002, 4, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Vassilev, P.M.; Li, X.; Kawanabe, Y.; Zhou, J. Native polycystin 2 functions as a plasma membrane Ca2+-permeable cation channel in renal epithelia. Mol. Cell. Biol. 2003, 23, 2600–2607. [Google Scholar] [CrossRef]
- Ma, R.; Li, W.P.; Rundle, D.; Kong, J.; Akbarali, H.I.; Tsiokas, L. PKD2 functions as an epidermal growth factor-activated plasma membrane channel. Mol. Cell. Biol. 2005, 25, 8285–8298. [Google Scholar] [CrossRef] [PubMed]
- Pelucchi, B.; Aguiari, G.; Pignatelli, A.; Manzati, E.; Witzgall, R.; Del Senno, L.; Belluzzi, O. Nonspecific cation current associated with native polycystin-2 in HEK-293 cells. J. Am. Soc. Nephrol. 2006, 17, 388–397. [Google Scholar] [CrossRef]
- Tsiokas, L. Function and regulation of TRPP2 at the plasma membrane. Am. J. Physiol. Renal. Physiol. 2009, 297, F1–F9. [Google Scholar] [CrossRef]
- Wang, Q.; Dai, X.Q.; Li, Q.; Wang, Z.; Cantero Mdel, R.; Li, S.; Shen, J.; Tu, J.C.; Cantiello, H.; Chen, X.Z. Structural interaction and functional regulation of polycystin-2 by filamin. PLoS ONE 2012, 7, e40448. [Google Scholar] [CrossRef]
- Xu, G.M.; González-Perrett, S.; Essafi, M.; Timpanaro, G.A.; Montalbetti, N.; Arnaout, M.A.; Cantiello, H.F. Polycystin-1 activates and stabilizes the polycystin-2 channel. J. Biol. Chem. 2003, 278, 1457–1462. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Gui, Y.; Hou, X.; Ye, L.; Wang, L. Transient Receptor Potential Channels, Natriuretic Peptides, and Angiotensin Receptor-Neprilysin Inhibitors in Patients With Heart Failure. Front. Cardiovasc. Med. 2022, 9, 904881. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Chen, L. Recent progress in structural studies on canonical TRP ion channels. Cell Calcium 2019, 83, 102075. [Google Scholar] [CrossRef]
- Hiraishi, K.; Kurahara, L.H.; Ishikawa, K.; Go, T.; Yokota, N.; Hu, Y.; Fujita, T.; Inoue, R.; Hirano, K. Potential of the TRPM7 channel as a novel therapeutic target for pulmonary arterial hypertension. J. Smooth Muscle Res. 2022, 58, 50–62. [Google Scholar] [CrossRef]
- Etchepare, L.; Gréa, H.; Durand, P.; Bouchet, D.; Groc, L. NMDA receptor membrane dynamics tunes the firing pattern of midbrain dopaminergic neurons. J. Physiol. 2021, 599, 2933–2951. [Google Scholar] [CrossRef]
- Perszyk, R.E.; Zheng, Z.; Banke, T.G.; Zhang, J.; Xie, L.; McDaniel, M.J.; Katzman, B.M.; Pelly, S.C.; Yuan, H.; Liotta, D.C.; et al. The Negative Allosteric Modulator EU1794-4 Reduces Single-Channel Conductance and Ca2+ Permeability of GluN1/GluN2A N-Methyl-d-Aspartate Receptors. Mol. Pharmacol. 2021, 99, 399–411. [Google Scholar] [CrossRef]
- Naz, R.; Khan, A.; Alghamdi, B.S.; Ashraf, G.M.; Alghanmi, M.; Ahmad, A.; Bashir, S.S.; Haq, Q.M.R. An Insight into Animal Glutamate Receptors Homolog of Arabidopsis thaliana and Their Potential Applications—A Review. Plants 2022, 11, 2580. [Google Scholar] [CrossRef] [PubMed]
- Gallo, S.; Vitacolonna, A.; Crepaldi, T. NMDA Receptor and Its Emerging Role in Cancer. Int. J. Mol. Sci. 2023, 24, 2540. [Google Scholar] [CrossRef]
- Kantamneni, S. Cross-talk and regulation between glutamate and GABAB receptors. Front. Cell. Neurosci. 2015, 9, 135. [Google Scholar] [CrossRef] [PubMed]
- Rozov, A.; Burnashev, N. Fast interaction between AMPA and NMDA receptors by intracellular calcium. Cell Calcium 2016, 60, 407–414. [Google Scholar] [CrossRef]
- Swanson, G.T.; Kamboj, S.K.; Cull-Candy, S.G. Single-channel properties of recombinant AMPA receptors depend on RNA editing, splice variation, and subunit composition. J. Neurosci. 1997, 17, 58–69. [Google Scholar] [CrossRef]
- Yelshanskaya, M.V.; Patel, D.S.; Kottke, C.M.; Kurnikova, M.G.; Sobolevsky, A.I. Opening of glutamate receptor channel to subconductance levels. Nature 2022, 605, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Miehl, C.; Gjorgjieva, J. Stability and learning in excitatory synapses by nonlinear inhibitory plasticity. PLoS Comput. Biol. 2022, 18, e1010682. [Google Scholar] [CrossRef]
- Chater, T.E.; Goda, Y. The role of AMPA receptors in postsynaptic mechanisms of synaptic plasticity. Front. Cell. Neurosci. 2014, 8, 401. [Google Scholar] [CrossRef] [PubMed]
- Formisano, L.; Guida, N.; Mascolo, L.; Serani, A.; Laudati, G.; Pizzorusso, V.; Annunziato, L. Transcriptional and epigenetic regulation of ncx1 and ncx3 in the brain. Cell Calcium 2020, 87, 102194. [Google Scholar] [CrossRef] [PubMed]
- Yao, A.; Su, Z.; Nonaka, A.; Zubair, I.; Lu, L.; Philipson, K.D.; Bridge, J.H.; Barry, W.H. Effects of overexpression of the Na+-Ca2+ exchanger on [Ca2+]i transients in murine ventricular myocytes. Circ. Res. 1998, 82, 657–665. [Google Scholar] [CrossRef]
- Khananshvili, D. Sodium-calcium exchangers (NCX): Molecular hallmarks underlying the tissue-specific and systemic functions. Pflugers Arch. 2014, 466, 43–60. [Google Scholar] [CrossRef]
- Khananshvili, D. Structure-Based Function and Regulation of NCX Variants: Updates and Challenges. Int. J. Mol. Sci. 2022, 24, 61. [Google Scholar] [CrossRef] [PubMed]
- Ottolia, M.; John, S.; Hazan, A.; Goldhaber, J.I. The Cardiac Na+-Ca2+ Exchanger: From Structure to Function. Compr. Physiol. 2021, 12, 2681–2717. [Google Scholar]
- Linck, B.; Qiu, Z.; He, Z.; Tong, Q.; Hilgemann, D.W.; Philipson, K.D. Functional comparison of the three isoforms of the Na+/Ca2+ exchanger (NCX1, NCX2, NCX3). Am. J. Physiol. 1998, 274, C415–C423. [Google Scholar] [CrossRef]
- Blaustein, M.P.; Lederer, W.J. Sodium/calcium exchange: Its physiological implications. Physiol. Rev. 1999, 79, 763–854. [Google Scholar] [CrossRef]
- Kofuji, P.; Lederer, W.J.; Schulze, D.H. Mutually exclusive and cassette exons underlie alternatively spliced isoforms of the Na/Ca exchanger. J. Biol. Chem. 1994, 269, 5145–5149. [Google Scholar] [CrossRef]
- Quednau, B.D.; Nicoll, D.A.; Philipson, K.D. Tissue specificity and alternative splicing of the Na+/Ca2+ exchanger isoforms NCX1, NCX2, and NCX3 in rat. Am. J. Physiol. 1997, 272 Pt 1, C1250–C1261. [Google Scholar] [CrossRef]
- Michel, L.Y.; Hoenderop, J.G.; Bindels, R.J. Towards Understanding the Role of the Na²⁺-Ca²⁺ Exchanger Isoform 3. Rev. Physiol. Biochem. Pharmacol. 2015, 168, 31–57. [Google Scholar]
- Michel, L.Y.M.; Verkaart, S.; Koopman, W.J.H.; Willems, P.; Hoenderop, J.G.J.; Bindels, R.J.M. Function and regulation of the Na+-Ca2+ exchanger NCX3 splice variants in brain and skeletal muscle. J. Biol. Chem. 2014, 289, 11293–11303. [Google Scholar] [CrossRef] [PubMed]
- Jeffs, G.J.; Meloni, B.P.; Bakker, A.J.; Knuckey, N.W. The role of the Na+/Ca2+ exchanger (NCX) in neurons following ischaemia. J. Clin. Neurosci. 2007, 14, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.L.; Yu, A.S.; Lytton, J. Tissue-specific expression of Na+-Ca2+ exchanger isoforms. J. Biol. Chem. 1994, 269, 14849–14852. [Google Scholar] [CrossRef]
- Ottolia, M.; Torres, N.; Bridge, J.H.; Philipson, K.D.; Goldhaber, J.I. Na/Ca exchange and contraction of the heart. J. Mol. Cell Cardiol. 2013, 61, 28–33. [Google Scholar] [CrossRef]
- Wier, W.G.; Egan, T.M.; López-López, J.R.; Balke, C.W. Local control of excitation-contraction coupling in rat heart cells. J. Physiol. 1994, 474, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Bridge, J.H.; Smolley, J.R.; Spitzer, K.W. The relationship between charge movements associated with ICa and INa-Ca in cardiac myocytes. Science 1990, 248, 376–378. [Google Scholar] [CrossRef] [PubMed]
- Bkaily, G.; Chahine, M.; Al-Khoury, J.; Avedanian, L.; Beier, N.; Scholz, W.; Jacques, D. Na+-H(+) exchanger inhibitor prevents early death in hereditary cardiomyopathy. Can. J. Physiol. Pharmacol. 2015, 93, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Bkaily, G.; Jacques, D. Na+-H(+) exchanger and proton channel in heart failure associated with Becker and Duchenne muscular dystrophies. Can. J. Physiol. Pharmacol. 2017, 95, 1213–1223. [Google Scholar] [CrossRef]
- Tappia, P.S.; Shah, A.K.; Ramjiawan, B.; Dhalla, N.S. Modification of Ischemia/Reperfusion-Induced Alterations in Subcellular Organelles by Ischemic Preconditioning. Int. J. Mol. Sci. 2022, 23, 3425. [Google Scholar] [CrossRef] [PubMed]
- Chahine, M.; Bkaily, G.; Nader, M.; Al-Khoury, J.; Jacques, D.; Beier, N.; Scholz, W. NHE-1-dependent intracellular sodium overload in hypertrophic hereditary cardiomyopathy: Prevention by NHE-1 inhibitor. J. Mol. Cell. Cardiol. 2005, 38, 571–582. [Google Scholar] [CrossRef]
- Iwamoto, T.; Pan, Y.; Nakamura, T.Y.; Wakabayashi, S.; Shigekawa, M. Protein kinase C-dependent regulation of Na+/Ca2+ exchanger isoforms NCX1 and NCX3 does not require their direct phosphorylation. Biochemistry 1998, 37, 17230–17238. [Google Scholar] [CrossRef]
- Iwamoto, T.; Pan, Y.; Wakabayashi, S.; Imagawa, T.; Yamanaka, H.I.; Shigekawa, M. Phosphorylation-dependent regulation of cardiac Na+/Ca2+ exchanger via protein kinase C. J. Biol. Chem. 1996, 271, 13609–13615. [Google Scholar] [CrossRef]
- Palty, R.; Hershfinkel, M.; Sekler, I. Molecular identity and functional properties of the mitochondrial Na+/Ca2+ exchanger. J. Biol. Chem. 2012, 287, 31650–31657. [Google Scholar] [CrossRef]
- Palty, R.; Silverman, W.F.; Hershfinkel, M.; Caporale, T.; Sensi, S.L.; Parnis, J.; Nolte, C.; Fishman, D.; Shoshan-Barmatz, V.; Herrmann, S.; et al. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc. Natl. Acad. Sci. USA 2010, 107, 436–441. [Google Scholar] [CrossRef]
- Kostic, M.; Sekler, I. Functional properties and mode of regulation of the mitochondrial Na+/Ca2+ exchanger, NCLX. Semin. Cell Dev. Biol. 2019, 94, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Pizzo, P.; Drago, I.; Filadi, R.; Pozzan, T. Mitochondrial Ca²⁺ homeostasis: Mechanism, role, and tissue specificities. Pflugers Arch. 2012, 464, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, A.; Kim, B.; Matsuoka, S. The mitochondrial Na+-Ca2+ exchanger, NCLX, regulates automaticity of HL-1 cardiomyocytes. Sci. Rep. 2013, 3, 2766. [Google Scholar] [CrossRef]
- Takeuchi, A.; Matsuoka, S. Physiological and Pathophysiological Roles of Mitochondrial Na+-Ca2+ Exchanger, NCLX, in Hearts. Biomolecules 2021, 11, 1876. [Google Scholar] [CrossRef]
- Mishra, J.; Jhun, B.S.; Hurst, S.; Csordás, G.; Sheu, S.S. The Mitochondrial Ca2+ Uniporter: Structure, Function, and Pharmacology. Handb. Exp. Pharmacol. 2017, 240, 129–156. [Google Scholar]
- Shattock, M.J.; Ottolia, M.; Bers, D.M.; Blaustein, M.P.; Boguslavskyi, A.; Bossuyt, J.; Bridge, J.H.; Chen-Izu, Y.; Clancy, C.E.; Edwards, A.; et al. Na+/Ca2+ exchange and Na+/K+-ATPase in the heart. J. Physiol. 2015, 593, 1361–1382. [Google Scholar] [CrossRef] [PubMed]
- Breault, N.M.; Wu, D.; Dasgupta, A.; Chen, K.H.; Archer, S.L. Acquired disorders of mitochondrial metabolism and dynamics in pulmonary arterial hypertension. Front. Cell Dev. Biol. 2023, 11, 1105565. [Google Scholar] [CrossRef]
- De Stefani, D.; Patron, M.; Rizzuto, R. Structure and function of the mitochondrial calcium uniporter complex. Biochim. Biophys. Acta 2015, 1853, 2006–2011. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Schulz, R.; Girao, H.; Kwak, B.R.; De Stefani, D.; Rizzuto, R.; Bernardi, P.; Di Lisa, F. Mitochondrial ion channels as targets for cardioprotection. J. Cell. Mol. Med. 2020, 24, 7102–7114. [Google Scholar] [CrossRef]
- Huang, C.; Deng, K.; Wu, M. Mitochondrial cristae in health and disease. Int. J. Biol. Macromol. 2023, 235, 123755. [Google Scholar] [CrossRef]
- Lozano, O.; Marcos, P.; Salazar-Ramirez, F.J.; Lázaro-Alfaro, A.F.; Sobrevia, L.; García-Rivas, G. Targeting the mitochondrial Ca2+ uniporter complex in cardiovascular disease. Acta Physiol. 2023, 237, e13946. [Google Scholar] [CrossRef] [PubMed]
- Ashok, D.; Papanicolaou, K.; Sidor, A.; Wang, M.; Solhjoo, S.; Liu, T.; O’Rourke, B. Mitochondrial Membrane Potential Instability on Reperfusion After Ischemia Does Not Depend on Mitochondrial Ca2+ Uptake. J. Biol. Chem. 2023, 104708. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Liu, C.; Zhou, J.; Yu, Q.; Duan, Y.; Zhang, T.; Li, Y.; Fu, G.; Sun, Y.; Tian, J.; et al. Upregulation of mitochondrial calcium uniporter contributes to paraquat-induced neuropathology linked to Parkinson’s disease via imbalanced OPA1 processing. J. Hazard. Mater. 2023, 453, 131369. [Google Scholar] [CrossRef] [PubMed]
- Romero-Garcia, S.; Prado-Garcia, H. Mitochondrial calcium: Transport and modulation of cellular processes in homeostasis and cancer (Review). Int. J. Oncol. 2019, 54, 1155–1167. [Google Scholar] [CrossRef]
- Tsai, C.W.; Liu, T.Y.; Chao, F.Y.; Tu, Y.C.; Rodriguez, M.X.; Van Keuren, A.M.; Ma, Z.; Bankston, J.; Tsai, M.F. Evidence supporting the MICU1 occlusion mechanism and against the potentiation model in the mitochondrial calcium uniporter complex. Proc. Natl. Acad. Sci. USA 2023, 120, e2217665120. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Chen, X.; Li, J.; Wang, F. CERS6 antisense RNA 1 promotes colon cancer via upregulating mitochondrial calcium uniporter. Eur. J. Clin. Investig. 2023, 53, e13951. [Google Scholar] [CrossRef]
- Gunter, T.E.; Pfeiffer, D.R. Mechanisms by which mitochondria transport calcium. Am. J. Physiol. 1990, 258 Pt 1, C755–C786. [Google Scholar] [CrossRef] [PubMed]
- Brini, M.; Carafoli, E. The plasma membrane Ca2+ ATPase and the plasma membrane sodium calcium exchanger cooperate in the regulation of cell calcium. Cold Spring Harb. Perspect. Biol. 2011, 3, a004168. [Google Scholar] [CrossRef] [PubMed]
- Krebs, J. Structure, Function and Regulation of the Plasma Membrane Calcium Pump in Health and Disease. Int. J. Mol. Sci. 2022, 23, 1027. [Google Scholar] [CrossRef]
- Schatzmann, H.J. ATP-dependent Ca++-extrusion from human red cells. Experientia 1966, 22, 364–365. [Google Scholar] [CrossRef] [PubMed]
- Chałupnik, P.; Szymańska, E. Kainate Receptor Antagonists: Recent Advances and Therapeutic Perspective. Int. J. Mol. Sci. 2023, 24, 1908. [Google Scholar] [CrossRef]
- Corti, E.; Duarte, C.B. The role of post-translational modifications in synaptic AMPA receptor activity. Biochem. Soc. Trans. 2023, 51, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Di Leva, F.; Domi, T.; Fedrizzi, L.; Lim, D.; Carafoli, E. The plasma membrane Ca2+ ATPase of animal cells: Structure, function and regulation. Arch. Biochem. Biophys. 2008, 476, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Golubeva, E.A.; Lavrov, M.I.; Radchenko, E.V.; Palyulin, V.A. Diversity of AMPA Receptor Ligands: Chemotypes, Binding Modes, Mechanisms of Action, and Therapeutic Effects. Biomolecules 2022, 13, 56. [Google Scholar] [CrossRef] [PubMed]
- Møller, J.V.; Juul, B.; le Maire, M. Structural organization, ion transport, and energy transduction of P-type ATPases. Biochim. Biophys. Acta 1996, 1286, 1–51. [Google Scholar] [CrossRef]
- Toyoshima, C.; Nakasako, M.; Nomura, H.; Ogawa, H. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 A resolution. Nature 2000, 405, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Guerini, D.; Coletto, L.; Carafoli, E. Exporting calcium from cells. Cell Calcium 2005, 38, 281–289. [Google Scholar] [CrossRef]
- Carafoli, E. The Ca2+ pump of the plasma membrane. J. Biol. Chem. 1992, 267, 2115–2118. [Google Scholar] [CrossRef] [PubMed]
- Carafoli, E. Calcium—A universal carrier of biological signals. Delivered on 3 July 2003 at the Special FEBS Meeting in Brussels. FEBS J. 2005, 272, 1073–1089. [Google Scholar] [CrossRef]
- Sanders, K.M. Invited review: Mechanisms of calcium handling in smooth muscles. J. Appl. Physiol. 2001, 91, 1438–1449. [Google Scholar] [CrossRef] [PubMed]
- Ebashi, S.; Lipmann, F. Adenosine triphosphate-linked concentration of calcium ions in a particulate fraction of rabbit muscle. J. Cell Biol. 1962, 14, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Chambers, P.J.; Juracic, E.S.; Fajardo, V.A.; Tupling, A.R. Role of SERCA and sarcolipin in adaptive muscle remodeling. Am. J. Physiol. Cell Physiol. 2022, 322, C382–C394. [Google Scholar] [CrossRef] [PubMed]
- Nemirovskaya, T.L.; Sharlo, K.A. Roles of ATP and SERCA in the Regulation of Calcium Turnover in Unloaded Skeletal Muscles: Current View and Future Directions. Int. J. Mol. Sci. 2022, 23, 6937. [Google Scholar] [CrossRef]
- Zhang, Y.; Inaba, K. Structural basis of the conformational and functional regulation of human SERCA2b, the ubiquitous endoplasmic reticulum calcium pump. Bioessays 2022, 44, e2200052. [Google Scholar] [CrossRef]
- Britzolaki, A.; Saurine, J.; Klocke, B.; Pitychoutis, P.M. A Role for SERCA Pumps in the Neurobiology of Neuropsychiatric and Neurodegenerative Disorders. Adv. Exp. Med. Biol. 2020, 1131, 131–161. [Google Scholar]
- Wu, K.D.; Bungard, D.; Lytton, J. Regulation of SERCA Ca2+ pump expression by cytoplasmic Ca2+ in vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 2001, 280, C843–C851. [Google Scholar] [CrossRef]
- Jaskulska, A.; Janecka, A.E.; Gach-Janczak, K. Thapsigargin-From Traditional Medicine to Anticancer Drug. Int. J. Mol. Sci. 2020, 22, 4. [Google Scholar] [CrossRef]
- Chemaly, E.R.; Troncone, L.; Lebeche, D. SERCA control of cell death and survival. Cell Calcium 2018, 69, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Dhalla, N.S.; Elimban, V.; Bartekova, M.; Adameova, A. Involvement of Oxidative Stress in the Development of Subcellular Defects and Heart Disease. Biomedicines 2022, 10, 393. [Google Scholar] [CrossRef]
- Fabiato, A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am. J. Physiol. 1983, 245, C1–C14. [Google Scholar] [CrossRef]
- Fabiato, A.; Fabiato, F. Calcium-induced release of calcium from the sarcoplasmic reticulum of skinned cells from adult human, dog, cat, rabbit, rat, and frog hearts and from fetal and new-born rat ventricles. Ann. N. Y. Acad. Sci. 1978, 307, 491–522. [Google Scholar] [CrossRef]
- Inui, M.; Saito, A.; Fleischer, S. Isolation of the ryanodine receptor from cardiac sarcoplasmic reticulum and identity with the feet structures. J. Biol. Chem. 1987, 262, 15637–15642. [Google Scholar] [CrossRef] [PubMed]
- Fill, M.; Coronado, R. Ryanodine receptor channel of sarcoplasmic reticulum. Trends Neurosci. 1988, 11, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Sutko, J.L.; Kenyon, J.L. Actions of ryanodine. J. Gen. Physiol. 1990, 96, 439–445. [Google Scholar] [CrossRef]
- Endo, M. Calcium release from the sarcoplasmic reticulum. Physiol. Rev. 1977, 57, 71–108. [Google Scholar] [CrossRef]
- Rampersad, P.; Mutawe, M.B.A.; Cook, T.; Gilchrist, J. Functional significance of ryanodine receptor-mediated calcium leaks in sarcoplasmic reticulum membranes. In Pathophysiology of Cardiovascular Disease; Dhalla, N.S.R., Angel, A., Pierce, G.N., Eds.; Kluwer Academic Publishers: Boston, MA, USA; Dordrecht, The Netherlands; London, UK, 2004; pp. 59–80. [Google Scholar]
- Pessah, I.N.; Zimanyi, I. Characterization of multiple [3H]ryanodine binding sites on the Ca2+ release channel of sarcoplasmic reticulum from skeletal and cardiac muscle: Evidence for a sequential mechanism in ryanodine action. Mol. Pharmacol. 1991, 39, 679–689. [Google Scholar]
- Laporte, R.; Hui, A.; Laher, I. Pharmacological modulation of sarcoplasmic reticulum function in smooth muscle. Pharmacol. Rev. 2004, 56, 439–513. [Google Scholar] [CrossRef]
- Neylon, C.B.; Richards, S.M.; Larsen, M.A.; Agrotis, A.; Bobik, A. Multiple types of ryanodine receptor/Ca2+ release channels are expressed in vascular smooth muscle. Biochem. Biophys. Res. Commun. 1995, 215, 814–821. [Google Scholar] [CrossRef]
- Rossi, D.; Sorrentino, V. Molecular genetics of ryanodine receptors Ca2+-release channels. Cell Calcium 2002, 32, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Protasi, F.; Girolami, B.; Serano, M.; Pietrangelo, L.; Paolini, C. Ablation of Calsequestrin-1, Ca2+ unbalance, and susceptibility to heat stroke. Front. Physiol. 2022, 13, 1033300. [Google Scholar] [CrossRef]
- Parys, J.B.; Bultynck, G.; Vervliet, T. IP(3) Receptor Biology and Endoplasmic Reticulum Calcium Dynamics in Cancer. Prog. Mol. Subcell. Biol. 2021, 59, 215–237. [Google Scholar]
- Worley, P.F.; Baraban, J.M.; Colvin, J.S.; Snyder, S.H. Inositol trisphosphate receptor localization in brain: Variable stoichiometry with protein kinase C. Nature 1987, 325, 159–161. [Google Scholar] [CrossRef] [PubMed]
- De Smedt, H.; Parys, J.B. Molecular and functional diversity of inositol triphosphate-induced Ca2+ release. Verh. K. Acad. Geneeskd. Belg. 1995, 57, 423–458. [Google Scholar]
- Patel, S.; Joseph, S.K.; Thomas, A.P. Molecular properties of inositol 1,4,5-trisphosphate receptors. Cell Calcium 1999, 25, 247–264. [Google Scholar] [CrossRef]
- Chalmers, S.; Olson, M.L.; MacMillan, D.; Rainbow, R.D.; McCarron, J.G. Ion channels in smooth muscle: Regulation by the sarcoplasmic reticulum and mitochondria. Cell Calcium 2007, 42, 447–466. [Google Scholar] [CrossRef]
- Ross, C.A.; Meldolesi, J.; Milner, T.A.; Satoh, T.; Supattapone, S.; Snyder, S.H. Inositol 1,4,5-trisphosphate receptor localized to endoplasmic reticulum in cerebellar Purkinje neurons. Nature 1989, 339, 468–470. [Google Scholar] [CrossRef] [PubMed]
- Streb, H.; Irvine, R.F.; Berridge, M.J.; Schulz, I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature 1983, 306, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Bosanac, I.; Alattia, J.R.; Mal, T.K.; Chan, J.; Talarico, S.; Tong, F.K.; Tong, K.I.; Yoshikawa, F.; Furuichi, T.; Iwai, M.; et al. Structure of the inositol 1,4,5-trisphosphate receptor binding core in complex with its ligand. Nature 2002, 420, 696–700. [Google Scholar] [CrossRef]
- Shuai, J.; Rose, H.J.; Parker, I. The number and spatial distribution of IP3 receptors underlying calcium puffs in Xenopus oocytes. Biophys. J. 2006, 91, 4033–4044. [Google Scholar] [CrossRef] [PubMed]
- Magistroni, R.; Mangolini, A.; Guzzo, S.; Testa, F.; Rapanà, M.R.; Mignani, R.; Russo, G.; di Virgilio, F.; Aguiari, G. TRPP2 dysfunction decreases ATP-evoked calcium, induces cell aggregation and stimulates proliferation in T lymphocytes. BMC Nephrol. 2019, 20, 355. [Google Scholar] [CrossRef]
- Guilbault, P.; Coraboeuf, E. Action of calcium ions on the duration of the action potential of the ventricular fiber of the rat and guinea pig. J. Physiol. 1965, 57, 618–619. [Google Scholar]
- Melville, K.I.; Shister, H.E.; Huq, S. Iproveratril: Experimental data on coronary dilatation and antiarrhythmic action. Can. Med. Assoc. J. 1964, 90, 761–770. [Google Scholar] [PubMed]
- Bean, B.P. Two kinds of calcium channels in canine atrial cells. Differences in kinetics, selectivity, and pharmacology. J. Gen. Physiol. 1985, 86, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Fleckenstein, A.; Fleckenstein-Grün, G.; Frey, M.; Thimm, F. Experimental antiarteriosclerotic effects of calcium antagonists. J. Clin. Pharmacol. 1990, 30, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Gasser, R.; Byon, Y.K.; Frey, G.; Fleckenstein-Grün, G.; Fleckenstein, A. Diltiazem and verapamil prevent vitamin D3-induced myocardial calcium overload in rat papillary muscle: Assessment with PVC ETH-123 calcium-selective microelectrodes. Cardiovasc. Drugs Ther. 1996, 10, 185–187. [Google Scholar] [CrossRef]
- Janis, R.A.S.; Triggle, D.J. Drug Action and Cellular Calcium Regulation. Adv. Drug Res. 1987, 16, 309–439. [Google Scholar]
- Alshaya, O.A.; Alhamed, A.; Althewaibi, S.; Fetyani, L.; Alshehri, S.; Alnashmi, F.; Alharbi, S.; Alrashed, M.; Alqifari, S.F.; Alshaya, A.I. Calcium Channel Blocker Toxicity: A Practical Approach. J. Multidiscip. Healthc. 2022, 15, 1851–1862. [Google Scholar] [CrossRef] [PubMed]
- Catterall, W.A.; Seagar, M.J.; Takahashi, M.; Nunoki, K. Molecular properties of dihydropyridine-sensitive calcium channels. Ann. N. Y. Acad. Sci. 1989, 560, 1–14. [Google Scholar] [CrossRef]
- Catterall, W.A.; Swanson, T.M. Structural Basis for Pharmacology of Voltage-Gated Sodium and Calcium Channels. Mol. Pharmacol. 2015, 88, 141–150. [Google Scholar] [CrossRef]
- Fleckenstein-Grün, G.; Frey, M.; Thimm, F.; Fleckenstein, A. Protective effects of various calcium antagonists against experimental arteriosclerosis. J. Hum. Hypertens 1992, 6 (Suppl. S1), S13–S18. [Google Scholar] [PubMed]
- Wallnöfer, A.; Cauvin, C.; Lategan, T.W.; Rüegg, U.T. Differential blockade of agonist- and depolarization-induced 45Ca2+ influx in smooth muscle cells. Am. J. Physiol. 1989, 257 Pt 1, C607–C611. [Google Scholar] [CrossRef]
- Tiruppathi, C.; Ahmmed, G.U.; Vogel, S.M.; Malik, A.B. Ca2+ signaling, TRP channels, and endothelial permeability. Microcirculation 2006, 13, 693–708. [Google Scholar] [CrossRef] [PubMed]
- Berrout, J.; Jin, M.; O’Neil, R.G. Critical role of TRPP2 and TRPC1 channels in stretch-induced injury of blood-brain barrier endothelial cells. Brain Res. 2012, 1436, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ippoliti, I.; Ancidoni, A.; Da Cas, R.; Pierantozzi, A.; Vanacore, N.; Trotta, F. Anti-dementia drugs: A descriptive study of the prescription pattern in Italy. Neurol. Sci. 2023, 44, 1587–1595. [Google Scholar] [CrossRef]
- Zakaria, E.M.; Abdel-Ghany, R.H.; Elgharbawy, A.S.; Alsemeh, A.E.; Metwally, S.S. A novel approach to repositioning memantine for metabolic syndrome-induced steatohepatitis: Modulation of hepatic autophagy, inflammation, and fibrosis. Life Sci. 2023, 319, 121509. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, H.; Shimohama, S.; Chachin, M.; Taniguchi, T.; Kimura, J. Ca2+-dependent and Ca2+-independent protein kinase C changes in the brain of patients with Alzheimer’s disease. J. Neurochem. 1996, 67, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Tamargo, J.; Ruilope, L.M. Investigational calcium channel blockers for the treatment of hypertension. Expert Opin. Investig. Drugs 2016, 25, 1295–1309. [Google Scholar] [CrossRef]
- Dolphin, A.C. The α2δ subunits of voltage-gated calcium channels. Biochim. Biophys. Acta 2013, 1828, 1541–1549. [Google Scholar] [CrossRef]
- House, S.J.; Potier, M.; Bisaillon, J.; Singer, H.A.; Trebak, M. The non-excitable smooth muscle: Calcium signaling and phenotypic switching during vascular disease. Pflugers Arch. 2008, 456, 769–785. [Google Scholar] [CrossRef]
- Striessnig, J.; Ortner, N.J.; Pinggera, A. Pharmacology of L-type Calcium Channels: Novel Drugs for Old Targets? Curr. Mol. Pharmacol. 2015, 8, 110–122. [Google Scholar] [CrossRef]
- Fan, G.; Cui, Y.; Gollasch, M.; Kassmann, M. Elementary calcium signaling in arterial smooth muscle. Channels 2019, 13, 505–519. [Google Scholar] [CrossRef]
- Meyer, M.R.; Field, A.S.; Kanagy, N.L.; Barton, M.; Prossnitz, E.R. GPER regulates endothelin-dependent vascular tone and intracellular calcium. Life Sci. 2012, 91, 623–627. [Google Scholar] [CrossRef]
- Holm, A.; Hellstrand, P.; Olde, B.; Svensson, D.; Leeb-Lundberg, L.M.; Nilsson, B.O. The G protein-coupled estrogen receptor 1 (GPER1/GPR30) agonist G-1 regulates vascular smooth muscle cell Ca²⁺ handling. J. Vasc. Res. 2013, 50, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Bkaily, G.; Simon, Y.; Normand, A.; Jazzar, A.; Najibeddine, H.; Khalil, A.; Jacques, D. Short-Communication: Short-Term Treatment with Taurine Prevents the Development of Cardiac Hypertrophy and Early Death in Hereditary Cardiomyopathy of the Hamster and Is Sex-Dependent. Nutrients 2022, 14, 3287. [Google Scholar] [CrossRef]
- Jacques, D.; Bkaily, G.; Jasmin, G.; D’Orléans-Juste, P.; Chahine, M. Isradipine prevents the development of spontaneously occurring cardiac necrosis in cardiomyopathic hamster. Can. J. Physiol. Pharmacol. 2003, 81, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Perez-Zoghbi, J.F.; Karner, C.; Ito, S.; Shepherd, M.; Alrashdan, Y.; Sanderson, M.J. Ion channel regulation of intracellular calcium and airway smooth muscle function. Pulm Pharmacol. Ther. 2009, 22, 388–397. [Google Scholar] [CrossRef]
- Quignard, J.F.; Frapier, J.M.; Harricane, M.C.; Albat, B.; Nargeot, J.; Richard, S. Voltage-gated calcium channel currents in human coronary myocytes. Regulation by cyclic GMP and nitric oxide. J. Clin. Investig. 1997, 99, 185–193. [Google Scholar] [CrossRef]
- Berridge, M.J. The versatility and complexity of calcium signalling. Novartis Found Symp. 2001, 239, 52–64; discussion 64–67, 150–159. [Google Scholar]
- Bager, J.E.; Manhem, K.; Andersson, T.; Hjerpe, P.; Bengtsson-Boström, K.; Ljungman, C.; Mourtzinis, G. Hypertension: Sex-related differences in drug treatment, prevalence and blood pressure control in primary care. J. Hum. Hypertens. 2023, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, P.M. Differential effects of calcium entry blockers on vascular smooth muscle. In New Perspectives on Calcium Antagonists; American Physiological Society: Bethesda, MD, USA, 1981; pp. 109–121. [Google Scholar]
- Rubin, R.P. Actions of calcium antagonists on secretory cells. In New Perspectives on Calcium Antagonists; American Physiological Society: Bethesda, MD, USA, 1981; pp. 147–158. [Google Scholar]
- Yingst, D.R.; Davis, J.; Schiebinger, R. Effects of extracellular calcium and potassium on the sodium pump of rat adrenal glomerulosa cells. Am. J. Physiol. Cell Physiol. 2001, 280, C119–C125. [Google Scholar] [CrossRef]
- Hirasawa, M.; Pittman, Q.J. Nifedipine facilitates neurotransmitter release independently of calcium channels. Proc. Natl. Acad. Sci. USA 2003, 100, 6139–6144. [Google Scholar] [CrossRef]
- Shalaeva, E.V.; Messerli, F.H. What is resistant arterial hypertension? Blood Press. 2023, 32, 2185457. [Google Scholar] [CrossRef]
- Bkaily, G.; Jazzar, A.; Normand, A.; Simon, Y.; Al-Khoury, J.; Jacques, D. Taurine and cardiac disease: State of the art and perspectives. Can. J. Physiol. Pharmacol. 2020, 98, 67–73. [Google Scholar] [CrossRef]
- Jazzar, A.; Jacques, D.; Bkaily, G. Insulin-Induced Cardiomyocytes Hypertrophy That Is Prevented by Taurine via beta-alanine-Sensitive Na+-Taurine Symporter. Nutrients 2021, 13, 3686. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.F.; Watanabe, Y.; Ono, K.; Iwamoto, T.; Yamashita, K.; Satoh, H.; Urushida, T.; Hayashi, H.; Kimura, J. Characterization of SN-6, a novel Na+/Ca2+ exchange inhibitor in guinea pig cardiac ventricular myocytes. Eur. J. Pharmacol. 2007, 573, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Bkaily, G.; Simon, Y.; Jazzar, A.; Najibeddine, H.; Normand, A.; Jacques, D. High Na+ Salt Diet and Remodeling of Vascular Smooth Muscle and Endothelial Cells. Biomedicines 2021, 9, 883. [Google Scholar] [CrossRef] [PubMed]
- Batiste, S.M.; Blackwell, D.J.; Kim, K.; Kryshtal, D.O.; Gomez-Hurtado, N.; Rebbeck, R.T.; Cornea, R.L.; Johnston, J.N.; Knollmann, B.C. Unnatural verticilide enantiomer inhibits type 2 ryanodine receptor-mediated calcium leak and is antiarrhythmic. Proc. Natl. Acad. Sci. USA 2019, 116, 4810–4815. [Google Scholar] [CrossRef]
- Murayama, T.; Kurebayashi, N.; Ishida, R.; Kagechika, H. Drug development for the treatment of RyR1-related skeletal muscle diseases. Curr. Opin. Pharmacol. 2023, 69, 102356. [Google Scholar] [CrossRef]
- Sabharwal, S.S.; Schumacker, P.T. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 2014, 14, 709–721. [Google Scholar] [CrossRef]
| Type | Subtype | Conductance (pS) | Localization | Function | Inorganic Antagonist | Antagonist Organic |
|---|---|---|---|---|---|---|
| VOCC-L | Cav1.1 | 25 | SKELETAL MUSCLE | CONTRACTION | Ba2+, Mn2+, Ca2+, Mg2+ | DHP, VERAPAMIL, DILTIAZEM |
| VOCC-L | Cav1.2 | 25 | CARDIAC, VSM, NEURON | CONTRACTION, SECRETION, TRANSCRIPTION | Ba2+, Mn2+, Ca2+, Mg2+ | VERAPAMIL HEART, DHPVSM |
| VOCC-L | Cav1.3 | 25 | ENDOCRINE, NEURONAL, ATRIA, PACEMAKER | SECRETION, CONDUCTION | Ba2+, Mn2+, Ca2+, Mg2+ | DHP, VERAPAMIL, DILTIAZEM |
| VOCC-L | Cav1.4 | 25 | RETINAL | VISION, PHOTORECEPTOR | Ba2+, Mn2+, Ca2+, Mg2+ | DHP |
| VOCC-P/Q | Cav2.1 | 10–20 | NEURONAL | SECRETION | W-AGATOXIN | |
| VOCC-N | Cav2.2 | 10–20 | NEURONAL | SECRETION | W-CONOTOXIN | |
| VOCC-R | Cav2.3 | 24 | ALL CELL TYPES | RESTING [Ca2+]i | - | LOW CONCENTRATION OF ISRADIPINE |
| VOCC-T | Cav3.1 | 8–12 | CARDIAC, NEURONAL | PACEMAKER | Ni2+, Ca2+ | MIBEFRADIL |
| VOCC-T | Cav3.2 | 8–9 | CARDIAC, NEURONAL | PACEMAKER | Ni2+, Ca2+ | MIBEFRADIL |
| VOCC-T | Cav3.3 | 8–9 | NEURONAL | PACEMAKER | Ni2+, Ca2+ | MIBEFRADIL |
| AMPAR | - | 8.2–37 | ALZHEIMER, PARKINSON. DEPRESSION, EPILEPSY, CARDIAC, SKELETAL | MEMORY | - | PERAMPANEL TALAMPANEL JSTX-3 and DNQX |
| TRPC | 1 | 25–138 | RENAL, | REFILLING OF ER/SR STORE | La3+,Cd3+ | SAR7334, SKF96365, MPEP |
| TRPP | 2 | 80–160 1.2–4.5 | RENAL, T-LYMPHOCYTE. VSM, VEC, ER/SR, CARDIAC | ENDOCRINE, PROLIFERATION, REFILLING ER, APOPTOSIS, SPERM FERTILISATION | La3+, Gd3+ | AMILORIDE LOE-908 |
| NMDAR | - | 23–89 | NEURONAL, CARDIAC, VEC | LEARNING, MEMORY, NEURONAL MIGRATION | Zn2+, Mg2+, and Pb2+ | APS MK-801 IFENPRODIL MEMANTINE |
| Ca2+-PUMP | PMCA, SERCA | - | ALL CELLS | MAINTAIN Ca2+, HEMOSTASIS INTRACELLULAR | - | OUABAIN, THAPSIGARGIN |
| Na+-Ca2+ EXCHANGER | 1 | - | ALL CELLS | REGULATE INTRACELLULAR Ca2+ AND Na+ HOMEOSTASIS ER/SR Calcium | Li+ | SN-6 |
| RyR | 1–3 | - | ALL CELLS | RELEASE CHANNEL | - | DANTROLENE |
| IP3R | 1–3 | - | ALL CELLS | RELEASE CHANNEL | - | 2-APB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bkaily, G.; Jacques, D. Calcium Homeostasis, Transporters, and Blockers in Health and Diseases of the Cardiovascular System. Int. J. Mol. Sci. 2023, 24, 8803. https://doi.org/10.3390/ijms24108803
Bkaily G, Jacques D. Calcium Homeostasis, Transporters, and Blockers in Health and Diseases of the Cardiovascular System. International Journal of Molecular Sciences. 2023; 24(10):8803. https://doi.org/10.3390/ijms24108803
Chicago/Turabian StyleBkaily, Ghassan, and Danielle Jacques. 2023. "Calcium Homeostasis, Transporters, and Blockers in Health and Diseases of the Cardiovascular System" International Journal of Molecular Sciences 24, no. 10: 8803. https://doi.org/10.3390/ijms24108803
APA StyleBkaily, G., & Jacques, D. (2023). Calcium Homeostasis, Transporters, and Blockers in Health and Diseases of the Cardiovascular System. International Journal of Molecular Sciences, 24(10), 8803. https://doi.org/10.3390/ijms24108803









