Natural Variation Confers ‘Aiyuan 38’ Citrus Mutant a New Color and Unique Flavor
Abstract
1. Introduction
2. Results and Discussion
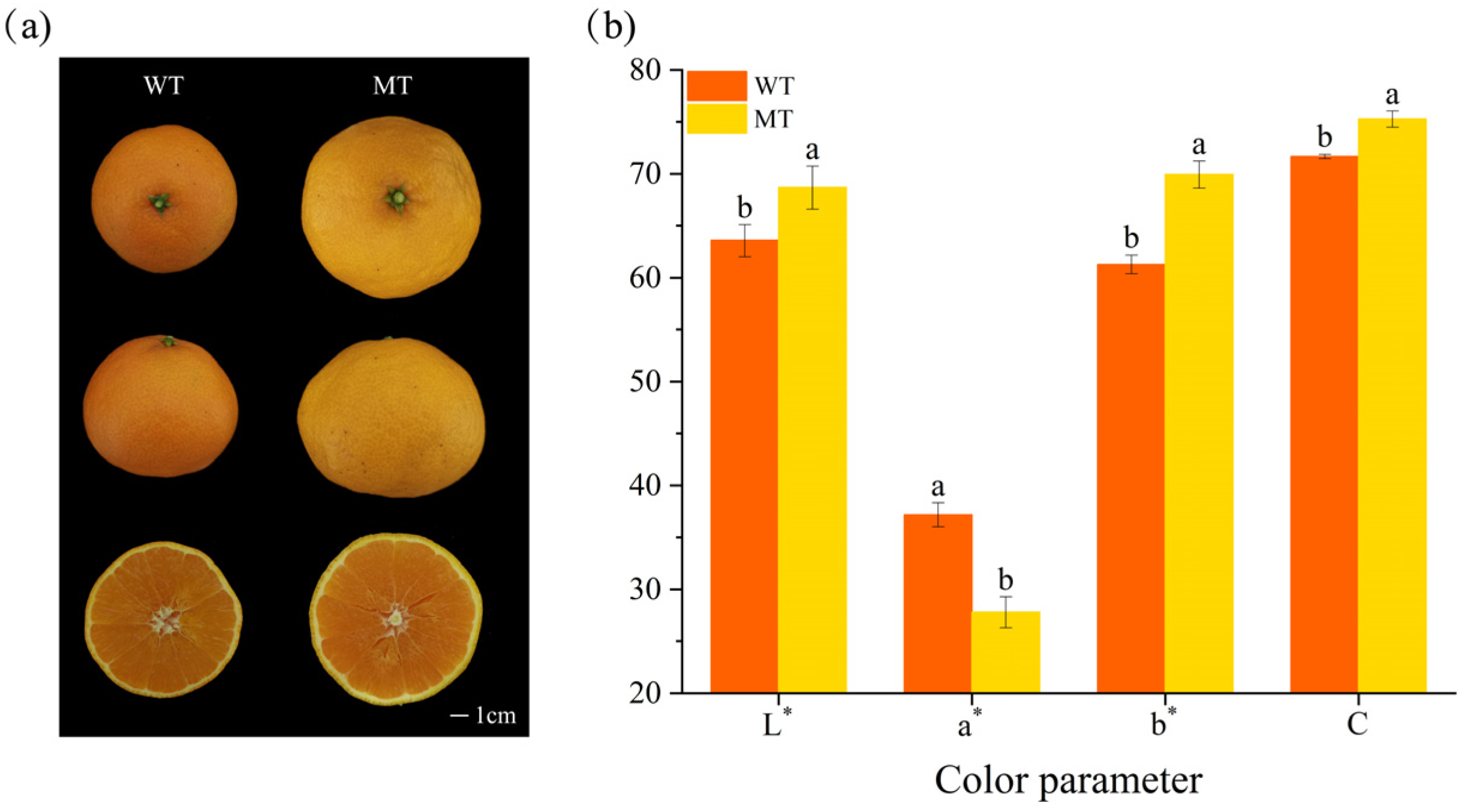
2.1. Fruit Color Variation
2.2. Standard Quality Parameters
2.3. Sugar and Acid Components
2.4. Total Flavonoid and Phenolic Content
2.5. Correlation Analysis
2.6. VOCs
2.6.1. Identification and Quantification of the VOCs in the Pulp of WT and MT
2.6.2. Identification and Quantification of the VOCs in the Peel of WT and MT
2.7. OAVs
2.8. Normalization of OAVs
3. Materials and Methods
3.1. Plant Materials and Treatment
3.2. Peel Color Measurement
3.3. TSS, TA, and Vitamin C (VC)
3.4. Sugar and Acid Compounds
3.5. Total Flavonoid and Phenolic Contents
3.6. VOC Identification and Quantification
3.7. Odor Activity Value (OAV) Calculation and Normalization
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palei, S.; Behera, S.K.; Sethy, P.K. A Systematic Review of Citrus Disease Perceptions and Fruit Grading Using Machine Vision. Procedia Comput. Sci. 2023, 218, 2504–2519. [Google Scholar] [CrossRef]
- Hu, L.P.; Yang, C.; Zhang, L.N.; Feng, J.; Xi, W.P. Effect of Light-Emitting Diodes and Ultraviolet Irradiation on the Soluble Sugar, Organic Acid, and Carotenoid Content of Postharvest Sweet Oranges (Citrus sinensis (L.) Osbeck). Molecules 2019, 24, 3440. [Google Scholar] [CrossRef] [PubMed]
- Perez-Roman, E.; Borreda, C.; Usach, A.L.G.; Talon, M. Single-nucleotide mosaicism in citrus: Estimations of somatic mutation rates and total number of variants. Plant Genome 2022, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S.; Abdullah, T.L.; Ahmad, Z.; Sahebi, M.; Azizi, P. Phenotypic and molecular effects of chronic gamma irradiation on Curcuma alismatifolia. Eur. J. Hortic. Sci. 2016, 81, 137–147. [Google Scholar] [CrossRef]
- Atay, A.N.; Atay, E.; Lauri, P.E.; Kunter, B.; Kantoglu, K.Y. Phenotyping gamma-ray-induced mutant population of ‘Amasya’ apple for architectural traits, precocity, floral phenology and fruit characteristics. Sci. Hortic. 2018, 233, 195–203. [Google Scholar] [CrossRef]
- Jaime Zacarías-García, P.J.C.; Gianfranco, D.; Lorenzo, Z.; María, J.R. A comprehensive analysis of carotenoids metabolism in two red-fleshed mutants of Navel and Valencia sweet oranges (Citrus sinensis). Front. Plant Sci. 2022, 13, 1034204. [Google Scholar] [CrossRef]
- Ruiz, D.; García-Gómez, B.E.; Egea, J.; Molina, A.; Martínez-Gómez, P.; Campoy, J.A. Phenotypical characterization and molecular fingerprinting of natural early-flowering mutants in apricot (Prunus armeniaca L.) and Japanese plum (P. salicina Lindl.). Sci. Hortic. 2019, 254, 187–192. [Google Scholar] [CrossRef]
- Testolin, R.; Messina, R.; Lain, O.; Cipriani, G. A natural sex mutant in kiwifruit (Actinidia deliciosa). N. Z. J. Crop Hortic. Sci. 2004, 32, 179–183. [Google Scholar] [CrossRef]
- Loewe, L.; Hill, W.G. The population genetics of mutations: Good, bad and indifferent. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 1153–1167. [Google Scholar] [CrossRef]
- Ni, X.P.; Ni, Z.J.; Ouma, K.O.; Gao, Z.H. Mutations in PmUFGT3 contribute to color variation of fruit skin in Japanese apricot (Prunus mume Sieb. et Zucc.). BMC Plant Biol. 2022, 22, 16. [Google Scholar] [CrossRef]
- Luo, T.; Xu, K.Y.; Luo, Y.; Chen, J.J.; Sheng, L.; Wang, J.Q.; Han, J.W.; Zeng, Y.L.; Xu, J.; Chen, J.M.; et al. Distinct Carotenoid and Flavonoid Accumulation in a Spontaneous Mutant of Ponkan (Citrus reticulata Blanco) Results in Yellowish Fruit and Enhanced Postharvest Resistance. J. Agric. Food Chem. 2015, 63, 8601–8614. [Google Scholar] [CrossRef] [PubMed]
- Hjernø, K.; Alm, R.; Canbäck, B.; Matthiesen, R.; Trajkovski, K.; Björk, L.; Roepstorff, P.; Emanuelsson, C. Down-regulation of the strawberry Bet v 1-homologous allergen in concert with the flavonoid biosynthesis pathway in colorless strawberry mutant. Proteomics 2006, 6, 1574–1587. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jang, S.J.; Jeong, H.B.; Lee, S.Y.; Venkatesh, J.; Lee, J.H.; Kwon, J.K.; Kang, B.C. A mutation in Zeaxanthin epoxidase contributes to orange coloration and alters carotenoid contents in pepper fruit (Capsicum annuum). Plant J. 2021, 106, 1692–1707. [Google Scholar] [CrossRef] [PubMed]
- Morales Alfaro, J.; Bermejo, A.; Navarro, P.; Quiñones, A.; Salvador, A. Effect of Rootstock on Citrus Fruit Quality: A Review. Food Rev. Int. 2021, 2021, 8093. [Google Scholar] [CrossRef]
- Cimen, B.; Yesiloglu, T.; Incesu, M.; Yilmaz, B. Studies on mutation breeding in citrus: Improving seedless types of ‘Kozan’ common orange by gamma irradiation. Sci. Hortic. 2021, 278, 10. [Google Scholar] [CrossRef]
- Lin, Y.X.; Hou, G.Y.; Jiang, Y.Y.; Liu, X.Y.; Yang, M.; Wang, L.X.; Long, Y.; Li, M.Y.; Zhang, Y.T.; Wang, Y.; et al. Joint Transcriptomic and Metabolomic Analysis Reveals Differential Flavonoid Biosynthesis in a High-Flavonoid Strawberry Mutant. Front. Plant Sci. 2022, 13, 17. [Google Scholar] [CrossRef] [PubMed]
- Calín-Sánchez, Á. Flavor and Aroma Analysis as a Tool for Quality Control of Foods. Foods 2021, 10, 224. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.L.; Yan, Z.Y.; Liu, Z.; Yao, Y.X. Early ripening events caused by bud mutation in Beni Shogun apple. Russ. J. Plant Physiol. 2011, 58, 439–447. [Google Scholar] [CrossRef]
- Zheng, T.; Zhang, S.H.; Leng, X.P.; Sadeghnezhad, E.; Li, T.; Pervaiz, T.; Liu, F.Q.; Jia, H.F.; Fang, J.G. Profiling Analysis of Volatile and Non-volatile Compounds in Vitis Vinifera Berries (cv. Chardonnay) and Spontaneous Bud Mutation. Front. Nutr. 2021, 8, 16. [Google Scholar] [CrossRef]
- Du, L.D.; Liu, Y.H.; Liu, J.Z.; Ding, X.Q.; Hong, B.; Hu, D.G.; Sun, C.H. Identification and Characterization of Petal Color Change from Pink to Yellow in Chrysanthemum morifolium ‘Pink Candy’ and Its Bud Variant. Agriculture 2022, 12, 1323. [Google Scholar] [CrossRef]
- Belasco, R.; Edwards, T.; Munoz, A.J.; Rayo, V.; Buono, M.J. The Effect of Hydration on Urine Color Objectively Evaluated in CIE L*a*b* Color Space. Front. Nutr. 2020, 7, 9. [Google Scholar] [CrossRef] [PubMed]
- Voss, D.H. Relating Colorimeter Measurement of Plant Color to the Royal Horticultural Society Colour Chart. HortScience 1992, 27, 1256–1260. [Google Scholar] [CrossRef]
- Isuzugawa, K.; Murayama, H.; Nishio, T. Characterization of a giant-fruit mutant exhibiting fruit-limited polyploidization in pear (Pyrus communis L.). Sci. Hortic. 2014, 170, 196–202. [Google Scholar] [CrossRef]
- Yamane, H.; Ichiki, M.; Tao, R.; Esumi, T.; Yonemori, K.; Niikawa, T.; Motosugi, H. Growth characteristics of a small-fruit dwarf mutant arising from bud sport mutation in Japanese persimmon (Diospyros kaki thunb.). Hortscience 2008, 43, 1726–1730. [Google Scholar] [CrossRef]
- Malladi, A.; Hirst, P.M. Increase in fruit size of a spontaneous mutant of ‘Gala’ apple (Malusxdomestica Borkh.) is facilitated by altered cell production and enhanced cell size. J. Exp. Bot. 2010, 61, 3003–3013. [Google Scholar] [CrossRef] [PubMed]
- Jideani, A.I.O.; Silungwe, H.; Takalani, T.; Omolola, A.O.; Udeh, H.O.; Anyasi, T.A. Antioxidant-rich natural fruit and vegetable products and human health. Int. J. Food Prop. 2021, 24, 41–67. [Google Scholar] [CrossRef]
- Conklin, P.L.; Pallanca, J.E.; Last, R.L.; Smirnoff, N. L-ascorbic acid metabolism in the ascorbate-deficient arabidopsis mutant vtc1. Plant Physiol. 1997, 115, 1277–1285. [Google Scholar] [CrossRef]
- Tao, J.J.; Wu, M.T.; Zhong, W.Q.; Jiao, X.D.; Chen, S.S.; Jia, H.M.; Jia, D.F.; Huang, C.H. Changes in Fruit Quality and Sugar Components of Wild Actinidia eriantha of Different Varieties (Lines) at the Ripening Stage. Horticulturae 2022, 8, 824. [Google Scholar] [CrossRef]
- Sdiri, S.; Bermejo, A.; Aleza, P.; Navarro, P.; Salvador, A. Phenolic composition, organic acids, sugars, vitamin C and antioxidant activity in the juice of two new triploid late-season mandarins. Food Res. Int. 2012, 49, 462–468. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S. Determination of volatile, phenolic, organic acid and sugar components in a Turkish cv. Dortyol (Citrus sinensis L. Osbeck) orange juice. J. Sci. Food Agric. 2011, 91, 1855–1862. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of Citrus Flavonoids, Naringin and Naringenin, on Metabolic Syndrome and Their Mechanisms of Action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef]
- Abad-Garcia, B.; Garmon-Lobato, S.; Berrueta, L.A.; Gallo, B.; Vicente, F. Online characterization of 58 phenolic compounds in Citrus fruit juices from Spanish cultivars by high-performance liquid chromatography with photodiode-array detection coupled to electrospray ionization triple quadrupole mass spectrometry. Talanta 2012, 99, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Hecker, K.D.; Bonanome, A.; Coval, S.M.; Binkoski, A.E.; Hilpert, K.F.; Griel, A.E.; Etherton, T.D. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002, 113 (Suppl. 9B), 71S–88S. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments: Anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- John, I.; Muthukumar, K.; Arunagiri, A. A review on the potential of citrus waste for D-Limonene, pectin, and bioethanol production. Int. J. Green Energy 2017, 14, 599–612. [Google Scholar] [CrossRef]
- Shin, S.D.; Kim, C.S.; Lee, J.H. Compositional characteristics and antibacterial activity of essential oils in citrus hybrid peels. Food Sci. Technol. 2022, 42, 9. [Google Scholar] [CrossRef]
- Khan, M.M.; Iqbal, M.; Hanif, M.A.; Mahmood, M.S.; Naqvi, S.A.; Shahid, M.; Jaskani, M.J. Antioxidant and Antipathogenic Activities of Citrus Peel Oils. J. Essent. Oil Bear. Plants 2012, 15, 972–979. [Google Scholar] [CrossRef]
- Asikin, Y.; Kawahira, S.; Goki, M.; Hirose, N.; Kyoda, S.; Wada, K. Extended aroma extract dilution analysis profile of Shiikuwasha (Citrus depressa Hayata) pulp essential oil. J. Food Drug Anal. 2018, 26, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Ortin, N.; Escudero, A.; Lopez, R.; Cacho, J. Chemical characterization of the aroma of Grenache rose wines: Aroma extract dilution analysis, quantitative determination, and sensory reconstitution studies. J. Agric. Food Chem. 2002, 50, 4048–4054. [Google Scholar] [CrossRef]
- Gemert, L.J. Odour Thresholds: Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter & Partners BV: Zeist, The Netherlands, 2011. [Google Scholar]
- Li, H.; Luo, L.; Ma, M.; Zeng, L. Characterization of Volatile Compounds and Sensory Analysis of Jasmine Scented Black Tea Produced by Different Scenting Processes. J. Food Sci. 2018, 83, 2718–2732. [Google Scholar] [CrossRef]
- Zeng, B.; Shen, J.; Wang, Q.; Dong, H. Analysis of Odorants in Cinnamomum burmannii Wood with Different Moisture Contents. Linye Kexue/Sci. Silvae Sin. 2021, 57, 133–141. [Google Scholar]
- Zareiyan, F.; Khajehsharifi, H. Analyzing Bioactive Compounds in Essential Oil of Citrus maxima and Citrus sinensis Peel. J. Essent. Oil Bear. Plants 2021, 24, 677–682. [Google Scholar] [CrossRef]
- Galvan-Lima, A.; Cunha, S.C.; Martins, Z.E.; Soares, A.G.; Ferreira, I.; Farah, A. Headspace volatolome of peel flours from citrus fruits grown in Brazil. Food Res. Int. 2021, 150, 11. [Google Scholar] [CrossRef] [PubMed]
- Paw, M.; Begum, T.; Gogoi, R.; Pandey, S.K.; Lal, M. Chemical Composition of Citrus limon L. Burmf Peel Essential Oil from North East India. J. Essent. Oil Bear. Plants 2020, 23, 337–344. [Google Scholar] [CrossRef]
- Giovanelli, S.; Ciccarelli, D.; Giusti, G.; Mancianti, F.; Nardoni, S.; Pistelli, L. Comparative assessment of volatiles in juices and essential oils from minor Citrusfruits (Rutaceae). Flavour Fragr. J. 2020, 35, 639–652. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S. Identification of phenolic compositions and the antioxidant capacity of mandarin juices and wines. J. Food Sci. Technol. Mysore 2014, 51, 1094–1101. [Google Scholar] [CrossRef]
- Babazadeh-Darjazi, B.; Jaimand, K. The Effect of Rootstocks on the Sugars, Acids, Carotenoids, Chlorophylls and Ethylene of Clementine Mandarin (Citrus clementina). J. Med. Plants By-Prod. 2018, 7, 91–98. [Google Scholar]
- Bi, X.; Liao, L.; Deng, L.; Jin, Z.; Huang, Z.; Sun, G.; Xiong, B.; Wang, Z. Combined Transcriptome and Metabolome Analyses Reveal Candidate Genes Involved in Tangor (Citrus reticulata & times; Citrus sinensis) Fruit Development and Quality Formation. Int. J. Mol. Sci. 2022, 23, 5457. [Google Scholar] [PubMed]
- Liao, L.; Dong, T.T.; Qiu, X.; Rong, Y.; Wang, Z.H.; Zhu, J. Nitrogen nutrition is a key modulator of the sugar and organic acid content in citrus fruit. PLoS ONE 2019, 14, 18. [Google Scholar] [CrossRef]
- Sulaiman, S.F.; Sajak, A.A.B.; Ooi, K.L.; Supriatno; Seow, E.M. Effect of solvents in extracting polyphenols and antioxidants of selected raw vegetables. J. Food Compos. Anal. 2011, 24, 506–515. [Google Scholar] [CrossRef]
- Molan, A.L.; Flanagan, J.; Wei, W.; Moughan, P.J. Selenium-containing green tea has higher antioxidant and prebiotic activities than regular green tea. Food Chem. 2009, 114, 829–835. [Google Scholar] [CrossRef]
- Shen, S.L.; Yin, X.R.; Zhang, B.; Xie, X.L.; Jiang, Q.; Grierson, D.; Chen, K.S. CitAP2.10 activation of the terpene synthase CsTPS1 is associated with the synthesis of (+)-valencene in ‘Newhall’ orange. J. Exp. Bot. 2016, 67, 4105–4115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Dai, Z.; Fan, X.J.; Liu, M.; Ma, J.F.; Shang, W.T.; Liu, J.G.; Strappe, P.; Blanchard, C.; Zhou, Z.K. A study on volatile metabolites screening by HS-SPME-GC-MS and HS-GC-IMS for discrimination and characterization of white and yellowed rice. Cereal Chem. 2020, 97, 496–504. [Google Scholar] [CrossRef]
- Feng, X.Y.; Wang, H.W.; Wang, Z.R.; Huang, P.M.; Kan, J.Q. Discrimination and characterization of the volatile organic compounds in eight kinds of huajiao with geographical indication of China using electronic nose, HS-GC-IMS and HS-SPME-GC-MS. Food Chem. 2022, 375, 11. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.L.; Wang, P.; Zhan, P.; Tian, H.L. Characterization of key aroma compounds in flat peach juice based on gas chromatography-mass spectrometry-olfactometry (GC-MS-O), odor activity value (OAV), aroma recombination, and omission experiments. Food Chem. 2022, 366, 9. [Google Scholar] [CrossRef]




| Fruit Type | Fruit Weight (g) | Vertical Diameter (mm) | Transverse Diameter (mm) | Fruit Shape Index | TSS (%) | TA (%) | TSS/TA Ratio | VC (mg/100 mL FW) |
|---|---|---|---|---|---|---|---|---|
| WT | 174.73 ± 5.09 b | 61.76 ± 1.93 b | 70.96 ± 1.32 b | 0.87 ± 0.03 a | 9.07 ± 0.06 a | 0.92 ± 0.01 a | 9.88 ± 0.12 a | 30.37 ± 0.56 a |
| MT | 223.70 ± 20.90 a | 68.42 ± 3.23 a | 77.75 ± 2.91 a | 0.88 ± 0.05 a | 8.97 ± 0.06 a | 0.89 ± 0.01 b | 10.08 ± 0.08 a | 27.91 ± 0.54 b |
| Compound Names | RI | WT | MT |
|---|---|---|---|
| Esters | |||
| cis-Carvyl acetate | 1336 | 1.10 ± 0.12 b | 4.12 ± 0.66 a |
| (1S,5S)-Carvyl acetate | 1363 | ND | 4.20 ± 0.12 a |
| Subtotal | 1.10 ± 0.12 b | 8.32 ± 0.60 a | |
| Alcohols | |||
| 1-Octanol | 1148 | 2.32 ± 0.24 a | 1.94 ± 0.46 a |
| Linalool B | 1099 | 41.82 ± 1.00 b | 72.75 ± 3.49 a |
| cis-p-Mentha-2,8-dien-1-ol | 1139 | 4.52 ± 0.17 b | 7.67 ± 0.38 a |
| Terpinen-4-ol | 1194 | 8.72 ± 1.27 b | 12.87 ± 2.20 a |
| α-Terpineol | 1215 | 3.68 ± 0.18 b | 5.31 ± 0.24 a |
| cis-Carveol | 1257 | 0.68 ± 0.06 b | 2.58 ± 0.01 a |
| Citronellol | 1280 | 3.91 ± 0.30 b | 6.60 ± 0.16 a |
| Subtotal | 65.65 ± 1.52 b | 109.72 ± 0.10 a | |
| Ketones | |||
| Thujone | 1115 | 0.63 ± 0.08 b | 0.99 ± 0.13 a |
| (+)-2-Bornanone | 1147 | ND | 0.94 ± 0.09 a |
| D-Dihydrocarvone | 1223 | 1.13 ± 0.18 b | 1.86 ± 0.17 a |
| D-Carvone | 1294 | 19.95 ± 0.25 b | 31.50 ± 3.96 a |
| cis-Geranylacetone | 1450 | 1.08 ± 0.04 a | 1.19 ± 0.20 a |
| Subtotal | 22.61 ± 0.65 b | 36.48 ± 4.38 a | |
| Aldehydes | |||
| Hexanal | 810 | 6.56 ± 0.16 b | 11.59 ± 1.25 a |
| Octanal | 1033 | 7.79 ± 1.93 b | 14.22 ± 1.34 a |
| Nonanal | 1141 | 2.95 ± 0.26 a | 3.25 ± 0.25 a |
| 6-Octenal, 3,7-dimethyl-, (R)- | 1166 | ND | 1.55 ± 0.01 a |
| Decanal | 1243 | ND | 4.79 ± 0.34 a |
| Perillal | 1342 | ND | 2.09 ± 0.41 a |
| Subtotal | 17.30 ± 2.06 b | 37.50 ± 3.15 a | |
| Alkenes | |||
| α-Pinene | 949 | 2.61 ± 0.08 a | 2.34 ± 0.30 a |
| Sabinen | 978 | 6.39 ± 0.81 a | 7.76 ± 0.65 a |
| β-Myrcene | 993 | 8.41 ± 1.20 b | 15.23 ± 0.87 a |
| (+)-4-Carene | 1013 | 1.31 ± 0.24 a | 1.58 ± 0.26 a |
| D-Limonene | 1025 | 545.86 ± 86.26 b | 900.98 ± 37.92 a |
| β-cis-Ocimene | 1044 | ND | 0.53 ± 0.01 a |
| γ-Terpinene | 1052 | 0.72 ± 0.17 b | 1.78 ± 0.29 a |
| p-Cymenene | 1084 | 1.38 ± 0.21 b | 3.24 ± 0.57 a |
| 1,3,8-p-Menthatriene | 1120 | 5.15 ± 0.02 b | 8.53 ± 0.61 a |
| Cosmene | 1229 | ND | 8.35 ± 0.67 a |
| 2,6-Octadiene, 2,6-dimethyl- | 1493 | ND | 3.14 ± 0.14 a |
| cis-β-Farnesene | 1450 | ND | 2.03 ± 0.30 a |
| Valencen | 1477 | 3.49 ± 0.50 a | 3.96 ± 0.88 a |
| Subtotal | 575.34 ± 87.02 b | 959.46 ± 41.11 a | |
| Others | |||
| 3,9-Epoxy-1-p-menthene | 1206 | ND | 1.41 ± 0.33 a |
| 3-Cyclohexen-1-ol, 5-methylene-6-(1-methylethenyl)- | 1210 | 4.25 ± 0.68 a | ND |
| Subtotal | 4.25 ± 0.68 a | 1.41 ± 0.33 b | |
| Total | 686.43 ± 86.05 b | 1152.89 ± 48.38 a |
| Compound Names | RI | WT | MT |
|---|---|---|---|
| Esters | |||
| Acetic acid, octyl ester | 1257 | 50.05 ± 2.99 a | 27.74 ± 5.52 b |
| Myrtenyl acetate | 1321 | 21.76 ± 3.74 a | 12.03 ± 0.90 b |
| cis-Carvyl acetate | 1337 | 251.02 ± 18.52 a | 202.37 ± 17.19 b |
| Carveol acetate | 1363 | 257.52 ± 22.93 a | 190.21 ± 9.20 b |
| Acetic acid, decyl ester | 1421 | 22.49 ± 2.52 a | 8.36 ± 1.27 b |
| Subtotal | 602.85 ± 47.68 a | 440.71 ± 29.40 b | |
| Alcohols | |||
| cis-4-Thujanol | 1062 | 102.86 ± 9.78 a | 47.87 ± 3.05 b |
| 1-Octanol | 1148 | 79.81 ± 0.54 a | 45.34 ± 0.76 b |
| Linalool B | 1104 | 3029.12 ± 102.04 a | 1672.41 ± 30.03 b |
| (E)-p-2,8-Menthadien-1-ol | 1122 | 101.26 ± 1.91 a | 39.46 ± 1.65 b |
| α-Terpineol | 1216 | 288.43 ± 13.09 a | 119.62 ± 1.01 b |
| cis-Carveol | 1261 | 125.32 ± 15.76 a | 61.50 ± 0.06 b |
| Carveol | 1280 | 67.41 ± 2.64 a | 39.62 ± 3.02 b |
| Citronellol | 1285 | 413.74 ± 24.51 a | 277.96 ± 5.39 b |
| trans-Nerolidol | 1562 | 9.73 ± 1.03 a | ND |
| Spathulenol | 1564 | 41.32 ± 3.86 a | 23.50 ± 2.74 b |
| Subtotal | 4258.98 ± 172.74 a | 2327.27 ± 38.17 b | |
| Ketones | |||
| Thujone | 1116 | 128.00 ± 2.42 a | 81.74 ± 3.46 b |
| Pinocarvone | 1173 | 11.63 ± 0.15 a | 6.10 ± 0.33 b |
| trans-3-Pinanone | 1188 | 50.07 ± 4.52 a | 32.56 ± 2.12 b |
| Dihydrocarvone | 1223 | 182.12 ± 3.26 a | 151.74 ± 11.44 b |
| D-Carvone | 1300 | 2750.73 ± 104.41 a | 2101.84 ± 26.89 b |
| Subtotal | 3122.55 ± 105.71 a | 2373.98 ± 43.21 b | |
| Aldehydes | |||
| Hexanal | 782 | 405.11 ± 11.27 a | 342.72 ± 1.32 b |
| 2-Hexenal, (E)- | 874 | 257.03 ± 4.35 a | 200.45 ± 7.20 b |
| Octanal | 1037 | 563.99 ± 1.55 a | 334.55 ± 16.76 b |
| Nonanal | 1144 | 79.17 ± 2.17 a | 49.61 ± 5.67 b |
| 6-Octenal, 3,7-dimethyl-, (R)- | 1167 | 391.79 ± 10.39 a | 253.91 ± 11.54 b |
| Decanal | 1244 | 466.33 ± 58.70 a | 295.44 ± 21.88 b |
| Perillal | 1342 | 370.09 ± 14.68 a | 227.24 ± 3.71 b |
| Citral | 1345 | 49.54 ± 2.08 a | 20.61 ± 1.78 b |
| Subtotal | 2583.06 ± 93.40 a | 1724.52 ± 63.39 b | |
| Alkenes | |||
| β-Thujene | 946 | 65.86 ± 2.46 a | 40.43 ± 3.91 b |
| (-)-α-Pinene | 949 | 245.76 ± 0.05 a | 166.05 ± 21.90 b |
| Sabinen | 979 | 1870.80 ± 15.86 a | 1242.81 ± 135.03 b |
| β-Myrcene | 995 | 1382.55 ± 28.33 a | 964.00 ± 93.37 b |
| (+)-4-Carene | 1018 | 29.54 ± 0.63 b | 38.19 ± 3.68 a |
| D-Limonene | 1037 | 44,406.08 ± 898.83 a | 31,706.31 ± 2936.91 b |
| β-cis-Ocimene | 1038 | 5.95 ± 0.26 b | 12.30 ± 2.98 a |
| β-Ocimene | 1046 | 96.31 ± 5.37 a | 45.80 ± 4.18 b |
| γ-Terpinene | 1054 | 57.76 ± 3.16 a | 33.97 ± 2.85 b |
| Terpinolene | 1084 | 62.92 ± 4.88 a | 41.47 ± 2.77 b |
| Cosmene | 1110 | 9.77 ± 0.64 a | 5.10 ± 0.47 b |
| Limonene oxide, cis- | 1137 | 452.88 ± 34.18 a | 370.48 ± 15.88 b |
| cis-p-Mentha-2,8-dien-1-ol | 1140 | 116.42 ± 4.04 a | 39.45 ± 0.84 b |
| (+)-(E)-Limonene oxide | 1143 | 426.38 ± 16.03 a | 377.07 ± 7.14 b |
| Camphene | 1238 | 28.80 ± 1.05 a | 11.31 ± 0.39 b |
| α-Cubebene | 1330 | 31.73 ± 3.37 a | 13.80 ± 1.39 b |
| 2,6-Octadiene, 2,6-dimethyl- | 1494 | 337.99 ± 29.39 a | 227.06 ± 15.49 b |
| 3-Carene | 1513 | 261.51 ± 22.36 a | 152.95 ± 11.15 b |
| β-Cubebene | 1371 | 60.45 ± 4.61 a | 29.72 ± 3.79 b |
| β-Elemene | 1374 | 77.00 ± 7.89 a | 37.20 ± 2.16 b |
| 4-Tetradecene, (E)- | 1382 | 13.86 ± 1.42 a | 6.21 ± 0.99 b |
| Zingiberene | 1393 | 78.20 ± 7.88 a | 35.54 ± 3.63 b |
| Aromandendrene | 1418 | 10.40 ± 1.54 a | 2.83 ± 0.36 b |
| α-Guaiene | 1422 | 4.62 ± 0.17 a | ND |
| Humulene | 1433 | 31.81 ± 3.02 a | 12.82 ± 0.90 b |
| cis-β-Farnesene | 1445 | 96.99 ± 9.13 a | 37.84 ± 4.83 b |
| (E)-β-Famesene | 1452 | 1286.95 ± 122.95 a | 524.50 ± 17.50 b |
| β-Copaene | 1464 | 26.75 ± 3.26 a | 8.02 ± 0.53 b |
| Longipinene | 1470 | 12.43 ± 1.38 a | ND |
| Curcumene | 1474 | 27.38 ± 2.57 a | 12.49 ± 0.84 b |
| Valencen | 1478 | 343.80 ± 37.64 a | 186.18 ± 8.84 b |
| Bicyclogermacrene | 1481 | 77.20 ± 14.63 a | 35.78 ± 2.93 b |
| α-Muurolene | 1488 | 25.44 ± 3.06 a | 13.70 ± 1.09 b |
| α-Bulnesene | 1492 | 9.24 ± 0.70 a | 4.49 ± 0.30 b |
| Pentadecane | 1499 | 6.01 ± 0.60 a | 4.64 ± 0.18 b |
| γ-Muurolene | 1501 | 42.09 ± 4.28 a | 17.29 ± 0.12 b |
| trans-Calamenene | 1511 | 11.04 ± 0.05 a | 4.07 ± 0.67 b |
| δ-Cadinene | 1513 | 104.62 ± 10.18 a | 40.58 ± 5.42 b |
| Sesquiphellandrene | 1516 | 54.70 ± 5.69 a | 15.57 ± 2.66 b |
| α-Farnesene | 1523 | 7.42 ± 0.25 a | 4.19 ± 0.22 b |
| Caryophyllene oxide | 1568 | 29.96 ± 2.15 a | 15.44 ± 1.32 b |
| Subtotal | 52,327.33 ± 1133.87 a | 36,537.65 ± 3204.70 b | |
| Total | 62,894.77 ± 1535.96 a | 43,404.12 ± 3330.26 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Xiong, B.; Zheng, Z.; Qin, Z.; Deng, L.; Zheng, W.; Zhang, M.; Sun, G.; He, S.; Wang, J.; et al. Natural Variation Confers ‘Aiyuan 38’ Citrus Mutant a New Color and Unique Flavor. Int. J. Mol. Sci. 2023, 24, 8816. https://doi.org/10.3390/ijms24108816
Wang T, Xiong B, Zheng Z, Qin Z, Deng L, Zheng W, Zhang M, Sun G, He S, Wang J, et al. Natural Variation Confers ‘Aiyuan 38’ Citrus Mutant a New Color and Unique Flavor. International Journal of Molecular Sciences. 2023; 24(10):8816. https://doi.org/10.3390/ijms24108816
Chicago/Turabian StyleWang, Tie, Bo Xiong, Zhendong Zheng, Zeyu Qin, Lijun Deng, Wei Zheng, Mingfei Zhang, Guochao Sun, Siya He, Jun Wang, and et al. 2023. "Natural Variation Confers ‘Aiyuan 38’ Citrus Mutant a New Color and Unique Flavor" International Journal of Molecular Sciences 24, no. 10: 8816. https://doi.org/10.3390/ijms24108816
APA StyleWang, T., Xiong, B., Zheng, Z., Qin, Z., Deng, L., Zheng, W., Zhang, M., Sun, G., He, S., Wang, J., & Wang, Z. (2023). Natural Variation Confers ‘Aiyuan 38’ Citrus Mutant a New Color and Unique Flavor. International Journal of Molecular Sciences, 24(10), 8816. https://doi.org/10.3390/ijms24108816






