Caveolin-1 and Atherosclerosis: Regulation of LDLs Fate in Endothelial Cells
Abstract
1. Introduction
1.1. Caveolae, Caveolin-1 and Atherosclerosis
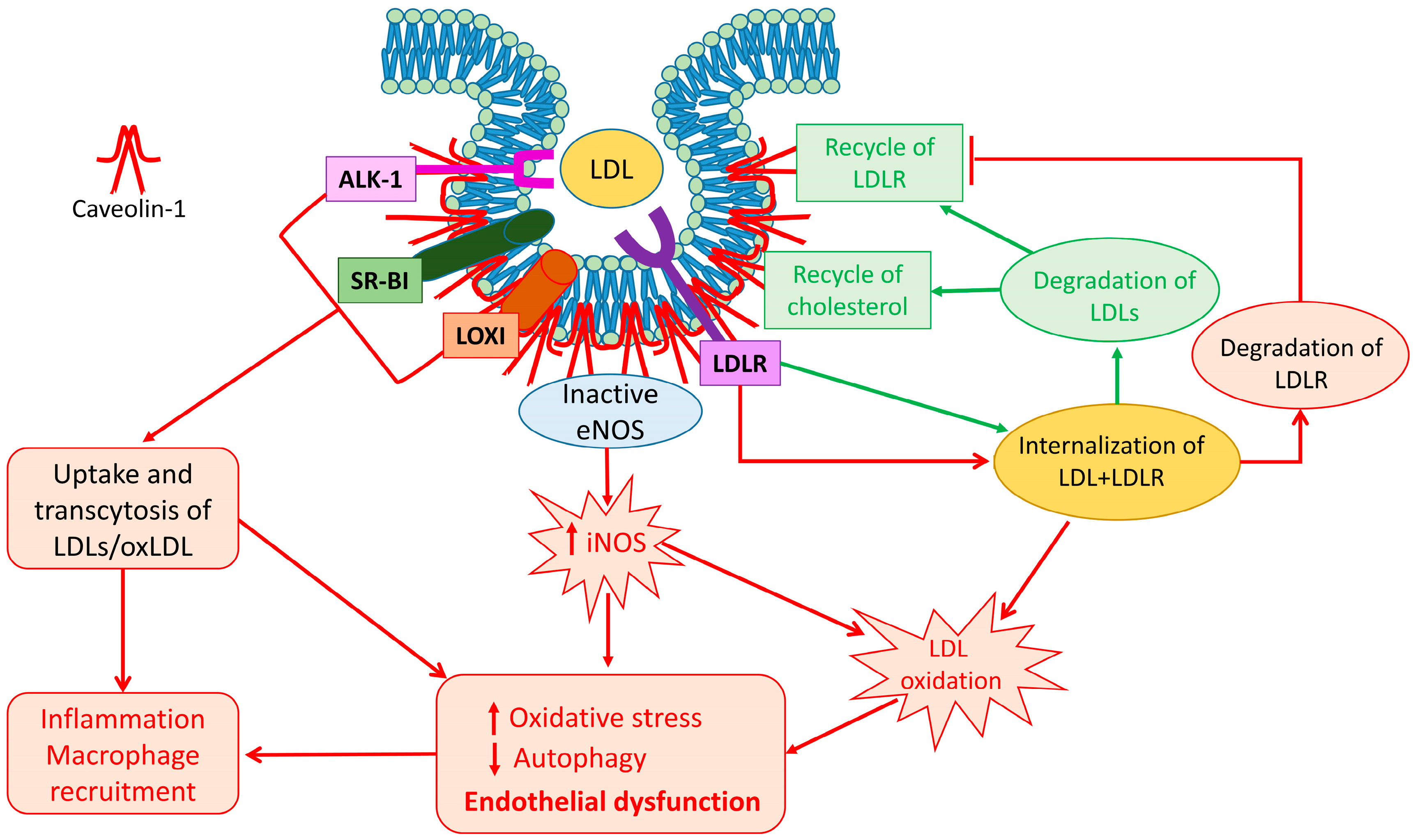
1.2. Cav-1 and LDL Uptake
| Endothelial Cell Function | Role of Caveolin-1 | References |
|---|---|---|
| eNOS Activity | Interaction between eNOS and Cav-1 results in inactivation of eNOS | [23,24,25,26] |
| Autophagy | Cav-1 affects expression of LC3BII | [31,32] |
| Cav-1 affects intracellular localization of ATG5 | ||
| Absence of Cav-1 promotes autophagy | ||
| Cholesterol Control | Cav-1 is involved in cholesterol exchange between lipoproteins and plasma membrane | [34,35] |
| Cav-1 may regulate intracellular cholesterol levels by mediating LDL endocytosis | ||
| Cav-1 mediates transport of cholesterol from ER to plasma membrane | ||
| Senescence | Cav-1 expression is increased in senescent cells. Cav-1 is involved in degradation of LDLR | [45,46] |
| LDLs Trafficking | Rise in Cav-1 expression increases oxidative stress and vascular permeability | [46,47,48,49,50,59] |
| Cav-1 is involved in endocytosis and transcytosis of LDLs | ||
| Cav-1 is involved in degradation of LDLR |
1.3. Cav-1 and LDL Metabolism
1.4. Cav-1 and LDL Transcytosis
2. Conclusions
3. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kovtun, O.; Tillu, V.A.; Ariotti, N.; Parton, R.G.; Collins, B.M. Cavin family proteins and the assembly of caveolae. J. Cell Sci. 2015, 128, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Li, X.A.; Everson, W.V.; Smart, E.J. Caveolae, lipid rafts, and vascular disease. Trends Cardiovasc. Med. 2005, 15, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Parton, R.G.; del Pozo, M.A. Caveolae as plasma membrane sensors, protectors and organizers. Nat. Rev. Mol. Cell Biol. 2013, 14, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.M.; Lisanti, M.P. The caveolin proteins. Genome Biol. 2004, 5, 214. [Google Scholar] [CrossRef] [PubMed]
- Couet, J.; Li, S.; Okamoto, T.; Ikezu, T.; Lisanti, M.P. Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J. Biol. Chem. 1997, 272, 6525–6533. [Google Scholar] [CrossRef] [PubMed]
- Zimnicka, A.M.; Husain, Y.S.; Shajahan, A.N.; Sverdlov, M.; Chaga, O.; Chen, Z.; Toth, P.T.; Klomp, J.; Karginov, A.V.; Tiruppathi, C.; et al. Src-dependent phosphorylation of caveolin-1 Tyr-14 promotes swelling and release of caveolae. Mol. Biol. Cell 2016, 27, 2090–2106. [Google Scholar] [CrossRef]
- Gokani, S.; Bhatt, L.K. Caveolin-1: A Promising Therapeutic Target for Diverse Diseases. Curr. Mol. Pharmacol. 2022, 15, 701–715. [Google Scholar] [CrossRef]
- Hou, K.; Li, S.; Zhang, M.; Qin, X. Caveolin-1 in autophagy: A potential therapeutic target in atherosclerosis. Clin. Chim. Acta Int. J. Clin. Chem. 2021, 513, 25–33. [Google Scholar] [CrossRef]
- Libby, P.; Tabas, I.; Fredman, G.; Fisher, E.A. Inflammation and its resolution as determinants of acute coronary syndromes. Circ. Res. 2014, 114, 1867–1879. [Google Scholar] [CrossRef]
- Mundi, S.; Massaro, M.; Scoditti, E.; Carluccio, M.A.; van Hinsbergh, V.W.M.; Iruela-Arispe, M.L.; De Caterina, R. Endothelial permeability, LDL deposition, and cardiovascular risk factors-a review. Cardiovasc. Res. 2018, 114, 35–52. [Google Scholar] [CrossRef]
- Robbins, C.S.; Hilgendorf, I.; Weber, G.F.; Theurl, I.; Iwamoto, Y.; Figueiredo, J.L.; Gorbatov, R.; Sukhova, G.K.; Gerhardt, L.M.; Smyth, D.; et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nat. Med. 2013, 19, 1166–1172. [Google Scholar] [CrossRef]
- Nakajima, A.; Libby, P.; Mitomo, S.; Yuki, H.; Araki, M.; Seegers, L.M.; McNulty, I.; Lee, H.; Ishibashi, M.; Kobayashi, K.; et al. Biomarkers associated with coronary high-risk plaques. J. Thromb. Thrombolysis 2022, 54, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.W.; Lin, Y.C.; Chang, T.Y.; Tsai, S.H.; Ho, H.C.; Chen, Y.T.; Yang, V.C. Caveolin-1 expression is associated with plaque formation in hypercholesterolemic rabbits. J. Histochem. Cytochem. 2006, 54, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Feo, J.A.; Hellings, W.E.; Moll, F.L.; De Vries, J.P.; van Middelaar, B.J.; Algra, A.; Sluijter, J.; Velema, E.; van den Broek, T.; Sessa, W.C.; et al. Caveolin-1 influences vascular protease activity and is a potential stabilizing factor in human atherosclerotic disease. PLoS ONE 2008, 3, e2612. [Google Scholar] [CrossRef] [PubMed]
- Schwencke, C.; Schmeisser, A.; Walter, C.; Wachter, R.; Pannach, S.; Weck, B.; Braun-Dullaeus, R.C.; Kasper, M.; Strasser, R.H. Decreased caveolin-1 in atheroma: Loss of antiproliferative control of vascular smooth muscle cells in atherosclerosis. Cardiovasc. Res. 2005, 68, 128–135. [Google Scholar] [CrossRef]
- Montecucco, F.; Lenglet, S.; Gayet-Ageron, A.; Bertolotto, M.; Pelli, G.; Palombo, D.; Pane, B.; Spinella, G.; Steffens, S.; Raffaghello, L.; et al. Systemic and intraplaque mediators of inflammation are increased in patients symptomatic for ischemic stroke. Stroke 2010, 41, 1394–1404. [Google Scholar] [CrossRef]
- Liang, M.; Puri, A.; Devlin, G. The vulnerable plaque: The real villain in acute coronary syndromes. Open Cardiovasc. Med. J. 2011, 5, 123–129. [Google Scholar] [CrossRef]
- Frank, P.G.; Lisanti, M.P. Caveolin-1 and caveolae in atherosclerosis: Differential roles in fatty streak formation and neointimal hyperplasia. Curr. Opin. Lipidol. 2004, 15, 523–529. [Google Scholar] [CrossRef]
- Frank, P.G.; Lee, H.; Park, D.S.; Tandon, N.N.; Scherer, P.E.; Lisanti, M.P. Genetic ablation of caveolin-1 confers protection against atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 98–105. [Google Scholar] [CrossRef]
- Fernandez-Hernando, C.; Yu, J.; Suarez, Y.; Rahner, C.; Davalos, A.; Lasuncion, M.A.; Sessa, W.C. Genetic evidence supporting a critical role of endothelial caveolin-1 during the progression of atherosclerosis. Cell Metab. 2009, 10, 48–54. [Google Scholar] [CrossRef]
- Fernandez-Hernando, C.; Yu, J.; Davalos, A.; Prendergast, J.; Sessa, W.C. Endothelial-specific overexpression of caveolin-1 accelerates atherosclerosis in apolipoprotein E-deficient mice. Am. J. Pathol. 2010, 177, 998–1003. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A., Jr.; Garcia-Cardena, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.J.; Restini, C.B.; Lunardi, C.N.; Neto Mdos, A.; Moreira, J.E.; Bendhack, L.M. Decreased number of caveolae in endothelial cells impairs the relaxation induced by acetylcholine in hypertensive rat aortas. Eur. J. Pharmacol. 2010, 627, 251–257. [Google Scholar] [CrossRef]
- Garcia-Cardena, G.; Martasek, P.; Masters, B.S.; Skidd, P.M.; Couet, J.; Li, S.; Lisanti, M.P.; Sessa, W.C. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J. Biol. Chem. 1997, 272, 25437–25440. [Google Scholar] [CrossRef] [PubMed]
- Michel, J.B.; Feron, O.; Sacks, D.; Michel, T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J. Biol. Chem. 1997, 272, 15583–15586. [Google Scholar] [CrossRef] [PubMed]
- Su, Y. Regulation of endothelial nitric oxide synthase activity by protein-protein interaction. Curr. Pharm. Des. 2014, 20, 3514–3520. [Google Scholar] [CrossRef]
- Ramirez, C.M.; Zhang, X.; Bandyopadhyay, C.; Rotllan, N.; Sugiyama, M.G.; Aryal, B.; Liu, X.; He, S.; Kraehling, J.R.; Ulrich, V.; et al. Caveolin-1 Regulates Atherogenesis by Attenuating Low-Density Lipoprotein Transcytosis and Vascular Inflammation Independently of Endothelial Nitric Oxide Synthase Activation. Circulation 2019, 140, 225–239. [Google Scholar] [CrossRef]
- Bharath, L.P.; Mueller, R.; Li, Y.; Ruan, T.; Kunz, D.; Goodrich, R.; Mills, T.; Deeter, L.; Sargsyan, A.; Anandh Babu, P.V.; et al. Impairment of autophagy in endothelial cells prevents shear-stress-induced increases in nitric oxide bioavailability. Can. J. Physiol. Pharmacol. 2014, 92, 605–612. [Google Scholar] [CrossRef]
- Fetterman, J.L.; Holbrook, M.; Flint, N.; Feng, B.; Breton-Romero, R.; Linder, E.A.; Berk, B.D.; Duess, M.A.; Farb, M.G.; Gokce, N.; et al. Restoration of autophagy in endothelial cells from patients with diabetes mellitus improves nitric oxide signaling. Atherosclerosis 2016, 247, 207–217. [Google Scholar] [CrossRef]
- Takagaki, Y.; Lee, S.M.; Dongqing, Z.; Kitada, M.; Kanasaki, K.; Koya, D. Endothelial autophagy deficiency induces IL6—Dependent endothelial mesenchymal transition and organ fibrosis. Autophagy 2020, 16, 1905–1914. [Google Scholar] [CrossRef]
- Bai, X.; Yang, X.; Jia, X.; Rong, Y.; Chen, L.; Zeng, T.; Deng, X.; Li, W.; Wu, G.; Wang, L.; et al. CAV1-CAVIN1-LC3B-mediated autophagy regulates high glucose-stimulated LDL transcytosis. Autophagy 2020, 16, 1111–1129. [Google Scholar] [CrossRef]
- Zhang, X.; Ramirez, C.M.; Aryal, B.; Madrigal-Matute, J.; Liu, X.; Diaz, A.; Torrecilla-Parra, M.; Suarez, Y.; Cuervo, A.M.; Sessa, W.C.; et al. Cav-1 (Caveolin-1) Deficiency Increases Autophagy in the Endothelium and Attenuates Vascular Inflammation and Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1510–1522. [Google Scholar] [CrossRef]
- Vasile, E.; Simionescu, M.; Simionescu, N. Visualization of the binding, endocytosis, and transcytosis of low-density lipoprotein in the arterial endothelium in situ. J. Cell Biol. 1983, 96, 1677–1689. [Google Scholar] [CrossRef]
- Frank, P.G.; Cheung, M.W.; Pavlides, S.; Llaverias, G.; Park, D.S.; Lisanti, M.P. Caveolin-1 and regulation of cellular cholesterol homeostasis. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H677–H686. [Google Scholar] [CrossRef]
- Tan, M.; Ye, J.; Zhao, M.; Ke, X.; Huang, K.; Liu, H. Recent developments in the regulation of cholesterol transport by natural molecules. Phytother. Res. PTR 2021, 35, 5623–5633. [Google Scholar] [CrossRef]
- Qin, L.; Zhu, N.; Ao, B.X.; Liu, C.; Shi, Y.N.; Du, K.; Chen, J.X.; Zheng, X.L.; Liao, D.F. Caveolae and Caveolin-1 Integrate Reverse Cholesterol Transport and Inflammation in Atherosclerosis. Int. J. Mol. Sci. 2016, 17, 429. [Google Scholar] [CrossRef]
- Cao, S.; Fernandez-Zapico, M.E.; Jin, D.; Puri, V.; Cook, T.A.; Lerman, L.O.; Zhu, X.Y.; Urrutia, R.; Shah, V. KLF11-mediated repression antagonizes Sp1/sterol-responsive element-binding protein-induced transcriptional activation of caveolin-1 in response to cholesterol signaling. J. Biol. Chem. 2005, 280, 1901–1910. [Google Scholar] [CrossRef]
- Babitt, J.; Trigatti, B.; Rigotti, A.; Smart, E.J.; Anderson, R.G.; Xu, S.; Krieger, M. Murine SR-BI, a high density lipoprotein receptor that mediates selective lipid uptake, is N-glycosylated and fatty acylated and colocalizes with plasma membrane caveolae. J. Biol. Chem. 1997, 272, 13242–13249. [Google Scholar] [CrossRef]
- Acton, S.; Rigotti, A.; Landschulz, K.T.; Xu, S.; Hobbs, H.H.; Krieger, M. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 1996, 271, 518–520. [Google Scholar] [CrossRef]
- Rhainds, D.; Brodeur, M.; Lapointe, J.; Charpentier, D.; Falstrault, L.; Brissette, L. The role of human and mouse hepatic scavenger receptor class B type I (SR-BI) in the selective uptake of low-density lipoprotein-cholesteryl esters. Biochemistry 2003, 42, 7527–7538. [Google Scholar] [CrossRef]
- Matveev, S.; van der Westhuyzen, D.R.; Smart, E.J. Co-expression of scavenger receptor-BI and caveolin-1 is associated with enhanced selective cholesteryl ester uptake in THP-1 macrophages. J. Lipid Res. 1999, 40, 1647–1654. [Google Scholar] [CrossRef]
- Wang, L.; Connelly, M.A.; Ostermeyer, A.G.; Chen, H.H.; Williams, D.L.; Brown, D.A. Caveolin-1 does not affect SR-BI-mediated cholesterol efflux or selective uptake of cholesteryl ester in two cell lines. J. Lipid Res. 2003, 44, 807–815. [Google Scholar] [CrossRef]
- Truong, T.Q.; Aubin, D.; Bourgeois, P.; Falstrault, L.; Brissette, L. Opposite effect of caveolin-1 in the metabolism of high-density and low-density lipoproteins. Biochim. Biophys. Acta 2006, 1761, 24–36. [Google Scholar] [CrossRef]
- Arai, T.; Wang, N.; Bezouevski, M.; Welch, C.; Tall, A.R. Decreased atherosclerosis in heterozygous low density lipoprotein receptor-deficient mice expressing the scavenger receptor BI transgene. J. Biol. Chem. 1999, 274, 2366–2371. [Google Scholar] [CrossRef]
- Voghel, G.; Thorin-Trescases, N.; Farhat, N.; Nguyen, A.; Villeneuve, L.; Mamarbachi, A.M.; Fortier, A.; Perrault, L.P.; Carrier, M.; Thorin, E. Cellular senescence in endothelial cells from atherosclerotic patients is accelerated by oxidative stress associated with cardiovascular risk factors. Mech. Ageing Dev. 2007, 128, 662–671. [Google Scholar] [CrossRef]
- Zou, H.; Stoppani, E.; Volonte, D.; Galbiati, F. Caveolin-1, cellular senescence and age-related diseases. Mech. Ageing Dev. 2011, 132, 533–542. [Google Scholar] [CrossRef]
- Jiang, R.; Cai, J.; Zhu, Z.; Chen, D.; Wang, J.; Wang, Q.; Teng, Y.; Huang, Y.; Tao, M.; Xia, A.; et al. Hypoxic trophoblast HMGB1 induces endothelial cell hyperpermeability via the TRL-4/caveolin-1 pathway. J. Immunol. 2014, 193, 5000–5012. [Google Scholar] [CrossRef]
- Wang, F.; Song, X.; Zhou, M.; Wei, L.; Dai, Q.; Li, Z.; Lu, N.; Guo, Q. Wogonin inhibits H2O2-induced vascular permeability through suppressing the phosphorylation of caveolin-1. Toxicology 2013, 305, 10–19. [Google Scholar] [CrossRef]
- Barale, C.; Melchionda, E.; Morotti, A.; Russo, I. PCSK9 Biology and Its Role in Atherothrombosis. Int. J. Mol. Sci. 2021, 22, 5880. [Google Scholar] [CrossRef]
- Jang, H.D.; Lee, S.E.; Yang, J.; Lee, H.C.; Shin, D.; Lee, H.; Lee, J.; Jin, S.; Kim, S.; Lee, S.J.; et al. Cyclase-associated protein 1 is a binding partner of proprotein convertase subtilisin/kexin type-9 and is required for the degradation of low-density lipoprotein receptors by proprotein convertase subtilisin/kexin type-9. Eur. Heart J. 2020, 41, 239–252. [Google Scholar] [CrossRef]
- Santibanez, J.F.; Blanco, F.J.; Garrido-Martin, E.M.; Sanz-Rodriguez, F.; del Pozo, M.A.; Bernabeu, C. Caveolin-1 interacts and cooperates with the transforming growth factor-beta type I receptor ALK1 in endothelial caveolae. Cardiovasc. Res. 2008, 77, 791–799. [Google Scholar] [CrossRef]
- Tao, B.; Kraehling, J.R.; Ghaffari, S.; Ramirez, C.M.; Lee, S.; Fowler, J.W.; Lee, W.L.; Fernandez-Hernando, C.; Eichmann, A.; Sessa, W.C. BMP-9 and LDL crosstalk regulates ALK-1 endocytosis and LDL transcytosis in endothelial cells. J. Biol. Chem. 2020, 295, 18179–18188. [Google Scholar] [CrossRef]
- Brown, M.S.; Goldstein, J.L. Receptor-mediated endocytosis: Insights from the lipoprotein receptor system. Proc. Natl. Acad. Sci. USA 1979, 76, 3330–3337. [Google Scholar] [CrossRef]
- Yamamoto, T.; Davis, C.G.; Brown, M.S.; Schneider, W.J.; Casey, M.L.; Goldstein, J.L.; Russell, D.W. The human LDL receptor: A cysteine-rich protein with multiple Alu sequences in its mRNA. Cell 1984, 39, 27–38. [Google Scholar] [CrossRef]
- Brown, M.S.; Goldstein, J.L. A receptor-mediated pathway for cholesterol homeostasis. Science 1986, 232, 34–47. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Brown, M.S. The LDL receptor. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 431–438. [Google Scholar] [CrossRef]
- Bist, A.; Fielding, P.E.; Fielding, C.J. Two sterol regulatory element-like sequences mediate up-regulation of caveolin gene transcription in response to low density lipoprotein free cholesterol. Proc. Natl. Acad. Sci. USA 1997, 94, 10693–10698. [Google Scholar] [CrossRef]
- Meddings, J.B.; Dietschy, J.M. Regulation of plasma levels of low-density lipoprotein cholesterol: Interpretation of data on low-density lipoprotein turnover in man. Circulation 1986, 74, 805–814. [Google Scholar] [CrossRef]
- Pavlides, S.; Gutierrez-Pajares, J.L.; Iturrieta, J.; Lisanti, M.P.; Frank, P.G. Endothelial caveolin-1 plays a major role in the development of atherosclerosis. Cell Tissue Res. 2014, 356, 147–157. [Google Scholar] [CrossRef]
- Kosenko, T.; Golder, M.; Leblond, G.; Weng, W.; Lagace, T.A. Low density lipoprotein binds to proprotein convertase subtilisin/kexin type-9 (PCSK9) in human plasma and inhibits PCSK9-mediated low density lipoprotein receptor degradation. J. Biol. Chem. 2013, 288, 8279–8288. [Google Scholar] [CrossRef]
- Kraehling, J.R.; Chidlow, J.H.; Rajagopal, C.; Sugiyama, M.G.; Fowler, J.W.; Lee, M.Y.; Zhang, X.; Ramirez, C.M.; Park, E.J.; Tao, B.; et al. Genome-wide RNAi screen reveals ALK1 mediates LDL uptake and transcytosis in endothelial cells. Nat. Commun. 2016, 7, 13516. [Google Scholar] [CrossRef] [PubMed]
- Mehta, J.L.; Chen, J.; Hermonat, P.L.; Romeo, F.; Novelli, G. Lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1): A critical player in the development of atherosclerosis and related disorders. Cardiovasc. Res. 2006, 69, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Adachi, H.; Tsujimoto, M. Endothelial scavenger receptors. Prog. Lipid Res. 2006, 45, 379–404. [Google Scholar] [CrossRef] [PubMed]
- Calvo, D.; Gomez-Coronado, D.; Suarez, Y.; Lasuncion, M.A.; Vega, M.A. Human CD36 is a high affinity receptor for the native lipoproteins HDL, LDL, and VLDL. J. Lipid Res. 1998, 39, 777–788. [Google Scholar] [CrossRef]
- Sun, S.W.; Zu, X.Y.; Tuo, Q.H.; Chen, L.X.; Lei, X.Y.; Li, K.; Tang, C.K.; Liao, D.F. Caveolae and caveolin-1 mediate endocytosis and transcytosis of oxidized low density lipoprotein in endothelial cells. Acta Pharmacol. Sin. 2010, 31, 1336–1342. [Google Scholar] [CrossRef]
- Matarazzo, S.; Quitadamo, M.C.; Mango, R.; Ciccone, S.; Novelli, G.; Biocca, S. Cholesterol-lowering drugs inhibit lectin-like oxidized low-density lipoprotein-1 receptor function by membrane raft disruption. Mol. Pharmacol. 2012, 82, 246–254. [Google Scholar] [CrossRef]
- Li, D.; Mehta, J.L. Upregulation of endothelial receptor for oxidized LDL (LOX-1) by oxidized LDL and implications in apoptosis of human coronary artery endothelial cells: Evidence from use of antisense LOX-1 mRNA and chemical inhibitors. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Le Master, E.; Paul, A.; Lazarko, D.; Aguilar, V.; Ahn, S.J.; Lee, J.C.; Minshall, R.D.; Levitan, I. Caveolin-1 is a primary determinant of endothelial stiffening associated with dyslipidemia, disturbed flow, and ageing. Sci. Rep. 2022, 12, 17822. [Google Scholar] [CrossRef]
- Henderson, J.M.; Weber, C.; Santovito, D. Beyond Self-Recycling: Cell-Specific Role of Autophagy in Atherosclerosis. Cells 2021, 10, 625. [Google Scholar] [CrossRef]
- Islam, M.M.; Hlushchenko, I.; Pfisterer, S.G. Low-Density Lipoprotein Internalization, Degradation and Receptor Recycling Along Membrane Contact Sites. Front. Cell Dev. Biol. 2022, 10, 826379. [Google Scholar] [CrossRef]
- Yang, H.X.; Zhang, M.; Long, S.Y.; Tuo, Q.H.; Tian, Y.; Chen, J.X.; Zhang, C.P.; Liao, D.F. Cholesterol in LDL receptor recycling and degradation. Clin. Chim. Acta Int. J. Clin. Chem. 2020, 500, 81–86. [Google Scholar] [CrossRef]
- Torisu, K.; Singh, K.K.; Torisu, T.; Lovren, F.; Liu, J.; Pan, Y.; Quan, A.; Ramadan, A.; Al-Omran, M.; Pankova, N.; et al. Intact endothelial autophagy is required to maintain vascular lipid homeostasis. Aging Cell 2016, 15, 187–191. [Google Scholar] [CrossRef]
- Trinh, M.N.; Brown, M.S.; Goldstein, J.L.; Han, J.; Vale, G.; McDonald, J.G.; Seemann, J.; Mendell, J.T.; Lu, F. Last step in the path of LDL cholesterol from lysosome to plasma membrane to ER is governed by phosphatidylserine. Proc. Natl. Acad. Sci. USA 2020, 117, 18521–18529. [Google Scholar] [CrossRef]
- Chang, T.Y.; Li, B.L.; Chang, C.C.; Urano, Y. Acyl-coenzyme A: Cholesterol acyltransferases. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E1–E9. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Leake, D.S. Low density lipoprotein undergoes oxidation within lysosomes in cells. Circ. Res. 2007, 100, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Yancey, P.G.; Jerome, W.G. Lysosomal cholesterol derived from mildly oxidized low density lipoprotein is resistant to efflux. J. Lipid Res. 2001, 42, 317–327. [Google Scholar] [CrossRef]
- Lin, F.; Pei, L.; Zhang, Q.; Han, W.; Jiang, S.; Lin, Y.; Dong, B.; Cui, L.; Li, M. Ox-LDL induces endothelial cell apoptosis and macrophage migration by regulating caveolin-1 phosphorylation. J. Cell. Physiol. 2018, 233, 6683–6692. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Gao, X.; Wang, R.; Sun, J.; Guo, B.; Wei, R.; Jia, Y. Adiponectin inhibits proliferation of vascular endothelial cells induced by Ox-LDL by promoting dephosphorylation of Caveolin-1 and depolymerization of eNOS and up-regulating release of NO. Int. Immunopharmacol. 2019, 73, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Wahid, A.; Chen, W.; Wang, X.; Tang, X. High-mobility group box 1 serves as an inflammation driver of cardiovascular disease. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 139, 111555. [Google Scholar] [CrossRef]
- Puddu, A.; Sanguineti, R.; Maggi, D. Caveolin-1 Down-Regulation Reduces VEGF-A Secretion Induced by IGF-1 in ARPE-19 Cells. Life 2021, 12, 44. [Google Scholar] [CrossRef]
- Gliozzi, M.; Scicchitano, M.; Bosco, F.; Musolino, V.; Carresi, C.; Scarano, F.; Maiuolo, J.; Nucera, S.; Maretta, A.; Paone, S.; et al. Modulation of Nitric Oxide Synthases by Oxidized LDLs: Role in Vascular Inflammation and Atherosclerosis Development. Int. J. Mol. Sci. 2019, 20, 3294. [Google Scholar] [CrossRef] [PubMed]
- Frank, P.G.; Pavlides, S.; Lisanti, M.P. Caveolae and transcytosis in endothelial cells: Role in atherosclerosis. Cell Tissue Res. 2009, 335, 41–47. [Google Scholar] [CrossRef]
- Predescu, S.A.; Predescu, D.N.; Malik, A.B. Molecular determinants of endothelial transcytosis and their role in endothelial permeability. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 293, L823–L842. [Google Scholar] [CrossRef]
- Tiruppathi, C.; Naqvi, T.; Wu, Y.; Vogel, S.M.; Minshall, R.D.; Malik, A.B. Albumin mediates the transcytosis of myeloperoxidase by means of caveolae in endothelial cells. Proc. Natl. Acad. Sci. USA 2004, 101, 7699–7704. [Google Scholar] [CrossRef] [PubMed]
- Frank, P.G.; Pavlides, S.; Cheung, M.W.; Daumer, K.; Lisanti, M.P. Role of caveolin-1 in the regulation of lipoprotein metabolism. Am. J. Physiol. Cell Physiol. 2008, 295, C242–C248. [Google Scholar] [CrossRef] [PubMed]
- Bian, F.; Yang, X.; Zhou, F.; Wu, P.H.; Xing, S.; Xu, G.; Li, W.; Chi, J.; Ouyang, C.; Zhang, Y.; et al. C-reactive protein promotes atherosclerosis by increasing LDL transcytosis across endothelial cells. Br. J. Pharmacol. 2014, 171, 2671–2684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, X.; Bian, F.; Wu, P.; Xing, S.; Xu, G.; Li, W.; Chi, J.; Ouyang, C.; Zheng, T.; et al. TNF-alpha promotes early atherosclerosis by increasing transcytosis of LDL across endothelial cells: Crosstalk between NF-kappaB and PPAR-gamma. J. Mol. Cell. Cardiol. 2014, 72, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.L.; Yang, X.Y.; Li, J.Y.; Ye, L.; Jia, X.; Xiong, Z.F.; Wang, Y.M.; Jin, S. Cavin-1 regulates caveolae-mediated LDL transcytosis: Crosstalk in an AMPK/eNOS/NF-kappaB/Sp1 loop. Oncotarget 2017, 8, 103985–103995. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, X.; Yang, X.; Bai, X.; Lu, Y.; Zhu, L.; Cheng, W.; Shu, M.; Zhu, Y.; Du, X.; et al. Deacetylation of Caveolin-1 by Sirt6 induces autophagy and retards high glucose-stimulated LDL transcytosis and atherosclerosis formation. Metab. Clin. Exp. 2022, 131, 155162. [Google Scholar] [CrossRef]
- Bai, X.; Jia, X.; Lu, Y.; Zhu, L.; Zhao, Y.; Cheng, W.; Shu, M.; Jin, S. Salidroside-Mediated Autophagic Targeting of Active Src and Caveolin-1 Suppresses Low-Density Lipoprotein Transcytosis across Endothelial Cells. Oxidative Med. Cell. Longev. 2020, 2020, 9595036. [Google Scholar] [CrossRef] [PubMed]
- Bian, F.; Cui, J.; Zheng, T.; Jin, S. Reactive oxygen species mediate angiotensin II-induced transcytosis of low-density lipoprotein across endothelial cells. Int. J. Mol. Med. 2017, 39, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fernandez-Hernando, C. The Janus-faced role of SR-BI in atherosclerosis. Nat. Metab. 2019, 1, 586–587. [Google Scholar] [CrossRef]
- Davis, C.G.; Lehrman, M.A.; Russell, D.W.; Anderson, R.G.; Brown, M.S.; Goldstein, J.L. The J.D. mutation in familial hypercholesterolemia: Amino acid substitution in cytoplasmic domain impedes internalization of LDL receptors. Cell 1986, 45, 15–24. [Google Scholar] [CrossRef]
- Tolleshaug, H.; Hobgood, K.K.; Brown, M.S.; Goldstein, J.L. The LDL receptor locus in familial hypercholesterolemia: Multiple mutations disrupt transport and processing of a membrane receptor. Cell 1983, 32, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.M.; Sugiyama, M.G.; Fung, K.Y.; Gao, Y.; Wang, C.; Levy, A.S.; Azizi, P.; Roufaiel, M.; Zhu, S.N.; Neculai, D.; et al. A novel assay uncovers an unexpected role for SR-BI in LDL transcytosis. Cardiovasc. Res. 2015, 108, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Chambliss, K.L.; Gao, X.; Yuhanna, I.S.; Behling-Kelly, E.; Bergaya, S.; Ahmed, M.; Michaely, P.; Luby-Phelps, K.; Darehshouri, A.; et al. SR-B1 drives endothelial cell LDL transcytosis via DOCK4 to promote atherosclerosis. Nature 2019, 569, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Kakava, S.; Schlumpf, E.; Panteloglou, G.; Tellenbach, F.; von Eckardstein, A.; Robert, J. Brain Endothelial Cells in Contrary to the Aortic Do Not Transport but Degrade Low-Density Lipoproteins via Both LDLR and ALK1. Cells 2022, 11, 3044. [Google Scholar] [CrossRef] [PubMed]
- Andreone, B.J.; Chow, B.W.; Tata, A.; Lacoste, B.; Ben-Zvi, A.; Bullock, K.; Deik, A.A.; Ginty, D.D.; Clish, C.B.; Gu, C. Blood-Brain Barrier Permeability Is Regulated by Lipid Transport-Dependent Suppression of Caveolae-Mediated Transcytosis. Neuron 2017, 94, 581–594.e5. [Google Scholar] [CrossRef]
- Lisanti, M.P.; Scherer, P.E.; Vidugiriene, J.; Tang, Z.; Hermanowski-Vosatka, A.; Tu, Y.H.; Cook, R.F.; Sargiacomo, M. Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: Implications for human disease. J. Cell Biol. 1994, 126, 111–126. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puddu, A.; Montecucco, F.; Maggi, D. Caveolin-1 and Atherosclerosis: Regulation of LDLs Fate in Endothelial Cells. Int. J. Mol. Sci. 2023, 24, 8869. https://doi.org/10.3390/ijms24108869
Puddu A, Montecucco F, Maggi D. Caveolin-1 and Atherosclerosis: Regulation of LDLs Fate in Endothelial Cells. International Journal of Molecular Sciences. 2023; 24(10):8869. https://doi.org/10.3390/ijms24108869
Chicago/Turabian StylePuddu, Alessandra, Fabrizio Montecucco, and Davide Maggi. 2023. "Caveolin-1 and Atherosclerosis: Regulation of LDLs Fate in Endothelial Cells" International Journal of Molecular Sciences 24, no. 10: 8869. https://doi.org/10.3390/ijms24108869
APA StylePuddu, A., Montecucco, F., & Maggi, D. (2023). Caveolin-1 and Atherosclerosis: Regulation of LDLs Fate in Endothelial Cells. International Journal of Molecular Sciences, 24(10), 8869. https://doi.org/10.3390/ijms24108869







