The Role of Inflammation in Age-Associated Changes in Red Blood System
Abstract
1. Introduction
2. Results
2.1. Participants and Body Composition
2.2. Hematological Variables
2.3. Biochemical Variables
2.4. Inflammatory Variables
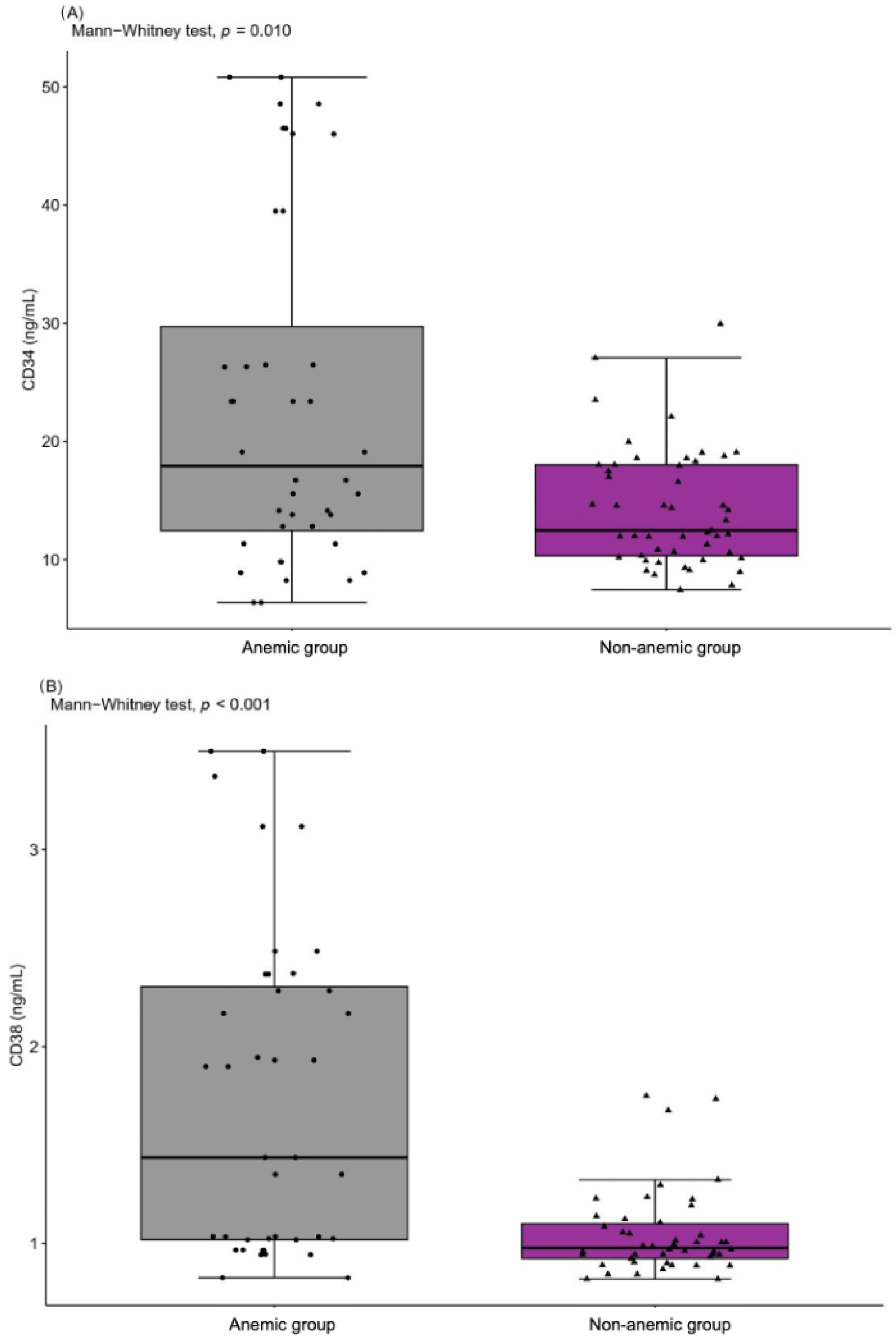
2.5. Peripheral Blood Mononuclear Cells
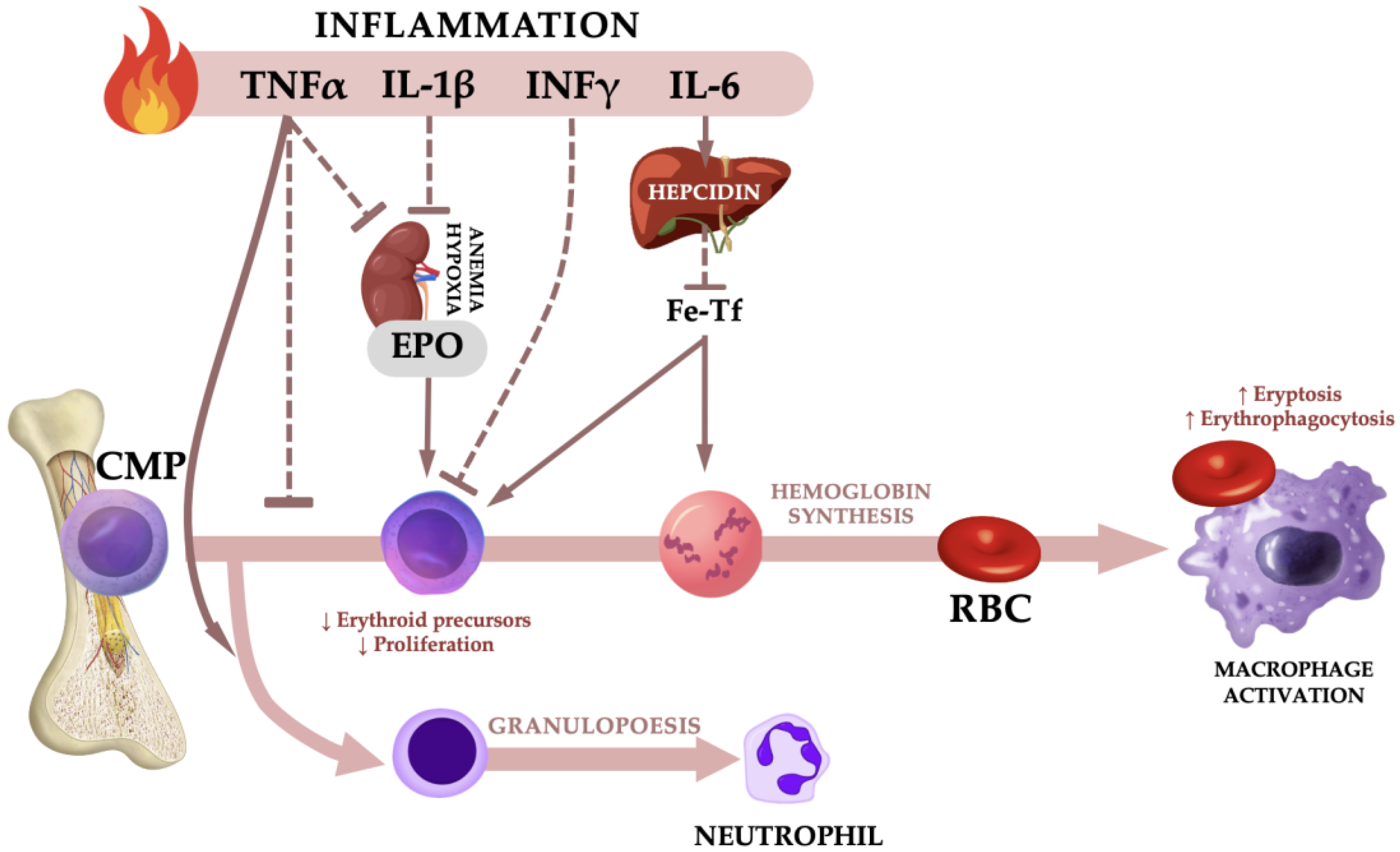
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Blood Sampling
4.3. Hematological Variables
4.4. Biochemical Variables
4.5. Inflammatory Variables and Peripheral Blood Mononuclear Cells
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shavelle, R.M.; MacKenzie, R.; Paculdo, D.R. Anemia and Mortality in Older Persons: Does the Type of Anemia Affect Survival? Int. J. Hematol. 2012, 95, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Beutler, E.; Waalen, J. The Definition of Anemia: What Is the Lower Limit of Normal of the Blood Hemoglobin Concentration? Blood 2006, 107, 1747–1750. [Google Scholar] [CrossRef] [PubMed]
- Stauder, R.; Valent, P.; Theurl, I. Anemia at Older Age: Etiologies, Clinical Implications, and Management. Blood 2018, 131, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Zakai, N.A.; Katz, R.; Hirsch, C.; Shlipak, M.G.; Chaves, P.H.M.; Newman, A.B.; Cushman, M. A Prospective Study of Anemia Status, Hemoglobin Concentration, and Mortality in an Elderly Cohort: The Cardiovascular Health Study. Arch. Intern. Med. 2005, 165, 2214–2220. [Google Scholar] [CrossRef] [PubMed]
- Culleton, B.F.; Manns, B.J.; Zhang, J.; Tonelli, M.; Klarenbach, S.; Hemmelgarn, B.R. Impact of Anemia on Hospitalization and Mortality in Older Adults. Blood 2006, 107, 3841–3846. [Google Scholar] [CrossRef] [PubMed]
- Wouters, H.J.C.M.; van der Klauw, M.M.; de Witte, T.; Stauder, R.; Swinkels, D.W.; Wolffenbuttel, B.H.R.; Huls, G. Association of Anemia with Health-Related Quality of Life and Survival: A Large Population-Based Cohort Study. Haematologica 2019, 104, 468–476. [Google Scholar] [CrossRef]
- Girelli, D.; Marchi, G.; Camaschella, C. Anemia in the Elderly. Hemasphere 2018, 2, e40. [Google Scholar] [CrossRef]
- Cappellini, M.D.; Motta, I. Anemia in Clinical Practice-Definition and Classification: Does Hemoglobin Change with Aging? Semin. Hematol. 2015, 52, 261–269. [Google Scholar] [CrossRef]
- Penninx, B.W.J.H.; Guralnik, J.M.; Onder, G.; Ferrucci, L.; Wallace, R.B.; Pahor, M. Anemia and Decline in Physical Performance among Older Persons. Am. J. Med. 2003, 115, 104–110. [Google Scholar] [CrossRef]
- Katsumi, A.; Abe, A.; Tamura, S.; Matsushita, T. Anemia in Older Adults as a Geriatric Syndrome: A Review. Geriatr. Gerontol. Int. 2021, 21, 549–554. [Google Scholar] [CrossRef]
- Woodman, R.; Ferrucci, L.; Guralnik, J. Anemia in Older Adults. Curr. Opin. Hematol. 2005, 12, 123–128. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Eisenstaedt, R.S.; Ferrucci, L.; Klein, H.G.; Woodman, R.C. Prevalence of Anemia in Persons 65 Years and Older in the United States: Evidence for a High Rate of Unexplained Anemia. Blood 2004, 104, 2263–2268. [Google Scholar] [CrossRef] [PubMed]
- Lanier, J.B.; Park, J.J.; Callahan, R.C. Anemia in Older Adults. Am. Fam. Physician 2018, 98, 437–442. [Google Scholar]
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69 (Suppl. S1), S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Wawrzyniak-Gramacka, E.; Hertmanowska, N.; Tylutka, A.; Morawin, B.; Wacka, E.; Gutowicz, M.; Zembron-Lacny, A. The Association of Anti-Inflammatory Diet Ingredients and Lifestyle Exercise with Inflammaging. Nutrients 2021, 13, 3696. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-Aging: An Evolutionary Perspective on Immunosenescence. Ann. N. Y. Acad. Sci. 2006, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Minciullo, P.L.; Catalano, A.; Mandraffino, G.; Casciaro, M.; Crucitti, A.; Maltese, G.; Morabito, N.; Lasco, A.; Gangemi, S.; Basile, G. Inflammaging and Anti-Inflammaging: The Role of Cytokines in Extreme Longevity. Arch. Immunol. Ther. Exp. 2016, 64, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Bissinger, R.; Bhuyan, A.A.M.; Qadri, S.M.; Lang, F. Oxidative Stress, Eryptosis and Anemia: A Pivotal Mechanistic Nexus in Systemic Diseases. FEBS J. 2019, 286, 826–854. [Google Scholar] [CrossRef]
- Weiss, G.; Ganz, T.; Goodnough, L.T. Anemia of Inflammation. Blood 2019, 133, 40–50. [Google Scholar] [CrossRef]
- Muckenthaler, M.U.; Rivella, S.; Hentze, M.W.; Galy, B. A Red Carpet for Iron Metabolism. Cell 2017, 168, 344–361. [Google Scholar] [CrossRef]
- La Ferla, K.; Reimann, C.; Jelkmann, W.; Hellwig-Bürgel, T. Inhibition of Erythropoietin Gene Expression Signaling Involves the Transcription Factors GATA-2 and NF-KappaB. FASEB J. 2002, 16, 1811–1813. [Google Scholar] [CrossRef] [PubMed]
- Rivkin, M.; Simerzin, A.; Zorde-Khvalevsky, E.; Chai, C.; Yuval, J.B.; Rosenberg, N.; Harari-Steinfeld, R.; Schneider, R.; Amir, G.; Condiotti, R.; et al. Inflammation-Induced Expression and Secretion of MicroRNA 122 Leads to Reduced Blood Levels of Kidney-Derived Erythropoietin and Anemia. Gastroenterology 2016, 151, 999–1010.e3. [Google Scholar] [CrossRef] [PubMed]
- Krajewski, J.; Batmunkh, C.; Jelkmann, W.; Hellwig-Bürgel, T. Interleukin-1beta Inhibits the Hypoxic Inducibility of the Erythropoietin Enhancer by Suppressing Hepatocyte Nuclear Factor-4alpha. Cell. Mol. Life Sci. 2007, 64, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, S.; Dai, C.H.; Price, J.O.; Krantz, S.B. Interferon Gamma Downregulates Stem Cell Factor and Erythropoietin Receptors but Not Insulin-like Growth Factor-I Receptors in Human Erythroid Colony-Forming Cells. Blood 1997, 90, 2244–2252. [Google Scholar] [CrossRef] [PubMed]
- Penninx, B.W.J.H.; Pluijm, S.M.F.; Lips, P.; Woodman, R.; Miedema, K.; Guralnik, J.M.; Deeg, D.J.H. Late-Life Anemia Is Associated with Increased Risk of Recurrent Falls. J. Am. Geriatr. Soc. 2005, 53, 2106–2111. [Google Scholar] [CrossRef]
- Penninx, B.W.J.H.; Pahor, M.; Cesari, M.; Corsi, A.M.; Woodman, R.C.; Bandinelli, S.; Guralnik, J.M.; Ferrucci, L. Anemia Is Associated with Disability and Decreased Physical Performance and Muscle Strength in the Elderly. J. Am. Geriatr. Soc. 2004, 52, 719–724. [Google Scholar] [CrossRef]
- Penninx, B.W.J.H.; Pahor, M.; Woodman, R.C.; Guralnik, J.M. Anemia in Old Age Is Associated with Increased Mortality and Hospitalization. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 474–479. [Google Scholar] [CrossRef]
- Stauder, R.; Thein, S.L. Anemia in the Elderly: Clinical Implications and New Therapeutic Concepts. Haematologica 2014, 99, 1127–1130. [Google Scholar] [CrossRef]
- Alvarez-Payares, J.C.; Rivera-Arismendy, S.; Ruiz-Bravo, P.; Sánchez-Salazar, S.M.; Manzur, R.A.; Ramirez-Urrea, S.I.; Puello, A. Unexplained Anemia in the Elderly. Cureus 2021, 13, e19971. [Google Scholar] [CrossRef]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation. World Health Organ. Tech. Rep. Ser. 2000, 894, 1–253. [Google Scholar]
- Wagner, K.-H.; Cameron-Smith, D.; Wessner, B.; Franzke, B. Biomarkers of Aging: From Function to Molecular Biology. Nutrients 2016, 8, E338. [Google Scholar] [CrossRef]
- Wyczalkowska-Tomasik, A.; Czarkowska-Paczek, B.; Zielenkiewicz, M.; Paczek, L. Inflammatory Markers Change with Age, but Do Not Fall Beyond Reported Normal Ranges. Arch. Immunol. Ther. Exp. 2016, 64, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Koelman, L.; Pivovarova-Ramich, O.; Pfeiffer, A.F.H.; Grune, T.; Aleksandrova, K. Cytokines for Evaluation of Chronic Inflammatory Status in Ageing Research: Reliability and Phenotypic Characterisation. Immun. Ageing 2019, 16, 11. [Google Scholar] [CrossRef]
- Ferrucci, L.; Semba, R.D.; Guralnik, J.M.; Ershler, W.B.; Bandinelli, S.; Patel, K.V.; Sun, K.; Woodman, R.C.; Andrews, N.C.; Cotter, R.J.; et al. Proinflammatory State, Hepcidin, and Anemia in Older Persons. Blood 2010, 115, 3810–3816. [Google Scholar] [CrossRef] [PubMed]
- Jelkmann, W. Proinflammatory Cytokines Lowering Erythropoietin Production. J. Interferon Cytokine Res. 1998, 18, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.V. Epidemiology of Anemia in Older Adults. Semin. Hematol. 2008, 45, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.G. The Sex Difference in Haemoglobin Levels in Adults—Mechanisms, Causes, and Consequences. Blood Rev. 2014, 28, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Lipschitz, D.A.; Mitchell, C.O.; Thompson, C. The Anemia of Senescence. Am. J. Hematol. 1981, 11, 47–54. [Google Scholar] [CrossRef]
- den Elzen, W.P.J.; Willems, J.M.; Westendorp, R.G.J.; de Craen, A.J.M.; Blauw, G.J.; Ferrucci, L.; Assendelft, W.J.J.; Gussekloo, J. Effect of Erythropoietin Levels on Mortality in Old Age: The Leiden 85-plus Study. Can. Med. Assoc. J. 2010, 182, 1953–1958. [Google Scholar] [CrossRef]
- den Elzen, W.P.J.; de Craen, A.J.M.; Wiegerinck, E.T.; Westendorp, R.G.J.; Swinkels, D.W.; Gussekloo, J. Plasma Hepcidin Levels and Anemia in Old Age. The Leiden 85-Plus Study. Haematologica 2013, 98, 448–454. [Google Scholar] [CrossRef]
- Kroot, J.J.C.; Tjalsma, H.; Fleming, R.E.; Swinkels, D.W. Hepcidin in Human Iron Disorders: Diagnostic Implications. Clin. Chem. 2011, 57, 1650–1669. [Google Scholar] [CrossRef]
- Traglia, M.; Girelli, D.; Biino, G.; Campostrini, N.; Corbella, M.; Sala, C.; Masciullo, C.; Viganò, F.; Buetti, I.; Pistis, G.; et al. Association of HFE and TMPRSS6 Genetic Variants with Iron and Erythrocyte Parameters Is Only in Part Dependent on Serum Hepcidin Concentrations. J. Med. Genet. 2011, 48, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Galesloot, T.E.; Vermeulen, S.H.; Geurts-Moespot, A.J.; Klaver, S.M.; Kroot, J.J.; van Tienoven, D.; Wetzels, J.F.M.; Kiemeney, L.A.L.M.; Sweep, F.C.; den Heijer, M.; et al. Serum Hepcidin: Reference Ranges and Biochemical Correlates in the General Population. Blood 2011, 117, e218–e225. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, L.; Bengtsson, C.; Lapidus, L.; Lindstedt, G.; Lundberg, P.A.; Hultén, L. Screening for Iron Deficiency: An Analysis Based on Bone-Marrow Examinations and Serum Ferritin Determinations in a Population Sample of Women. Br. J. Haematol. 1993, 85, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Mast, A.E.; Blinder, M.A.; Gronowski, A.M.; Chumley, C.; Scott, M.G. Clinical Utility of the Soluble Transferrin Receptor and Comparison with Serum Ferritin in Several Populations. Clin. Chem. 1998, 44, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.H.; Tan, H.L.; Lai, H.C.; Kuperan, P. Accuracy of Various Iron Parameters in the Prediction of Iron Deficiency in an Acute Care Hospital. Ann.-Acad. Med. Singap. 2005, 34, 437–440. [Google Scholar] [PubMed]
- Guyatt, G.H.; Patterson, C.; Ali, M.; Singer, J.; Levine, M.; Turpie, I.; Meyer, R. Diagnosis of Iron-Deficiency Anemia in the Elderly. Am. J. Med. 1990, 88, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Rohr, M.; Brandenburg, V.; Brunner-La Rocca, H.-P. How to Diagnose Iron Deficiency in Chronic Disease: A Review of Current Methods and Potential Marker for the Outcome. Eur. J. Med. Res. 2023, 28, 15. [Google Scholar] [CrossRef] [PubMed]
- Pei, X. Who Is Hematopoietic Stem Cell: CD34+ or CD34−? Int. J. Hematol. 1999, 70, 213–215. [Google Scholar]
- Naeim, F.; Nagesh Rao, P.; Song, S.X.; Phan, R.T. Principles of Immunophenotyping. In Atlas of Hematopathology; Elsevier: Amsterdam, The Netherlands, 2018; pp. 29–56. ISBN 978-0-12-809843-1. [Google Scholar]
- Salati, S.; Zini, R.; Bianchi, E.; Testa, A.; Mavilio, F.; Manfredini, R.; Ferrari, S. Role of CD34 Antigen in Myeloid Differentiation of Human Hematopoietic Progenitor Cells. Stem Cells 2008, 26, 950–959. [Google Scholar] [CrossRef] [PubMed][Green Version]
- AlbeniZ, I.; Türker-Şener, L.; Baş, A.; KaleliOğlu, İ.; Nurten, R. Isolation of Hematopoietic Stem Cells and the Effect of CD38 Expression during the Early Erythroid Progenitor Cell Development Process. Oncol. Lett. 2012, 3, 55–60. [Google Scholar] [CrossRef]
- Jiang, Z.; Wu, D.; Lin, S.; Li, P. CD34 and CD38 Are Prognostic Biomarkers for Acute B Lymphoblastic Leukemia. Biomark Res. 2016, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- Jing, D.; Wobus, M.; Poitz, D.M.; Bornhauser, M.; Ehninger, G.; Ordemann, R. Oxygen Tension Plays a Critical Role in the Hematopoietic Microenvironment in Vitro. Haematologica 2012, 97, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Boslett, J.; Hemann, C.; Christofi, F.L.; Zweier, J.L. Characterization of CD38 in the Major Cell Types of the Heart: Endothelial Cells Highly Express CD38 with Activation by Hypoxia-Reoxygenation Triggering NAD(P)H Depletion. Am. J. Physiol.-Cell Physiol. 2018, 314, C297–C309. [Google Scholar] [CrossRef] [PubMed]
- Bruserud, Ø.; Vo, A.K.; Rekvam, H. Hematopoiesis, Inflammation and Aging—The Biological Background and Clinical Impact of Anemia and Increased C-Reactive Protein Levels on Elderly Individuals. J. Clin. Med. 2022, 11, 706. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.r-project.org/ (accessed on 18 April 2023).
| Anemic n = 47 | Non-Anemic n = 66 | Anemic vs. Non-Anemic p Level | |
|---|---|---|---|
| Mean ± SD (Me) | Mean ± SD (Me) | ||
| Age (years) | 77.3 ± 5.0 (78.0) | 71.8 ± 7.6 (71.0) | <0.001 |
| Weight (kg) | 74.7 ± 12.7 (73.0) | 69.7 ± 10.5 (67.5) | <0.001 |
| Height (cm) | 167.3 ± 6.5 (168.0) | 160.2 ± 6.0 (159.0) | <0.001 |
| BMI (kg/m2) | 26.6 ± 3.6 (26.1) | 27.3 ± 3.7 (27.1) | 0.765 |
| Reference Values | Anemic n = 47 | Non-Anemic n = 66 | Anemic vs. Non-Anemic p Level | |
|---|---|---|---|---|
| Mean ± SD (Me) | Mean ± SD (Me) | |||
| WBC (103/µL) | 4.0–10.2 | 6.37 ± 2.12 (5.67) | 6.25 ± 1.72 (6.08) | 0.937 |
| Neutrophils (103/µL) | 2.0–6.9 | 4.14 ± 1.87 (3.90) | 3.59 ± 1.44 (3.29) | 0.052 |
| Lymphocytes (103/µL) | 0.6–3.4 | 1.80 ± 0.77 (1.66) | 1.89 ± 0.63 (1.84) | 0.207 |
| Monocytes (103/µL) | 0.00–0.90 | 0.44 ± 0.19 (0.42) | 0.56 ± 0.29 (0.51) | 0.055 |
| RBC (106/µL) | F 4.0–5.5 M 4.5–6.6 | 4.20 ± 0.39 (4.24) | 4.69 ± 0.53 (4.64) | <0.001 |
| Hb (g/dL) | F 12.5–16.0 M 13.5–18.0 | 12.50 ± 0.98 (12.70) | 14.26 ± 0.90 (14.05) | <0.001 |
| Hct% | F 37–47 M 40.0–51.0 | 36.25 ± 3.56 (37.0) | 42.58 ± 2.87 (42.25) | <0.001 |
| MCV (fL) | 82–92 | 86.42 ± 6.30 (88.00) | 91.30 ± 4.99 (91.95) | <0.001 |
| MCH (pg) | 27–33 | 29.81 ± 1.56 (29.80) | 30.58 ± 1.61 (30.75) | <0.001 |
| MCHC (g/dL) | 32–36 | 34.61 ± 1.93 (33.70) | 33.52 ± 1.16 (33.35) | 0.015 |
| RDW% | 11.5–14.5 | 14.56 ± 2.44 (14.30) | 14.75 ± 0.98 (14.85) | <0.001 |
| Platelets (103/µL) | 140–420 | 219 ± 56 (211) | 229 ± 57 (226) | 0.417 |
| MPV (fL) | 7.5–10.5 | 8.85 ± 1.77 (9.10) | 7.26 ± 1.16 (7.11) | <0.001 |
| Iron (µg/dL) | F 37–145 M 58–158 | 78.22 ± 24.76 (76.20) | 104.21 ± 30.27 (101.60) | <0.001 |
| Ferritin (ng/mL) | F 10–200 M 15–400 | 16.04 ± 21.82 (8.60) | 38.27 ± 44.91 (23.43) | <0.001 |
| Tf (mg/dL) | 200–400 | 393 ± 184 (306) | 302 ± 99 (298) | 0.096 |
| TfS% | 20–50 | 23.40 ± 11.24 (21.14) | 37.09 ± 15.90 (37.07) | <0.001 |
| EPO (mU/mL) | 4.3–29 | 12.77 ± 4.32 (11.02) | 8.05 ± 3.04 (7.24) | 0.197 |
| Reference Values | Anemic n = 47 | Non-Anemic n = 66 | Anemic vs. Non-Anemic p Level | |
|---|---|---|---|---|
| Mean ± SD (Me) | Mean ± SD (Me) | |||
| TG (mg/dL) | <150 | 114.43 ± 36.69 (117.40) | 143.78 ± 31.77 (139.23) | <0.001 |
| TC (mg/dL) | <200 | 194.80 ± 48.87 (187.96) | 228.90 ± 38.67 (228.70) | <0.001 |
| LDL (mg/dL) | <130 | 91.37 ± 35.93 (90.58) | 87.89 ± 30.84 (89.05) | 0.864 |
| oxLDL (mg/dL) | – | 0.031 ± 0.034 (0.016) | 0.038 ± 0.041 (0.014) | 0.822 |
| HDL (mg/dL) | desirable > 60 | 61.25 ± 13.74 (60.70) | 71.48 ± 19.49 (69.59) | <0.001 |
| non-HDL (mg/dL) | <130 | 133.56 ± 45.62 (125.16) | 157.42 ± 43.37 (160.04) | <0.001 |
| Glucose (mg/dL) | 60–115 | 94.44 ± 28.17 (86.50) | 93.33 ± 20.61 (93.40) | <0.001 |
| Bilirubin (mg/dL) | <1.0 | 0.24 ± 0.18 (0.22) | 0.12 ± 0.13 (0.07) | <0.001 |
| Anemic n = 47 | Non-Anemic n = 66 | Anemic vs. Non-Anemic p Level | |
|---|---|---|---|
| Mean ± SD (Me) | Mean ± SD (Me) | ||
| CRP (mg/L) | 3.12 ± 2.34 (3.12) | 2.92 ± 2.29 (2.54) | 0.843 |
| NPT (nmol/L) | 23.16 ± 11.30 (22.88) | 19.95 ± 5.86 (19.95) | 0.205 |
| HPC (ng/mL) | 16.84 ± 13.88 (15.60) | 6.42 ± 3.29 (6.08) | <0.001 |
| IL-1β (ng/mL) | 11.69 ± 9.83 (8.19) | 2.55 ± 3.64 (1.21) | <0.001 |
| IL-6 (pg/mL) | 97.22 ± 68.28 (75.78) | 74.98 ± 27.27 (74.72) | 0.195 |
| TNFα (ng/mL) | 121.50 ± 69.82 (112.00) | 85.65 ± 59.64 (82.41) | <0.001 |
| Variables | AUC | Cut-off Value | Sensitivity (%) | Specificity (%) | OR | 95%CI |
|---|---|---|---|---|---|---|
| Iron (µg/dL) | 0.745 | 88.6 | 68.2 | 72.3 | 5.510 | 2.292–13.937 |
| Ferritin (ng/mL) | 0.652 | 16.4 | 60.6 | 74.5 | 4.424 | 1.845–11.193 |
| Tf (mg/mL) | 0.622 | 3.2 | 78.4 | 48.9 | 3.422 | 1.208–10.546 |
| TfS (mg/dL) | 0.756 | 32.5 | 62.2 | 83.0 | 7.76 | 2.64–25.23 |
| EPO (mU/mL) | 0.583 | 8.48 | 59.1 | 48.9 | 0.725 | 0.318–1.643 |
| HPC (ng/mL) | 0.735 | 9.4 | 84.8 | 59.6 | 8.069 | 3.131–22.397 |
| IL-1β (ng/mL) | 0.922 | 5.3 | 87.9 | 91.5 | 72.374 | 19.688–354.366 |
| IL-6 (pg/mL) | 0.523 | 75.5 | 65.2 | 51.1 | 1.939 | 0.848–4.489 |
| TNFα (ng/mL) | 0.685 | 97.7 | 74.2 | 66.0 | 5.488 | 2.292–13.741 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wacka, E.; Wawrzyniak-Gramacka, E.; Tylutka, A.; Morawin, B.; Gutowicz, M.; Zembron-Lacny, A. The Role of Inflammation in Age-Associated Changes in Red Blood System. Int. J. Mol. Sci. 2023, 24, 8944. https://doi.org/10.3390/ijms24108944
Wacka E, Wawrzyniak-Gramacka E, Tylutka A, Morawin B, Gutowicz M, Zembron-Lacny A. The Role of Inflammation in Age-Associated Changes in Red Blood System. International Journal of Molecular Sciences. 2023; 24(10):8944. https://doi.org/10.3390/ijms24108944
Chicago/Turabian StyleWacka, Eryk, Edyta Wawrzyniak-Gramacka, Anna Tylutka, Barbara Morawin, Marzena Gutowicz, and Agnieszka Zembron-Lacny. 2023. "The Role of Inflammation in Age-Associated Changes in Red Blood System" International Journal of Molecular Sciences 24, no. 10: 8944. https://doi.org/10.3390/ijms24108944
APA StyleWacka, E., Wawrzyniak-Gramacka, E., Tylutka, A., Morawin, B., Gutowicz, M., & Zembron-Lacny, A. (2023). The Role of Inflammation in Age-Associated Changes in Red Blood System. International Journal of Molecular Sciences, 24(10), 8944. https://doi.org/10.3390/ijms24108944






